Abstract
A satisfactory account of human cognitive evolution will explain not only the psychological mechanisms that make our species unique, but also how, when, and why these traits evolved. To date, researchers have made substantial progress toward defining uniquely human aspects of cognition, but considerably less effort has been devoted to questions about the evolutionary processes through which these traits have arisen. In this article, I aim to link these complementary aims by synthesizing recent advances in our understanding of what makes human cognition unique, with theory and data regarding the processes of cognitive evolution. I review evidence that uniquely human cognition depends on synergism between both representational and motivational factors and is unlikely to be accounted for by changes to any singular cognitive system. I argue that, whereas no nonhuman animal possesses the full constellation of traits that define the human mind, homologies and analogies of critical aspects of human psychology can be found in diverse nonhuman taxa. I suggest that phylogenetic approaches to the study of animal cognition—which can address questions about the selective pressures and proximate mechanisms driving cognitive change—have the potential to yield important insights regarding the processes through which the human cognitive phenotype evolved.
Keywords: cognitive evolution, human evolution, comparative psychology, human uniqueness, cognition
Human minds seem unlike those of any other species. We participate in large-scale institutions, wage wars over beliefs, imagine the distant future, and communicate about these processes using syntax and symbols. What aspects of human cognition allow us to accomplish these seemingly unique feats, and are these processes qualitatively different from those of other animals? Equally importantly, how and why did such a peculiar psychology evolve? What was it about early human lifestyles that favored these flexible forms of cognition, and how did natural selection sculpt these features from a nonhuman ape-like foundation? The questions above address different levels of explanation (1, 2) for human cognitive uniqueness, but ultimately a satisfactory account of human cognitive evolution will explain not only the mechanisms that make our species unique, but also how, when, and why these traits evolved. To date, scientists have made substantial progress toward defining uniquely human aspects of cognition, but considerably less effort has been devoted to questions about the evolutionary processes through which these traits have arisen. In this article, I aim to link these unique but complementary aims by first highlighting recent advances in our understanding of how human psychology differs from that of other extant taxa. I then turn to the less well-understood questions of how, when, and why these traits evolved and underscore the importance of understanding evolutionary processes, not just their products, for a comprehensive understanding of human cognitive evolution.
What Makes Human Cognition Unique?
At first glance, the cognitive differences between humans and all other animals seem to be enormous. Humans alone do calculus, travel in machines with global positioning systems, search for life beyond our planet, and store information about how to do so in digital repositories accessible around the world. But none of these feats are hardwired in the human brain, nor were any of them invented de novo by a single enterprising individual. Instead, all of these accomplishments depended on the accretion of thousands of years of incremental progress and a cognitive and cultural system that allowed (and motivated) individuals to acquire and transmit accumulated knowledge and skills (3). Tomasello and Rakoczy (4) highlight this point by noting that “if we imagine a human child born onto a desert island, somehow magically kept alive by itself until adulthood, it is possible that this adult’s cognitive skills would not differ very much—perhaps a little, but not very much—from those of other great apes (ref. 4, p. 121)”. Therefore, there is an important distinction between trying to understand how cumulative culture has influenced the collective cognition of our species and trying to understand how humans became such prolifically cultural beings in the first place.
For clues regarding the origins of human cultural cognition, scientists have turned attention to human development, and the fundamental aspects of human cognition that allow us to communicate with, share, and acquire information from others (5). These processes emerge early in human ontogeny and are supported by a nascent understanding of others as intentional agents. Within the first year of life, human children begin to relate to others in new ways, tuning into others’ attention through processes such as gaze following and exchanging information with others through simple acts of referential gesture (6). These basic skills for communication and shared attention provide the social foundation for a variety of forms of cultural learning, including the initial stages of language acquisition (7, 8). For example, by 2 y of age, these perspective-taking skills allow human children to make pragmatic inferences linking new words with the (inferred) target of another’s attention (9). Thus, already in the first years of life, human children begin to experience the world not only through their own eyes, but also together with others, and these abilities for reasoning about others’ minds—collectively termed “theory of mind”—provide children with powerful mechanisms for acquiring and sharing cultural information, including language, social norms, and societal beliefs. Around the age of four, human children reach another milestone in their understanding of others as intentional agents, explicitly interpreting others’ behavior as the output of a belief–desire psychology and also reasoning about the goals and beliefs not only of other individuals, but also of their cultural group more broadly (4). Recent cross-cultural studies reveal that these early-emerging skills for reasoning about others’ minds develop at approximately the same age across diverse cultures (10, 11) and represent critical milestones on the path to uniquely human cultural cognition. Of course, the acquisition of a symbolic language further propels human cognition, possibly by providing a new representational medium that permits novel forms of abstract and relational reasoning, as well as unprecedented forms of communicative flexibility (12–15). However, the critical point is that the acquisition and use of a human-like language is simply neither possible, nor useful, for a species without prerequisite skills for reasoning about other minds. Are these foundational sociocognitive skills unique to humans?
Until recently, there was a general consensus that humans were indeed unique in their understanding of others as intentional agents (16, 17). However, research in the last 15 y has called this black-and-white interpretation into question (but see ref. 18), revealing that some nonhuman species do exploit information about others’ perception, knowledge, and intentions (19). The revised thinking about a nonhuman theory of mind has unfolded in parallel with changes in experimental methods that now emphasize studies of animals under more ecologically relevant conditions. For example, the first positive evidence for perspective taking in humans’ closest living relatives emerged when chimpanzees were tested in competitive situations with conspecifics rather than in cooperative contexts with humans (20, 21). Since these initial studies, several species besides apes have shown similar skills in the context of social competition (22–24). This social–cognitive flexibility in competitive but not cooperative contexts aligns well with the “Machiavellian intelligence” hypothesis, which proposes that primate minds were shaped by an evolutionary arms race in which skills for manipulating and deceiving others were paramount (25, 26).
If the ability to reason about others’ minds is not entirely unique to humans, then what accounts for the uniquely human forms of culture and communication that begin to emerge around children’s’ first birthdays? One explanation places a special emphasis on the combination of perspective-taking skills and a cooperative motivation for sharing psychological states with others, including joint goals and intentions (27). Unlike nonhuman apes, who exploit others’ perspectives primarily for their own purposes (28), human infants put their perspective-taking skills to work in the contexts of sharing attention with others and communicating cooperatively with one another. Importantly, human children also expect their social partners to be similarly motivated, creating a reciprocally cooperative framework for communicative and collaborative endeavors. For example, around their first birthdays, human children begin to produce pointing gestures simply to call others’ attention to objects of interest, and, when others point for them, children assume a cooperative motive relevant to the common ground between the two communicators (29). In contrast, whereas great apes can learn to point imperatively, for example when requesting food (30), they do not produce pointing gestures simply to share information with others, and, when others point cooperatively for them (e.g., to indicate the location of hidden food), nonhuman apes tend to perform poorly, most likely because they do not understand their partner’s cooperative intention. Shortly after 1 y of age, human prosocial and cooperative motives begin to evidence themselves more explicitly through acts of (unsolicited) instrumental helping, which again are critically supported by the ability to infer others’ intentions, knowledge, and desires (31). Therefore, unlike nonhuman apes, human cognition seems to be most tailored for cooperative and prosocial rather than Machiavellian purposes (32).
Importantly, neither the understanding of others as intentional agents nor prosocial and cooperative attitudes alone can support uniquely human cultural cognition. Rather, it is the synergy between motivations to engage in collaborative activities with shared goals and psychological processes for representing the underlying “we” intentionality (33) that allows humans to create the cultural products that differ so substantially from those of other species (34). How and why then did this unusual constellation of traits evolve? Were these motivational and representational changes evolutionarily coupled or do they have independent evolutionary origins?
Becoming Human
Attempts to reconstruct human cognitive evolution in the last 5–7 million y require inferences about the characteristics of our last common ancestor (LCA) with our closest living relatives—bonobos and chimpanzees. Because bonobos and chimpanzees are equally related to humans, but differ from one another morphologically, behaviorally, and cognitively, there has been active debate regarding which (if either) of the two Pan species serves as the best living model of our LCA (35–38). Although there is no strong consensus on this issue, studies comparing cranial development in great apes reveal that bonobos deviate from the conserved pattern found in gorillas and chimpanzees and indicate that bonobos may be relatively derived due to neotenic development (39). These changes in the timing of development are thought to have had cascading effects on diverse aspects of bonobo biology, leading to derived aspects of social behavior and cognition that differ from chimpanzees, and presumably also from the LCA of bonobos, chimpanzees, and humans (37, 40).
Assuming a more chimpanzee-like last common ancestor, evolutionary changes in temperament and specifically a shift toward more tolerant and less aggressive social behavior may have been critical prerequisites for the evolution of human forms of cultural behavior and cognition. In wild chimpanzees, within-group aggression among both males and females can be extreme, and intergroup aggression is often lethal (41, 42). Although chimpanzees cooperate in contexts such as group hunting, the successful captor typically retains the majority of the spoils and provides scraps to others predominantly in response to harassment (43). Therefore, although they are skillful cooperators in some contexts, it seems that chimpanzees are motivated primarily by individual goals, are relatively intolerant sharing partners, and have little regard for the equitable distribution of resources arising from cooperative efforts (44). The constraining role of social tolerance on chimpanzee cooperation and cognition has also been well-documented through experimental studies. For example, chimpanzees are more successful in instrumental cooperation tasks when the reward is physically dispersed than when it is clumped and individually monopolizable (45), and intolerance between individuals can preclude social learning in model–observer paradigms (46). Therefore, social intolerance between individuals can present an emotional barrier that significantly impedes potential for cooperation and social learning.
Relative to chimpanzees, bonobos—who are equally related to humans—are characterized by less intense forms of aggression both within and between social groups (47). Bonobos cofeed with one another more tolerantly than chimpanzees (ref. 48; but see refs. 49 and 50) and voluntarily share food with conspecifics (51) (Fig. 1), including strangers (52). This tolerance and willingness to share allows bonobos to outperform chimpanzees in some cooperative tasks, particularly when rewards are potentially monopolizable (48). The relatively lower levels of aggression and increased social tolerance in bonobos relative to chimpanzees have been hypothesized to result from a process of “self-domestication” (40). This hypothesis is supported by data revealing a syndrome of behavioral, morphological, and psychological traits in bonobos that are similar to those found in artificially domesticated species. The cooccurrence of these traits in bonobos and domesticated species is thought to result from selection (natural or artificial) against aggression, which has led to changes in developmental timing and neurophysiology, including alterations to the hypothalamic–pituitary–adrenal axis, androgen levels, and the serotonergic system (40, 53).
Fig. 1.
Two bonobos share a piece of fruit. Bonobos share food more tolerantly than chimpanzees, allowing them to collaborate successfully even when rewards are potentially monopolizable. Selection for increased social tolerance and concern for the equitable distribution of resources was likely an important precursor to large-scale cooperation in humans. Figure courtesy of Jingzhi Tan (photographer).
Why might natural selection have favored these behavioral changes in bonobos but not chimpanzees? One plausible explanation emphasizes differences in feeding ecology between the regions north and south of the Congo River (54). Whereas chimpanzees and gorillas share habitat and compete for vegetation north of the river, bonobos are restricted to regions south of the river that do not overlap with those of chimpanzees or gorillas. Due to high levels of scramble competition, chimpanzee females resort to foraging alone, do not form strong alliances with other females, and are vulnerable to sexual coercion by males (55–57). In contrast, bonobo females face lower levels of scramble competition and form alliances with other females that can effectively deter male coercion, and male bonobos benefit through affiliative rather than aggressive behavior toward females (47, 58). Under these conditions, selection likely favored males with a reduced propensity for aggression, ultimately leading to a self-domesticated phenotype (40).
Data from artificially domesticated species provide further evidence for how a reduction in aggression can potentially transform not only temperament, but also aspects of social cognition, including some of the cooperative–communicative skills believed to be critical in human cognitive development. For example, comparisons of domesticated species and their wild forebearers—including dogs and wolves, experimentally domesticated sliver foxes and a control lineage, and domestic and wild ferrets—reveal that domesticated forms display an increased sensitivity to cooperative communication, as well as alterations in other social behaviors such as the willingness to sustain eye contact (59–62). Importantly, these changes in cooperative behavior are thought to have arisen as a byproduct of changes in temperament (63), illustrating how emotional evolution can release constraints on social tolerance and effectively permit new forms of social engagement (64). Was this type of temperamental transformation also a key step in human cognitive evolution? Was Darwin correct in conjecturing that “[m]an in many respects may be compared with those animals which have long been domesticated” (ref. 65, p. 172)?
One major morphological trend in human evolution has been a reduction in sexual dimorphism in both body mass and canine tooth size. Comparative analyses with extant primates have linked these morphological traits to variance in social systems and suggest that high levels of dimorphism are associated with intense male–male competition and polygynous mating systems (66). Thus, the reduction in sexual dimorphism in the human lineage may reflect a transition from a more chimpanzee-like mating system, with high levels of male–male violence and sexual coercion of females, toward monogamy and cooperative breeding. This shift in social systems—which may have begun as early as 3 million y ago (67)–likely favored an initial reduction in aggression and increased tolerance between individuals (68). Within the last 200,000 y, additional changes in human craniofacial morphology raise the intriguing possibility of a second wave of selection against aggression that coincided with the emergence of behavioral modernity (69). Specifically, Cieri et al. documented increased feminization of human crania from the Middle Pleistocene through the present—evidenced by a reduction in brow ridge projection and a shortening of the upper facial region (70). These anatomical changes are hypothesized to result from a reduction in androgen activity and are consistent with the well-documented effects of testosterone on craniofacial masculinization. Given that testosterone is linked to dominance and aggressive behavior in men (71), these changes may reflect a more recent wave of selection for increased social tolerance that allowed humans to work productively with conspecifics in new ways (72), as suggested by the proliferation of cultural artifacts 20–70 thousand years ago (kya) (69, 73, 74).
In addition to its effects on temperament, a reduction in testosterone may also have directly affected aspects of human cognition, including processes related to communication and the theory of mind. Evidence for this possibility stems from the systemizing–empathizing theory, developed by Baron-Cohen et al. (75) and proposed as an explanation for the deficits in theory of mind observed in autism spectrum disorder (76). In brief, the theory proposes that cognitive differences between males and females arise in part due to differential prenatal androgen exposure. Baron-Cohen reviews evidence that, at the population level, human males outperform females on tasks involving visuospatial and spatiotemporal abilities (77), presumably reflecting “systemizing” skills—or the tendency to analyze the world in terms of lawful and deterministic rules (78). In contrast, females exhibit an advantage with language (77), when interpreting facial expressions and engaging in interactive social exchange—including sharing and turn taking—and girls reach certain theory-of -mind milestones earlier in development than do boys (79). Although some of these effects may be culturally driven, similar differences have been reported in nonhuman animals, some of which are diminished when males are castrated, or females are artificially androgenized (78). The systemizing–empathizing hypothesis proposes that high levels of androgen exposure during prenatal development can cause an extreme masculinization of the brain, leading to a (pathological) bias toward systemizing and resulting in deficits in empathic processes, such as the theory of mind. Baron-Cohen and coworkers suggest that autism spectrum disorders—which are characterized by deficits in theory of mind—are caused by an “extreme male brain” but also present data that, even in typically developing humans, prenatal androgen exposure is predictive of autistic traits measured later in childhood (80).
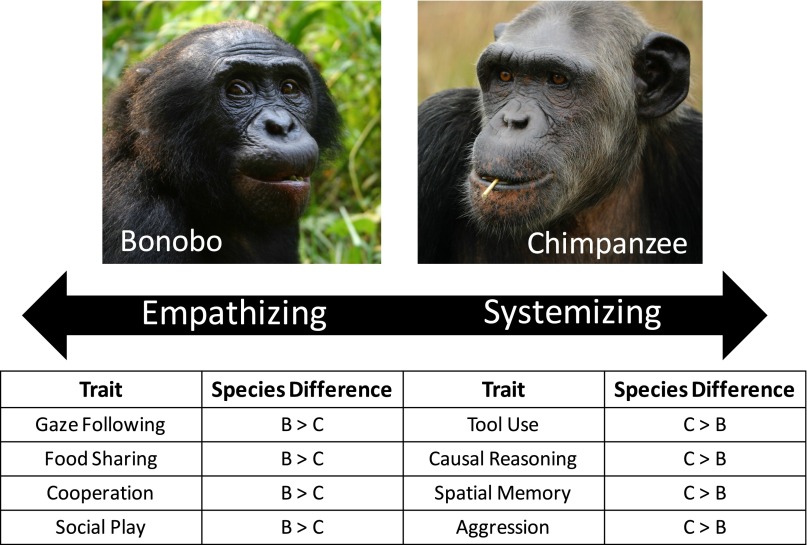
Although the systemizing–empathizing hypothesis was proposed to account for intraspecific variation in modern humans, its predictions may also partially account for species differences in the social cognition of great apes and key changes in human cognitive evolution. For example, bonobos—who exhibit signatures of lower prenatal androgen exposure than chimpanzees (81)—outperform chimpanzees on some measures of theory of mind and cooperation (48, 82), attend to the face and eyes more often than chimpanzees when viewing social images (83), and share food and play socially more often as adults than chimpanzees (refs. 48 and 84; but see refs. 49 and 50)—all traits associated with empathizing. In contrast, chimpanzees outperform bonobos in tests of tool use, causal reasoning, and spatial memory (82, 85)—cognitive traits associated with systemizing—and chimpanzees exhibit more severe aggression than bonobos (47), consistent with the predictions of the systemizing–empathizing hypothesis (Fig. 2). Comparative brain imaging studies with chimpanzees and bonobos also reveal that bonobos have more gray matter in regions implicated in empathy and more robust neural pathways relating to the inhibition of aggression (86), with bonobos having approximately twice the serotonergic innervation of the amygdala in comparison with chimpanzees (53).
Fig. 2.
Cognitive and behavioral differences between chimpanzees and bonobos that align with the predictions of the systemizing–empathizing hypothesis. B, bonobo; C, chimpanzee.
Assuming that human ancestors were endowed with the basic perspective-taking skills found in other great apes, reductions in androgen activity in recent human evolution may have been a catalyst both for the elaboration of these abilities and for their application to new types of cooperative social interactions. Therefore, under this scenario, critical aspects of human-typical social motivation (e.g., tolerance and gregariousness) and social cognition (e.g., aspects of the theory of mind) may have evolved in parallel, due in part to similar biological mechanisms regulating both sets of traits.
Testing Hypotheses About Cognitive Evolution
The evolutionary scenarios described above are speculative and draw on data from relatively few (but phylogenetically informative) taxa to make inferences about key processes in human cognitive evolution. Although human cognition evolved only once and represents the final product of myriad incremental evolutionary changes, comparative research with nonhuman animals provides an opportunity to explicitly test hypotheses about how and why some of these changes may have occurred (2). To date, few studies have adopted this approach—mainly due to the challenges of compiling large and high-quality interspecific datasets on animal cognition—but several recent studies illustrate the value of explicitly comparative approaches for research in cognitive evolution. For example, MacLean et al. (87) conducted tests of self-control in 36 species of vertebrates and uncovered robust links between absolute (but not relative) brain size and skills for self-control. These data raise the possibility that increases in absolute brain size, a defining feature in human evolution, may have yielded improved abilities for self-regulation, possibly supporting the increased social tolerance (e.g., through the inhibition of aggression) that is critical for human cooperation. Interestingly, in this study, there was no relationship between species-typical social group size and self-control, suggesting that merely living in larger social groups is not sufficient to favor these abilities. However, in another experiment with lemurs, MacLean et al. (88) found that larger species-typical social group sizes were associated with increased skill relevant to visual perspective taking (a key component of the theory of mind), corroborating the hypothesis that life in complex social groups was a driver for cognitive skills that allow individuals to outcompete others for access to food and mates. Therefore, living in larger and more complex social networks may have favored the initial evolution of some components of the theory of mind, which in humans have been repurposed in novel cooperative contexts. With regard to the evolution of human cooperative and prosocial motives, Burkart et al. (89) tested 15 primate species in a series of proactive prosociality tasks and examined a range of socioecological predictors of species differences. In this case, the extent of allomaternal care (care for young provided by individuals other than the mother) was the best predictor of species differences in prosocial behavior. These findings are consistent with the idea that a shift from polygamy to cooperative breeding may have been critical for the evolution of uniquely human forms of cooperative psychology (90). Importantly, because early humans were most likely already endowed with many components of theory of mind, the motivational changes accompanying cooperative breeding may have provided a catalyst for the application of these skills to the cooperative settings in which shared intentionality became adaptive (68). In contrast, other cooperatively breeding primates (e.g., callitrichids) may lack human-like shared intentions because they possess the motivational, but not the representational, foundations for these processes, and vice versa for extant great apes who possess some of the requisite representational abilities but may lack the level of prosocial motivation found in cooperative breeders (68).
The studies above highlight the utility of phylogenetic comparative approaches for inferring how and why particular aspects of psychology evolve—including psychological traits believed to be critical for the human cognitive phenotype. However, few such studies have been conducted, and many of the hypotheses outlined throughout this article remain ripe for comparative study. For example, do social tolerance and skills for cooperation or social learning covary across nonhuman species? Closely related taxa that differ substantially in social tolerance—for example, the macaque radiation (91)—provide powerful opportunities for assessing whether these traits may be functionally linked. Similarly, if the influence of prenatal androgen exposure yields systematic differences in cognition related to systemizing and empathizing, one would predict that these effects should be evident in a range of taxa outside the great apes. For these types of questions, comparative studies will be critical for assessing whether hypotheses about human cognitive evolution align with the patterns observed in other taxa. There is no doubt that human cognition is unique and composed of a constellation of traits that collectively may not cooccur in any other species. However, many important aspects of human cognition have homologies—and often, more interestingly, analogies (resulting from convergent evolution)—in other taxa, creating rich opportunities to make inferences about when, how, and why these traits evolve.
Lastly, in cases where aspects of human cognition seem radically different from those of other species, phylogenetic approaches can be used to assess whether humans should be considered an evolutionary outlier (92, 93). For example, recent analyses have shown that, despite having many more neurons than any other primate brain, the number of neurons in human brains is not remarkable given the volume of the human brain and data on the general cellular scaling rules of primate brains (94, 95). Similar analyses can be undertaken with cognitive traits to assess whether apparently outlying observations in humans represent an extreme but predictable occurrence, taking into consideration primate phylogeny and a set of predictor variables that covary with the trait of interest across taxa. When human traits can be partially explained by broader evolutionary patterns, comparative approaches will be particularly useful for addressing questions about how, when, and why these traits evolved in humans. In cases where humans deviate substantially from broad-scale evolutionary patterns, these findings suggest that humans could be considered an evolutionary outlier (96) and demand great caution in reconstructing how and why these aspects of human cognition may have evolved.
Conclusions
Humans are unusual animals in many respects, but it is in our species-typical cognition that human uniqueness evidences itself most prominently. The precise ways in which human cognition differs from that of other species remains a topic of intense debate (14), but many data currently support the hypothesis that it is an early emerging set of social skills for reasoning about conspecifics as intentional agents, coupled with a distinctly cooperative and prosocial motivation, that fuels many of our most remarkable cognitive achievements (97). Although the scientific literature is replete with attempts to identify single capacities that make human cognition unique, it is likely that human cognition is more than the sum of its parts and is dependent on synergy between a unique combination of representational and motivation traits. Therefore, although the whole of human cognition may be unparalleled in the animal kingdom, key components of our cognitive phenotype can be found in other taxa, including not only great apes, but also more distantly related species bearing cognitive resemblances to humans as a result of convergent evolution. Accordingly, new lines of research integrating phylogenetic comparative methods with the study of animal minds will play an essential role in our quest to determine not only what makes human cognition unique, but also how and why these traits evolved.
Acknowledgments
I thank B. Hare, J. Tan, C. Krupenye, and two anonymous reviewers for helpful comments on an earlier draft of this manuscript, J. Tan for sharing the photograph in Fig. 1, and the Stanton Foundation for financial support during the time the manuscript was written.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Tinbergen N. On aims and methods of ethology. Z Tierpsychol. 1963;20:410–433. [Google Scholar]
- 2.MacLean EL, et al. How does cognition evolve? Phylogenetic comparative psychology. Anim Cogn. 2012;15(2):223–238. doi: 10.1007/s10071-011-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasello M, Kruger AC, Ratner HH. Cultural learning. Behav Brain Sci. 1993;16(3):495–511. [Google Scholar]
- 4.Tomasello M, Rakoczy H. What makes human cognition unique? From individual to shared to collective intentionality. Mind Lang. 2003;18(2):121–147. [Google Scholar]
- 5.Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- 6.Tomasello M. The Cultural Origins of Human Cognition. Harvard Univ Press; Cambridge, MA: 1999. [Google Scholar]
- 7.Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr Soc Res Child Dev. 1998;63(4):i–vi, 1–143. [PubMed] [Google Scholar]
- 8.Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Dev Sci. 2005;8(6):535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar N, Carpenter M, Tomasello M. The role of discourse novelty in early word learning. Child Dev. 1996;67(2):635–645. [Google Scholar]
- 10.Callaghan T, et al. Early social cognition in three cultural contexts. Monogr Soc Res Child Dev. 2011;76(2):1–142. doi: 10.1111/j.1540-5834.2011.00603.x. [DOI] [PubMed] [Google Scholar]
- 11.Shahaeian A, Peterson CC, Slaughter V, Wellman HM. Culture and the sequence of steps in theory of mind development. Dev Psychol. 2011;47(5):1239–1247. doi: 10.1037/a0023899. [DOI] [PubMed] [Google Scholar]
- 12.Gentner D. Language in Mind: Advances in the Study of Language and Thought. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- 13.Clark A. Language, embodiment, and the cognitive niche. Trends Cogn Sci. 2006;10(8):370–374. doi: 10.1016/j.tics.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31(2):109–130, discussion 130–178. doi: 10.1017/S0140525X08003543. [DOI] [PubMed] [Google Scholar]
- 15.Vygotsky L, Hanfmann E, Vakar G. Thought and Language. MIT Press; Cambridge, MA: 2012. [Google Scholar]
- 16.Tomasello M, Call J. Primate Cognition. Oxford Univ Press; New York: 1997. [Google Scholar]
- 17.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Univ of Chicago Press; Chicago: 1990. [Google Scholar]
- 18.Penn DC, Povinelli DJ. On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind’. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):731–744. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Call J, Tomasello M. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn Sci. 2008;12(5):187–192. doi: 10.1016/j.tics.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Anim Behav. 2001;61(1):139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- 21.Hare B, Call J, Agnetta B, Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim Behav. 2000;59(4):771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- 22.Dally JM, Emery NJ, Clayton NS. Food-caching western scrub-jays keep track of who was watching when. Science. 2006;312(5780):1662–1665. doi: 10.1126/science.1126539. [DOI] [PubMed] [Google Scholar]
- 23.Flombaum JI, Santos LR. Rhesus monkeys attribute perceptions to others. Curr Biol. 2005;15(5):447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 24.Sandel AA, MacLean E, Hare B. Evidence from four lemur species that ringtailed lemur social cognition converges with that of haplorhine primates. Anim Behav. 2011;81(5):925–931. [Google Scholar]
- 25.Byrne RW, Whiten A, editors. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Clarendon Press; Oxford: 1988. [Google Scholar]
- 26.Whiten A, Byrne RW, editors. Machiavellian Intelligence II: Extensions and Evaluations. Cambridge Univ Press; Cambridge, UK: 1997. [Google Scholar]
- 27.Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behav Brain Sci. 2005;28(5):675–691, discussion 691–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- 28.MacLean EL, Hare B. Bonobos and chimpanzees infer the target of another’s attention. Anim Behav. 2012;83(2):345–353. [Google Scholar]
- 29.Tomasello M, Carpenter M, Liszkowski U. A new look at infant pointing. Child Dev. 2007;78(3):705–722. doi: 10.1111/j.1467-8624.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 30.Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Dev Psychol. 1998;34(5):813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warneken F. Precocious prosociality: Why do young children help? Child Dev Perspect. 2015;9(1):1–6. [Google Scholar]
- 32.Moll H, Tomasello M. Cooperation and human cognition: The Vygotskian intelligence hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362(1480):639–648. doi: 10.1098/rstb.2006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuomela R. The Importance of Us: A Philosophical Study of Basic Social Notions. Stanford Univ Press; Stanford, CA: 1995. [Google Scholar]
- 34.Tomasello M. A Natural History of Human Thinking. Harvard Univ Press; Cambridge, MA: 2014. [Google Scholar]
- 35.de Waal FBM, Lanting F. Bonobo: The Forgotten Ape. Univ of California Press; Berkeley, CA: 1997. [Google Scholar]
- 36.Zihlman AL, Cronin JE, Cramer DL, Sarich VM. Pygmy chimpanzee as a possible prototype for the common ancestor of humans, chimpanzees and gorillas. Nature. 1978;275(5682):744–746. doi: 10.1038/275744a0. [DOI] [PubMed] [Google Scholar]
- 37.Wrangham R, Pilbeam D. African apes as time machines. In: Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. All Apes Great and Small. Kluwer; New York: 2001. pp. 5–18. [Google Scholar]
- 38.Prüfer K, et al. The bonobo genome compared with the chimpanzee and human genomes. Nature. 2012;486(7404):527–531. doi: 10.1038/nature11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shea BT. Paedomorphosis and neoteny in the pygmy chimpanzee. Science. 1983;222(4623):521–522. doi: 10.1126/science.6623093. [DOI] [PubMed] [Google Scholar]
- 40.Hare B, Wobber V, Wrangham R. The self-domestication hypothesis: Evolution of bonobo psychology is due to selection against aggression. Anim Behav. 2012;83(3):573–585. [Google Scholar]
- 41.Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Adv Stud Behav. 2005;35:275–331. [Google Scholar]
- 42.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513(7518):414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 43.Gilby IC. Meat sharing among the gombe chimpanzees: Harassment and reciprocal exchange. Anim Behav. 2006;71(4):953–963. [Google Scholar]
- 44.Hamann K, Warneken F, Greenberg JR, Tomasello M. Collaboration encourages equal sharing in children but not in chimpanzees. Nature. 2011;476(7360):328–331. doi: 10.1038/nature10278. [DOI] [PubMed] [Google Scholar]
- 45.Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Anim Behav. 2006;72:275–286. [Google Scholar]
- 46.Horner V, Whiten A, Flynn E, de Waal FB. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. Proc Natl Acad Sci USA. 2006;103(37):13878–13883. doi: 10.1073/pnas.0606015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuichi T. Female contributions to the peaceful nature of bonobo society. Evolutionary Anthropology: Issues. News Rev (Melb) 2011;20(4):131–142. doi: 10.1002/evan.20308. [DOI] [PubMed] [Google Scholar]
- 48.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr Biol. 2007;17(7):619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 49.Jaeggi AV, Stevens JM, Van Schaik CP. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am J Phys Anthropol. 2010;143(1):41–51. doi: 10.1002/ajpa.21288. [DOI] [PubMed] [Google Scholar]
- 50.Cronin KA, De Groot E, Stevens JM. Bonobos show limited social tolerance in a group setting: A comparison with chimpanzees and a test of the relational model. Folia Primatol (Basel) 2015;86(3):164–177. doi: 10.1159/000373886. [DOI] [PubMed] [Google Scholar]
- 51.Hare B, Kwetuenda S. Bonobos voluntarily share their own food with others. Curr Biol. 2010;20(5):R230–R231. doi: 10.1016/j.cub.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 52.Tan J, Hare B. Bonobos share with strangers. PLoS One. 2013;8(1):e51922. doi: 10.1371/journal.pone.0051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stimpson CD, et al. Differential serotonergic innervation of the amygdala in bonobos and chimpanzees. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrangham RW. The evolution of sexuality in chimpanzees and bonobos. Hum Nat. 1993;4(1):47–79. doi: 10.1007/BF02734089. [DOI] [PubMed] [Google Scholar]
- 55.Wrangham RW, Smuts BB. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J Reprod Fertil Suppl. 1980;1980(Suppl 28):13–31. [PubMed] [Google Scholar]
- 56.Wrangham RW, Peterson D. Demonic Males: Apes and the Origins of Human Violence. Houghton Mifflin Harcourt; New York: 1997. [Google Scholar]
- 57.Goodall J. Belknap Press of Harvard Univ Press; Cambridge, MA: 1986. The Chimpanzees of Gombe: Patterns of Behavior. [Google Scholar]
- 58.Kano T. The Last Ape: Pygmy Chimpanzee Behavior and Ecology. Stanford University Press; Stanford, CA: 1992. [Google Scholar]
- 59.Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2002;298(5598):1634–1636. doi: 10.1126/science.1072702. [DOI] [PubMed] [Google Scholar]
- 60.Hare B, et al. Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Curr Biol. 2005;15(3):226–230. doi: 10.1016/j.cub.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 61.Hernádi A, Kis A, Turcsán B, Topál J. Man’s underground best friend: Domestic ferrets, unlike the wild forms, show evidence of dog-like social-cognitive skills. PLoS One. 2012;7(8):e43267. doi: 10.1371/journal.pone.0043267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miklósi A, et al. A simple reason for a big difference: Wolves do not look back at humans, but dogs do. Curr Biol. 2003;13(9):763–766. doi: 10.1016/s0960-9822(03)00263-x. [DOI] [PubMed] [Google Scholar]
- 63.Hare B, Tomasello M. Human-like social skills in dogs? Trends Cogn Sci. 2005;9(9):439–444. doi: 10.1016/j.tics.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Porges SW. 2011. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation, Norton Series on Interpersonal Neurobiology (Norton, New York)
- 65.Darwin C. The Descent of Man, and Selection in Relation to Sex. Appleton; New York: 1871. p. 2 v. [Google Scholar]
- 66.Plavcan JM, van Schaik CP. Interpreting hominid behavior on the basis of sexual dimorphism. J Hum Evol. 1997;32(4):345–374. doi: 10.1006/jhev.1996.0096. [DOI] [PubMed] [Google Scholar]
- 67.Reno PL, Meindl RS, McCollum MA, Lovejoy CO. Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc Natl Acad Sci USA. 2003;100(16):9404–9409. doi: 10.1073/pnas.1133180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burkart JM, Hrdy SB, Van Schaik CP. Cooperative breeding and human cognitive evolution. Evol Anthropol. 2009;18(5):175–186. [Google Scholar]
- 69.Klein RG. Anatomy, behavior, and modern human origins. J World Prehist. 1995;9(2):167–198. [Google Scholar]
- 70.Cieri RL, Churchill SE, Franciscus RG, Tan J, Hare B. Craniofacial feminization, social tolerance, and the origins of behavioral modernity. Curr Anthropol. 2014;55(4):419–443. [Google Scholar]
- 71.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21(3):353–363, discussion 363–397. [PubMed] [Google Scholar]
- 72.Hill KR, Wood BM, Baggio J, Hurtado AM, Boyd RT. Hunter-gatherer inter-band interaction rates: Implications for cumulative culture. PLoS One. 2014;9(7):e102806. doi: 10.1371/journal.pone.0102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derex M, Boyd R. The foundations of the human cultural niche. Nat Commun. 2015;6:8398. doi: 10.1038/ncomms9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powell A, Shennan S, Thomas MG. Late Pleistocene demography and the appearance of modern human behavior. Science. 2009;324(5932):1298–1301. doi: 10.1126/science.1170165. [DOI] [PubMed] [Google Scholar]
- 75.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 76.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 77.Miller DI, Halpern DF. The new science of cognitive sex differences. Trends Cogn Sci. 2014;18(1):37–45. doi: 10.1016/j.tics.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 79.Christov-Moore L, et al. Empathy: Gender effects in brain and behavior. Neurosci Biobehav Rev. 2014;46(Pt 4):604–627. doi: 10.1016/j.neubiorev.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auyeung B, et al. Fetal testosterone and autistic traits. Br J Psychol. 2009;100(Pt 1):1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- 81.McIntyre MH, et al. Bonobos have a more human-like second-to-fourth finger length ratio (2D:4D) than chimpanzees: A hypothesized indication of lower prenatal androgens. J Hum Evol. 2009;56(4):361–365. doi: 10.1016/j.jhevol.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Herrmann E, Hare B, Call J, Tomasello M. Differences in the cognitive skills of bonobos and chimpanzees. PLoS One. 2010;5(8):e12438. doi: 10.1371/journal.pone.0012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kano F, Hirata S, Call J. Social attention in the two species of pan: Bonobos make more eye contact than chimpanzees. PLoS One. 2015;10(6):e0129684. doi: 10.1371/journal.pone.0129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palagi E, Cordoni G. The right time to happen: Play developmental divergence in the two pan species. PLoS One. 2012;7(12):e52767. doi: 10.1371/journal.pone.0052767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosati AG, Hare B. Chimpanzees and bonobos exhibit divergent spatial memory development. Dev Sci. 2012;15(6):840–853. doi: 10.1111/j.1467-7687.2012.01182.x. [DOI] [PubMed] [Google Scholar]
- 86.Rilling JK, et al. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc Cogn Affect Neurosci. 2012;7(4):369–379. doi: 10.1093/scan/nsr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacLean EL, et al. The evolution of self-control. Proc Natl Acad Sci USA. 2014;111(20):E2140–E2148. doi: 10.1073/pnas.1323533111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maclean EL, et al. Group size predicts social but not nonsocial cognition in lemurs. PLoS One. 2013;8(6):e66359. doi: 10.1371/journal.pone.0066359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burkart JM, et al. The evolutionary origin of human hyper-cooperation. Nat Commun. 2014;5:4747. doi: 10.1038/ncomms5747. [DOI] [PubMed] [Google Scholar]
- 90.Hrdy SB. Mothers and Others. Harvard Univ Press; Cambridge, MA: 2009. [Google Scholar]
- 91.Thierry B. Unity in Diversity: Lessons from Macaque Societies. Evol Anthropol. 2007;16(6):224–238. [Google Scholar]
- 92.Nunn CL, Zhu L. Phylogenetic prediction to identify “evolutionary singularities.”. In: Garamszegi LZ, editor. Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. Springer; Berlin: 2014. pp. 481–514. [Google Scholar]
- 93.Organ C, Nunn CL, Machanda Z, Wrangham RW. Phylogenetic rate shifts in feeding time during the evolution of Homo. Proc Natl Acad Sci USA. 2011;108(35):14555–14559. doi: 10.1073/pnas.1107806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nunn CL. The Comparative Approach in Evolutionary Anthropology and Biology. Univ of Chicago Press; Chicago: 2011. [Google Scholar]
- 97.Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317(5843):1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]