Significance
Nucleosome assembly is regulated at multiple levels to impact distinct cellular processes. Mutations in factors involved in nucleosome assembly, such as histone chaperones and histone variants, result in genome instability and gene expression defects that, in turn, promote the development of human disease including cancer and aging. Therefore, it is important to determine how nucleosome assembly of H3.3 is regulated. Our findings demonstrate a role for O-linked N-acetylglucosamine (GlcNAc) transferase in regulating H3.3 deposition/exchange and establish the O-GlcNAc modification of HIRA as a previously unidentified mechanism regulating nucleosome assembly of H3.3 and cellular senescence.
Keywords: histone H3.3, HIRA, OGT, nucleosome assembly, cellular senescence
Abstract
The histone chaperone HIRA complex, consisting of histone cell cycle regulator (HIRA), Ubinuclein1 (UBN1), and calcineurin binding protein 1 (CABIN1), deposits histone variant H3.3 to genic regions and regulates gene expression in various cellular processes, including cellular senescence. How HIRA-mediated nucleosome assembly of H3.3–H4 is regulated remains not well understood. Here, we show that O-linked N-acetylglucosamine (GlcNAc) transferase (OGT), an enzyme that catalyzes O-GlcNAcylation of serine or threonine residues, interacts with UBN1, modifies HIRA, and promotes nucleosome assembly of H3.3. Depletion of OGT or expression of the HIRA S231A O-GlcNAcylation–deficient mutant compromises formation of the HIRA–H3.3 complex and H3.3 nucleosome assembly. Importantly, OGT depletion or expression of the HIRA S231A mutant delays premature cellular senescence in primary human fibroblasts, whereas overexpression of OGT accelerates senescence. Taken together, these results support a model in which OGT modifies HIRA to regulate HIRA–H3.3 complex formation and H3.3 nucleosome assembly and reveal the mechanism by which OGT functions in cellular senescence.
In eukaryotic cells, genetic information (DNA) is packaged into an organized complex known as chromatin. Chromatin governs a number of cellular processes, including DNA replication, DNA repair, and gene transcription. The basic repeating unit of chromatin is the nucleosome (1), consisting of 147 base pairs of DNA wrapped around a core histone octamer composed of a central histone (H3–H4)2 tetramer and two histone H2A-H2B dimers (2–5). Nucleosome assembly is a key regulatory step in the establishment and maintenance of distinct chromatin states (4, 6–8), and deregulation of nucleosome assembly is linked to aging and cancer (4, 9, 10). Therefore, it is important to determine how nucleosome assembly pathways are regulated.
In mammalian cells, canonical histone H3.1/H3.2 and histone H3 variant H3.3 differ by four or five amino acid residues (5) and are assembled into nucleosomes via either replication-coupled (H3.1/H3.2) or replication-independent (H3.3) nucleosome assembly pathways orchestrated by distinct histone chaperones. For instance, along with H4, canonical histone H3.1/H3.2 is deposited by the histone chaperone CAF-1 during S phase, whereas H3.3 is assembled into nucleosomes at pericentric and telomeric regions by DAXX/ATRX, and at genic regions by the HIRA complex (11–18). In addition to histone chaperones, posttranslational modifications on newly synthesized histones impact nucleosome assembly, in part by regulating the interactions between histone chaperones and their histone cargo. For example, histone H4 acetylation regulates nuclear import of new H3–H4 (14, 19–21). In budding yeast, acetylation of histone H3 lysine 56, as well as acetylation at the N terminus of H3, contribute DNA replication-coupled nucleosome assembly, in part, through promoting the interaction of histone chaperones and histones (14, 22, 23). Ubiquitylation of histone H3 by the E3 ubiquitin ligase, Rtt101Mms1 in yeast and Cul4/DDB1 in human cells, promotes the hand off of H3–H4 from the histone chaperone Asf1 to downstream chaperones CAF-1 and HIRA for nucleosome assembly (24). In humans, phosphorylation of histone H4 serine 47, catalyzed by the PAK2 kinase, promotes nucleosome assembly of H3.3–H4 by increasing the binding affinity of HIRA to H3.3–H4 (25). Therefore, nucleosome assembly is regulated at multiple levels, which likely impact downstream cellular processes by using histones and histone chaperones.
The HIRA complex, composed of HIRA, Ubinuclein1 (UBN1), and CABIN1, assembles H3.3 at genic regions, thereby regulating H3.3 dynamics for different cellular processes (5, 6, 26). For instance, HIRA-mediated H3.3 incorporation at gene promoters destabilizes nucleosomes and increases nucleosome dynamics for transcriptional activation and ongoing transcription (15, 27–29). Conversely, HIRA regulates transcriptional gene silencing in yeast (25, 30, 31), and the HIRA complex and H3.3 mediate formation of senescence-associated heterochromatin foci (SAHF) during oncogene-induced proliferation arrest of primary human fibroblasts (32, 33). Thus, the trimeric HIRA complex is an important chromatin regulator involved in both gene activation and transcriptional silencing (15, 27–29, 34). However, how HIRA complex-mediated nucleosome assembly of H3.3 is regulated remains unclear.
Cellular senescence represents an irreversible cell proliferation arrest state leading to loss of tissue function in mammals and is linked to organismal aging (35–41). Cellular senescence was initially used to describe the progressive attrition of chromosomal ends and cellular arrest in cultured human fibroblasts over time (replicative senescence) (42–45). Cellular senescence can also be induced prematurely by cellular stresses, termed premature cellular senescence. For instance, oncogene activation in normal human cells leads to premature cellular senescence (32, 38, 46). Specifically, it has been shown that exogenous expression of oncogenic Ras or expression of activated MEK in IMR90 human fibroblasts induces cellular senescence. Senescent cells characteristically display one large nucleolus with tiny spots at the nuclear periphery as visualized by DAPI staining, called SAHF (32, 38). SAHF formation may be largely due to augmented nucleosome density (47). It has been shown that the HIRA complex incorporates H3.3 during senescence in a replication-independent manner, and ectopic expression of HIRA or H3.3 induces premature cellular senescence (33, 48). Therefore, understanding how the HIRA-mediated nucleosome assembly pathway is regulated will likely provide insight into the regulation of cellular senescence.
Herein, we describe the first link to our knowledge among the O-GlcNAcylation of HIRA, nucleosome assembly, and cellular senescence. We identify O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) as a binding partner of the HIRA complex by using mass spectrometry. We show that OGT interacts with UBN1, modifies HIRA with O-GlcNAc, and impacts the formation of HIRA–H3.3 complex. Cells expressing a HIRA O-GlcNAcylation site mutant exhibited defects in formation of HIRA–H3.3 complex and H3.3 nucleosome assembly. Furthermore, we show that OGT and HIRA O-GlcNAcylation are involved in cellular senescence. Together, these studies reveal that HIRA is O-GlcNAcylated, and this modification regulates HIRA-mediated H3.3 nucleosome assembly and cellular senescence.
Results
OGT Interacts with the HIRA Complex Through UBN1.
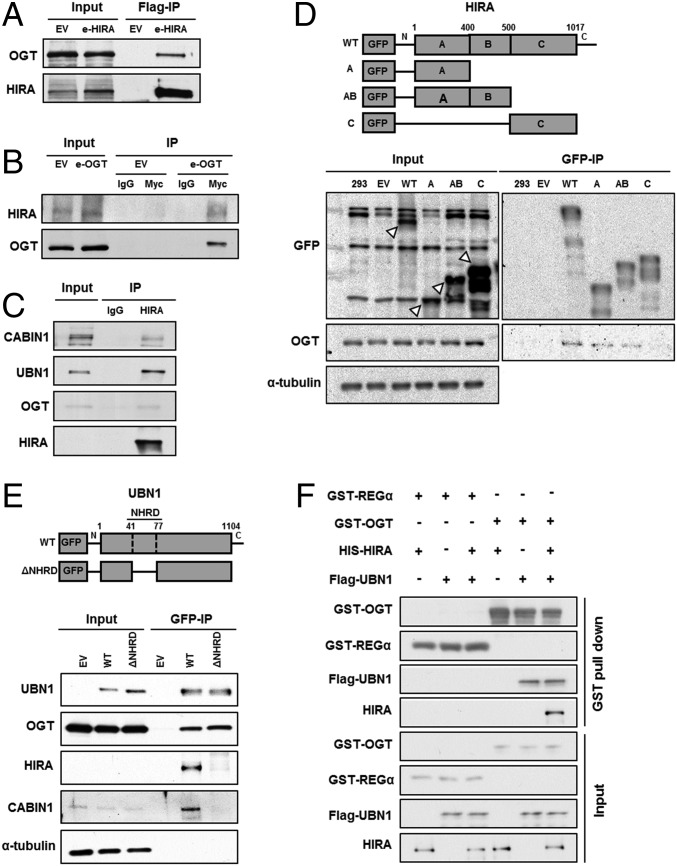
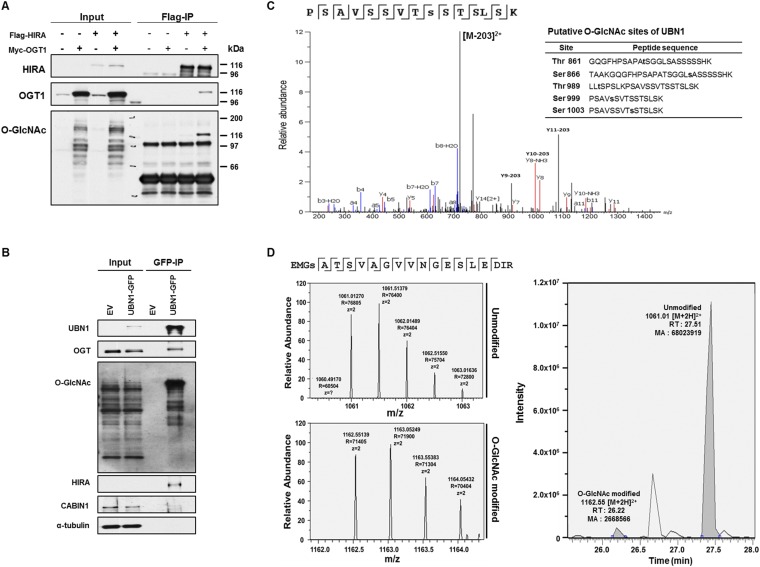
The HIRA complex consists of three subunits, HIRA, UBN1, and CABIN1, and deposits H3.3–H4 at genic regions. To understand how HIRA-mediated nucleosome assembly is regulated, we established HEK293T cell lines stably expressing Flag-tagged HIRA, purified the HIRA complex from cell extracts, and identified HIRA-interacting proteins by mass spectrometry. In addition to the three subunits of the HIRA complex and ASF1a, a histone chaperone that delivers H3.3–H4 to the HIRA complex (14), we identified several potential binding partners, including OGT (Table S1). We chose to focus our studies on OGT, an enzyme that catalyzes O-GlcNAcylation of serine or threonine residues of cytoplasmic and nuclear proteins, because OGT had no known role in nucleosome assembly, a process which uses protein posttranslational modification as a regulatory mechanism. To verify this interaction, we immunoprecipitated HIRA or OGT from cells transiently expressing Flag-tagged HIRA or Myc-tagged OGT. Endogenous OGT coimmunoprecipitated with Flag-HIRA (Fig. 1A), and endogenous HIRA was associated with Myc-OGT in the reciprocal immunoprecipitation (Fig. 1B). The physical interaction of the OGT–HIRA complex was further confirmed by immunoprecipitation of endogenous HIRA (Fig. 1C). These results indicate that OGT interacts with the HIRA complex.
Table S1.
OGT is a putative HIRA binding protein
| HIRA purification protein | Molecular mass, Da | No. of unique peptides | Coverage, % |
| ASF1A_HUMAN | 22,968.40 | 7 | 44.1 |
| CABIN_HUMAN | 246,356.20 | 53 | 27.4 |
| UBN1_HUMAN | 146,091.70 | 16 | 14.9 |
| HIRA_HUMAN | 111,836.20 | 49 | 56.7 |
| OGT_HUMAN | 116,928.00 | 2 | 1.72 |
| DCD_HUMAN | 11,284.10 | 2 | 20 |
| HSP71_HUMAN | 70,054.00 | 2 | 42.4 |
| M3K7_HUMAN | 67,196.50 | 2 | 4.79 |
| SAMH1_HUMAN | 72,202.70 | 2 | 3.51 |
| F263_HUMAN | 59,609.80 | 6 | 12.7 |
| RUVB1_HUMAN | 50,229.40 | 6 | 17.5 |
| BAG5_HUMAN | 51,201.00 | 2 | 5.37 |
| DSG1_HUMAN | 113,748.90 | 3 | 3.72 |
| LEG7_HUMAN | 15,074.70 | 2 | 18.4 |
| PM14_HUMAN | 14,585.60 | 2 | 16.8 |
HIRA and its associated proteins were purified by immunoprecipitation and analyzed by mass spectrometry.
Fig. 1.
OGT interacts with the HIRA complex. (A) Exogenously expressed HIRA associates with endogenous OGT. Exogenously expressed Flag-HIRA (e-HIRA) was immunoprecipitated (IP) with Flag M2 beads from 293T cells, and proteins at input and IP samples were analyzed by Western blotting. EV, empty vector. (B) Exogenously expressed OGT (e-OGT) associates with endogenous HIRA. The experiment was performed as described in A except that Myc-OGT was immunoprecipitated with antibodies against the Myc epitope. (C) Endogenous HIRA associates with endogenous OGT. HIRA was immunoprecipitated with antibodies against HIRA and proteins in input, and IP were analyzed by Western blotting using the indicated Abs. (D) OGT interacts with the A domain of HIRA. GFP-tagged full length or different domains of HIRA (A, AB, and C, Upper) were expressed in 293T cells. Total lysates were subject to IP with antibodies against GFP, followed by Western blotting using the indicated Abs. White arrows indicates GFP-tagged full length or different domains of HIRA. (E) UBN1 mediates the OGT–HIRA interactions. GFP-tagged, full-length UBN1 or a UBN1 mutant deficient in HIRA binding was expressed in 293T cells and immunoprecipitated by antibodies against GFP. (F) OGT interacts with UBN1 in vitro. Full-length HIS-HIRA and Flag-UBN1 were expressed alone or in combination in Sf9 insect cells and were tested for the ability to bind recombinant GST-OGT. GST-OGT bound proteins were pulled down and were analyzed by Western blotting using the indicated antibodies. GST-REGα, a mammalian proteasome binding protein, was used as a negative control.
The HIRA complex consists of three subunits, HIRA, UBN1, and CABIN1. To determine which subunit interacted with OGT, we first used HIRA mutants to define the OGT–HIRA protein–protein interaction domain in vivo. HIRA contains three domains: the HIRA A region (residues 1–400) that consists of WD40 repeats and interacts with the N terminus of UBN1 (49, 50); the HIRA B domain (residues 401–500) that interacts with ASF1a (33, 51, 52); and the HIRA C domain (residues 501–1017) that binds CABIN1 (28) (Fig. 1D, Top). GFP-tagged wild-type HIRA or various deletion mutants (HIRA-A only, HIRA-AB domains, and HIRA-C) were expressed in HEK293T cells and immunoprecipitated by using antibodies against GFP. The HIRA A domain, alone or in combination with the B domain, was sufficient for OGT binding, whereas the HIRA C domain exhibited a significant reduction in OGT binding, indicating that HIRA interacts with OGT through the HIRA A domain, either directly or indirectly (Fig. 1D).
Because both UBN1 and OGT associate with the same region of HIRA, we tested whether the HIRA–OGT interaction was mediated by UBN1. We performed immunoprecipitation assays by using wild-type UBN1 and a UBN1 mutant lacking the HIRA-binding domain (NHRD; amino acids 41–77) (Fig. 1E, Top). Wild-type UBN1 coprecipitated with OGT (Fig. 1E). In addition, whereas OGT bound to the UBN1 NHRD deletion mutant, neither HIRA nor CABIN1 did. This result suggests that the association of OGT with the HIRA complex is likely mediated by UBN1. Consistent with this interpretation, recombinant GST-OGT pulled down UBN1 from Sf9 cell extracts expressing UBN1 or coexpressing UBN1-HIRA, but not from extracts expressing HIRA alone in vitro (Fig. 1F). Therefore, our results suggest that OGT is a binding partner of the HIRA complex and associates with HIRA, most likely through UBN1.
OGT Is Required for de Novo Deposition of H3.3.
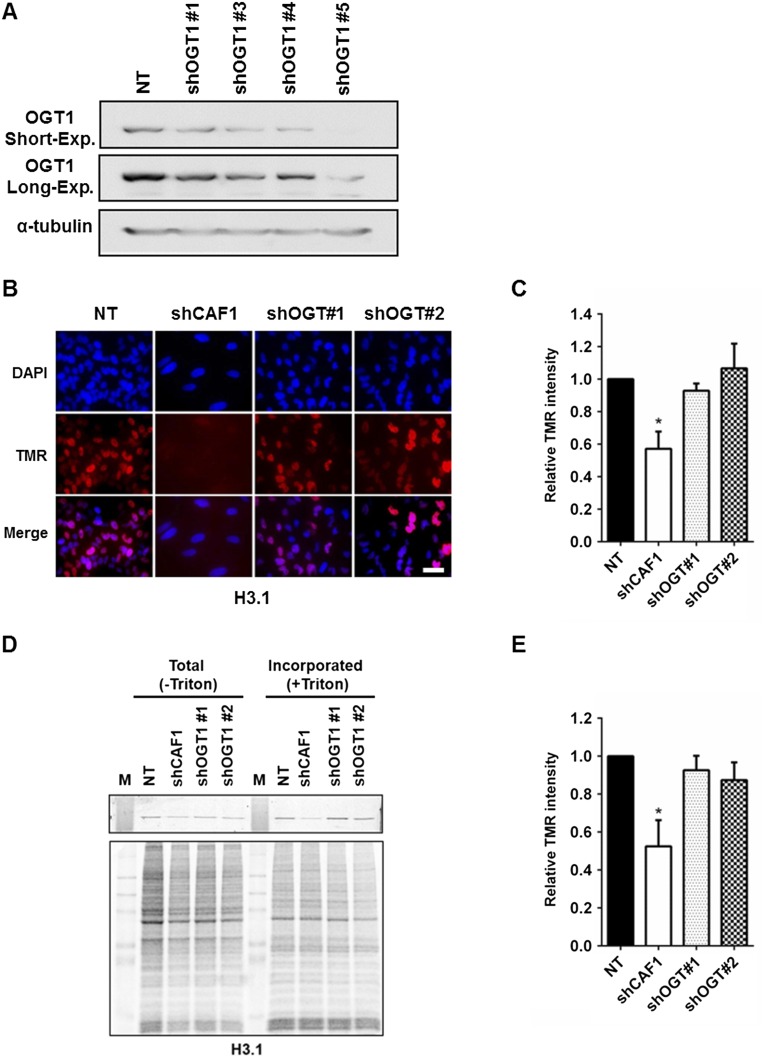
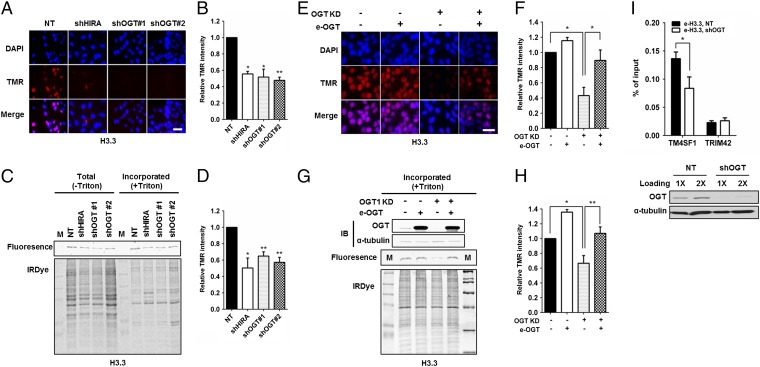
HIRA mediates H3.3–H4 nucleosome assembly at both active and poised genes. To explore the biological implication of the physical interaction between OGT and the HIRA complex, we tested whether OGT is involved in H3.3–H4 nucleosome assembly in human cells. Briefly, we used HeLa cells expressing H3.3 tagged with SNAP, a mutant form of O6-guanine nucleotide alkyltransferase that covalently reacts with benzyl-guanine (BG). After chasing existing H3.3-SNAP with a cell-permeable, nonfluorescent BG derivative, we labeled newly synthesized H3.3-SNAP with TMR (tetramethylrhodamine)-BG and detected newly synthesized H3.3-SNAP by either quantifying the fluorescence intensity of individual cells or analyzing H3.3-SNAP chromatin incorporation by using chromatin fractionation assays. In this way, we were able to monitor the deposition of newly synthesized H3.3. Two different small hairpin RNAs (shRNAs) (Fig. S1A) were used to deplete OGT in H3.3-SNAP–tagged HeLa cell lines (53). Analysis of the fluorescence intensity of individual cells revealed that depletion of OGT, like that of HIRA, resulted in a marked reduction in H3.3 deposition (approximately 40%) compared with control cells (Fig. 2 A and B). Consistently, depletion of OGT led to a significant decrease in H3.3 incorporation into chromatin compared with control cells (Fig. 2 C and D) based on analysis of chromatin-bound new H3.3-SNAP. In contrast, depletion of OGT had little effect on de novo H3.1 deposition (Fig. S1 B–E), suggesting that OGT is specifically required for efficient deposition of new H3.3. To confirm these results, we performed rescue experiments by transiently overexpressing OGT from an shRNA-resistant plasmid in OGT-depleted cells (Table S2). OGT expression rescued the H3.3 deposition defects in OGT-depleted cells as monitored by H3.3 deposition in individual cells (Fig. 2 E and F) and H3.3 incorporation into chromatin using the chromatin binding assay (Fig. 2 G and H). Taken together, these results indicate that OGT is required for the efficient deposition of new H3.3 in human cells.
Fig. S1.
Depletion of OGT does not affect deposition of histone H3.1. (A) Knockdown efficiency of five shRNA targeting OGT. Lentivirus for each of the five shRNAs targeting OGT, as well as a nontargeting control were transduced into HEK293T cells. Knockdown efficiency was monitored by Western blot. (B–E) OGT depletion has no apparent effect on de novo deposition of histone H3.1. HeLa cells expressing H3.1-SNAP cells were transduced with shRNA targeting OGT or CAF-1p150 (control). Nucleosome assembly of new H3.1 was monitored by using SNAP staining of individual cells (B and C) or chromatin fractionation (D and E) 72 h after selection. (Scale bar: 20 µm.) Values represent mean ± SEM (*P < 0.05; **P < 0.01; n = 3 biological replicates; Student’s t test).
Fig. 2.
OGT is required for de novo deposition of histone H3.3. (A–D) OGT depletion compromised the deposition of newly synthesized H3.3. OGT1 was depleted from H3.3-SNAP–tagged HeLa cells. After old H3.3-SNAP was blocked by using a nonfluorescent blocker, new H3.3-SNAP was marked with TMR-STAR and visualized by using a fluorescence microscopy (A). The SNAP-TMR signaling intensity was quantified and reported as mean ± SEM of three experiments (B; *P < 0.05; **P < 0.01). Depletion of HIRA was used as a control. (C and D) Deposition of new H3.3 was monitored by a chromatin fractionation assay. New H3.3-SNAP proteins on chromatin were detected by using a Typhoon FLA 7000 (C, Top), and total proteins detected by IRDye Blue Protein Stain (C, Bottom). (D) The relative SNAP intensity of H3.3-SNAP over total proteins was calculated and reported as the mean ± SEM of three independent experiments. (E–H) Expression of shRNA-resistant OGT rescues H3.3 deposition defect caused by OGT depletion. (E) Representative images. (F) Quantification of the SNAP-H3 intensity from three independent experiments (mean ± SEM, *P < 0.05). (G and H) Deposition of new H3.3 was monitored by using the chromatin fractionation assay shown in G. Results from three independent experiments were shown in H (mean ± SEM, *P < 0.05; **P < 0.01). (I) Depletion of OGT results in reduced H3.3 occupancy at an H3.3 enriched gene locus. H3.3-Flag ChIP was performed in cells with or without OGT depletion and ChIP DNA was analyzed by real-time PCR. Results from three independent experiments were shown (mean ± SEM, *P < 0.05). I, Bottom shows the OGT knockdown efficiency. (Scale bars: 20 µm.)
Table S2.
The sequences of primer pairs used for construction of the resistant OGT plasmid against shOGT and ChIP-PCR
| Primer name | Direction | Primer sequence |
| shRNA-resistant OGT | For. | 5′-GACAGATCTGCTCACTTTAGTACTCTGGCAATTAAACAGA-3′ |
| Rev. | 5′-TCTGTTTAATTGCCAGAGTACTAAAGTGAGCAGATCTGTC-3′ | |
| TTS of TM4SF1 | For. | 5′-AAGACAGGAAGCCGTTAGCAA-3′ |
| Rev. | 5′-GGCAGGAGGACCACGAGGAA-3′ | |
| TSS of TRIM42 | For. | 5′-AGTTTCCACCAACATACCAGC-3′ |
| Rev. | 5′-TCC-CAGGACTCTTGATGCCT-3′ |
For., forward; Rev., reverse; TSS, transcription start site; TTS, transcription terminal site.
Using a chromatin immunoprecipitation (ChIP) assay, we have shown that HIRA is important for H3.3 occupancy at the transcription terminal site of TM4SF1, but not the transcription starting site of TRIM42, which is marked by H3.1 (54). Depletion of OGT resulted in a significant reduction in H3.3 occupancy at TM4SF1 compared with control cells (Fig. 2I). This result provides additional support for the idea that OGT regulates the HIRA-dependent deposition of newly synthesized H3.3.
OGT Regulates the Integrity of the HIRA–H3.3 Complex.
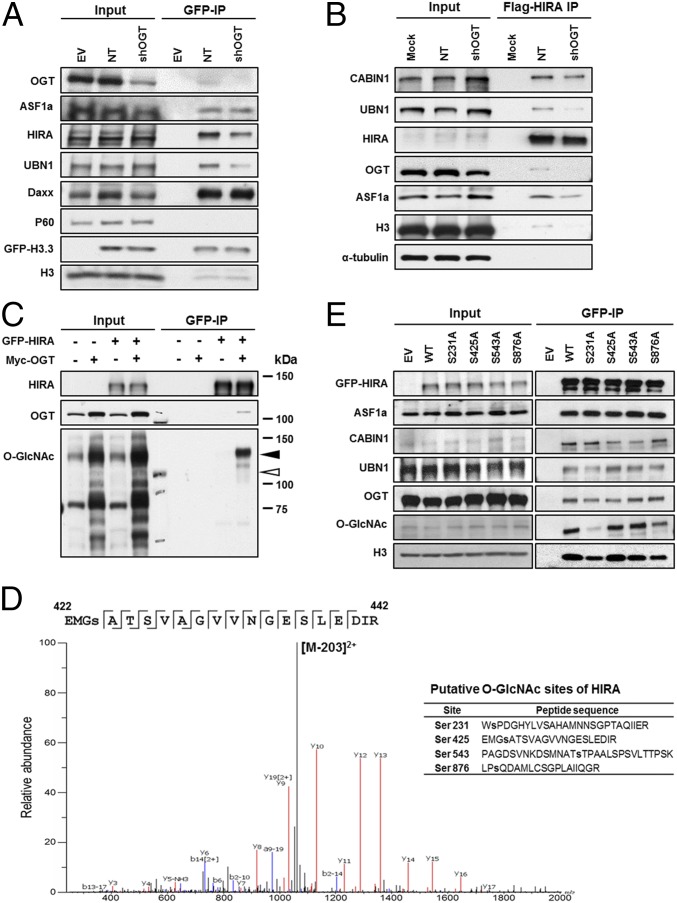
To understand how OGT regulates H3.3 deposition, we asked whether OGT was required for the efficient association of H3.3–H4 with its chaperones. GFP-tagged H3.3 was purified from 293T cells with or without OGT depletion, and copurified proteins were detected by Western blot (Fig. 3A). Consistent with published reports, histone chaperones, ASF1a, Daxx, and HIRA, but not CAF-1 (p60), copurified with H3.3 (15). Interestingly, less HIRA and UBN1 copurified with H3.3 in OGT-depleted cells than in control cells (Fig. 3A), whereas depletion of OGT had little effect on the association of H3.3 with Asf1a and Daxx.
Fig. 3.
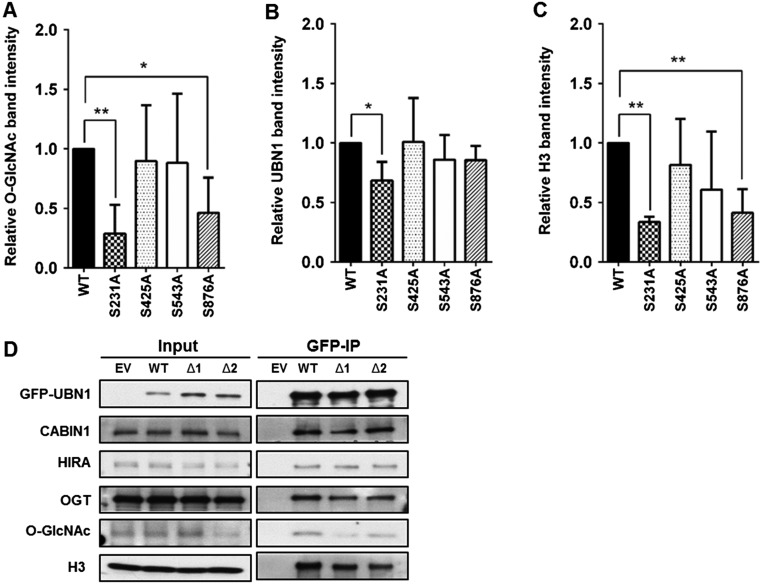
OGT modifies HIRA and O-GlcNAcylation of HIRA regulates the integrity of HIRA-H3.3 complex in vivo. (A) OGT depletion results in reduced association of HIRA with H3.3. HEK293T cells stably expressing GFP-tagged histone H3.3 (GFP-H3.3) were transduced with viruses for nontarget (NT) or shRNA against OGT. GFP-H3.3 was purified by using antibodies against GFP and copurified proteins were analyzed by Western blot. (B) OGT depletion leads to reduced association of HIRA with UBN1. Stably expressed Flag-HIRA from nontargeting control (NT) or OGT depleted cells (shOGT) was immunoprecipitated (IP) with Flag M2 beads in 293T cells and input and IP proteins were analyzed by Western blotting using the indicated Abs. (C) HIRA is O-GlcNAcylated. GFP-tagged HIRA were expressed in 293T cells with or without expression of Myc-tagged OGT. GFP-HIRA was immunoprecipitated, and proteins in IP and input were analyzed by Western blot. Black and white arrowheads on the O-GlcNAc blot denote the expected HIRA and OGT molecular masses, respectively. Kilodaltons (kDa) are a measure of molecular mass. (D) Identification of potential O-GlcNAcylation sites of HIRA. GFP-HIRA was purified as described in C and subjected to nano-flow liquid chromatography electrospray tandem mass spectrometry. (E) Mutations at a putative O-GlcNAcylation residue of HIRA affect O-GlcNAcylation and the integrity of the HIRA–H3.3 complex. GFP-tagged full length HIRA (WT) or single mutant HIRA (S231A, S425A, S543A, and S876A) were expressed in 293T cells and immunoprecipitated by antibodies against GFP.
Next, we analyzed how depletion of OGT affected the integrity of the HIRA complex by immunoprecipitating Flag-HIRA from OGT-depleted or not targeting control cells. Less UBN1, CABIN1, and H3.3 copurified with HIRA in OGT-depleted cells compared with control cells (Fig. 3B). These results suggest that OGT is required for the formation of the HIRA–H3.3 complex.
HIRA O-GlcNAcylation Is Important for the Integrity of the HIRA–H3.3 Complex.
OGT catalyzes the addition of N-acetylglucosamine to serine or threonine of a target protein. We therefore asked whether OGT modified HIRA and UBN1, two key components of the HIRA complex. GFP-tagged HIRA was immunoprecipitated under denaturing conditions in the presence of exogenously expressed Myc-tagged OGT, and precipitated proteins were analyzed by Western blot. We found that both HIRA and UBN1 were O-GlcNAcylated (Fig. 3C and Fig. S2 A and B). Next, we used mass spectrometry and identified four amino acid residues (S231, S425, S593, and S878) of HIRA and five amino acids (T861, S866, T989, S999, and S1003) of UBN1 that were likely O-GlcNAcylated (Fig. 3D and Fig. S2C). These results indicate that HIRA and UBN1 are likely modified with O-GlcNAc in vivo. In addition, we estimated that O-GlcNAc–modified HIRA was ∼4% of unmodified HIRA under conditions when OGT was overexpressed (Fig. S2D). It is likely that a small fraction of HIRA will also be modified by OGT under normal cellular conditions, potentially suggesting that this modification is transient and may have a regulatory role.
Fig. S2.
HIRA and UNB1 are O-GlcNAcylated. (A) HIRA is O-GlcNAcylated. Flag-tagged HIRA and Myc-tagged OGT were expressed in HEK293T cells. Flag-tagged HIRA and associated proteins were immunoprecipitated with FLAG M2 beads. Immunoprecipitated (IP) proteins and total cell extracts (input) were analyzed by Western blot analysis using the indicated antibodies. (B and C) UBN1 is O-GlcNAcylated by OGT. GFP-tagged UBN1 and Myc-tagged OGT were expressed in HEK293T. UBN1-GFP was immunoprecipitated, and associated proteins were analyzed by Western blotting using the indicated antibodies (B) or by mass spectrometry (C). (D) Quantification of modified and unmodified peptide of HIRA. Selected ion chromatograms of 1,061.01 [M+2H]2+ and 1,162.55 [M+2H]2+ that correspond to the unmodified and the O-GlcNac modified, respectively, are shown (Left). The peak area of the O-GlcNAc–modified peptide ions is ∼4% of the unmodified ion peak area (Right).
To determine which potential O-GlcNAcylated residue(s) of HIRA were functionally relevant, we mutated each residue (S231, S425, S543, and S876) to alanine, expressed each HIRA mutant tagged with GFP in 293T cells, and analyzed how each mutation affected GlcNAcylation. Mutations at S231 and S876 consistently exhibited the most dramatic effect on HIRA GlcNAcylation, suggesting that these sites are modified in cells (Fig. 3E and Fig. S3A). Less UBN1 copurified with HIRA S231A mutant than wild-type HIRA. Furthermore, both HIRA S231A and S876A mutants also exhibited reduced association with H3 compared with HIRA, whereas two other HIRA mutants (S425A and S543A) had no apparent effect on the formation of the HIRA–H3.3 complex (Fig. 3E and Fig. S3 B and C). These results indicate that O-GlcNAcylation of HIRA impacts the interaction of the HIRA complex with H3.3, and to a lesser extent, the interaction of the HIRA subunit with UBN1.
Fig. S3.
HIRA S231A mutation affects O-GlcNAcylation of HIRA, the HIRA–UBN1 interaction, and the formation of HIRA–H3.3 complex. (A–C) Quantification of Western blot in Fig. 3E by densitometric analysis from three independent experiments. Western blot band intensities were obtained by using ImageJ. Band intensities were normalized to GFP-IP band intensity. Relative intensity is to that of WT HIRA value. Values represent mean ± SEM (*P < 0.05; **P < 0.01; n = 3 biological replicates; Student’s t test). (D) Deletion of potential UBN1 O-GlcNAcylation sites does not affect the integrity of the HIRA–H3.3 complex. GFP-tagged full-length or UBN1 deletion mutants [T861-S866 (Δ1) and T989-S1003 (Δ2)] were expressed in HEK293T cells along with Myc-tagged OGT. Total lysates were subjected to IP with GFP antibodies followed by Western blot analysis of associated proteins as indicated.
We also tested how mutating potential UBN1 O-GlcNAcylation sites affected O-GlcNAcylation and formation of the HIRA–H3.3 complex. Because the five residues (T861, S866, T989, S999, and S1003) are close, we made two deletion mutants (from T861 to S866, Δ1, and from T989 to S1003, Δ2) and tested how these two deletion mutants affected the O-GlcNAcylation of UBN1 and its interaction with H3–H4. As shown in Fig. S3D. UBN1 O-GlcNAcylation was reduced in both deletion mutants. However, these deletion mutants did not show notable effects on UBN1 association with HIRA, CABIN1, or histone H3, suggesting that UBN1 O-GlcNAcylation is dispensable for the HIRA complex and H3 interaction and likely has other functions.
HIRA O-GlcNAcylation Affects de Novo Deposition of H3.3.
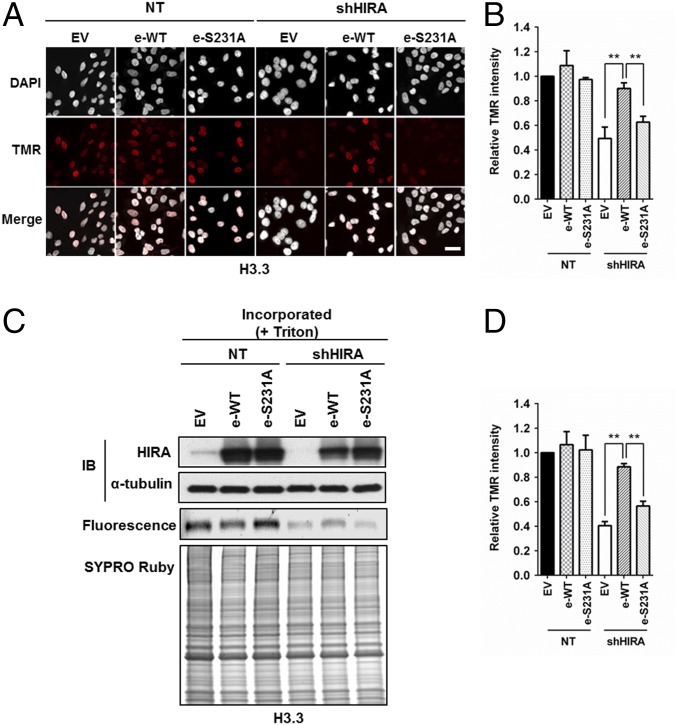
The HIRA complex regulates H3.3 deposition, and depletion of HIRA results in reduced deposition of new H3.3–H4. To investigate whether HIRA O-GlcNAcylation affects H3.3 deposition, we performed complementation experiments by expressing WT HIRA and the O-GlcNAcylation HIRA mutant (S231A) in HeLa cells depleted of HIRA, and analyzed deposition of H3.3 in individual cells using immunofluorescence (Fig. 4 A and B) and in cell populations using chromatin fractionation (Fig. 4 C and D). By both assays, we observed that depletion of HIRA led to a reduction in H3.3 deposition. Expression of shRNA-resistant wild-type HIRA, but not the HIRA S231A mutant, rescued the effect of HIRA depletion on H3.3 deposition (Fig. 4). Because wild-type HIRA and HIRA S231A mutant were expressed at a similar level (Fig. 4C), the inability of the HIRA S231A mutant to rescue nucleosome assembly defects is likely due to reduced O-GlcNAcylation and compromised ability to form the HIRA–H3.3 complex. These experiments suggest that the O-GlcNAc modification of HIRA is required for the efficient incorporation of histones H3.3–H4 into chromatin.
Fig. 4.
HIRA S231A mutant exhibits defects in de novo histone H3.3 deposition. (A and B) Exogenously expressed HIRA, but not HIRA S231A mutant, rescues the H3.3 defect caused by HIRA depletion. Endogenous HIRA was depleted from HeLa cells expressing H3.3-SNAP, and HIRA or HIRA S231A mutant was reexpressed. The effect of H3.3 deposition was monitored at individual cells by using microscopy. (A) Representative DAPI and H3.3-SNAP staining images. EV, empty vector. (Scale bar: 20 μm.) (B) Quantification of H3.3-SNAP staining intensity at individual cells (mean ± SEM, **P < 0.01, n = 3 biological replicates). (C and D) HIRA S231A mutant fails to complement H3.3 deposition defects as detected by chromatin fractionation assays. The experiments were performed as described above except that chromatin fractionation assays were used to monitor H3.3 deposition. Proteins in chromatin fractions were analyzed. C, Top shows analysis of HIRA depletion and expression of exogenous wild type HIRA (e-WT) or HIRA S231A mutant (e-S231A). H3.3-SNAP florescence was detected by phosphoImager (C, Middle) and total proteins by SYPRO Ruby staining (C, Bottom). Relative TMR intensity was calculated by normalizing H3.3-SNAP signals against whole protein levels (D, mean ± SEM, **P < 0.01, n = 3 biological replicates).
OGT Is Involved in Cellular Senescence.
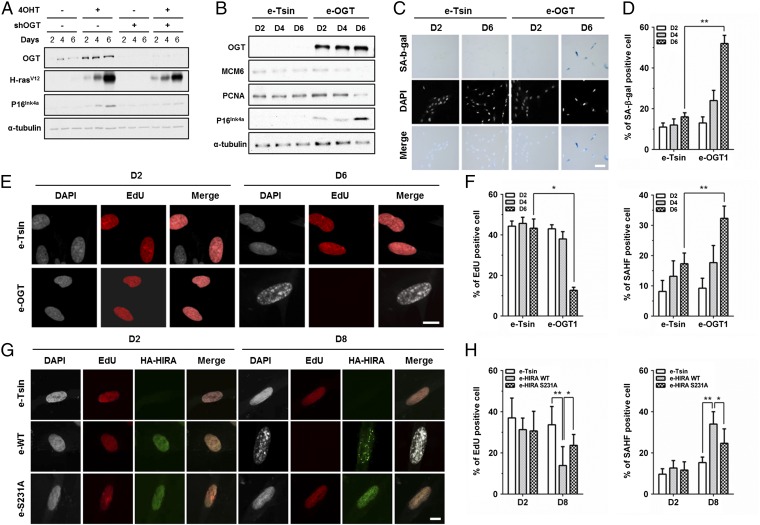
HIRA-mediated deposition of H3.3 regulates chromatin dynamics in senescent cells and is, in part, responsible for the formation of SAHF (29, 33, 48). SAHF, in turn, repress the transcription of proliferation-related genes, including Cyclin A, minichromosome maintenance complex 6 (MCM6), and PCNA, thereby maintaining permanent cell-cycle arrest in senescent cells (32, 55). In addition to SAHF formation, the cellular transition from proliferation to senescence is marked by increased expression of the cell cycle inhibitor p16INK4A and enhanced staining with SA β-gal. To determine whether OGT has a role in senescence, we first monitored p16INK4A and OGT expression in primary human IMR90 fibroblasts undergoing premature senescence induced by oncogenic Ras expression. IMR90 cells with OGT depletion (shRNA targeting OGT, shOGT) were transduced with an H-RasV12 overexpression construct under the control of the estrogen promoter. After transduction and selection, cells were treated with 4-hydroxytamoxifen (4OHT) to induce H-RasV12 expression and, thereby, trigger cellular senescence. Lysates were prepared from cells between 0 and 6 d after selection, and p16INK4A expression was examined by Western blot (Fig. 5A). As expected, p16INK4A increased with increased expression of oncogenic Ras. Importantly, depletion of OGT compromised the accumulation of p16INK4A compared with control cells. We also observed that OGT expression increased with H-RasV12 induction (Fig. 5A). These results suggest that OGT is required for oncogene-induced cellular senescence.
Fig. 5.
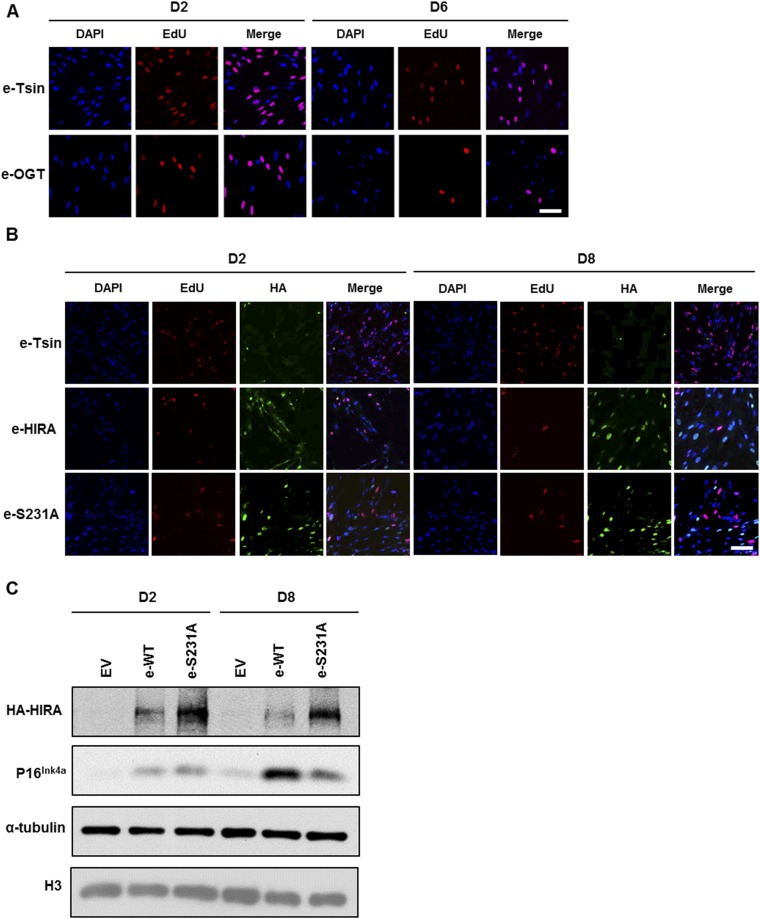
OGT is involved in cellular senescence. (A) OGT is required for oncogene-induced senescence. OGT was depleted by using shOGT from IMR90 cells with or without expression of H-RasV12 under control of 4OHT. The level of p16INK4A was analyzed by Western blotting. (B–F) Overexpression of OGT results in increased cellular senescence and reduced cell proliferation. (B) Analysis of p16INK4a, MCM6, and PCNA by Western blotting using lysates from IMR90 cells without (empty vector, e-Tsin) and with overexpression of OGT (e-OGT). α-Tubulin was used as a loading control. (C) IMR90 cells in the absence or presence of OGT overexpression were stained for SA-β-gal activity followed by DAPI staining. (D) Quantification of percentage cells with SA-β-gal staining (mean ± SEM, **P < 0.01, n = 3 biological replicates). (E) Confocal microscopy imaging of DAPI and EdU (red) staining of IMR90 cells. (F) Percentage of SAHF-positive cells (DAPI-dense foci, right) or cells with EdU staining (Left) was calculated (mean ± SEM, *P < 0.05, **P < 0.01, n = 3 biological replicates). (G and H) Overexpression of HIRA, but not HIRA S231 mutant promotes cellular senescence. (G) Representative images of DAPI, EdU (red), and HA (green) images obtained using confocal microscopy were shown. (H) Quantification of EdU and SAHF positive cells at the indicated time postselection (mean ± SEM, *P < 0.05, **P < 0.01, n = 3 biological replicates). (Scale bars: C, 50 μm; E and G, 10 μm.)
Next, we investigated whether ectopic expression of OGT was sufficient to drive cellular senescence in the absence of activated oncogenic Ras signaling. Early-passage IMR90 fibroblasts were transduced with a lentivirus encoding an empty vector or a vector expressing wild-type OGT. To monitor senescence, we analyzed p16INK4A expression, SA β-gal staining, SAHF formation, and incorporation of the deoxyribonucleotide precursor EdU (5-ethynyl-2′-deoxyuridine) as an indicator of cell proliferation. Ectopic expression of OGT (e-OGT) resulted in increased expression of p16 (Fig. 5B), with a concomitant reduction in the levels of the E2F-target genes MCM6 and PCNA. In addition, OGT overexpression resulted in an increase in the percentage of SA–β-gal positive cells on day 6 compared with controls (Fig. 5 C and D). Finally, at day 6 after OGT overexpression, the percentage of EdU-positive cells was dramatically reduced compared with control cells, with a concomitant increase in the percentage of cells with SAHF as monitored by DAPI staining (Fig. 5 E and F and Fig. S4A). In summary, these results strongly support the notion that OGT regulates cellular senescence.
Fig. S4.
Overexpression of OGT and HIRA S231A mutant affect cellular senescence. (A) OGT overexpression inhibits proliferation. EdU staining was performed at the indicated time points in IMR90 cells expressing empty vector (e-Tsin) or OGT (e-OGT). The experiment was performed as described in Fig. 5E. (B) Expression of the HIRA S231A mutant had a reduced effect on inhibition of proliferation during cellular senescence compared with wild-type HIRA. Wild-type HIRA or HIRA S231A was transduced in IMR90 cells, and EdU staining was performed at the indicated time points. (C) Expression of the HIRA S231A mutant has reduced effect on p16INK4A expression compared with wild-type HIRA. Wild-type HIRA (e-WT) or mutant HIRA S231A (e-S231A) was expressed in IMR90 cells, and p16INK4A expression was detected by Western blot. EV, empty vector. (Scale bars: 20 µm.)
Next, we explored whether mutation at HIRA O-GlcNAcylation is important in cellular senescence. To test this idea, early-passage IMR90 fibroblasts were transduced with a lentivirus encoding an empty vector, wild-type HIRA (e-HIRA), or HIRA S231A mutant (e-S231A), and cell senescence was monitored by examining p16INK4A expression (Fig. S4C), EdU incorporation, and SAHF formation. Cells expressing e-HIRA showed increased p16INK4A expression at 8 d after transduction compared with day 2 (Fig. S4C). HIRA overexpression also resulted in a decrease in proliferation and a dramatic increase in the number of cells with SAHF (Fig. 5 G and H and Fig. S4B). Compared with wild-type HIRA, overexpression of the HIRA S231A mutant led to reduced expression of p16INK4A, increased proliferation, and reduced SAHF formation at the same time points. Taken together, these results strongly support the idea that HIRA O-GlcNAcylation is important for HIRA’s ability to promote SAHF formation and regulate cellular senescence.
Discussion
Here, we report a previously uncharacterized association between the histone H3.3–H4 chaperone HIRA and a key cell sensor protein OGT. By mass spectrometry, we identified OGT as a binding partner of the HIRA complex, and we show that OGT associates with HIRA, most likely through UBN1. Depletion of OGT leads to reduced deposition of newly synthesized H3.3 onto chromatin and compromises the integrity of the HIRA–H3.3 complex. Furthermore, mutations at S231, a potential GlcNAcylation site on HIRA, result in similar defects in nucleosome assembly as OGT depletion. Importantly, depletion of OGT compromises oncogenic stress-induced premature senescence. In contrast, overexpression of OGT or HIRA promotes cellular senescence in human fibroblasts, whereas overexpression of HIRA S231A does not efficiently promote premature cellular senescence. Therefore, these results support a model whereby OGT binds and modifies HIRA and promotes HIRA-mediated nucleosome assembly of H3.3–H4 and cellular senescence.
OGT Regulates HIRA-Mediated Nucleosome Assembly of H3.3–H4.
Histone variant H3.3 is assembled into nucleosomes at distinct chromatin domains in a replication-independent process (11–18). In addition, histone H3.3 is mutated in high-grade pediatric brain tumors, chondroblastoma, and giant cell tumors (56–58). Therefore, it is important to determine how nucleosome assembly of H3.3 is regulated. In this work, we present the following lines of evidence supporting the idea that OGT O-GlcNAcylates HIRA and regulates HIRA-mediated H3.3–H4 nucleosome assembly. First, we show that OGT interacts with the HIRA histone chaperone complex, most likely through the UBN1 subunit of the HIRA complex. Second, OGT is required for the efficient deposition of H3.3. Third, OGT O-GlcNAcylates both HIRA and UBN1, and mutations at a putative HIRA O-GlcNAcylated residue compromise H3.3–H4 nucleosome assembly. Together, these results support the idea that OGT regulates HIRA-mediated nucleosome assembly.
How does OGT regulate HIRA-mediated nucleosome assembly? One possibility is that O-GlcNAc modification of HIRA influences the expression of the HIRA complex. However, unlike depletion of HIRA complex subunits that affects the stability of other subunits (28, 49), depletion of OGT does not affect the expression of three HIRA subunits (Fig. 3 A and B). In addition, UBN1 and CABIN1 protein levels are not affected by mutation of HIRA O-GlcNAcylation sites (Fig. 3E). This result suggests that the role of OGT in nucleosome assembly is most likely not due to its impact on the expression of the HIRA complex components UBN1 and CABIN1. Instead, we observed that depletion of OGT compromises the formation of the HIRA–H3.3 complex (Fig. 3B). In addition, blocking HIRA O-GlcNAcylation at S231 dramatically reduced HIRA–H3.3 binding and overall HIRA O-GlcNAcylation (Fig. 3E). Because S231 of HIRA resides in the HIRA–UBN1 interaction domain, it is possible that O-GlcNAcylation of this residue directly mediates the interaction of UBN1-HIRA in cells. Alternatively, O-GlcNAc is a bulkier posttranslational modification that has the potential to induce conformational changes. Thus, O-GlcNAcylation of HIRA by OGT in vivo enables HIRA–UBN1–CABIN1–H3.3 complex formation, which in turn regulates H3.3 nucleosome assembly.
The Role of HIRA O-GlcNAcylation and OGT in Cellular Senescence.
Recent studies show that changes in OGT and O-GlcNAc of nucleocytoplasmic proteins are linked to aging-associated diseases such as Alzheimer’s (59–61). In rodent models, O-GlcNAc levels increase in multiple aged tissues (62). However, the molecular basis of the function of OGT in aging has not been clearly elucidated. In this study, we present the following lines of evidence indicating that O-GlcNAcylation of HIRA by OGT may regulate cellular senescence. We observed that the level of OGT increases during cell senescence induced by oncogenic Ras. Moreover, depletion of OGT in human primary fibroblasts compromises premature cellular senescence, whereas overexpression of OGT promotes cellular senescence and SAHF formation. In senescent cells, SAHF formation is largely due to augmented nucleosome density, and these changes to nuclear architecture are specific to the senescent state (47). Therefore, our results strongly support a function for OGT in cellular senescence. Given that OGT promotes the deposition of H3.3 through HIRA O-GlcNAcylation, and expression of a HIRA mutant deficient in O-GlcNAcylation compromises the ability of HIRA to promote senescence, OGT’s role in senescence is likely at least in part mediated through its role in H3.3 deposition.
Although OGT is known to modify a variety of cytosolic substrates, accumulating evidence indicates that OGT also has a role in chromatin regulation. For instance, OGT is essential for PcG repression of homeotic genes (63, 64). Moreover, OGT modifies Ezh2, the catalytic subunit of PRC2 complex (65). Recently, it has been shown that OGT interacts with the Ten-Eleven-Translocation (TET) enzymes, which catalyze DNA demethylation (66). The OGT–TET interaction affects H2B O-GlcNAcylation and HCF1. HCF1 is a component of the SET1/COMPASS complex, and HCF1 O-GlcNAcylation is important for the SET1 complex integrity (67). Our studies reveal a previously unidentified role for OGT in chromatin dynamics and cellular senescence. Future studies should focus on how this modification of HIRA affects H3.3 incorporation/nucleosome assembly in various biological contexts and how misregulation of OGT and nucleosome assembly may promote aging and/or the development of human disease.
Materials and Methods
SI Materials and Methods provides a detailed discussion of the materials and methods used in this study.
Cell Culture.
HEK293T (ATCC), HeLa, and IMR90 (ATCC) cells were maintained according to standard protocols. Information of stable cell line and cell transfections are described in SI Materials and Methods.
HIRA Protein Complex Purification.
HIRA were purified by tandem immunoprecipitation as outlined in SI Materials and Methods.
Immunoprecipitation and Western Blotting.
A detailed protocol is provided in SI Materials and Methods.
In Vitro GST-Pulldown Assays.
Recombinant GST-OGT fusion protein was purified by using glutathione agarose beads and used for pull down assays as described in SI Materials and Methods.
H3-SNAP Staining and Chromatin Immunoprecipitation Assay and Real-Time PCR.
These assays were performed as described in SI Materials and Methods. Primer sets used for ChIP-PCR are listed in Table S1.
Immunofluorescence and Senescence Assays.
A detailed protocol is provided in SI Materials and Methods.
SI Materials and Methods
Cell Culture and Gene Transfer.
HEK293T (ATCC), HeLa and IMR90 (ATCC) cells were grown in DMEM (Cellgro) supplemented with 10% (vol/vol) FBS (Sigma) and 1% penicillin/streptomycin (GIBCO), maintained at 37 °C with 5% (vol/vol) CO2. To generate stable cell lines, HEK293T or HeLa cells were transduced by lentiviral vectors encoding H3.3 (pTsin-puro-H3.3) and selected with 2 µg/mL puromycin. Clones with low expression levels were used in experiments and maintained in medium with 1 µg/mL puromycin. HeLa stable cell lines expressing H3.3-SNAP were grown in the presence of 250 µg/mL G418. Transient transfections were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Lentivirus expressing OGT and wild-type and mutant HIRA were produced in HEK293T following the same procedures. The retrovirus expressing H-RasV12 (pLNC-neo ER::RASV12) and HIRA (pBABE-Flag-His-HIRA) vectors were packaged in Phoenix cells and transduced into targeting cells following protocols described (68).
Plasmid Construction and Description.
Plasmids for expressing H3-SNAP were constructed as described (24). Plasmids for GFP-H3.3 expression were constructed by cloning the cDNA into pEGFP-C1 mammalian expressing vector (Addgene) at XhoI and EcoRI restriction enzyme sites. Myc-OGT plasmids were provided by Xiaochun Yu, University of Michigan Health System, Ann Arbor, MI. GST-OGT plasmids were provided by El Bachir Affar, University of Montréal, Montreal. Plasmids for HIRA expression were generated in the EGFP-N1 vector. Plasmids for UBN1 expression were generated by using the EGFP-C1 vector. The cDNAs encoding OGT and HIRA were subcloned into the pTsin vector for lentivirus production and infection. Plasmids for expression of HIRA putative O-GlcNAcylation site mutants (S231A, S425A, S543A, and S876A) were generated by site-directed mutagenesis and were subcloned into pEGFP-N1. All site-directed mutagenesis experiments were carried out with Phusion polymerase (New England Biolabs). All plasmid constructs were confirmed by Sanger DNA sequencing.
shRNA Interference and Lentivirus Package.
The shRNAs against OGT and HIRA were purchased from Sigma-Aldrich and validated for knockdown efficiency. shRNAs: shOGT#1, TRCN0000286200; shOGT#3, TRCN0000293652; shOGT#4, TRCN0000286201; shOGT#5, TRCN0000286199 and shHIRA, TRCN0000232156. Lentiviral particles were produced by using HEK293T cells.
HIRA Protein Complex Purification.
To purify HIRA from 293T cells, a stable line expressing HIRA tagged with Flag and HIS tags was isolated. Proteins were purified from cytosolic S100 and nuclear extracts using affinity chromatography. Cells were harvested by trypsin digestion and washed once with cold PBS. The cell pellet was resuspended in 2× the pellet volume of hypo buffer and incubated on ice for 10 min. Cells were homogenized 14 strokes with a B type pestle (glass Dounce homogenizer). After incubation on ice for 10 min, the hypo buffer extract was separated from the nuclei by centrifugation at 1,000 × g for 10 min. The supernatant was further spun at 20,000 × g for 30 min. The nuclei were resuspended with an equal volume of nuclear extraction buffer and incubated at 4 °C for 30 min. The nuclear extract was spun at 20,000 × g for 30 min. To purify HIRA containing complexes, the S100 and nuclear extracts were dialyzed in A100 buffer [25 mM Tris, pH7.5, 1 mM EDTA, 0.01% Nonidet P-40, 10% (vol/vol) glycerol, 100 mM NaCl, 1 mM DTT, 1 mM benzamidine, 0.5 mM PMSF, 10 mM NaF]. After centrifugation at 100,000 × g for 30 min, the supernatants were incubated with M2 beads overnight. Proteins were eluted with 2 mg/mL Flag peptide in A100 at 16 °C for 30 min. Proteins eluted from M2 beads were incubated with Ni-NTA beads for further purification of HIRA at 4 °C for 4 h, and protein complexes were eluted with SDS sample buffer, resolved via SDS/PAGE and subjected to analysis by mass spectrometry (69) or Western blot.
Immunoprecipitation and Western Blotting.
To immunoprecipitate proteins using GBP (anti-GFP) beads, 293T cells were lysed by using lysis buffer containing 50 mM Hepes–KOH (pH 7.4), 100 mM NaCl, 1% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, 1 mM DTT and proteinase inhibitors (Roche). After clarification by centrifugation, lysates were incubated with 30 µL of GBP (anti-GFP) beads at 4 °C overnight. The beads were washed by using washing buffer containing 50 mM Hepes–KOH (pH 7.4), 150 mM NaCl, 0.01% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA for 5 min × four times. Proteins were eluted by using SDS sample buffers and analyzed by Western blot. For immunoprecipitation of Flag-HIRA using M2 beads, cells were lysed by using the lysis buffer [50 mM Hepes-KOH, pH7.4, 200 mM NaCl, 0.5% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1 mM Benzamidine] and dounced by 30 passages. After clarification by centrifugation, the lysates were incubated with 30 µL of M2 (anti-Flag) beads at 4 °C for overnight. The beads were washed by using washing buffer [50 mM Hepes-KOH, pH 7.4, 100 mM NaCl, 0.01% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, and protease inhibitors] for 5 min × six times. Purified proteins were eluted with 2 mg/mL Flag peptide. The eluted proteins were precipitated by using TCA, dissolved with 1× SDS sample buffer, and analyzed by Western blot.
To perform Western blot analyses, SDS/PAGE gels were transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked in Tris-buffered saline containing 5% (wt/vol) skim milk powder and were then probed with the various antibodies used in this study: p60 (Abcam, ab8133), Asf1a (homemade, MC2114), anti-Flag (Sigma, M2), rabbit anti-EGFP (Abcam, ab6556), α-tubulin (DSHB, 12G10), OGT (Cell Signaling, 5368), HIRA (Millipore, WC119), Daxx (Abcam, ab2017), UBN1 (Abcam, ab84953), CABIN1 (Abcam, ab3349), O-GlcNAc (Sigma, O7764, CTD110.6 clone), H3 (Abcam, ab1791), HA (Abcam, 12CA5 clone, ab16918), H-RAS (Santa Cruz Biotechnology, sc-29), PCNA (Abcam, PC10, ab29), MCM6 (Abcam, ab184147), P16 (Santa Cruz Biotechnology, sc-468).
GST-OGT Pull-Down Assay.
Recombinant GST-OGT fusion protein was expressed in BL21 (DE3) and purified by using Glutathione Sepharose 4 Fast Flow beads (GE Healthcare) as described (70). Cell extracts from Sf9 insect cells individually infected or coinfected with baculoviruses to express recombinant full-length HIS-HIRA and Flag-UBN1 were prepared by Dounce homogenization in 50 mM Hepes–KOH (pH 7.4), 200 mM NaCl, 1% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, 1 mM DTT and proteinase inhibitors (Roche). For in vitro binding assay, 2–3 μg of beads containing recombinant GST-OGT were incubated with full-length HIRA, UBN1, and HIRA–UBN1 complex extracts for 6–8 h at 4 °C. The beads were washed by using washing buffer containing 50 mM Hepes–KOH (pH 7.4), 100 mM NaCl, 0.01% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA for 5 min × three times. Proteins were eluted by using SDS sample buffers and analyzed by Western blot.
Analysis of Deposition of New H3.1 or H3.3 Using H3-SNAP Staining.
Detection of new H3.1 and H3.3 deposition using the SNAP staining was performed as described (24, 53, 71). Briefly, 10 µM SNAP block reagent (New England Biolabs) was added to culture medium at 37 °C for 30 min to quench old H3.1-SNAP or H3.3-SNAP in HeLa cells. Cells were then washed with fresh medium three times and incubated in fresh medium for another 30 min. After chasing for 8 h, 2 µM SNAP-TMR-Star (New England Biolabs) was added to the medium for 20 min at 37 °C. Cells were then preextracted with 0.5% Triton X-100 (Sigma) and fixed in paraformaldehyde. A fluorescence microscope (40×) was used to record fluorescent images of SNAP staining, and ImageJ was used to quantify the SNAP fluorescence intensity. For each experiment, >200 cells were counted. Alternatively, a chromatin fraction assay was performed to analyze the incorporation of new H3.1 or H3.3 into chromatin (72). After SNAP staining, the cells were collected by trypsin and washed with PBS. Cells were extracted with CSK buffer (10 mM Pipes pH = 7, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2) on ice for 5 min followed by high-speed centrifugation (18,000 × g). The chromatin pellet was washed with PBS and dissolved with 1× SDS sample buffer. Proteins were separated by SDS/PAGE, and SANP-tagged proteins were detected by a Typhoon 7900. Total proteins were visualized by IRDye Blue/SYPRO Ruby Protein Stain and used for normalization controls.
Chromatin Immunoprecipitation Assay and Real-Time PCR.
ChIP-PCR assays were performed as described (25). Briefly, cells were cross-linked with 1% (vol/vol) formaldehyde for 10 min at room temperature and quenched by the addition of 0.125 M glycine. After quenching with glycine, cells were resuspended in lysis buffer [50 mM Hepes pH 7.5, 1% Triton X-100, 140 mM NaCl, 1 mM EDTA, 0.1% (wt/vol) sodium deoxycholate, and protease inhibitors] and digested by MNase (NEB; M0247S, 0.5 units per 1,000 cells) to achieve a mean DNA fragment size of 150–300 bp. After clarification by centrifugation, supernatants were incubated with M2 (anti-Flag) beads (1.5 mg/1 × 107 cells) overnight at 4 °C. The beads were washed extensively, and the DNA was recovered by using elution buffer (10 mM Tris at pH 8.0, 10 mM EDTA at pH 8.0, 1% SDS, 150 mM NaCl, 5 mM DTT). DNA was subsequently purified by using Qiagen Mini Elute PCR purification kit. Immunoprecipitated DNA was analyzed on a Bio-Rad real-time PCR machine with iQ SYBRgreen PCR mastermix (Bio-Rad) and normalized against input DNA.
Immunofluorescence.
Immunofluorescence was performed as described (73, 74). Cells grown on chamber slides (Nunc) or coverslips were washed briefly with PBS followed by fixation with 3% (wt/vol) paraformaldehyde at room temperature for 15 min. Cells were permeabilized with 0.5% Triton X-100 solution for 5 min at room temperature and blocked with 5% (vol/vol) normal goat serum for 1 h. Cells were stained in the same buffer with primary and secondary antibodies before mounting onto slides. Images were acquired by using a Zeiss Axioplan Fluorescence microscope or an LSM 780 confocal system (Carl Zeiss) with 40×, 100× oil-immersion lens. HA (Abcam; 12CA5 clone, ab16918, 1:1,000) antibody was used for wild-type and mutant HIRA staining. DAPI (1:2,000, Sigma-Aldrich) was used to visualize SAHF.
Senescence Assays.
Oncogene-induced senescence was performed as described (48, 75, 76). A retrovirus expressing a tamoxifen-inducible pLNC-neo ER::RASV12 construct was packaged in Phoenix cells and transduced into IMR90 cells as described above. A final concentration of 100 nM 4OHT (Sigma-Aldrich) was used to induce expression of RASV12. Cells were collected at different days of induction, and cellular senescence was analyzed by measuring SA-β-gal activities, EdU incorporation, and formation of senescence-induced heterochromatin foci. Detection of SA-β-gal activity was performed by using a Senescence β-Galactosidase Staining Kit (Cell Signaling Technology; 9860) following the manufacturer’s protocol. Images were captured by a fluorescence microscope Leica DM 4000 equipped with HC plan APO 20×/0.7 objective, DFC 450C color camera and LAS V4.4 software (Leica). For cell cycle arrest analyses, cells were labeled with EdU (5-ethyl-2′-deoxyuridine) by using the Click-it EdU Alexa Fluor 555 imaging kit (Invitrogen). Briefly, 13 h after changing the medium, EdU was added at a final concentration of 10 µM, and 3 h later, the cells were fixed for fluorescence staining of EdU, followed by DAPI for nuclear staining. Deoxyuridine incorporation was revealed with the click-it chemistry according to the manufacturer’s instructions. Each experiment was repeated three times, and approximately 200 cells were counted for each replicate.
Acknowledgments
We thank Benjamin Madden for technical assistance in the mass spectrometry analyses; Xiaochun Yu and El Bachir Affar for the generous gift of OGT constructs; Rebecca Burgess, Georges Mer, Chang Hoon Cho, Debra Evans, and Hyoungjun Ham for discussion and critical reading of the manuscript; and all members of the Z.Z. laboratory for helpful discussion. J.-S.L. acknowledges fellowship funding from the Mayo Graduate School, and this work is supported by NIH Grant CA154789.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.K.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600509113/-/DCSupplemental.
References
- 1.Kornberg RD. Chromatin structure: A repeating unit of histones and DNA. Science. 1974;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 2.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 3.Luger K. Dynamic nucleosomes. Chromosome Res. 2006;14(1):5–16. doi: 10.1007/s10577-005-1026-1. [DOI] [PubMed] [Google Scholar]
- 4.Ransom M, Dennehey BK, Tyler JK. Chaperoning histones during DNA replication and repair. Cell. 2010;140(2):183–195. doi: 10.1016/j.cell.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsaesser SJ, Goldberg AD, Allis CD. New functions for an old variant: No substitute for histone H3.3. Curr Opin Genet Dev. 2010;20(2):110–117. doi: 10.1016/j.gde.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szenker E, Ray-Gallet D, Almouzni G. The double face of the histone variant H3.3. Cell Res. 2011;21(3):421–434. doi: 10.1038/cr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128(4):721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Morrison AJ, Shen X. Chromatin remodelling beyond transcription: The INO80 and SWR1 complexes. Nat Rev Mol Cell Biol. 2009;10(6):373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459(7243):113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polo SE, et al. Chromatin assembly factor-1, a marker of clinical value to distinguish quiescent from proliferating cells. Cancer Res. 2004;64(7):2371–2381. doi: 10.1158/0008-5472.can-03-2893. [DOI] [PubMed] [Google Scholar]
- 11.Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell. 1986;45(4):555–565. doi: 10.1016/0092-8674(86)90287-4. [DOI] [PubMed] [Google Scholar]
- 12.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 13.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116(1):51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 14.Burgess RJ, Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nat Struct Mol Biol. 2013;20(1):14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg AD, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140(5):678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banaszynski LA, et al. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell. 2013;155(1):107–120. doi: 10.1016/j.cell.2013.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pchelintsev NA, et al. Placing the HIRA histone chaperone complex in the chromatin landscape. Cell Reports. 2013;3(4):1012–1019. doi: 10.1016/j.celrep.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orsi GA, et al. Drosophila Yemanuclein and HIRA cooperate for de novo assembly of H3.3-containing nucleosomes in the male pronucleus. PLoS Genet. 2013;9(2):e1003285. doi: 10.1371/journal.pgen.1003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci USA. 1995;92(4):1237–1241. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parthun MR, Widom J, Gottschling DE. The major cytoplasmic histone acetyltransferase in yeast: Links to chromatin replication and histone metabolism. Cell. 1996;87(1):85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Han J, Kang B, Burgess R, Zhang Z. Human histone acetyltransferase 1 protein preferentially acetylates H4 histone molecules in H3.1-H4 over H3.3-H4. J Biol Chem. 2012;287(9):6573–6581. doi: 10.1074/jbc.M111.312637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, et al. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315(5812):653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, et al. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell. 2008;134(2):244–255. doi: 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J, et al. A Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assembly. Cell. 2013;155(4):817–829. doi: 10.1016/j.cell.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang B, et al. Phosphorylation of H4 Ser 47 promotes HIRA-mediated nucleosome assembly. Genes Dev. 2011;25(13):1359–1364. doi: 10.1101/gad.2055511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbert PB, Henikoff S. Histone variants--ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol. 2010;11(4):264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- 27.Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21(12):1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rai TS, et al. Human CABIN1 is a functional member of the human HIRA/UBN1/ASF1a histone H3.3 chaperone complex. Mol Cell Biol. 2011;31(19):4107–4118. doi: 10.1128/MCB.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai TS, et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 2014;28(24):2712–2725. doi: 10.1101/gad.247528.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman PD, Cohen JL, Osley MA. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol. 1998;18(8):4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamane K, et al. Asf1/HIRA facilitate global histone deacetylation and associate with HP1 to promote nucleosome occupancy at heterochromatic loci. Mol Cell. 2011;41(1):56–66. doi: 10.1016/j.molcel.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8(1):19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden GW, et al. Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet. 2007;39(2):251–258. doi: 10.1038/ng1949. [DOI] [PubMed] [Google Scholar]
- 35.Campisi J, d’Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 36.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 39.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker DJ, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36(7):744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 41.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 43.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 44.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 45.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 46.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6(6):472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27(6):2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte LF, et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat Commun. 2014;5:5210. doi: 10.1038/ncomms6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banumathy G, et al. Human UBN1 is an ortholog of yeast Hpc2p and has an essential role in the HIRA/ASF1a chromatin-remodeling pathway in senescent cells. Mol Cell Biol. 2009;29(3):758–770. doi: 10.1128/MCB.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, et al. Identification of an ubinuclein 1 region required for stability and function of the human HIRA/UBN1/CABIN1/ASF1a histone H3.3 chaperone complex. Biochemistry. 2012;51(12):2366–2377. doi: 10.1021/bi300050b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirov N, Shtilbans A, Rushlow C. Isolation and characterization of a new gene encoding a member of the HIRA family of proteins from Drosophila melanogaster. Gene. 1998;212(2):323–332. doi: 10.1016/s0378-1119(98)00143-7. [DOI] [PubMed] [Google Scholar]
- 52.Tang Y, et al. Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly. Nat Struct Mol Biol. 2006;13(10):921–929. doi: 10.1038/nsmb1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray-Gallet D, et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell. 2011;44(6):928–941. doi: 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Jin C, et al. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet. 2009;41(8):941–945. doi: 10.1038/ng.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanigan F, Geraghty JG, Bracken AP. Transcriptional regulation of cellular senescence. Oncogene. 2011;30(26):2901–2911. doi: 10.1038/onc.2011.34. [DOI] [PubMed] [Google Scholar]
- 56.Behjati S, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45(12):1479–1482. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 58.Wu G, et al. St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24(4):1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673(1-2):13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761(5-6):599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Fülöp N, et al. Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology. 2008;9(3):139–151. doi: 10.1007/s10522-007-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gambetta MC, Oktaba K, Müller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325(5936):93–96. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- 64.Sinclair DA, et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc Natl Acad Sci USA. 2009;106(32):13427–13432. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu CS, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA. 2014;111(4):1355–1360. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493(7433):561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deplus R, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32(5):645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCurrach ME, Lowe SW. Methods for studying pro- and antiapoptotic genes in nonimmortal cells. Methods Cell Biol. 2001;66:197–227. doi: 10.1016/s0091-679x(01)66010-2. [DOI] [PubMed] [Google Scholar]
- 69.Zhou H, Madden BJ, Muddiman DC, Zhang Z. Chromatin assembly factor 1 interacts with histone H3 methylated at lysine 79 in the processes of epigenetic silencing and DNA repair. Biochemistry. 2006;45(9):2852–2861. doi: 10.1021/bi0521083. [DOI] [PubMed] [Google Scholar]
- 70.Daou S, et al. Crosstalk between O-GlcNAcylation and proteolytic cleavage regulates the host cell factor-1 maturation pathway. Proc Natl Acad Sci USA. 2011;108(7):2747–2752. doi: 10.1073/pnas.1013822108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Wang Z, Zhang Z. PP1α, PP1β and Wip-1 regulate H4S47 phosphorylation and deposition of histone H3 variant H3.3. Nucleic Acids Res. 2013;41(17):8085–8093. doi: 10.1093/nar/gkt583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Méndez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20(22):8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan KM, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan KM, Zhang H, Malureanu L, van Deursen J, Zhang Z. Diverse factors are involved in maintaining X chromosome inactivation. Proc Natl Acad Sci USA. 2011;108(40):16699–16704. doi: 10.1073/pnas.1107616108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 76.Corpet A, Olbrich T, Gwerder M, Fink D, Stucki M. Dynamics of histone H3.3 deposition in proliferating and senescent cells reveals a DAXX-dependent targeting to PML-NBs important for pericentromeric heterochromatin organization. Cell Cycle. 2014;13(2):249–267. doi: 10.4161/cc.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]