In PNAS, Li et al. (1) report the pathological findings in a single patient with Parkinson’s disease (PD) who received transplantation of embryonic dopamine grafts derived from four donors placed into the right putamen 24 y before death. The patient was 59 y of age at the time of surgery and was reported to have experienced marked clinical improvement after a latency of ∼12 mo (originally reported in ref. 2). Bilateral benefits (predominantly on the left side) were noted with respect to rigidity, bradykinesia, rest tremor, and motor fluctuations, and were of sufficient magnitude that levodopa was withdrawn 32 mo following surgery. Levodopa was reinstated at 64 mo at a dose one-third of that used preoperatively and remained unchanged for the life of the patient. Still motor benefits were maintained for ∼10 y. Thereafter, the patient experienced progressive deterioration in motor function and developed a progressive dementia at year 14. At postmortem examination, the investigators reported the survival of ∼42,000 grafted dopaminergic neurons with extensive putamenal dopaminergic innervation. Approximately 11–12% of residual grafted neurons contained α-synuclein aggregates. This case suggests that the original goals of cell replacement therapy for PD, namely, survival of implanted dopamine neurons, striatal reinnervation, and clinical benefit, were met.
This is the longest postgrafting interval reported for any PD patient who has undergone a postmortem examination. It is a fascinating case that indicates that grafted fetal dopaminergic cells can survive for almost a quarter of a century and provide extensive striatal innervation. Previous reports have demonstrated similar findings at postmortem performed 18 mo (3) and 4–16 y after grafting (4–7); the present study suggests that these findings can endure for the life of the patient. Importantly, the authors report that fetal nigral grafting in this patient was associated with long-term motor benefit sufficient to withdraw levodopa, albeit this was based on an open-label assessment. Although short-term benefits have been previously reported in several open-label studies (7), they have not been confirmed in double-blind trials, even with evidence of improvement on fluorodopa–PET and graft survival with striatal innervation equal to or greater than described in the present case (8, 9).
It is remarkable that such robust motor improvement, sufficient to stop medical therapy for several years, was achieved, particularly when only one hemisphere was treated with dopaminergic grafts. Although PD may present unilaterally, it is virtually always a bilateral disorder that is inexorably progressive. Unilateral surgical treatments for PD such as lesions or deep-brain stimulation (DBS) can be associated with ipsilateral benefits (10), but it is very unusual to see such dramatic improvement and slowing of progression bilaterally as reported in the present case. This begs the question as to whether this patient had straightforward sporadic PD, as there is no mention in the paper as to whether the diagnosis was confirmed pathologically. Indeed, this patient displayed extensive white-matter atrophy, as shown in their figure 4A, which is a pathology not typically found in PD.
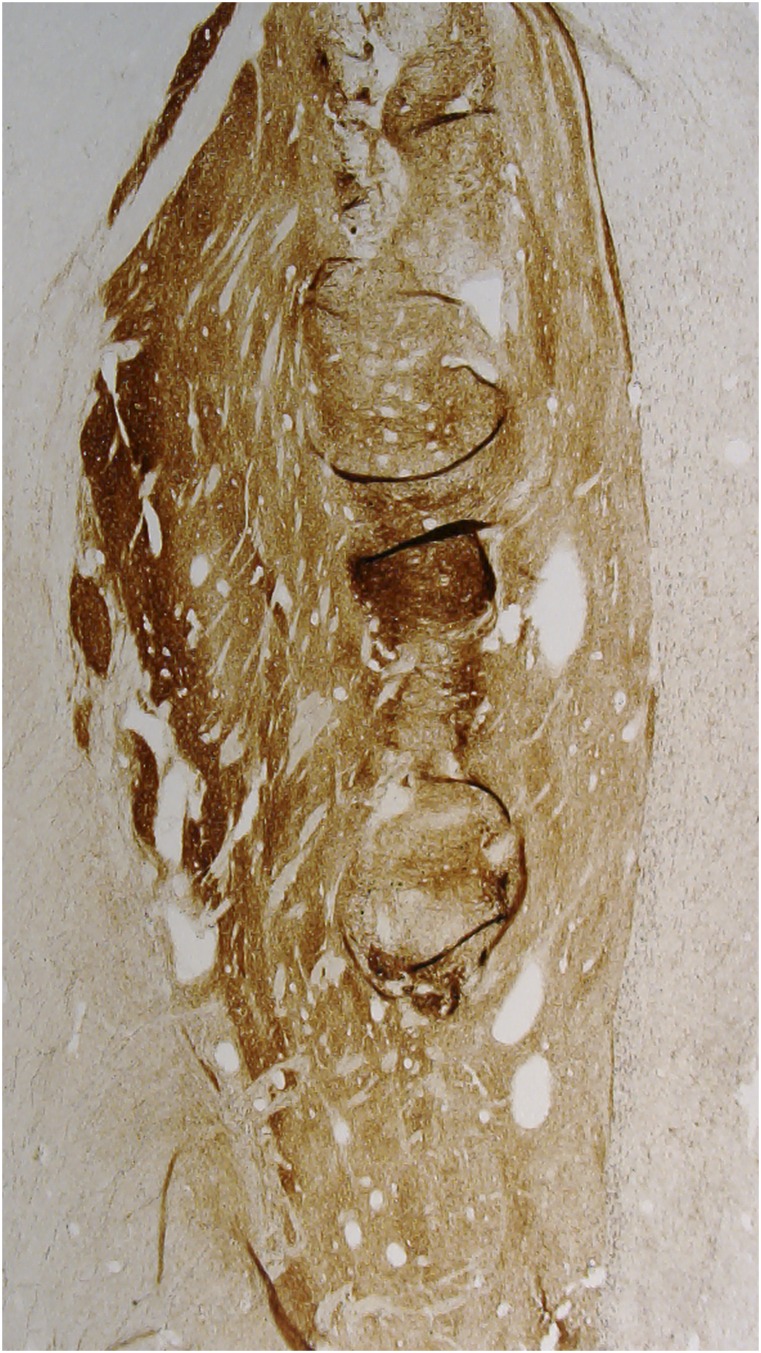
An important question, critical to the success of cell transplantation in PD, is how many surviving grafted cells are needed to achieve motor benefit. Clinical improvement has been described with bilateral transplantation and survival in each putamen of 72,000 and 120,000 grafted dopamine neurons in one patient at 18 mo (3) and 90,000 and 200,000 neurons at 4 y in another report (7, 11). In the present report, motor benefits were observed with survival of ∼40,000 grafted dopaminergic cells at 24 y. The authors suggest that 40,000 neurons may be sufficient to provide motor benefit and propose that the decline in function was the result of degeneration of nonnigrostriatal brain regions. However, which brain regions are responsible for the loss of function is not presently elucidated. This number may, however, be an underestimate of the number of cells that provided benefit at earlier time points, as many cells may have degenerated over the years, possibly accounting for the loss of motor benefit. Alternatively, loss of benefit may have resulted from grafted cells becoming dysfunctional over time secondary to the accumulation of pathological serine-129–phosphorylated α-synuclein aggregates, as evidenced by a reduction in tyrosine hydroxylase (TH) staining. Indeed, we and others have previously reported the presence of Lewy pathology in grafted neurons when patients survive for more than 10 y, but not at earlier time points (e.g., refs. 5 and 6), and have suggested the possibility that these cells have become affected in a prion-like manner (12). It should also be noted that, in each of these, cases were evaluated in an open-label manner. In double-blind studies, some cases in which we did not see clinical benefit, similar or greater numbers of surviving grafted neurons and striatal innervation were observed (4) (Fig. 1).
Fig. 1.
TH-immunostained section from a PD patient receiving a fetal graft 4 y before death. This patient had a similar number of TH-ir grafted neurons and innervation as the patient in the report by Li et al. but failed to demonstrate clinical recovery in a blinded assessment.
Although scientists tend to focus on the number of surviving grafted neurons, the more essential parameter is striatal dopamine reinnervation, as it is the binding of dopamine to host postsynaptic receptors, and not just the preservation of nigral perikarya, that mediates functional recovery (13). The authors state that the putamen, 24 y after grafting, was “completely reinnervated.” However, one has to be judicious in this interpretation. Although the density and intensity of TH-immunoreactive (TH-ir) fiber staining in regions of the dorsal putamen proximal to the graft appear to approach what is typically seen in a normal person of similar age, figures 2 and 3 of their paper clearly illustrate that dopaminergic reinnervation of the ventral half of the putamen is of a density and intensity that more closely resembles an ungrafted putamen in a PD patient. It is possible that complete normalization of striatal innervation is not required for functional benefit, but the paucity of dopaminergic reinnervation in the ventral half of the putamen is noteworthy and may be responsible for the decline in motor benefit ultimately seen in this patient. As with the number of grafted neurons, it is not possible to determine with certainty whether there was a loss of fiber innervation over time.
It is important to note that this patient eventually developed dementia, a common finding in advanced PD patients, and a
Li et al. teach us what cellular replacement approaches can do in PD and highlight the critical importance of encouraging patients to participate in research and brain donation programs to evaluate and develop new therapies.
major source of disability and nursing home placement. This study demonstrates that dopamine cell grafting does not protect against the development of this disabling feature. The hypothesis that restoration of striatal dopamine innervation might have been of value in preventing or treating dementia is not supported in this study, and implies that nondopaminergic pathology is responsible for these nonmotor and nonlevodopa responsive symptoms.
Cellular replacement strategies, and particularly stem cells, continue to remain of great interest as a possible therapy for PD and should continue to be vigorously and rigorously pursued. This important manuscript indicates that long-term graft survival with innervation is possible and suggests the potential for long-term clinical benefits, possibly even without the need for levodopa. However, the clinical observations are open label and the long-standing bilateral effects are somewhat difficult to understand. Pathological changes in grafted neurons may interfere with function and may limit the benefits that can be achieved with this technology. Importantly, the patient developed dementia, illustrating that, even in cases that are thought to represent the best that can be obtained with cell therapy, grafted DA neurons cannot prevent the development of nondopaminergic features such as dementia, which in the present era represent the major source of disability and nursing home placement for PD patients. It is also unclear where cell transplantation strategies, even if successful, will fit in the PD treatment paradigm. There are already procedures such as DBS and continuous intraintestinal levodopa infusion that provide rapid and sustained benefits for advanced PD patients comparable to what is sought with transplant procedures (13–15). Further, less invasive methods of delivering levodopa are currently being investigated and may provide an opportunity to obtain all of the benefits of the drug without motor complications or complications related to a surgical procedure.
Still, this excellent report remains of great value. Li at al. teach us what cellular replacement approaches can do in PD and highlight the critical importance of encouraging patients to participate in research and brain donation programs to evaluate and develop new therapies.
Acknowledgments
J.H.K. is supported in part by a grant from the Parkinson’s Disease Foundation.
Footnotes
Conflict of interest statement: J.H.K. receives consulting fees from NsGene and Brainstorm, Inc., and has an NIH grant (NS070577) on induced pluripotent stem cells. C.W.O. is a shareholder in Clintrex. Clintrex provides consulting services for AstraZeneca, Accorda/Civitas/Biotie, Britannia, Corium, Cynapsus, Forward Pharma, Intec, Michael J. Fox Foundation, Lundbeck, Lysosomal Therapeutics, Neuroderm, Neurmedix, Orion, Otsuka/INC, Prana, Pharma2B, Pfizer, Raptor, Remedy, Sanofie/Genzyme, Serina, Sunovian, Synagile, Upsher Smith, Teva, Titan, USWorldMed, Vaccinex, Vertex, Weston Foundation, and Zambon.
See companion article on page 6544.
References
- 1.Li W, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci USA. 2016;113:6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindvall O, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35(2):172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 3.Kordower JH, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995;332(17):1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 4.Olanow CW, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 5.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 6.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 7.Mendez I, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(Pt 7):1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 1996;19(3):102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 9.Freed CR, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 10.Germano IM, et al. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: A double-blind 12-month evaluation study. J Neurosurg. 2004;101(1):36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 11.Kordower JH, et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: Chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol. 1996;370(2):203–230. doi: 10.1002/(SICI)1096-9861(19960624)370:2<203::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA. 2009;106(31):12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20(12):4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deep-Brain Stimulation for Parkinson’s Disease Study Group Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 15.Olanow CW, et al. LCIG Horizon Study Group Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi: 10.1016/S1474-4422(13)70293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]