Significance
The activity of some proteins requires dramatic conformational movements. The ability to undergo these movements can be enabled by folding of these proteins to constrained higher free energy or metastable states. Metastable proteins are particularly susceptible to misfolding and are therefore associated with a number of pathologies. Here the cellular folding pathway of the serpin antithrombin III (ATIII), which inhibits proteases involved in the coagulation cascade, was determined. ATIII uses a large conformational movement in a mousetrap-like mechanism to bind and distort its target protease, resulting in protease inhibition. This work establishes that folding to an active, cocked state requires early stabilization of the C-terminal region, which is the last sequence translated, explaining how the serpin or mousetrap is set.
Keywords: cellular protein folding, endoplasmic reticulum, serpins, antithrombin, thrombosis
Abstract
Although proteins generally fold to their thermodynamically most stable state, some metastable proteins populate higher free energy states. Conformational changes from metastable higher free energy states to lower free energy states with greater stability can then generate the work required to perform physiologically important functions. However, how metastable proteins fold to these higher free energy states in the cell and avoid more stable but inactive conformations is poorly understood. The serpin family of metastable protease inhibitors uses large conformational changes that are downhill in free energy to inhibit target proteases by pulling apart the protease active site. The serpin antithrombin III (ATIII) targets thrombin and other proteases involved in blood coagulation, and ATIII misfolding can thus lead to thrombosis and other diseases. ATIII has three disulfide bonds, two near the N terminus and one near the C terminus. Our studies of ATIII in-cell folding reveal a surprising, biased order of disulfide bond formation, with early formation of the C-terminal disulfide, before formation of the N-terminal disulfides, critical for folding to the active, metastable state. Early folding of the predominantly β-sheet ATIII domain in this two-domain protein constrains the reactive center loop (RCL), which contains the protease-binding site, ensuring that the RCL remains accessible. N-linked glycans and carbohydrate-binding molecular chaperones contribute to the efficient folding and secretion of functional ATIII. The inability of a number of disease-associated ATIII variants to navigate the folding reaction helps to explain their disease phenotypes.
Irreversible switching from one conformation to another allows proteins to perform mechanical work without external energy sources such as ATP. Large conformational movements, up to ∼100 Å, can be triggered by proteolysis or changes in environmental conditions, such as pH, initiating processes including membrane fusion for viral infection, protease activation, or inhibition (1). To facilitate these processes, proteins must fold to kinetically trapped metastable states with relatively high free energy. How proteins fold to these states and avoid more thermodynamically stable conformations is poorly understood.
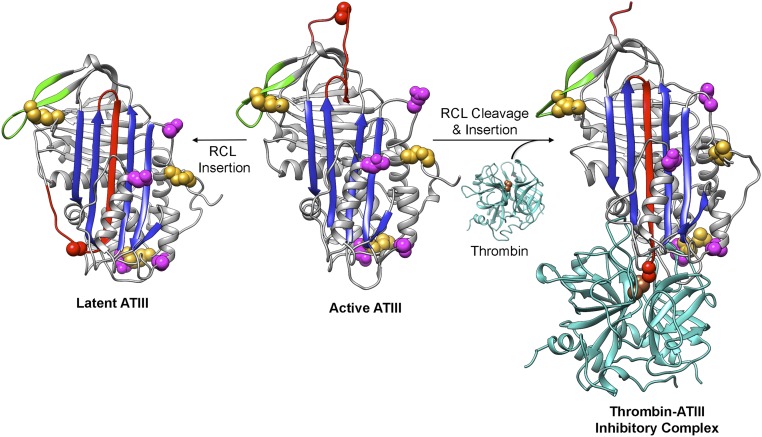
The serpin family of serine protease inhibitors exemplifies this type of metastable protein, and its mechanism of folding presents a conundrum. The native, active serpin fold positions the target protease-binding site on a loosely structured, accessible stretch of sequence termed the reactive center loop (RCL). Once the protease forms a covalent acyl intermediate in the scissile bond in the RCL and cleaves the bond, the serpin undergoes a major conformational change like the springing of a mousetrap, and the protease is carried ∼70 Å to the opposite side of the serpin, thereby inactivating the protease by mechanical deformation (2, 3) (Fig. S1). Strikingly, the conformational landscape of serpins has an alternate fold that is more stable than the functionally required “cocked mousetrap” fold. In this alternative fold, called the latent state, the intact RCL is inserted as an additional strand into the central β-sheet, resulting in a more stable but inactive state (4). Encoding this gymnastic ability in the folding landscape of serpins comes at a risk: Many mutations in serpins cause misfolding and are associated with diseases called serpinopathies (5).
Fig. S1.
ATIII conformational changes. The RCL (red) can spontaneously insert into β-sheet A (the central sheet in blue), resulting in irreversibly inactivated latent ATIII. Cleavage of active, metastable ATIII by target proteases (cyan) at Arg393 (red space fill) leads to insertion of the N-terminal end of the cleaved RCL into sheet A, translocation of the protease relative to the serpin, and trapping of the serine protease acyl-enzyme intermediate with a covalent bond between ATIII Arg393 and thrombin Ser195 (brown space fill), the serine residue in the catalytic triad. Cys residues and the sites of N-linked glycosylation are shown as gold and magenta space filling representations, respectively. The active (heparin bound) and latent ATIII structures are from Protein Data Bank (PDB) ID code 1E05, and the thrombin structure is from PDB ID code 1PPB. The ATIII–thrombin inhibitory complex was modeled using the α1-antitrypsin–trypsin inhibitory complex structure (PDB ID code1EZX). The sequences of residues 3–393 of human ATIII (from Uniprot P01008) along with a 2.0 Å distance constraint for each of the N-terminal disulfide bonds were submitted to I-TASSER (37) (zhanglab.ccmb.med.umich.edu/I-TASSER/) along with the A chain of 1EZX as the template. Models of loop-inserted ATIII were generated, and the top model, the structure of the ATIII C-terminal residues 394–431 from PDB ID code 1E05, and the structure of α-thrombin (PDB ID code 1PPB) were structurally aligned with the α1-antitrypsin–trypsin inhibitory complex structure (PDB ID code 1EZX) using UCSF Chimera (38). The resulting model of the ATIII–thrombin inhibitory complex was then adjusted manually. All structure figures were made using UCSF Chimera (38).
The serpin antithrombin III (ATIII) plays an essential role in blood clotting by regulating the activity of thrombin and other serine proteases in the coagulation cascade. Numerous misfolding mutations of ATIII are linked to thrombosis (6, 7). The cellular folding process of ATIII, including its traversal of the secretory pathway, facilitates its folding to the functional metastable high free energy state. The differing outcomes of unassisted refolding of purified ATIII and its cellular folding underline the profound difference between protein folding reactions in isolation and in cells and invite further exploration of the key players and steps that make cellular folding so successful (8, 9). The fact that ATIII and other serpins must adopt metastable states to function and that their misfolding is implicated in several pathologies further raises the importance of understanding their cellular folding pathway.
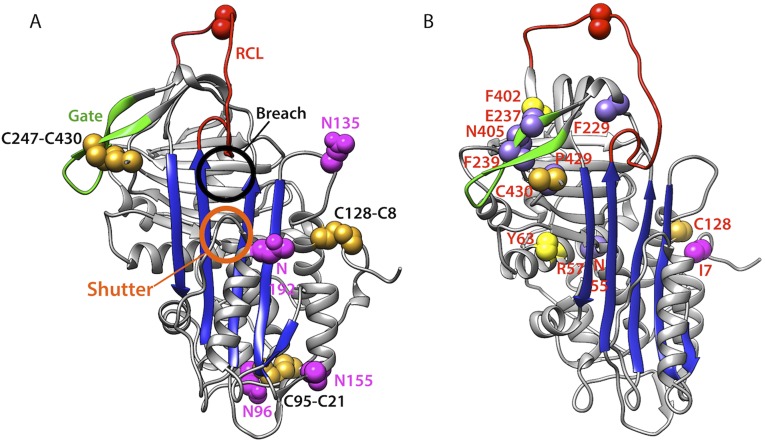
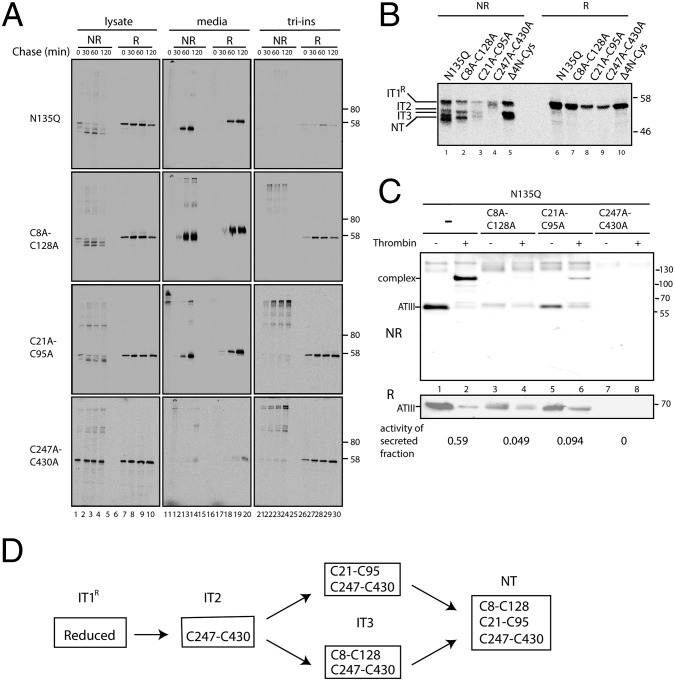
Because disulfide bonding requires two Cys residues to be positioned within a few angstroms of each other, the intramolecular disulfide bond reaction is tightly coupled to formation of native protein topology. Hence, the order of disulfide bond formation reveals the progress of its folding; in fact, disulfides have been used as reporters of folding states to map the folding pathway for a number of proteins both for purified components and in cells (10–14). ATIII was chosen as a substrate to map the folding pathway for serpins as it is one of the few serpin proteins that possess intramolecular disulfides. Its three disulfide bonds are optimally positioned to report on the evolution of the ATIII structure (Fig. S2A). ATIII comprises three β-sheets and nine α-helices combined into two nonsequential, intertwined domains with a central β-sheet both forming the core of the α/β domain and bridging the two domains. One disulfide, between Cys-8 and Cys-128, links the flexible N terminus to a later helix in the N-terminal, largely helical subdomain; a second, between Cys-21 and Cys-95, connects two helical segments that flank the central bridging β-sheet; and the third, between Cys-247 and Cys-430, creates a large loop around the C-terminal third of the molecule (Fig. 1A).
Fig. S2.
Location of ATIII disease mutations. (A) The structure of active ATIII (PDB ID code 1E05) showing the gate (green), disulfide bonds (gold), N-linked glycosylation sites (magenta), RCL (red), and the central β-sheet (blue). The breach and shutter regions are indicated. (B) Mutations are designated on the ATIII structure using the following color code: mutated from Cys (goldenrod), mutated to Cys (yellow), mutation adds an additional N-linked glycosylation site (magenta), and all other mutations (light purple).
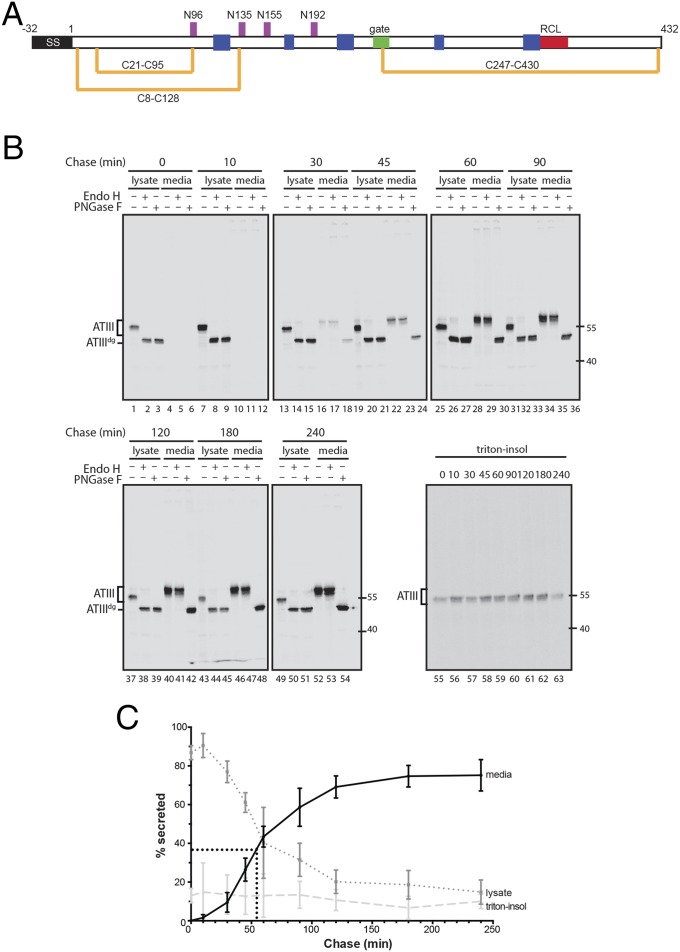
Fig. 1.
ATIII is efficiently secreted from cells. (A) Schematic depiction of ATIII. The N-terminal signal sequence (SS) is shown in black. The positions of the four N-linked glycosylation sites (magenta) and three disulfide bonds (gold) are shown. The ATIII construct also contains a C-terminal myc-His tag. (B) Pulse–chase time course of ATIII secretion. CHO cells were pulsed with [35S]-Met/Cys for 30 min and chased for the indicated times. Cell and media populations were collected for each time point. The immunoprecipitated ATIII was enzymatically deglycosylated with endoglycosidases Endo H or PNGase F. ATIIIdg indicates deglycosylated ATIII. (C) Quantification of ATIII secretion over time. Secretion at each time point was calculated as a percentage of the sum of the 0 min lysate, media, and triton-insoluble fractions plotted against each chase time. Error bars indicate SEM from three independent experiments.
To address the long-standing question of how metastable proteins can fold to higher energy states in the cell and avoid lower energy forms, we mapped the folding pathway of ATIII by determining the order in which its disulfides formed. Interestingly, although the C terminus is translated last, it is the first domain to lock into place. This constraint is key, as it positions the RCL or protease bait loop in an exposed orientation that keeps it from inserting into an earlier translated sheet where it would reach a lower energy but inactive state referred to as the latent state. Interestingly, a number of disease-associated, single-site missense mutations in ATIII derail the protein folding program, providing an explanation for some cases of thrombosis.
Results
ATIII Folds Rapidly and Efficiently in Cells.
We initially determined the timing of ATIII maturation in live cells. Chinese hamster ovary (CHO) cells transiently expressing a tagged construct of ATIII were pulsed with [35S]-Met/Cys and chased for various times (Fig. 1B). Secretion of ATIII into the media occurred with a half-time of 54 min and reached a maximum secretion level of 75% (Fig. 1C). Secreted ATIII was able to form a covalent complex with thrombin, confirming its successful folding to an active, metastable, and native state (see Fig. 3B, lanes 1 and 2). Secreted ATIII was resistant to endoglycosidase H (Endo H), which cleaves the high mannose N-glycans attached in the ER but not complex carbohydrates that are added in the Golgi, consistent with its having traversed both the ER and Golgi (Fig. 1B). The cellular lysate fraction of ATIII was distributed between soluble ATIII that was sensitive to Endo H and therefore ER-localized (15%) and triton-insoluble protein aggregates that presumably arose from protein that misfolded (10%) (Fig. 1C). Together these results show that wild-type ATIII is rapidly and efficiently secreted from CHO cells in a properly folded, native, and active state.
Fig. 3.
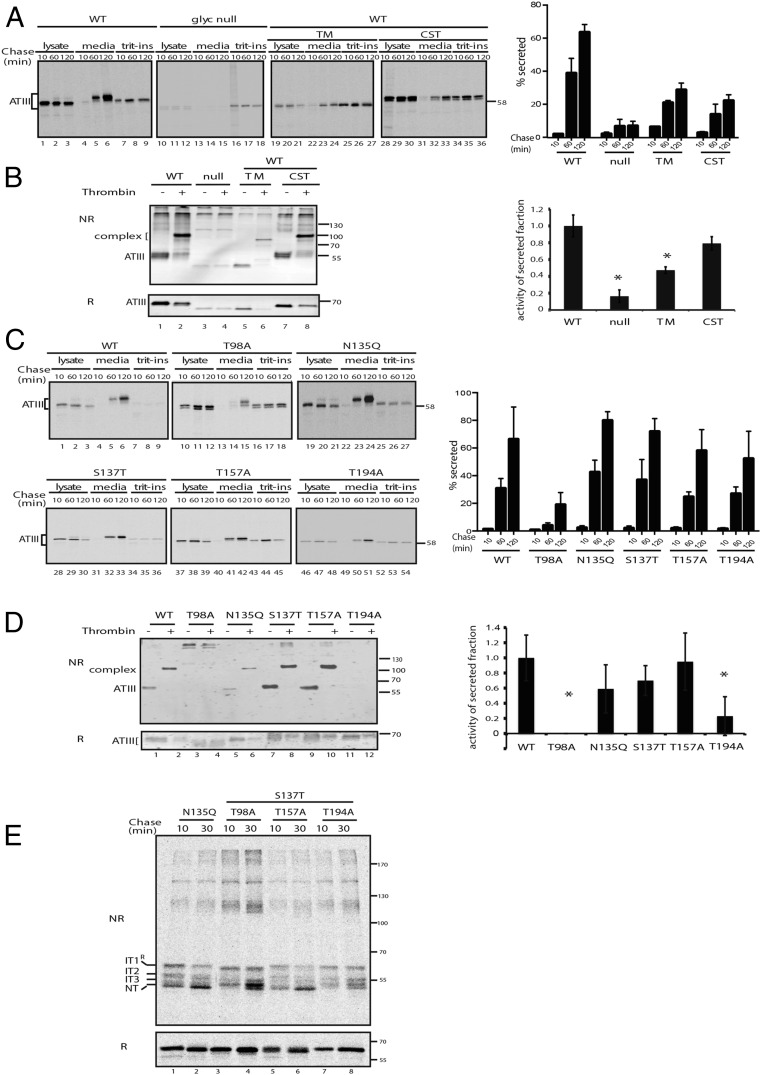
N-linked glycans are required for efficient ATIII secretion. (A) Pulse–chase time course examining the intrinsic and extrinsic effect of glycans on ATIII secretion. Cells were transfected with WT ATIII or a glyc-null mutant. Cells were pulsed for 30 min and chased for the indicated times. Cells were treated during the pulse and chase periods with 5 μg/mL TM or 1 mM CST where indicated. The bar graph shows the quantification of ATIII media secretion. Secretion at each time point was quantified as a percentage of the 10-min lysate fraction for each treatment condition. Error bars indicate SEM from three independent experiments. (B) Thrombin inhibitory activity was analyzed as in Fig. 2C. (C) Cells expressing wild-type and single glycan ATIII mutants were pulsed for 30 min and chased for the indicated times. Secretion amounts for each mutant were compared with WT. Secretion at each time point was quantified as above. (D) The thrombin inhibitory activity for the glycan deletion mutants of ATIII was analyzed as described in Fig. 2C and Experimental Procedures. (E) ATIII glycan mutants that had each of the four sites mutated individually were expressed. Cells were pulsed for 15 min and chased for the indicated times. ATIII was resolved by nonreducing (NR) and reducing (R) SDS/PAGE.
The C-Terminal Disulfide of ATIII Forms First and Is Sufficient for Secretion.
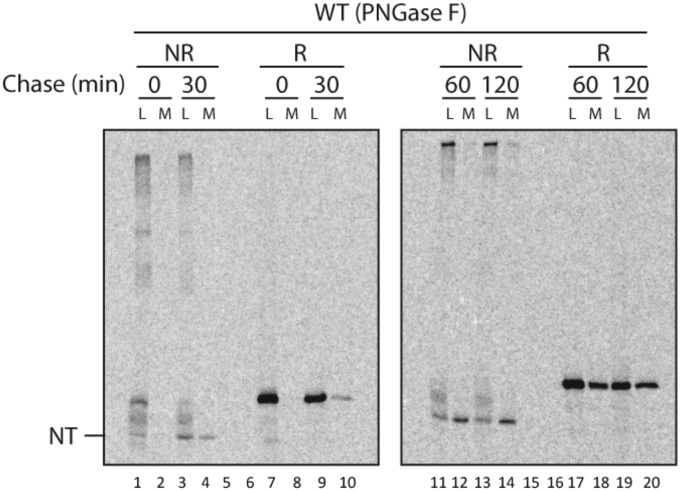
We next dissected the development of native topology in ATIII as it proceeded through the secretory pathway by monitoring the order of formation of its three disulfide bonds. The homogeneously glycosylated N135Q variant of ATIII was used for these experiments, as glycosylation of Asn-135 is inefficient and leads to protein heterogeneity (15). This variant was secreted with the same efficiency as the wild type (Fig. 3C). Immediately after a 30-min [35S]-Met/Cys pulse, several discrete ATIII bands can be observed in the cell lysate under nonreducing conditions (Fig. 2B, lane 1). With time and using a shorter 15-min radiolabel pulse, the population of protein in the higher mobility bands increased until the final native oxidized form (NT) was reached, as disulfides formed progressively and caused the protein to become more compact (Fig. 2A, lanes 1–4). Consistent with this interpretation, under reducing conditions a single band is observed that migrates slowly and corresponds to the t = 0 band under oxidizing conditions; we thus attribute this band to the most unfolded or reduced intermediate of ATIII and call it IT1R. ATIII in the medium behaved similarly to NT, except for a slight increase in mass caused by the addition of complex carbohydrates in the Golgi. Indeed, after treatment with peptide-N-glycosidase F (PNGase F), which removes complex carbohydrates from N-linked glycans, oxidized ATIII from the medium had mobility comparable to the fastest migrating form from the cell lysate, indicating that these forms both corresponded to the fully oxidized native conformation (Fig. S3).
Fig. 2.
Iterative oxidation of ATIII. (A) CHO cells were transfected with ATIII containing all three disulfides (ATIII-N135Q) or mutant variants that both had Cys from an individual disulfide pair mutated to Ala residues. The oxidation and secretion of ATIII was examined by pulse–chase analysis. Cells were pulsed with [35S]-Met/Cys for 15 min and chased for the indicated times. ATIII was immunoprecipitated from cell lysates and media fractions and resolved on SDS/PAGE under nonreducing (NR) or reducing (R) conditions. (B) Comparison of the folding intermediate species in N135Q and the paired disulfide mutants. Postnuclear supernatants were collected after a 30-min pulse, immunoprecipitated for ATIII, and resolved by nonreducing (NR) or reducing (R) SDS/PAGE. In addition to the constructs used in A, a mutant in which all four N-terminal Cys were mutated to Ala was also analyzed (Δ4N-Cys). The various oxidative intermediates are denoted as IT1R, IT2, and IT3, with native protein termed NT. (C) Media from cells transfected with ATIII was collected 20 h posttransfection and analyzed for inhibitory activity. Inhibitory activity against thrombin was quantified with the activity of the secreted fraction displayed below the corresponding gel lanes. (D) The in-cell oxidative reaction scheme for ATIII is depicted starting with the reduced protein (ITR) and culminating with the formation of the fully oxidized native protein (NT).
Fig. S3.
Oxidized ATIII from media comigrates with NT from cell lysates. CHO cells transfected with wild-type ATIII were pulsed for 30 min with [35S]-Met/Cys and either analyzed directly or chased for 30 min. Both cell lysates and media were collected at each time point. ATIII was isolated by immunoprecipitation with anti-myc sera. Pellets were treated with PNGase F to remove any heterogeneity caused by glycosylation, and samples were resolved by both nonreducing (NR; lanes 1–4 and 11–14) and reducing (R; lanes 7–10, and 17–20) SDS/PAGE. NT denotes the fully oxidized native form of ATIII found both in the cell lysate (L) and media (M).
To assign the observed intermediates in ATIII maturation and thereby deduce its cellular folding mechanism, we used variants lacking the native disulfide pairs. Strikingly, mutation of the C-terminal disulfide pair (C247A–C430A) completely abolished both folding (as indicated by the lack of higher mobility species) and secretion of ATIII (Fig. 2A). Instead, ATIII lacking the Cys residues necessary to form the C-terminal intrachain disulfide bond accumulated in a band that comigrated with fully reduced ATIII (see IT1R). Importantly, this observation strongly suggests that the other, more N-terminal disulfides do not form unless the C237–C430 disulfide forms (Fig. 2A, compare lane 4 to lanes 7–10). Reciprocally, a mutant lacking all four N-terminal cysteines (Δ4N-Cys), and thus unable to form either N-terminal disulfide, still populated a species with relatively high mobility under oxidizing conditions, indicating that formation of the C-terminal C247–C430 disulfide was accompanied by significant compaction, and therefore folding (Fig. 2B, lane 5; intermediate 2, or IT2).
In contrast with the behavior of the variant lacking the C-terminal disulfide, ATIII variants lacking either of the N-terminal disulfides (C8A–C128A or C21A–C95A) under oxidizing conditions both populated IT1R early, then IT2, and ultimately a third, faster mobility band (termed IT3) that migrated only slightly slower than NT and therefore represented a state with near-native compaction (Fig. 2B, lanes 2 and 3). Because IT3 accumulated for both the C8A–C128A and C21A–C95A variants, it appears that there is not a stringent order of formation for the two N-terminal disulfides. An intriguing finding is that variants lacking either of the N-terminal disulfides were secreted at similar levels to wild-type ATIII (with all three native disulfides) (Fig. 2A). Thus, formation of the N-terminal disulfides is not essential to the cell’s treatment of the protein as a folded entity. In other words, the quality control machinery of the ER views these incompletely folded but substantially compacted proteins as adequately folded and allows them to proceed through the secretory pathway. Nonetheless, ATIII variants missing either of the two N-terminal disulfides were inactive when secreted, as measured by their compromised ability to form a complex with thrombin (Fig. 2C), and each had an increased propensity to aggregate, as evidenced by a lower yield of secreted protein and concomitantly a larger triton-insoluble fraction in the cell lysates (Fig. 2A).
Taken together, these results paint the following picture of ATIII in-cell folding: The C-terminal disulfide C247–C430 must obligatorily form before formation of the N-terminal disulfides, and there is likely not a stringent order of formation of the two N-terminal disulfides (Fig. 2D). In an unexpected finding, formation of the C-terminal disulfide of ATIII is sufficient for secretion. However, ATIII lacking a full complement of its native disulfides is inactive and thus incapable of attaining the native, active state.
N-Glycans Direct the Efficient Cellular Maturation of ATIII.
The remodeling of the four N-linked glycans of ATIII also acts as a reporter for protein maturation. Carbohydrate modification and trimming occur at different locations along the secretory pathway and enable binding of lectin chaperones and other quality-control factors. Insight into the cellular management of ATIII folding and quality control is revealed by controlling the carbohydrate status of ATIII during passage through the secretory pathway.
Treatment of ATIII-transfected cells with tunicamycin (TM), an N-glycosylation inhibitor, diminished ATIII secretion by more than 50% (Fig. 3A, lanes 19–27). As TM disrupts the N-glycosylation of all glycoproteins by inhibiting the production of carbohydrate-dolichol phosphate precursors and stresses cells by activating the unfolded protein response cellular stress pathway (16), the effect of disrupting only ATIII glycosylation was tested by constructing an ATIII glycan-null variant. This variant was nonfunctional (Fig. 3B) and almost completely retained in the cell (Fig. 3A, lanes 10–18), demonstrating that the N-glycans were required for ATIII secretion.
We hypothesized that the ER carbohydrate-binding chaperones calnexin and calreticulin may be coconspirators in high-fidelity cellular folding of ATIII. We tested this hypothesis by treatment of ATIII producing cells with the glucosidase inhibitor castanospermine (CST), which prevents the formation of monoglucosylated glycoproteins and thereby inhibits lectin chaperone binding (17, 18). In the presence of CST, the level of ATIII secreted was reduced by a factor of three (Fig. 3A, lanes 28–36). The activity of the secreted triglucosylated protein was also modestly lower than untreated ATIII (Fig. 3B). Together, these results demonstrate the importance of glucosidase trimming and subsequent lectin chaperone binding for the efficient maturation of functional ATIII.
To determine which ATIII glycosylation sites influence its maturation and secretion, pulse–chase analysis of ATIII glycosylation mutants missing each of the four individual sites was performed (Fig. 3C). Secretion of ATIII missing the glycan at position Asn-96 was significantly reduced, whereas the absence of glycans at positions Asn-135, Asn-155, and Asn-192 had little influence on secretion levels. Glycosylation at Asn-96 was also required for activity, as was the C-terminal glycan (Asn-192) but at a more modest level (Fig. 3D). The two glycans at Asn-96 and -192 were also needed for efficient oxidation and folding, as in their absence neither mutant efficiently reached the NT state after a 30-min chase (Fig. 3E, lanes 4 and 8). Although the two central N-linked glycans at Asn-135 and 155 were largely dispensable for folding, secretion, and activity, glycans at Asn-96 and -192 were required for efficient and proper folding of active ATIII.
Folding, Secretion, and Activity of Disease-Associated ATIII Mutants.
ATIII is a major inhibitor of blood coagulation, as it is a potent inhibitor of thrombin and factor Xa (19). A large number of missense ATIII mutations have been discovered in patients suffering from thrombosis (20–22). To further test the importance of ordered disulfide bond formation and N-linked glycans for in-cell folding, secretion, and function, we monitored the effects of known ATIII mutations that add or subtract Cys residues and one mutation that adds an N-linked glycosylation site using the cellular assays developed in this study (Table S1). Our results also suggest that the gate (Fig. S2A) and C-terminal region may be particularly important for proper ATIII folding, and we therefore also monitored the effect of a number of mutations in these critical regions.
Table S1.
Locations, residue conservation, and type for ATIII disease-associated mutations
| Mutation | Location in ATIII structure (domain*) | ATII/serpin conservation† | Disease type‡ | References§ |
| I7N “Rouen-III” | N-terminal loop (α β domain) | No/no | Type II, heparin binding | (39) |
| N55Δ/R57C | Helix A (α β domain) | No/no | Type I | (39) |
| Y63C | Helix A (α β domain) | No/no | Type I | (39) |
| C128Y | Helix D (α β domain) | Yes/no | Type I | (39) |
| F229L | Loop from strand 3A to strand 4C (interdomain loop) | Yes/yes | Pleiotropic reduced circulating levels and functional defects | (22) |
| E237K, Truro | Gate/strand 4C (mainly β domain) | No/no | Type II, heparin binding | (39) |
| F239S | Gate/strand 4C (mainly β domain) | Yes/yes | Type I | (40) |
| F402C, Rosny | Strand 1C (mainly β domain) | Yes/no | Type II, pleiotropic | (39) |
| N405K, La Rochelle | Loop strand 1C to strand 4B (mainly β domain) | No/no | Type II, pleiotropic | (39) |
| P429L, Budapest | C-terminal loop (mainly β domain) | Yes/yes | Type II, pleiotropic | (39) |
| C430F | C-terminal loop | Yes/no | Type I | (39) |
Domains are as defined by CATH (Class Architecture Topology Homology) (www.cathdb.info), where the mainly β domain is CATH superfamily 2.30.39.10 and the α β domain is CATH superfamily 3.30.497.10.
Conservation within the ATIII family is based on a 59-sequence in-house, curated ATIII alignment and a published ATIII phylogeny (41), where positions showing ≥90% identity in the ATIII alignments were considered conserved. Conservation within the serpin superfamily is based on the serpin phylogeny of Irving et al. (33) and the associated list of 50 well-conserved serpin residues.
Disease type is based on the references and, for those mutations listed in the antithrombin mutation database, the type assigned in the database (https://www1.imperial.ac.uk/departmentofmedicine/divisions/experimentalmedicine/haematology/coag/antithrombin/) (39). Type I mutations result in low circulating ATIII levels and are found in ∼80% of symptomatic patients, whereas type II mutations are associated with reduced activity and, in some pleiotropic cases, reduced plasma levels (19).
Due to space limitations in the main text, the references describing the ATIII mutations are listed only for mutations not found in the antithrombin mutation database (39). For all other mutations, please refer to the database for additional information.
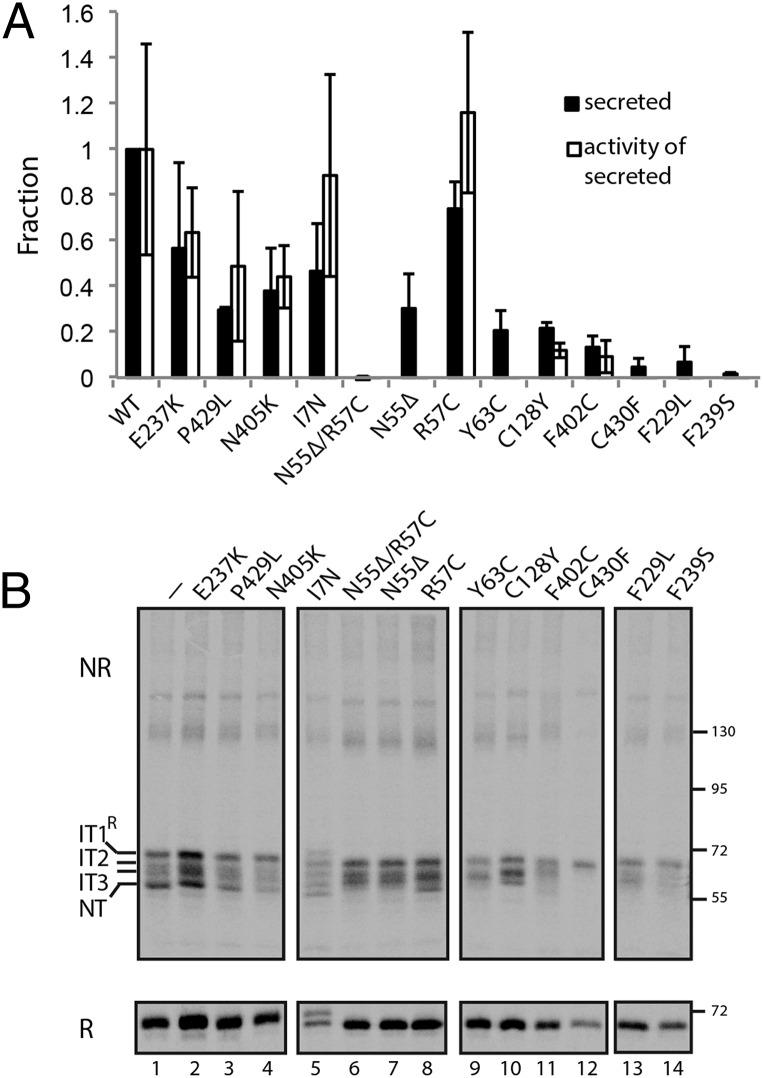
The effects of these mutations in cellular assays may be predicted based on our findings mentioned earlier and/or the classifications of mutations in patients. Human ATIII mutations are classified as either type I, secretion defects leading to low circulating levels of ATIII, or type II, activity defects where ATIII plasma levels are at or near normal levels but activity is low. Based on our results for disulfide bond mutants, we predicted that the Cys mutations would significantly perturb ATIII folding and secretion. As expected, four of the five mutations that involved Cys residues (Y63C, C128Y, F402C, and C430F) resulted in CHO cell secretion levels of less than 20% relative to wild-type levels of ATIII, consistent with their classification as type I and secretion mutants (Fig. 4A). The other Cys mutation, R57C, was found in a patient as a N55Δ/R57C double mutation (23). Although N55Δ/R57C was poorly secreted, consistent with its type I classification, the single R57C mutant did not exhibit a significant defect in secretion or activity. However, the N55Δ deletion mutation significantly impaired secretion. Also, the I7N variant that created an additional partially recognized glycosylation site (see the protein doublet in Fig. 4B, lane 9, Lower) did not significantly alter secretion, consistent with its type II classification (24). Heparin increases the rate at which ATIII inhibits target serine proteases and some type II mutations, including I7N, interfere with heparin binding (24, 25). Because the activity assays were performed without added heparin, they were relatively insensitive to the effects of such type II mutations, and thus in our activity assays, I7N and wild-type ATIII had similar activities (Fig. 4A).
Fig. 4.
The folding, secretion, and activity of disease-associated ATIII mutants. (A) Media from CHO cells transfected with wild type or mutant ATIII was collected 20 h posttransfection and analyzed for ATIII levels or inhibitory activity as in Fig. 2C. The secretion level compared with wild type and the activity of the secreted fraction are plotted. (B) The ATIII mutants were expressed in CHO cells. Cells were pulsed for 15 min and chased for 10 min. ATIII was resolved by nonreducing (NR) and reducing (R) SDS/PAGE. Folding intermediates are indicated.
The C-terminal disulfide bond is between Cys247 in the gate, formed by strands 3C and 4C, and Cys430 located almost at the C terminus of this 432-residue-long protein (Fig. S2A). The importance of the gate and the C-terminal region led us to investigate the effects on in-cell folding of disease-associated mutations in these regions. We chose three disease-associated mutations in or near the gate (F229L, E237K, and F239S) and two mutations in or near the C terminus (N405K and P429L) (Fig. S2B). The results of the cellular assays correlate with circulating levels in patients. Type I mutations F229L and F239S resulted in secretion levels of less than 20% relative to wild type and were largely inactive. In contrast, secretion levels of the type II mutants (E237K, P429L, and N405K) were all above 20%, with 40% or more of the secreted protein being active. These results demonstrated that CHO cell expression of the ATIII mutants recapitulated the approximate disease phenotypes observed in plasma of patients.
To determine if the secretion levels correlated with proper folding, the ATIII mutants were studied using a pulse–chase approach and analyzed by nonreducing and reducing SDS/PAGE. The folding or oxidation of the type I mutants was found to be blocked at a variety of states depending upon the specific mutation. F239S and C430F were arrested at the earliest oxidative intermediate (IT1R), indicating that none of the disulfides were able to form for these two mutants, demonstrating early derailment of the folding program due to disruption of the C-terminal disulfide (Fig. 4B, lanes 7 and 12). N55Δ/R57C, N55Δ, Y63C, C128Y, F239S, F229L, and F402C were partially oxidized, as they formed more compact folding intermediates but were unable to reach NT after the 10-min chase, indicative of misfolding later in the folding process. In contrast, the four type II mutants all showed significant amounts of NT. Thus, the more severe type I defective mutants were commonly associated with ATIII misfolding, providing an explanation for the diminished levels of ATIII in patient plasma.
Discussion
ATIII and other serpins must fold to a metastable or kinetically trapped state so that they can then use a large conformational movement initiated by peptide bond cleavage to inhibit target proteases. We have found that in cells ATIII folds to this higher free energy state by positioning the RCL at the C terminus early on in the folding process even though it is the last region to be synthesized. Exposing a loop like the RCL, particularly one that has an alternative topological position in which many hydrogen bonds may form (i.e., as a strand in the central β-sheet), requires stabilizing interactions flanking the loop (Fig. S1). Formation of the C-terminal disulfide is a key lynchpin, allowing the gate region to stabilize the RCL and keep it from prematurely inserting into the central β-sheet (Fig. S2A). Constraining the gate itself may also be important, as conformational changes in this region have been implicated in pathological serpin polymerization (26), which can occur in the ER during serpin maturation. For the serpins α1-antitrypsin and ovalbumin, both of which lack C-terminal disulfide bonds, purified protein-folding studies have demonstrated the importance of the C-terminal region for proper folding and function (27–30). These results highlight the importance of constraining conformationally labile regions early in serpin folding to efficiently reach the functionally required metastable structure.
Do cellular factors conspire to ensure the order of formation of ATIII disulfides? Or is the observed essentiality of formation of the C-terminal disulfide favored by sequence-encoded folding information? ATIII appears to rely on the lectin chaperone system comprised of calnexin, calreticulin, as well as the UDP-glucose, glycoprotein glucosyltransferase 1, which directs rebinding and ER retention of nonnative substrates (31, 32). The secretion level of ATIII was reduced by two-thirds in the absence of intervention by the lectin chaperone network; however, this diminished secreted fraction was active. Thus, although efficient ATIII maturation required the lectin chaperones, a small fraction of ATIII folded properly in their absence either unassisted or possibly helped by other chaperone systems that reside in the ER.
Interestingly, although the formation of the C-terminal disulfide is sufficient to pass the ER quality-control interrogation test, all three disulfides are required for ATIII to fold properly and acquire activity. Evidently the reliance on the lectin chaperone system supports efficient maturation of the wild-type protein, but the quality-control system fails to recognize the two N-terminal Cys double mutants. These inactive mutants should have been retained in the ER and targeted for degradation but were instead allowed to progress through the secretory pathway as inactive proteins. It will be of future interest to determine the nature of the mutant proteins and why they were incorrectly evaluated by the ER quality-control system.
Because of the oxidative nature of the ER lumen, unpaired Cys residues caused by deletions or additions of Cys are particularly sensitive for maturation and quality control. As expected based on the oxidation studies and disulfide bond mutants, most mutations leading to either the loss (C128Y and C430F) or gain (Y63C and F402C) of a Cys residue result in ATIII secretion defects (Fig. 4A) and type I mutations. However, by itself R57C did not significantly perturb secretion or activity. Unlike Tyr63 or Phe402, which are both buried residues, Arg57 is solvent-exposed, suggesting that solvent exposure in the final structure can modulate the deleterious effects of unpaired Cys residues. The effects of buried Cys mutations on folding correlate with their location relative to the C terminus with both C430F, which directly disrupts the C-terminal C247/C430 bond, and F402C, which adds a Cys residue near the C terminus, causing disruptions early in oxidative folding, whereas mutations at or near the N-terminal disulfide bonds, C128Y and Y63C, disrupt later steps in folding (Fig. 4B).
The type I F239S mutation involves a conserved residue in the gate. Phe239 is part of a conserved hydrophobic cluster in serpins (33) and is located within 2 Å of Cys247. As might be expected from its proximity to the C-terminal disulfide bond, F239S disrupts early oxidative folding events (Fig. 2D). Pro429 is both conserved in serpins and precedes C430 in the ATIII sequence (33); thus, one might expect that Pro429 mutations would lead to a type I secretion defect. However, although P429L secretion is lower than that of wild-type ATIII, it folds to NT (Fig. 2D), and the observed decrease in activity (Fig. 4A) is consistent with reports that its rate constant for thrombin inhibition is only 36% that of wild-type ATIII (34). Thus, proximity to the C-terminal disulfide is useful but not sufficient for predicting the effects of ATIII mutations on oxidative folding.
Serpin activity requires insertion of the RCL into the central β sheet, sheet A, and this conformational flexibility makes serpins particularly susceptible to mutation (35). RCL insertion requires opening of both the breach at the top of sheet A and the shutter region near the middle of sheet A (36). Phe229 forms part of a conserved hydrophobic cluster in the breach (33) in a loop that connects the two serpin domains, and the type I F229L mutation is postulated to lead to a cavity in this region (22). Interestingly, despite its interactions with both domains, the F229L mutation perturbs later steps in oxidative folding allowing ATIII to fold to at least IT2. Both Asn55 and Arg57 are in helix A, and Asn55 is within 5 Å of the shutter. Mutations in the shutter are often associated with serpin misfolding and polymerization in the ER (5). Deletion of Asn55 resulted in ∼30% secretion relative to wild type, but the secreted protein was inactive, whereas the R57C mutation did not significantly perturb secretion or activity, making it likely that Asn55 deletion and the resulting disruption in the register of helix A are largely responsible for perturbing folding. Interestingly, both F229L and N55Δ allow some oxidative folding but not full progression to NT, again supporting the idea that the early steps in ATIII oxidative folding involve the β rich serpin domain containing sheets B and C and the C terminus and that later steps consolidate folding of the central β-sheet A in the α/β serpin domain.
Although secretion levels of the type II mutants of ATIII were higher than the type I mutants, the four type II ATIII mutants all had reduced secretion levels compared with wild type. These mutations generally involved polar solvent-exposed residues or the addition of a large hydrophilic N-linked glycan (I7N), explaining their tapered impact on disrupting folding, secretion, and activity.
Our data point to the essentiality of key steps in ATIII folding and serve as a jumping-off point to explore both in vitro and in vivo folding of related serpins, particularly those that lack disulfides. What structural features and folding information play roles comparable to the ATIII C-terminal disulfide and N-terminal glycans? How do these other serpins successfully navigate their folding landscape to produce adequate amounts of functional metastable protein and avoid deleterious aggregation? The present results point to possible answers to some of these questions, which will be tested in future work.
Experimental Procedures
Pulse–Chase [35S]-Met/Cys Radiolabeling.
CHO cells were starved in DMEM without Met and Cys for 1 h and then pulsed with 65 μCi [35S]-Met/Cys for pulse times as indicated. Cells were rinsed with 1× PBS (137 mM NaCl, 4.3 mM Na2HPO4, 2.7 mM KCl, 1.4 mM KH2PO4) and chased with cold α-MEM as indicated. Cells were treated with or without inhibitors (1 mM CST or 5 μg/mL TM) during starvation, pulse, and chase time points. Media was collected for each chase time point, and cells were rinsed in PBS once.
ATIII Activity Assay.
For the protease inhibition assay, 100-mm dishes of near-confluent CHO cells were transfected with 7 μg plasmid and 11.4 μg poly(ethylenimine) (PEI) incubated in 420 μL Opti-MEM with media collected 20 h posttransfection. Media was spun at 16,000 × g for 5 min with the supernatant isolated and split into two fractions. To one fraction, 1.5 units of thrombin (EMD Bioscience, Inc.) was added and incubated at 37 °C for 1 h. Samples were precipitated with 20% (vol/vol) TCA and spun at 14,000 × g for 5 min. The pellets were washed with 0.5 mL of acetone before resolution by 8% nonreducing and reducing SDS/PAGE. Immunoblots were processed using a primary anti-myc sera, a secondary IRDye 800CW-goat-anti mouse antibody, and a LI-COR Odyssey CLx. The ATIII-thrombin complex was detected at ∼100 kDa (see, e.g., Fig. 2C), and band intensities were quantified using Image Studio Ver 4.0 from Li-Cor. The inhibitory activity of secreted ATIII was calculated as the ATIII–thrombin band intensity divided by the band intensity of secreted ATIII in the absence of thrombin. Error bars represent SD for at least three independent experiments. Detailed materials and methods are presented in SI Experimental Procedures.
SI Experimental Procedures
Cell Culture and Plasmid Transfection.
CHO cells were maintained in α-MEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated at 37 °C in 5% CO2. ATIII cDNA was cloned into pcDNA3.1 with a flexible C-terminal linker region (18 amino acids in length) followed by the –myc epitope and a 6×His tag. Plasmid transfections were performed using near-confluent cells in 35-mm dishes transfected with PEI (Polysciences, Inc.). We incubated 1 μg plasmid and 3.25 μg PEI in 60 μL Opti-MEM and added it drop-wise to cells. Transfections were incubated 16 h before pulse labeling and harvesting cells.
Immunoprecipitations.
Cells were lysed in cold buffer (0.5% Triton X-100, 20 mM Mes, 100 mM NaCl, 30 mM Tris·HCl, pH 7.5) containing freshly added protease inhibitors (20 mM N-ethyl maleimide, 400 μM PMSF, 50 μM N-acetyl-l-leucyl-l-leucyl-leucyl-l-norleucinal, 1 μM pepstatin A, 20 μM leupeptin, and 1.5 μM aprotinin). Postnuclear supernatants and media fractions were cleared with 10% zysorbin for 1 h at 4 °C. Clarified supernatant and media samples were incubated with α-ATIII (Abcam) or α-myc (Cell Signaling Technology, Inc.) antibody and protein A-Sepharose and rotated for 16 h at 4 °C. Immune complexes were washed twice with a wash buffer (0.05% triton X-100, 0.1% SDS, 300 mM NaCl, 10 mM Tris·HCl, pH 8.6), resuspended in sample buffer (9% SDS, 15% glycerol, 30 mM Tris, pH 6.8, 0.05% bromophenol blue), and resolved on SDS/PAGE. Reduced samples were supplemented with 100 mM DTT. The percent of ATIII secreted was quantified using a phosphorimager as follows: (secreted [35S]-Met/Cys-labeled ATIII at the indicated chase time)/(sum of the secreted, lysate, and triton-insoluble fractions of [35S]-Met/Cys-labeled ATIII at the initial chase time) × 100. Error bars represent SEM for at least three independent experiments.
Acknowledgments
This work was supported by National Institutes of Health Grants GM094848 (to A.G., L.M.G., and D.N.H.) and GM086874 (to D.N.H.) and American Heart Association Postdoctoral Fellowship 11POST7570018 (to K.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603386113/-/DCSupplemental.
References
- 1.Ferreiro DU, Komives EA, Wolynes PG. Frustration in biomolecules. Q Rev Biophys. 2014;47(4):285–363. doi: 10.1017/S0033583514000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407(6806):923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 3.Dementiev A, Dobó J, Gettins PG. Active site distortion is sufficient for proteinase inhibition by serpins: Structure of the covalent complex of alpha1-proteinase inhibitor with porcine pancreatic elastase. J Biol Chem. 2006;281(6):3452–3457. doi: 10.1074/jbc.M510564200. [DOI] [PubMed] [Google Scholar]
- 4.Wardell MR, et al. Preparative induction and characterization of L-antithrombin: A structural homologue of latent plasminogen activator inhibitor-1. Biochemistry. 1997;36(42):13133–13142. doi: 10.1021/bi970664u. [DOI] [PubMed] [Google Scholar]
- 5.Gooptu B, Lomas DA. Conformational pathology of the serpins: Themes, variations, and therapeutic strategies. Annu Rev Biochem. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- 6.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102(12):4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 7.Perry DJ, Carrell RW. Molecular genetics of human antithrombin deficiency. Hum Mutat. 1996;7(1):7–22. doi: 10.1002/(SICI)1098-1004(1996)7:1<7::AID-HUMU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Fish WW, et al. Denaturation behavior of antithrombin in guanidinium chloride. Irreversibility of unfolding caused by aggregation. Biochemistry. 1985;24(6):1510–1517. doi: 10.1021/bi00327a033. [DOI] [PubMed] [Google Scholar]
- 9.Sun XJ, Chang JY. Re-formation of disulphide bonds in reduced antithrombin III. Biochem J. 1990;269(3):665–669. doi: 10.1042/bj2690665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creighton TE. Disulfide bonds as probes of protein folding pathways. Methods Enzymol. 1986;131:83–106. doi: 10.1016/0076-6879(86)31036-x. [DOI] [PubMed] [Google Scholar]
- 11.Weissman JS, Kim PS. Reexamination of the folding of BPTI: Predominance of native intermediates. Science. 1991;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- 12.Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci USA. 1995;92(14):6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansens A, van Duijn E, Braakman I. Coordinated nonvectorial folding in a newly synthesized multidomain protein. Science. 2002;298(5602):2401–2403. doi: 10.1126/science.1078376. [DOI] [PubMed] [Google Scholar]
- 15.Picard V, Ersdal-Badju E, Bock SC. Partial glycosylation of antithrombin III asparagine-135 is caused by the serine in the third position of its N-glycosylation consensus sequence and is responsible for production of the beta-antithrombin III isoform with enhanced heparin affinity. Biochemistry. 1995;34(26):8433–8440. doi: 10.1021/bi00026a026. [DOI] [PubMed] [Google Scholar]
- 16.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 17.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert DN, Molinari M. Flagging and docking: Dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci. 2012;37(10):404–410. doi: 10.1016/j.tibs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik MM, Moll S. Inherited antithrombin deficiency: A review. Haemophilia. 2008;14(6):1229–1239. doi: 10.1111/j.1365-2516.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 20.Lane DA, et al. Pleiotropic effects of antithrombin strand 1C substitution mutations. J Clin Invest. 1992;90(6):2422–2433. doi: 10.1172/JCI116133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheffield WP, Castillo JE, Blajchman MA. Intracellular events determine the fate of antithrombin Utah. Blood. 1995;86(9):3461–3467. [PubMed] [Google Scholar]
- 22.Picard V, et al. Antithrombin Phe229Leu: A new homozygous variant leading to spontaneous antithrombin polymerization in vivo associated with severe childhood thrombosis. Blood. 2003;102(3):919–925. doi: 10.1182/blood-2002-11-3391. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury V, et al. Identification of nine novel mutations in type I antithrombin deficiency by heteroduplex screening. Br J Haematol. 1993;84(4):656–661. doi: 10.1111/j.1365-2141.1993.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 24.Brennan SO, et al. New carbohydrate site in mutant antithrombin (7 Ile-Asn) with decreased heparin affinity. FEBS Lett. 1988;237(1-2):118–122. doi: 10.1016/0014-5793(88)80183-2. [DOI] [PubMed] [Google Scholar]
- 25.Olson ST, Björk I, Shore JD. Kinetic characterization of heparin-catalyzed and uncatalyzed inhibition of blood coagulation proteinases by antithrombin. Methods Enzymol. 1993;222:525–559. doi: 10.1016/0076-6879(93)22033-c. [DOI] [PubMed] [Google Scholar]
- 26.Irving JA, et al. An antibody raised against a pathogenic serpin variant induces mutant-like behaviour in the wild-type protein. Biochem J. 2015;468(1):99–108. doi: 10.1042/BJ20141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolmer K, Gettins PG. How the serpin α1-proteinase inhibitor folds. J Biol Chem. 2012;287(15):12425–12432. doi: 10.1074/jbc.M111.315465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onda M, et al. Cleaved serpin refolds into the relaxed state via a stressed conformer. J Biol Chem. 2008;283(25):17568–17578. doi: 10.1074/jbc.M709262200. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui Y, Dela Cruz R, Wintrode PL. Folding mechanism of the metastable serpin α1-antitrypsin. Proc Natl Acad Sci USA. 2012;109(12):4467–4472. doi: 10.1073/pnas.1109125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stocks BB, Sarkar A, Wintrode PL, Konermann L. Early hydrophobic collapse of α₁-antitrypsin facilitates formation of a metastable state: Insights from oxidative labeling and mass spectrometry. J Mol Biol. 2012;423(5):789–799. doi: 10.1016/j.jmb.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 32.Hebert DN, Lamriben L, Powers ET, Kelly JW. The intrinsic and extrinsic effects of N-linked glycans on glycoproteostasis. Nat Chem Biol. 2014;10(11):902–910. doi: 10.1038/nchembio.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: Implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10(12):1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- 34.Olds RJ, et al. Antithrombin III Budapest: A single amino acid substitution (429Pro to Leu) in a region highly conserved in the serpin family. Blood. 1992;79(5):1206–1212. [PubMed] [Google Scholar]
- 35.Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995;2(2):96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- 36.Whisstock JC, Skinner R, Carrell RW, Lesk AM. Conformational changes in serpins: I. The native and cleaved conformations of alpha(1)-antitrypsin. J Mol Biol. 2000;296(2):685–699. doi: 10.1006/jmbi.1999.3520. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 39.Lane DA, et al. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Antithrombin mutation database: 2nd (1997) update. Thromb Haemost. 1997;77(1):197–211. [PubMed] [Google Scholar]
- 40.Martínez-Martínez I, et al. The infective polymerization of conformationally unstable antithrombin mutants may play a role in the clinical severity of antithrombin deficiency. Mol Med. 2012;18:762–770. doi: 10.2119/molmed.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Bhandari A, Sarde SJ, Goswami C. Sequence, phylogenetic and variant analyses of antithrombin III. Biochem Biophys Res Commun. 2013;440(4):714–724. doi: 10.1016/j.bbrc.2013.09.134. [DOI] [PubMed] [Google Scholar]