Abstract
The exhibition of increasingly intensive and complex niche construction behaviors through time is a key feature of human evolution, culminating in the advanced capacity for ecosystem engineering exhibited by Homo sapiens. A crucial outcome of such behaviors has been the dramatic reshaping of the global biosphere, a transformation whose early origins are increasingly apparent from cumulative archaeological and paleoecological datasets. Such data suggest that, by the Late Pleistocene, humans had begun to engage in activities that have led to alterations in the distributions of a vast array of species across most, if not all, taxonomic groups. Changes to biodiversity have included extinctions, extirpations, and shifts in species composition, diversity, and community structure. We outline key examples of these changes, highlighting findings from the study of new datasets, like ancient DNA (aDNA), stable isotopes, and microfossils, as well as the application of new statistical and computational methods to datasets that have accumulated significantly in recent decades. We focus on four major phases that witnessed broad anthropogenic alterations to biodiversity—the Late Pleistocene global human expansion, the Neolithic spread of agriculture, the era of island colonization, and the emergence of early urbanized societies and commercial networks. Archaeological evidence documents millennia of anthropogenic transformations that have created novel ecosystems around the world. This record has implications for ecological and evolutionary research, conservation strategies, and the maintenance of ecosystem services, pointing to a significant need for broader cross-disciplinary engagement between archaeology and the biological and environmental sciences.
Keywords: biodiversity, extinctions, invasive species, novel ecosystems, Anthropocene
The reshaping of global biodiversity is one of the most significant impacts humans have had on Earth’s ecosystems. As our planet experiences its sixth “mass extinction event” (1), the effect of anthropogenic landscape modification, habitat fragmentation, overexploitation, and species invasions could not be more apparent (2, 3). These transformations are linked largely to the industrial economies, burgeoning populations, and dense transport networks of contemporary human societies. Accordingly, the human-mediated alteration of species distributions has been characterized as a modern phenomenon with limited, and largely insignificant, historical antecedents. This conventional understanding fails to account for several decades of archaeological, paleoecological, and genetic research that reveal a long and widespread history of human transformation of global biodiversity (4–6). The evolutionary trajectory of Homo sapiens has seen growing capacities for advanced cognition and demographic and geographic expansion, along with an exponential increase in the scope and impact of human niche constructing activities (7) that have culminated in fundamental changes to planetary ecosystems.
Drawing upon findings from a range of new methods and datasets, including new cross-disciplinary research programs, we explore this uniquely human trajectory and reveal a pattern of significant long-term, anthropogenic shaping of species distributions on all of the earth’s major occupied continents and islands. We show that, even before the Age of Discovery, cumulative human activities over millennia resulted in dramatic changes to the abundance and geographic range of a diverse array of organisms across taxonomic groups. Few, if any, regions can be characterized as pristine. Extinction has been the starkest of these anthropogenic impacts, but widespread changes to species abundance, composition, community structure, richness, and genetic diversity as a result of human niche construction are also increasingly demonstrable and of equally lasting impact.
We highlight the role of new classes of data, such as ancient DNA (aDNA), stable isotopes, and microfossils, as well as new approaches, including powerful morphometric, chronometric, computational, and statistical methods, for understanding changes to species distributions at various scales (Fig. 1). The increasingly systematic application of traditional environmental archaeology methods in the last few decades is also yielding new insights. While acknowledging that human engagement in niche construction has very early origins, we focus on examples from four key phases of more recent and wide-reaching anthropogenic change: the Late Pleistocene near-global dispersal of H. sapiens; the emergence and spread of agriculture beginning in the Early Holocene; the colonization of the world’s islands; and the premodern expansion of urbanization and trade beginning in the Bronze Age. Although not exhaustive, our review highlights key trends, including the significant prehistoric and historic reorganization of species distributions at local, regional, and intercontinental scales; a broadly accelerating but uneven rate of alien species introductions across multiple geographical regions; and the involvement of a wide range of species, including plant and animal domesticates, as well as a diverse array of wild, commensal, invasive, and pathogenic species. We emphasize the role of these cumulative changes in contributing to the creation of novel ecosystems over the long term. We conclude by considering the implications of an archaeologically informed perspective on contemporary biodiversity for how we understand, study, and conserve the earth’s biomes, as well as how we comprehend the evolutionary pressures exerted by human ecosystem engineering.
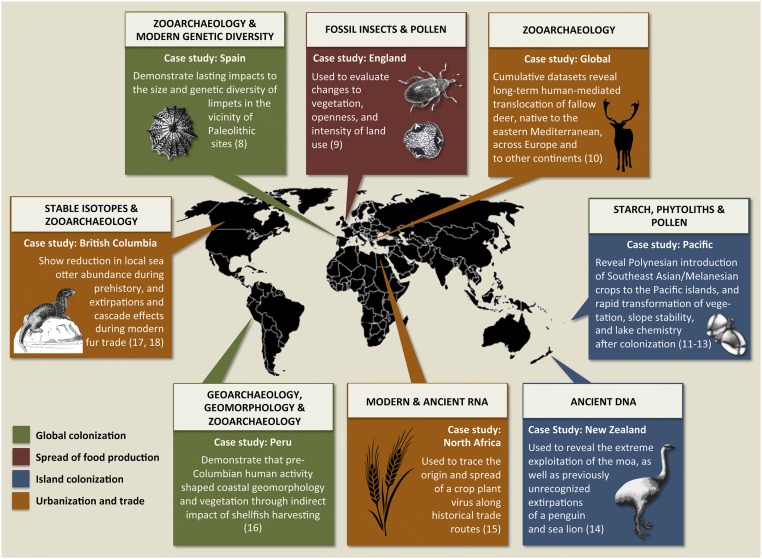
Fig. 1.
Case studies in the application of archaeological science methods to understand past human-mediated biological translocations and transformations relating to the following: global colonization, origins and spread of food production, island colonization, and trade and urbanization (8–18).
Four Key Phases of Anthropogenic Transformation
Global Colonization.
Fossil evidence demonstrates that H. sapiens was present ∼195,000 y ago (195 ka) in East Africa (19) and that, by 12 ka, our species had dispersed to the far corners of Eurasia, Australia, and the Americas (20). Mounting evidence indicates that these Late Pleistocene dispersals, and the increase in global human populations with which they are associated, were linked in complex ways with a variety of species extinctions, extirpations, translocations, and new modes of niche modification. Evaluating Pleistocene anthropogenic impacts remains challenging, but novel methods and approaches are providing solutions to long-standing problems posed by limited preservation and chronological resolution.
New data link the geographic and demographic expansion of H. sapiens to fire regime change and transformations to plant community composition. For example, pollen and microcharcoal records indicate that the early colonists of New Guinea deliberately burned and disturbed tropical rainforests to promote the growth of useful plants, especially gap colonizers like yams (Dioscorea spp.), which have been identified from microscopic starch residues extracted from some of the region’s earliest stone tools (21). (For species other than H. sapiens, this manuscript employs common species names, although the scientific name for each species discussed is also provided at first mention. For humans, the scientific name is further specified when it is important to distinguish from other hominid species.) Vegetation burning also enhanced hunting opportunities by drawing game and other faunal resources to new plant growth. A human contribution to the shaping of early fire regimes has been demonstrated for Africa and, after human arrival, in Borneo, Australia, and the Americas (22–25).
The human-mediated translocation of species now dates back to the Late Pleistocene. For example, the northern common cuscus (Phalanger orientalis), endemic to New Guinea, was transported to eastern Indonesia, the Solomon Islands, and the Bismarck Archipelago beginning ∼20–23 ka, becoming a key subsistence species (26, 27). Other taxa were also moved; together with a species of bandicoot (Echymipera kalubu) and the Admiralty cuscus (Spilocuscus kraemeri), the Canarium indicum tree was introduced to Manus by ∼13 ka, followed a few millennia later by the rat Rattus praetor (26). Translocation patterns mirror patterns of maritime obsidian exchange in Melanesia in the Late Pleistocene and Early Holocene (26).
Evidence of human overexploitation has been suggested for some Late Pleistocene faunal sequences. Diverse archaeological assemblages, from Africa, Europe, and South Asia, for example, document the Late Pleistocene appearance of small, quick, and difficult-to-catch game, such as fish, birds, rabbits, rodents and monkeys, that may signal anthropogenic impacts to resource availability (28, 29). Other studies document decreases in the size of certain species, such as limpets and tortoises, that may also reflect resource overexploitation (e.g., refs. 8 and 30). Some of these changes may result from the expansion of bone, stone, shell, fiber, and other tool repertoires in the Late Pleistocene, enabling new forms of intensive exploitation (e.g., refs. 31 and 32).
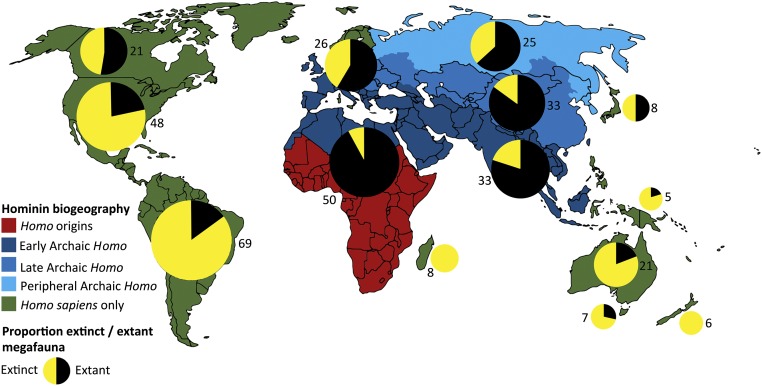
One of the most significant impacts of the Late Pleistocene expansion of our species may have been on megafauna (Fig. S1). The human role in the Late Quaternary extinction episode, which saw at least 101 of 150 genera of Earth’s megafauna (animals larger than 44 kg) go extinct between 50 and 10 ka (33), has long been contentious (e.g., refs. 34–36). Recent analyses support at least a partial anthropogenic impetus in numerous regions, and a dominant human role in others (37, 38). Of particular importance are new global analyses drawing on higher resolution data and computational modeling approaches. These studies indicate an important role for humans and an inverse relationship between severity of extinction and duration of hominin–megafauna coevolution, with uniformly high extinction rates in areas where H. sapiens was the first hominin to arrive (39, 40) (Fig. S1).
Fig. S1.
Proportions of megafauna known to be extinct in each region of the globe relative to the length of coevolution and contact with humans (genus Homo) (adapted from figure 1C in ref. 39 and figure 1 in ref. 40). The numbers next to each pie chart indicate the total number of megafauna genera originally present within each region.
New regional analyses support these findings. For example, recent high-resolution paleoecological and stable isotope data from Australia, where no hominins existed before ∼55 ka, show that megafaunal collapse occurred during a period of climatic stability and most closely correlates with human arrival (41). Improved chronologies for various Australian and Tasmanian sites (e.g., refs. 42 and 43) support anthropogenic rather than climatic explanations for megafaunal extinctions. Chronometric resolution remains poor for South America, although recent studies support a human role in megafaunal extinction in Patagonia (44), whereas data from aDNA studies suggest that climatic extinction drivers were more influential in northern regions (e.g., ref. 45). Implicating humans in Late Pleistocene megafaunal extinctions suggests an anthropogenic role in subsequent and major biosphere transformations that followed their demise (33, 46, 47). Megafauna were keystone species whose disappearance had dramatic effects on ecosystem structure, fire regimes, seed dispersal, land surface albedo, and nutrient availability (41, 46, 48) (Fig. 2A).
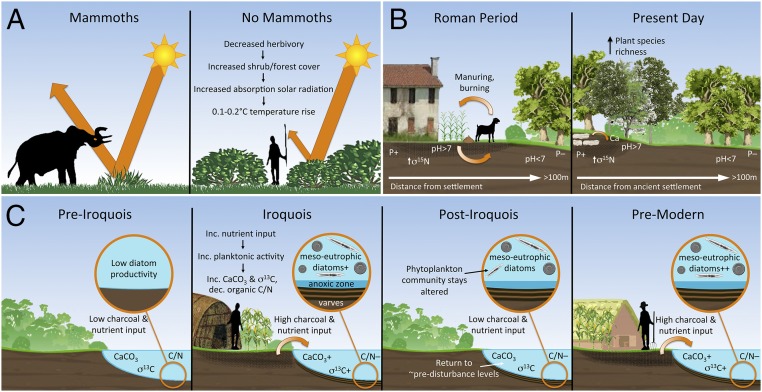
Fig. 2.
Cascade effects of changes to species, showing long-term transformation of landscapes. (A) Impact of eliminating large herbivores (49). (B) Long-term effects of ancient agriculture on soil geochemistry and plant biodiversity in forests (50–52). (C) Limnological responses to cultural disturbance of lake watershed (53, 54).
Emergence and Spread of Agriculture and Pastoralism.
The beginning of the Holocene (<11.7 ka) witnessed fundamental shifts in climatic and geological regimes globally, as well as in human societies. The Early to Middle Holocene in many regions worldwide saw the beginning of agricultural economies, placing new evolutionary pressures on plants, animals, and microbes, and resulting in major demographic expansions for humans (55). This Neolithic period opened the way for a radical transformation in the human capacity for niche construction, increasingly demonstrated through the accumulation of zooarchaeological and archaeobotanical data, as well as the application of biomolecular techniques.
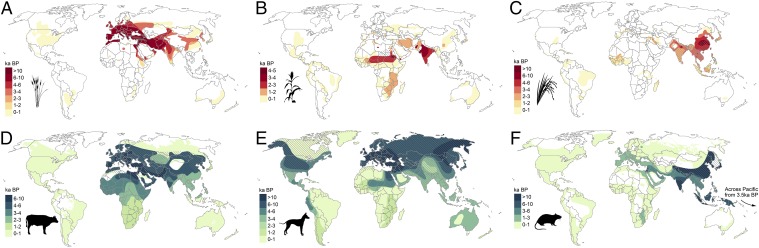
One of the major outcomes of the Neolithic was the inexorable spread of agriculture from ∼14–20 centers of early domestication (56) to encompass large swaths of the Old and New Worlds. This expansion had unprecedented and enduring impacts on species distributions. Key among these transformations was the promotion and expansion of a range of human-favored taxa, including newly created species (and subspecies) of domesticated crops and animals. Cumulative archaeological data show that crops and animals saw significant prehistoric and historic range expansion (Fig. 3). The scale of agriculture and land use in some regions was significant; for example, expansion of land area used for livestock and rice (Oryza sativa) paddy agriculture was sufficient to increase atmospheric methane emissions between 4,000 and 1,000 y B.P. (57) whereas deforestation and tillage are suggested to have contributed to increasing CO2 over the past 8,000 y (58).
Fig. 3.
Global spread of selected food crops (red) and domesticated and commensal animals (blue) through time. (A) Wheat (Triticum spp.). (B) Sorghum (Sorghum bicolor). (C) Rice (Oryza sativa, Oryza glaberrima). (D) Cattle (Bos taurus, Bos indicus). (E) Dog (Canis familiaris). (F) Rat (Rattus rattus, Rattus tanezumi, Rattus norvegicus, Rattus exulans). The major spread of rats to global islands beginning by 3 ka is not apparent at the scale shown. (Note that maps use different temporal scales, appropriate to individual species and their temporality of spread; hatching indicates natural distribution.)
Modern and aDNA studies are shedding light on patterns of genetic adaptation and hybridization that shaped crop dispersal (e.g., ref. 59) whereas plant microfossil and genetic studies are beginning to clarify the spread of tropical species (e.g., refs. 60 and 61). The geographic expansion of agricultural crops was a complex process that carried along other species and transformed local ecosystems in diverse ways (Fig. 3 A–C). Crops often moved as part of ecological packages that included nondomesticated or weed species. In the European Neolithic, for example, some crop weeds derived ultimately from the Near East whereas others were European plants promoted by anthropogenic disturbance and the novel ecologies of cultivated plots (e.g., ref. 62). Such weeds came to be important components of regional wild vegetation, in some cases becoming more common in regions where they were introduced than in their zones of origin. This naturalization occurred to such a degree that, for many of the most widespread weeds, it is unclear where in the world they originated (63).
Domesticated animals also dispersed across the world’s landmasses. New high resolution aDNA, protein, isotope, and geometric-morphometric techniques join standard archaeobiological methods to reveal the expansion of different livestock species across the globe (Fig. 3 D and E). Sheep (Ovis aries), goat (Capra hircus), and cattle (Bos taurus) were domesticated in the Near East ∼10.5 ka and arrived in Europe, Africa, and South Asia within a few millennia (57, 64). Chickens (Gallus gallus) were domesticated in East Asia (although the specific timing and location remains contentious), reached Britain by the second half of the last millennium before the common era (B.C.E.), and now outnumber people by more than three to one (65). Wild boar (Sus spp.) populations in East Asia and Anatolia were domesticated independently, and, like all major animal domesticates, pigs (Sus scrofa) are now associated with humans well outside their natural Old World distribution (66). Dogs (Canis familiaris), the only animal domesticated before the emergence of agricultural societies, are now the most abundant and ubiquitous carnivore, with an estimated 700 million to 1 billion dogs worldwide (67). The biomass of wild vertebrates is now vanishingly small compared with that of domestic animals (68).
Neolithic dispersals also featured pathogens. Ancient DNA, stable isotope, and other studies are clarifying the spread of pathogens favored by shifts in diet, lifestyle, mobility, and human–animal relationships with the onset of agriculture. Ancient DNA from Yersinia pestis and Mycobacterium tuberculosis has been identified from Neolithic human skeletons (e.g., refs. 69 and 70) and linked to large-scale population movements (69, 71). Plant and animal pathogens also spread in the Neolithic. The northwest European elm decline (3700–3600 B.C.E.) may have been caused in part by the spread of a pathogen, such as the fungal disease Ophiostoma, carried by the elm bark beetle (Scolytus scolytus), which saw habitat expansion with clearance for agriculture (72).
The spread of human populations and the species they favored altered the distributions of existing species, sometimes in synergy with Holocene climatic changes. Numerous regional studies demonstrate the link between Neolithic agriculture and the creation of more open landscapes, facilitated through various means from fire to the cutting and coppicing of trees (73, 74). For example, the early Neolithic corresponded with shifts away from deciduous tree cover in various regions of central and northern Europe (e.g., ref. 74). The spread of farmers into central Africa caused an encroachment on rainforest by some expanded savannah species (75). Early rice cultivation in the coastal wetlands of eastern China was linked to clearance of alder-dominated wetland scrub (76).
Early to Middle Holocene forest clearance correlates with a variety of broader species and habitat impacts. The transformation of forests and tall grassland into pastures that began 7–8 ka in central and northern Eurasia is linked to radically increased herbivore load due to the grazing of introduced species (77, 78). Together with forest burning, this activity significantly accentuated climate-induced vegetation change, with resultant changes in albedo in Tibet suggested to have impacted the monsoon system (78). Forest removal and agricultural activities increased erosion and impacted lake biota, including lacustrine microfloras and microfaunas (e.g., diatoms, macrophytes, and foraminifera) (Fig. 2C). Paleolimnological studies in lowland Europe, for example, suggest human-mediated increases in mesotrophic–eutrophic planktonic diatoms, including Asterionella formosa and Fragilaria crotonensis, by 5,000 y B.P. (79).
Island Colonization.
The colonization of islands was a feature of H. sapiens expansion from the Late Pleistocene onwards but accelerated significantly in the Holocene as maritime technological advances enabled humans to reach increasingly remote oceanic islands (80). Evidence from global island-focused research programs suggests that ancient humans had major impacts on island ecosystems that often lacked the resilience of continental biomes (81, 82). Island ecologies are often characterized by high endemism, naive and/or disharmonic fauna, and low functional redundancy (83). Thus, the overall impact on islands of human-transported species, anthropogenic fire, deforestation, and predation was often the radical restructuring of island ecosystems.
Species translocations to islands were so common in the past that archaeologists often speak of “transported landscapes” (84). These new landscapes included a broad range of domesticated animals, commensals, crops, weeds, microbes, and other species carried by humans. For example, Neolithic colonizers who arrived on Cyprus brought domestic cereals, pulses, sheep, goat, cattle, pigs, domestic dogs, and cats (Felis catus), as well as mainland game animals such as fallow deer (Dama dama), fox (Vulpes vulpes), and wild boar beginning 10.6 ka (64, 85). Polynesian people, expanding across the Pacific after ∼3,500 y B.P. (84), introduced a broad range of domesticated species, including the crops taro (Colocasia esculenta), yam (Dioscorea spp.), and banana (Musa spp.), and such animals as the domestic pig, chicken, dog, and Pacific rat (Rattus exulans). In the Caribbean, Archaic and Ceramic period peoples introduced a variety of species, including wild avocado (Persea americana), manioc/cassava (Manihot esculenta), maize (Zea mays), tobacco (Nicotiana rustica), and various trees, as well as dogs, opossums (Didelphis sp.), guinea pigs (Cavia porcellus), and shrews (Nesophontes edithae) (86).
Such introductions played a role in making islands more habitable for humans. Before human habitation, Cyprus had a low density of food animals (85), and the islands of the Pacific often lacked edible plants and possessed limited nonmarine fauna (87). In island Southeast Asia, humans transported a range of domesticates, as well as various species of deer, primate, civet, cuscus, wallaby, bird, shrew, rat, and lizard to generate habitats more favorable to human sustenance (27). Anthropogenic landscapes were created through species introductions, as well as habitat modification, including fire and other means, which reshaped the composition and abundance of native species. On the Pacific island of Tonga, Polynesians introduced at least 40 plant species, mostly trees, shrubs, and herbaceous cultigens (88). They burned and cleared indigenous rainforests, altering the abundance and distribution of species to favor useful native plants such as Canarium harveyi, Casuarina equisetifolia, Erythrina variegata, and Pandanus tectorius (88). Not all translocated plants were introduced for subsistence; paper mulberry (Broussonetia papyrifera), for example, is a fiber crop introduced across the Pacific in prehistory for making barkcloth (89).
Numerous species were unintentionally introduced to islands, including commensal and parasitic species adapted to the human niche. Although a variety of plants were deliberately carried to the subtropical islands of Polynesia in the pre-European era, at least 17 were unintentionally introduced weed species (90). Pacific rats and black rats (Rattus rattus) were widely introduced to global islands as accidental stowaways on boats beginning in the Middle Holocene (Fig. 3C), as were house mice (Mus musculus), various commensal shrews and lizards, and numerous insects and land snails, with the movements of many now clarified through genetic and aDNA studies. Genetic data demonstrate that Helicobacter pylori, a human pathogen, moved with prehistoric populations expanding through Melanesia and into the Pacific (91).
Extinctions and extirpations were a common consequence of island colonization in prehistory. Thousands of bird populations in the Pacific went extinct after Polynesian colonization (92). One recent study of nonpasserine birds on 41 Pacific islands shows that two-thirds went extinct between initial prehistoric colonization and European contact (93). Bird species extinctions impact important ecosystem processes like decomposition, pollination, and seed dispersal, leading to trophic cascades (94). Human impacts have been primarily responsible for the extinction of four genera of giant sloths in the Caribbean, as well as nine taxa of snakes, lizards, bats, birds, and rodents on Antigua between 2350 and 550 B.C.E. (82, 95). Floral extinctions have not been as well-studied, but a range of island plant species went extinct on islands in prehistoric times. Pollen and wood charcoal analyses demonstrate at least 18 plant extinctions on Rapanui (Easter Island), for example, and show dense palm forest disappearing within 200 y of human settlement (96).
New chronometric data are revealing the rapidity with which prehistoric extinctions sometimes unfolded (80). New Zealand saw numerous vertebrate extinctions after Polynesian arrival (e.g., refs. 80 and 92), including the elimination of various species of moa (Dinornis) within two centuries of human colonization (97). Recent studies of sea lion and penguin aDNA show that several New Zealand species once thought to have survived early human impacts were extirpated soon after human arrival and replaced within a few centuries by nonindigenous lineages from the subantarctic region (98).
Extinction and extirpation rates underestimate human impacts because not all species under pressure went extinct. Although Hawaiian geese (Branta sandvicensis), unlike other species, survived the prehistoric colonization of Hawaii by humans, aDNA research points to a drastic reduction in their genetic diversity after human arrival (99). Zooarchaeological data from the Caribbean point to the overharvesting and decline of a variety of island marine species beginning ∼2,000 y ago, with biomass, mean trophic level, and average size all radically altered (86). Research on California’s Channel Islands points to similar impacts on a broad range of marine animals as a result of overexploitation by prehistoric hunter-gatherers (81, 82), patterns increasingly recognized on islands around the world.
Urbanization and the Elaboration of Trade Networks.
By the Middle to Late Holocene, agriculture and the production of food surpluses paved the way for the emergence of larger human populations, increasingly dense, urbanized settlements, and more complex and intensive networks of trade, travel, and dispersal in many parts of the world. Cultural niche construction became intense and elaborate, with dramatic implications for species diversity and distributions.
Multidisciplinary datasets reveal that agricultural intensification, in response to factors like growing populations and emerging markets, was a major driver of ecological change across the Old World from the Bronze Age onwards (100). In the Near East, Bronze Age datasets reveal pervasive turnover from deciduous to evergreen oak and replacement of indigenous forest with cultivated orchard crops like olive (Olea europea), grape (Vitis vinifera), and fig (Ficus carica) (e.g., refs. 101 and 102). Cereal crops and vegetation indicative of grazing and other anthropogenic disturbance (e.g., Rumex, Plantago, and Artemisia) increased. Archaeological study of wood charcoal points to a decline in tree taxa richness from the Middle Bronze Age to the Late Iron Age (103). By 1000 B.C.E., one archaeologically tested model suggests that 80–85% of areas suited to agriculture in much of the Near East were cultivated (104).
Similar trends can be seen for all early urban societies that have been studied. Increased deforestation, linked to agricultural intensification and urbanization in the Iron Age, is evident in diverse sedimentary and paleoecological records in China (e.g., refs. 105 and 106). European and Near Eastern landscapes in the Roman period also saw significant transformation, with expansion of cultivation into previously marginal areas, growth of the cash crop industry, and a new emphasis on high yield agro-pastoralism (100). Sedimentary sequences across the eastern Mediterranean record the highest Holocene rates of soil erosion and sedimentation during the Classical era (102). Population growth and political expansion in lowland Mayan civilization have been linked to forest removal and erosion (107, 108).
Deforestation and the expansion of species favoring anthropogenic disturbance were not continual processes, and many sequences reveal temporary reversals in these long-term trends. For example, the arrival of plague in Europe at several points from the Late Neolithic onwards, as now confirmed by recovery of Yersinia pestis aDNA from human skeletons (69, 109), seems to have been linked to episodes of forest regrowth due to abandonment of agricultural fields (104, 110). By the Iron Age and sometimes earlier, however, changing species compositions were often irreversible. Recent multidisciplinary research in “ancient” forests in France demonstrated a strong correlation between Roman sites and present-day forest plant diversity, with areas altered by Roman agriculture and settlement favoring nitrogen-demanding and ruderal species (e.g., refs. 50 and 51) (Fig. 2C). Grassland diversity in present-day Estonia maps closely to Late Iron Age human population density (111).
Defaunation is another enduring legacy of ancient human activities. The emergence of socially stratified urban societies in the Near East and Egypt, for instance, was linked to the extirpation of a number of wild animal species. Onager (Equus hemionus), Persian gazelle (Gazella subgutturosa), hartebeest (Alcelaphus buselaphus), Arabian oryx (Oryx leucoryx), and ostrich (Struthio camelus) were all extirpated from the southern Levant, largely through ungulate mass kills, by the second millennium B.C.E. (112). Ancient urbanization contributed to a major reduction in large-bodied mammal species in Egypt, from 37 in the Late Pleistocene/Early Holocene to only 8 today (113). Roman era hunting and acquisition of wild animals for arena and other events led to species reductions and extirpations across Europe and North Africa (114). Stable isotope analysis of archaeological fish remains from northern and western Europe demonstrates that overexploitation of local fish reserves prompted increasing globalization of the fishing industry as early as the 13th to 14th century of the common era (C.E.) (115).
Despite such trends, and contrary to popularized narratives of overexploitation-fueled environmental and cultural collapse (e.g., ref. 116), recent studies also demonstrate that agricultural and other practices of early civilizations helped maintain ecosystem services. Intensification through human practices shifted carrying capacity upwards (5). Parts of the Amazon, a region long viewed as pristine tropical forest, are now known to have supported densely settled, highly productive, and powerful regional polities for millennia before European arrival (117). These societies created areas of fertile anthropogenically modified soil that enabled cultivation and the growth of populations in regions viewed today as marginal (118). Although caution is needed regarding claims of basin-wide anthropogenic alterations (119), it is clear that, in some regions, forests were converted into patchy, managed landscapes that included large-scale transformations to forest plants, animals, and wetlands (117). The Maya also created highly managed landscapes and forest gardens that enabled significant population growth and political complexity (107, 108, 120, 121). Studies in Africa demonstrate an anthropogenic role in forest creation (122), with prehistoric parallels suggested for several regions globally, including the Fertile Crescent, where oak parkland with wild cereals is argued to be ancient but largely anthropogenic (123).
The increasingly intensive long-distance translocation of species from the Bronze Age onwards was part of this wider picture of habitation transformation that was sometimes destructive and other times promoted the provision of ever-increasing human populations. Widespread translocation of invasive species like the black rat and house mouse with improved maritime and terrestrial transport systems, as revealed by zooarchaeological and molecular genetic studies (124), led to negative ecosystem and disease impacts (e.g., refs. 80 and 96). Plant pathogens and pests also spread. In Britain, for example, a range of nonnative but now established synanthropic beetle grain pests (e.g., Sitophilus granarius, Oryzaephilus surinamensis, Laemophloeus ferrugineus) first appeared in Roman times, probably carried over with grain imports (125).
On the other hand, through time, the extraordinary diversification of food economies based on widespread circulation of new plant and animal domesticates contributed to more diverse diets, many of which were impoverished by the prehistoric shift from foraging to food production (126). A recent estimate from Britain, where some of the most systematic archaeobotanical studies have been carried out, indicates that at least 50 new plant foods (mostly fruits, herbs, and vegetables) were introduced in the Roman period alone, with many entering into cultivation (127). Genetic and aDNA studies have revealed that various domesticates were transported between the Pacific Islands and South America, including coconut (Cocos nucifera) and chicken (128, 129). In short order, these taxa became key food species in their new homelands, enriching human diets and transforming ecologies. Coconut palm, for example, widely dispersed by prehistoric humans, has important impacts on the floristic, structural, and soil characteristics of forests (130). Genetic and archaeological studies demonstrate that the medieval Indian Ocean saw the circulation and adoption of a broad array of new plant and animal domesticates, many of which improved nutrient availability and agricultural resilience (e.g., ref. 131). Nondomesticated species also continued to spread in this period, with zooarchaeological and genetic (including aDNA) studies of species, ranging from snails and geckos to birds and deer, indicating anthropogenic alterations to range distributions as a result of increasing globalization (132, 133).
Broad Patterns of Ancient Anthropogenic Change
A review of global archaeological, paleoecological, and historical datasets, distilled here into key trends and examples, suggests a number of general patterns concerning the long-term human shaping of biodiversity. First, human niche construction activities have had a major impact on the abundance, composition, distribution, and genetic diversity—as well as extinction rates and translocation pathways—of species globally. Late Pleistocene human impacts are the most difficult to assess, but, placed in the context of longer-term trends, they seem highly likely, especially given that even conservative estimates of anthropogenic contribution to megafaunal extinctions, extirpations, and depletions imply significant ecosystem impacts (38, 47).
Second, there is a strong link between present-day patterns of biodiversity and historical processes (Fig. 2). The combined effects of human activity over the millennia include the creation of extensively altered, highly cosmopolitan species assemblages on all landmasses. “Pristine” landscapes simply do not exist and, in most cases, have not existed for millennia. Most landscapes are palimpsests shaped by repeated episodes of human activity over multiple millennia (5, 36, 100).
Third, there is widespread evidence for increasing rates of human-mediated species translocation, extinction, and ecosystem and biodiversity reshaping through time. This acceleration is not constant but is characterized by pulses and pauses that reflect cultural, ecological, and climatic transformations at local, regional, and global scales. These changes have increasingly concentrated biomass into particular sets of human-favored plants and animals (134).
Fourth, archaeological and paleoecological data are critical to identifying and understanding the deep history and pervasiveness of such human impacts (6, 36, 135). Ecologists and other researchers are often insufficiently aware of archaeological and other historical datasets. The continued default position among many researchers is that a landscape or seascape that does not have obvious, contemporary human alterations has experienced little human manipulation (136). In fact, as exemplified by the revelation of dense prehistoric human settlements in parts of the Amazon, the more appropriate default expectation is one of anthropogenic transformation, regardless of how pristine a modern landscape may superficially seem.
Finally, negative consequences of human activity, such as extinction, reduced biodiversity, and habitat destruction, tend to receive more attention from researchers than examples of resilience and sustainability (100), probably because these transformations are more dramatic and visible in the archaeological record (137). The anthropogenic reshaping of species distributions, however, has been central to the creation of landscapes capable of supporting increasingly dense human populations through time. Domesticated ecosystems enhance human food supplies, reduce exposure to predators and natural dangers, and promote commerce (138). The creation of novel ecosystems (139) has enabled the provision of ecological goods and services, not just in the modern era but throughout the Holocene and in the Late Pleistocene as well (5, 100, 140).
These broad historical patterns have implications not only for how we understand the past, but also for how we address the present and the future. This realization calls for archaeologists and other historical scientists to weigh in on key ecological and political debates. One of these controversies concerns the date for the start of the Anthropocene, the current, human-dominated phase of Earth’s geological sequence (141). Even the partial and coarse-grained historical datasets currently available suggest that widespread reshaping of global biodiversity probably began in the Late Pleistocene or Early Holocene, with attendant geomorphological, atmospheric, oceanic, and biogeochemical changes (6, 141, 142). The assertion that preindustrial societies had only local and transitory environmental impacts is mistaken and reflects lack of familiarity with a growing body of archaeological data.
Another important consideration is the role of human niche construction as a major evolutionary force on the planet. Processes of human niche creation have reshaped, and continue to influence, the evolutionary trajectories of a broad array of species. Except for studies of domestication and antibiotic/pesticide resistance, however, investigation into processes of gene-culture coevolution has otherwise minimally explored the role of human culture in driving evolution in nonhuman species. However, human activities have exerted novel selection pressures that have had important evolutionary consequences not just for humans but also for the rest of the natural world (143).
Recognizing the long-term human shaping of global biodiversity is also key to understanding contemporary human–ecology interactions and to predictive modeling of future transformations. Present day landscape processes cannot be fully understood without recognizing past processes that have shaped terrestrial and aquatic ecosystems around the world for millennia. Determining the consequences of past ecological change will also inform predictions of how modern communities may respond to ongoing anthropogenic or climatic factors (113, 144). Archaeological data can additionally help prioritize conservation efforts by enabling assessment of how enduring specific types of changes to biodiversity are over the long term (145).
If an archaeological perspective is key to conservation efforts, it also challenges elements of their foundation. If change is the only constant in human–ecology relationships, it remains unclear what “natural” targets ecological restoration should aim for (139). The wholesale appropriation of land and resources for environmental ends—“green grabbing”—at the expense of the needs and livelihoods of local and indigenous groups (often seen as destructive of pristine ecologies) is also further problematized (146). Appreciation of historical data shifts conservation ecology away from concern with a return to original ecological conditions, suggesting the need for pragmatic solutions that acknowledge the integral role humans have long played in shaping natural systems (36, 139). The impact of human agency on ecosystems is neither completely avoidable nor entirely undesirable. People have inhabited a growing range of environments at ever increasing densities only through the continual anthropogenic transformation of ecosystems. Rather than an impossible return to pristine conditions, what is needed is the historically informed management of emerging novel ecosystems to ensure the maintenance of ecological goods and services (139). Such efforts need to account for the needs of all stakeholders and balance local livelihoods against first world agendas.
Historical datasets not only caution against unrealistic goals, but also provide clues for shaping more resilient domesticated landscapes. Although anthropogenic processes have certainly had catastrophic ecological impacts through time, they have also played a significant role in generating sustainable ecosystems (138). Humans may have contributed to the Late Pleistocene megafaunal extinctions that disrupted biogeochemical cycling in the Amazon (48), for instance, but they also created extraordinary terra preta soils that supported productive agriculture and large human populations on nutrient-poor Amazon soils by 2,000 y B.P. (118). The fertile terra preta anthrosols created by indigenous Amazonians have been the focus of attempts to understand, and replicate, their unique chemistry and microbial communities to promote sustainable agriculture and long-term CO2 sequestration (147). A variety of ancient anthropogenic ecosystems in the Mediterranean, Americas, Africa, and elsewhere are attracting attention for similar reasons (e.g., refs. 140 and 148).
Archaeologists have an obligation to share their increasing knowledge of the major anthropogenic role in shaping global species distributions, as well as other ecosystem properties. Present-day changes to the diversity, composition, and distribution of species are part of long-term processes that need to be factored into programs of research, planning, conservation, and management. The urgent challenges of mediating and managing present-day anthropogenic forces demand a fully informed approach that recognizes that today’s societies possess exceptional but not unique capacities for reshaping global ecosystems. Highlighting a long-term human role in shaping biodiversity does not absolve present-day populations of taking responsibility for Earth’s environments. Instead, it reaffirms the human capacity for ecological transformation that is denied by those interest groups that challenge scientific evidence for anthropogenic global warming, and suggests that we should own up to our role in transforming ecosystems and embrace responsible policies befitting a species that has engaged in millennia of ecological modification.
Acknowledgments
We thank Ardern Hulme-Beaman and Heidi Eager for information regarding Rattus distributions. We thank the Fyssen Foundation (Paris) for supporting the symposium from which this paper emerged and thank the other participants for their stimulating contributions. This paper reflects the output of European Research Council funding to N.L.B. (Grant 206148-SEALINKS), D.Q.F. (Grant 323842-COMPAG), G.L. (Grant 337574-UNDEAD), and M.D.P. (Grant 295719-PALAEODESERTS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525200113/-/DCSupplemental.
References
- 1.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 2.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328(5982):1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 3.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 4.Boivin N, Crassard R, Petraglia M, editors. , Species Movements in Human History: From Prehistory to the Present. Cambridge Univ Press; Cambridge, UK: in press. [Google Scholar]
- 5.Ellis EC, et al. Used planet: A global history. Proc Natl Acad Sci USA. 2013;110(20):7978–7985. doi: 10.1073/pnas.1217241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erlandson JM, Braje TJ, eds (2013) When humans dominated the Earth: Archaeological perspectives on the Anthropocene. Anthropocene 4(December):1–122.
- 7.Laland KN, Odling-Smee J, Feldman MW. Cultural niche construction and human evolution. J Evol Biol. 2001;14(1):22–33. doi: 10.1046/j.1420-9101.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 8.Turrero P, Muñoz-Colmenero M, Pola IG, Arbizu M, García-Vázquez E. Morphological, demographic and genetic traces of Upper Palaeolithic human impact on limpet assemblages in North Iberia. J Quat Sci. 2012;27(3):244–253. [Google Scholar]
- 9.Smith D, Whitehouse N, Bunting M, Chapman H. Can we characterise ‘openness’ in the Holocene palaeoenvironmental record? Modern analogue studies of insect faunas and pollen spectra from Dunham Massey deer park and Epping Forest, England. Holocene. 2010;20(2):215–229. [Google Scholar]
- 10.Sykes N, Carden R, Harris K. Changes in the size and shape of fallow deer: Evidence for the movement and management of a species. Int J Osteoarchaeol. 2013;23(1):55–68. [Google Scholar]
- 11.Crowther A. Starch residues on undecorated Lapita pottery from Anir, New Ireland. Archaeol Ocean. 2005;40(2):62–66. [Google Scholar]
- 12.Horrocks M, Bedford S, Spriggs M. A short note on banana (Musa) phytoliths in Lapita, immediately post-Lapita and modern period archaeological deposits from Vanuatu. J Archaeol Sci. 2009;36(9):2048–2054. [Google Scholar]
- 13.McWethy DB, Wilmshurst JM, Whitlock C, Wood JR, McGlone MS. A high-resolution chronology of rapid forest transitions following polynesian arrival in New Zealand. PLoS One. 2014;9(11):e111328. doi: 10.1371/journal.pone.0111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskam C, et al. Ancient DNA analyses of early archaeological sites in New Zealand reveal extreme exploitation of moa (Aves: Dinornithiformes) at all life stages. Quat Sci Rev. 2012;52:41–48. [Google Scholar]
- 15.Smith O, et al. A complete ancient RNA genome: Identification, reconstruction and evolutionary history of archaeological Barley Stripe Mosaic Virus. Sci Rep. 2014;4:4003. doi: 10.1038/srep04003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belknap DF, Sandweiss DH. Effect of the Spanish Conquest on coastal change in Northwestern Peru. Proc Natl Acad Sci USA. 2014;111(22):7986–7989. doi: 10.1073/pnas.1404568111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szpak P, Orchard T, McKechnie I, Gröcke D. Historical ecology of late Holocene sea otters (Enhydra lutris) from northern British Columbia: Isotopic and zooarchaeological perspectives. J Archaeol Sci. 2012;39(5):1553–1571. [Google Scholar]
- 18.Szpak P, Orchard T, Salomon A, Gröcke D. Regional ecological variability and impact of the maritime fur trade on nearshore ecosystems in southern Haida Gwaii (British Columbia, Canada): Evidence from stable isotope analysis of rockfish (Sebastes spp.) bone collagen. Archaeol Anthropol Sci. 2013;5(2):159–182. [Google Scholar]
- 19.McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433(7027):733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 20.Gamble C. Settling the Earth: The Archaeology of Deep Human History. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 21.Summerhayes GR, et al. Human adaptation and plant use in highland New Guinea 49,000 to 44,000 years ago. Science. 2010;330(6000):78–81. doi: 10.1126/science.1193130. [DOI] [PubMed] [Google Scholar]
- 22.Miller GH, et al. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science. 2005;309(5732):287–290. doi: 10.1126/science.1111288. [DOI] [PubMed] [Google Scholar]
- 23.Pinter N, Fiedel S, Keeley JE. Fire and vegetation shifts in the Americas at the vanguard of Paleoindian migration. Quat Sci Rev. 2011;30(3):269–272. [Google Scholar]
- 24.Archibald S, Staver AC, Levin SA. Evolution of human-driven fire regimes in Africa. Proc Natl Acad Sci USA. 2012;109(3):847–852. doi: 10.1073/pnas.1118648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt CO, Gilbertson DD, Rushworth G. A 50,000-year record of late Pleistocene tropical vegetation and human impact in lowland Borneo. Quat Sci Rev. 2012;37:61–80. [Google Scholar]
- 26.Summerhayes GR. Island Melanesian pasts—a view from archaeology. In: Friedlaender JS, editor. Genes, Language, and Culture History in the Southwest Pacific. Oxford Univ Press; Oxford: 2007. pp. 10–35. [Google Scholar]
- 27. Heinsohn TE (2010) Marsupials as introduced species: Long-term anthropogenic expansion of the marsupial frontier and its implications for zoogeographic interpretation. Altered Ecologies: Fire, Climate and Human Influence on Terrestrial Landscapes, Terra Australis 32, eds Haberle S, Stevenson J, Prebble M (ANU E Press, Canberra, ACT, Australia), pp. 133–176.
- 28.Haws JA. Paleolithic socionatural relationships during MIS 3 and 2 in central Portugal. Quat Int. 2012;264:61–77. [Google Scholar]
- 29.McCall GS, Thomas JT. Still Bay and Howiesons Poort foraging strategies: Recent research and models of culture change. Afr Archaeol Rev. 2012;29(1):7–50. [Google Scholar]
- 30.Stiner MC, Munro ND, Surovell TA, Tchernov E, Bar-Yosef O. Paleolithic population growth pulses evidenced by small animal exploitation. Science. 1999;283(5399):190–194. doi: 10.1126/science.283.5399.190. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor S, Ono R, Clarkson C. Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science. 2011;334(6059):1117–1121. doi: 10.1126/science.1207703. [DOI] [PubMed] [Google Scholar]
- 32.Shea J, Sisk M. Complex projectile technology and Homo sapiens dispersal into western Eurasia. PaleoAnthropol. 2010;2010:100–122. [Google Scholar]
- 33.Barnosky AD. Colloquium paper: Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11543–11548. doi: 10.1073/pnas.0801918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wroe S, et al. Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea) Proc Natl Acad Sci USA. 2013;110(22):8777–8781. doi: 10.1073/pnas.1302698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook BW, et al. Lack of chronological support for stepwise prehuman extinctions of Australian megafauna. Proc Natl Acad Sci USA. 2013;110(36):E3368. doi: 10.1073/pnas.1309226110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grayson DK. The archaeological record of human impacts on animal populations. J World Prehist. 2001;15(1):1–68. [Google Scholar]
- 37.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 38.Braje TJ, Erlandson JM. Human acceleration of animal and plant extinctions: A Late Pleistocene, Holocene, and Anthropocene continuum. Anthropocene. December 2013;4:14–23. [Google Scholar]
- 39.Sandom C, Faurby S, Sandel B, Svenning J-C. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc Biol Sci. 2014;281(1787):20133254. doi: 10.1098/rspb.2013.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett LJ, et al. Robustness despite uncertainty: Regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography. 2015;39(2):152–161. [Google Scholar]
- 41.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 42.Turney CSM, et al. Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction. Proc Natl Acad Sci USA. 2008;105(34):12150–12153. doi: 10.1073/pnas.0801360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grün R, et al. ESR and U-series analyses of faunal material from Cuddie Springs, NSW, Australia: Implications for the timing of the extinction of the Australian megafauna. Quat Sci Rev. 2010;29(5):596–610. [Google Scholar]
- 44.Villavicencio NA, et al. Combination of humans, climate, and vegetation change triggered Late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography. 2016;39(2):125–140. [Google Scholar]
- 45.Lorenzen ED, et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479(7373):359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CN. Ecological consequences of Late Quaternary extinctions of megafauna. Proc Biol Sci. 2009;276(1667):2509–2519. doi: 10.1098/rspb.2008.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doughty CE, et al. Global nutrient transport in a world of giants. Proc Natl Acad Sci USA. 2016;113(4):868–873. doi: 10.1073/pnas.1502549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 2013;6:761–764. [Google Scholar]
- 49.Doughty CE, Wolf A, Field CB. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human-induced global warming? Geophys Res Lett. 2010;37(15):L15703. [Google Scholar]
- 50.Dambrine E, et al. Present forest biodiversity patterns in France related to former Roman agriculture. Ecology. 2007;88(6):1430–1439. doi: 10.1890/05-1314. [DOI] [PubMed] [Google Scholar]
- 51.Dupouey JL, Dambrine E, Laffite JD, Moares C. Irreversible impact of past land use on forest soils and biodiversity. Ecology. 2002;83(11):2978–2984. [Google Scholar]
- 52.Plue J, et al. Persistent changes in forest vegetation and seed bank 1,600 years after human occupation. Landsc Ecol. 2008;23(6):673–688. [Google Scholar]
- 53.Ekdahl E, et al. Diatom assemblage response to Iroquoian and Euro-Canadian eutrophication of Crawford Lake, Ontario, Canada. J Paleolimnol. 2007;37(2):233–246. [Google Scholar]
- 54.Ekdahl E, et al. Prehistorical record of cultural eutrophication from Crawford Lake, Canada. Geology. 2004;32(9):745–748. [Google Scholar]
- 55.Bocquet-Appel J-P. When the world’s population took off: The springboard of the Neolithic Demographic Transition. Science. 2011;333(6042):560–561. doi: 10.1126/science.1208880. [DOI] [PubMed] [Google Scholar]
- 56.Larson G, et al. Current perspectives and the future of domestication studies. Proc Natl Acad Sci USA. 2014;111(17):6139–6146. doi: 10.1073/pnas.1323964111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuller DQ, et al. The contribution of rice agriculture and livestock pastoralism to prehistoric methane levels: An archaeological assessment. Holocene. 2011;21(5):743–759. [Google Scholar]
- 58.Kaplan JO, et al. Holocene carbon emissions as a result of anthropogenic land cover change. Holocene. 2011;21(5):775–791. [Google Scholar]
- 59.Jones G. Phylogeographic analysis of barley DNA as evidence for the spread of Neolithic agriculture through Europe. J Archaeol Sci. 2012;39(10):3230–3238. [Google Scholar]
- 60.Perrier X, et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc Natl Acad Sci USA. 2011;108(28):11311–11318. doi: 10.1073/pnas.1102001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ball T, et al. Phytoliths as a tool for investigations of agricultural origins and dispersals around the world. J Archaeol Sci. April 2016;68:32–45. [Google Scholar]
- 62.Coward F, Shennan S, Colledge S, Conolly J, Collard M. The spread of Neolithic plant economies from the Near East to northwest Europe: A phylogenetic analysis. J Archaeol Sci. 2008;35(1):42–56. [Google Scholar]
- 63.Baker HG. The continuing evolution of weeds. Econ Bot. 1991;45(4):445–449. [Google Scholar]
- 64.Zeder MA. Out of the Fertile Crescent: The dispersal of domestic livestock through Europe and Africa. In: Boivin N, Crassard R, Petraglia M, editors. Species Movements in Human History: From Prehistory to the Present. Cambridge Univ Press; Cambridge, UK: in press. [Google Scholar]
- 65.Lawler A. Why Did the Chicken Cross the World? The Epic Saga of the Bird That Powers Civilization. Duckworth Overlook; London: 2015. [Google Scholar]
- 66.Frantz L, et al. The evolution of Suidae. Annu Rev Anim Biosci. 2016;4:61–85. doi: 10.1146/annurev-animal-021815-111155. [DOI] [PubMed] [Google Scholar]
- 67.Ritchie EG, Letnic CR, Vanak AT. Dogs as predators and trophic regulators. In: Gompper ME, editor. Free-Ranging Dogs and Wildlife Conservation. Oxford Univ Press; Oxford: 2014. pp. 55–68. [Google Scholar]
- 68.Smil V. Harvesting the biosphere: The human impact. Popul Dev Rev. 2011;37(4):613–636. doi: 10.1111/j.1728-4457.2011.00450.x. [DOI] [PubMed] [Google Scholar]
- 69.Rasmussen S, et al. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell. 2015;163(3):571–582. doi: 10.1016/j.cell.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hershkovitz I, et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS One. 2008;3(10):e3426. doi: 10.1371/journal.pone.0003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Comas I, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45(10):1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waller M. Drought, disease, defoliation and death: Forest pathogens as agents of past vegetation change. J Quat Sci. 2013;28(4):336–342. [Google Scholar]
- 73.Yerkes RW, Khalaily H, Barkai R. Form and function of early neolithic bifacial stone tools reflects changes in land use practices during the neolithization process in the levant. PLoS One. 2012;7(8):e42442. doi: 10.1371/journal.pone.0042442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Innes JB, Blackford JJ, Rowley-Conwy PA. Late Mesolithic and early Neolithic forest disturbance: A high resolution palaeoecological test of human impact hypotheses. Quat Sci Rev. 2013;77:80–100. [Google Scholar]
- 75.Neumann K, et al. First farmers in the Central African rainforest: A view from southern Cameroon. Quat Int. 2012;249:53–62. [Google Scholar]
- 76.Zong Y, et al. Fire and flood management of coastal swamp enabled first rice paddy cultivation in east China. Nature. 2007;449(7161):459–462. doi: 10.1038/nature06135. [DOI] [PubMed] [Google Scholar]
- 77.Miehe G, et al. How old is pastoralism in Tibet? An ecological approach to the making of a Tibetan landscape. Palaeogeogr Palaeoclimatol Palaeoecol. 2009;276(1):130–147. [Google Scholar]
- 78.Schlütz F, Lehmkuhl F. Holocene climatic change and the nomadic Anthropocene in eastern Tibet: Palynological and geomorphological results from the Nianbaoyeze Mountains. Quat Sci Rev. 2009;28(15):1449–1471. [Google Scholar]
- 79.Wilkinson IP, Poirier C, Head MJ, Sayer CD, Tibby J. Microbiotic signatures of the Anthropocene in marginal marine and freshwater palaeoenvironments. Geol Soc Lond Spec Publ. 2014;395(1):185–219. [Google Scholar]
- 80.Anderson A. The rat and the octopus: Initial human colonization and the prehistoric introduction of domestic animals to Remote Oceania. Biol Invasions. 2009;11(7):1503–1519. [Google Scholar]
- 81.Erlandson JM, Rick TC. Archaeology meets marine ecology: The antiquity of maritime cultures and human impacts on marine fisheries and ecosystems. Annu Rev Mar Sci. 2010;2:231–251. doi: 10.1146/annurev.marine.010908.163749. [DOI] [PubMed] [Google Scholar]
- 82.Rick TC, Kirch PV, Erlandson JM, Fitzpatrick SM. Archeology, deep history, and the human transformation of island ecosystems. Anthropocene. December 2013;4:33–45. [Google Scholar]
- 83.O’Dowd DJ, Green PT, Lake PS. Invasional ‘meltdown’ on an oceanic island. Ecol Lett. 2003;6(9):812–817. [Google Scholar]
- 84.Kirch PV. The Lapita Peoples: Ancestors of the Oceanic world. Blackwell; Oxford: 1997. [Google Scholar]
- 85.Vigne J-D, et al. First wave of cultivators spread to Cyprus at least 10,600 y ago. Proc Natl Acad Sci USA. 2012;109(22):8445–8449. doi: 10.1073/pnas.1201693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzpatrick SM, Keegan WF. Human impacts and adaptations in the Caribbean Islands: An historical ecology approach. Earth Environ Sci Trans Roy Soc Edinb. 2007;98(1):29–45. [Google Scholar]
- 87.Spriggs M. Landscape catastrophe and landscape enhancement: Are either or both true in the Pacific. In: Kirch PV, Hunt TL, editors. Historical Ecology in the Pacific Islands: Prehistoric Environmental and Landscape Change. Yale Univ Press; New Haven, CT: 1997. pp. 80–104. [Google Scholar]
- 88.Fall PL. 2010. Pollen evidence for plant introductions in a Polynesian tropical island ecosystem, Kingdom of Tonga. Altered Ecologies: Fire, Climate and Human Influence on Terrestrial Landscapes, Terra Australis 32, eds Haberle S, Stevenson J, Prebble M (ANU E Press, Canberra, ACT, Australia), pp 253–271.
- 89.Chang C-S, et al. A holistic picture of Austronesian migrations revealed by phylogeography of Pacific paper mulberry. Proc Natl Acad Sci USA. 2015;112(44):13537–13542. doi: 10.1073/pnas.1503205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prebble M. 2008. No fruit on that beautiful shore: What plants were introduced to the subtropical Polynesian islands prior to European contact. Islands of Inquiry: Colonisation, Seafaring and the Archaeology of Maritime Landscapes, Terra Australis 29, eds Clark GR, Leach F, O’Connor S (ANU E Press, Canberra, ACT, Australia), pp 227–251.
- 91.Moodley Y, et al. The peopling of the Pacific from a bacterial perspective. Science. 2009;323(5913):527–530. doi: 10.1126/science.1166083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steadman DW. Extinction and Biogeography of Tropical Pacific Birds. Univ of Chicago Press; Chicago: 2006. [Google Scholar]
- 93.Duncan RP, Boyer AG, Blackburn TM. Magnitude and variation of prehistoric bird extinctions in the Pacific. Proc Natl Acad Sci USA. 2013;110(16):6436–6441. doi: 10.1073/pnas.1216511110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J. Cascading effects of bird functional extinction reduce pollination and plant density. Science. 2011;331(6020):1068–1071. doi: 10.1126/science.1199092. [DOI] [PubMed] [Google Scholar]
- 95.Steadman DW, et al. Asynchronous extinction of late Quaternary sloths on continents and islands. Proc Natl Acad Sci USA. 2005;102(33):11763–11768. doi: 10.1073/pnas.0502777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prebble M, Dowe JL. The late Quaternary decline and extinction of palms on oceanic Pacific islands. Quat Sci Rev. 2008;27(27-28):2546–2567. [Google Scholar]
- 97.Perry GLW, Wheeler AB, Wood JR, Wilmshurst JM. A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes) Quat Sci Rev. 2014;105:126–135. [Google Scholar]
- 98.Collins CJ, et al. Extinction and recolonization of coastal megafauna following human arrival in New Zealand. Proc Biol Sci. 2014;281(1786):20140097. doi: 10.1098/rspb.2014.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paxinos EE, et al. Prehistoric decline of genetic diversity in the nene. Science. 2002;296(5574):1827. doi: 10.1126/science.296.5574.1827. [DOI] [PubMed] [Google Scholar]
- 100.Butzer KW. Environmental history in the Mediterranean world: Cross-disciplinary investigation of cause-and-effect for degradation and soil erosion. J Archaeol Sci. 2005;32(12):1773–1800. [Google Scholar]
- 101.Fall PL, Falconer SE, Lines L. Agricultural intensification and the secondary products revolution along the Jordan Rift. Hum Ecol. 2002;30(4):445–482. [Google Scholar]
- 102.Dusar B, Verstraeten G, Notebaert B, Bakker J. Holocene environmental change and its impact on sediment dynamics in the Eastern Mediterranean. Earth Sci Rev. 2011;108(3):137–157. [Google Scholar]
- 103.Wright NJ, Fairbairn AS, Faith JT, Matsumura K. Woodland modification in Bronze and Iron Age central Anatolia: An anthracological signature for the Hittite state? J Archaeol Sci. March 2015;55:219–230. [Google Scholar]
- 104.Kaplan JO, Krumhardt KM, Zimmermann N. The prehistoric and preindustrial deforestation of Europe. Quat Sci Rev. 2009;28(27):3016–3034. [Google Scholar]
- 105.Shen J, Jones RT, Yang X, Dearing JA, Wang S. The Holocene vegetation history of Lake Erhai, Yunnan province southwestern China: The role of climate and human forcings. Holocene. 2006;16(2):265–276. [Google Scholar]
- 106.Atahan P, et al. Holocene-aged sedimentary records of environmental changes and early agriculture in the lower Yangtze, China. Quat Sci Rev. 2008;27(5):556–570. [Google Scholar]
- 107.Dunning NP, Beach TP, Luzzadder-Beach S. Kax and kol: Collapse and resilience in lowland Maya civilization. Proc Natl Acad Sci USA. 2012;109(10):3652–3657. doi: 10.1073/pnas.1114838109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennett DJ, Beach TP. Archeological and environmental lessons for the Anthropocene from the Classic Maya collapse. Anthropocene. December 2013;4:88–100. [Google Scholar]
- 109.Wagner DM, et al. Yersinia pestis and the plague of Justinian 541-543 AD: A genomic analysis. Lancet Infect Dis. 2014;14(4):319–326. doi: 10.1016/S1473-3099(13)70323-2. [DOI] [PubMed] [Google Scholar]
- 110.Ruddiman WF. The anthropogenic greenhouse era began thousands of years ago. Clim Change. 2003;61(3):261–293. [Google Scholar]
- 111.Pärtel M, Helm A, Reitalu T, Liira J, Zobel M. Grassland diversity related to the Late Iron Age human population density. J Ecol. 2007;95(3):574–582. [Google Scholar]
- 112.Tsahar E, Izhaki I, Lev-Yadun S, Bar-Oz G. Distribution and extinction of ungulates during the Holocene of the southern Levant. PLoS One. 2009;4(4):e5316. doi: 10.1371/journal.pone.0005316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeakel JD, et al. Collapse of an ecological network in Ancient Egypt. Proc Natl Acad Sci USA. 2014;111(40):14472–14477. doi: 10.1073/pnas.1408471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hughes JD. Europe as consumer of exotic biodiversity: Greek and Roman times. Landsc Res. 2003;28(1):21–31. [Google Scholar]
- 115.Barrett JH, et al. Interpreting the expansion of sea fishing in medieval Europe using stable isotope analysis of archaeological cod bones. J Archaeol Sci. 2011;38(7):1516–1524. [Google Scholar]
- 116.Diamond JM. Collapse: How Societies Choose to Fail or Succeed. Penguin; New York: 2006. [Google Scholar]
- 117.Heckenberger MJ, Russell JC, Toney JR, Schmidt MJ. The legacy of cultural landscapes in the Brazilian Amazon: Implications for biodiversity. Philos Trans R Soc Lond B Biol Sci. 2007;362(1478):197–208. doi: 10.1098/rstb.2006.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arroyo-Kalin M. The Amazonian formative: Crop domestication and anthropogenic soils. Diversity (Basel) 2010;2(4):473–504. [Google Scholar]
- 119.Piperno DR, McMichael C, Bush MB. Amazonia and the Anthropocene: What was the spatial extent and intensity of human landscape modification in the Amazon Basin at the end of prehistory? Holocene. 2015;25(10):1588–1597. [Google Scholar]
- 120.Ford A, Nigh R. Origins of the Maya forest garden: Maya resource management. J Ethnobiol. 2009;29(2):213–236. [Google Scholar]
- 121.McNeil CL, Burney DA, Burney LP. Evidence disputing deforestation as the cause for the collapse of the ancient Maya polity of Copan, Honduras. Proc Natl Acad Sci USA. 2010;107(3):1017–1022. doi: 10.1073/pnas.0904760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fairhead J, Leach M. Misreading the African Landscape: Society and Ecology in a Forest-Savanna Mosaic. Cambridge Univ Press; Cambridge, UK: 1996. [Google Scholar]
- 123.Asouti E, Kabukcu C. Holocene semi-arid oak woodlands in the Irano-Anatolian region of Southwest Asia: Natural or anthropogenic? Quat Sci Rev. 2014;90:158–182. [Google Scholar]
- 124.Jones EP, Eager HM, Gabriel SI, Jóhannesdóttir F, Searle JB. Genetic tracking of mice and other bioproxies to infer human history. Trends Genet. 2013;29(5):298–308. doi: 10.1016/j.tig.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 125.King GA, Kenward H, Schmidt E, Smith D. Six-legged hitchhikers: An archaeobiogeographical account of the early dispersal of grain beetles. J North Atl. 2014;23(1):1–18. [Google Scholar]
- 126.Larsen CS. The agricultural revolution as environmental catastrophe: Implications for health and lifestyle in the Holocene. Quat Int. 2006;150(1):12–20. [Google Scholar]
- 127.van der Veen M, Livarda A, Hill A. New plant foods in Roman Britain: Dispersal and social access. Environ Archaeol. 2008;13(1):11–36. [Google Scholar]
- 128.Baudouin L, Lebrun P. Coconut (Cocos nucifera L.) DNA studies support the hypothesis of an ancient Austronesian migration from Southeast Asia to America. Genet Resour Crop Evol. 2008;56(2):257–262. [Google Scholar]
- 129.Thomson VA, et al. Using ancient DNA to study the origins and dispersal of ancestral Polynesian chickens across the Pacific. Proc Natl Acad Sci USA. 2014;111(13):4826–4831. doi: 10.1073/pnas.1320412111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young HS, Raab TK, McCauley DJ, Briggs AA, Dirzo R. The coconut palm, Cocos nucifera, impacts forest composition and soil characteristics at Palmyra Atoll, Central Pacific. J Veg Sci. 2010;21(6):1058–1068. [Google Scholar]
- 131.Boivin N, Crowther A, Prendergast M, Fuller DQ. Indian Ocean food globalisation and Africa. Afr Archaeol Rev. 2014;31(4):547–581. [Google Scholar]
- 132.Sykes N. Worldviews in transition: The impact of exotic plants and animals on Iron Age/Romano-British landscapes. Landscapes. 2010;10(2):19–36. [Google Scholar]
- 133.Forcina G, et al. Impacts of biological globalization in the Mediterranean: Unveiling the deep history of human-mediated gamebird dispersal. Proc Natl Acad Sci USA. 2015;112(11):3296–3301. doi: 10.1073/pnas.1500677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Williams M, et al. The Anthropocene biosphere. Anthropocene Rev. 2015;2(3):196–219. [Google Scholar]
- 135.Briggs JM, et al. Why ecology needs archaeologists and archaeology needs ecologists. Front Ecol Environ. 2006;4(4):180–188. [Google Scholar]
- 136.Wohl E. Wilderness is dead: Whither critical zone studies and geomorphology in the Anthropocene? Anthropocene. October 2013;2:4–15. [Google Scholar]
- 137.Hayashida FM. Archaeology, ecological history, and conservation. Annu Rev Anthropol. 2005;34(4):43–65. [Google Scholar]
- 138.Kareiva P, Watts S, McDonald R, Boucher T. Domesticated nature: Shaping landscapes and ecosystems for human welfare. Science. 2007;316(5833):1866–1869. doi: 10.1126/science.1140170. [DOI] [PubMed] [Google Scholar]
- 139.Jackson ST, Hobbs RJ. Ecological restoration in the light of ecological history. Science. 2009;325(5940):567–569. doi: 10.1126/science.1172977. [DOI] [PubMed] [Google Scholar]
- 140.Blondel J. The ‘design’ of Mediterranean landscapes: A millennial story of humans and ecological systems during the Historic Period. Hum Ecol. 2006;34(5):713–729. [Google Scholar]
- 141.Ruddiman WF, Ellis EC, Kaplan JO, Fuller DQ. Defining the epoch we live in. Science. 2015;348(6230):38–39. doi: 10.1126/science.aaa7297. [DOI] [PubMed] [Google Scholar]
- 142.Smith BD, Zeder MA. The onset of the Anthropocene. Anthropocene. December 2013;4:8–13. [Google Scholar]
- 143.Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293(5536):1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 144.Hofman CA, Rick TC, Fleischer RC, Maldonado JE. Conservation archaeogenomics: Ancient DNA and biodiversity in the Anthropocene. Trends Ecol Evol. 2015;30(9):540–549. doi: 10.1016/j.tree.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 145.Redman CL. Resilience theory in archaeology. Am Anthropol. 2005;107(1):70–77. [Google Scholar]
- 146.Fairhead J, Leach M, Scoones I. Green grabbing: A new appropriation of nature? J Peasant Stud. 2012;39(2):237–261. [Google Scholar]
- 147.Glaser B, Birk JJ. State of the scientific knowledge on properties and genesis of anthropogenic dark earths in Central Amazonia (terra preta de Índio) Geochim Cosmochim Acta. 2012;82:39–51. [Google Scholar]
- 148.Young KR. Andean land use and biodiversity: Humanized landscapes in a time of change. Ann Mo Bot Gard. 2009;96(3):492–507. [Google Scholar]