Abstract
The biologically active form of vitamin D3, calcitriol (1,25-(OH)2D3), plays a key role in mineral homeostasis and bone formation and dietary vitamin D3 deficiency is a major cause of bone disorders in poultry. Supplementary dietary cholecalciferol (25-hydroxyvitamin D, 25-OH), the precursor of calcitriol, is commonly employed to combat this problem; however, dosage must be carefully determined as excess dietary vitamin D can cause toxicity resulting in a decrease in bone calcification, hypercalcinemia and renal failure. Despite much research on the therapeutic administration of dietary vitamin D in humans, the relative sensitivity of avian species to exogenous vitamin D has not been well defined. In order to determine the effects of exogenous 1,25-(OH)2D3 during avian osteogenesis, chicken bone marrow-derived mesenchymal stem cells (BM-MSCs) were exposed to varying doses of 1,25-(OH)2D3 during in vitro osteogenic differentiation and examined for markers of early proliferation and osteogenic induction. Similar to humans and other mammals, poultry BM-MSCs were found to be highly sensitive to exogenous 1,25-(OH)2D3 with super pharmacological levels exerting significant inhibition of mineralization and loss of cell proliferation in vitro. Strain related differences were apparent, with BM-MCSs derived from layers strains showing a higher level of sensitivity to 1,25-(OH)2D3 than those from broilers. These data suggest that understanding species and strain specific sensitivities to 1,25-(OH)2D3 is important for optimizing bone health in the poultry industry and that use of avian BM-MSCs are a useful tool for examining underlying effects of genetic variation in poultry.
Keywords: vitamin D, poultry, calcitriol, osteogenic differentiation, mesenchymal stem cell
INTRODUCTION
The active metabolite of vitamin D, calcitriol (1,25-(OH)2D3), plays a well-established dose-dependent role in calcium and phosphorous homeostasis during endochondral ossification (DeLuca, 2004). Although the role of Vitamin D in calcium homeostasis has been well characterized in mammals, there is little information available on the role of 1,25-(OH)2D3 in osteogenesis in avian species. Commercial poultry suffer from bone growth defects associated with 25-hydroxyvitamin D deficiency (Riddell, 1981; Pines, 2007), and controlling levels of dietary vitamin D in commercial flocks is critical to prevent abnormalities of both bone and shell development in this species (Ameenuddin et al., 1987; Elliot and Edwards Jr, 1997).
Formation of the long bones differs between birds and mammals, and it cannot be assumed that these disparate species respond in the same manner to exogenous dietary 25-hydroxyvitamin D and its active metabolite. In avian species, the developing long bone contains a cartilaginous growth plate without a secondary centre of ossification until hatching. The only secondary ossification centers are found in the proximal and distal end of the tibia and distal end of the metatarsus (Hogg, 1980; Yahyaei, 2013). Compared to the mammalian growth plate, there are more cells in each zone of avian growth plate and metaphyseal blood vessels penetrate deeply into hypertrophic cartilage of the avian growth plate (Riddell, 1981; Pines and Hurwitz, 1991). In addition, layer strains of poultry have also been shown to express unique vitamin D binding proteins involved in targeting vitamin D to the yolk of the egg, suggesting that both sensitivity and mobilization of vitamin D may be strain dependent (Wittow 2000, de Matos, 2008).
Although maintaining appropriate biological levels of 1,25-dihydroxyvitamin D3 is known to be crucial for normal bone growth and repair, high levels of this active metabolite have been shown to inhibit osteoblast differentiation, osteocyte mineralization and inhibit normal cell proliferation during osteogenic differentiation (Artaza et al., 2010; Chen et al., 2012; Yang et al., 2013). Proliferation and differentiation of osteoprogenitor cells, and their subsequent maturation into osteoblasts are critical for normal bone development and calcium homeostasis (Wittow, 2000; Neve et al., 2011), and increasing concentrations of exogenous 1,25-dihydroxyvitamin D3 have been shown to inhibit osteogenesis in vitro in a dose-dependent manner (Ecarot and Desbarats, 1999; Atmani et al., 2002).
To limit adverse effects resulting from excessive intake of exogenous dietary 25-hydroxyvitamin D, a tolerable upper daily intake level for adult humans has been suggested to be 10,000 IU (Hathcock JN, 2007). The dietary requirement for the active 25-hydroxyvitamin D3 metabolite in chickens is suggested to be 300 IU/kg of feed for egg type white breeders and between 1,200 to 2,800 IU/kg of feed for broiler breeders (Atencio et al., 2006). A tolerable upper intake level for poultry has not been established and ranges from 4 to 100 times the recommended daily intake have been suggested to be toxic (de Matos, 2008).
Normal serum levels of calcidiol (25-OH vitamin D) have been established in rodents ranging between 20 to 50nM (Rojanasathit and Haddad, 1977), whilst the range in poultry has been identified to be narrower, between 20 to 30nM (Sedrani, 1984). Serum ranges of the active metabolite calcitriol (1,25-(OH)2D3) in poultry have been shown to be between 57 to 338pM with the highest values found in adult females (Sedrani, 1984). To investigate the effect of high levels of the active metabolite of vitamin D, 1,25-(OH)2D3, in poultry we examined the effect of different doses (2.4nM and 24nM) of 1,25-(OH)2D3, during in vitro osteogenic differentiation of avian mesenchymal stem cells (MSCs) derived from the bone marrow of three poultry strains: the commercial broiler strain, Ross 308, commercial layer strains Hy-Line, and an SPF layer strain, Lohmann.
Bone marrow-derived mesenchymal stem cells (MSCs) possess both self-renewal capacity and multi-lineage differentiation potential, including the ability to differentiate to osteogenic lineages making them a useful tool for the study of mineralization during osteogenesis (Pittenger et al., 1999). Osteogenic differentiation of bone marrow-derived MSCs has been well characterized in the rat (Maniatopoulos et al., 1988), mouse (Tropel et al., 2004), human (Frank et al., 2002), and poultry (Khatri et al., 2009). However, there is a paucity of information on osteogenic differentiation of MSCs from different commercial strains of poultry. In this study, MSCs from three strains of commercial poultry were isolated and induced to differentiation to osteogenic lineages in the presence or absence of varying concentrations of 1,25(OH)2D3. Effects on mineralization and cell proliferation were examined in vitro.
MATERIALS AND METHODS
Isolation of Bone Marrow-derived MSCs from Different Commercial Strains of Poultry
All procedures involving animals and birds were approved by the Charles Sturt University Animal Care and Ethics Committee. Bone marrow-derived mesenchymal stem cells (BM-MSC) were isolated from the femur of 21-day-old juvenile Wistar rats and 19-day-old chicken embryos as previously described (Khatri et al., 2009). Three strains of poultry were used: the commercial boiler strain Ross 308 (Baida Hatcheries, Griffith, Australia), the commercial layer strain Hy-line, (Hy-Line Australia Pty Ltd, Bendigo, Australia), and an SPF layer strain (Lohmann LSL, Australian SPF Services Pty Ltd, Australia). A minimum of 12 embryos was used per strain for each derivation.
Osteogenic Differentiation
Chicken BM-MSCs were seeded in 4 or 24-well plates (Thermo Scientific; CELLSTAR®, Greiner bio-one) at a density of 2.5 × 104 cells/cm2 and cultured in complete media (CM, Dulbecco's modified Eagle Media (DMEM, Life Technologies) containing 10% heat-inactivated fetal bovine serum, 0.3 mg/mL glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin and 1.8mM calcium chloride) until cells reached 100% confluence. Rat BM-MSCs were used as a positive control and for comparison. Osteogenic differentiation was induced by supplementing the cells with osteogenic induction media (IM), complete media containing 10mM β-glycerol (Sigma-Aldrich), 0.05mM ascorbic acid (Sigma-Aldrich) and 100nM dexamethasone (Sigma-Aldrich). Media was changed every 3 d alternating between induction and maintenance media containing 2.4mM (IM+2.4nM) or 24nM (IM+24nM) of the active vitamin D3 metabolite 1,25(OH)2D3, Sigma-Aldrich). Control cultures for both chicken and rat BM-MSCs were designated as those cultured only in complete media (CM) containing no inducing compounds. No Treatment control cultures received induction media (IM) only and contained no exogenous 1,25(OH)2D3. Chicken and rat cells were cultured for a maximum of 7 and 21 d post induction, respectively.
Analysis of Calcium Deposition and Early Osteogenic Differentiation
Chicken MSCs were examined for calcium deposition by Alizarin Red staining (Merck) (2% aqueous solution) after day 3, 5, and 7 of culture as per published protocols (Song et al., 2011). Rat cultures were only stained after day 21. Cultures were photographed using a Leica DMLB inverted light microscope. Six images were obtained from each well for image analysis. Calcium deposition was quantified histomorphometrically using Image J™ software (http://rsb.info.nih.gov/ij/). Images were at threshold to define mineralization before the area fraction of the darkly stained regions corresponding to calcium deposition was obtained. Threshold data was analyzed using GraphPad® Prism Software, Inc. USA.
Alkaline Phosphatase (AP) is an early marker of an osteoblastic cell phenotype (Yamamoto et al., 2002). Chicken BM-MSCs were examined for AP expression using histochemical staining on day 3, 5, or 7 of in vitro osteogenic differentiation. Cells were fixed in cold 4% paraformaldehyde (PFA) and cells were stained using an alkaline phosphatase detection kit according to manufacturer's instructions (SCR004, Merck Millipore). Cultures were photographed using standard light microscopy.
Analysis of BM-MSCs Cell Proliferation During Osteogenic Induction
Cell proliferation was determined by measuring incorporation of the thymidine analogue Bromodeoxyuridine (BrDU) a synthetic nucleoside into host DNA (Taupin, 2007). All chicken BM-MSCs were plated in a complete culture medium at a density of 2.5×104 cells/cm2. Cells grown in the presence of CM, IM, and IM containing 2.4nM or 24nM 1,25(OH)2D3 were exposed to an acute pulse of BrDU (10mg/ml) for two h on day 0 of osteogenic induction and then exposed to the different culture conditions for a further 3 or 5 d. Cells were then fixed with cold 4% PFA and visualized for the presence of BrDU incorporation by immunocytochemistry (Jackson and Cook, 2008). To quantify the number of BrDU positive cells, cells were counterstained with Harris hematoxylin and photographed using standard light microscopy. Six randomly selected images per well were analyzed for the presence or absence of BrDU labelling.
Statistical Analysis
A generalized linear model was used to analyze the data for calcium deposition or cell proliferation as dependent variable and strain, treatments, and their interaction as fixed effects. When the analysis of variance suggested a significant treatment, treatment*strain effect for a variable, the means were compared using post hoc tests. All data generated was analyzed using SPSS™ version 21 software. P values < 0.05 were considered statistically significant.
RESULTS
Exogenous 1,25(OH)2D3 Inhibits Mineral Deposition in Rodent and Poultry Mesenchymal Stem Cells Undergoing Osteogenic Differentiation
To compare effects of high levels of exogenous 1,25(OH)2D3 on osteogenic induction in BM-MSCs derived from layer or broiler strains of poultry, poultry BM-MSCs were induced to an osteogenic lineage in the presence of super pharmacological concentrations of 1,25(OH)2D3 and compared to rat BM-MSCs undergoing the same protocol. Deposition of calcium was confirmed by Alizarin Red staining.
Addition of exogenous 1,25(OH)2D3 significantly inhibited calcium deposition in rat MSCs in a dose-dependent manner (Figure 1A-C). Quantitative analysis of calcium deposition in rat BM-MSCs after 21 d of osteogenic induction showed that treatment of rat MSCs with the highest doses (IM+24nM) of 1,25(OH)2D3 significantly inhibited calcium deposition (IM area fraction mean: 20.10 ± 6.23; 24nM 1,25(OH)2D3 mean: 2.92 ± 0.82; n = 3, P = 0.026) (Figure 1J). There was a trend towards a significant decrease in calcium deposition in 2.4nM treated cultures (2.4nM 1,25(OH)2D3 area fraction mean: 7.09 ± 1.86; NI mean: 20.10 ± 6.23; n = 3, P = 0.058) (Figure 1J) and between IM+2.4nM and IM+24nM cultures (2.4nM 1,25(OH)2D3 mean: 7.09 ± 1.86; 24nM 1,25(OH)2D3 mean: 2.92 ± 0.82; n = 3, P = 0.055) although this difference did not reach significance. Surprisingly, chicken BM-MSCs undergoing the same protocol showed exceptionally high levels of mineral deposition in untreated control cultures with extensive mineralization observed across the wells at all time points examined from 9 d post induction (Figure 1D-F). The extent of mineralization in these cultures prohibited colorimetric image analysis and earlier time points post induction were examined for maximum efficacy of analysis.
Figure 1.
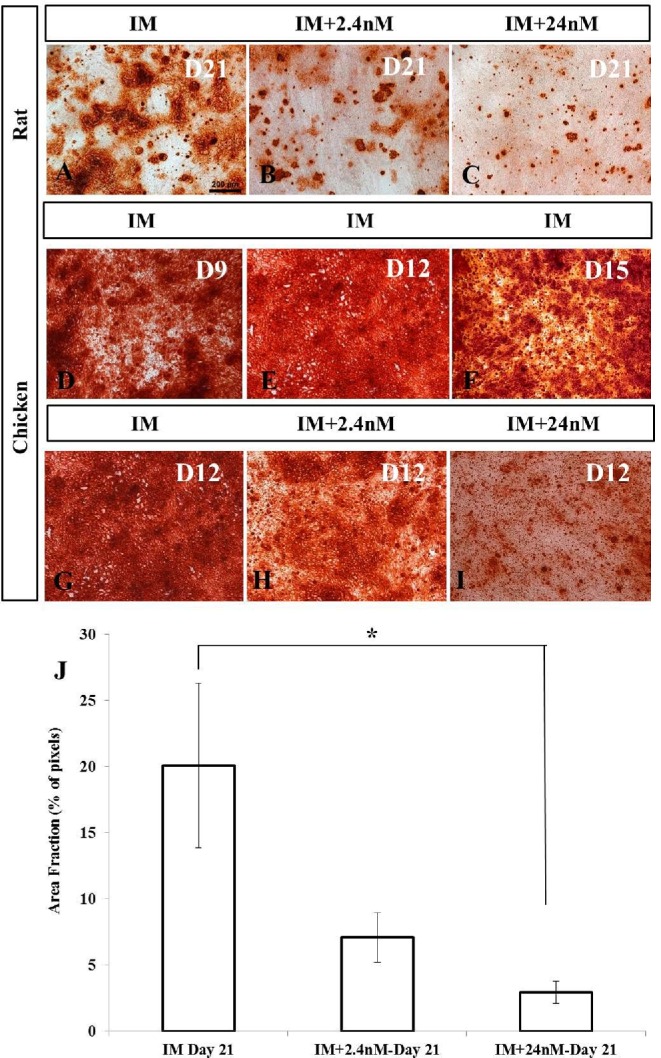
Mineral deposition in mesenchymal stem cells isolated from mouse and broiler chickens induced to an osteogenic phenotype in vitro. (A-C) Alizarin Red staining of rat MSCs treated with or without 1,25(OH)2D3 for 21 d during osteogenic differentiation. Calcium deposition was reduced after treatment of rat MSCs with 24nM of 1,25(OH)2D3 (C) when compared to no treatment control cultures (A). (D-F) Alizarin Red staining of broiler MSCs at different intervals of osteogenic differentiation treated without 1,25(OH)2D3 showing early evidence of calcium deposition compared to no treatment control cultures of rat MSCs. (G-I) Reduction in calcium deposition after treatment of broiler MSCs with 24nM of 1,25(OH)2D3 (I) compared to cultures containing no exogenous 1,25(OH)2D3 (G) at day 12 of osteogenic differentiation. J). Quantitative analysis of calcium deposition in rat MSCs expressed as area fraction (% of pixels). In all cases, values are mean ± SEM (n = 3). Comparisons marked with an asterisk (*) are significantly different (p < 0.05). Scale bar = 200μm. Abbreviations: IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
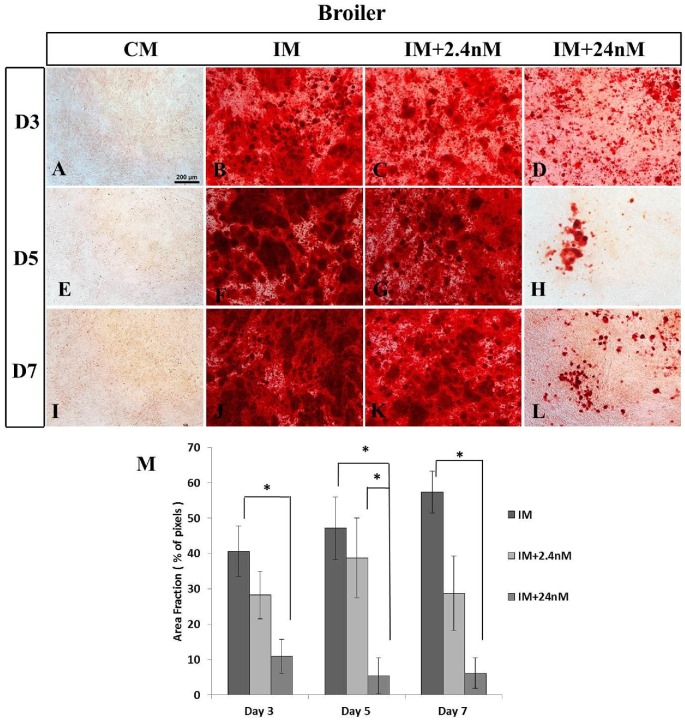
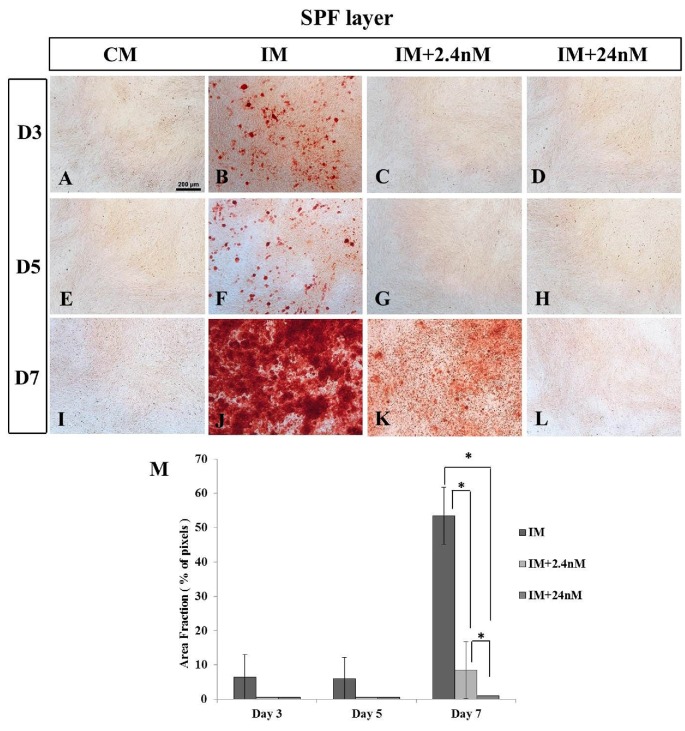
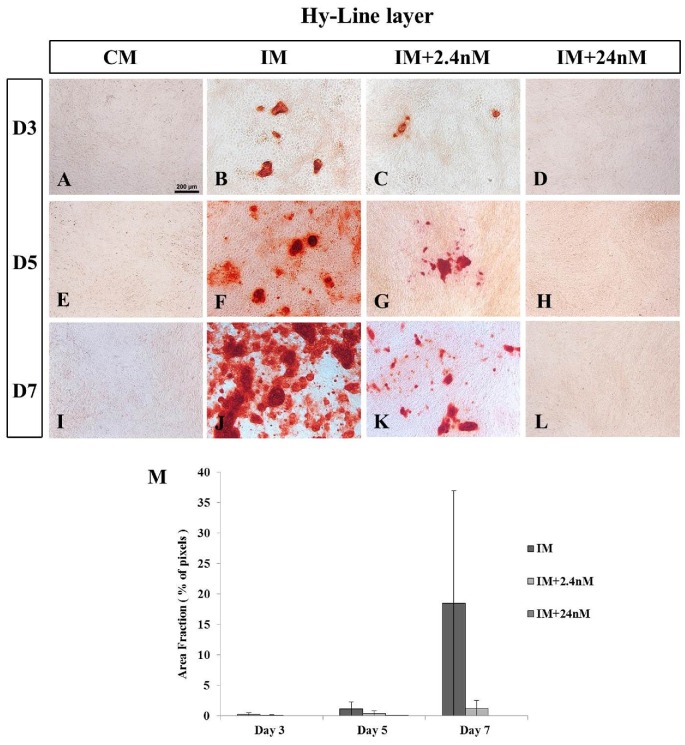
Mineralization in poultry BM-MSCs undergoing osteogenic induction was analyzed in three strains of chicken BM-MSCs (commercial broiler strain Ross 308, SPF layer strain Lohmann LSL, and commercial layer strain Hy-Line) at day three (D3), day five (D5) or day seven (D7) of osteogenic induction. Calcium deposition was confirmed using Alizarin Red staining. Calcium deposition was clearly apparent as early as (D3) of osteogenic induction in MSCs derived from poultry strains with the strongest mineralization observed in cells derived from the broiler strain Ross 308 (Figure 2B-D) when compared to the two layer strains examined: SPF (Figure 3) and Hy-Line (Figure 4). Analysis of later time points was not possible due to high levels of cell confluence and the significant presence of mineralization in most cultures. Similar to that observed in mammalian cells (Figure 1J) 1,25(OH)2D3 was found to inhibit mineral deposition in all poultry strains examined in a dose-dependent manner (Figures 2M, 3M and 4). No significant incidence of cell death was noted in any cultures in poultry BM-MSC cultures during early stages of osteogenic induction (Days 3 and 5, data not shown).
Figure 2.
Inhibition of calcium deposition in broiler MSCs undergoing osteogenic differentiation in the presence of 24nM and 2.4nM concentrations of 1,25(OH)2D3 in vitro. Broiler MSCs were grown in complete, osteogenic induction media with or without 2.4nM (C, G, K) and 24nM (D, H, L) concentrations of 1,25(OH)2D3. (B, C, F, G, J, K) Cultures were stained with Alizarin Red to show mineral deposition on days 3, 5, or 7 of osteogenic differentiation. Extensive mineralization is observed in all cultures treated with no, or 2.4nM concentrations of 1,25(OH)2D3 at all time points examined. (D, H, L, M) A significant inhibition of mineralization is observed in cultures treated with high concentrations of 1,25(OH)2D3. (A, E, I) Broiler MSCs grown in the absence of osteogenic inducing factors show no mineralization on any day examined. (M) Quantitative analysis of calcium deposition expressed as area fraction (% of pixels) Values are mean ± SEM (Day 3, n = 11; Day 5, n = 6 and Day 7, n = 7). Comparisons marked with an asterisk (*) are significantly different (p < 0.05). Scale bar = 200μm Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
Figure 3.
Inhibition of calcium deposition in SPF-Layer (Lohmann LSL) MSCs undergoing osteogenic differentiation in the presence of high and low concentrations of 1,25(OH)2D3 in vitro. Mineralization was observed at all time points in cells undergoing osteogenic induction in the absence of exogenous 1,25(OH)2D3 (B, F, J). No mineralization is observed at Day 3 or 5 in the presence of 2.4nM, (C, G) or 24nM (D, H) 1,25(OH)2D3. (K) Only small foci of mineralization are observed in low 2.4nM 1,25(OH)2D3 at Day 7 of osteogenic differentiation A, E, I) MSCs grown in the absence of osteogenic inducing factors show no mineralization on any day examined. M) Quantitative analysis of calcium deposition expressed as area fraction (% of pixels). Values are mean ± SEM (n = 4 on all days examined). Comparisons marked with an asterisk (*) are significantly different (p < 0.05). Scale bar = 200μm. Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
Figure 4.
(B, F, J) Mineralization of Hy-Line layer MSCs undergoing in vitro osteogenic differentiation. (D, H, L) No mineralization is observed in Hy-Line layer MSCs undergoing osteogenic induction in the presence of 24nM concentrations of 1,25(OH)2D3 at any time point examined. (C, G, K) Low levels of mineralization are observed in cells undergoing osteogenic differentiation in the presence of 2.4nm concentrations of exogenous 1,25(OH)2D3. (A, E, I) MSCs grown in the absence of osteogenic inducing factors (CM) show no mineralization on any day examined. Values are mean ± SEM (n = 2 on all days examined). Scale bar = 200μm. Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
In broiler BM-MSCs cells, high levels of 1,25(OH)2D3 significantly inhibited mineralization by Day 3 of osteogenic induction compared to no treatment control cultures (NI mean: 40.64 ± 7.17; 24nM 1,25(OH)2D3 mean: 10.94 ± 4.83; n = 11, P = 0.001) (Figure 2C, D, M). A similar effect was observed at Day 5 and Day 7 of osteogenic induction (Day 5: Figure 2F-H, M; Day 7: Figure 2J-L, M).
SPF layer MSCs showed significantly greater sensitivity to super pharmacological concentrations of 1,25(OH)2D3 compared to broiler cells (Figure 3). No mineralization was detected in SPF layer BM- MSCs on either Day 3 or Day 5 of osteogenic induction at both 2.4nM and 24nM concentrations of 1,25(OH)2D3 (Figure 3C, D, G, H, M) with only limited mineralization observed in untreated controls (Figure 3B, F). By Day 7 some small foci of calcium deposition were observed in SPF layer cells undergoing osteogenic induction in the presence of 2.4nM 1,25(OH)2D3 (Figure 3K), but this was significantly reduced compared to SPF layer no treatment controls (Figure 3J, M).
Consistent with observations in Broiler Ross 308 BM-MSCs, mineralization was observed on Day 3 of osteogenic differentiation in cells derived from the commercial layer strain Hy-Line (Figure 4B) although this was markedly reduced compared to broiler counterparts. Mineralization was observed to increase between Days 5 and 7, with a coincident inhibition of mineralization in the presence of 2.4nM (Figure 4C, G. K) and 24nM 1,25(OH)2D3 (Figure 4D, H, L). Similar to that observed in Lohmann-SPF layers cells, 24nM 1,25(OH)2D3 completely inhibited mineralization of Hy-Line BM-MSCs at all time points examined (Figure 4D, H, L). Together, these data suggest that MSCs derived from layer strains of poultry are more sensitive to super biological levels of exogenous 1,25(OH)2D3 than their broiler counterparts.
Differentiation of BM-MSC-derived Cells with an Osteoblastic Phenotype are Observed in the Presence of Increasing Concentrations of Exogenous 1,25(OH)2D3
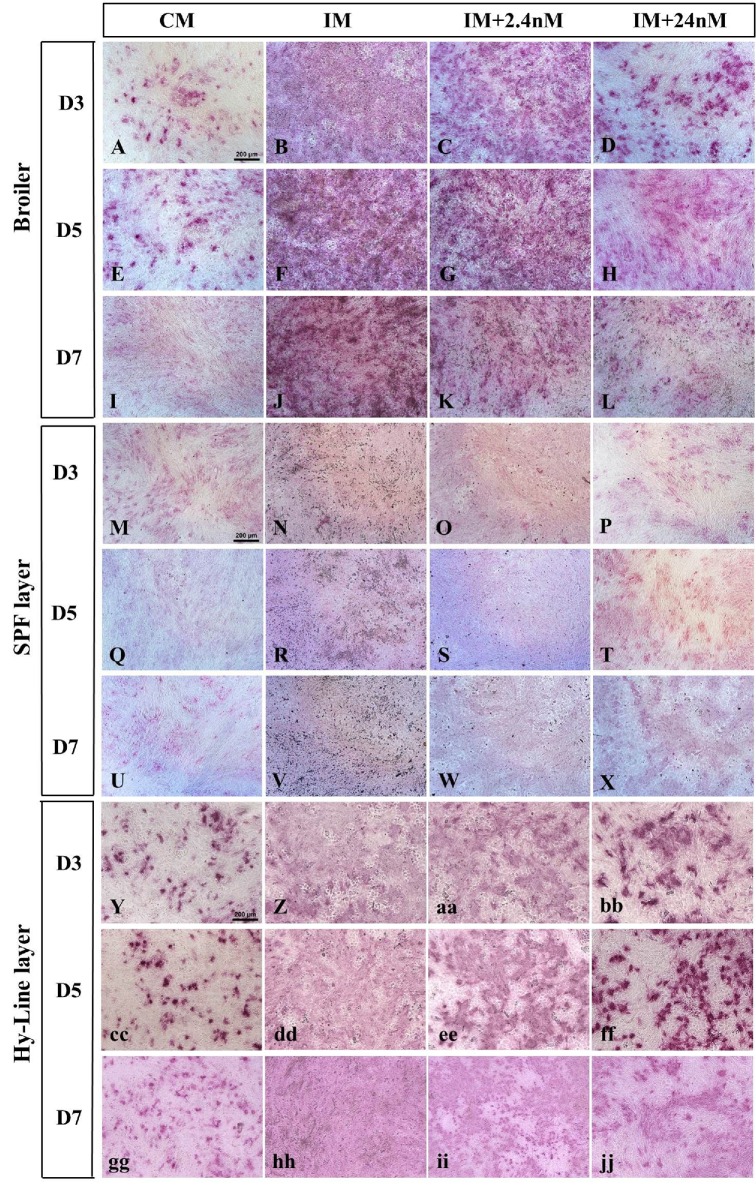
To determine whether the reduction in mineral deposition observed in our cultures was as a direct result of inhibition of osteoblastic phenotype, the first definitive cell type produced during osteogenic differentiation, poultry BM-MSCs were examined for the presence of alkaline phosphatase (AP), as the marker of early osteoblastic differentiation on Days 3, 5 and 7 of osteogenic differentiation (Figure 5). Alkaline phosphatase is widely used as a marker of early osteoblastic phenotype and is essential for bone matrix formation (Atmani et al., 2003; Beck et al., 1998; Malaval et al., 1994).
Figure 5.
(A-jj) Histochemical localisation of the marker of osteoblastic phenotype, alkaline phosphatase(AP), in Broiler Ross 308 (A-L), SPF layer – Lohmann LSL (M-X) and Hy-Line layer-derived (Y-jj) BM-MSCs cultured in the presence or absence of 2.4nM and 24nM 1,25(OH)2D3 for Days 3, 5, or 7. In the presence of osteogenic inducing factors only (IM: B, F, J, N, R, V, Z, dd, hh) all strains show a diffuse and widespread pattern of AP staining indicative of large numbers of osteoblastic cells. A dose-dependent reduction in the proportion of cells expressing AP is observed in the presence of high concentrations of 1,25(OH)2D3 at all time points examined (Broiler: D, H, L; SPF layer: P, T, X and Hy-Line layer: bb, ff, jj). AP expression is highly focal in the absence of osteogenic inducing factors, and exogenous 1,25(OH)2D3 (A, E, I, M, Q, U, Y, cc,gg). Values are mean ± SEM, n = 3 for all days and strains examined. Scale = bar 200μm. Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
Poultry BM-MSCs cultured in the absence of the osteogenic inducing factors β-glycerol, ascorbic acid and dexamethazone (IM) were observed to express AP in all three strains of poultry MSCs examined (Figure 5: Broiler Ross 308: A, E, I; Lohmann SPF LSL layer: M, Q, U; Hy-line layer: Y, cc, gg) indicative of cells of a stem-like nature being present in these undifferentiated cultures. In the presence of osteogenic inducing media and the absence of exogenous 1,25(OH)2D3, AP staining was observed to be intense and widespread in cells derived from all three poultry strains with the highest intensity of staining observed in Ross 308 broiler cells (Figure 4B, F, J). A diffuse and widespread pattern of AP staining was observed at Day 3 of osteogenic induction compared to un-induced controls, suggestive of an increase in AP-positive osteoblasts in these cultures (Figure 4B, N, Z). Exposure to increasing concentrations of 1,25(OH)2D3 reduced the intensity of AP staining and changed the pattern of staining to one of a focal nature. This staining pattern was found to be similar in all three poultry strains and at all time points examined (Figure 5). Together this data suggests that cells with an osteoblastic phenotype are present from Day 3 of osteogenic induction in cultures containing all concentrations of 1,25(OH)2D3 examined (0nM, 2.4nM and 24nM) but that AP-positive cells numbers are reduced in the presence of increasing concentrations of 1,25(OH)2D3.
Super Biological Levels of 1,25(OH)2D3 Inhibits Cell Proliferation in Poultry BM-MSCs Undergoing Osteogenic Differentiation in vitro
Exposure to high concentrations of 1,25(OH)2D3 has been shown previously to inhibit cell cycle progression in both murine mesenchymal stem cells (Artaza et al., 2010) and human tumor cell lines (Jensen et al., 2001; Gumireddy et al., 2003). To examine whether the presence of super biological concentrations of 1,25(OH)2D3 was exerting an inhibitory influence on osteoblastic cell proliferation in avian cells, BM-HSCs were labelled with the thymidine analogue bromodeoxyuridine (BrDU), a marker of proliferating cells, in the presence or absence of exogenous 1,25(OH)2D3. Using this method, any cells that were in S phase of the cell cycle at the time of BrDU exposure on Day 0 of osteogenic induction, and their daughter cells, will show dense localisation of BrDU labelling in the nucleus by immunohistochemical staining. BM-MSCs of all three poultry strains were pulsed acutely with BrDU on Day 0 of osteogenic induction and allowed to continue to differentiate in the presence or absence of exogenous 1,25(OH)2D3. Numbers of BrDU positive cells were quantified on Day 3 or Day 5 of the induction protocol (Figure 6).
Figure 6.
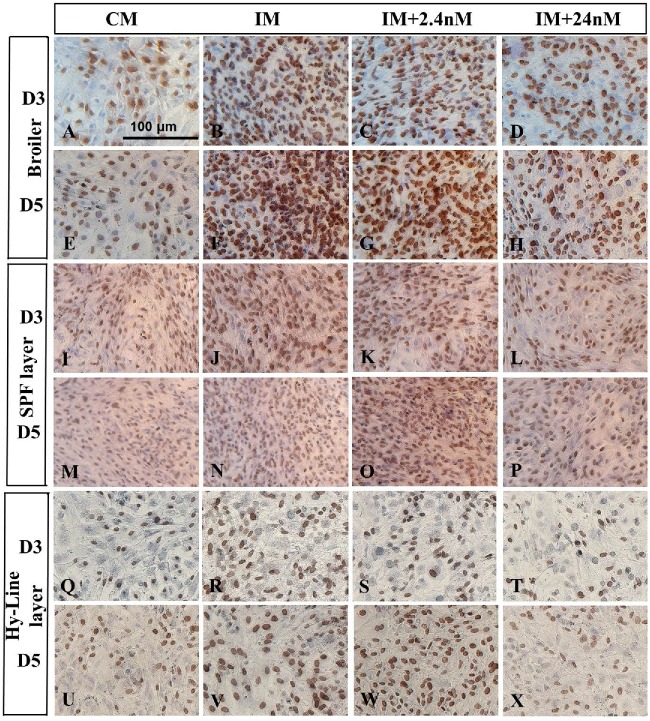
Photomicrograph of BrDU immunostaining of broiler Ross 308 (A-H), SPF layer – Lohmann LSL (I-P) and Hy-Line layer (Q-X) MSCs after day 3 or 5 of incubation in complete media (no induction control) and osteogenic induction media with or without 1,25(OH)2D3 (2.4nM and 24nM). Cells were pulsed with BrDU for 2 hours on day 0 and cells stained using immunohistochemistry for the presence of BrDU (10mg/ml) at days 3 or 5 of osteogenic differentiation. Cells, which had been in S phase of the cell cycle on Day 0 of osteogenic induction, as well as their daughter cells, are labelled with BrDU. BrDU positive cells were observed to be present in all strains of BM-MSCs examined, however, a reduced number of BrDU labelled nuclei are observed in BM-MSCs in the presence of 24nM concentrations of 1,25(OH)2D3 (D, H, L, P. T, X). Cells cultured in the absence of osteogenic induction factors showed the fewest labelled cell nuclei (A, E, I, M, Q, U). Broiler (n = 4), SPF layer (n = 3) and Hy-Line layer (n = 2). Scale bar = 200μm. Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3; D, Day.
All strains showed discrete BrDU labelling on all days examined (Figure 6). The most intense labelling was observed in those cultures undergoing osteogenic induction in the absence of exogenous 1,25(OH)2D3 (Figure 6, NT cultures: Broiler, B, F; Lohmann SPF LSL layer, J, N; Hy-Line layer, R, V). The greatest proportions of BrDU labelled cells were observed in Broiler and SPF layer cultures at Day 5 of induction, indicative of an increase in cell number over time in these cultures. Generally, fewer labelled cells were apparent in all Hy-Line cultures than in the other strains examined (Q - X) indicating that these cells may have reduced proliferative capacity compared to their strain counterparts.
Total proportions of BrDU labeled versus unlabeled cells were determined in all strains examined (Figure 7). In all cases, in the absence of exogenous 1,25(OH)2D3, the proportion of BrDu labeled cells present on Day 3 and Day 5 of culture was found to be greater in number in the presence of osteogenic inducing factors than their un-induced counterparts (Figure 6). Proportions of BrDU positive cells were observed to be significantly reduced on both Day 3 and Day 5 of osteogenic induction in all poultry strains treated with super pharmacological levels of 1,25(OH)2D3 compared to untreated controls (Figure 7). Both 2.4nM and 24nM concentrations of 1,25(OH)2D3 induced a significant reduction in BrDU positive cells compared to their untreated counterparts in cells derived from broiler strain Ross 308 and SPF layer strain Lohmann (Figure 7A, B). Hy-Line layer derived cells also showed a similar trend with 1,25(OH)2D3 treated cultures showing a marked reduction in the proportion of BrDU labelled cells on both day 3 and day 5 of osteogenic induction (Figure 7C). Together, these data suggest that increasing concentrations of 1,25(OH)2D3 inhibit osteoblast proliferation in BM-MSCs derived from all strains examined during in vitro osteogenic differentiation.
Figure 7.
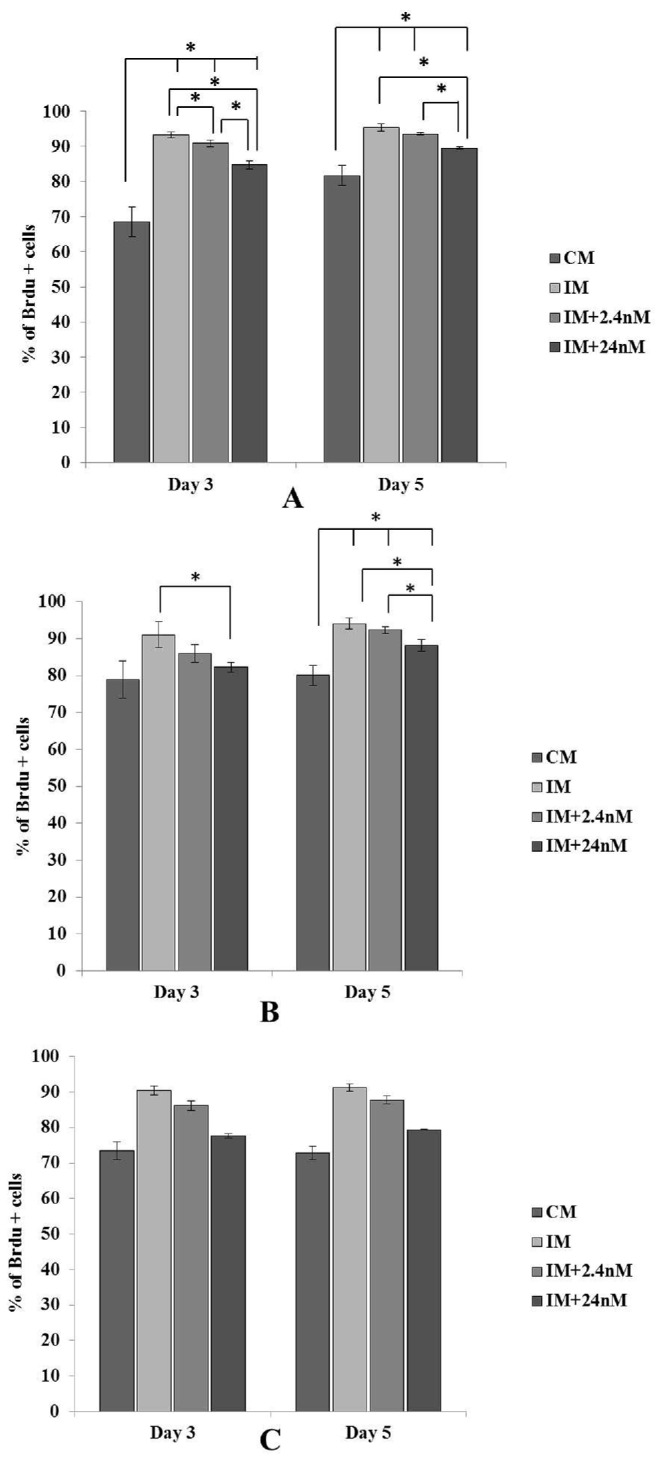
Proportions of BrDU labelled cells from three strains of chicken MSCs (A) Broiler Ross 308, (B) SPF layer, Lohmann LSL and (C) Hy-Line layer cultured in the presence or absence of 1,25(OH)2D3 and osteogenic inducing factors. Cells were pulsed with BrDU (10mg/ml) for 2 h and the effect of 1,25(OH)2D3 on cell proliferation examined by quantitating proportions of BrDU labelled cells on Day 3 or 5 of osteogenic differentiation. Numbers of BrDU-positive cells were reduced in all strains examined, in the presence of both 2.4nM and 24nM concentrations of 1,25(OH)2D3 on Day 3 and Day 5 of osteogenic induction compared cells cultures in induction media alone (IM). A relative decrease in BrDU positive cells was also observed in cell cultures in the absence of osteogenic induction factors. Values are expressed as mean ± SEM. Broiler (n+4), SPF layer (n = 3), Hy-Line layer (n = 2). Comparisons marked with an asterisk (*) are significantly different (P < 0.05).Abbreviations: CM, control media containing no exogenous 1,25(OH)2D3; IM, induction media; IM+2.4nM, induction media containing 2.4nM 1,25(OH)2D3; IM+24nM, induction media containing 24nM 1,25(OH)2D3.
Levels of Mineralization, But Not Proliferation, Differ between Avian Strains in BM-MSCs Undergoing Osteogenic Differentiation in the Presence of Exogenous 1,25(OH)2D3
To quantify differences between layer and broiler strains, values for % area of mineralization and proportions of BrDU-labeled cells were compared between strains. Statistical analysis showed calcium deposition in broiler strains to be significantly higher than SPF layer counterparts at Day 3 and Day 5 of osteogenic induction (mean area fraction% pixilation: Day 3: Broiler, 26.60 ± 3.15; SPF layer, 2.167 ± 5.236, P = 0.002; Day 5: Broiler, 30.43 ± 4.27; SPF layer, 2.023 ± 5.236, P = 0.002). Conversely, proportions of proliferating cells were not observed to be significantly different between the two strains at the same time points (mean BrDU positive cells: Day 3: Broiler, 84.39 ± 1.06; SPF layer, 84.55 ± 1.22, P = 0.902; Day 5: Broiler, 90.06 ± 1.06; SPF layer, 88.66 ± 1.22, P = 0.390). These data suggest that layer strains show significantly greater inhibition of mineralization in the presence of super-biological concentrations of 1,25(OH)2D3 during the earliest phases of mineral induction during osteogenic induction in vitro, but that proliferation of osteogenic precursors was not adversely affected in this process.
DISCUSSION
Chicken MSCs Show Evidence of Precocious Mineralization Compared to Mammals
Onset of mineralization during osteogenic induction of MSCs has not been examined previously in poultry, either in relation to different poultry strains or by comparison to mammalian cells lines. In this study, evidence of an early onset of mineralization was apparent in all strains of chicken MSCs examined. One possible explanation for the observed early onset of mineralization in poultry MSCs compared to mammalian MSCs may be that avian bone contains developmentally more matured osteoblasts and fewer pre-osteoblast cells than cells isolated from mammalian species.
Osteoblasts express osteocalcin and its expression is maximal during mineralization, hence, osteocalcin is considered as a maker of matured osteoblasts (Lian et al., 1989; Owen et al., 1990). The level of osteocalcin in chick osteoblasts isolated from calvaria was found to be almost five times higher than that found in 21 d rat calvaria osteoblasts (Lian et al., 1982; Hauschka et al., 1983; Lian et al., 1985). It is therefore possible that avian cells may require less time to form matured osteoblasts, initiate extra cellular matrix maturation and form bone nodules than their mammalian counterparts (Owen et al., 1990), a hypothesis supported by the findings in this study.
Calcium Deposition Was Inhibited by the Super Pharmacological Active Metabolite of Vitamin D3 in Rat and Chicken MSCs in vitro
High doses of 1,25(OH)2D3 have been shown to have a potent inhibitory action on mineralization in many species in vitro (Broess et al., 1995; Ecarot and Desbarats, 1999; Fromigué et al., 1997; Yang et al., 2013). In rodents, inhibition of mineralization has been previously reported in vitro at concentrations of at 10nM and 100nM 1,25(OH)2D3 where these concentrations were observed to significantly inhibited mineralization in the pre-osteoblastic cell line MC3T3-E1 and primary mouse bone marrow stromal cells (Yamaguchi and Weitzmann, 2012). Acute and chronic treatment of osteoblasts derived from chicken calvaria with 10nM 1,25(OH)2D3 markedly decreased mineralization of the extracellular matrix (Broess et al., 1995). This study shows that poultry MSCs are sensitive to lower levels of 1,25(OH)2D3 with significant reduction in mineralization observed in poultry cells undergoing osteogenic induction in the presence of 2.4nM 1,25(OH)2D3, a finding that was not mirrored in rat cells exposed to the same concentrations, with layer MSCs being most sensitive of all poultry cells examined
Mineralization of the extracellular matrix is a function of mature osteoblasts and a final step in osteoblast differentiation (Quarles et al., 1992). It is well known that osteocalcin, osteopontin and phex are markers of differentiated osteoblasts, and their expression is associated with matrix mineralization (Beck et al., 1998; Ecarot and Desbarats, 1999). The 1,25(OH)2D3 treatment of rat, chicken and mouse osteoblast cultures has been shown to inhibit expression of collagen type I, osteocalcin, osteopontin, and phex with subsequent inhibition of mineral deposition (Broess et al., 1995; Ecarot and Desbarats, 1999; Owen et al., 1991). These studies suggest highly conserved pathways governing mineralization during osteogenesis in these species. The poultry BM-MSC model system used in this study could provide a tool to further dissect genetic and epigenetic factors influencing mineralization in poultry-derived osteocytes in vitro.
Early Osteoblastic Phenotype is Inhibited by 1,25(OH)2D3 in Poultry MSCs in vitro
The process of osteogenic differentiation occurs in three temporally regulated phases of differentiation; proliferation, matrix maturation, and mineralization. During each phase, distinct markers are expressed by cells undergoing osteoblastic lineage specification and differentiation (Olsen et al., 2000). Alkaline phosphatase is a marker of early osteoblast differentiation (Malaval et al., 1994), and in previous studies, the presence of exogenous 1,25(OH)2D3 has been shown to have both an inhibitory (Yang et al., 2013) and stimulatory (Matsumoto et al., 1991; Fromigué et al., 1997) action on AP expression in non-avian species, suggesting multiple effects during osteogenesis.
The results of this study identified a dose-dependent reduction in AP-positive cells in all chicken strains examined in the presence of 1,25(OH)2D3, consistent with previous studies in rodents (Li et al., 2008) where treatment of primary chicken osteoblasts isolated from embryonic calvaria exposed to 0.1nM and 10nM doses of 1,25(OH)2D3 for 30 days showed downregulation of AP enzyme activity (Broess et al., 1995). Findings presented in this study are consistent with 1,25(OH)2D3 inhibition of the earliest phases of osteocyte differentiation, with potentially a similar mechanism underlying this effect in both mammalian and avian species.
Calcitriol Exerts an Anti-proliferative Effect on Chicken MSCs Undergoing Osteogenic Differentiation
Increasing concentrations of 1,25(OH)2D3 were observed to exert a potent anti-proliferative effect during in vitro osteogenic differentiation of poultry MSCs in this study. This finding also is consistent with studies in humans and other mammals where bone marrow stromal cells (Fromigué et al., 1997; Geng et al., 2011), mouse C3H10T1/2 mesenchymal cells (Artaza et al., 2010), mouse bone marrow stromal cells (Li et al., 2008) and human keratinocytes (Takahashi et al., 2003) showed reduced cell proliferation in the presence of exogenous 1,25(OH)2D3. An anti-proliferative effect for 1,25(OH)2D3 is also well documented in many tumor cell lines, for example, addition of 1,25(OH)2D3 to neuroblastoma cell lines and the human breast cancer cell line MCF-7 resulted in a decrease in the number of proliferating cells in vitro (Jensen et al., 2001; Gumireddy et al., 2003). Our study suggests that avian cells show a conserved mechanism of response to 1,25(OH)2D3 in terms proliferation.
It has been suggested that the inhibitory effect of 1,25(OH)2D3 on osteoblast cell proliferation is dependent on the maturation state of the cells used for the study, and direct effects on markers of cell proliferation have been observed (Lian and Stein, 1992). A biphasic effect of 1,25(OH)2D3 has been demonstrated in rat osteoblast cultures where 1,25(OH)2D3 downregulated the cell growth controlling histone gene expression during the proliferative period (days 8 to 15) whereas no significant effect was observed in histone mRNA levels at later time points (days 15 to 30) (Owen et al., 1991). This suggests osteoblasts exhibit differential sensitivity to the anti-proliferative effect of 1,25(OH)2D3 during their maturation, a sensitivity, which is potentially markedly increased in cells derived from layer strains of poultry in this study. Overall, a combinatorial effect of high concentrations of 1,25(OH)2D3 on inhibition of expansion of the osteogenic pool, and inhibition in their differentiation to a mineralized cell phenotype, with a greater sensitivity to 1,25(OH)2D3 observed in poultry, suggest that avoidance of excessive dietary intake in poultry species is critical.
CONCLUSION
This study has identified poultry MSCs, in particular those derived from layer strains, to be highly sensitive to the inhibitory effects of vitamin D3 metabolite 1,25(OH)2D3 during bone development. Super pharmacalogical 1,25(OH)2D3 inhibited cell proliferation, osteoblast differentiation, and calcium deposition in a strain-specific and concentration-dependent manner in avian mesenchymal stem cells undergoing osteogenic differentiation. To our knowledge, this is the first study that has explored strain-specific differences in birds to exogenous 1,25(OH)2D3 and establishes a novel mechanism to look at the downstream targets of vitamin D during osteogenesis in poultry to better understand effects of feeding exogenous Vitamin D to both parental layers and young stock in different commercial strains of poultry.
REFERENCES
- Ameenuddin S., Sunde M. L., DeLuca H. F. Lack of Response of Bone Mineralization of Chicks Fed Egg Yolks from Hens on Dietary 1,25 Dihydroxycholecalciferol. Poult. Sci. 1987;66:1829–1834. doi: 10.3382/ps.0661829. [DOI] [PubMed] [Google Scholar]
- Artaza J. N., Sirad F., Ferrini M. G., Norris K. C. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J. Steroid. Biochem. Mol. Biol. 2010;119:73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio A., Edwards H., Jr., Pesti G., Ware G. The vitamin D3 requirement of broiler breeders. Poult. Sci. 2006;85:674–692. doi: 10.1093/ps/85.4.674. [DOI] [PubMed] [Google Scholar]
- Atmani H., Audrain C., Mercier L., Chappard D., Basle M. F. Phenotypic effects of continuous or discontinuous treatment with dexamethasone and/or calcitriol on osteoblasts differentiated from rat bone marrow stromal cells. J. Cell. Biochem. 2002;85:640–650. doi: 10.1002/jcb.10165. [DOI] [PubMed] [Google Scholar]
- Atmani H., Chappard D., Basle M. F. Proliferation and differentiation of osteoblasts and adipocytes in rat bone marrow stromal cell cultures: Effects of dexamethasone and calcitriol. J. Cell. Biochem. 2003;89:364–372. doi: 10.1002/jcb.10507. [DOI] [PubMed] [Google Scholar]
- Beck G. R., Sullivan E. C., Moran E., Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J. Cell. Biochem. 1998;68:269–280. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Broess M., Riva A., Gerstenfeld L. C. Inhibitory effects of 1,25(OH)2 vitamin D3 on collagen type I, osteopontin, and osteocalcin gene expression in chicken osteoblasts. J. Cell. Biochem. 1995;57:440–451. doi: 10.1002/jcb.240570310. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Ninomiya T., Hosoya A., Hiraga T., Miyazawa H., Nakamura H. 1α,25-Dihydroxyvitamin D3 inhibits osteoblastic differentiation of mouse periodontal fibroblasts. Arch. Oral Biol. 2012;57:453–459. doi: 10.1016/j.archoralbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- de Matos R. Calcium metabolism in birds. Vet. Clin. North Am. Exot. Anim. Pract. 2008;11:59–82. doi: 10.1016/j.cvex.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Ecarot B., Desbarats M. 1,25-(OH)2D3 down-regulates expression of Phex, a marker of the mature osteoblast. Endocrinology. 1999;140:1192–1199. doi: 10.1210/endo.140.3.6593. [DOI] [PubMed] [Google Scholar]
- Elliot M., Edwards H. M., Jr Effect of 1,25-dihydroxycholecalciferol, cholecalciferol, and fluorescent lights on the development of tibial dyschondroplasia and rickets in broiler chickens. Poult. Sci. 1997;76:570–580. doi: 10.1093/ps/76.4.570. [DOI] [PubMed] [Google Scholar]
- Frank O., Heim M., Jakob M., Barbero A., Schäfer D., et al. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J. Cell. Biochem. 2002;85:737–746. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- Fromigué O., Marie P. J., Lomri A. Differential effects of transforming growth factor β2, dexamethasone and 1,25-dihydroxyvitamin D on human bone marrow stromal cells. Cytokine. 1997;9:613–623. doi: 10.1006/cyto.1997.0209. [DOI] [PubMed] [Google Scholar]
- Geng S., Zhou S., Glowacki J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J. Bone Miner. Res. 2011;26:1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumireddy K., Ikegaki N., Phillips P. C., Sutton L. N., Reddy C. D. Effect of 20-epi-1α,25-dihydroxyvitamin D3 on the proliferation of human neuroblastoma: role of cell cycle regulators and the Myc–Id2 pathway. Biochem. Pharmacol. 2003;65:1943–1955. doi: 10.1016/s0006-2952(03)00205-3. [DOI] [PubMed] [Google Scholar]
- Hathcock J. N. S. A, Vieth R., Heany R. Risk assessment for Vitamin D. Am. J. Clin. Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Frenkel J., DeMuth R., Gundberg C. M. Presence of osteocalcin and related higher molecular weight 4-carboxyglutamic acid-containing proteins in developing bone. J. Biol. Chem. 1983;258:176–182. [PubMed] [Google Scholar]
- Hogg D. A. A re-investigation of the centres of ossification in the avian skeleton at and after hatching. J. Anat. 1980;130:725–743. [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Cook P. R. Analyzing DNA Replication I: Labeling Animals, Tissues, and Cells with Bromodeoxyuridine (BrdU) Cold Spring Harbor Protocols. 2008;2008:1–4. doi: 10.1101/pdb.prot5031. [DOI] [PubMed] [Google Scholar]
- Jensen S. S., Madsen M. W., Lukas J., Binderup L., Bartek J. Inhibitory effects of 1α,25-dihydroxyvitamin D3 on the G1–S phase-controlling machinery. Mol. Endocrinol. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- Khatri M., T. D. O'Brien, Sharma J. M. Isolation and differentiation of chicken mesenchymal stem cells from bone marrow. Stem Cells Dev. 2009;18:1485–1492. doi: 10.1089/scd.2008.0223. [DOI] [PubMed] [Google Scholar]
- Li Y., Bäckesjö C. M., Haldosén L. A., Lindgren U. Species difference exists in the effects of 1α,25(OH)2D3 and its analogue 2-methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 (2MD) on osteoblastic cells. J. Steroid Biochem. Mol. Biol. 2008;112:110–116. doi: 10.1016/j.jsbmb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., et al. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proceedings of the National Academy of Sciences. 1989;86:1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. B., Coutts M., Canalis E. Studies of hormonal regulation of osteocalcin synthesis in cultured fetal rat calvariae. J. Biol. Chem. 1985;260:8706–8710. [PubMed] [Google Scholar]
- Lian J. B., Roufosse A., Reit B., Glimcher M. Concentrations of osteocalcin and phosphoprotein as a function of mineral content and age in cortical bone. Calcif. Tissue Int. 1982;34:S82–S87. [PubMed] [Google Scholar]
- Lian J. B., Stein G. S. Concepts of Osteoblast Growth and Differentiation: Basis for Modulation of Bone Cell Development and Tissue Formation. Crit. Rev. Oral Biol. Med. 1992;3:269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- Malaval L., Modrowski D., Gupta A. K., Aubin J. E. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J. Cell. Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- Maniatopoulos C., Sodek J., Melcher A. H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell. Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Igarashi C., Takeuchi Y., Harada S., Kikuchi T., et al. Stimulation by 1,25-Dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991;12:27–32. doi: 10.1016/8756-3282(91)90051-j. [DOI] [PubMed] [Google Scholar]
- Neve A., Corrado A., Cantatore F. Osteoblast physiology in normal and pathological conditions. Cell. Tissue Res. 2011;343:289–302. doi: 10.1007/s00441-010-1086-1. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Reginato A. M., Wang W. Bone development. Annu. Rev. Cell Dev. Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Owen T. A., Aronow M. S., Barone L. M., Bettencourt B., Stein G. S., Lian J. B. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: Dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology. 1991;128:1496–1504. doi: 10.1210/endo-128-3-1496. [DOI] [PubMed] [Google Scholar]
- Owen T. A., Aronow M., Shalhoub V., Barone L. M., Wilming L., et al. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell. Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Pines M. Proceedings of the 19th Australian Poultry Science Symposium, Sydney, New South Wales, Australia, 12–14 February 2007. Poultry Research Foundation; 2007. pp. 110–121. [Google Scholar]
- Pines M., Hurwitz S. The Role of the Growth Plate in Longitudinal Bone Growth. Poult. Sci. 1991;70:1806–1814. doi: 10.3382/ps.0701806. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quarles L. D., Yohay D. A., Lever L. W., Caton R., Wenstrup R. J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Riddell C. Skeletal deformities in poultry. Adv. Vet. Sci. Comp. Med. 1981;25:277–310. [PubMed] [Google Scholar]
- Rojanasathit S., Haddad J. G. Ontogeny and Effect of Vitamin-D Deprivation on Rat Serum 25-Hydroxyvitamin-D Binding-Protein. Endocrinology. 1977;100:642–647. doi: 10.1210/endo-100-3-642. [DOI] [PubMed] [Google Scholar]
- Sedrani S. H. Changes in Serum Levels of 1,25-Dihydroxyvitamin-D3, Calcium and Phosphorus with Age and Vitamin-D Status in Chickens. Br. J. Nutr. 1984;52:329–334. doi: 10.1079/bjn19840099. [DOI] [PubMed] [Google Scholar]
- Song I., Kim B.-S., Kim C.-S., Im G.-I. Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem. Biophys. Res. Commun. 2011;408:126–131. doi: 10.1016/j.bbrc.2011.03.135. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Ibe M., Kinouchi M., Ishida Yamamoto A., Hashimoto Y., Iizuka H. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J. Dermatol. Sci. 2003;31:21–28. doi: 10.1016/s0923-1811(02)00136-6. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tropel P., Noël D., Platet N., Legrand P., Benabid A. L., Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp. Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Wittow G. C. Chapter 18 – The Parathyroids, Calcitonin, and Vitamin D. In: Wittow G. C., editor. Sturkie's Avian Physiology. 5th ed. Orlando, FL, USA: Academic Press; 2000. pp. 473–488. [Google Scholar]
- Yahyaei B., Gilanpour H., Veshkini A. Study of the ossification centers and skeletal development of pelvic limb in quail after hatching. Advances in Environmental Biology. 2013;7:2074–2080. [Google Scholar]
- Yamaguchi M., Weitzmann M. N. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int. J. Mol. Med. 2012;29:934–938. doi: 10.3892/ijmm.2012.900. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Furuya K., Hanada K. Progressive development of the osteoblast phenotype during differentiation of osteoprogenitor cells derived from fetal rat calvaria: model for in vitro bone formation. Biol. Pharm. Bull. 2002;25:509–515. doi: 10.1248/bpb.25.509. [DOI] [PubMed] [Google Scholar]
- Yang D., Atkins G. J., Turner A. G., Anderson P. H., Morris H. A. Differential effects of 1,25-dihydroxyvitamin D on mineralisation and differentiation in two different types of osteoblast-like cultures. J. Steroid. Biochem. Mol. Biol. 2013;136:166–170. doi: 10.1016/j.jsbmb.2012.11.016. [DOI] [PubMed] [Google Scholar]