Abstract
Anaplasma phagocytophilum is an emerging tick-borne zoonosis with extensive increased interest. Epidemiological data are available in several regions of the USA, Europe and Asia in contrast to other parts of the world such as North Africa. Blood samples of 261 healthy individuals divided in two groups i.e., dog handlers and blood donors were analysed. Indirect immunofluorescent assay using a commercial kit was performed to detect specific A. phagocytophilum IgG. Two dilutions were used to assess the prevalence of seroreactive samples. Demographic variables were assessed as potential risk factors using exact logistic regression. Seropositivity rates reached 37% and 27% in dog handlers and 36% and 22% in blood donors. No statistically significant differences were found in the prevalence rates between the two groups. Analysis of risk factors such as gender, age groups, outdoor activities, self-reported previous exposure to ticks, or contact with domestic animals (dogs, cats, ruminants and horses) did not shown any significant difference. A. phagocytophilum exposure was common in both high-risk population and blood donors in Morocco.
Introduction
Anaplasma phagocytophilum is an obligate intracellular gram negative bacterium that infects neutrophils. The bacterium causes an emerging zoonotic tick-borne disease (TBD) called granulocytic anaplasmosis [1]. A. phagocytophilum is mostly transmitted to humans through the bites of ticks of the Ixodes genus. However, other modes of transmission have been described including transplacental transmission, percutaneous exposure or inhalation of the contaminated blood of deer, nosocomial infection following direct contact with blood and respiratory secretions and through blood transfusions [2, 3].
Human granulocytic anaplasmosis (HGA) is an unspecific flu-like illness that is typically characterized by the acute onset of fever, headache, chills, myalgia, malaise, nausea, and cough. Depending on several risk factors, which include advanced age, immunosuppression, co-morbidities and delays in the onset of treatment, HGA can be mild or fatal [4, 5]. Life-threatening complications occur in 3% of cases. Consequently, half of the HGA cases are hospitalized and up to 17% of patients require admission to intensive care units, especially when the diagnosis and treatment are delayed [1, 4]. Therefore, the Infectious Diseases Society of America recommends that antimicrobial therapy be given to every person suspected of having HGA on the basis of their clinical presentation, so as not to delay the treatment [6]. Due to the potentially serious outcome and the difficulty of the diagnosis, epidemiological data on the prevalence and distribution of human cases within a country are important to increase awareness of physicians and to develop adapted public health strategies to prevent and control this disease [7].
HGA commonly occurs in the USA and Europe, and it is increasingly diagnosed in some Asian countries [6, 8]. In the USA, at least 15,952 HGA cases were reported since 1995 and a 12-fold increased incidence has been observed between 2001 and 2011 [4]. In China, the exposure to A. phagocytophilum has continuously increased from 8.8% to 59.2% in high-risk populations between 2006 and 2009 [3]. Despite a moderate to high seroprevalence in several countries, HGA is still unrecognized and rarely diagnosed due to several factors including limited epidemiological information, difficult diagnosis, asymptomatic or subclinical infections and the lack of awareness among physicians and the public [2, 3, 6]. Moreover, the occurrence of HGA is unknown in many regions of the world such as Oceania, South America, Africa, and in large regions of Asia. To the author’s knowledge, no data are available in North Africa on either the occurrence of HGA or the prevalence of human exposure to A. phagocytophilum. However, ticks are abundant in this region and might represent a hazard for both animal and human public health [9]. Therefore, we carried out a cross-sectional epidemiological serologic survey to investigate the potential human exposure to A. phagocytophilum in Morocco.
Materials and Methods
Study population
Between June and September 2015, 261 healthy individuals from two groups were sampled from three cities of Morocco. The first group included 144 military and police dog handlers from the first kenel of the Royal Forces Army of Benslimane and the kenel of the Royal Gendarmerie of Temara. This group was considered to be at a high risk for TBDs because of their regular contact with dogs and outdoor occupational activities. The data collected on this group included age and exposure to ticks. The second group included 117 blood donors from the Regional Transfusion Centre of Rabat. All of the blood donors were informed on the purpose of the survey and signed informed consent forms before enrollment. An epidemiological report was completed for each blood donor containing data on the age, city of residence, occupation, travels outside Morocco during the previous year, outdoor activities, tick exposure and potential contact with dogs and other domestic animals (i.e., cats, horses and ruminants). The study protocol was approved by the Ethical Committee for Biomedical Research of the Mohammed V University of Rabat (n°698; July 10, 2014) and the Ministry of Health of Morocco (n°965; June 12, 2014).
Blood sampling
For each person included in the study, 5 ml of non-anticoagulated blood was collected from the elbow groove veins. Blood samples were centrifuged at 3500 rpm during 10 min at 15°C and sera were aliquoted and stored at -32°C until shipment to the National Reference Laboratory for A. phagocytophilum in Belgium.
Serological tests
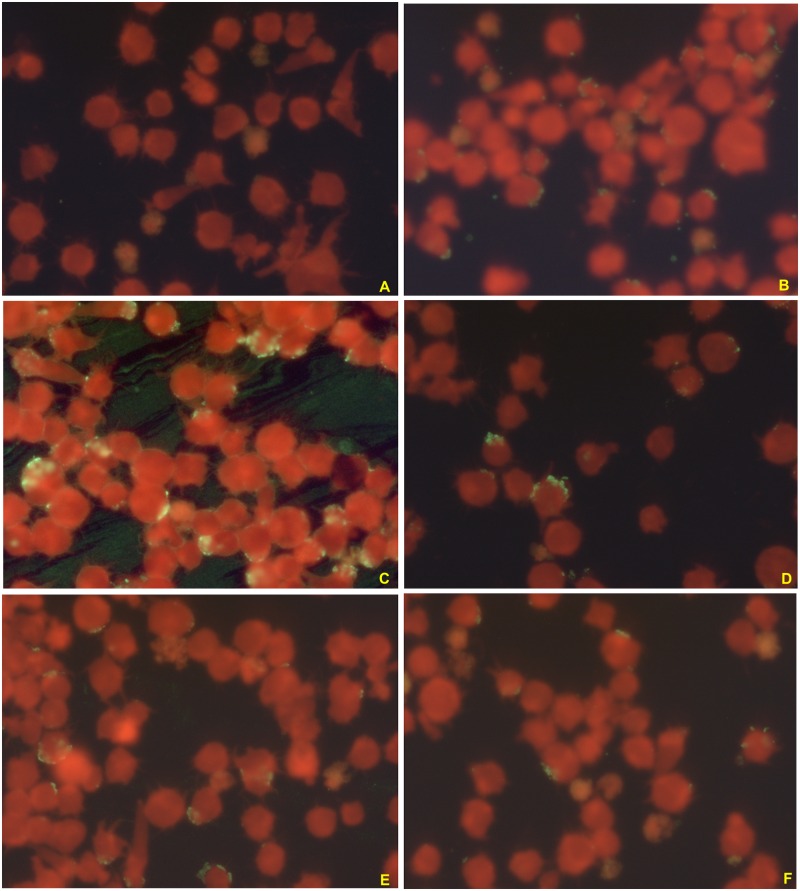
Immunoglobuline G (IgG) antibodies were detected against A. phagocytophilum by a semi-quantitative indirect immunofluorescent assay (IFA) using a commercial kit (Focus Diagnostics, Cypress, California, USA) containing HL60 cells infected with a human isolate of A. phagocytophilum HGE-1 according to the manufacturer’s instructions. Briefly, 5 μL of serum were diluted in 315 μL of phosphate-buffer saline (PBS) (0.01 M, pH = 7.2±0.1). The positive IgG control was also diluted into the following five dilutions: 1:2, 1:4, 1:8, 1:16 and 1:32. Then, 25 μL of diluted sera were added in the wells of each slide. The first line of the first slide contained the negative IgG control and the five dilutions of the positive IgG control. The slides were incubated in humid chambers between 35 and 36.5°C for 30 min, then they were washed with the PBS solution followed by distilled water to eliminated non conjugated serum antibodies. Next, 25 μL of the conjugate containing human IgG and fluorescein were added to each well. The slides were incubated again and washed as described above. Finally, the slides were dried and coverslipped using a mounting medium and were examined under ultraviolet light microscopy (×400). The titer was defined as the reciprocal of the highest dilutions of serum with the homogeneously stained cytoplasmic morulae (Fig 1). A serum titer of ≥ 1:64 was considered as positive for A. phagocytophilum IgG according to the instructions provided by the manufacturer. Samples that were positive at the first dilution of 1:64 were then further diluted to 1:128 and those remaining positive at the second dilution were then titered at 1:256 and 1:512. Ten samples were reassessed by a blinded technician from the laboratory at a dilution of 1:64 and the results were confirmed in all cases.
Fig 1. Photographs of ultraviolet light microscopy (×400) of A. phagocytophilum IgG semi-quatitative IFA measurement using a commercial kit (Focus Diagnostics, Cypress, California, USA) showing a negative control (A), a positive control (B) and for positive dilutions i.e., 1:64 (C), 1:128 (D); 1:256 (E) and 1:516 (F) from the same patient.
The positivity is set on the observation of green morulae surrounding the cell’s cytoplasmic membrane.
Statistical analyse
A statistical analysis was performed using SAS version 6.4 (SAS Institute Inc., Car, NC, USA). The exact logistic regression model was fitted to compare seroreactivity rates between both dog handler and blood donor groups and between gender, the presence or absence of outdoor activities, exposure to ticks, dogs or other domestic animals inside the blood donor group. The statistical significance was set at 5%. The results were summarized in terms of the odds ratio with a 95% confidence interval.
Results
Eight samples were excluded due to hemolysis that could interfere with the results according to the manufacturer instructions. A total of 138 dog handlers (54.5%) and 115 blood donors (45.4%) were included in the study. The majority of blood donors (105/115) lived in Rabat or the surrounding cities whereas nine blood donors were from other cities (Table 1).
Table 1. Distribution of the number of blood donors and of positive samples for both dilutions according to city.
| Administrative region | City | Distance to Rabat (km) | Number of blood donors | Number of positive IgG 1: 64 | Number of positive IgG 1: 128 |
|---|---|---|---|---|---|
| Rabat-Salé-Kénitra | Rabat | 55 | 19 | 10 | |
| Salé | 5.2 | 32 | 11 | 6 | |
| Temara | 8.0 | 13 | 5 | 2 | |
| Ain El Aouda | 30.0 | 1 | 1 | 1 | |
| Sidi Allal El Bahraoui | 37.7 | 1 | 0 | 0 | |
| Kenitra | 55.0 | 1 | 0 | 0 | |
| Casablanca-Settat | Bouznika | 39.6 | 1 | 1 | 1 |
| Benslimane | 60.0 | 1 | 0 | 0 | |
| Béni Mellal-Khénifra | Khenifra | 237.4 | 1 | 1 | 1 |
| Beni Mellal | 233.1 | 1 | 1 | 1 | |
| Tanger-Tétouan-Al Hoceima | Tangier | 250.0 | 1 | 1 | 1 |
| Souss-Massa-Drâa | Tinghir | 477.0 | 1 | 0 | 0 |
| Agadir | 547.0 | 3 | 1 | 1 | |
| Tizi n'Tichka | 1 | 0 | 0 | ||
| Guelmim-Oued Noun | Sidi Ifni | 686.0 | 1 | 0 | 0 |
Abbreviations: IgG, immunoglobuline G.
The city of origin was unavailable for one blood donor. All dog handlers were men between 21 and 51 years of age (average age: 33 years). The blood donors group included 63 men (54.8%) and 52 women (42.2%), and their ages ranged from 18 to 61 years (average age: 39 years). The distribution according to the epidemiological variables in the two groups is summarized in Table 2.
Table 2. Distribution of age, sex, exposure to ticks, contact with dogs or other domestic animals and travel history outside Morocco in both dog handlers (n = 138) and blood donors (n = 115) groups.
| Variables | Dog handlers (%) | Blood donors (%) | |
|---|---|---|---|
| Sex | Men | 138 (100) | 63 (54.8) |
| Women | 0 (0.0) | 52 (45.2) | |
| Age (years-old) | ≤20 | 1 (0.7) | 7 (6.1) |
| 21–30 | 78 (56.5) | 26 (22.6) | |
| 31–40 | 47 (34.1) | 27 (23.5) | |
| 41–50 | 10 (7.2) | 36 (31.3) | |
| >50 | 1 (0.7) | 19 (16.5) | |
| Exposure to ticks | 2 (1.4) | 7 (6.1) | |
| Outdoor activities | 138 (100) | 86 (74.8) | |
| Contact with dogs | 138 (100) | 11 (9.6) | |
| Contact with other domestic animals | - | 17 (14.8) | |
| Travel | 0 (0.0) | 18 (15.7) | |
Outdoor activities in the forest or rural areas were either occupational or for leisure (picnic, hiking, jogging, walking or hunting). More than half of the blood donors (47/86) reported their outdoor activities in the region of Rabat-Salé-Kenitra région and 20.9% (18/86) reported their activities in other Moroccan regions including Moulay Bousselham, Gharb region, Tangier, Ouazzane, Rif mountain, Ifrane, Azrou, Khouribga, Oued Zem, Nador, Taza, Oujda, El Jadida, Safi, Essaouira, Agadir, Tiznit, Dakkhla, Beni Mellal, Azilal, Marrakech, Ait Baha, Zagora, Taroudant and the High Atlas mountains. The remaining 21 blood donors (24.4%) reported their outdoor activities in both Rabat-Salé-Kenitra and other regions. Previous exposure to ticks was recorded in 1.4 (2/138) and 6.1% (7/117) of dog handlers and blood donors, respectively. Travel outside of Morocco was recorded in 15.6% (18/115) of blood donors and ten traveled to two or more countries.
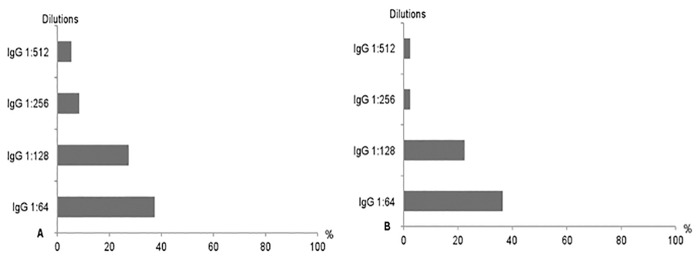
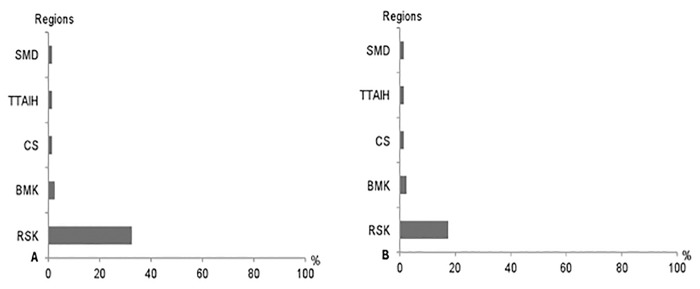
The seropositivity rates for A. phagocytophilum IgG at the first dilution reached 37.0% (51/138) and 35.7% (41/115) in dog handlers and blood donors, respectively (Table 3 and Fig 2). At the second dilution, 27.5% (38/138) and 21.7% (25/115) of sera were still reactive in the dog handlers and the blood donors groups, respectively (Table 3 and Fig 2). Most seropositive blood donors for both dilutions (i.e., 1:64 and 1:128) were from the region of Rabat-Salé-Kénitra (Fig 3).
Table 3. Number of seropositive samples in both dog handlers (n = 138) and blood donors (n = 115) groups at the four different dilution.
| Variables | Dog handlers (%) | Blood donors (%) | OR | 95%CI | P value |
|---|---|---|---|---|---|
| IgG 1:64 | 51 (37.0) | 41 (35.7) | 1.05 | 0.61–1.83 | 0.90 |
| IgG 1:128 | 38 (27.5) | 25 (21.7) | 1.37 | 0.74–2.56 | 0.31 |
| IgG 1:256 | 11 (8.0) | 2 (1.7) | - | - | - |
| IgG 1:512 | 7 (5.1) | 2 (1.7) | - | - | - |
Abbreviations: IgG, immunoglobuline G; OR, odds ratio; 95%CI, 95% confidence interval.
Fig 2. Distribution of positivity rates for the four A. phagocytophilum IgG dilutions (i.e, 1:64, 1:128, 1:256 and 1:516) in both dog handlers (A) and blood donors (B) groups.
Fig 3. Distribution of A. phagocytophilum IgG positivity rates in blood donors according to the region of living the Rabat-Salé-Kénitra (RSK), Casablanca-Settat (CS), Tangier-Tétouane-Al Hoceima (TTAlH) and Souss-Massa-Drâa (SMD) regions and for both 1:64 (A) and 1:128 (B) dilutions.
No statistically significant differences were found between the two groups considering the seroreactivity rates at both dilutions (Table 3). Similarly, no statistically significant differences were found in the blood donor group when comparing between gender, age groups, the presence of outdoor activities, exposure to ticks, and contact with dogs or other domestic animals at both dilutions (Table 4). In the dog handlers group, 11 (8.0%) and 7 (5.1%) of the sera were still positive when further diluted to 1:256 and 1:512, respectively (Table 3 and Fig 2). Only two of the samples remained positive at both 1:256 and 1:512 in the blood donors group (Table 3 and Fig 2).
Table 4. Distribution of seropositive samples according to the gender, age group, presence of outdoor activities, contact with ticks, dogs or other animals and travel history in the blood donor group for both 1:64 (n = 41) and 1:128 (n = 25) dilutions.
| Varaiables | IgG 1:64 | IgG 1:128 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) | OR | 95%CI | P value | Number (%) | OR | 95%CI | P value | ||
| Gender | Men | 23 (56.1) | 1.08 | 0.47–2.52 | 0.85 | 16 (64.0) | 1.62 | 0.60–4.62 | 0.37 |
| Women | 18 (43.9) | 9 (36.0) | |||||||
| Age (years-old) | ≤20 | 2 (4.9) | 1.03 | 0.99–1.06 | 0.12 | 2 (8.0) | 1.00 | 0.96–1.03 | 0.85 |
| 21–30 | 9 (22.0) | 7 (28.0) | |||||||
| 31–40 | 6 (14.6) | 6 (24.0) | |||||||
| 41–50 | 14 (34.1) | 3 (12.0) | |||||||
| >50 | 10 (24.4) | 7 (28.0) | |||||||
| Exposure to ticks | 2 (4.9) | 1.41 | 0.22–15.45 | 1.00 | 1 (4.0) | 1.71 | 0.19–82.09 | 1.00 | |
| Outdoor activities | 30 (73.2) | 1.44 | 0.56–3.63 | 0.51 | 17 (68.0) | 1.73 | 0.59–4.89 | 0.31 | |
| Contact with dogs | 5 (12.2) | 0.64 | 0.15–2.84 | 0.52 | 2 (12.0) | 0.72 | 0.15–4.55 | 0.70 | |
| Contact with other domestic animals | 4 (9.8) | 1.96 | 0.55–8.87 | 0.41 | 2 (8.0) | 2.29 | 0.47–22.08 | 0.36 | |
| Travel | 7 (17.1) | - | - | - | 5 (20.0) | - | - | - | |
Abbreviations: IgG, immunoglobuline G; OR, odds ratio; 95%CI, 95% confidence interval.
Discussion
To the author’ s knowledge, this is the first report investigating human exposure to A. phagocytophilum in Africa. In Europe, the USA and Asia, several reports have investigated the prevalence of human exposure in blood donors [10–19] and in high-risk populations including people living in forest areas and forestry workers [10, 12, 16, 20–25], people living in rural areas and farmers [3, 8, 19, 26–28], hunters [8, 11], national parks rangers [29–30], military personnel [31], people in close contact with domestic animals [7, 21], and people at high risk of exposure or previously exposed to ticks [7, 32, 33]. The prevalences recorded in high-risk populations or in endemic areas were up to 32%, 35.6%, and 33.7% in Europe [23], the USA [34], and China [35], respectively. However, several serological methods and cutoffs were used, which made the comparison between these studies difficult [31]. When comparing the results of military dog handlers obtained in this study at the threshold of 1:64, i.e., 37%, with other Chinese [24, 35] and European [12, 21, 23, 25, 28, 32, 36] reports using the same method and the same cutoff, it appears that the prevalence in Morocco is higher. The highest prevalences recorded in both China and Europe were 20% [7] and 9.6% [25], repectively. One study from Cyprus reported a prevalence of 32% with a cutoff of 1:128 [23] which is slightly higher than the results found in Morocco at the same cut off (27.5%). When comparing the results of this study with high-risk populations from other European Mediterranean countries such as Italy (8.8%) [22], Portugal (5.4%) [17], and Spain (1.4%) [21], the prevalence found in Moroccan dog handlers is higher at both the first and second dilutions. Moreover, the prevalence in Moroccan dog handlers is even higher than the prevalence found in patients with clinical signs and history of tick bites in Belgium [5].
Because high-risk populations were shown to have a significantly higher prevalence of A. phagocytophilum exposure [3,16, 31] they may not reflect the true exposure of the general population of the same country [7]. Therefore, a more representative sample including blood donors with more diverse social and intellectual levels, occupational and leisure activities would be a better sample to estimate the prevalence of A. phagocytophilum exposure in Morocco. In addition, the high seroprevalence rates in blood donors of some geographic locations, the potential asymptomatic or subclinical evolution of the disease, the survival of A. phagocytophilum in refrigerated blood products and documented transfusion-transmitted HGA cases, provide further reasons to screen blood donors in Morocco. Although only a few cases of transfusion-transmitted anaplasmosis have been reported, A. phagocytophilum infection is among the TBDs that are considered to represent a potential risk for transmission by blood transfusion. In addition, because sharing blood products between different areas is growing, such an acute illness after blood transfusion should be included in the differential diagnosis even in nonendemic areas [2, 37]. Our results showed that even in the blood donor group, high prevalences of 35.7% and 21.7% at both the 1:64 and 1:128 dilutions, respectively, were recorded. When compared to European prevalences in blood donors using the same method and the same cutoffs, these results are higher than those published in Poland (2%) [12], and Austria (9%) [15], but they are similar to those from Greece (21.4%) [18]. Without taking into account the method and the cutoffs, the results from Moroccan blood donors are even higher than those from several US [13, 14] and European reports [10, 11, 16, 17, 19, 33, 38]. In several reports that compared the seroreactivity rates of blood donors to those of high-risk populations, significant differences were found [10,16, 17]; these findings are in contrast to our report. These differences could be due to the relatively high proportion of the blood donors that report outdoor activities, which could then increase the possible exposure to ticks and thus predispose them to A. phagocytophilum infection.
No risk factors were identified in this survey in either group. Similarly, some reports failed to identify specific demographic variables as potential risk factors [12, 17, 23, 25, 28]. In contrast, other reports demonstrated that seropositivity rates were significantly higher in men [3, 23, 30], in age groups from 20 to 40 [3, 23] and 40 to 65 years of age [32] and the rates increased with age [29]. Seropositivity rates among Moroccan blood donors were higher in men especially for the dilution of 1:128 and lower in the age group ≤20 year-old, although this was not statistically significant. No statistically significant associations between seroreactivity rates and the contact with animals or outdoor activities were found in this study. However, the chance of coming into contact with infected ticks depends on several epidemiological and ecological factors, such as the environment, the presence of appropriate hosts and reservoirs. Consequently, outdoor activities that are especially related to wooded areas, meadow habitats and grasslands are considered to be some of the major risk factors for acquiring TBDs [39]. Moreover, a large number of participants to a study from Germany mentioned contracting their most recent tick bite in their gardens and half of the participants with past exposure to A. phagocytophilum listed gardening as a regular leisure activity; despite a comparatively low risk of exposure associated with this activity. Therefore, public health measures to increase awareness for TBDs should also target the large portion of the population who are involved in comparatively low risk outdoor activities such as gardening, cycling or walking [32]. Although not statistically significant, a high proportion (74.8%) of the blood donors mentioned participating in outdoor activities. Consequently, the obvious popularity of outdoor activities may predispose a large number of people to the risk of infection by A. phagocytophilum. Only a small portion (3.6%) of the tested population had a history of tick exposure without any significant difference between both groups. Similarly, several surveys did not find any association between self-reported exposure to ticks and the seroreactivity rates of A. phagocytophilum [3, 10, 12, 25, 28, 40]. Moreover, a range of studies demonstrated seropositivity among the blood donors and the control populations without a specific history of a tick bite [32]. Another report described the highest seropositivity among persons who denied having tick bites [25]. A study investigating the risk of acquiring a tick-borne pathogen after a tick bite failed to identify a significant difference between the group of persons bitten by ticks infected with A. phagocytophilum and the group bitten by uninfected ticks [40]. The possible explanations for this oversight could be that the stage of feeding ticks as nymphs and larvae may not be detectable because of their small size or that the capacity of ticks to modulate host immune and inflammatory responses may also decrease the chance of detection [12, 28]. Further, several persons from the blood donor group that were questioned about previous contact with ticks were not familiar with these parasites. Therefore, A. phagocytophilum infection should not be ruled out in the absence of self-reported previous tick exposure [4].
Most of the epidemiological surveys about A. phagocytophilum have used only the indirect IFA or the enzyme linked immunosorbent assay (ELISA). Either technique used alone with the standard cutoffs may overestimate the prevalence of antibodies [28, 40]. The World Health Organization guidelines set the cutoff at the 98th percentile i.e., at 1:128, to fulfil the requirements for seroepidemiological studies. This cutoff should reduce the overestimation of the seroprevalence and therefore provide reliable information with regard to previous infections [15, 18]. The overestimation of the seroreactivity with IFA testing might be due to false-positive results secondary to potential cross-reactions [40]. These results can be observed with several other vector-borne pathogens including tick-borne encephalitis virus (6.7%), Rickettsia conorii (8%), Coxiella burnetii (10%), Borrelia burgdorferi (16.7%) and Bartonella quintana (70%) [5]. The Epstein–Barr virus infection, autoimmune disorders and Ehrlichia species may also induce cross-reactivity [3, 15, 33, 40]. However, two studies have failed to demonstrate an increased reactivity to A. phagocytophilum in samples that were seropositive to Epstein-Barr virus, cytomegalovirus, parvovirus B19, Toxoplasma gondii, Borrelia burgdorferi sensu lato, Coxiella burnettii, Rickettsia conorii and E. chaffeensis [15, 21]. Moreover, IFA based on HL60-cells infected with a human isolate of A. phagocytophilum are considered to be both sensitive [15] and highly specific for the investigation of seroreactivity [5]. According to the manufacturer, the specificity of this test reaches 100%, and the sensitivity depends on the period between the moment of sampling and the begining of the clinical signs, which ranges from 66.7% to 100%.
Clinical data were not recorded in our study; thus it is unknown whether the subjects who were seropositive to A. phagocytophilum experienced any clinical signs before the date of sampling. Although one previous report has found a positive association between fever in the last two years and a high seroprevalence of A. phagocytophilum [3], all or almost all seropositive persons denied any clinical symptoms of HGA in several epidemiological surveys especially in Europe [18, 25], suggesting that a high proportion of the infections could be subclinical [31]. Other possible reasons for the discrepancy between a high seroprevalence and a low incidence of the disease include underdiagnosis or misdiagnosis due to the unawareness of physicians, the circulation of variants that are non-pathogenic for humans, which may cause only transient infections without the relevant clinical signs and the potential serologic cross-reactivity with other bacteria [32, 40]. Despite a low incidence rate and because the severity of the disease is closely linked to delayed diagnosis and treatment, some authors have emphasised the importance of clinicians awareness to promptly diagnose this infection especially in high-risk areas and even in persons without a self-reported history of a tick bite [28]. At least, A. phagocytophilum should be considered in the differencial diagnosis of flu-like syndromes, febrile patients especially from high-risk areas, febrile illness of unknown aetiology or in those who are not responding to beta-lactam antibiotics or macrolides [16, 20, 21]. Only one serum sample was performed for each participant; samples were not paired and IgM were not measured. Therefore, it was not possible to estimate the incidence of seroconversion and evaluate a potential acute exposure [28]. IgM antibodies are detectable during the first 40 days after infection and IgG seroconversion occurs approximately 20–40 days after the onset of symptoms and persists for several months to years postinfection. Therefore, with a single positive IgG titer, it is not possible to distinguish between current and past exposure to A. phagocytophilum [35]. In addition, serological testing close to the onset of symptoms is usually negative [4, 6, 32]. However, IgM testing is reported to be less sensitive than IgG detection, even during early stages of infection.4 Sampling took place only in two cities of Morocco and subjects’ deployment histories were unavailable in the dog handler group. Therefore, no valuable data were available on human exposure in other regions of the country or the distribution or the presence of specific foci within some regions. A more comprehensive and representative study should be conducted to better estimate the prevalence of this bacterium in Morocco.
Conclusion
To the author’s knowledge, this study is the first evidence of human exposure to A. phagocytophilum or to an antigenically similar bacterium in Morocco. The very high prevalence rate found in both high-risk populations and blood donors indicated the necessity for large-scale serologic surveys to better estimate the prevalence of this bacterium in Morocco. We hope that this study can serve as an indicator to Moroccan physicians that A. phagocytophilum infection is present and that this will help raise awareness of the potential occurrence of TBDs. Further studies especially those based on the isolation of the causative agent from patients with clinical signs compatible with HGA are warranted to clearly confirm the presence of the bacterium and to assess its role in causing disease in Morocco. Investigations of the epidemiology and the ecology of the bacterium in Morocco are also needed.
Acknowledgments
We would like to address our gratitude to the General Hosni Benslimane for the authorization to sample military and police dog handlers. We would like to thank Dr. Walter Heuninckx and Mrs. Pierrette Parmentier fo their help in IFAT dosage. We also would like to thank Dr. Souad Boutayeb and Dr. Khadija Hajjout for their help in blood donors sampling and samples processing. We are grateful to Pr. Aicha Hafidi for her help in obtaining all the authorizations requested. We also thank the Emergency Medical Help Services of the Royal Gendarmery, the first kenel of the Royal Forces Army (Benslimane, Morocco) and the kenel of the Royal Gendarmerie (Témara, Morocco).
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005; 11: 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annen K, Friedman K, Eshoa C, Horowitz M, Gottschall J, Straus T. Two cases of transfusion-transmitted Anaplasma phagocytophilum. Am J Clin Pathol. 2012; 137: 562–565. 10.1309/AJCP4E4VQQQOZIAQ [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Liu H, Xu B, Zhang Z, Jin Y, Li W, et al. Rural residents in China are at increased risk of exposure to tick-borne pathogens Anaplasma phagocytophilum and Ehrlichia chaffeensis. Biomed Res Int. 2014; 2014: 313867 10.1155/2014/313867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin N Am. 2015; 29: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyman P, Cochez C, Bigaignon G, Guillaume B, Zizi M, Vandenvelde C. Human granulocytic ehrlichiosis in Belgium: an underestimated cause of disease. J Infect. 2003; 47: 129–132. [DOI] [PubMed] [Google Scholar]
- 6.Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, et al. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther. 2010; 8: 33–50. 10.1586/eri.09.118 [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Hai R, Li W, Li G, Lin G, He J, et al. Seroprevalence of human granulocytotropic anaplasmosis in Central and Southeastern China. Am J Trop Med Hyg. 2009; 81: 293–295. [PubMed] [Google Scholar]
- 8.Zhang L, Cui F, Wang L, Zhang L, Zhang J, Wang S, et al. Investigation of anaplasmosis in Yiyuan County, Shandong Province, China. Asian Pac J Trop Med. 2011; 4: 568–572. 10.1016/S1995-7645(11)60148-X [DOI] [PubMed] [Google Scholar]
- 9.Seng P, Sarih M, Socolovschi C, Boudebouch N, Hassar M, Parola P, et al. Detection of Anaplasmataceae in ticks collected in Morocco. Clin Microbiol Infect. 2009; 15 (Suppl 2): 86–87. 10.1111/j.1469-0691.2008.02251.x [DOI] [PubMed] [Google Scholar]
- 10.Fingerle V, Goodman JL, Johnson RC, Kurtti TJ, Munderloh UG, Wilske B. Human granulocytic ehrlichiosis in Southern Germany: increased seroprevalence in high-risk groups. J Clin Microbiol. 1997; 35: 3244–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pusterla N, Weber R, Wolfensberger C, Schfir G, Zbinden R, Fierz W, et al. Serological evidence of human granulocytic ehrlichiosis in Switzerland. Eur J Clin Microbiol Infect Dis. 1998; 17: 207–209. [DOI] [PubMed] [Google Scholar]
- 12.Grzeszczuk A, Stanczak J, Kubica-Biernat B. Serological and molecular evidence of human granulocytic ehrlichiosis focus in the Bialowieza Primeval forest (Puszcza Bialowieska), Northeastern Poland. Eur J Clin Microbiol Infect Dis. 2002; 21: 6–11. [DOI] [PubMed] [Google Scholar]
- 13.Aguero-Rosenfeld ME, Donnarumma L, Zentmaier L, Jacob J, Frey M, Noto R, et al. Seroprevalence of antibodies that react with Anaplasma phagocytophila, the agent of human granulocytic ehrlichiosis, in different populations in Westchester County, New York. J Clin Microbiol. 2002; 40: 2612–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leiby DA, Chung AP, Cable RG, Trouern-Trend J, McCullough J, Homer MJ, et al. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp.) in blood donors. Transfusion 2002; 42: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 15.Walder G, Tiwald G, Dierich MP, Würzner R. Serological evidence for human granulocytic ehrlichiosis in Western Austria. Eur J Clin Microbiol Infect Dis. 2003; 22: 543–547. [DOI] [PubMed] [Google Scholar]
- 16.Cisak E, Chmielewska-Badora J, Zwoliński J, Wójcik-Fatla A, Polak J, Dutkiewicz J. Risk of tick-borne bacterial diseases among workers of Roztocze National Park (south-eastern Poland). Ann Agric Environ Med. 2005; 12: 127–132. [PubMed] [Google Scholar]
- 17.Santos AS, Bacellar F, Dumler JS. Human exposure to Anaplasma phagocytophilum in Portugal. Ann N Y Acad Sci. 2006; 1078: 100–105. [DOI] [PubMed] [Google Scholar]
- 18.Chochlakis D, Papaeustathiou A, Minadakis G, Psaroulaki A, Tselentis Y. A serosurvey of Anaplasma phagocytophilum in blood donors in Crete, Greece. Eur J Clin Microbiol Infect Dis. 2008; 27: 473–475. 10.1007/s10096-007-0457-3 [DOI] [PubMed] [Google Scholar]
- 19.Chmielewska-Badora J, Moniuszko A, Żukiewicz-Sobczak W, Zwoliński J, Piątek J, Pancewicz S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann Agric Environ Med. 2012; 19: 271–274. [PubMed] [Google Scholar]
- 20.Groen J, Koraka P, Nur YA, Avsic-Zupanc T, Goessens WHF, Ott A, et al. Serologic evidence of ehrlichiosis among humans and wild animals in the Netherlands. Eur J Clin Microbiol Infect Dis. 2002; 21: 46–49. [DOI] [PubMed] [Google Scholar]
- 21.Oteo JA, Gil H, Barral M, Perez A, Jimenez S, Blanco JR, et al. Presence of granulocytic ehrlichia in ticks and serological evidence of human infection in La Rioja, Spain. Epidemiol Infect. 2001; 127: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinco M, Barbone F, Grazia Ciufolini MG, Mascioli M, Anguero Rosenfeld M, Stefanel P, et al. Seroprevalence of tick-borne infections in forestry rangers from northeastern Italy. Clin Microbiol Infect. 2004; 10: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 23.Chochlakis D, Ioannou I, Kokkini I, Tselentis Y, Psaroulaki A. Seroprevalence of Anaplasma phagocytophilum in a high-risk human population. J Infect. 2009; 58: 87–88. 10.1016/j.jinf.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 24.Hao Q, Geng Z, Hou XX, Tian Z, Yang XJ, Jiang WJ, et al. Seroepidemiological investigation of Lyme disease and human granulocytic anaplasmosis among people living in forest areas of eight provinces in China. Biomed Environ Sci. 2013; 26: 185–189. 10.3967/0895-3988.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 25.Stanczak J, Grzeszczuk A. Seroprevalence of Anaplasma phagocytophilum among forestry rangers in northern and northeastern Poland. Ann N Y Acad Sci. 2006; 1078: 89–91. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Shan A, Mathew B, Yin J, Fu X, Zhang J, et al. 2008. Rickettsial seroepidemiology among farm workers, Tianjin, People's Republic of China. Emerg Infect Dis. 2008; 14: 938–940. 10.3201/eid1406.071502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XC, Zhang LX, Li WH, Wang SW, Sun YL, Wang YY, et al. Ehrlichiosis and zoonotic anaplasmosis in suburban areas of Beijing, China. Vector Borne Zoonotic Dis. 2012; 12: 932–937. 10.1089/vbz.2012.0961 [DOI] [PubMed] [Google Scholar]
- 28.Thomas DR, Sillis M, Coleman TJ, Kench SM, Ogden NH, Salmon RL, et al. Low rates of ehrlichiosis and Lyme borreliosis in English farmworkers. Epidemiol Infect. 1998; 121: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adjemian J, Weber IB, McQuiston J, Griffith KS, Mead PS, Nicholson W, et al. Zoonotic infections among employees from Great Smoky Mountains and Rocky Mountain national parks, 2008–2009. Vector Borne Zoonotic Dis. 2012; 12: 922–931. 10.1089/vbz.2011.0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Żukiewicz-Sobczak W, Zwoliński J, Chmielewska-Badora J, Galińska EM, Cholewa G, Krasowska E, et al. Prevalence of antibodies against selected zoonotic agents in forestry workers from eastern and southern Poland. Ann Agric Environ Med. 2014; 21: 767–770. 10.5604/12321966.1129930 [DOI] [PubMed] [Google Scholar]
- 31.Graf PCF, Chretien JP, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis. 2008; 46: 70–77. 10.1086/524018 [DOI] [PubMed] [Google Scholar]
- 32.Von Wissmann B, Hautmann W, Sing A, Hizo-Teufel C, Fingerle V. Assessing the risk of human granulocytic anaplasmosis and lyme borreliosis after a tick bite in Bavaria, Germany. Int J Med Microbiol. 2015; 305: 736–741. 10.1016/j.ijmm.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 33.Brouqui P, Dumler JS, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet 1995; 346: 782–783. [DOI] [PubMed] [Google Scholar]
- 34.Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006; 1078: 236–247. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Wang S, Shi Y, Yu H, Cao M, Mei L, et al. Anaplasmosis in farmers and domestic animals in Anhui province, China. Asian Pac J Trop Dis. 2012; 2: 27–30. [Google Scholar]
- 36.Mączka I, Roguska U, Tylewska-Wierzbanowska S. Prevalence of rickettsioses in Poland in 2006–2012. Przegl Epidemiol. 2013; 67: 633–636. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Anaplasma phagocytophilum transmitted through blood transfusion: Minnesota, 2007. MMWR Morb Mortal Wkly Rep. 2008; 57: 1145–1148. [PubMed] [Google Scholar]
- 38.Hjetland R, Henningsson AJ, Vainio K, Dudman SG, Grude N, Ulvestad E. Seroprevalence of antibodies to tick-borne encephalitis virus and Anaplasma phagocytophilum in healthy adults from western Norway. Infect Dis. 2015; 47: 52–56. [DOI] [PubMed] [Google Scholar]
- 39.Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Cascio A, Cazzin S, et al. Ticks infesting humans in Italy and associated pathogens. Parasit Vectors. 2014; 7: 328 10.1186/1756-3305-7-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henningsson AJ, Wilhelmsson P, Gyllemark P, Kozak M, Matussek A, Nyman D, et al. Low risk of seroconversion or clinical disease in humans after a bite by an Anaplasma phagocytophilum-infected tick. Ticks Tick Borne Dis. 2015; 6: 787–792. 10.1016/j.ttbdis.2015.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.