Abstract
The aim of the present study was to optimize the purification of mycelia selenium polysaccharides (MSPS) from Agrocybe cylindracea SL-02 and characterize their in vitro antioxidant and in vivo anti-ageing activities. The Box-Behnken experimental design (BBD) was evaluated, which showed that the optimum conditions included an extraction temperature of 94.99°C, a pH of 9 and a precipitation temperature of 12°C, and the predicted yield was 11.036 ± 0.31%. The in vitro antioxidant assay demonstrated that MSPS had potential effects on scavenging and enhanced the reducing power of reactive oxygen species. The in vivo anti-ageing evaluation showed that MSPS significantly reduced the malonaldehyde (MDA) contents and total cholesterol (CHOL) levels, and remarkably improved the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) in mice in response to D-galactose-induced ageing. Furthermore, the characteristic analysis of MSPS indicated a selenium content of 1.76 ± 0.10 mg/g at a concentration of 6 μg/mL in liquid media and a monosaccharide composition of rhamnose, arabinose, mannose, glucose and galactose at a molar ratio of 29:3:1:18.8:2.7. These results suggest that MSPS might be suitable for functional foods and natural drugs on preventing the ageing progress induced by toxic chemicals.
Introduction
Ageing, an inevitable process for all living organisms, damages the cell structure and promotes the disorder of physiological functions [1]. Several studies have reported that the ageing process involves many factors, including the accumulation of genomic mutations, toxic metabolites and free radicals; the hyposecretion of hormones; and the cross-linking of macromolecules under glycation [2]. However, the detailed mechanisms remain poorly understood [3]. One of the most popular theories for explaining the ageing process is the free radical theory [4], and an increasing number of studies have demonstrated that oxidative stress, followed by the overproduction of free radicals, plays a vital role in ageing [5]. As the balance of reactive oxygen species (ROS) production and antioxidant defence could determine the degree of ROS, oxidant intake and both dietary and synthetic antioxidants are beneficial to reduce the degree of ROS and confer protection against ageing. Nevertheless, synthetic antioxidants are restricted, reflecting the side effects of these compounds [6]. Hence, it is necessary to identify natural antioxidants with highly efficient and safe antioxidant properties to delay the ageing process. Therefore, the focus has recently changed to identifying harmless natural antioxidants from edible materials [7].
Currently, healthy diets have drawn increasing attention for their ability to retard the ageing process. Furthermore, the popularity of edible mushrooms has increased, as the polysaccharides of fungi possess immunoregulatory, antitumour, hypoglycaemic, antihyperlipidaemic and antioxidant activities [8]. Agrocybe cylindracea, one of the most precious edible and medicinal mushrooms industrially cultivated in China, contains high nutritional values and an attractive flavour. Recent studies have revealed that A. cylindracea has beneficial physiological activities, such as antitumour, anti-fungal, nerve tonic, lipid peroxidation inhibitory, anti-hypercholesterolaemia and anti-hyperlipidaemia activities [9]. However, there is a limited number of studies concerning the polysaccharide from A. cylindracea mycelia and its structure-function relationship.
Selenium, one of the essential trace elements in the human body, is involved in the synthesis of at least 30 antioxidant enzymes, particularly glutathione peroxidases (GSH-Px) [10]. Reflecting the low content of selenium in nature and common foods, selenium deficiency diseases are abundant worldwide. Therefore, there is a considerable demand for complementary and alternative medicines for the treatment of selenium deficiency. Interestingly, organic selenium generated through the biotransformation of mushrooms has received increasing attention as a result of its high bioavailability and low toxicity [11,12]. Because these mushrooms have higher bioaccumulation, the selenium content in mushroom-derived products can be improved through artificial cultivation on growth substrates with inorganic selenium. Although many reports have focused on the cultivation of fruit bodies [9,13,14], this strategy is time consuming and expensive. Although previous studies have reported that the bioaccumulation ability of fungi is species-specific and element-dependent [15], there are few studies concerning the combination of selenium with polysaccharides in mushrooms and the biological activities of selenium-polysaccharides.
In the present study, the conditions for purifying mycelia selenium polysaccharides (MSPS) from A. cylindracea were optimized using a Box-Behnken experimental design (BBD), a mathematical model that represents the relationship between the response and variables. The anti-ageing effects were analysed in vitro and in vivo, and the selenium accumulation rate, selenium content and monosaccharide composition were also processed.
Experimental methods
Chemicals and reagents
DEAE-52 cellulose, hydrogen peroxide (H2O2), ferrozine, 1,1-diphenyl-2-picrylhydrazyl (DPPH) and standard monosaccharides were purchased from Sigma Chemicals Company (St. Louis, USA). All other chemicals and reagents were analytical grade and purchased from local chemical suppliers in China.
Fungal strains and culture conditions
The fungus strain A. cylindracea SL-02 was provided from Shandong Agricultural University and maintained on potato dextrose agar (PDA) slants (potato 200 g/L, dextrose 20 g/L, agar 20 g/L, MgSO4 1 g/L and KH2PO4 1.5 g/L). Liquid fermentation technology was used to produce A. cylindracea mycelia. After 14 days at 25°C, the A. cylindrace in Petri dishes was inoculated into 500 mL Erlenmeyer flasks containing 250 mL of medium and incubated on a rotary shaker at 120 rpm for 10 days at 25°C.
Optimization of the Na2SeO3-concentration
Different concentrations of sodium selenite (Na2SeO3) (2, 4, 6 and 8 mg/L) were added to the substrates. After incubation, the mycelia of A. cylindracea were collected and weighed to obtain the best concentration. The mycelia were filtered and washed three times with deionized water, followed by constant drying at 50°C to measure the biomass (g/L). The mycelia (0.1 g) were nitrified through the addition of 2 mL of perchloric acid and 8 mL of nitric acid at room temperature for 12 h. The final 2 mL of nitrification liquor, determined through continuous heating, was mixed with 23 mL of double-distilled water for further flame atomic absorption spectrometry analysis (FAAS, nov AA® 300, Analytik Jena AG, Jena, Germany).
BBD optimization for MSPS extraction
Three parameters that significantly affect MSPS yields, including pH, extraction temperature and precipitation time, were selected for optimization through BBD. The test factors were coded according to the following equation:
| (1) |
where xi and Xi represent the coded and actual values of independent variables, X0 is the actual value of the independent variable at the centre point and ΔXi is the step change value. To correlate the response variable to the independent variable, the following quadratic polynomial equation was applied to fit the response variable to a quadratic model:
| (2) |
where Y is the predicted response value; β0, βi, βii and βij represent the intercept, linear, squared and interaction term, respectively; and xi and xj represent the coded levels of independent variables.
Preparation of mycelia polysaccharides
The mycelia polysaccharides (MPS) and mycelia selenium polysaccharides (MSPS) were prepared as previously reported [16]. After washing three times with deionized water, the homogeneous mycelia powder was dried to constant weight at 50°C, pulverized using a mill and sieved through a 200-mesh screen. The MPS and MSPS were extracted in a water bath under the optimization conditions described above. Subsequently, the supernatant was centrifuged (3000 rpm, 10 min), concentrated and ethanol precipitated (1:4, v/v) at 4°C overnight. The polysaccharide precipitates were collected after centrifugation (3000 rpm, 10 min) and quantified using a phenol-sulfate method [17]. After lyophilization, the MPS and MSPS were collected for further analysis.
Determination of monosaccharide composition
The monosaccharide composition was determined through gas chromatography (GC) (GC-2010, Shimadzu, Japan) on an Rtx-1 capillary column (30 m × 0.25 mm × 0.25 μm) according to Sheng et al. [18], with slight modifications. Briefly, the samples were hydrolysed with trifluoroacetic acid (TFA, 2 M, 110°C) for 4 h. After acetylation with hydroxylamine hydrochloride and pyridine, the hydrolysed supernatant (1 μL) was injected onto a column equipped with a flame ionization detector. Sugar identification was confirmed through comparison with standard monosaccharides of mannose, rhamnose, glucose, galactose, arabinose, D-ribose, xylose and inositol. The relative molar ratios were calculated using an area normalization method according to the chromatogram.
Experiment of antioxidant properties in vitro
Scavenging assay of DPPH
The DPPH scavenging activity was determined according to Sun and Ho [19]. The reaction mixture contained DPPH-ethanol (2 mL, 0.1 mM) and sample (2 mL, 0–3000 mg/L). After shaking vigorously and incubating in the dark for 30 min, the absorbance was measured at 517 nm against a mixture of ethanol (2 mL) and distilled water (2 mL) as a blank.
The DPPH scavenging ability was expressed as:
| (3) |
Where A is the absorbance of the tested sample, and A0 is the absorbance of the blank.
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity was determined according to Smirnoff and Cumbes [20], with some modifications. The H2O2 (1 mL, 8.8 mM) was added to initiate the reaction containing FeSO4 (1 mL, 9 mM), sodium salicylate-ethanol (1 mL, 9 mM) and sample (1 mL, 0–3000 mg/L) at 37°C for 0.5 h. After centrifugation (3000 rpm, 10 min), the absorbance was measured at 510 nm, with distilled water as a blank. For the control group, an equal amount of distilled water replaced the sample.
The hydroxyl radical scavenging activity was expressed as:
| (4) |
where A0 is the absorbance of the control group, and A1 is the absorbance of the sample.
Reducing power assay
The reducing power of MPS and MSPS was measured according to Oyaizu [21], with slight modifications. The reaction mixtures, containing 1 mL sample (0–3000 mg/L), 2.5 mL phosphate buffer (pH 6.6, 0.2 M) and 1 mL potassium ferricyanide (1%, w/v), were incubated at 50°C for 20 min and terminated after adding 2 mL trichloroacetic acid (10%, w/v). After centrifugation (1200 rpm, 10 min), the supernatant was collected and incubated with ferric trichloride (0.1%, 1.2 mL) for 15 min at room temperature. The absorbance was measured at 700 nm using distilled water as a blank.
Anti-ageing in vivo experiments
Sixty Kunming mice (20 ± 2 g) were purchased from Taibang Biological Products Ltd. Co. (Taian, China), and the animal experiments were approved through the institutional animal care and use committee of Shandong Agricultural University in accordance with the Animals (Scientific Procedures) Act of 1986 (amended 2013). The mice were acclimatized for 7 d under controlled conditions (20–25°C, lights on 12 h daily) with diet and water ad libitum. All mice were randomly allocated into three control groups: normal (NC, n = 10), model (MC, n = 10) and test (n = 40) groups. The test group was further randomly and equally divided into low-dose (200 mg/kg) groups of MPS and MSPS (LM, LS) and high-dose (600 mg/kg) groups of MPS and MSPS (HM, HS). The normal and test groups were treated with 0.2 mL of distilled water through gastric gavage, and the test group was gavaged with 0.2 mL of different polysaccharides daily. Simultaneously, the normal group was administered physiological saline, and other groups were administered D-galactose (D-gal) (150 mg/kg) through intraperitoneal injection. After 20 days, the mice were fasted overnight and sacrificed through exsanguination under diethyl ether anaesthesia. The blood samples were obtained from the orbital sinus and centrifuged at 14000 rpm (4°C, 10 min) to afford the required serum. The livers were rapidly removed, weighed and immediately homogenized (1:9, w/v) in phosphate-buffered solutions (0.2 M, pH 7.4, 4°C). After centrifugation (5000 rpm, 4°C) for 20 min, the supernatants were collected for further biochemical analysis.
The GSH-Px activity, total antioxidant capacity (T-AOC), malonaldehyde (MDA) and total cholesterol (CHOL) contents in the liver and superoxide dismutase (SOD) activity in the serum were assayed using commercially available diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
All experiments were performed in triplicate, and the results are presented as the means ± standard deviation (SD). The results were analysed using one-way analysis of variance (ANOVA) with the IBM SPSS Statistical software package programme. P < 0.05 was considered statistically significant.
Results and Discussion
Determination of the Na2SeO3 concentration
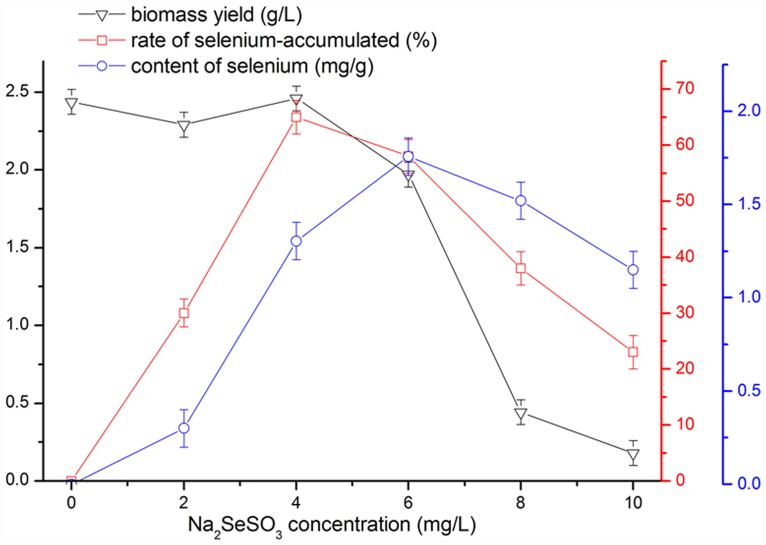
The Na2SeO3 concentration in the liquid medium was optimized and defined according to three indices: biomass yield, selenium accumulation rate and selenium content. As depicted in Fig 1, the three indices all presented increasing trends with increasing Na2SeO3 concentrations, peaking at 2.46 ± 0.08 g/L, 65.00 ± 3.00% and 1.76 ± 0.10 mg/g at the concentration of 4, 4 and 6 μg/mL, respectively. However, the trends were sharply reversed with increasing concentrations, potentially associated with mycotoxicity at high concentrations of elements, resulting in the inhibition of mycelial growth [22,23]. To determine the highest amount of selenium in the mycelia, the optimal Na2SeSO3 concentration of 6 μg/mL was used, and the biomass yield, selenium accumulation rate and selenium content were 1.97 ± 0.07 g/L, 58.00 ± 3.00%, and 1.76 ± 0.10 mg/g, respectively.
Fig 1. The biomass yield, selenium content and selenium accumulation rate of MSPS at different Na2SeO3 concentrations.
BBD optimization of MSPS extraction
The BBD matrix and the experimental and predicted MSPS data are shown in Table 1, and the results of the ANOVA analysis are shown in Table 2. Using a multiple regression analysis, the polynomial model for the empirical relationship between the response and variables was expressed as
| (5) |
where Y is the predicted response (Yields of MSPS, %), and x1, x2, and x3 represent the coded test variables for pH, extraction temperature (°C), and precipitation temperature (°C), respectively.
Table 1. Experimental and predicted values of MSPS based on central composite.
| Run | X1 a | X2 b | X3 c | MSPS yield (%) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | -1 (5) | -1 (75) | 0 (8) | 6.10 ± 0.23 | 6.21 |
| 2 | 1 (9) | -1 | 0 | 7.35 ± 0.31 | 7.39 |
| 3 | -1 | 1 (95) | 0 | 8.60 ± 0.25 | 8.56 |
| 4 | 1 | 1 | 0 | 9.93 ± 0.32 | 9.82 |
| 5 | -1 | 0 (85) | -1 (4) | 8.39 ± 0.28 | 8.31 |
| 6 | 1 | 0 | -1 | 8.81 ± 0.26 | 8.80 |
| 7 | -1 | 0 | 1 (12) | 8.38 ± 0.33 | 8.39 |
| 8 | 1 | 0 | 1 | 10.26 ± 0.35 | 10.34 |
| 9 | 0 (7) | -1 | -1 | 7.31 ± 0.28 | 7.28 |
| 10 | 0 | 1 | -1 | 9.71 ± 0.31 | 9.83 |
| 11 | 0 | -1 | 1 | 8.37 ± 0.35 | 8.25 |
| 12 | 0 | 1 | 1 | 10.44 ± 0.34 | 10.48 |
| 13 | 0 | 0 | 0 | 8.98 ± 0.31 | 8.86 |
| 14 | 0 | 0 | 0 | 8.81 ± 0.28 | 8.86 |
| 15 | 0 | 0 | 0 | 8.89 ± 0.26 | 8.86 |
| 16 | 0 | 0 | 0 | 8.81 ± 0.24 | 8.86 |
| 17 | 0 | 0 | 0 | 8.81 ± 0.29 | 8.86 |
a: pH
b: Extraction temperature (°C)
c: Precipitation temperature (°C)
Table 2. ANOVA for the evaluation of the quadratic model.
| Source | Coefficients | S.E. | Sum of squares | Mean square | F-value | P |
|---|---|---|---|---|---|---|
| Model | - | - | 18.92 | 2.1 | 157.69 | <0.0001 |
| Intercept | 8.86 | 0.052 | - | - | - | - |
| x1 | 0.61 | 0.041 | 2.98 | 2.98 | 223.58 | <0.0001 |
| x2 | 1.19 | 0.041 | 11.4 | 11.4 | 855.36 | <0.0001 |
| x3 | 0.4 | 0.041 | 1.3 | 1.3 | 97.69 | <0.0001 |
| x1x2 | 0.021 | 0.058 | 1.71E-03 | 1.71E-03 | 0.13 | 0.7306 |
| x1x3 | 0.36 | 0.058 | 0.53 | 0.53 | 39.79 | 0.0004 |
| x2x3 | -0.083 | 0.058 | 0.027 | 0.027 | 2.06 | 0.1948 |
| x12 | -0.43 | 0.056 | 0.79 | 0.79 | 59.41 | 0.0001 |
| x22 | -0.43 | 0.056 | 0.79 | 0.79 | 59.41 | 0.0001 |
| x32 | 0.53 | 0.056 | 1.2 | 1.2 | 90.29 | <0.0001 |
| Lack-of-fit | 0.072 | 0.024 | 4.19 | 0.1001 | ||
| Residual | 0.094 | 0.013 | ||||
| Pure error | 0.023 | |||||
| Cor total | 18.99 | |||||
| Mean | 8.7 | |||||
| c.v.% | 1.33 | |||||
| Adeq Precision | 47.837 | |||||
| R-squared | 0.995 | |||||
| Adj R-squared | 0.9886 | |||||
| Pred R-squared | 0.9378 |
The results of the ANOVA, a statistical technique used to subdivide the total variations into component parts associated with specific sources of variation to examine hypotheses on the parameters, are shown in Table 2. The linear term regression coefficients (x1, x2, and x3), quadratic coefficients (x12, x22, and x32) and interaction coefficient (x1x3 and x2x3) were significant at the 1% level, indicating that pH, extraction temperature and precipitation temperature were all significantly correlated with the MSPS yield. The large model F-value (157.69) and the low Lack-of-Fit F-value (4.19) suggest that most of the variation in MSPS yield reflects this regression equation, demonstrating that the developed quadratic models were significant to predict the MSPS yield.
In addition, the variance analysis, including the mean, coefficient of variation (C.V., %), Adeq precision, R-squared, Adj R-squared and pred R-squared values were calculated to assess the adequacy and accuracy of the developed models. The R-squared value showed the proportion of the total variation in the response predicted using the model. A high R-squared value of 0.9950 in the present study ensured satisfactory fitness to represent the actual relationship between the responses and the variables. The Adj R-squared and pred R-squared values represented the amount of variation around the mean explained by the model, adjusting for the number of terms in the model. The current Adj R-squared and pred R-squared values indicated that the selected terms significantly contributed to the model, and almost 93.78% of the variability in predicting new observations in the design space could be explained in this model. Furthermore, as a significant method to measure the unexplained or residual variability of the data as a percentage of the mean of the response variable, a low C.V.% value of 1.33 in the present study indicated a high degree of precision and a good deal of experimental values reliability [24,25]. In conclusion, the model equation was appropriate to predict the MSPS yield under any combination of values.
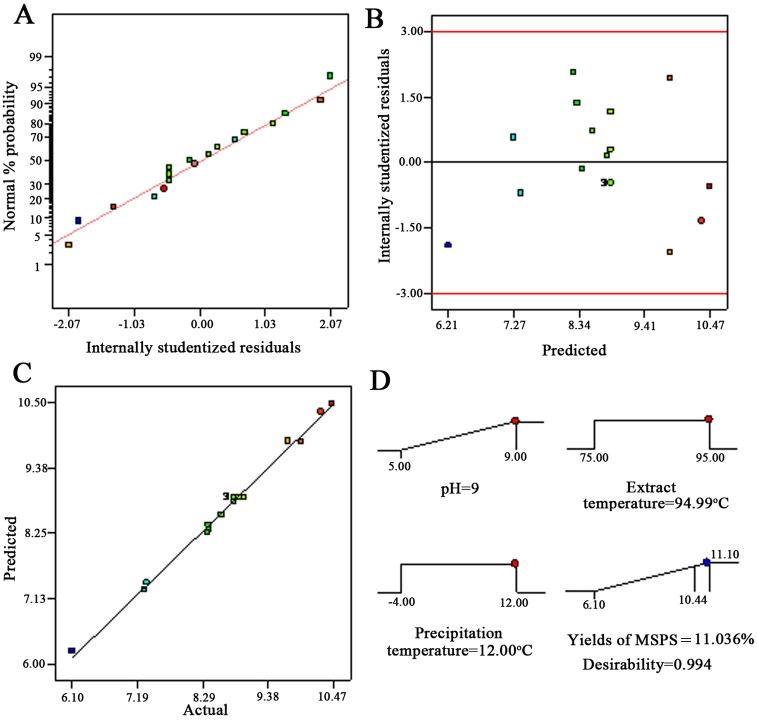
The adequacy of the model was also evaluated after inspecting the diagnostic plots of the residuals, residuals vs. predicted and predicted vs. actual values (Fig 2). The data were analysed to assess the normality of the residuals to the determination coefficient. Hifney et al. [25] showed that residuals following a normal distribution should form a straight line when the values are fitness under the theorized model. As shown in Fig 2(A), these valued formed a straight line, and the normal plot of residuals for MSPS yields was normally distributed, indicating no deviation of the variance. No clear patterns were observed in the residuals vs. predicted plot, validating the initial assumption of constant variance (Fig 2(B)). In addition, the predicted vs. actual values plots also showed excellent agreement (Fig 2(C)). Hence, the adequacy of the present model was well established.
Fig 2. Diagnostic plots for the Box-Behnken model adequacy.
(A) Normal plot of residuals, (B) plot of internally studentized residuals vs. predicted response, (C) plot of internally studentized residuals vs. actual and (D) desirability ramp plot for optimization.
By solving Eq 5, the optimal conditions for obtaining the maximum MSPS yield (11.036 ± 0.31%) were pH of 9, 94.99°C extraction temperature and 12°C precipitation temperature, and the ramp desirability figure showed 0.994 desirability, a value close to 1, indicating that this model could make significant contributions to an economically advantageous factor for extracting MSPS.
Monosaccharide composition
The HPLC chromatograms of MSPS and MPS are shown in Fig 3. Glucose was the major component of all polysaccharides. MSPS comprised rhamnose, arabinose, mannose, glucose and galactose at a molar ratio of 29:3:1:18.8:2.7, while MPS comprised rhamnose, arabinose, mannose and glucose at a molar ratio of 29.2:1.8:3:4. These results showed that the major monosaccharide component in MSPS and MPS was glucose, and galactose was present only in MSPS, indicating that both glucose and galactose could maintain the antioxidant activities of polysaccharides. Capek et al. reported similar results, showing that galactose had superior abilities of enhancing antioxidant activities [26].
Fig 3. HPLC chromatograms of polysaccharides.
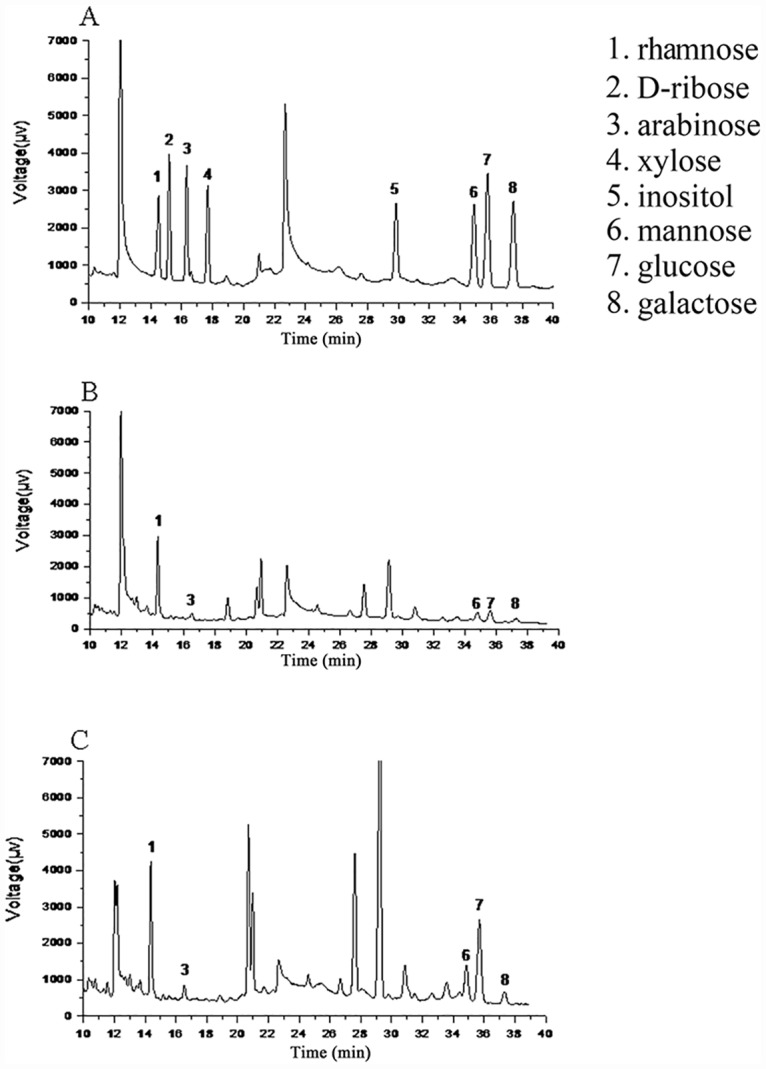
(A) Standard, (B) MSPS, and (C) MPS.
Antioxidant properties in vitro
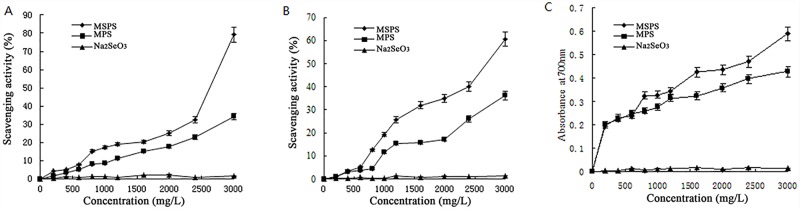
Previous studies have reported that microelements potentially enhance the abilities of biomacromolecules, such as polysaccharides for scavenging radicals [27] and proteins for antioxidation [10]. Because free radicals are the major cause of ageing, antioxidant scavenging could be an indicator of anti-ageing activities. Selenium is an essential dietary trace element that plays an important role in a number of ageing processes, and a recent widely publicized study showed that selenium supplements delay senescence [28,29]. The aim of the present study was to evaluate the absorbance of selenium by fungi and the anti-ageing properties of selenium-polysaccharide. As depicted in Fig 4, three typical indices were selected to determine the antioxidant abilities of MSPS and MPS using Na2SeO3 as the control material.
Fig 4. Antioxidant capacities of the polysaccharides in vitro.
(A) DPPH radical-scavenging activity, (B) hydroxyl radical-scavenging activity and (C) reducing power.
DPPH, a relatively stable radical widely used to investigate the scavenging activity of some antioxidants, can accept an electron or hydrogen atom to become a stable diamagnetic molecule [30]. The radical form of DPPH could be scavenged by an antioxidant into a non-radical DPPH form, thereby reducing the absorbance [31]. As shown in Fig 4(A), the scavenging ability of MSPS reached 79.13 ± 0.23% at 3000 mg/L, which was 131.6% higher than the MPS concentration. The effect of MSPS on DPPH scavenging was better than that of the hot water A. cylindracea extracts [32].
Hydroxyl radical (HO·), a natural by-product, can attack biological molecules, such as lipids, proteins, enzymes, DNA and RNA, leading to cell or tissue injury associated with degenerative diseases [33,34]. As illustrated in Fig 4(B), the scavenging ability of MSPS reached 60.54% (67.3% higher than that of MPS), indicating that MSPS has potential antioxidant abilities in vitro.
Reducing agents might serve as significant indicators of potential antioxidant activity [35]. As shown in Fig 4(C), the reducing power of MPS and MSPS exhibited a dose-dependent effect. The reducing power of MSPS reached 0.61 ± 0.09 at 3000 mg/L, which was 69.4% higher than that of MPS, indicating that MSPS had better potential antioxidant properties than MPS in vitro.
In addition, as an inorganic compound, Na2SeO3 showed scarce antioxidant abilities at any concentration, demonstrating the prominent roles of fungi in the biotransformation of elements.
Anti-ageing abilities
As selenium has a narrow range between dietary deficiency (< 40 μg/day) and toxic levels (> 400 μg/day), the current recommended daily dietary intake of selenium for humans is 57 μg/d [36]. Although the demand for selenium is low, the content of this element in food is typically insufficient, and the chemical form of selenium is limited. For the mice used in the present study, the daily dietary intake of polysaccharides was 600 and 200 mg/kg.
D-gal has been widely used to induce age-related damage in rodents, based on the production of free radicals and the acceleration of senescence [37]. The natural ageing process in humans has been associated with free radicals, which severely damage adjacent biomolecules, such as proteins, DNA, fatty acids and nucleic acids. The pathogenesis of ageing through D-gal is oxidative damage. Antioxidants might play an important role in preventing free radical damage associated with ageing, interfering directly in the generation or scavenging of radicals. Previous studies have indicated that mushroom polysaccharides, as antioxidants, can limit the degree of ROS [38]. Furthermore, the co-production of selenium and other antioxidants show antioxidant effects on radicals and lipid peroxidation production [39]. Therefore, we examined the co-effect of selenium and mycelia polysaccharides on anti-ageing.
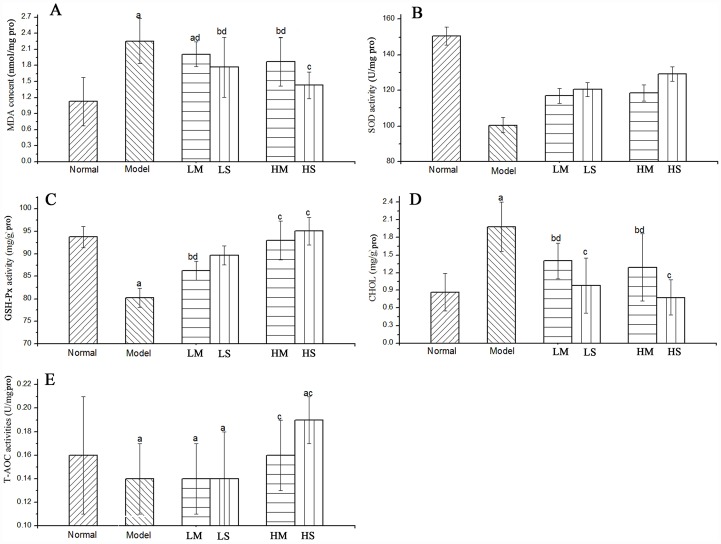
As shown in Fig 5(A), compared with the model control group, the MDA contents in LS, HS, LM and HM in the liver were reduced 21.7%, 36.7%, 11.1% and 17.3% in dose-dependent patterns at the tested concentrations, respectively. The SOD activities in LS, HS, LM and HM were increased 20.0%, 28.6%, 16.5% and 18.1%, respectively, compared with the model group. The GSH-Px activities of HS were significantly increased 5.89% compared with HM, indicating that MSPS had higher activities than MPS. The liver cholesterol levels (Fig 5(D)) in LS, HS, LM and HM were 50.5%, 60.6%, 29.3% and 34.8% lower, respectively, than in the model group. As shown in Fig 5(E), neither LM nor LS showed differences from the model group in the T-AOC index; however, the levels of T-AOC in HM and HS were higher than in the model control group and increased in a dose-dependent manner. HS and HM were 35.7% and 14.3% higher, respectively, than the model group. HS was 18.8% higher than the normal group, showing that MSPS has stronger antioxidant activity.
Fig 5. Hepatic parameters of (A) MDA content, (C) GSH-Px activity, (D) CHOL level and (E) T-AOC activity and serum parameters of (B) SOD activity a p < 0.01 compared with the normal control group.
The overproduction of radicals induced through D-gal increased the lipid peroxide levels and decreased enzyme activity, preventing lipid peroxidation in the tissues. For the analysis of lipid peroxide, MDA, an indicator of oxidative stress, is the main decomposition product of peroxides derived from polyunsaturated fatty acids, which determines the lipid peroxidation levels [40]. A significant increase in the MDA content (Fig 5(A)) was observed in the D-gal model control group compared with the normal control group, indicating that high-dose MSPS could relieve the mice undergoing D-gal treatment. For the analysis of enzymatic reactions, SOD is the first and most important antioxidant enzyme defence system against oxidative stress, converting the superoxide radical to H2O2 through GSH-Px [41,42]. In addition, SOD catalyses the dismutation of superoxide radicals into oxygen and hydrogen peroxide, thereby participating with other antioxidant enzymes in the enzymatic defence against oxidative injury [43]. GSH-Px, with selenium as an essential factor, is located in the cytosol of most cells and is responsible for the reduction of hydro and organic peroxides during senescence. Selenium plays an antioxidant role with GSH-Px, as this co-factor reduces hydrogen peroxides, lipids and phospholipid hydroperoxides [44]. Accordingly, T-AOC represents an original enzymatic and non-enzymatic antioxidant in mice. Compared with the normal group, a significant decrease of T-AOC was observed in the model group, indicating that that the model was successfully established. Cholesterol is a waxy, fat-like substance naturally occurring in all parts of the body; when in excess, cholesterol can cause heart disease. In summary, these observations indicate that MSPS has anti-ageing activity and could significantly counteract increased oxidative stress through the promotion of enzymatic and non-enzymatic antioxidant activities, thereby reducing levels of lipid peroxides.
Conclusion
The Na2SeO3 concentration in the liquid medium used to produce MSPS of Agrocybe cylindracea SL-02 was determined, and BBD was a successful tool for optimizing the extraction MSPS. In addition, MSPS exhibited anti-ageing activities in vivo and antioxidant activities in vitro. In summary, the selenium-enriched mycelia of A. cylindracea represent a novel dietary source of bioavailable supplemental selenium.
Supporting Information
(DOCX)
Acknowledgments
Thanks are due to Taibang Biologic products, Inc. for providing the mice and The Central hospital of Taian for providing the places for animal experiments.
Abbreviations
- ANOVA
Analysis of variance
- BBD
Box-Behnken experimental design
- CHOL
Total cholesterol
- D-gal
D-galactose
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- GC
Gas chromatography
- GSH-Px
Glutathione peroxidase
- HO
Hydroxyl radical
- H2O2
Hydrogen peroxide
- MDA
Malonaldehyde
- MPS
Mycelia polysaccharide
- MSPS
Mycelia selenium polysaccharides
- MC
Model control group
- NC
Normal control group
- Na2SeO3
Sodium selenite
- PDA
Potato dextrose agar
- ROS
Reactive oxygen species
- SD
Standard deviation
- SOD
Superoxide dismutases
- T-AOC
Total antioxidant capacity
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by Mushroom Technology System of Shandong Province (SDAIT-07-05), Le Jia, Presenter.
References
- 1.Farage MA, Miller KW, Elsner P, Maibach HI. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008;20: 195–200. 10.1007/BF03324769 [DOI] [PubMed] [Google Scholar]
- 2.Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96: 291–302. 10.1016/S0092-8674(00)80567-X [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Prakash A, Dogra S. Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. J Asian Nat Prod Res. 2011;13: 42–55. 10.1080/10286020.2010.544253 [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11: 298–300. 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 5.Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br Med Bull. 1993;49: 653–667. [DOI] [PubMed] [Google Scholar]
- 6.Zhou YC, Zheng RL. Phenolic compounds and an analog as superoxide anion scavengers and antioxidants. Biochem Pharmacol. 1991;42: 1177–1179. 10.1016/0006-2952(91)90251-Y [DOI] [PubMed] [Google Scholar]
- 7.Li B, Liu S, Xing R, Li K, Li R, Qin Y, et al. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr Polym. 2013;92: 1991–1996. 10.1016/j.carbpol.2012.11.088 [DOI] [PubMed] [Google Scholar]
- 8.Ye M, Chen WX, Qiu T, Yuan RY, Ye YW, Cai JM. Structural characterisation and anti-ageing activity of extracellular polysaccharide from a strain of Lachnum sp. Food Chem. 2012;132: 338–343. 10.1016/j.foodchem.2011.10.087 [DOI] [PubMed] [Google Scholar]
- 9.Kim HO, Lim JM, Joo JH, Kim SW, Hwang HJ, Choi JW, et al. Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea. Bioresour Technol. 2005;96: 1175–1182. 10.1016/j.biortech.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 10.Maseko T, Callahan DL, Dunshea FR, Doronila A, Kolev SD, Ng K. Chemical characterisation and speciation of organic selenium in cultivated selenium-enriched agaricus bisporus. Food Chem. 2013;141: 3681–3687. 10.1016/j.foodchem.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Luo XY, Wu Y, Chen YM, Xu WJ, Yi XX. Optimization of submerged fermentation of Thelephora ganbajun zang. J Basic Microbiol. 2014;54: 866–872. 10.1002/jobm.201200757 [DOI] [PubMed] [Google Scholar]
- 12.Falandysz J. Review: on published data and methods for selenium in mushrooms. Food Chem. 2013;138: 242–250. 10.1016/j.foodchem.2012.10.046 [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Tsai S, Mau J. Antioxidant properties of methanolic extracts from Agrocybe cylindracea. LWT Food Sci Technol. 2006;39: 379–387. 10.1016/j.lwt.2005.02.012 [DOI] [Google Scholar]
- 14.Tsai S, Tsai H, Mau J. Antioxidant properties of agaricus blazei, Agrocybe cylindracea, and boletus edulis. LWT Food Sci Technol. 2007;40: 1392–1402. 10.1016/j.lwt.2006.10.001 [DOI] [Google Scholar]
- 15.Thomet U, Vogel E, Krähenbühl U. The uptake of cadmium and zinc by mycelia and their accumulation in mycelia and fruiting bodies of edible mushrooms. Eur Food Res Technol. 1999;209: 317–324. 10.1007/s002170050502 [DOI] [Google Scholar]
- 16.Gan D, Ma L, Jiang C, Xu R, Zeng X. Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis Bi. Carbohydr Polym. 2011;84: 997–1003. 10.1016/j.carbpol.2010.12.058 [DOI] [Google Scholar]
- 17.Chaplin MF, Kennedy JF. Carbohydrate analysis: A practical approach. New York: IRL Press Ltd; 1994. [Google Scholar]
- 18.Sheng J, Yu F, Xin Z, Zhao L, Zhu X, Hu Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007;105: 533–539. 10.1016/j.foodchem.2007.04.018 [DOI] [Google Scholar]
- 19.Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90: 743–749. 10.1016/j.foodchem.2004.04.035 [DOI] [Google Scholar]
- 20.Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28: 1057–1060. 10.1016/0031-9422(89)80182-7 [DOI] [Google Scholar]
- 21.Oyaizu M. Studies on product of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J Nutr. 1980;44: 307–315. [Google Scholar]
- 22.Figlas D, Oddera M, Curvetto N. Bioaccumulation and bioavailability of copper and zinc on mineral-enriched mycelium of Grifola frondosa. J Med Food. 2010;13: 469–475. 10.1089/jmf.2008.0284 [DOI] [PubMed] [Google Scholar]
- 23.da Silva MCS, Naozuka J, da Luz JMR, de Assunção LS, Oliveira PV, Vanetti MCD. Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks. Food Chem. 2012;131: 558–563. 10.1016/j.foodchem.2011.09.023 [DOI] [Google Scholar]
- 24.Wu Z, Li H, Yang Y, Tan H. Ultrasonic extraction optimization of L. macranthoides polysaccharides and its physicochemical properties. Int J Biol Macromol. 2015;74: 224–231. 10.1016/j.ijbiomac.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 25.Hifney AF, Fawzy MA, Abdel-Gawad KM, Gomaa M. Industrial optimization of fucoidan extraction from sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocolloids. 2016;54: 77–88. 10.1016/j.foodhyd.2015.09.022 [DOI] [Google Scholar]
- 26.Capek P, Machová E, Turjan J. Scavenging and antioxidant activities of immunomodulating polysaccharides isolated from Salvia officinalis L. Int J Biol Macromol. 2009;44: 75–80. 10.1016/j.ijbiomac.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 27.Zheng L, Liu M, Zhai GY, Ma Z, Wang LQ, Jia L. Antioxidant and anti-aging activities of mycelia zinc polysaccharide from Pholiota nameko SW-03. J Sci Food Agric. 2014;95: 3117–3126. 10.1002/jsfa.7048 [DOI] [PubMed] [Google Scholar]
- 28.Balaban H, Nazıroğlu M, Demirci K, Övey İS. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2016. March 28 10.1007/s12035-016-9835-0 [DOI] [PubMed] [Google Scholar]
- 29.Jain RB, Choi YS. Normal reference ranges for and variability in the levels of blood manganese and selenium by gender, age, and race/ethnicity for general U.S. population. J Trace Elem Med Bio. 2015;30: 142–152. 10.1016/j.jtemb.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 30.Kacuráková M, Capek P, Sasinkova V, Wellner N, Ebringerova A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2000;43: 195–203. 10.1016/S0144-8617(00)00151-X [DOI] [Google Scholar]
- 31.You Q, Yin X, Zhang S, Jiang Z. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr Polym. 2014;99: 1–10. 10.1016/j.carbpol.2013.07.088 [DOI] [PubMed] [Google Scholar]
- 32.Tsai S, Huang S, Mau J. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006;98: 670–677. 10.1016/j.foodchem.2005.07.003 [DOI] [Google Scholar]
- 33.Dong CH, Yao YJ. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. LWT Food Sci Technol. 2008;41: 669–677. 10.1016/j.lwt.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye CL, Huang Q. Extraction of polysaccharides from herbal Scutellaria barbata D Don (Ban-Zhi-Lian) and their antioxidant activity. Carbohydr Polym. 2012;89: 1131–1137. 10.1016/j.carbpol.2012.03.084 [DOI] [PubMed] [Google Scholar]
- 35.Soares JR, Dinis TC, Cunha AP, Almeida LM. Antioxidant activities of some extracts of thymus zygis. Free Radic Res. 1997;26: 469–478. 10.3109/10715769709084484 [DOI] [PubMed] [Google Scholar]
- 36.Falandysz J, Borovička J. Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol. 2013;97: 477–501. 10.1007/s00253-012-4552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh HM, Wu WM, Hu ML. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol. 2009;47: 625–632. 10.1016/j.fct.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 38.Zhong W, Liu N, Xie Y, Zhao Y, Song X, Zhong W. Antioxidant and anti-aging activities of mycelial polysaccharides from Lepista sordida. Int J Biol Macromol. 2013;60: 355–359. 10.1016/j.ijbiomac.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 39.Naziroğlu M, Karaoğlu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195: 221–230. 10.1016/j.tox.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 40.Jayakumar T, Thomas PA, Sheu JR, Geraldine P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res Int. 2011;44: 851–861. 10.1016/j.foodres.2011.03.015 [DOI] [Google Scholar]
- 41.Xiao N, Wang XC, Diao YF, Liu R, Tian KL. Effect of initial fluid resuscitation on subsequent treatment in uncontrolled hemorrhagic shock in rats. Shock. 2004;21: 276–280. 10.1097/01.shk.0000110622.42625.cb [DOI] [PubMed] [Google Scholar]
- 42.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33: 337–349. 10.1016/S0891-5849(02)00905-X [DOI] [PubMed] [Google Scholar]
- 43.Luo A, He X, Zhou S, Fan Y, Luo A, Chun Z. Purification, composition analysis and antioxidant activity of the polysaccharides from dendrobium nobile Lindl. Carbohydr Polym. 2010;79: 1014–1019. 10.1016/j.carbpol.2009.10.033 [DOI] [Google Scholar]
- 44.Rayman MP. The importance of selenium to human health. Lancet. 2000;356: 233–241. 10.1016/S0140-6736(00)02490-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.