Abstract
Background
The retinal pigment epithelium serves as a defensive barrier to the retina in the same way that the choroid plexus functions in the brain. Previous studies have shown that lead sequestration in the choroid plexus reduces the production and secretion of transthyretin by the choroid plexus. The purpose of this study was to investigate the distribution of lead and transthyretin in human eyes and to explore the potential effect of lead on transthyretin in human eyes.
Methods
Eight pairs of human eyes were obtained from the New York City Eyebank within 24 hours of postmortem. The eyes were dissected to obtain the aqueous, vitreous, retina, and choroid. Lead and transthyretin concentrations in ocular tissues and liquids were determined by atomic absorption spectrophotometry for lead and radioimmunoassay for transthyretin. Lead accumulated in the retina at concentrations that were 166, 739, and 5 times higher than those in the aqueous, vitreous, and choroid, respectively (p < 0.05 for all comparisons). Lead concentrations in ocular tissues or intraocular liquids did not change as a function of age or gender. The concentration of transthyretin in the vitreous (14.4 ± 5.1SE μg/mL) was twice as much as in the aqueous (7.0 ± 1.1SE μg/mL) and was significantly correlated to that in the retina (r = 0.93, p < 0.005).
Conclusions
This study indicates that lead accumulates in human ocular tissues, particularly in the retina. The markedly elevated retina lead level and its relationship to ocular transthyretin and other macromolecules bear further investigation.
INTRODUCTION
Lead (Pb)-induced toxicities continue to be a major public health concern. In addition to its toxic effect on the central nervous system (CNS), Pb toxicity has been linked to renal dysfunction, hypertensive cardiovascular disease, and auditory disturbances and visual disability.1 Recent studies from a rat retinal model indicate that low level exposure to Pb produces scotopic visual deterioration, characterized by diminished rod-mediated electro-retinogram, rod and bipolar apoptotic cell death, and inhibited cyclic guanine monophosphate phosphodiesterase (cGMP PDE).2 While Pb toxicity has been linked to visual dysfunction in mammals, few studies have been performed to quantitate Pb distribution in human eyes. Even less has been learned about molecular and cellular mechanisms underlying Pb-induced visual disturbance.
The retinal pigment epithelium (RPE) serves as a defensive barrier to the retina in the same way as the choroid plexus (CP) functions to the brain.3 Our previous studies have shown that the CP sequesters Pb, resulting in reduced secretion of transthyretin (TTR) to the cerebrospinal fluid (CSF).4,5 TTR is a 55,000-Dalton protein and functions as a carrier protein for thyroid hormones, retinal, and retinol-binding protein. In the eyes, TTR plays a critical role in transporting retinol to the photo-receptor for the phototransduction process.3 However, little quantitative information is available concerning the distribution of TTR in human eyes.
This study was therefore conducted to investigate (1) whether the Pb accumulated in human eyes and what the relative distribution of Pb was throughout the aqueous, vitreous, retina, and choroid; (2) whether the Pb distribution in the eyes was age-related as is in the CP; and (3) what the distribution pattern of TTR was in human eyes, and whether this was affected by the Pb deposit in the eyes.
METHODS
Chemicals were obtained from the following sources: Ultrex HNO3 and NH4 H2 PO4 from Aldrich Chemical Co.(Milwaukee, WI); AA standards of Pb from Alfa Products (Danvers, MA); 125Iodide (specific activity: 17 mCi/μg) from Du Pont (Boston, MA); lactoperoxidase, purified human plasma TTR, and goat anti-human TTR antiserum from Sigma Chemical Co. (St. Louis, MO). All reagents were of analytical-grade, HPLC-grade, or the best available pharmaceutical-grade.
Sample Collection
Eye samples were obtained from New York City Eye-bank within 24 hours of death and immediately frozen at −80°C. For various clinical reasons, these eyes were considered inappropriate for corneal transplantation. The globes were thawed at room temperature. Aqueous aspirates of each globe were obtained through a peripheral corneal stab incision with a 25-gauge needle on a 3-cc syringe into the anterior chamber. Vitreous aspirates were collected with an 18-gauge needle on a 10-cc syringe after myringotomy blade incision over the pars plana, about 5.5 mm from the surgical limbus. The anterior segment of the globe was dissected from the posterior segment using a curved Stevens scissors and a Bishop-Harmon forceps. The retina was then detached with the pigment epithelium in its entirety from the choroid using Castroviejo 0.3 forceps. The choroid was then separated and removed from the sclera. The entire dissection procedure was completed within 15 minutes to avoid redistribution of TTR and Pb to other compartments.
Atomic Absorption Spectrophotometry (AAS) Analysis
To determine Pb in the aqueous or vitreous, samples were separately diluted with 0.7% Ultrex HNO3 containing 0.2% ammonium phosphate, in a 1:1 ratio. If necessary, the samples were further diluted after the initial analysis. For tissue Pb, portions of retina and choroid were combusted in acid-washed crucibles at 800°C for 4 hours. The crucibles were then rinsed with 200 μL of 0.5% HNO3/0.2% NH4 H2 PO4 and transferred to auto-sampler vials. The blank samples without tissues were routinely run in the same manner to ensure a Pb-free operation. Standards for all determinations were freshly prepared daily from a lead nitrate AA working stock solution in 0.5% Ultrex HNO3. Reference materials for blood Pb from the New York State Department of Health were used as internal quality control standards. A Perkin-Elmer Model 3030 Zeeman atomic absorption spectrophotometer (wavelength at 283.3 nm), equipped with an HGA-600 graphite furnace, was used for quantification.5
Radioimmunoassay (RIA) of TTR
RIA procedures for determining TTR concentrations in body fluids and tissues have been well established in Dr. Zheng’s laboratory according to the method of Blaner.4,7 The RIA for TTR employs purified human plasma TTR (both as a standard and for use as 125I-TTR) and a monospecific goat anti-human TTR antibody. A standard displacement curve was established by plotting the percentage of maximal binding of 125I-TTR with known amounts of homogeneously purified human plasma TTR for a standard dilution of anti-TTR. The purified human TTR was iodinated by the lactoperoxidase procedure as described by Blaner.7 TTR concentrations in the aqueous and vitreous were quantitated from the standard curve. To determine TTR concentrations in tissues, the retina and choroid were homogenized in 4 volumes (v/w) of RIA buffer containing 1% Triton X-100. Appropriate dilution was made to ensure the values conformed to the range of the standard curve.
Statistical Analyses
The statistical analyses were carried out by two-tailed Student t-test for single comparison and by linear regression for associations between Pb and TTR in the aqueous, vitreous, retina, and choroid, and as a function of age.
RESULTS
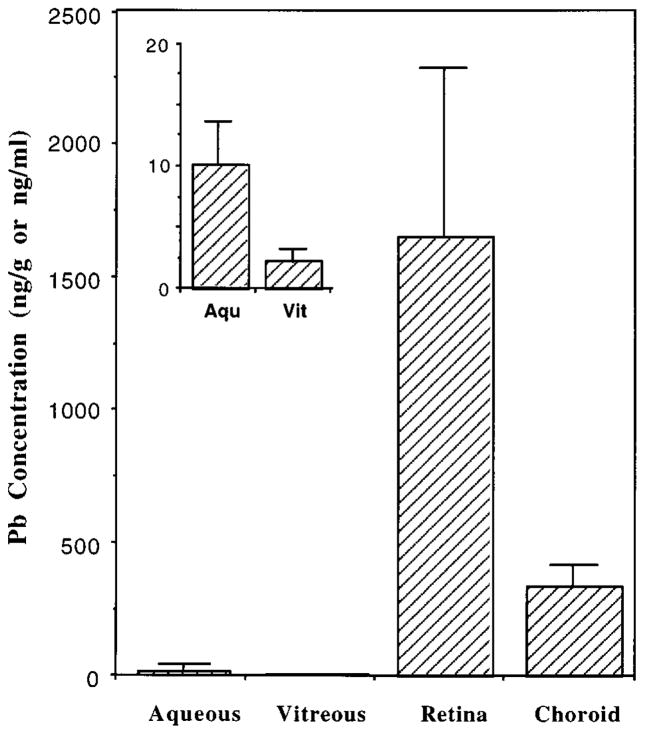
The ages of eye donors ranged from 19 to 90 years old. Table 1 summarizes the disease status of these patients at the time of death. Our results clearly demonstrated that the Pb accumulated in human eye tissues to a great extent, particularly in the retina (Figure 1). For example, the Pb concentration in the retina was 166 and 739 times greater than that in the aqueous and vitreous, respectively. Within the tissues, the retinal concentration of Pb was significantly associated with the choroid Pb (r = 0.851, p < 0.01). Moreover, the retina contained an average Pb about 5 times higher than the choroid. It is also noteworthy that the average concentration of Pb was 5 times greater in the aqueous than in the vitreous (Table 2). The extent to which the Pb distributed to the aqueous, vitreous, retina, and choroid, however, was not statistically significant in association with age. Nor was the gender difference found among these samples.
Table 1.
Characteristics of the Study Population
| Study # | Patient I.D. | Age | Sex | Causes of Death |
|---|---|---|---|---|
| 1 | 1662–97 | 74 | F | Colon cancer |
| 2 | 1642–97 | 83 | F | Congestive heart failure |
| 3 | 1583–97 | 75 | M | Prostate cancer |
| 4 | 1527–97 | 90 | F | Advanced sarcoma |
| 5 | 0187–97 | 65 | M | Lung cancer |
| 6 | 0215–97 | 31 | M | Cerebral infarction |
| 7 | 0131–98 | 19 | F | Hodgkin’s disease |
| 8 | 0157–98 | 72 | F | Diabetic ketoacidosis |
Figure 1.
Pb accumulation in human eyes. Eye samples from the residents of New York City were dissected to obtain aqueous, vitreous, retina, and choroid within 24 h of death. Samples were assayed by AAS for Ph and RIA for TTR. Data represent Mean ± SE (n = 7 − 8). *p < 0.01 compared to other tissues or fluids.
Table 2.
Distribution of Pb and TTR in Human Eyes
| Pb (ng/mL or ng/g) | TTR (μg/mL or μg/g) | |
|---|---|---|
| Aqueous | 9.97 ± 3.63 | 7.01 ± 1.08 |
| Vitreous | 2.24 ± 0.96 | 14.35 ± 5.13 |
| Retina | 1655.3 ± 640.2 | 24.97 ± 10.66 |
| Choroid | 341.8 ± 78.26 | 21.15 ± 5.81 |
Data represent Mean ± SEM (n = 7 − 8). Units for the aqueous and vitreous are expressed as ng/mL or μg/mL of ocular fluid; for retina and choroid, units are expressed as ng/g or μg/g of tissue.
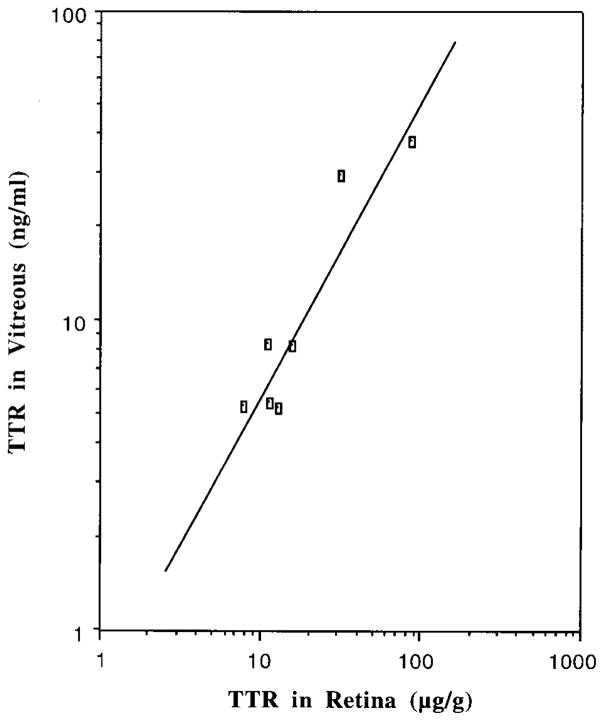
Our data corroborated that human ocular tissues and liquids contained TTR. The concentrations of TTR in the vitreous were positively correlated to those in retinal tissues (r = 0.929, p < 0.005) (Figure 2). Unlike TTR in CSF, which declines as age advances, TTR concentrations in both ocular tissues and liquids did not appear to be affected by age. This appears to be due to limited sample numbers and uneven distribution of the subjects’ age. The Pb accumulation in the retina did not seem to affect TTR concentrations in the vitreous or aqueous. Nor was there a statistical relation between Pb and TTR in the retina.
Figure 2.
Positive correlation of TTR concentrations between the retina and vitreous. A simple linear regression was used for statistic analyses. Correlation coefficient: r = 0.9291; two-tailed probability: p < 0.005.
DISCUSSION
The results of this study show that regardless of age, Pb accumulated in the human retina at considerably higher levels than the remainder of the eye. Because we do not have blood Pb information from these subjects, it is uncertain whether the high retina Pb levels in these samples reflect the gradual accumulation of Pb or perhaps an elevated current exposure. Previous studies by Fox et al.2 on animal models demonstrate that long-term Pb exposure in rats induces functional alterations in the retina. Our data further suggest that the retina has the unique capacity in accumulating Pb ions.
The subcellular ligand(s) to which Pb ions bind in the retina remain unknown. Several studies have suggested that the RPE melanosomes have a high affinity for metal ions. For example, Potts and Au8 found that RPE melanosomes were capable of binding to various metals according to the atomic weight and volume; e.g., the percent binding of calcium was 30%, zinc 37%, iron 64.5%, mercury 72%, and lead 62%. Ulshafer et al.,9 using RPE metal X-ray spectra in block human tissue, observed an accumulation of aluminum, selenium, and mercury in RPE melanosomes, although an energy dispersive pattern for Pb was not presented. Thus, it appears likely that Pb ions, upon entering the ocular circulation, may be sequestered by the cellular ligands, such as melanosomes, in the RPE. Such a sequestration may serve as the first line of defense against Pb ions from further entry into the neural retina. Quantitatively, the retina accumulates Pb to a much greater extent than does the choroid. Thus, the retina may serve as a Pb collection site, either short-term such as blood, or long-term such as bone or choroid plexus.
Accumulation of Pb in the retina may provoke the functional injury to the eyes. Clinically, Pb has been associated with vasculitis and degenerative changes in the optic nerve as well as abnormal central visual fields under mesopic, but not photopic, light adaptation.10 The similar outcomes that Pb primarily affects the rods but not cones were also observed under different experimental settings.11,12 Subcellularly, Fox and associates2,13 have linked Pb-induced retinal degeneration to decreased mitochondrial oxygen consumption as well as inhibited rod and bipolar cGMP PDE. Our earlier studies on the choroid plexus, which produces and secretes TTR to the CNS, indicate that sequestration of Pb in this blood-CSF barrier activates protein kinase C and reduces TTR levels in the CSF.4,5,14 Because the RPE is a unique site of TTR synthesis in the eye,3,6 it appears plausible that a high accumulation of Pb may influence TTR production by the retina. A disturbance in TTR homeostasis in the eyes could then contribute to the Pb-induced optical dysfunction, because TTR has been suggested as having a role in the intraocular cycling of the retinol as well as thyroxin.3,6 Our current results, however, did not reveal an association between Pb and TTR in the retina, possibly due to the small sample number. Therefore, we can not completely exclude the possible role of Pb in the distortion of ocular TTR homeostasis. A larger study population is therefore needed in order to test this hypothesis.
It is unclear as yet whether TTR in the more anterior portions of the eye is derived from the plasma or from the retina. Our results show that the distribution of TTR in the vitreous was directly correlated with that of retinal tissues (r = 0.929, p < 0.005). This tight correlation suggests a local distribution or transport of TTR from the retina to the vitreous. It is also interesting to note that the TTR levels in the vitreous were at twice the aqueous levels. Thus, a concentration gradient appears to present for TTR from retina to vitreous and to aqueous. How this gradient, by serving to deliver the useful molecules, e.g., thyroxin, help maintain metabolic activity of the thinning peripheral retina, and how the environmental insults due to toxic metal exposure alter this gradient, deserve further investigation.
Acknowledgments
We wish to thank Dr. Qiuqu Zhao for her technical assistance in radioimmunoassay of transthyretin. We are also greatly indebted to Dr. Donald A. Fox for his critical review and many helpful suggestions for this manuscript.
This manuscript was supported by National Institute of Environmental Health Sciences Grant RO1-ES08146, grants from the Department of Ophthalmology, Mount Sinai School of Medicine, and private funds donated to Joseph Eichenbaum.
Contributor Information
Joseph W. Eichenbaum, Mount Sinai School of Medicine, New York, New York
Wei Zheng, Columbia University, New York, New York.
References
- 1.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidmiologic studies of chronic toxicity: Conceptual paradigms. Env Health Persp. 1998;105:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox DA, Campbell ML, Blocker YS. Functional alterations and apoptotic cell death in the retina following developmental or adult lead exposure. Neurotoxicology. 1997;18:645–664. [PubMed] [Google Scholar]
- 3.Cavallaro T, Martone RL, Dwork AJ, Schon EA, Herbert J. The retinal pigment epithelium is the unique site of transthyretin synthesis in the rat eye. Invest Ophthal Vis Sci. 1990;31:497–501. [PubMed] [Google Scholar]
- 4.Zheng W, Shen H, Blaner WS, et al. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: The role of the choroid plexus. Toxicol Appl Pharmacol. 1996;139:445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng W, Blaner WS, Zhao Q. Inhibition by Pb of production and secretion of transthyretin in the choroid plexus: Its relationship to thyroxine transport at the blood-CSF barrier. Toxicol Appl Pharmacol. 1999;155:24–31. doi: 10.1006/taap.1998.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwork AJ, Cavallaro I, Martone RL, Goodman DS, Schon EA, Herbert J. Distribution of transthyretin in the rat eye. Invest Ophthal Vis Sci. 1990;31:489–496. [PubMed] [Google Scholar]
- 7.Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods Enzymol. 1990;189:270–281. doi: 10.1016/0076-6879(90)89298-v. [DOI] [PubMed] [Google Scholar]
- 8.Potts AM, Au PC. The affinity of melanin for inorganic ions. Exp Eye Res. 1976;22:487–491. doi: 10.1016/0014-4835(76)90186-x. [DOI] [PubMed] [Google Scholar]
- 9.Ulshafer RJ, Allen CB, Rubin ML. Distributions of elements in the human retinal pigment epithelium. Arch Ophthal. 1990;108:113–117. doi: 10.1001/archopht.1990.01070030119041. [DOI] [PubMed] [Google Scholar]
- 10.Fox DA. Sensory system alterations following occupational exposure to chemicals. In: Manzo L, Costa LG, editors. Occupational Neurotoxicology. Boca Raton: CRC Press; 1998. pp. 169–184. [Google Scholar]
- 11.Fox DA, Sillman AJ. Heavy metals affect rod, but not cone photoreceptors. Science. 1979;206:78–80. doi: 10.1126/science.314667. [DOI] [PubMed] [Google Scholar]
- 12.Bushnell PJ, Bowman RE. Scotopic vision deficits in young monkeys exposed to lead. Science. 1977;196:333–335. doi: 10.1126/science.403610. [DOI] [PubMed] [Google Scholar]
- 13.Medrano CJ, Fox DA. Oxygen consumption in the rat outer and inner retina: Light and pharmacologically-induced inhibition. Exp Eye Res. 1995;61:273–284. doi: 10.1016/s0014-4835(05)80122-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, Slavkovich V, Zheng W. Lead exposure promotes translocation of protein kinase C activity in rat choroid plexus in vitro, but not in vivo. Toxicol Appl Pharmacol. 1998;149:99–106. doi: 10.1006/taap.1997.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]