Abstract
Lead (Pb) exposure reportedly modulates PKC activity in brain endothelial preparations, which may underlie Pb-induced damage at the blood–brain barrier. Our previous work indicates that Pb accumulates in the choroid plexus and causes dysfunction of this blood–cerebrospinal fluid (CSF) barrier. The present studies were undertaken to test the hypothesis that Pb in the choroid plexus may alter PKC activity and thus affect the functions of the blood–CSF barrier. When choroidal epithelial cells in a primary culture were exposed to Pb (10 μM in culture medium), the membrane-bound PKC activity increased by 5.2-fold, while the cytosolic PKC activities decreased, an indication of the induction of PKC translocation by Pb. The effect of Pb on cellular PKC was concentration dependent in the range of 0.1–10 μM. We further evaluated PKC activity of the choroid plexus in rats chronically exposed to Pb in the drinking water (control, 50 or 250 μg Pb/ml) for 30, 60, or 90 days. Two-way analysis of variance revealed a significant age-related decline of PKC activities in both cytosol and membrane of the choroid plexus. However, Pb treatment did not alter plexus PKC activities. In addition, we found that short-term, acute Pb exposure in rats did not significantly change PKC activities nor did it affect the expression of PKC isoenzymes in the choroid plexus. Our results suggest that Pb exposure may promote the translocation of PKC from cytosol to membrane in rat blood–CSF barrier in vitro, but not in vivo.
The mammalian blood–cerebrospinal fluid (CSF) barrier resides primarily in the choroid plexus and plays a pivotal role in regulating the chemical stability of the central nervous system (Johanson, 1995; Davson and Segal, 1996). Toxic metals, especially lead (Pb), have been known to accumulate in the choroid plexus (Friedheim et al., 1983; Manton et al., 1984; O’Tuama et al., 1976; Zheng et al., 1991; Zheng, 1996). Our recent work revealed that accumulation of Pb in the choroid plexus is associated with a significant reduction in CSF concentrations of transthyretin (TTR), a major thyroxine-carrying protein in brain (Zheng et al., 1996). We postulated that Pb accumulation in the choroid plexus might also alter key enzymes that are apparently targeted by cellular Pb toxicity, such as the protein kinase C (PKC).
This hypothesis was based on two sets of findings: (1) PKC plays a critical role in transduction of cellular signals, in regulation of membrane functions, and in control of cell proliferation (Gamard et al., 1994; Nishizuka, 1986, 1992; Stabel and Parker, 1991); and (2) low concentrations of Pb exposure modulate PKC activity in the blood–brain barrier and other tissues (Markovac and Goldstein, 1988a,b; Long et al., 1994). PKC represents a family of Ca2+- and phospholipid-dependent protein kinases that catalyze the transfer of the γ-phosphate of ATP to phosphoacceptor serine or threonine residues of protein and peptide substrates. PKC is activated as a consequence of receptor-dependent increases in intracellular [Ca2+] and diacylglycerol (DAG). Activation leads to the translocation of the enzyme from the soluble to membrane-associated particulate component of the cell (Gainer, 1985; Huang and Huang, 1993; Stabel and Parker, 1991). Studies using specific PKC activators have shown that PKC activation is closely associated with the loss of epithelial barrier function (Gainer, 1985; Ojakian, 1981), increase in transendothelial permeability (Lynch et al., 1991), and inhibition of astroglia-induced endothelial differentiation (Laterra et al., 1992). PKC is presumably present in nearly all types of tissues (Nishizuka, 1986; Stabel and Parker, 1991); however, the forms and activities of PKC in mammalian choroid plexus to our knowledge have never been characterized.
Several lines of evidence indicate that Pb stimulates PKC activity. Markovac and Goldstein (1988a) demonstrated that picomolar concentrations of Pb activate partially purified PKC from rat brain. Using isolated brain microvessels, they further reported that Pb treatment promotes the translocation of PKC activity from the cytosol to membrane, indicating a possible role of PKC in Pb-induced cerebral microvascular dysfunction (Laterra et al., 1992; Markovac and Goldstein, 1988b). In vitro activation of PKC by low level of Pb exposure was also seen in peripheral blood vessels (Chai and Webb, 1988) and in brain extracts (Long et al., 1994). Inasmuch as brain capillaries serve as the target for Pb toxicity (Bradbury and Deane, 1988; Bressler and Goldstein, 1991; Clasen et al., 1973; Laterra et al., 1992; Press, 1985), it is not surprising that alterations in PKC activity by Pb would likely occur in the choroid plexus, for this highly vascularized tissue is a significant brain compartment for Pb sequestration (Zheng, 1996).
The present studies were undertaken to characterize PKC isoform expression in the choroid plexus and to determine whether Pb exposure influences PKC activity. A model of primary cultures of choroidal epithelial cells developed in this laboratory (Zheng et al., 1997) was employed for in vitro studies, and results were compared with those reported by others in the literature. Since little information is available with respect to the in vivo interactions between Pb and PKC, we also set out to examine the effect of Pb on plexus PKC in a long-term, low-dose Pb exposure model in rats. We further performed a short-term, acute study designed to evaluate the influence of a high dose of Pb on PKC isozyme expression in vivo.
MATERIALS AND METHODS
Materials and animals
Chemicals were obtained from the following sources: phenylmethyl sulfonyl fluoride (PMSF), phosphatidyl-L-serine (PS), phorbol 12-myristate 13-acetate (PMA), histone III-S, adenosine 5′-triphosphate (ATP), protein kinase inhibitor (PKI, rabbit sequence), epidermal growth factor (EGF), gentamicin, aprotinin, leupeptin, and Pb acetate from Sigma Chemical Co. (St. Louis, MO); Dulbecco’s modified essential medium (DMEM), Hanks’ balanced salt solution (HBSS), fetal bovine serum, medium 199, antibiotic-antimycin, and polyclonal antibody against PKC ζ from Gibco–BRL (Grand Island, NY); monoclonal antibodies against PKC α and β from Seikagaku (Rockville, MD); Na acetate from Fisher Scientific Co. (Fair Lawn, NJ); NH4H2PO4 from Aldrich Chemical Co. (Milwaukee, WI); atomic absorption standards of Pb from Alfa Products (Danvers, MA); [γ-32P]ATP (specific activity: 3 Ci/μmol) from Du Pont NEN (Boston, MA). Polyclonal antibody against PKC ε was a gift from Dr. Weinstein at Columbia University. All reagents were of analytical grade, HPLC grade, or the best-available pharmaceutical grade.
Sprague–Dawley rats of both sexes were purchased from Harlan Inc. (Indianapolis, IN) and were (unless otherwise indicated) 4–6 weeks old (80–90 g) at the time of experiments. The animals were housed in a temperature-controlled, 12:12 light/dark room and were allowed free access to tap water and food.
Choroidal epithelial cell culture and in vitro Pb exposure
Choroidal epithelial cells were cultured using a method recently established in this laboratory (Zheng et al., 1997). In short, the choroid plexuses were dissected, washed in DMEM, chopped with scissors, and digested in Hanks’ buffer containing 0.2% pronase at 37°C for 5 min. The dissociated cells were washed in PBS twice and were resuspended in DMEM supplemented with 10% fetal bovine serum, antibiotics, and EGF. The cells were placed in 35-mm dishes and incubated at 37°C in a 5% CO2 atmosphere under 90% humidity. The culture medium was replaced 2 days after initial seeding and every other day thereafter. The culture from 5-week-old rats showed a dominant polygonal type of epithelial cells for at least 7–10 days with a doubling time of about 3–4 days. Six-day-old cultures were used in this study.
Prior to Pb exposure, the cells were washed three times with medium-199 containing 25 mM HEPES. The cells were then exposed to Pb (as Pb acetate) dissolved in medium-199 at final concentrations of 0.1, 1, or 10 μM at 37°C for 60 min. The Pb concentrations were chosen as such because our previous in vivo work has found 1.5 μM of Pb in the CSF following Pb exposure in rats (Zheng et al., 1991). At the end of exposure, the cells were rinsed with homogenizing buffer, detached by scraping with a rubber policeman, and resuspended in ice-cold homogenizing buffer for PKC assay as described below.
In order to exclude the possible interference due to the cell killing by Pb cytotoxicity, we used the Trypan-blue exclusion test to monitor cell viability with or without Pb treatment. No significant cell death was observed when the cultured cells were exposed to Pb in a concentration range of 0.1–10 μM. The cell viability was 88 ± 2.5% (mean ± SD) for the Pb-treated group (10 μM) vs 90 ± 2.3% for the control group.
In vivo animal studies
The dosing paradigm of Cory-Slechta et al., (1983) and Cohn and Cory-Slechta (1993) was adapted for our chronic Pb exposure study. Male Sprague–Dawley rats aged 20–22 days, weighing 30–50 g upon arrival, were assigned to three groups such that the group mean body weights were comparable. The animals had free access to pelleted Purina semipurified rat chow (Purina Mills Test Diet, 5755C; Purina Mills, Richmond, IN) as well as pre-prepared drinking water. On the third day (age 22–24 days) after arrival, the animals started to receive Pb in drinking water. The drinking water was prepared by dissolving Pb acetate in distilled, deionized water (50 or 250 μg Pb/ml). Pb concentrations were verified by graphite furnace atomic absorption spectrophotometry. For the control group, sodium acetate with an acetate concentration equivalent to the high dose of Pb acetate was prepared in the same manner.
At 30, 60, and 90 days following initiation of Pb exposure, 10 rats from each group were anesthetized with pentobarbital (50 mg/kg ip). The brain was removed, the choroid plexus was dissected, and the wet weight was recorded. The choroid plexuses from three rats were pooled and used for each PKC assay.
For an acute Pb exposure study, rats received ip injections of Pb acetate solution dissolved in deionized water once daily for 5 consecutive days at a dose of 4 mg Pb/kg/day (about 1/20th of the ip LD50). The dose was chosen in order to minimize animal morbidity during the experiment and to ensure sufficient distribution of Pb to the choroid plexus (Zheng et al., 1991). The animals in the control group received equal volumes of deionized water. Twenty-four hours after the last injection, rats were euthanized, and the brain tissues were dissected for PKC assay and Western blot analysis.
Western blot analysis
The choroid plexus and frontal cortex were homogenized (1:10, wt/vol) in a buffer composed of 20 mM Tris (pH 7.5), 2 mM EGTA, 2 mM EDTA, 15 mM 2-mercaptoethanol, 0.2% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.25 M PMSF on ice. Following centrifugation at 13,600g at 4°C for 1 h, aliquots of supernatant of the homogenate and molecular weight marker were applied to an 8.5% SDS-polyacrylamide gel, and electrophoresed under constant current of 30 mA per gel. At the end of the electrophoresis, the proteins were transferred onto a nitrocellulose membrane and immunoblotted with monoclonal anti-PKC antibodies (1:500–1000 dilution) (Luo and Weinstein, 1993). Membranes were washed five times with a blocking buffer consisting of 50 mM Tris–HCl (pH 7.5), 200 mM NaCl, and 0.2% Triton X-100. The bound anti-PKC antibodies were detected by incubating at room temperature for 1 h with 125I-labeled goat anti-mouse F(ab′)2 fragments of the secondary antibodies at a final concentration of 0.25 μCi/ml. The membranes were washed five times with the blocking buffer, dried, and autoradiographed with Kodak Biomax MR film for 3–5 days in an intensifying screen at −70°C.
PKC assay
PKC activity was assayed using a modified procedure of O’Brian et al., (1984). Tissues or cultured cells were washed and homogenized in buffer containing 20 mM Tris (pH 7.5), 2 mM EGTA, 2 mM EDTA, 1 mM DTT, 1 μg/ml aprotinin, 5 μg/ml leupeptin, and 0.25 mM PMSF. The homogenate was then sonicated on ice using Sonifer Model 250 at duty cycle 20% and output 3.5 for 25 pulses and centrifuged at 100,000g at 4°C for 1 h. The supernatant was used to determine cytosolic PKC. The pellet was further solubilized in homogenizing buffer containing 0.2% Triton X-100, sonicated on ice for 15 pulses, and centrifuged at 100,000g at 4°C for 1 h. The supernatant from this preparation was used to determine the membrane-bound (particulate) PKC.
PKC activity was assayed by incorporation of radiolabel from [γ-32P]ATP into lysine-rich histone III-S in a total volume of 120 μl. Our preliminary studies indicated that the reaction system with 1.2 mM Ca2+ and 3–5 μg of unpurified (or 1–1.5 μg semipurified) sample proteins produces an ideal PKC activation in the linear range. Thus, the reaction mixture consisted of 20 mM Tris (pH 7.5), 5 mM MgCl2, 0.1 mM DTT, 1.2 mM CaCl2, 83 μg/ml PS, 100 ng/ml PMA, 0.5 mg/ml histone, and 0.5 μM PKI. Addition of PKI into the assay system should eliminate the interference due to the phosphorylation by cyclic AMP-dependent protein kinases. The reaction was initiated by adding 5 μg unpurified (or 1.0–1.5 μg semipurified) proteins in samples and 10 μl of 66 μM ATP (with 4 μCi [γ-32P]ATP) into the above mixture. The reaction continued at 30°C for 10 min. At the end of the reaction, a 40-μl aliquot of reaction mixture was spotted onto a P81 phosphocellulose filter (Millipore, Bedford, MA) to stop the reaction. The filters were then washed five times with deionized water. Radioactivity retained on the filters was quantified using a Packard Tri-Carb Model 2100TB liquid scintillation counter. All assays were carried out in duplicate. PKC activity was calculated by determining the difference in the activity between the samples in the presence and absence of PKC activators (PS and PMA) and expressed as phosphate incorporation into histone (nmol/mg protein/min at 30°C).
For the partial purification of PKC, both cytosolic and membranous fractions were loaded onto a DEAE-Sephacel (Sigma) anion-exchange column (1 ml) equilibrated with the elution buffer containing 20 mM Tris (pH 7.5), 1.0 mM DTT, 2 mM EDTA, and 2 mM EGTA. The column was sequentially washed with (i) 10 ml of the elution buffer, (ii) 1.5 ml of the elution buffer containing 150 mM NaCl, and (iii) 1.5 ml of the elution buffer containing 250 mM NaCl. Fraction II (150 mM NaCl) typically had the most PKC activity and thus was used for PKC assay as described above (Rybin and Steinberg, 1996).
The method of Bradford (1976), using bovine serum albumin as reference, was used for all protein determinations.
Statistics
Since the mass of the choroid plexus is small in rats, the choroid plexus tissues collected from two to three rats who received the same treatment were pooled and designated as one sample (n = 1). Three to four such samples were defined as one group for statistical evaluation. PKC activities in tissues as affected by time and Pb treatment were analyzed by analysis of variance (ANOVA). When ANOVA revealed an overall treatment effect, contrasting analyses were performed at individual time points using Scheffe’s test (Scheffe, 1967). Differences between two groups were regarded as significant if p values were less than or equal to 0.05.
RESULTS
Characterization of PKC in the choroid plexus
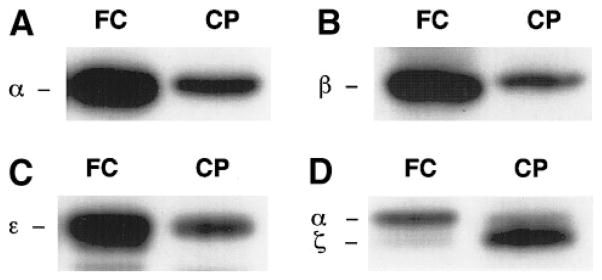
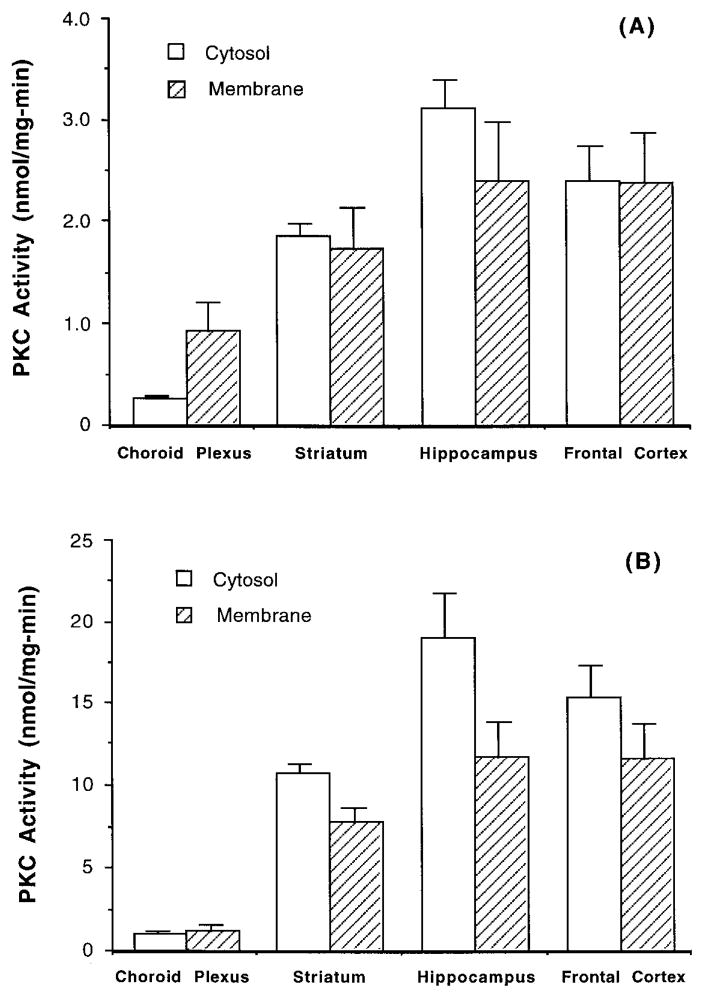
Using specific antibodies against PKC isozymes (α, β, ζ, and ε) in Western blot analysis, we found that rat choroid plexus expressed all four subtypes of PKC (Fig. 1). On the basis of the photodensity of resulting bands on the same lane, it is noted that the expression of ζ subtype in the choroid plexus appeared to be higher than that of α subtype, while the frontal cortex expressed more α than ζ subtype (Fig. 1D). When tissue PKC was activated by PS and PMA, the hippocampus had the highest PKC activities, and the choroid plexus had the lowest (Fig. 2).
FIG. 1.
Expression of PKC subtypes in rat choroid plexus and brain cortex. (A) PKCα; (B) PKCβ; (C) PKCε; (D) PKCζ. FC, frontal cortex; CP, choroid plexus. There were 20–100 μg proteins of tissue homogenates loaded on each lane.
FIG. 2.
PKC activities in the choroid plexus, frontal cortex, hippocampus, and striatum of rat aged 4–6 weeks. (A) Cytosolic and membranous fractions were directly assayed for PKC activity; (B) cytosolic and membranous fractions were partially purified for PKC proteins prior to enzymatic assay. Data represent means ± SE (n = 3–5).
The specificity of the PKC assay relies on how selectively the activators act on PKC enzyme to incorporate 32Pi from [γ-32P]ATP to lysine-rich substrates. It is possible that 32Pi released in the assay system might result from nonspecific Pb activation of other phosphorylation enzymes present in cell homogenates. To eliminate the possible nonspecific interference, we adapted a procedure to partially purify PKC proteins by running the cell homogenates through an anion-exchange column prior to the enzymatic assay. This procedure proved useful, although it diluted the samples and introduced considerable variability. Nonetheless, following partial purification, the specific PKC activities in cortex, hippocampus, and striatum were increased by 5- to 6-fold in cytosol and 4- to 5-fold in membrane (Fig. 2B). The purification procedure also raised cytosolic and membranous PKC activities about 3.7- and 1.3-fold, respectively, in choroid plexus tissue. Therefore, in subsequent in vivo studies, we routinely purified PKC proteins prior to enzymatic assay.
In vitro studies
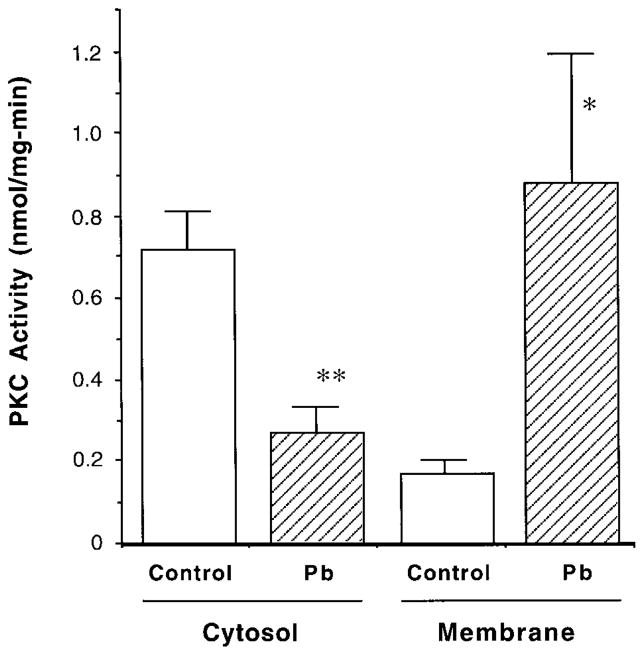
The purification procedure, however, did not improve the specific activity of PKC when it was applied to the cultured cells. This was apparently due to the relatively uniform, less contaminated cell type used in the study. By using direct PKC assay for cultured choroidal epithelial cells, we found that Pb exposure significantly promoted the translocation of PKC from the cytosol to membrane (Fig. 3). Following incubation of the cells with 10 μM Pb in culture medium, the membrane-bound PKC activities were significantly increased by 5.2-fold compared to the control, while the cytosolic PKC activities were decreased in parallel, the pattern of typical PKC activation (Fig. 3) (Huang and Huang, 1993; Stabel and Parker, 1991). We also noted that exposure of the cells to Pb for less than 30 min did not produce notable changes in cellular PKC activities. Concentrations of Pb in the cultured cells following 60-min exposure to 10 μM Pb in culture medium, as estimated from a separate set of experiments using the same experimental approaches, approximated 0.19 ± 0.02 and 26.8 ± 1.06 nmol/mg (SE) protein of cell homogenates in the controls and Pb-treated groups, respectively.
FIG. 3.
Pb promotes the translocation of PKC activity in cultured choroidal epithelial cells. Cells were exposed to 10 μM Pb in medium for 60 min. Cytosolic and membrane-bound fractions were separated and directly assayed for PKC activity without purification. Data represent means ± SE (n = 5). **p < 0.01, *p < 0.05 compared to the control.
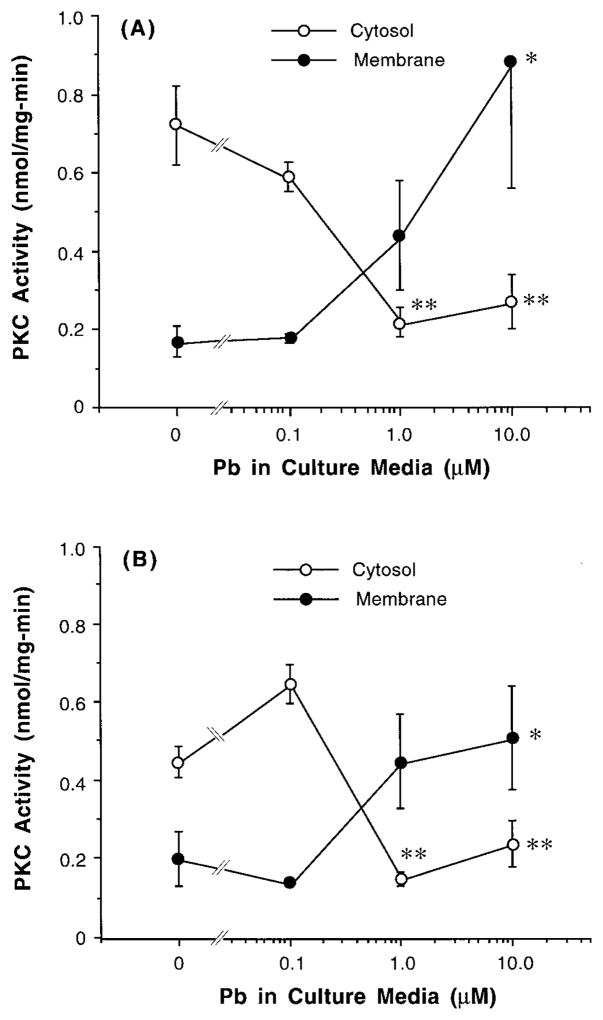
In addition, the action of Pb on PKC was Pb concentration dependent. Initial attempts to increase Pb concentrations in the culture medium yielded a visible Pb-induced precipitation, slightly at 40 μM, and severely at 50 μM. Thus, we used low-range Pb concentrations in our in vitro study. ANOVA analyses of concentration–response curves presented in Fig. 4A revealed a significant Pb concentration-related decline of cytosolic PKC; yet the association between membranous PKC and Pb concentration in culture media was not significant (p = 0.08). The experiments from the semipurification procedure showed similar outcomes (Fig. 4B).
FIG. 4.
Dose–response relationship of Pb effect on PKC activity in cultured choroidal epithelial cells. Cells were exposed to Pb in medium for 60 min. Data represent means ± SE (n = 3–5). **p < 0.01, *p < 0.05 compared to the control (0 μM Pb). (A) Cytosolic and membranous fractions were directly assayed for PKC activity; (B) cytosolic and membranous fractions were partially purified for PKC proteins prior to enzymatic assay.
In vivo studies
The effect of Pb on choroid plexus PKC was further evaluated in rats chronically exposed to Pb in drinking water (control or 50 or 250 μg Pb/ml) for 30, 60, or 90 days. In those rats, we have previously reported that Pb is dose dependently sequestered by the choroid plexus (Zheng et al., 1996). For example, at day 90, Pb concentration in the choroid plexus of control rats was 0.21 ± 0.01 nmol/mg (SE) of plexus proteins, while it was 0.39 ± 0.10 and 0.53 ± 0.22 nmol/mg in the low- and high-dose groups, respectively. Two-way ANOVA showed that Pb treatment did not significantly modulate PKC activities in either the cytosol or membrane fractions of the choroid plexus (Table 1); however, there indeed existed a highly significant age-related decline of PKC activities in both cytosol and membrane at all three dose groups (Table 1).
TABLE 1.
PKC Activity in Rat Choroid Plexus Following Long-Term, Chronic Exposure to Pb in Drinking Water
| Control | Low dose (50 μg/ml) | High dose (250 μg/ml) | |
|---|---|---|---|
| Cytosolic (nmol/mg/min) | |||
| Day 30 | 1.00 ± 0.075 | 1.27 ± 0.233 | 1.24 ± 0.236 |
| Day 60 | 1.05 ± 0.143 | 1.28 ± 0.171 | 1.01 ± 0.185 |
| Day 90 | 0.79 ± 0.020 | 0.69 ± 0.035 | 0.87 ± 0.091 |
| Membrane bound (nmol/mg/min) | |||
| Day 30 | 0.85 ± 0.517 | 0.83 ± 0.337 | 0.84 ± 0.405 |
| Day 60 | 0.65 ± 0.082 | 0.46 ± 0.067 | 0.57 ± 0.107 |
| Day 90 | 0.48 ± 0.0638 | 0.49 ± 0.046 | 0.41 ± 0.037 |
Note. Pb acetate was dissolved in drinking water. Data represent means ± SD; n = 5 for the day 30 groups, n = 3 for all other groups.
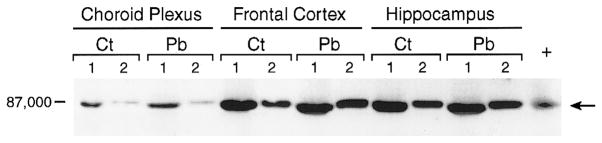
Since the animals following long-term Pb exposure could develop a compensatory mechanism to down-regulate PKC activity by reducing its protein synthesis, it was necessary to use a short-term exposure scheme to examine the effect of Pb on PKC expression in the choroid plexus. Thus, a group of rats were acutely exposed to Pb by ip injections of Pb solution for 5 consecutive days, and the tissue homogenates were used to estimate total PKC proteins by Western blot analysis. Acute Pb exposure did not seem to alter the density of PKC bands by Western blot analysis (Fig. 5) nor did it alter PKC activities in plexus by the enzymatic assay (data not shown). Thus, both acute and chronic studies demonstrated that in vivo Pb exposure did not have a distinct effect on choroid plexus PKC. However, our results do not rule out the possibility that Pb may modulate PKC activity in other brain regions. In addition, we noted that the bands corresponding to hippocampal PKC seemed darkened following Pb treatment (Fig. 5).
FIG. 5.
Expression of PKC proteins in choroid plexus and brain tissues following acute Pb exposure. Rats (male, 4 weeks old) received injection of 4 mg Pb/kg ip once daily for 5 days. PKC expression in tissue homogenates was assessed on Day 6. Arrows indicate the bands corresponding to PKCβ. Amounts of proteins on the gel: lane 1, 388 μg; lane 2, 78 μg. +, Positive control from rabbit brain preparation.
DISCUSSION
Regulatory enzyme PKC is thought to be present in almost any type of tissue and to play a pivotal role in a wide variety of cellular processes (Zidovetzki and Lester, 1992; Huang and Huang, 1993). The present work clearly indicates that the choroid plexus, a brain tissue with vigorous metabolic activities, expresses PKC enzymes, although the activity expressed as per milligram of tissue proteins appears to be lower in the choroid plexus than in other brain regions. In the absence of Pb, cultured choroidal epithelial cells display a PKC distribution pattern similar to that observed in endothelium-dominant brain capillary, namely, high in cytosol and low in membrane (Laterra et al., 1992).
Our in vitro results demonstrated that Pb exposure significantly promoted the translocation of PKC activity from the cytosol to membrane in cultured plexus cells. These observations confirm the results of previous studies suggesting a possible role of cellular PKC in Pb-induced cytotoxicity (Goldstein, 1993; Long et al., 1994). However, some studies have found that the activation of PKC could be achieved by much lower Pb concentrations (10−14–10−10 M) in partially purified brain extracts and in the suspension of cultured endothelial cells (Markovac and Goldstein, 1988a,b; Laterra et al., 1992). Others have also reported the activation of brain PKC by monitoring concentrations of free Pb2+ in the range of 3.6 × 10−11 to 5 × 10−9 M (Long et al., 1994). In the latter study, the activation was apparent in the presence of free Pb2+ alone even without Ca2+. We failed to reproduce these results when Pb concentrations in our studies fell bellow 1 μM in the culture medium. As the choroid plexus has a high capacity to sequester Pb (Friedheim et al., 1983; Zheng et al., 1991), it is possible that Pb ions may bind to intracellular components in epithelial cells, which may consequently reduce intracellular free Pb ions available for PKC activation. It is known that Pb can form the stable complexes with a variety of macromolecules that are rich in sulfhydryl groups (Goering, 1993).
Pb modulation of cellular PKC activity remains a controversial subject. Direct mixing of Pb with highly purified PKC proteins results in an obvious inhibition of the enzyme (Lison et al., 1990; Murakami et al., 1993; Rajanna et al., 1995). In contrast, exposure of cultured cells to Pb produces distinct activation (Markovac and Goldstein, 1988a,b; Laterra et al., 1992; Long et al., 1994). Clearly, the assay system to which the purified enzymes are or are not used makes a fundamental difference. At the molecular level, the polypeptides of PKC contain several cysteine-rich domains. Binding of diacylglycerol and Ca2+ to these domains induces the conformational change of the enzyme that is essential for cytosolic PKC to translocate to the membrane (Zidovetzki and Lester, 1992). In a purified PKC preparation, Pb ions, which possess a strong affinity to thiol groups, seem likely to directly interact with the catalytic domain of PKC (Murakami et al., 1993) and therefore inhibit subsequent translocation. In fact, when many other soft metals, including Zn2+, Cd2+, Al3+, Co2+, Cu2+, Hg+, Mn2+, and CH3Hg, were directly incubated with purified PKC, all these metals displayed various degrees of inhibition of the enzyme (Lison et al., 1990; Luo and Weinstein, 1993; Murakami et al., 1993; Rajanna et al., 1995). Thus, incubation of purified PKC with excess amounts of toxic metal ions would likely repress the enzyme activity.
Why then, in the current study, did Pb activate PKC when intact cultured cells were used? Several reasons may account for this discrepancy. First, the sequence of the enzymatic assay was different and may provide a clue. When purified enzyme was used, there was no need to separate cytosolic PKC from membranous PKC, and the activity was directly accessed in a ‘‘reconstructed’’ system. In contrast, the initial step in our culture studies included the separation of membranous fraction from cytosol after the cells were exposed to Pb. The separated fractions were then used to determine the enzyme activity. Thus, what we measured was essentially the translocated PKC in the membrane and the posttranslocated, remaining PKC in the cytosol. Evidently, treatment with Pb promoted such a translocation.
Second, Pb in cell culture may not directly act on the catalytic domain of PKC as suggested for its action on the purified PKC. Rather, Pb ions may act on the cascade of PKC activation processes, particularly intracellular Ca2+. While it remains uncertain at present how exactly PKC is activated, it is generally accepted that there are three specific molecular events involving PKC activation: (i) binding of Ca2+ to soluble PKC to initiate translocation, (ii) interaction of Ca2+–PKC complex with phospholipid, and (iii) attachment of diacylglycerol to this complex leading to a fully active enzyme conformation (Huang and Huang, 1993; Zidovetzki and Lester, 1992). Increased intracellular Ca2+, as the result of Ca2+ influx or release from its internal storage, is the key step toward the promotion of the interactions between Ca2+–PKC and membrane phosphatidylserine (Huang and Huang, 1993). Pb2+ is known to disturb intracellular Ca2+ homeostasis (Pounds, 1984; Simons, 1993). The rise in total intracellular Ca2+ by Pb treatment is thought to be due to Pb stimulation of Ca2+ entry or inhibition of Ca2+ exit/storage (Goldstein, 1977; Long and Rosen, 1992; Pounds et al., 1982; Rosen and Pounds, 1989; Schanne et al., 1989). From our own preliminary studies, we also found that the Ca concentration in the assay system was critical to PKC activation. As we did not determine the intracellular Ca2+ content, we could not conclude whether Pb indeed alters intracellular Ca2+ in our in vitro studies. Yet, it seems likely that the promotive effect of Pb on PKC translocation in cultured epithelial cells may be secondary to Pb-induced alterations of intracellular Ca2+. It is also remotely possible that Pb may form the bridge to link the PKC to the membrane.
In contrast to our in vitro studies, in no case were the associations between in vivo Pb exposure and the alteration of either cytosolic or membranous PKC activities in rat plexus statistically significant. Studies using various PKC activators strongly support the view that chronic activation of PKC in vivo could initiate the down-regulation by accelerating the rate of PKC degradation (Ballester and Rosen, 1985; Kishimoto et al., 1989; Stabel and Parker, 1991; Young et al., 1987). Thus, it would be possible that the long-term, low-dose Pb exposure in our study might induce a similar compensatory mechanism so as to offset the escalated activity of PKC. However, our short-term, acute studies did not provide any evidence of activation and/or altered protein expression of PKC in the choroid plexus by Pb treatment. Thus, we cannot assume that Pb exposure induces the down-regulation of PKC in our in vivo study. In addition, it is noteworthy that the ages of animals at the initiation of exposure were about 22–24 days old. According to a recent report by Guilarte’s group (Jett et al., 1997), Pb-induced neurobehavioral toxicity in rats would occur earlier at weanling age (PN21), but not at an older age. Thus, a future study of PKC with earlier or even maternal Pb exposure is desirable. Finally, it is necessary to point out that Pb concentrations in the choroid plexus as normalized by protein contents were about 50-fold less in our in vivo studies than in the cultured cells. This difference may partially explain the discrepancy between the in vitro and in vivo results.
In conclusion, in vitro Pb exposure results in a significant translocation of PKC activity from the cytosol to membrane in the cultured choroidal epithelial cells, suggesting a possible role of PKC in Pb-induced cytotoxicity in vitro. While the mechanism of activation remains obscure, modulation of PKC activity due to Pb interaction with intracellular Ca2+ is postulated. Acute or chronic Pb exposure in vivo does not produce any significant alterations in both cytosolic and membrane-bound PKC activities in rat choroid plexus. The inclusion of earlier or maternal Pb exposure is necessary for future in vivo study of Pb interaction on PKC.
Acknowledgments
We are grateful to Dr. Jianhua Luo and Mr. Sean Ren for technical assistance and to Dr. Susan F. Steinberg for her advice in PKC assay and her criticism in the preparation of this manuscript. This research was supported in part by NIEHS Grants ES07042 and ES06831.
References
- Ballester R, Rosen OM. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985;260:15194–15199. [PubMed] [Google Scholar]
- Bradbury MWB, Deane R. Brain endothelium and interstitium as sites for effects of lead. Ann NY Acad Sci. 1988;529:1–8. doi: 10.1111/j.1749-6632.1988.tb51414.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991;41:479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Chai SS, Webb RC. Effects of lead on vascular reactivity. Environ Health Perspect. 1988;78:85–89. doi: 10.1289/ehp.887885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen RA, Hartmann JF, Starr AJ, Coogan PS, Pandolfi S, Liang I, Becker R, Hass GM. Electron microscopic and chemical studies of the vascular changes and edema of lead encephalopathy. Am J Pathol. 1973;74:215–240. [PMC free article] [PubMed] [Google Scholar]
- Cohn J, Cory-Slechta DA. Subsensitivity of lead-exposed rats to the accuracy-impairing and rate-altering effects of MK-801 on a multiple schedule of repeated learning and performance. Brain Res. 1993;600:208–218. doi: 10.1016/0006-8993(93)91375-3. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicol Appl Pharmacol. 1983;71:342–352. doi: 10.1016/0041-008x(83)90021-2. [DOI] [PubMed] [Google Scholar]
- Davson H, Segal MB. Physiology of the CSF and Blood-Brain Barrier. CRC Press; New York: 1996. [Google Scholar]
- Friedheim E, Corvi C, Graziano JH, Donnelli T, Breslin D. Choroid plexus as protective sink for heavy metals? Lancet. 1983;i(8331):981–982. doi: 10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- Gainer M. Diacylglycerol inhibits gap junction communication in cultured epithelial cells: Evidence for a role of protein kinase C. Biochim Biophys Res Commun. 1985;126:1109–1113. doi: 10.1016/0006-291x(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Gamard CJ, Blobe GC, Hannun YA, Obeid LM. Specific role for protein kinase C b in cell differentiation. Cell Growth Differ. 1994;5:405–409. [PubMed] [Google Scholar]
- Goering PL. Lead-protein interactions as a basis for lead toxicity. Neurotoxicology. 1993;14:45–60. [PubMed] [Google Scholar]
- Goldstein GW. Lead encephalopathy: The significance of lead inhibition of calcium uptake by brain mitochondria. Brain Res. 1977;136:185–188. doi: 10.1016/0006-8993(77)90145-7. [DOI] [PubMed] [Google Scholar]
- Goldstein GW. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology. 1993;14:97–102. [PubMed] [Google Scholar]
- Huang KP, Huang FL. How is protein kinase C activated in CNS? Biochem Int. 1993;22:417–433. doi: 10.1016/0197-0186(93)90037-6. [DOI] [PubMed] [Google Scholar]
- Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR. Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol Biochem Behav. 1997;57:271–279. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Ventricles and cerebrospinal fluid. In: Conn PM, editor. Neuroscience in Medicine. JB Lippincott; Philadelphia: 1995. pp. 171–196. [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka SI, Tommaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (cal-pain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- Laterra J, Bressler JP, Indurti RR, Belloni-Olivi L, Goldstein GW. Inhibition of astroglia-induced endothelial differentiation by inorganic lead: A role for protein kinase C. Proc Natl Acad Sci USA. 1992;89:10748–10752. doi: 10.1073/pnas.89.22.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lison D, Raguzzi F, Lauwerys R. Comparison of the effects of auranofin, heavy metals and retinoids on protein kinase C in vitro and on a protein kinase C mediated response in macrophages. Pharmacol Toxicol. 1990;67:239–242. doi: 10.1111/j.1600-0773.1990.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Long GJ, Rosen JF. Lead perturbs epidermal growth factor (EGF) modulation of intracellular calcium metabolism and collagen synthesis in clonal rat osteoblastic (ROS 17/2.8) cells. Toxicol Appl Pharmacol. 1992;114:63–70. doi: 10.1016/0041-008x(92)90097-c. [DOI] [PubMed] [Google Scholar]
- Long GJ, Rosen JF, Schanne FAX. Lead activation of protein kinase C from rat brain. J Biol Chem. 1994;269:834–837. [PubMed] [Google Scholar]
- Luo JH, Weinstein IB. Calcium-dependent activation of protein kinase C: The role of C2 domain in divalent cation selectivity. J Biol Chem. 1993;268:23580–23584. [PubMed] [Google Scholar]
- Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinases S activation. J Clin Invest. 1991;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton WI, Kirkpatrick JB, Cook JD. Does the choroid plexus really protect the brain from lead? Lancet. 1984;ii(8398):351. doi: 10.1016/s0140-6736(84)92719-3. [DOI] [PubMed] [Google Scholar]
- Markovac J, Goldstein GW. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988a;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- Markovac J, Goldstein GW. Lead activates protein kinase C in immature rat brain microvessels. Toxicol Appl Pharmacol. 1988b;96:14–23. doi: 10.1016/0041-008x(88)90242-6. [DOI] [PubMed] [Google Scholar]
- Murakami K, Feng G, Chen SG. Inhibition of brain protein kinase C subtypes by lead. J Pharmacol Exp Ther. 1993;264:757–761. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase c. Science. 1992;258:609–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- O’Brian CA, Lawrence DS, Kaiser ET, Weinstein IB. Protein kinase C phosphorylates the synthetic peptide ARG-ARG-LYS-ALA-SER-GLY-PRO-PRO-VAL in the presence of phospholipid plus either Ca2+ or a phorbol ester tumor promoter. Biochem Biophys Res Commun. 1984;124:269–302. doi: 10.1016/0006-291x(84)90951-3. [DOI] [PubMed] [Google Scholar]
- Ojakian GK. Tumor promoter-induced changes in the permeability of epithelial cell tight junctions. Cell. 1981;23:95–98. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- O’Tuama LA, Kim CS, Gatzy JT, Krigman MR, Mushak P. The distribution of inorganic lead in Guinea pig brain and neural barrier tissues in control and lead-poisoned animals. Toxicol Appl Pharmacol. 1976;36:1–9. doi: 10.1016/0041-008x(76)90021-1. [DOI] [PubMed] [Google Scholar]
- Pounds JG. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: A review. Neurotoxicology. 1984;5:295–332. [PubMed] [Google Scholar]
- Pounds JG, Wright R, Morrison D, Casciano DA. Effect of lead on calcium homeostasis in the isolated rat hepatocyte. Toxicol Appl Pharmacol. 1982;63:389–401. doi: 10.1016/0041-008x(82)90268-x. [DOI] [PubMed] [Google Scholar]
- Press MF. Lead-induced permeability changes in immature vessels of the developing cerebellar microcirculation. Acta Neuropathol. 1985;67:86–95. doi: 10.1007/BF00688128. [DOI] [PubMed] [Google Scholar]
- Rajanna B, Chetty CS, Rajanna S, Hall E, Fail S, Yallapragada PR. Modulation of protein kinase C by heavy metals. Toxicol Lett. 1995;81:197–203. doi: 10.1016/0378-4274(95)03433-1. [DOI] [PubMed] [Google Scholar]
- Rosen JF, Pounds JG. Quantitative interactions between Pb2+ and Ca2+ homeostasis in cultured osteoclastic bone cells. Toxicol Appl Pharmacol. 1989;98:530–543. doi: 10.1016/0041-008x(89)90181-6. [DOI] [PubMed] [Google Scholar]
- Rybin V, Steinberg SF. Thyroid hormone represses protein kinase C isoform expression and activity in rat cardiac myocytes. Circ Res. 1996;79:388–398. doi: 10.1161/01.res.79.3.388. [DOI] [PubMed] [Google Scholar]
- Schanne FAX, Dowd TL, Gupta RK, Rosen JF. Lead increases free Ca2+ concentration in cultured osteoblastic bone cells: Simultaneous detection of intracellular free Pb2+ by 19F NMR. Proc Natl Acad Sci USA. 1989;86:5133–5135. doi: 10.1073/pnas.86.13.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffe H. The Analysis of Variance. John Wiley & Sons; New York: 1967. [Google Scholar]
- Simons TJB. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology. 1993;14:77–86. [PubMed] [Google Scholar]
- Stabel S, Parker PJ. Protein kinase C. Pharmacol Ther. 1991;51:71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Young S, Parker PJ, Ullrich A, Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987;244:775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. The choroid plexus and metal toxicities. In: Chang LW, editor. Toxicology of Metals. CRC Press; New York: 1996. pp. 609–626. [Google Scholar]
- Zheng W, Perry DF, Nelson DL, Aposhian HV. Protection of cerebrospinal fluid against toxic metals by the choroid plexus. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- Zheng W, Shen H, Blaner SB, Zhao Q, Ren X, Graziano JH. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: The role of the choroid plexus. Toxicol Appl Pharmacol. 1996;139:445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Graziano JH. Primary culture of rat choroidal epithelial cells: A model for in vitro study of the blood-cerebrospinal fluid barrier. In Vitro Cell Biol Dev. 1998;34:40–45. doi: 10.1007/s11626-998-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Lester DS. The mechanism of activation of protein kinase C: A biophysical perspective. Biochim Biophys Acta. 1992;1134:261–272. doi: 10.1016/0167-4889(92)90185-e. [DOI] [PubMed] [Google Scholar]