Abstract
Biomaterials used in the context of tissue engineering or wound repair are commonly designed to be “non-immunogenic”. However, previously it has been observed that self-assembled peptide nanofiber materials are non-inflammatory despite their immunogenicity, suggesting that they may be appropriate for use in wound healing contexts. To test this hypothesis, mice were immunized with epitope-containing peptide self-assemblies until they maintained high antibody titers against the material, then gels of the same peptide assemblies were applied within full-thickness dermal wounds. In three different murine dermal wounding models with different baseline healing rates, even significantly immunogenic peptide assemblies did not delay healing. Conversely, adjuvanted peptide assemblies, while raising similar antibody titers to unadjuvanted assemblies, did delay wound healing. Analysis of the healing wounds indicated that compared to adjuvanted peptide assemblies, the unadjuvanted assemblies exhibited a progression of the dominant T cell subset from CD4+ to CD8+ cells in the wound, and CD4+ cell populations displayed a more Th2-slanted response. These findings illustrate an example of a significant anti-biomaterial adaptive immune response that does not adversely affect wound healing despite ongoing antibody production. This material would thus be considered “immunologically compatible” in this specific context rather than “non-immunogenic”, a designation that is expected to apply to a range of other protein and peptide-based biomaterials in wound healing and tissue engineering applications.
Keywords: peptide nanofibers, scaffold, T cell phenotype, immunogenic materials, wound
1. Introduction
Cells of the adaptive immune system are centrally involved in the process of wound healing [1-12]. However, the design of biomaterials for wound healing applications has largely avoided engaging the adaptive immune system, commonly taking the stance that any anti-biomaterial immune response is likely to be harmful. In this report, we describe vigorous anti-biomaterial immune responses that were entirely consistent with good performance of the material in dermal wounds in mice. The study employed peptide self-assemblies that raise strong and epitope-specific B cell and T cell responses in the absence of measurable inflammation [13-15]. This platform provided a simple experimental system where the immunogenicity of the material could be turned on and off based on the presence of T cell epitopes and B cell epitopes in the peptides.
Our interest in studying anti-biomaterial immune responses in wound healing contexts was prompted in part by other previous reports where adaptive immune responses have played positive and important roles in wound healing. T cells are prevalent in healing wounds, with their numbers peaking at day 7 [16]. Until recently it has been thought that they do not play major roles in wound healing, but recent work has indicated their involvement [9]. In previous studies where T cells were depleted using monoclonal antibodies, global depletion of T cells diminished wound healing, leading to decreases in both wound strength and collagen synthesis [8], yet in subsequent studies with subtype-specific antibodies, the beneficial T cell subset was challenging to identify [7, 17]. In a another study, T helper versus T suppressor subsets appeared to have opposing roles in the regulation of wound healing, where CD4+ T helper cells were associated with improved healing and CD8+ T suppressor with diminished healing in a rat model [18]. However again the specific temporal and subpopulation details underlying these findings have not yet been fully elucidated.
Beyond the T cell arm of the adaptive immune response, a few studies have suggested that antibody/B cell responses may also have positive roles in wound healing, even though B cells are not significantly recruited to the wound beds themselves [5, 12]. Antibodies directed against wounded tissue components have been found to enhance the healing of skin wounds in mice [12]. In humans, xenograft decellularized tissues such as small intestinal submucosa (SIS) perform well even though they raise substantial antibody responses [19]. Although this finding may not necessarily indicate a causal relationship between antibodies and proper healing, it does indicate that such antibody responses can exist and still maintain good clinical performance of the material. Additionally, SIS appears to elicit a Th2-restricted immune response [20], suggesting that this phenotype may be particularly well suited for biomaterials in wound healing situations; however, any detailed mechanism underlying these observations is not yet understood. Elucidating such a mechanism is challenging with decellularized tissues because they are composed of complicated mixtures of different antigens and epitopes that are difficult if not impossible to control experimentally. These examples indicate that the complexity of adaptive immune involvement in wound healing is only beginning to be understood. Encouragingly however, they also indicate that there may be particular immune phenotypes of anti-biomaterials responses that could be acceptable for the material's use in wound healing applications.
Here we report a fully synthetic material raising an immune response with such a phenotype: fibrillar peptide self-assemblies [13-15, 21-23]. We focused on the short self-assembling peptide Q11 (QQKFQFQFEQQ), which forms β-sheet nanofibers at subgelation concentrations (around 2 mM) and hydrogels above this concentration [15, 24, 25]. In their assembled state, these peptides elicit high-titer antibody responses and moderate T cell responses in the absence of any supplemental adjuvants. Importantly, they do not elicit measurable inflammation at their sites of delivery [13]. We hypothesized that this non-inflammatory but immunogenic material could be appropriate for use in wound healing contexts.
Unlike biologically sourced materials such as decellularized matrices, the modularity of peptide self-assemblies makes it straightforward to adjust the content and identity of a range of incorporated peptide sequences, including B cell epitopes, T cell epitopes, and other biologically active ligands [24-26]. These properties make Q11-based self-assembled nanofibers useful as a platform for vaccine development [26, 27], but they have not yet been investigated in the context of scaffolds for wound healing. Gels of Q11-based peptides function well as substrates for 3D cell culture [25, 28, 29], as do other peptide-based fibrillar matrices [30-33], but these materials have not been explored immunologically in wound healing contexts. Thus, owing to previous findings of the involvement of the adaptive immune response in wound healing and the availability of an experimentally convenient biomaterial capable of raising non-inflammatory but epitope-specific T cell and B cell responses, we sought to test the hypothesis that the specific immune phenotype raised by peptide self-assemblies would not interfere with dermal wound healing.
2. Materials and Methods
2.1. Peptide, vaccine, and scaffold formulation
All peptides were synthesized as previously reported [13-15, 24] with standard Fmoc solid-phase chemistry. Peptides used in this study included the self-assembling peptide Q11 (Ac-QQKFQFQFEQQ-Am) and Q11 extended N-terminally with the ovalbumin peptide OVA323-339 (ova), which contained both B cell and T cell epitopes (ovaQ11, N-ISQAVHAAHAEINEAGR-SGSGQQKFQFQFEQQ-Am). The OVA peptide lacking the Q11 domain will be designated as “ova.” Peptides were purified by HPLC, verified with MALDI-MS, and stored as lyophilized powders [24, 34]. Immunization and scaffold materials were prepared from lyophilized ovaQ11 or Q11 as 8 mM solutions in sterile water and stored at 4 °C overnight. For ovaQ11 vaccines this stock solution was brought to a working concentration of 2 mM with 1xPBS. For materials adjuvanted with complete Freund's adjuvante (CFA) or incomplete Freund's adjuvant (IFA), peptides were brought to 4 mM in 1xPBS and then formulated 1:1 (vol) with Imject CFA or IFA (Pierce) by vortexing, according to the manufacturer's instructions. Peptides in the final formulations for immunizations were below their gelation concentrations, whereas peptide materials applied to wounds were above their gelation concentrations. Peptides were applied to wounds at a final working concentration of 5 mM in PBS, at which they produced self-supporting gels. Endotoxin levels of the peptides were measured with the LAL chromogenic endpoint assay; all materials contained less than 1.5 EU/mL endotoxin, measured immediately prior to use in vivo.
2.2. Animals and immunizations
Wild type C57BL/6 mice were purchased from Harlan Sprague Dawley. Leptin deficient mice (ob/ob) were purchased from the Jackson Laboratory (B6.Cg-Lepob/J, stock No: 000632). These mice are diabetic, obese, and exhibit delayed wound closure [35-38]. Mice were housed in a centralized animal facility at the University of Chicago. Procedures were approved by the University of Chicago Institutional Animal Care and Use Committee, and the NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed. Immunizations in C57BL/6 mice were performed subcutaneously with one 100 μL injection of 2 mM ovaQ11 in PBS followed by boosting at 4 weeks and 6 weeks with half doses (50 μL of 2 mM ovaQ11). Owing to difficulties in placing subcutaneous injections in the obese ob/ob mice, for this strain immunizations were performed via intraperitoneal injections with one 200 μL injection of either ovaQ11 or CFA-ovaQ11 and boosted with half doses of 100 μL of ovaQ11 or IFA-ovaQ11 respectively at 4 weeks and 6 weeks. Mice in “PBS” negative control groups received PBS injections of the same volume as the experimental groups. Serum samples were collected via the submandibular vein and analyzed by ELISA.
2.3. Wounding Models
To study the influence of peptide immunogenicity in multiple contexts, dermal wounding was performed in three mouse models with increasingly delayed healing. These included a 5 mm-diameter wound in C57BL\6 mice (fastest healing), an 8mm wound in ob/ob mice (intermediate healing rate), and a 5 mm splinted wound in ob/ob mice (slowest healing). Mice were wounded when adequate and stable antibody responses were elicited against the peptide immunizations in the experimental groups, which was typically achieved at 6 to 8 weeks after primary immunization. For wounding, mice were anesthetized using isoflurane (2-2.5%), and 0.125% bupivacaine was injected subcuatenously 5 minutes prior to the procedure. Hair was removed from an area of the back using clippers and a chemical depilatory, and sterile technique was used for the remainder of the procedure. A full-thickness excisional round wound was created on the upper back of each mouse using a biopsy punch with a 5 mm or 8 mm diameter, as indicated. For splinted wounds, a 5 mm punch was made and a 0.5 mm thick autoclaved silicone splint was subsequently fixed to the skin by an immediate-bonding adhesive followed by an interrupted 6-0 nylon suture to secure the splint as described by other research groups [39-42]. For unsplinted wounds in the ob/ob mouse demonstrating intermediate healing rates, an 8 mm punch was used [35-38]. Materials delivered into wounds were prepared as described above to a final concentration of 5mM in PBS, and 100 μL of the material was injected intradermally at the edges of the wound. A pre-trimmed Tegaderm dressing was used to cover the wound bed. Wounds were photographed over the course of healing and wound area was measured using ImageJ software.
2.4. Histology and Immunohistochemistry
The 8mm unsplinted ob/ob model was used to investigate wound histology at day 17. Mice were sacrificed and the wounded tissue was excised, fixed in 10% buffered formalin, and embedded on-edge in paraffin. Immunostaining was performed on 5 μm-thick sections by standard techniques. Primary antibodies against murine CD3 (1:100 dilution, ab5690), B220 (1:500, Cat#14-0452-81, clone: RA3-6B2, eBioscience) and F4/80 (1:200, MCA497GA, AbD Serotec) were used with biotinylated secondary antibody( anti-rat IgG, BA-4001 or anti-rabbit IgG, BA-1000 from Vector laboratories accordingly). The antigen-antibody binding was detected by Elite kit (PK-6100, Vector Laboratories) and DAB sytem (DAKO, K3468). Cells were counted at 20x magnification. Due to the abundance of F4/80+ cells, rather than counting them directly, Image-J software was used to calculate the percentage of positively stained area. CD3+ and B220+ cells were quantified by average counts per field. Granulation tissue thickness was measured at two points on each slide at the edge of the wound.
2.5. T lymphocyte isolation and flow cytometry
To study T lymphocyte recruitment the C57BL/6 unsplinted 5mm model was used. All mice were immunized against ovaQ11 and wounds were treated with Q11 lacking the ova epitope (non-inflammatory and non-immunogenic), ovaQ11 (non-inflammatory yet immunogenic) or IFA/ovaQ11 (both inflammatory and immunogenic). Mice were sacrificed at day 5 and day 10, and 10 mm punch biopsies of the wound were obtained. The tissue was digested in 1mL RPMI 1640 with 1 mg/mL collagenase D for 30 minutes at 37°C while shaking. Samples were immediately cooled in ice to slow digestion. Samples were passed through a 70 μm nylon mesh strainer and centrifuged (300 g) for 20 minutes at 4 °C. Cells were resuspended in blocking buffer (2% FBS/PBS with anti-CD16/32 antibiody at 1:100 dilution) and stained on ice. Cytokine stains were performed after stimulating cells with phorbol dibutyrate and ionomycin and subsequent permeabilization of the cells. Washed cells were resupsended in flow buffer and flow cytometry was performed using a BD LSR-II Blue instrument. Analysis was performed using FlowJo Software. All antibodies were purchased from BioLegend with the exception of IL4 from BD Biosciences. Surface staining was performed for CD3 (Alexa700 clone 17A2), CD4 (PE dazzle 594, clone RM4-5), CD8 (BV605, clone 53-6.7), CD44 (APC Cy7, clone IM7), and IFNγ (APC, clone XMG 1.2) and intracellular staining was performed for IL4 (PE, clone 11B11).
2.6. Statistical Analysis
Groups were statistically compared using 1- or 2-way ANOVA with Tukey post-hoc tests.
3. Results
3.1. Significant antibody responses were produced from peptide assemblies with or without adjuvant
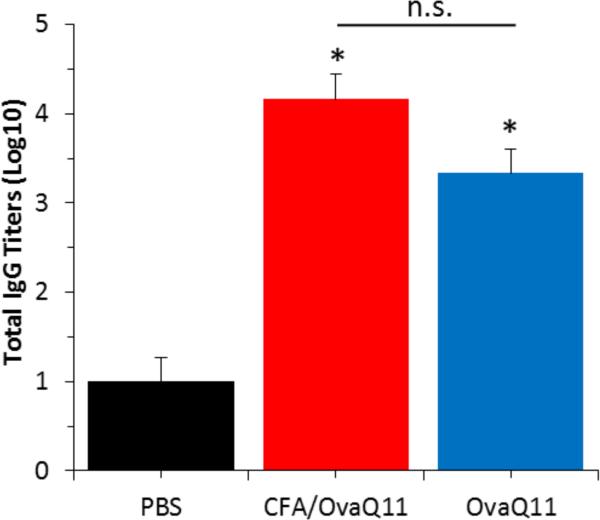
To produce different immune phenotypes with similar levels of antibody titers, we immunized mice with ovaQ11 with or without complete Freund's adjuvant (CFA). Previous studies indicated that ovaQ11 raises adaptive immune responses with essentially undetectable inflammatory responses [13], whereas CFA is known to be significantly inflammatory [43]. Negative control mice were injected with phosphate buffered saline. Mice were immunized and boosted at week 4 and week 6. By week 8 the two immunized groups had similar titers of IgG against ovaQ11 (Figure 1). Q11 not bearing an epitope has been previously shown to be unable to raise antibody responses [14, 15].
Figure 1. Antibody responses to unadjuvanted and adjuvanted peptide assemblies were similar.
Serum total IgG titers against ovaQ11 were not significantly different for mice immunized with ovaQ11 versus CFA/ovaQ11. Mice were immunized at week zero and received booster immunizations at week 4 and week 6. Titers for total IgG against ovaQ11 were measured by ELISA at week 8. n=5 mice per group. Each bar represents mean +/− standard error. *p <0.05 compared to negative control (PBS). n.s., not statistically different.
3.2. Wound healing in the presence of immunogenic peptide nanofibers
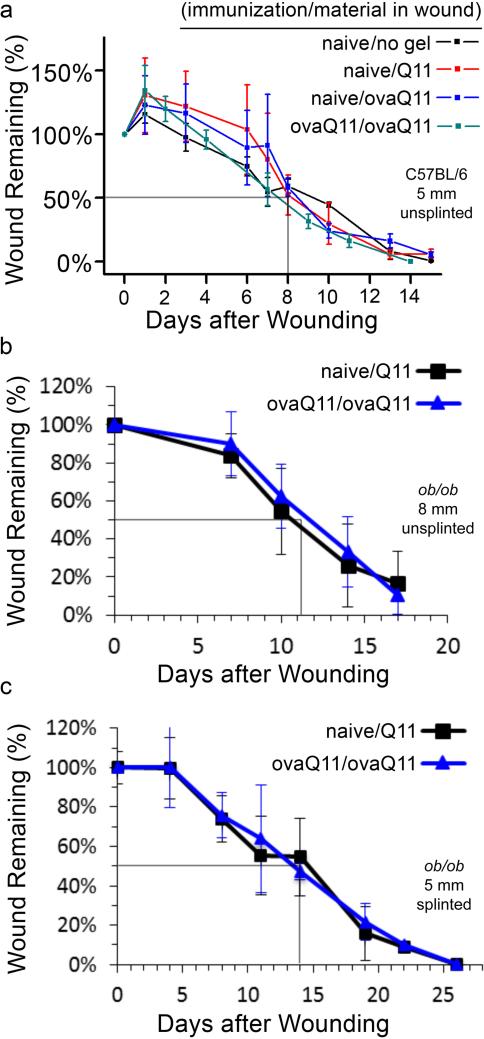
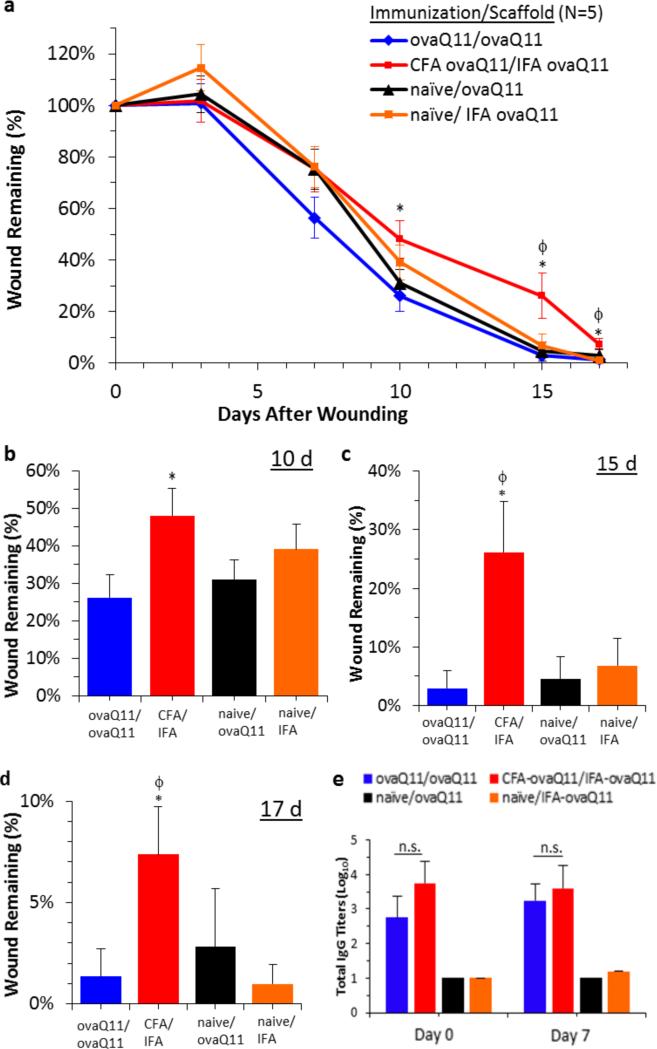
In three different mouse models with increasingly delayed healing (unsplinted C57BL/6, unsplinted ob/ob, and splinted ob/ob), we assessed whether an ongoing antibody response to the self-assembled peptide scaffold would delay wound healing, and whether the phenotype of the immune response had a measurable effect. Mice were either immunized against ovaQ11 prior to wounding or were naïve to ovaQ11 until it was placed in the wounds. In the wounds, subsets of mice received either non-immunogenic Q11, immunogenic ovaQ11, or PBS. Here the naming of the groups follows the convention of “(vaccination material/wound material)”. The rate of wound healing was first measured in C57BL/6 mice with 5mm unsplinted wounds. In all groups, it took around 8 days for the wounds to be about 50% closed (Figure 2a). In this model, there was no difference in the rate of healing between naïve mice receiving non-immunogenic peptide (naïve/Q11) and mice previously vaccinated with ovaQ11 receiving ovaQ11 in their wounds (ovaQ11/ovaQ11) (Figure 2a). Additionally there was no significant difference between vaccinated and unvaccinated mice receiving ovaQ11 in their wounds (ovaQ11/ovaQ11) versus (naïve/ovaQ11) (Figure 2a). Unsplinted ob/ob mice healed more slowly than C57BL/6 mice (Figure 2b), with 50% closure occurring around 11 days for all groups, yet there was still no significant difference between naïve/Q11 and ovaQ11/ovaQ11 groups. Finally, splinted ob/ob mice healed the slowest, with 50% closure occurring around 14 days, yet naïve/Q11 and ovaQ11/ovaQ11 groups remained statistically similar (Figure 2c). In sum, whether splinted or not, and in a range of models with varying healing times, ovaQ11/ovaQ11 groups healed at similar rates as naïve/Q11 groups. Collectively these results indicated that immunogenic peptide scaffolds did not have a negative effect on healing rates in these models. Next we investigated whether the phenotype of the immune responses could be altered to perturb the immunologically compatible phenotype observed with peptide self-assemblies so far. To do this, we immunized mice with ovaQ11 in complete Freund's adjuvant (CFA) and then applied ovaQ11 in incomplete Freund's adjuvant (IFA) in the wound (CFA-ovaQ11; IFA-ovaQ11 group). Here the model with the intermediate healing rate, the 8 mm unsplinted ob/ob mouse model was used. For comparison, two negative control groups remaineded unimmunized, with one unimmunized group receiving ovaQ11 in their wounds and the other receiving IFA-ovaQ11 in the wounds. These two control groups were included to measure the independent effect of the immunization and of IFA in the wounds, respectively. The rate of wound healing is shown in Figure 3a for the four conditions. Interestingly, the rate of healing for the CFA-ovaQ11/IFA-ovaQ11 group was significantly slower than the other groups at day 10, 15, and 17 (Figure 3a-d). IFA in the wound did not appear to have a significant effect by itself, as healing in the naïve/IFA-ovaQ11 group was statistically similar to the naïve/ovaQ11 group and the ovaQ11/ovaQ11 group at all time points (Figure 3a-d). Antibody titers were also similar between the ovaQ11/ovaQ11 group and the CFA-ovaQ11/IFA-ovaQ11 group (Figure 3e), indicating that the adjuvanted and unadjuvanted antibody responses were of similar strength. Collectively, these results indicated that whereas ovaQ11 peptide nanofibers raised an immune response that did not adversely affect wound healing, this phenotype could be shifted to one that does adversely affect healing, through the use of inflammatory adjuvants (CFA/IFA).
Figure 2. The rate of wound healing is unaffected by high antibody titers against the scaffold.
Wound closure rates are shown for 3 different models of increasingly delayed healing. Gray lines indicate approximate times to 50% healing. Naming convention: “(immunization material/scaffold material)” (a) The 5 mm unsplinted C57BL/6 model demonstrated no significant difference in wound healing rate between (ovaQ11/ovaQ11), (naïve/Q11), (naïve/PBS), or (naïve/ovaQ11). n= 3 mice per group, except for ovaQ11/ovaQ11 for which n=2 because one mouse died during anesthesia. (b) 8 mm unsplinted ob/ob model demonstrated no significant difference in wound healing rates between (naïve/Q11) and (ovaQ11/ovaQ11). n= 9 mice per group. (c) Splinted 5 mm ob/ob wound model demonstrated no significant difference in wound healing rates between (naïve/Q11) and (ovaQ11/ovaQ11), n=6 mice per group.
Figure 3. Healing rate was negatively affected by CFA-adjuvanted immunizations but not unadjuvanted peptide assemblies.
(a) In unsplinted 8 mm wounds in ob/ob mice, healing was delayed for CFA-ovaQ11/IFAovaQ11 compared to ovaQ11/ovaQ11, naïve/IFA-ovaQ11, and naïve/ovaQ11 (immunization/material in wound). The latter three groups did not differ significantly from each other. (b-d) Comparisons between wounds at day 10, 15, and 17. (e) Serum titers at the time of wounding at day 0 and day 7 after wounding, against ovaQ11 as measured by ELISA. All subpanels indicate mean with standard error. n=10 wounds per group (5 mice with 2 wounds per mouse). *p<0.05 comparing CFAovaQ11/IFAovaQ11 to ovaQ11/ovaQ11. ϕ p <0.05 comparing CFAovaQ11/IFAovaQ11 with naïve/IFAovaQ11. n.s, not statistically different.
3.3. Cell Recruitment to the Wound Bed
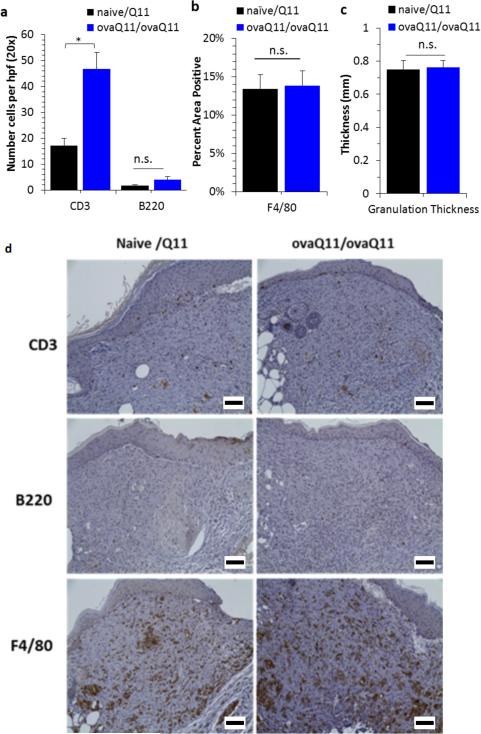
Because no significant differences in healing rates were seen grossly between immunized and non-immunized mice when ovaQ11 was used as the immunizing agent and the scaffold, we examined the wounds histologically to determine if there were differences in CD3+ cells (T cells), B220+ cells (B cells), or F4/80+ cells (macrophages) between immunogenic and nonimmunogenic materials. At day 17, immunohistochemistry demonstrated significantly more CD3+ T cells in the wound bed for ovaQ11/ovaQ11 mice compared to naïve/Q11 mice (Figure 4a). Negligible B220+ cells and significant numbers of F4/80+ cells were found in the wound bed for both groups, but the groups were not statistically different from one another (Figure 4a, 4b). Granulation tissue thickness did not vary between groups (Figure 4c). These results indicated that ovaQ11 scaffolds recruited significant numbers of T cells but their recruitment of B cells and macrophages was not significantly different compared to non-immunogenic scaffolds.
Figure 4. Immunogenic scaffolds recruited more T cells than non-immunogenic scaffolds.
Histologic analysis at day 17 (8 mm wounds in unsplinted ob/ob mice). Naïve/ovaQ11 groups were compared with ovaQ11/ovaQ11 (immunization/wound material). (a) Immunohistochemistry demonstrated significantly greater CD3+ (T cell) recruitment in the wound for ovaQ11/ovaQ11 mice compared naïve/Q11 mice. B220+ (B cells) were scant and not significantly different between the two groups. (b) F4/80+ cells were abundant in both groups and not significantly different. (c) The amount of granulation tissue was not significantly different between the groups. (d) Representative images of immunohistochemistry of the wound beds stained for CD3, B220 and F4/80.*p<0.05 by Student's t-test; n.s, not statistically different. n=9 mice per group. Scale bars 200 μm.
3.4. T cell phenotpyes
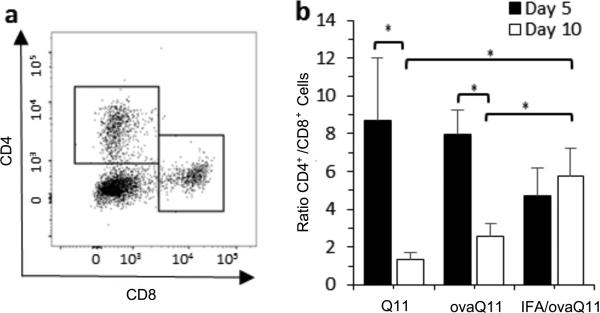
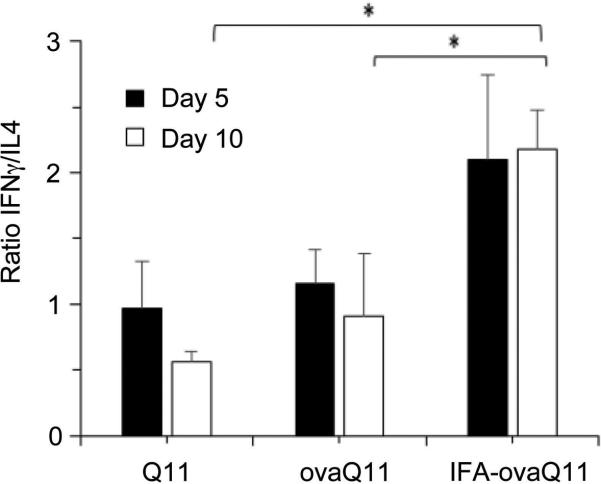
Several groups have demonstrated a significant role for T cells in wound healing [5, 7]. This previous work along with our observation of increased T cells in wound beds of immunogenic scaffolds led us to evaluate T cell phenotype. The dermal wound bed was harvested and digested, and T cell populations were analyzed by flow cytometry. In this experiment all mice were immunized against OVA-Q11. In C57BL/6 mice, 5 mm unsplinted wounds were created on immunized mice, and into the wounds were placed either Q11 (non-immunogenic and non-inflammatory), ovaQ11 (immunogenic and non-inflammmatory) or IFA-ovaQ11 (immunogenic and inflammatory). CD3+ lymphocytes were isolated from the wound bed tissue at 5 days and 10 days post-wounding. At Day 5, there were significantly more CD4+ cells in the wound beds than CD8+ cells for all groups (Figure 5). By 10 days after wounding, however, the CD4/CD8 ratio was more balanced for mice receiving Q11 and ovaQ11 in their wounds. For mice receiving IFA-ovaQ11 in their wounds, the predominance of CD4+ cells persisted. Further, CD4+ cells were additionally investigated with respect to their Th1/Th2 polarization by measuring IFNγ (Th1) and IL4 (Th2) by flow cytometry. At both Day 5 and Day 10, mice receiving Q11 and ovaQ11 in their wounds had T cell populations with similar numbers of IFNγ- and IL4-producing cells (Figure 6). In contrast, mice receiving adjuvanted materials exhibited a predominance of cells producing IFNγ compared to IL4 at both time periods. This indicated that the adjuvanted materials produced a T cell phenotype that was slanted more towards a Th1 response than the unadjuvanted peptides.
Figure 5. CD4+ helper and CD8+ cytotoxic lymphocyte balance within wounds.
Mice immunized against ovaQ11 were treated with Q11, ovaQ11 or IFA/ovaQ11 in their wounds, and lymphocytes were isolated from wounds at days 5 and 10 (5 mm wounds in C57BL/6 mice). (a) Flow cytometry gating for CD4+ (top left box) and CD8+ (lower right box) lymphocytes. (b) In wounds treated with Q11 or ovaQ11, CD4+ T cells predominated at Day 5 but were more balanced with CD8+ T cells at Day 10. Wounds treated with adjuvanted ovaQ11 retained their predominance of CD4+ T cells at Day 10. n= 6 wounds per time point (3 mice, 2 wounds per mouse). *p<0.05 by ANOVA with Tukey HSD post hoc test.
Figure 6. Cytokine Production of CD4+ cells: Th1 versus Th2.
Mice immunized against ovaQ11 received dermal wounds treated with Q11, ovaQ11 or IFA-ovaQ11 (5 mm wounds in C57BL/6 mice). T cells expressing IFNγ and IL4 were counted by flow cytometry. At Days 5 and 10 post-wounding, mice receiving Q11 or ovaQ11 in their wounds exhibited balanced Th1/Th2 T cell responses of similar amounts of IFNγ and IL4-producing cells, whereas mice receiving CFA-ovaQ11 had greater numbers of IFNγ-producing CD4+ T cells compared to IL4-producing CD4+ T cells, indicating a response that was comparatively more slanted towards Th1. n = 6 wounds per time point, 3 mice and 2 wounds per mouse. *p<0.05 by ANOVA with Tukey HSD post hoc test.
4. Discussion
Here we describe self-assembled peptide nanofiber biomaterials that raise strong immune responses yet still perform well in dermal wounds, not delaying closure in three different murine models. In three wound models spanning a range of overall rates of closure (wounds in C57BL/6 mice and both splinted and unsplinted wounds in ob/ob mice), these peptide biomaterials did not delay closure despite significant ongoing immune responses. Wounds containing the immunogenic ovaQ11 healed at the same rate as non-immunogenic Q11, even when mice had been immunized previously against ovaQ11. In contrast, mice that had been immunized using adjuvants that provoke an immune response with a more inflammatory phenotype demonstrated significant delayed closure in comparison, despite similar antibody titers to unadjuvanted materials.
The implications for self-assembled peptide biomaterials are significant. This class of materials has been gaining interest for a wide variety of in vivo biomedical applications including tissue engineering, cell delivery, and tissue repair [30], but in separate studies self-assembled peptides have also recently been found to elicit robust immune responses [15, 21]. Whether a self-assembled peptide is immunogenic or not is highly dependent on its epitope content [14, 26], and to our knowledge no self-assembled peptide had yet been studied explicitly with respect to its epitope-specific immunogenicity in tissue engineering or wound healing applications. As such, although there have been numerous reports of this class of materials being used successfully in vivo [44-49], it has remained an open question whether the immune responses observed for Q11 and other self-assembling peptides would be compatible, harmful, or beneficial when the materials are used as scaffolds for tissue repair. Here we report that even when an epitope-specific immune response is pre-established against these materials, they still function well in dermal wounds. This bodes well for the use of not only Q11 but other fibrillar peptide assemblies materials in other tissue repair contexts, regardless of whether an immunogenic epitope (B cell epitope or T cell epitope) is included in the peptide sequence. More broadly, this work indicates that there may be a specific type of anti-biomaterial immune response phenotype, or a range of phenotypes, that are consistent with biomaterials’ use in vivo. If borne out with ongoing and future work, this would constitute an interesting update of the current thinking in the biomaterials field that any anti-biomaterial immune response is necessarily harmful. In the work described in this paper, these productively immunogenic peptide assemblies were associated with a more Th2-slanted response throughout the duration of healing compared to adjuvanted materials that impeded wound healing, which exhibited a more Th1-slanted charater based on higher IFNγ secretion. The compatible phenotype of peptide nanofibers was also associated with a progression from a CD4+ T cell-dominated response to a more balanced CD4+/CD8+ T cell response by day 10 after healing. Further phenotypic characterization of these T cells may shed light on the progression of not only Th1/Th2 bias but also regulatory and tolerogenic phenotypes.
The delayed healing of adjuvanted groups was attributable to immune responses beyond the local effects of the adjuvant (IFA) in the wound bed, because mice that did not receive presensitizing immunizations of nanofibers in CFA healed more quickly than those that had been pre-immunized with the CFA-adjuvanted nanofibers. This adjuvanted immune phenotype represents one that is not as compatible with wound healing compared with unadjuvanted peptide nanofibers, despite the fact that the two formulations raise similar antibody titers. If specific immune phenotypes compatible with tissue repair such as the one raised by peptide nanofibers can be clearly defined, it would be helpful in the design of next-generation biomaterials composed of potentially immunogenic components such as peptides, proteins, or bioconjugates. Of course, it will be important to assess whether the phenomena we have observed in mice hold true for humans and for other defects sites beyond skin. However, it is encouraging that previous work with xenogeneic scaffolds such as SIS show that in humans even immunogenic materials can perform quite well clinically [19, 50].
A number of observations in this study prompt additional questions. For example, despite a strong antibody response to ovaQ11, there were very few B cells found in the wounds containing it. Previous studies by others have also found minimal B cell presence in healing wounds, so the role of B cells in such contexts has been believed to be small [5, 51]. However, B cells in lymphoid tissues remote from the wound are responsible for secreting the high antibody titers observed and as shown by previous work such antibody responses may play a role in healing [12]. B cells could have a remote influence on the course of wound healing, for example by secreting antibodies that bind to the material and influence both its clearance and the immunological phenotype around the biomaterial. This relationship would be interesting to study in future work, by investigating the development of anti-biomaterial responses in the spleen and lymph nodes during the course of healing in the presence of self-assembled biomaterials. Additionally, our work here poses interesting questions as to whether there may be an interaction between the Th1/Th2 polarization of T cells and the M1/M2 polarization of macrophages. In the field of Biomaterials, the understanding of these relationships is still early, yet it is apparent that many of the cytokines involved in the Th1/Th2 axis have strong influences in M1/M2 polarization [9, 10, 52]. Owing to the important role of macrophages in wound healing, this represents another potentially interesting area for future study.
5. Conclusions
We found that self-assembling peptide biomaterials in wound healing contexts can be highly immunogenic yet not impede normal wound healing. For these materials, their use in tissue repair and wound healing is not dependent on rendering them non-immunogenic. The immune response raised by these specific materials, while significant, is of a phenotype that does not have a significant adverse effect on healing. In comparison, adjuvanted peptide assemblies did have a deleterious effect on the rate of healing. Collectively, the results indicate that peptide self-assemblies may be appropriate as scaffolds for tissue repair or wound healing, and that the immune responses that they raise do not necessarily need to be attenuated for these applications.
Acknowledgements
The research described in this article was supported by the National Institutes of Health: The National Institute of Biomedical Imaging and Bioengineering (NIBIB) under grant number 1R01EB009701, and the National Institute of Allergy and Infectious Diseases (NIAID) under grant number 1R01AI118182. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
Footnotes
None of the authors have financial conflicts of interest to disclose.
References
- 1.Gawronska-Kozak B, Bogacki M, Rim JS, Monroe WT, Manuel JA. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006;14:265–76. doi: 10.1111/j.1743-6109.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyce DE, Ciampolini J, Ruge F, Murison MS, Harding KG. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg. 2001;54:511–6. doi: 10.1054/bjps.2001.3638. [DOI] [PubMed] [Google Scholar]
- 3.Adolph VR, DiSanto SK, Bleacher JC, Dillon PW, Krummel TM. The potential role of the lymphocyte in fetal wound healing. J Pediatr Surg. 1993;28:1316–20. doi: 10.1016/s0022-3468(05)80320-3. [DOI] [PubMed] [Google Scholar]
- 4.Barbul A, Regan MC. The regulatory role of T lymphocytes in wound healing. J Trauma. 1990;30:S97–100. doi: 10.1097/00005373-199012001-00021. [DOI] [PubMed] [Google Scholar]
- 5.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–6S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 6.Martin CW, Muir IF. The role of lymphocytes in wound healing. Br J Plast Surg. 1990;43:655–62. doi: 10.1016/0007-1226(90)90185-3. [DOI] [PubMed] [Google Scholar]
- 7.Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, Barbul A. Wound healing and T-lymphocytes. J Surg Res. 1990;48:460–3. doi: 10.1016/0022-4804(90)90013-r. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JM, Barbul A, Breslin RJ, Wasserkrug HL, Efron G. Significance of T-lymphocytes in wound healing. Surgery. 1987;102:300–5. [PubMed] [Google Scholar]
- 9.Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20:517–27. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 11.Iwata Y, Yoshizaki A, Komura K, Shimizu K, Ogawa F, Hara T, et al. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am J Pathol. 2009;175:649–60. doi: 10.2353/ajpath.2009.080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishio N, Ito S, Suzuki H, Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology. 2009;128:369–80. doi: 10.1111/j.1365-2567.2009.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, Sun T, et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–85. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–64. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–7. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishel RS, Barbul A, Beschorner WE, Wasserkrug HL, Efron G. Lymphocyte participation in wound healing. Morphologic assessment using monoclonal antibodies. Ann Surg. 1987;206:25–9. doi: 10.1097/00000658-198707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbul A, Breslin RJ, Woodyard JP, Wasserkrug HL, Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479–83. doi: 10.1097/00000658-198904000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis PA, Corless DJ, Aspinall R, Wastell C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. Br J Surg. 2001;88:298–304. doi: 10.1046/j.1365-2168.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 19.Ansaloni L, Cambrini P, Catena F, Di Saverio S, Gagliardi S, Gazzotti F, et al. Immune response to small intestinal submucosa (surgisis) implant in humans: preliminary observations. J Invest Surg. 2007;20:237–41. doi: 10.1080/08941930701481296. [DOI] [PubMed] [Google Scholar]
- 20.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–40. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 21.Hudalla GA, Sun T, Gasiorowski JZ, Han H, Tian YF, Chong AS, et al. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat Mater. 2014;13:829–36. doi: 10.1038/nmat3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trent A, Ulery BD, Black MJ, Barrett JC, Liang S, Kostenko Y, et al. Peptide amphiphile micelles self-adjuvant group A streptococcal vaccination. AAPS J. 2015;17:380–8. doi: 10.1208/s12248-014-9707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black M, Trent A, Kostenko Y, Lee JS, Olive C, Tirrell M. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv Mater. 2012;24:3845–9. doi: 10.1002/adma.201200209. [DOI] [PubMed] [Google Scholar]
- 24.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–10. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung JP, Moyano JV, Collier JH. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr Biol (Camb) 2011;3:185–96. doi: 10.1039/c0ib00112k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pompano RR, Chen J, Verbus EA, Han H, Fridman A, McNeely T, et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv Healthc Mater. 2014;3:1898–908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, et al. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–84. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 30.Matson JB, Stupp SI. Self-assembling peptide scaffolds for regenerative medicine. Chem Commun (Camb) 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian YF, Hudalla GA, Han H, Collier JH. Controllably degradable β-sheet nanofibers and gels from self-assembling depsipeptides. Biomater Sci. 2013:1. doi: 10.1039/C3BM60161G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian YF, Devgun JM, Collier JH. Fibrillized peptide microgels for cell encapsulation and 3D cell culture. Soft Matter. 2011;7:6005–11. doi: 10.1039/C1SM05504F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29:2143–51. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ring BD, Scully S, Davis CR, Baker MB, Cullen MJ, Pelleymounter MA, et al. Systemically and topically administered leptin both accelerate wound healing in diabetic ob/ob mice. Endocrinology. 2000;141:446–9. doi: 10.1210/endo.141.1.7373. [DOI] [PubMed] [Google Scholar]
- 36.Tsuboi R, Shi CM, Rifkin DB, Ogawa H. A wound healing model using healing-impaired diabetic mice. J Dermatol. 1992;19:673–5. doi: 10.1111/j.1346-8138.1992.tb03757.x. [DOI] [PubMed] [Google Scholar]
- 37.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–46. [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan SR, Underwood RA, Gibran NS, Sigle RO, Usui ML, Carter WG, et al. Validation of a model for the study of multiple wounds in the diabetic mouse (db/db). Plast Reconstr Surg. 2004;113:953–60. doi: 10.1097/01.prs.0000105044.03230.f4. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Ge J, Tredget EE, Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc. 2013;8:302–9. doi: 10.1038/nprot.2013.002. [DOI] [PubMed] [Google Scholar]
- 40.Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–92. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 41.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–9. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 43.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25:3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 44.Jung JP, Gasiorowski JZ, Collier JH. Fibrillar peptide gels in biotechnology and biomedicine. Biopolymers. 2010;94:49–59. doi: 10.1002/bip.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segers VF, Lee RT. Local delivery of proteins and the use of self-assembling peptides. Drug Discov Today. 2007;12:561–8. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Semino CE. Self-assembling peptides: from bio-inspired materials to bone regeneration. J Dent Res. 2008;87:606–16. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]
- 47.Kopecek J, Yang J. Peptide-directed self-assembly of hydrogels. Acta Biomater. 2009;5:805–16. doi: 10.1016/j.actbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamley IW. Peptide fibrillization. Angew Chem Int Ed Engl. 2007;46:8128–47. doi: 10.1002/anie.200700861. [DOI] [PubMed] [Google Scholar]
- 49.Ulijn RV, Smith AM. Designing peptide based nanomaterials. Chem Soc Rev. 2008;37:664–75. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]
- 50.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez A, Voskerician G, Meyerson H, MacEwan SR, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J Biomed Mater Res A. 2008;85:556–65. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- 52.Strom TB, Roy-Chaudhury P, Manfro R, Zheng XX, Nickerson PW, Wood K, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–93. doi: 10.1016/s0952-7915(96)80087-2. [DOI] [PubMed] [Google Scholar]