Abstract
Introduction
Dilated cardiomyopathy (DCM) is the most common cardiomyopathy and occurs often in families. As an inherited disease, understanding the significance of diagnostic procedures and genetic screening within families is of utmost importance.
Areas covered
Genetic studies have shown that in 30–40% of familial DCM (FDC) cases a causative genetic mutation can be identified. Successful genetic analysis is highly dependent on close examination of patient and family history, and clinical guidelines exist recommending genetic testing to aid in the evaluation of family members at risk of developing FDC. Clinical genetic testing offers a resource for families to identify the etiology of their disease, and in some cases may provide clinical prognostic insight.
Expert Opinion
As an inherited disease, future FCD studies will focus on elucidating the remaining 60–70% of genetic causes in inherited cases and the pathogenic mechanisms leading to the phenotype. Specifically, a focus on regulatory regions, copy number variation, genetic and environmental modifiers and functional confirmatory investigations will be essential.
I. Introduction: Familial Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a disease of the heart muscle characterized by left ventricle dilation and impaired systolic function. The wall of the left ventricle progressively weakens and thins, and the heart cannot pump blood efficiently to meet physiological needs. DCM is the most common form of cardiomyopathy1 and a leading cause of heart failure, transplantation, and death.2–4 Primary DCM has been described using a mixed model, meaning that it has either acquired or genetic etiology.4 An acquired dilated phenotype may result from a variety of factors including hypertension, valvular heart disease, autoimmune response, myocarditis, excessive alcohol consumption, drug usage, toxicity, and congenital heart disease.5 Primary DCM results when all acquired factors have been excluded, and can be either ‘idiopathic’ or ‘familial’.5, 6
Screening of relatives, in particular first degree relatives, of primary DCM cases using electrocardiogram (ECG) and echocardiography to measure left ventricle size and function allow the identification of familial DCM (FDC), where two or more family members are affected by cardiomyopathy. FDC can be identified in 20–35% of DCM cases, while the remaining are classified as ‘idiopathic’. However, the frequency of familial forms is likely underestimated due to small pedigrees and families with undiagnosed members, where an underlying genetic cause may be less apparent and more difficult to elucidate due to variable expressivity and reduced penetrance of the disease gene.7–10 Of the FDC cases, 30–40% of them have an identified genetic origin attributed to over 50 genes, a number that is constantly increasing as new genes are discovered.11, 12 While FDC is primarily an autosomal dominant disease, autosomal recessive and X-linked forms have also been described.13
DCM, which includes FDC, was originally considered to be a cytoskeletal disease, but it has since been shown that mutations in a large number of genes encoding various cardiac components can lead to disease. To date, it has been demonstrated that defects in the sarcomere, Z-disk, cytoskeleton, nuclear skeleton, mitochondria, desmosomes, sodium and potassium channels, and lysosomal membranes all play a role in DCM.11, 14 This indicates that DCM can result from interrupting a variety of pathways, which has made studying the pathogenesis of DCM a complex process.
II. Clinical Diagnosis and Management
DCM in general, despite its underlying etiology, is defined by the presence of two major clinical criteria: left ventricular (LV) fractional shortening less than 25% and/or LV ejection fraction less than 45% with LV end diastolic diameter greater than 117% of the predicted value corrected for age and body surface area based on Henry's formula (corresponding to two standard deviations (SD) from the expected normal limit plus 5%).9 These criteria are detailed in Table 1. DCM is only diagnosed in the absence of any other known cause of myocardial disease,9, 15, 16 and a diagnosis of idiopathic DCM is only assigned after all other possible explanations have been excluded.11
Table 1.
DCM and FDC diagnostic criteria.
| DCM |
|
| FDC |
|
| FDC in family members of proband |
|
| FDC minor criteria* |
|
SD = standard deviation
Family members of a proband are considered affected if they meet at least three minor criteria. If they meet one or two criteria, they are considered unknown.
In the context of familial DCM, these two major criteria are used to diagnose the proband in a family, the person who first presents in the clinic with the disease, while diagnosis of other family members follows less stringent criteria. This accounts for the fact that early signs of disease are more difficult to capture but also are more likely to occur when another family member has already been diagnosed with the disease. DCM can then be considered familial (FDC) when two or more affected relatives with DCM meet the major criteria of DCM, or when a first-degree relative of a diagnosed DCM patient dies inexplicably and suddenly before the age of 35.9 Other than a positive family history, there are no clinical phenotypes to distinguish between FDC and idiopathic DCM.3 Conversely, a negative family history does not eliminate the possibility of DCM due to genetic etiology. The individual could have a de novo mutation, or there could be cardiomyopathy within the pedigree that has not yet been recognized.11
In familial cardiomyopathies the pedigree is one of the most important tools in clinical care.11, 17 Optimally, a pedigree will include at least three generations of individuals.17, 18 A well-annotated family history sets the stage for determining disease status: family members in the pedigree are classified as affected, unaffected, or unknown. All first-degree relatives of a proband diagnosed with idiopathic DCM are strongly recommended to undergo clinical screening that includes an ECG and echocardiography. After family members have been screened, affected individuals are defined by one of three possibilities: the presence of the two major DCM criteria, the second major DCM criterion (LVEDD >117%) plus one minor criteria, or three minor criteria.9, 16 Table 1 outlines all the clinical diagnostic criteria of FDC, including the minor criteria.
Family members with any of the following conditions are typically excluded from being considered as affected: (1) consistently documented blood pressure greater than 160/110 mmHg; (2) obstruction (greater than 50%) of a major branch of the coronary artery; (3) alcohol intake greater than 100 g/day; (4) persistent supraventricular arrhythmia; (5) systemic diseases; (6) pericardial diseases; (7) congenital heart diseases; (8) pulmonary heart disease; (9) myocarditis. Some caveats in family member inclusion or exclusion include the fact that myocarditis and peripartum cardiomyopathy also occur in familial settings and rendering it hard to classify the exact cause of dilation and systolic dysfunction.16, 19 It has been suggested that peripartum cardiomyopathy in particular can be found more often in families with FDC, and may be a consequence of genetic etiology.20 Additionally, exclusion based upon alcohol consumption may be controversial as it has been shown previously that patients formerly diagnosed as having DCM secondary to alcohol consumption had affected first-degree relatives, implying a FDC diagnosis instead.21
Due to the variable expressivity of FDC, disease classification is not always straightforward. Inter- and intra-familial variation exists, especially in phenotypic severity and age of onset.2, 22 Age of onset is typically between the ages of 20–50, and while it can occur earlier or later, it is rarely diagnosed in the elderly.13, 22 Family members may be classified as unknown to indicate that they exhibit subtle cardiac abnormalities that may be relevant, but their symptoms are not sufficient for a definitive diagnosis at that time.9, 15 Unknown individuals are classified as having 1 or 2 minor criteria, but not meeting the threshold to be considered affected.16 This could reflect an individual that has not yet fully developed the disease, but also exhibits a need for more sensitive clinical criteria in order to more accurately diagnose affected relatives as quickly as possible.5 Even as a frequently autosomal dominant disease, FDC tends to demonstrate reduced penetrance, meaning that not all family members that you would expect to have the disease actually display signs of it. For example, an unaffected individual can have both an affected parent as well as an affected child. In cases where the specific genetic mutation has been identified, there may be individuals with the identified causal mutation that do not present with disease.3, 5 Additionally, there have been some cases of FDC where affected relatives actually present with hypertrophic cardiomyopathy (HCM) instead. This may be due to the genetic overlap seen in these two diseases, and it is possible that the same mutation could present differently due to environmental factors or other genetic contributions.21
Aside from clinical diagnostic criteria, other symptoms are characteristic of DCM and may vary between and within families. These can be cardiac-related problems, like conduction system abnormalities, valvular deformations, left ventricular non-compaction, and segmental hypokinesia, or the result of heart failure, like dyspnea, fatigue, and palpitations.8, 11 Abnormalities in other systems may occur as well, such as lipodystrophies, sensorineural deafness, and skeletal myopathies, the latter of which may be indicative of a multi-systemic disease like Emery-Dreifuss muscular dystrophy, Barth Syndrome, and Duchenne’s muscular dystrophy. These multi-systemic signs or symptoms are important and should be considered ‘red flags’ useful for the identification of the underlying genetic disorder.11 Alternatively, in DCM and FDC, many individuals may remain asymptomatic.2, 3, 13
Those family members identified as clinically affected should receive standard pharmacological management according to their symptoms and severity and according to current guidelines. For asymptomatic family members, especially younger offspring of the proband who may have not yet presented with the disease as well as family members who were categorized as unknown, it is recommended that there should be periodic follow-up appointments for cardiac screening.3, 23 The frequency of follow-up appointments should be determined on a familial basis, taking into account the typical age of onset and the minor symptoms presented in unknown individuals.3 Typically, if no mutation is known or present, these23 follow-up appointments should occur every 3–5 years, and if a mutation is present appointments should occur more frequently, yearly in childhood and very 1–3 years in adults. At the initial visit, creatine kinase (CK) levels of skeletal and muscle isoforms (MM) should be taken.23 At each visit thereafter, standard ECG and echocardiography should be performed to assess even minor changes (such as increases in left ventricle size and contractile function impairment) that could suggest early disease stages.3 A detailed history should be taken, with special attention given to assess arrhythmia, pre-syncope, and syncope symptoms. Physical examination should consider changes in both heart and skeletal muscle function.23 Other important tools more recently introduced in the clinical practice that may allow for better risk stratification in the progression of heart failure and sudden death include cardiac magnetic resonance (CMR)24 and Holter monitoring.25
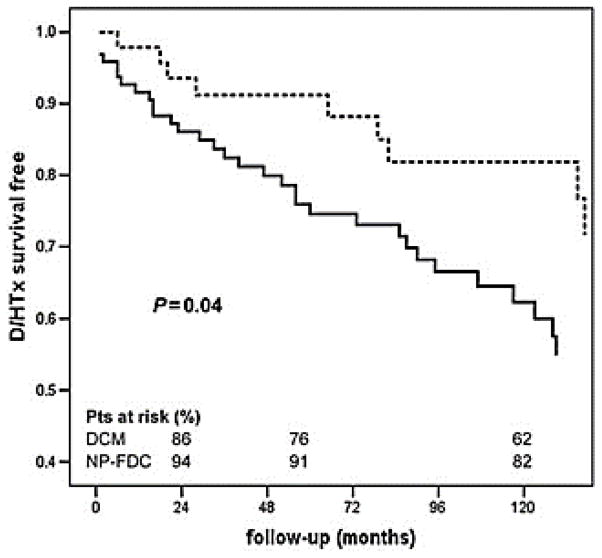
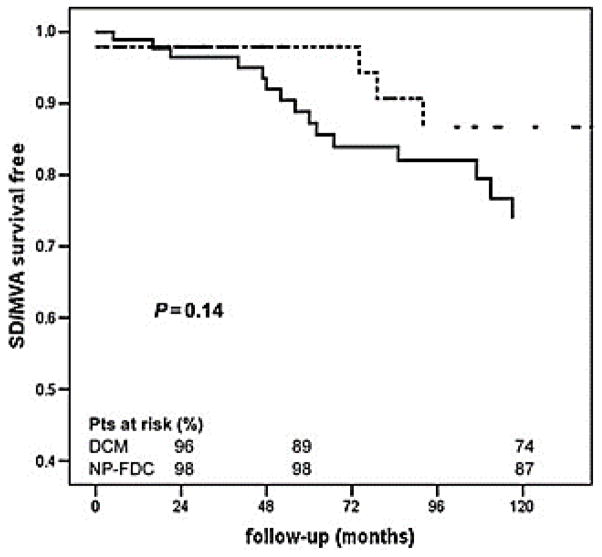
Longitudinal studies have demonstrated that approximately 1 in 10 of the individuals showing mild changes in left ventricle dilation and contraction develop DCM within five years.3, 26, 27 Family screening in FDC helps to identify patients at earlier stages of disease than in sporadic cases, and it has been suggested that this early identification can improve survival rates as compared to sporadic DCM cases.28 Patients identified via familial screening are less likely to undergo heart transplant or death resulting from FCM (Figure 1), but at this point has not been shown to protect against sudden death or life-threatening arrhythmias, for which FCM patients and sporadic DCM patients are equally susceptible (Figure 2).28
Figure 1. Patient survival in sporadic and familial DCM.
Analysis of survival free from death and heart transplant (D/HTx) in sporadic (solid line) and non-proband familial DCM (NP-FDC) (dotted line). From Moretti et al., with permission.28
Figure 2. Patient survival in sporadic and familial DCM.
Analysis of survival free from sudden death and major ventricular arrhythmias (SD/MVA) in sporadic (solid line) and non-proband familial DCM (NP-FDC) (dotted line). From Moretti et al., with permission.28
III. Prevalence
Prevalence data for DCM has been limited due to lack of large-scale, rigorous, population-based studies. Initial estimations originated from a study carried out by Codd et al from 1975 to 1984 in Olmstead County, Minnesota, USA. This frequently cited prospective study identified 46 individuals with idiopathic DCM who were evaluated via echocardiography (n=41) and/or angiography (n=16) with 4 individuals identified during autopsy. This number of affected individuals results in a prevalence of 36.5/100,000 individuals, or 1 in 2,700 with a male to female ratio of 3.4. The group also evaluated the frequency of hypertrophic cardiomyopathy (HCM), which is characterized by thickening of the heart muscle. They identified 21 individuals, which was half as many as DCM, giving HCM a prevalence of 19.7/100,000 or 1 in 5,100.29 Since this study’s publication in 1989, the prevalence of HCM has been reassessed to 1 in 500 individuals, while reports of the prevalence of DCM have remained inconsistent.30
Reports of the incidence and prevalence of idiopathic DCM have been highly variable, partially due to variation in diagnostic criteria but also possibly due to ethnic differences between populations studied.31, 32 Around the same time the Olmstead County study was taking place, another study found a prevalence of 8.3/100,000 in two regions in England.33 A two-year study from Trieste, Italy found 4.5/100,000 cases per year via autopsy, with an additional 2.5/100,000 cases per year clinically, for a total incidence of 7.0/100,000.34 A more recent 2002 study from Japan cites the incidence at 3.6/100,000, with an estimated crude prevalence of 14/100,000, or about 1 in 7000, which is remarkably less than the Codd study.32 These studies have been summarized in Table 2. Without a large-scale, epidemiological study based on modern imaging technology and diagnostic criteria, the true occurrence of DCM has yet to be determined.
Table 2.
Summary of prevalence/incidence studies referenced.
Though additional formal studies have yet to be done, many researchers and clinicians agree that the prevalence of idiopathic DCM is likely more common than 1 in 2,700, and that it may be underdiagnosed.5, 12, 16 A 2013 review by Hershberger et al. evaluated the history of DCM prevalence and questions the estimates that have been made.5 The authors clearly outline three approaches that can be used to extrapolate a more accurate estimate of idiopathic DCM prevalence: (1) evaluating the DCM:HCM ratio, (2) considering the clinical data of heart failure patients, and (3) using the more well-documented estimates of left ventricular dysfunction as a proxy for DCM.5
The first approach uses the Olmstead County study as its basis. In the study, the ratio of DCM:HCM was approximately 2:1. Assuming this ratio is accurate, and considering the fact that the prevalence of HCM has since been revised from 1 in 5100 to 1 in 500, then the prevalence of idiopathic DCM should be roughly half of that of HCM: 1 in 250. While these ideas are evaluated in detail in the DCM review by Hershberger et al,5 the main conclusion is that the original estimate of 1:2,700 is a grossly underestimated prevalence of idiopathic DCM, and existing data can be used to extrapolate an estimate that is closer to 1 in 500 or even 1 in 250. A well-designed large-scale epidemiological study of DCM will need to be carried out in order to estimate the true prevalence.
While a large-scale study has yet to be done, a large-scale meta-analysis has provided some insight into the prevalence issue. The study by Petretta et al undertook the task of cataloging all relevant FDC reports published over three decades (1980–2010), which resulted in a meta-analysis of 23 studies. This analysis resulted in a combined prevalence estimate of 23%, though the large range (2%–65%) indicated a significant heterogeneity in reported studies. Because the estimate of FDC correlated with the year of publication, the authors attribute the large prevalence range to inconsistent use of diagnostic criteria, which has ameliorated recently due to more systematic clinical screening. This meta-analysis provides a useful example of the necessity of standardized criteria in FDM studies.35
IV. Genetics and Screening
The advent of modern sequencing technology has helped to elucidate the genetic etiology of FDC and identify over 50 genes involved.11, 12 Table 3 lists many of these genes along with their protein name and mode of inheritance. While this review does not include extensive information on the genetics of FDC, comprehensive reviews of the subject can be found elsewhere.5, 12, 15, 36 The identification of disease-causing variants is a clinically relevant tool because mutations in certain genes have been associated with severity, prognosis, and survival rates.16, 36 For example, mutations in SCN5A, which encodes for a voltage gated sodium channel, have been associated with severe arrhythmias, including atrial fibrillation and ventricular tachycardia.37, 38 Similarly, mutations in LMNA, which encodes for an important structural component of the nuclear membrane, can lead to conduction system abnormalities. LMNA mutations account for approximately 8% of DCM patients and convey a high risk of malignant ventricular arrhythmias and sudden death.38 In this case, implantable cardioverter-defibrillators (ICDs) can be a preemptive life-saving tool that decreases the risk of sudden death.39
Table 3.
Genes involved in FDC that can be clinically screened.
| Gene | Gene Name | Inheritance | OMIM Phenotype |
|---|---|---|---|
| ABCC9 | ATP-binding cassette, sub-family C, member 9 | AD | 608569 |
| ACTC1 | actin, alpha, cardiac muscle 1 | AD | 613424 |
| ACTN2 | actinin, alpha 2 | AD | 612158 |
| BAG3 | BCL2-associated athanogene 3 | AD | 613881 |
| CRYAB | crystallin, alpha B | AD | 615184 |
| CSRP3 | cysteine and glycine-rich protein 3 (cardiac LIM protein) | AD | 607482 |
| DES | desmin | AD | 604765 |
| DMD | dystrophin | X-linked | 302045 |
| DSG2 | desmoglein 2 | AD | 612877 |
| DSP | desmoplakin | AD, AR | 615821(AD), 605676(AR) |
| EYA4 | eyes absent homolog 4 | AD | 605362 |
| FKTN | fukutin | AD | 611615 |
| GATAD1 | GATA zinc finger domain containing 1 | AD, AR | 614672 |
| LAMA4 | laminin, alpha 4 | AD | 615235 |
| LAMP2 | lysosome-associated membrane protein 2 | X-linked | 300257 |
| LDB3 | LIM domain binding 3 | AD | 601493 |
| LMNA | lamin A/C | AD | 115200 |
| MYBPC3 | myosin binding protein C, cardiac | AD | 615396 |
| MYH6 | myosin, heavy chain 6, cardiac muscle, alpha | AD | 613252 |
| MYH7 | myosin, heavy chain 7, cardiac muscle, beta | AD | 613426 |
| MYPN | myopalladin | AD | 615248 |
| NEXN | nexilin | AD | 613122 |
| PLN | phospholamban | AD | 609909 |
| PRDM16 | PR domain containing 16 | AD | 615373 |
| PSEN1 | presenilin 1 | AD | 613694 |
| PSEN2 | presenilin 2 | AD | 613697 |
| RAF1 | RAF proto-oncogene serine/threonine-protein kinase | AD | 615916 |
| RBM20 | RNA binding motif protein 20 | AD | 613172 |
| SCN5A | sodium channel, voltage-gated, type V, alpha subunit | AD | 601154 |
| SDHA | succinate dehydrogenase complex, subunit A | AD | 613642 |
| SGCD | sarcoglycan, delta (dystrophin-associated glycoprotein) | AD | 606685 |
| TAZ | tafazzin | X-linked | 302060 |
| TCAP | titin-cap (telethonin) | AD | 607487 |
| TMPO | thymopoietin | AD | 613740 |
| TNNC1 | cardiac troponin C type 1 | AD | 611879 |
| TNNI3 | cardiac troponin I type 3 | AD, AR | 613286(AD), 611880(AR) |
| TNNT2 | cardiac troponin T type 2 | AD | 601494 |
| TPM1 | tropomyosin 1 | AD | 611878 |
| TTN | titin | AD, AR | 604145 |
| VCL | vinculin | AD | 611407 |
AD = autosomal dominant, AR = autosomal recessive
Clinical genetic testing in DCM currently has a sensitivity of 40%, meaning that a pathogenic variant is identifiable in nearly half of cases.18 Genetic testing for DCM can test up to nearly 40 genes, and the targeted gene panels are evolving rapidly in response to advances in cardiovascular research. Table 3 lists a majority of the genes that have been associated with DCM, all of which can be found in various DCM gene panels. In the case of an unknown type of cardiomyopathy or when the results of another disease panel are negative, pan cardiomyopathy panels allow for testing of 80 or more heart function related genes.15, 18, 34
One of the biggest obstacles in DCM gene variant identification in clinical testing is the immense genetic heterogeneity in FDC. The majority of mutations previously associated with FDC are ‘private’ mutations, meaning that they have only been seen in one family. Herman et al showed that truncating mutations in TTN, which encodes the sarcomeric protein titin, are involved in 25% of FDC cases; in 47 individuals they identified 44 unique truncating mutations.40 This high prevalence of unique mutations makes the interpretation of genetic testing more difficult, because unreported pathogenic mutations must be validated, which takes time and delays the screening of other family members.22 In the context of reported sequencing data, one caveat is that the frequency of variants classified as ‘pathogenic’ within the population far exceeds the prevalence of DCM. This raises a number of questions about disease and variant classification, which have been reviewed in more detail in Hershberger et al and Ingles et al.5, 41
A recent report by Harakalova et al performed an extensive review of the literature, including PubMed, Embase, and OMIM, in order to catalog every gene that has been implicated in DCM thus far. While approximately 40 genes are currently included in genetic testing, an additional 68 genes have been implicated but are not included in genetic testing. While mutations in many of these 68 genes have only been found in individuals or in single families, this raises the awareness that the remaining 60% of cases that have not been explained by genetic testing likely still have a causative variant that can be identified.42 The Harakalova study provides ample foundation for future genetic analysis in DCM cohorts to further characterize the extensive genetic heterogeneity of this disease. With further characterization, these genes could eventually be added to clinical panels in order to increase the rate of positive identification, thus providing individuals and families with a more accurate genetic screening.
V. Expert Opinion
To date, the genetic causes of DCM have been discovered largely by identifying families with FDC followed by sequencing candidate genes or performing whole-exome sequencing to identify new candidate genes and causal mutations. Despite the past success in discovering genetic causes of FDC, identified mutations account for only 40% of all familial cases, suggesting many more genetic causes remain unknown. The onset of Next-Generation sequencing has introduced an unprecedented amount of genomic data that has dramatically expanded our ability to rapidly elucidate genetic causes, but in some cases has left more questions than answers. As ‘novel’ mutations continue to be discovered, there is an increased need for functional studies to validate novel variants by identifying pathway involvement and causal disease mechanisms.
For cases where a genetic cause has yet to be identified, the analysis may venture beyond the idea of a single pathogenic mutation into a more complex paradigm that could involve an unexpected gene, two or more mutations, copy number variation, enhancer region mutations, and intronic variants. These elements remain largely unexplored areas of inherited cardiomyopathy. A recent study found that in a cohort of 639 DCM patients screened for 84 cardiomyopathy genes, showing that possible pathogenic variants in DCM overlap frequently with other cardiomyopathies, in some cases even sharing the same mutation. Additionally, a significant number of patients had multiple mutations in different genes, suggesting that some cases of DCM may require multiple hits in order to cause disease.43
Another complication presented in familial cardiomyopathies is the clinical diversity related to incomplete penetrance and variable expressivity within families. This has made clinical categorization difficult and genetic characterization ambiguous, especially when classifying affected individuals within pedigrees. Identification of specific environmental factors or modifier genes that affect severity and phenotypic expression will aid in understanding the mechanisms involved in FDC and may help explain cases of incomplete penetrance. With the ultimate goal of understanding the role of genetic variance in FDC, future research in the field will rely on the continued identification of disease causing genetic variation, not only from continued candidate gene and whole-exome analysis, but also in the form a more in depth genomic analysis. This will lead to more accurate diagnoses and improved patient care.
Article Highlights.
Familial dilated cardiomyopathy (FDC) occurs when two or more family members are diagnosed with DCM
Though DCM occurs in at least 1:2700 individuals, it is likely much more common, probably 1:500
30–40% of FDC cases have been attributed to a genetic mutation
To date, >50 genes have been attributed to DCM and can be tested clinically
Further investigation into the molecular genetics of DCM will help to identify the remaining 60% of FDC cases
Acknowledgments
Support was received from the NIH grants 1R01HL116906, UL1 RR025780, R01 HL69071 to LM; K23 JL067915 and 1R01HL109209-01A1 to MRGT, and the Trans-Atlantic Network of Excellence grant 14-CVD03 from the Leducq Foundation.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015 Jan 27;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Fatkin D. Familial dilated cardiomyopathy: Current challenges and future directions. Global cardiology science & practice. 2012;2012(1):8. doi: 10.5339/gcsp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Fatkin D members of the CCGDCWG. Guidelines for the diagnosis and management of familial dilated cardiomyopathy. Heart, lung & circulation. 2011 Nov;20(11):691–3. doi: 10.1016/j.hlc.2011.07.008. Overview of current clinical guidelines for FDC. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006 Apr 11;113(14):1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 5**.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature reviews Cardiology. 2013 Sep;10(9):531–47. doi: 10.1038/nrcardio.2013.105. Review of the genetics and phenomics of DCM. [DOI] [PubMed] [Google Scholar]
- 6.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. Journal of the American College of Cardiology. 2005 Apr 5;45(7):969–81. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 7.Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, Driscoll DJ, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. The New England journal of medicine. 1992 Jan 9;326(2):77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 8.Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. Journal of the American College of Cardiology. 1998 Jan;31(1):186–94. doi: 10.1016/s0735-1097(97)00434-8. [DOI] [PubMed] [Google Scholar]
- 9.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, et al. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. European heart journal. 1999 Jan;20(2):93–102. doi: 10.1053/euhj.1998.1145. [DOI] [PubMed] [Google Scholar]
- 10.Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. Journal of the American College of Cardiology. 2011 Apr 19;57(16):1641–9. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinagra G, Mestroni L, Camerini F. Genetic Cardiomyopathies: A Clinical Approach. Milan, Italy: Springer; 2013. [Google Scholar]
- 12*.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. The Journal of clinical investigation. 2013 Jan;123(1):19–26. doi: 10.1172/JCI62862. Molecular genetics of FDC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teekakirikul P, Kelly MA, Rehm HL, Lakdawala NK, Funke BH. Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. The Journal of molecular diagnostics : JMD. 2013 Mar;15(2):158–70. doi: 10.1016/j.jmoldx.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Karkkainen S, Peuhkurinen K. Genetics of dilated cardiomyopathy. Annals of medicine. 2007;39(2):91–107. doi: 10.1080/07853890601145821. [DOI] [PubMed] [Google Scholar]
- 15.Mestroni L, Tharp CA, Sweet ME, Graw SL, Taylor MR. Molecular Genetics of Dilated Cardiomyopathy. eLS. 2014 [Google Scholar]
- 16.Taylor MR, Carniel E, Mestroni L. Cardiomyopathy, familial dilated. Orphanet journal of rare diseases. 2006;1:27. doi: 10.1186/1750-1172-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Morales A, Cowan J, Dagua J, Hershberger RE. Family history: an essential tool for cardiovascular genetic medicine. Congestive heart failure. 2008 Jan-Feb;14(1):37–45. doi: 10.1111/j.1751-7133.2008.08201.x. Using family history to better understand inherited cardiomyopathies. [DOI] [PubMed] [Google Scholar]
- 18**.Sturm AC, Hershberger RE. Genetic testing in cardiovascular medicine: current landscape and future horizons. Current opinion in cardiology. 2013 May;28(3):317–25. doi: 10.1097/HCO.0b013e32835fb728. Advantages and limitations of genetic testing in inherited cardiomyopathy. [DOI] [PubMed] [Google Scholar]
- 19.Mestroni L, Rocco C, Gregori D, Sinagra G, Di Lenarda A, Miocic S, et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. Journal of the American College of Cardiology. 1999 Jul;34(1):181–90. doi: 10.1016/s0735-1097(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh N, Haddad H. Recent progress in the genetics of cardiomyopathy and its role in the clinical evaluation of patients with cardiomyopathy. Current opinion in cardiology. 2011 Mar;26(2):155–64. doi: 10.1097/HCO.0b013e3283439797. [DOI] [PubMed] [Google Scholar]
- 21.Monserrat L, Hermida M, Bouzas B, Mosquera I, Mahon N, Peteiro J, et al. Familial dilated cardiomyopathy in patients transplanted for idiopathic dilated cardiomyopathy. Revista espanola de cardiologia. 2002 Jul;55(7):725–32. doi: 10.1016/s0300-8932(02)76691-8. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby D, McKenna WJ. Genetics of inherited cardiomyopathy. European heart journal. 2012 Feb;33(3):296–304. doi: 10.1093/eurheartj/ehr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA, et al. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. Journal of cardiac failure. 2009 Mar;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. European heart journal cardiovascular Imaging. 2015 Mar;16(3):307–15. doi: 10.1093/ehjci/jeu181. [DOI] [PubMed] [Google Scholar]
- 25.Zecchin M, Di Lenarda A, Gregori D, Merlo M, Pivetta A, Vitrella G, et al. Are nonsustained ventricular tachycardias predictive of major arrhythmias in patients with dilated cardiomyopathy on optimal medical treatment? Pacing and clinical electrophysiology : PACE. 2008 Mar;31(3):290–9. doi: 10.1111/j.1540-8159.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- 26.Fatkin D, Yeoh T, Hayward CS, Benson V, Sheu A, Richmond Z, et al. Evaluation of left ventricular enlargement as a marker of early disease in familial dilated cardiomyopathy. Circulation Cardiovascular genetics. 2011 Aug 1;4(4):342–8. doi: 10.1161/CIRCGENETICS.110.958918. [DOI] [PubMed] [Google Scholar]
- 27.Mahon NG, Murphy RT, MacRae CA, Caforio AL, Elliott PM, McKenna WJ. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Annals of internal medicine. 2005 Jul 19;143(2):108–15. doi: 10.7326/0003-4819-143-2-200507190-00009. [DOI] [PubMed] [Google Scholar]
- 28.Moretti M, Merlo M, Barbati G, Di Lenarda A, Brun F, Pinamonti B, et al. Prognostic impact of familial screening in dilated cardiomyopathy. European journal of heart failure. 2010 Sep;12(9):922–7. doi: 10.1093/eurjhf/hfq093. [DOI] [PubMed] [Google Scholar]
- 29.Codd MB, Sugrue DD, Gersh BJ, Melton LJ., 3rd Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989 Sep;80(3):564–72. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995 Aug 15;92(4):785–9. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 31.Manolio TA, Baughman KL, Rodeheffer R, Pearson TA, Bristow JD, Michels VV, et al. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop. The American journal of cardiology. 1992 Jun 1;69(17):1458–66. doi: 10.1016/0002-9149(92)90901-a. [DOI] [PubMed] [Google Scholar]
- 32.Miura K, Nakagawa H, Morikawa Y, Sasayama S, Matsumori A, Hasegawa K, et al. Epidemiology of idiopathic cardiomyopathy in Japan: results from a nationwide survey. Heart. 2002 Feb;87(2):126–30. doi: 10.1136/heart.87.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DG, Olsen EG. Prevalence of overt dilated cardiomyopathy in two regions of England. British heart journal. 1985 Aug;54(2):153–5. doi: 10.1136/hrt.54.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakar S, Sinagra G, Di Lenarda A, Poletti A, Bussani R, Silvestri F, et al. Epidemiology of dilated cardiomyopathy. A prospective post-mortem study of 5252 necropsies. The Heart Muscle Disease Study Group. European heart journal. 1997 Jan;18(1):117–23. doi: 10.1093/oxfordjournals.eurheartj.a015092. [DOI] [PubMed] [Google Scholar]
- 35.Petretta M, Pirozzi F, Sasso L, Paglia A, Bonaduce D. Review and metaanalysis of the frequency of familial dilated cardiomyopathy. The American journal of cardiology. 2011 Oct 15;108(8):1171–6. doi: 10.1016/j.amjcard.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Mestroni L, Taylor MR. Genetics and genetic testing of dilated cardiomyopathy: a new perspective. Discovery medicine. 2013 Jan;15(80):43–9. [PMC free article] [PubMed] [Google Scholar]
- 37.McNair WP, Sinagra G, Taylor MR, Di Lenarda A, Ferguson DA, Salcedo EE, et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. Journal of the American College of Cardiology. 2011 May 24;57(21):2160–8. doi: 10.1016/j.jacc.2010.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. Journal of the American College of Cardiology. 2012 Jan 31;59(5):493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 39.Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D. Primary prevention of sudden death in patients with lamin A/C gene mutations. The New England journal of medicine. 2006 Jan 12;354(2):209–10. doi: 10.1056/NEJMc052632. [DOI] [PubMed] [Google Scholar]
- 40.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. The New England journal of medicine. 2012 Feb 16;366(7):619–28. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingles J, Zodgekar PR, Yeates L, Macciocca I, Semsarian C, Fatkin D, et al. Guidelines for genetic testing of inherited cardiac disorders. Heart, lung & circulation. 2011 Nov;20(11):681–7. doi: 10.1016/j.hlc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Harakalova M, Kummeling G, Sammani A, Linschoten M, Baas AF, van der Smagt J, et al. A systematic analysis of genetic dilated cardiomyopathy reveals numerous ubiquitously expressed and muscle-specific genes. European journal of heart failure. 2015 May;17(5):484–93. doi: 10.1002/ejhf.255. [DOI] [PubMed] [Google Scholar]
- 43.Haas J, Frese KS, Peil B, Kloos W, Keller A, Nietsch R, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. European heart journal. 2014 Aug 27; doi: 10.1093/eurheartj/ehu301. [DOI] [PubMed] [Google Scholar]