Abstract
The Old World bollworm, Helicoverpa armigera (Hübner), was recently introduced into Brazil, where it has caused extensive damage to cotton and soybean crops. MON 87701 × MON 89788 soybean, which expresses the Bt protein Cry1Ac, was recently deployed in Brazil, providing high levels of control against H. armigera. To assess the risk of resistance to the Cry1Ac protein expressed by MON 87701 × MON 89788 soybean in Brazil, we conducted studies to evaluate the baseline susceptibility of H. armigera to Cry1Ac, in planta efficacy including the assessment of the high-dose criterion, and the initial resistance allele frequency based on an F2 screen. The mean Cry1Ac lethal concentration (LC50) ranged from 0.11 to 1.82 μg·mL−1 of diet among all H. armigera field populations collected from crop seasons 2013/14 to 2014/15, which indicated about 16.5-fold variation. MON 87701 × MON 89788 soybean exhibited a high level of efficacy against H. armigera and most likely met the high dose criterion against this target species in leaf tissue dilution bioassays up to 50 times. A total of 212 F2 family lines of H. armigera were established from field collections sampled from seven locations across Brazil and were screened for the presence of MON 87701 × MON 89788 soybean resistance alleles. None of the 212 families survived on MON 87701 × MON 89788 soybean leaf tissue (estimated allele frequency = 0.0011). The responses of H. armigera to Cry1Ac protein, high susceptibility to MON 87701 × MON 89788 soybean, and low frequency of resistance alleles across the main soybean-producing regions support the assumptions of a high-dose/refuge strategy. However, maintenance of reasonable compliance with the refuge recommendation will be essential to delay the evolution of resistance in H. armigera to MON 87701 × MON 89788 soybean in Brazil.
Introduction
Helicoverpa armigera (Hübner) is considered to be one of the most important agricultural pests in the world [1]. This polyphagous species is widespread throughout Europe, Africa, Asia, and Australia, where it causes extensive damage to a wide range of crops [2–4]. The ability of H. armigera to persist in agricultural areas and adapt to changes in farming practices is one of the major factors contributing to the pest status of this species [5].
In the Old World and in Australia, where H. armigera is a major pest of cotton, this species is the primary target of genetically modified cotton containing insecticidal proteins from Bacillus thuringiensis (Bt), which has been widely adopted by growers to overcome insecticide resistance issues [6–8]. Recently this species was identified in South America, especially in Brazil [9, 10]. Several studies also indicated the risk of a successful invasion of this species into North America [11, 12] and the Animal and Plant Health Inspection Service of the United States Department of Agriculture (USDA-APHIS) reported detection of H. armigera in Puerto Rico in 2014 [13] and in the continental United States in 2015 [14]. In Brazil, H. armigera has been detected on a variety of crops, particularly dicots and to lesser extent monocots [15, 16]. Among these host crops, soybean and cotton represent the largest areas in Brazil, totaling 30 million and 1 million hectares per year, respectively [1].
In different parts of the world, H. armigera represents a significant insect management challenge to cotton and soybean growers [6, 17–19]. Likewise, the widespread distribution of H. armigera in Brazil was identified as a risk to the effective and sustainable use of Bt crops in South America. Bt cotton has been widely used with success in many countries including Brazil [20, 21] where a Bt soybean product expressing the protein Cry1Ac (MON 87701 × MON 89788) was recently approved for cultivation [22] and commercially launched in crop season 2013/14. The high efficacy of MON 87701 × MON 89788 soybean against major lepidopteran pests demonstrated its potential to become part of an Integrated Pest Management (IPM) program aimed at reducing insecticide use [23, 24–25]. Although soybean looper, Chrysodeixis includens (Walker), stands out as the predominant soybean pest in Brazil, H. armigera has recently become more prevalent in Brazilian soybean-growing areas [26] and the risk of resistance evolution in this species to Bt soybean must be considered in Insect Resistance Management (IRM) programs.
As anticipated, MON 87701 × MON 89788 soybean has been rapidly adopted by growers in Brazil, likely due to the high levels of control provided against the main lepidopteran pests, especially C. includens, Anticarsia gemmatalis Hübner, Chloridea virescens (F.) and H. armigera [23, 24, 27–29]. As with other Bt crops, the primary threat to the sustainable use of MON 87701 × MON 89788 soybean is the evolution of resistance by target pests [30]. The most effective strategy for managing insect resistance to Bt crops, the high-dose/refuge strategy, is based on the assumptions that resistance alleles to a Bt protein are rare; that a Bt protein is consistently produced by a plant at a highly toxic concentration, reducing the selective differential between susceptible (SS) and heterozygous (RS) target insects, therefore making resistance alleles “functionally recessive”; and that refuge areas with non-Bt plants are cultivated to allow the development of susceptible (i.e., unselected) insects [30, 31]. Three key factors are consistently associated with the cases of field-evolved resistance reported thus far [32–37]: the deployment of single-mode-of-action Bt products, failure to meet the high-dose criterion, and poor refuge compliance [31, 38].
Previous studies reported high toxicity of MON 87701 × MON 89788 soybean against H. armigera [28, 29]. In this paper, we describe baseline susceptibility curves to Cry1Ac of several field H. armigera populations sampled from soybean and cotton fields and present data regarding toxicological attributes of MON 87701 × MON 89788 soybean against H. armigera generated in the laboratory and screenhouse, including a high-dose assessment. Furthermore, we present results of an F2 screen [39] for rare Cry1Ac resistance alleles carried out using Bt soybean leaf tissue to improve the sensitivity of estimates of resistance allele frequencies. Finally, we discuss the IRM implications of having large areas in Brazil cultivated with Bt technologies targeting H. armigera.
Results
Baseline susceptibility to Cry1Ac
The baseline susceptibility of H. armigera documented using a diet-incorporated bioassay for insects sampled in crop seasons 2013/14 and 2014/15 indicated neonate larvae were highly susceptible to Cry1Ac with LC50 values (i.e., concentrations that caused 50% mortality of H. armigera larvae) ranging from 0.11 (populations MS-1 and SP-1) to 1.82 μg Cry1Ac mL−1 diet (population BA-2) (Table 1) and EC50 values (i.e., concentrations that caused 50% growth inhibition) ranging from 0.0029 (population MS-1) to 0.0165 μg Cry1Ac mL−1 diet (population BA-4) (Table 2). There were overlapping responses (LC50 and EC50) and relative low variability across populations; the most susceptible and most tolerant H. armigera populations differed by approximately 16.5-fold and 5.7-fold for the LC50 and EC50 values, respectively.
Table 1. Summary of concentration-mortality (lethal concentration [LC]) of H. armigera neonates exposed to Cry1Ac protein incorporated into artificial diet.
| Seasona | Population | Generation | nb | Slope ± SE | LC50 (μg mL−1 diet)c | LC90 (μg mL−1 diet)c | Goodness of fit | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 95% CI | Concentration | 95% CI | χ2 | dfd | |||||
| 2013/14 | LAB | F4 | 688 | 1.59 ± 0.13 | 1.22 | 0.92–1.56 | 7.83 | 5.88–11.1 | 0.79 | 5 |
| MS-1 | F3 | 896 | 1.94 ± 0.13 | 0.11 | 0.08–0.13 | 1.43 | 1.02–2.15 | 6.79 | 5 | |
| SP-1 | F2 | 896 | 1.53 ± 0.10 | 0.11 | 0.08–0.14 | 3.15 | 2.07–5.29 | 7.84 | 5 | |
| BA-1 | F2 | 896 | 2.00 ± 0.13 | 1.37 | 0.95–1.81 | 5.99 | 4.55–8.56 | 4.94 | 5 | |
| PR-1 | F2 | 899 | 1.89 ± 0.12 | 0.12 | 0.09–0.15 | 1.76 | 1.25–2.67 | 3.88 | 5 | |
| MT-1 | F2 | 896 | 1.64 ± 0.17 | 0.41 | 0.32–0.52 | 8.96 | 5.90–15.0 | 2.20 | 5 | |
| GO-1 | F2 | 896 | 2.04 ± 0.13 | 0.19 | 0.15–0.22 | 2.22 | 1.60–3.28 | 5.21 | 5 | |
| PR-2 | F2 | 896 | 2.17 ± 0.18 | 0.32 | 0.26–0.39 | 3.35 | 2.44–4.90 | 4.15 | 5 | |
| BA-2 | F2 | 1152 | 2.11 ± 0.24 | 1.82 | 1.16–2.49 | 7.89 | 5.71–12.5 | 3.46 | 4 | |
| BA-3 | F3 | 720 | 2.74 ± 0.11 | 0.32 | 0.26–0.39 | 3.35 | 2.44–4.90 | 1.54 | 5 | |
| 2014/15 | MT-2 | F2 | 896 | 1.50 ± 0.12 | 0.20 | 0.22–0.36 | 1.66 | 1.36–2.02 | 3.45 | 5 |
| SP-2 | F2 | 896 | 1.87 ± 0.13 | 0.40 | 0.36–0.59 | 0.60 | 0.43–0.91 | 1.43 | 5 | |
| MT-3 | F2 | 896 | 1.66 ± 0.13 | 0.28 | 0.18–0.48 | 1.28 | 0.56–1.64 | 1.07 | 5 | |
| BA-4 | F2 | 768 | 1.90 ± 0.13 | 0.44 | 0.35–0.55 | 15.35 | 9.13–30.1 | 1.27 | 5 | |
| PR-3 | F2 | 896 | 1.43 ± 0.09 | 0.32 | 0.25–0.41 | 10.76 | 6.69–19.6 | 4.79 | 5 | |
a Season when populations were collected.
b Number of individuals tested.
c LC50 and LC90 are the concentrations of Cry1Ac protein (μg mL−1 diet) that cause death or inhibit molting beyond first instar of 50% and 90% of individuals, respectively, after 7 days of bioassay. CI, confidence interval.
d Degrees of freedom.
Table 2. Summary of effective concentration (EC, or growth inhibition concentration) of H. armigera neonates exposed to Cry1Ac protein incorporated into artificial diet.
| Seasona | Population | Generation | nb | EC50 (μg mL−1 diet)c | EC90 (μg mL−1 diet)c | ||
|---|---|---|---|---|---|---|---|
| Concentration | 95% CI | Concentration | 95% CI | ||||
| 2013/14 | LAB | F4 | 523 | 0.0028 | 0.0018–0.0049 | 0.0189 | 0.0062–0.0249 |
| MS-1 | F3 | 447 | 0.0029 | 0.0021–0.0037 | 0.0421 | 0.0344–0.0526 | |
| SP-1 | F2 | 466 | 0.0034 | 0.0031–0.0038 | 0.0407 | 0.0374–0.0444 | |
| BA-1 | F2 | 468 | 0.0072 | 0.0038–0.0082 | 0.0468 | 0.0422–0.2120 | |
| PR-1 | F2 | 451 | 0.0055 | 0.0049–0.0060 | 0.0341 | 0.0301–0.0389 | |
| MT-1 | F2 | 593 | 0.0079 | 0.0038–0.0128 | 0.1848 | 0.0894–0.4885 | |
| GO-1 | F2 | 507 | 0.0036 | 0.0020–0.0052 | 0.0547 | 0.0371–0.0871 | |
| PR-2 | F2 | 563 | 0.0033 | 0.0024–0.0042 | 0.0658 | 0.0261–0.0842 | |
| BA-2 | F2 | 478 | 0.0032 | 0.0018–0.0050 | 0.0602 | 0.0274–0.0644 | |
| BA-3 | F3 | 436 | 0.0050 | 0.0041–0.0059 | 0.0554 | 0.0452–0.0695 | |
| 2014/15 | MT-2 | F2 | 307 | 0.0032 | 0.0002–0.0040 | 0.0581 | 0.0470–0.0738 |
| SP-2 | F2 | 353 | 0.0030 | 0.0026–0.0038 | 0.0590 | 0.0500–0.0708 | |
| MT-3 | F2 | 316 | 0.0032 | 0.0002–0.0038 | 0.0590 | 0.0501–0.0708 | |
| BA-4 | F2 | 454 | 0.0165 | 0.0128–0.0209 | 0.2724 | 0.1676–0.4939 | |
| PR-3 | F2 | 418 | 0.0160 | 0.0153–0.0168 | 0.1143 | 0.1030–0.1275 | |
a Season when populations were collected.
b Number of individuals tested.
c EC50 and EC90 are the effective concentrations of protein required to cause 50% and 90% growth inhibition, respectively, at 7 days. CI, confidence interval.
Efficacy trials—Leaf disc and screenhouse
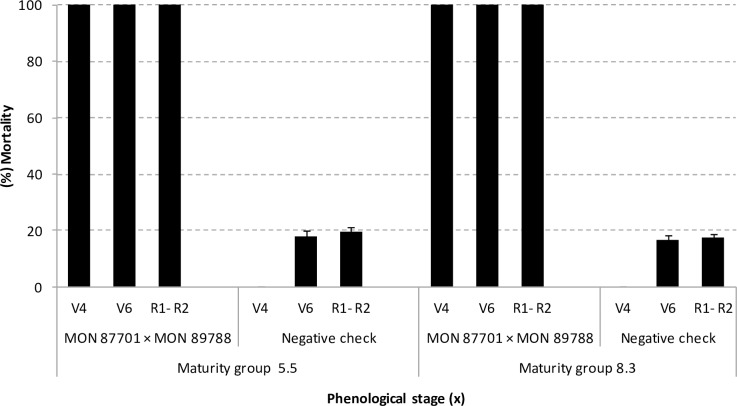
The Cry1Ac protein expressed in leaves of MON 87701 × MON 89788 soybean of maturity groups 5.5 and 8.3 was highly toxic to H. armigera neonates in all phenological stages evaluated, resulting in 100% mortality after five days of leaf disc bioassay (Fig 1).
Fig 1. H. armigera mortality on leaf discs of MON 87701 × MON 89788 and near isogenic negative checkschecks soybeans at different phenological stages.
The mean percentage of mortality of H. armigera neonates was assessed after 5 days on leaf discs of MON 87701 × MON 89788 soybean and near-isogenic negative checks of maturity groups 5.5 and 8.3. There were statistically significant differences (t-test, P ≤ 0.05) between MON 87701 × MON 89788 soybean and near-isogenic negative checks at all phenological stages evaluated (df = 10; P < 0.0001).
Evaluation in screenhouse with intense artificial infestation has shown that no H. armigera larvae were able to establish on MON 87701 × MON 89788 soybean plants, in contrast to a large number of larvae per meter observed on negative checks (Table 3). Reductions in damage on MON 87701 × MON 89788 soybean were observed as a consequence of H. armigera larvae control. Thirty-five days after infestation, accumulated defoliation reached the maximum defined level (50% defoliation) on negative checks, significantly higher than on MON 87701 × MON 89788 soybean of either maturity group tested.
Table 3. Larval incidence and plant damage on MON 87701 × MON 89788 and control soybean under high-pressure H. armigera infestation in screenhouse trials.
| Material | Larvae per meter | Defoliation (%) | Pods/plant | Pods damaged (%) |
|---|---|---|---|---|
| Maturity group 5.5 | ||||
| MON 87701 × MON 89788 | 0.0 ± 0.0* | 0.0 ± 0.0* | 30.0 ± 1.1 | 0.0 ± 0.0* |
| Negative checks | 480.6 ± 32.2 | 50.0 ± 0.0 | 37.5 ± 1.0 | 37.2 ± 1.9 |
| Maturity group 8.3 | ||||
| MON 87701 × MON 89788 | 0.0 ± 0.0* | 0.0 ± 0.0* | 72.6 ± 4.1* | 0.0 ± 0.0* |
| Negative checks | 463.3 ± 25.9 | 50.0 ± 0.0 | 0.7 ± 0.2 | 94.6 ± 11.2 |
Values of Larval incidence were measured as larvae per meter row. Damage was measured on vegetative (percentage defoliation) and reproductive structures (pods per plant and pods damaged). For each measurement, MON 87701 × MON 89788 soybean of maturity groups 5.5 and 8.3 was compared to non-Bt checks of the same maturity groups.
* There were statistically significant differences (t-test; P ≤ 0.05) between MON 87701 × MON 89788 soybean and negative checks of both maturity groups for larvae per meter, defoliation, and pods damaged, and in maturity group 8.3 for pods/plant (df = 6, P < 0.0001).
In addition to its effects on vegetative tissues, H. armigera has podworm behavior and can damage reproductive structures including flowers and pods. In the case of non-Bt soybean with a longer life cycle (maturity group 8.3), the plants were starting to flower at the time that H. armigera eggs hatched. H. armigera larvae fed on these flowers and consequently reduced the number of pods developed on negative check plants by ~99% relative to that of MON 87701 × MON 89788 soybean in the same maturity group (df = 6, P < 0.0001; Table 3). In contrast, the number of pods on non-Bt soybean with a shorter life cycle (maturity group 5.5) was not significantly different from that of MON 87701 × MON 89788 soybean of the same maturity group (df = 6, P = 0.066). However, for both maturity groups tested, the developed pods of MON 87701 × MON 89788 soybean were significantly less damaged than those of the corresponding negative checks.
High-dose assessment
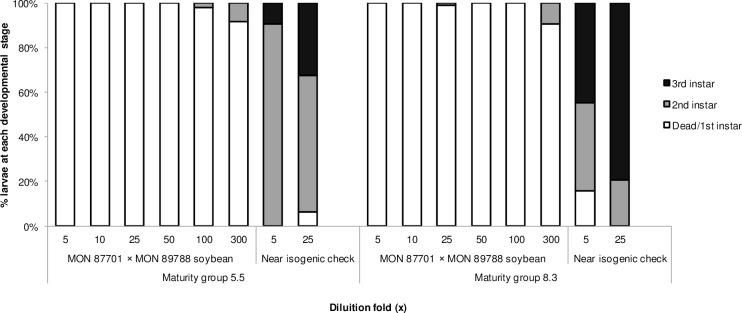
As described previously for other Bt soybean target pests such as C. includens, A. gemmatalis and C. virescens [23, 24], lyophilized leaf tissue of MON 87701 × MON 89788 soybean of maturity groups 5.5 and 8.3 caused high mortality (larvae dead or not molting beyond first instar) and stunting (larvae not molting to third instar) of H. armigera when diluted up to 25-fold in artificial diet (Fig 2). In contrast, near isogenic negative checks tissue diluted 5- and 25-fold in artificial diet caused low levels of mortality, ranging from 6.25% to 15.62% across the two maturity groups. Overall, most of the larvae exposed to artificial diet containing non-Bt soybean tissue reached the second instar after seven days, while most of the larvae on a diet containing lyophilized tissue of MON 87701 × MON 89788 soybean did not survive or develop past the first instar.
Fig 2. H. armigera development on diet containing MON 87701 × MON 89788 or control soybean leaf.
Mortality and larval development of H. armigera neonates was assessed after 7 days of feeding on artificial diet containing lyophilized leaf tissue at serial dilutions of 5- to 300-fold relative to fresh leaf tissue for MON 87701 × MON 89788 and 5- to 25-fold relative to the fresh tissue for the near-isogenic negative checks. Bars indicate mean percentages of dead or first-instar larvae (% mortality) and of second- and third-instar larvae.
Frequency of resistance alleles to MON 87701 × MON 89788 soybean
Of the 870 single pairs of H. armigera moths obtained from larvae collected from crop production field, only 212 (24%) successfully progressed to the stage where F2 neonate larvae were exposed to MON 87701 × MON 89788 soybean leaf tissue expressing the Cry1Ac protein (Table 4). A total of 212 F2 two-parent lines (originating from 424 feral individuals) of H. armigera were screened for resistance to Cry1Ac using fresh leaf tissue of MON 87701 × MON 89788 soybean. Among these lines, no surviving H. armigera larvae were found after four days, indicating that none of the 212 families carried major resistance alleles to Cry1Ac. The estimated resistant allele frequency was 0.0011 (95% confidence interval of 0–0.0043) (Table 4).
Table 4. Initial frequency of MON 87701 × MON 89788 soybean resistance alleles in H. armigera from Brazil.
| Population code | Single pairs (F0) | F1parental isoline families | F2 parental isoline families | Estimated R frequency (95% CI)a | |
|---|---|---|---|---|---|
| Screened | Positive | ||||
| BA-1 | 127 | 99 | 11 | 0 | 0.0194 (0–0.0701) |
| BA-2 | 145 | 120 | 51 | 0 | 0.0047 (0–0.0174) |
| BA-3 | 64 | 85 | 37 | 0 | 0.0064 (0–0.0236) |
| BA-4 | 120 | 100 | 37 | 0 | 0.0064 (0–0.0236) |
| MT-1 | 83 | 100 | 12 | 0 | 0.0180 (0–0.0652) |
| MT-2 | 104 | 88 | 28 | 0 | 0.0084 (0–0.0306) |
| SP-2 | 81 | 42 | 36 | 0 | 0.0066 (0–0.0242) |
| Total | 870 | 684 | 212 | 0 | 0.0011 (0–0.0043) |
a Frequency estimates for each population and for the pooled data (Total). CI, confidence interval.
Discussion
The overlap in the LC50 and EC50 values obtained for populations from different regions in Brazil indicated low intra-specific variability (Table 1) [40]. Although H. armigera populations were sampled across diverse regions, recent invasions and geographic expansion coupled with high mobility may sustain low levels of intra-specific variability in Brazil [16]. In contrast, variation in susceptibility to Cry1Ac of up to 50-fold previously has been described across different world areas and may be associated with natural variation in heliothine pest populations and with several other factors that may affect the outcome of bioassays, such as the source of Cry1Ac protein and the bioassay technique [41–44].
The responses of H. armigera populations sampled across Brazil are comparable to those obtained with a Cry1Ac (MVPII) diet-incorporation bioassay from cotton-growing areas in India two years prior to the commercial launch of Bt cotton. In those tests, the LC50 ranged between 0.11 and 0.61 μg mL−1 diet and the LC90 ranged between 1.17 and 6.94 μg mL−1 diet [45]. Susceptibility of H. armigera to Cry1Ac protein in Brazil also was comparable to the Cry1Ac susceptibility of C. includens and C. virescens, both additional key target pests of MON 87701× MON 89788 soybean [23, 24, 46].
Due to the importance of the Cry1Ac protein for Bt cotton globally, susceptibility of H. armigera to Cry1Ac has been well documented through baseline susceptibility studies in China, India, Australia and Africa [43, 47–49]. Nevertheless, establish in regional susceptibility baselines allows to detect shifts in susceptibility due to the evolution of resistance [50]. Resistance monitoring programs using phenotypic methods consist of collecting insect populations in the field and exposing their offspring to diagnostic concentrations established using baseline susceptibility data [51, 52]. The use of diagnostic concentrations to monitor resistance has the advantage of being more efficient for detecting low frequencies of resistance because all individuals are tested at an appropriate concentration, requiring fewer individuals and less time than complete concentration-mortality tests, and enabling a greater number of populations to be tested [51, 53]. Despite of the limitations of this method, such as limited sensitivity to detect recessive resistant alleles, this type of bioassay is compatible with a large-scale procedure for monitoring the susceptibility of MON 87701× MON 89788 soybean target pests [24, 41]. The response criterion that was used herein represented not only mortality but also larval stunting (i.e., first-instar larvae were considered dead). The use of this response criterion more accurately represents field responses and produces more appropriate diagnostic concentrations that can detect small changes in population susceptibility [54] and makes the monitoring of susceptibility faster and more practical [55]. The results presented in this paper provide a robust base for establishing and validating diagnostic concentrations to be used in a program to monitor the susceptibility of H. armigera populations to Cry1Ac in Brazil.
Major factors associated with the sustained usefulness of Bt crops are a high concentration of the Bt protein, causing practically complete mortality (e.g., ≥99.99%) of an insect pest at the field level and thus rendering inheritance of resistance to be functionally recessive; a low initial frequency of resistance alleles; and abundant refuges of non-Bt host plants available in proximity to the Bt crop [38, 56]. Several studies have reported the expected efficacy of MON 87701 × MON 89788 against the main soybean pests in South America, such as A. gemmatalis, C. includens, another soybean looper (Rachiplusia nu), the soybean shoot borer (Crocidosema aporema [Walsingham, 1914]), and C. virescens [23, 24, 27]. High efficacy of MON 87701 × MON 89788 against H. armigera was reported by Azambuja et al. [29], who documented 100% mortality of later-instar H. armigera larvae feeding on MON 87701 × MON 89788 soybean leaf tissue. Effective protection of MON 87701 × MON 89788 soybean against H. armigera was also reported by Yu et al. [28]; however, these authors measured survival of 2nd-instar larvae and larval weight only after four days of feeding on the tested material, whereas Azambuja et al. [29] infested with neonate larvae and checked for larval mortality up to six days. In the latter case, all larvae fed on MON 87701 × MON 89788 soybean leaf tissue died four to six days after infestation. The most direct approach to test the high-dose assumption is to allow resistant and susceptible adults to mate in the laboratory and measure the survival of their hybrid progeny on Bt plants. However, given that suitable resistant strains for direct tests are not available, indirect tests were used [56]. High mortality and stunting of H. armigera obtained with a range of leaf tissue dilutions (5 to 100x) indicated that the expression of Cry1Ac protein in MON 87701 × MON 89788 soybean should be capable of controlling most heterozygous resistant insects (i.e., individuals carrying one copy of the resistance allele), causing resistance to be functionally recessive [30, 38, 56–58].
Results obtained from the series of experiments presented herein demonstrated the high efficacy of MON 87701 × MON 89788 soybean against H. armigera. The high efficacy against H. armigera reflects both the relative susceptibility of H. armigera to Cry1Ac and very high Cry1Ac expression levels. The Cry1Ac protein expressed in MON 87701 × MON 89788 is identical to the Bt protein expressed in MON 531 cotton (Bollgard) and MON 15985 cotton (Bollgard II), which display virtually complete efficacy against tobacco budworm (C. virescens) and pink bollworm (Pectinophora gossypiella [Saunders]) and moderate activity against Helicoverpa zea (Boddie) and H. armigera [59,60]. However, the Cry1Ac expression levels in leaves of Bt cotton are significantly lower (around 5μg/g dry weight) [61] than the levels found in MON 87701 × MON 89788 soybean (from 50 to 200 μg/g fresh weight) [27].
The initial frequency of resistance alleles estimated herein (0.0011 (95% confidence interval of 0–0.0043)) overlaps with values obtained in Cry1Ac resistance monitoring programs in Australia using F2 screening over seven years for H. armigera (0.0006 (95% confidence interval of 0.0001–0.002)) [17]. In Brazil, a comparable Cry1Ac resistance allele frequency (0.0004) was recently estimated for C. includens using the same methods [46]. Although the F2 screen method provides sensitive estimates of resistance allele frequency in field populations, it requires more efforts than the use of diagnostic concentrations when used as tool in a routine resistance monitoring program [39]. The high toxicity of Bt soybean against H. armigera and the low estimated frequency of resistance allele meet the requirements for the high-dose/refuge IRM strategy [30, 38, 57].
The other critical element of the high-dose refuge strategy is that sufficient refuge exists in the form of plants that can serve as hosts for the pests but do not produce Bt proteins to allow the survival of susceptible insects [30]. H. armigera is a highly polyphagous species that develops on many cultivated and non-cultivated plants. In China, for instance, refuges of non-Bt cotton have not been required for Bt cotton that produces Cry1Ac [8, 62, 63]. The approach implemented in China was based on the premise that abundant non-Bt host plants of H. armigera in China would provide sufficient natural refuges to delay the evolution of resistance. Although several studies have reported small shifts in the frequency of H. armigera resistance to Cry1Ac, Bt cotton producing Cry1Ac has continued to provide substantial control of this pest in China [63]. In addition to the availability of a high proportion of non-Bt host plants, the small size of farms in China coupled with the high mobility of H. armigera are typically pointed out as factors contributing to the success of the natural refuge strategy for H. armigera in China [8, 64, 65].However, recent modeling suggests that natural refuges delayed resistance to Cry1Ac in H. armigera in China but may not have been as effective as an equivalent area of non-Bt cotton refuge, and that switching to Bt cotton producing two or more Bt proteins and integrating other control tactics could slow further resistance evolution [20].
The agricultural landscape in Brazil poses additional challenges for managing resistance to Bt crops. Warmer temperatures and longer growing seasons than in temperate regions allow for a build-up of insect pest populations, particularly polyphagous species [21]. Soybean cultivation is characterized by economies of scale and despite variation in farm size across the landscape, the average farm in the important Center-West soybean-producing region is six times the Brazilian average [66]. Bt proteins are available in Brazil in three main row crops that can host H. armigera (soybean, cotton, and maize), creating a cross-crop scenario whose outcome depends on the ecological interaction between the pest and its host plants and the efficacy of the different Bt crops in the system. There is no information on the spatial and temporal variability of host plant use by H. armigera in Brazil. These data could improve help in the design of IRM programs for Brazil [67]. MON 87701 × MON 89788 soybean was recently approved for cultivation in Brazil [22] and has been rapidly adopted by growers due to the high levels of control provided against the main lepidopteran pests of soybean [68]. Recognizing the risk of resistance evolution in this challenging landscape, growers in Brazil are recommended to plant and manage a structured refuge of non-Bt soybean equivalent to at least 20% of the total soybean area being cultivated. Seed mixtures are not recommended because studies indicated that no target pests survive on non-Bt plants interspersed in a Bt soy field (data not published). Maintenance of sufficient compliance with this structured refuge recommendation at the farm level is essential to delay the evolution of resistance in H. armigera and other target pests of MON 87701 × MON 89788 soybean. The planting of structured refuges for Bt crops is not mandatory in Brazil, thus engaging key stakeholders across the soybean production chain is critical to effectively reach growers and ensure facilitate the planting of refuges. In addition to effective implementation of refuges, the deployment of technologies pyramiding insect-protection traits is a strategy capable of slowing the evolution of insect resistance, and this approach should be pursued in Brazil.
Materials and Methods
Insect rearing and field collections
Permit access to collect material used in our research at various crop sites was granted by Sistema de Autorização e Informação em Biodiversidade (Sisbio) from the Brazilian Ministry of Environment to SGS Gravena (Sisbio License # 10018–1) and PROMIP (Sisbio License # 40380–2). Number of caterpillars collected and location are listed in Table 5.The insect colony used for characterizing the toxicological parameters of MON 87701 × MON 89788 soybean against H. armigera (leaf disc, screenhouse and dilution bioassays) was sampled on soybean in Goiás State during crop season 2013/14 and established in the laboratory as a reference population (LAB). Field populations were sampled on non-Bt soybean and non-Bt cotton from crop season 2013/14 to 2014/15 from multiple regions across Brazil to establish Cry1Ac baseline susceptibility and to perform an F2 screen (Table 5). Approximately 800–1,200 larvae were collected in each sampling area and kept on an artificial diet adapted from Kasten et al. [69]. Mortality caused mainly by parasitism and entomopathogens resulted in approximately 40% adult viability. Remaining adults were maintained in oviposition cages. To guarantee the absence of H. zea individuals in the populations, all adult males (F0) of each population were captured after mating and their genitalia were dissected and identified [2]. Populations were not used unless all individuals sampled were identified as H. armigera.
Table 5. Locations, host plant and crop season of H. armigera field populations sampled.
| Season | Code | County/State | Host | Latitude | Longitude |
|---|---|---|---|---|---|
| LAB | Montividiu, GO | Soybean | 17°22'28.8"S | 51°13'23.3"W | |
| MS-1 | Chapadão do Sul, MS | Soybean | 18°44'38.5"S | 52°35'17.8"W | |
| SP-1 | Jaboticabal, SP | Soybean | 21°12'27.8"S | 48°19'37.0"W | |
| BA-1 | Luis Eduardo Magalhães, BA | Cotton | 12°00'56.9"S | 45°49'19.8"W | |
| PR-1 | Ponta Grossa, PR | Soybean | 25°05'13.5"S | 49°59'18.7"W | |
| 2013/14 | MT-1 | Primavera do Leste, MT | Soybean | 15°26'22.4"S | 54°10'44.7"W |
| GO-1 | Rio Verde, GO | Soybean | 17°44'27.0"S | 50°55'55.4"W | |
| PR-2 | Rolândia, PR | Soybean | 23°14'34.9"S | 51°26'14.7"W | |
| BA-2 | Correntina, BA | Soybean | 13°21'57.6"S | 45°28'36.2"W | |
| BA-3 | Luis Eduardo Magalhães, BA | Soybean | 11°48'26.6"S | 45°59'01.3"W | |
| MT-2 | Primavera do Leste, MT | Cotton | 15°20'18.6"S | 54°20'23.0"W | |
| SP-2 | Jaboticabal, SP | Soybean | 21°12'50.7"S | 48°15'58.8"W | |
| 2014/15 | MT-3 | Lucas do Rio Verde, MT | Soybean | 12°58'15.7"S | 56°08'20.9"W |
| BA-4 | Luis Eduardo Magalhães, BA | Soybean | 12°11'27.9"S | 45°46'28.5"W | |
| PR-3 | Londrina, PR | Soybean | 23°26'38.2"S | 51°06'04.8"W |
Baseline susceptibility to Cry1Ac
A synthetic Cry1Ac protein formulated product (MVP II, Pseudomonas encapsulated Cry1Ac from Dow Chemicals, San Diego, CA, USA, containing 11.14% of active Cry1Ac protein) was incorporated into the artificial diet, without formalin and antibiotics, when the diet temperature reached 56°C. To establish the baseline curves, five to seven concentrations were used, ranging from 0.01 to 10 μg of active protein ml−1 of diet. A 1-ml aliquot of diet containing the protein was poured into each cell of a 16-cell square area of a 128-cell bioassay tray. The trays were sealed with self-adhesive plastic sheets (BIO-CV-16; CD International Inc., Pitman, NJ, USA) that allowed gas exchange with the external environment and then placed in a climatic chamber (temperature 27 ± 2°C, 60 ± 10% relative humidity, and a 14-h photoperiod). The experimental design was completely randomized, with seven to eight replicates per concentration (16 larvae per replicate). Mortality (individuals dead and inhibited from molting beyond first instar) and the weight of the surviving larvae were recorded after seven days to estimate two toxicological parameters: lethal concentration (LC) and effective concentration (EC) [70]. Toxicological parameters LC50, LC90, EC50, and EC90 and the respective confidence intervals (CI 95%) were estimated using JMP® v10.0 (SAS Institute Inc, Cary, NC, USA).
Efficacy trials—Leaf disc and screenhouse
Efficacy of MON 87701 × MON 89788 soybean plants against H. armigera was assessed by using leaf disc and screenhouse trials as previously described [23, 24]. MON 87701 × MON 89788 soybean and near isogenic negative checks of maturity groups 5.5 and 8.3 (different rates of growth and development) were grown in a greenhouse. Completely expanded leaves were removed from the upper third of the plants when they reached phenological stages V4, V6, and R1–R2. Leaf discs 1.2 cm in diameter were cut using a metallic cutter and placed on mixture of water–agar at 2.5% (1 mL cell−1) separated by a filter paper disc in 24-well acrylic plates (Costar®; Corning, Tewksbury, MA, USA). The experimental design was completely randomized with five replicates per treatment (24 neonates per replicate). Larval mortality was recorded at five days after infestation.
For the screenhouse trials, 5,000 H. armigera pupae were used to infest a containment system consisting of nylon net cages (16×18 mesh; 13.0 m length × 3.5 m width × 2.9 m height) when plants reached the R1 reproductive stage. Evaluations of larval incidence, defoliation, and damaged pods were taken at 32 days after adult emergence.
JMP® v10.0 (SAS Institute Inc, Cary, NC, USA) was used to perform statistical analysis. Data were transformed prior to statistical analysis to meet the assumption of normality. Mortality data obtained from laboratory bioassays (x) were transformed into and screenhouse data (x) were transformed into (x + 0.1)0.5. Statistical evaluation was performed using Student's t-test.
High-dose assessment
To assess the high-dose concept for MON 87701 × MON 89788 soybean against H. armigera, lyophilized and macerated leaf tissue at several dilutions was incorporated into artificial diet as previously described [23, 24]. To produce tissue, MON 87701 × MON 89788 soybean and near isogenic negative checks of maturity groups 5.5 and 8.3 were grown in a greenhouse. Leaves from the upper canopy were removed from MON 87701 × MON 89788 soybean and near isogenic negative checks plants at phenological stage R1–R2 and macerated to be incorporated into artificial diet. Dilutions tested in the diet bioassays ranged from 5- to 300-fold relative to the fresh tissue for MON 87701 × MON 89788 and 5- to 25-fold relative to the fresh tissue for the near isogenic negative checks controls. Bioassay and evaluation procedures were performed as described previously for baseline susceptibility.
Frequency of resistance alleles to MON 87701 × MON 89788 soybean
The F2 screen was conducted according to Huang et al. [71]. Pupae of H. armigera derived from individuals sampled on non-Bt soybean and non-Bt cotton plants were sexed and individually placed in cylindrical plastic cages (20 cm height × 10 cm diameter) until emergence. Adults were matched and allowed to mate and oviposit. Each single-pair mating represented an insect family line. Approximately 100 F1 progeny larvae of each single-pair mating were then reared on artificial diet to the pupal stage, as described above. F1 adults (30 couples) from each single-pair mating were sib-mated in plastic cages (20 cm height × 10 cm diameter) to produce F2 offspring. Offspring produced from a single-pair mating was considered as a two-parent family line. The F2 screen was conducted in 32-well trays (Advento do Brasil, São Paulo, Brazil). Leaf tissue was excised from leaves of MON 87701 × MON 89788 soybean plants at vegetative stages V4 until V6 and placed in each well. For each insect family line, 128 F2 neonates were screened. The number of surviving larvae was recorded and leaf tissue was replaced every four days until pupation. Family lines that presented survivors four days after infestation were retested as described above with additional F2 eggs from those lines. Allele frequency was estimated using the algorithm described in equation 1 of Andow and Alstad [39]. Confidence intervals (95% CI) were estimated by equation 5 if no resistant family lines were detected or by equation 7 if resistant family lines were detected. The resistance allele frequency was calculated using the function binom.bayes from the package binom in R 3.1.0.
Acknowledgments
We thank SGS Gravena (SISBIO License # 10018–1) and PROMIP (SISBIO License # 40380–2) for collecting insect samples.
Data Availability
All relevant data are within the paper.
Funding Statement
Monsanto provided support in the form of salaries for PMD, FBB, RAC, SM and GPH, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tay WT, Soria MF, Walsh T, Thomazoni D, Silvie P, Behere GT, et al. A Brave New World for an Old World Pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. Plos One. 2013; 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pogue MG. A New Synonym of Helicoverpa zea (Boddie) and Differentiation of Adult Males of H. zea and H. armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Annals of the Entomological Society of America. 2004;97(6):1222–6. [Google Scholar]
- 3.Zalucki M, Daglish G, Firempong S, Twine P. The Biology and Ecology of Heliothis-Armigera (Hubner) and Heliothis-Punctigera Wallengren (Lepidoptera, Noctuidae) in Australia—What Do We Know. Australian Journal of Zoology. 1986;34(6):779–814. [Google Scholar]
- 4.Cunningham JP, Zalucki MP. Understanding heliothine (Lepidoptera: Heliothinae) pests: what is a host plant? J Econ Entomol. 2014;107(3):881–96. [DOI] [PubMed] [Google Scholar]
- 5.Fitt GP. The ecology of Heliothis species in relation to agroecossystems. Annua Rev Entomol. 1989;34:17–52. [Google Scholar]
- 6.Downes S, Mahon R. Evolution, ecology and management of resistance in Helicoverpa spp. to Bt cotton in Australia. Journal of invertebrate pathology. 2012;110(3):281–6. 10.1016/j.jip.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 7.Kranthi KR, Jadhav DR, Kranthi S, Wanjari RR, Ali SS, Russell DA. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 2002;21(6):449–60. [Google Scholar]
- 8.Wu KM, Guo YY. The evolution of cotton pest management practices in China. Annu Rev Entomol. 2005;50:31–52. [DOI] [PubMed] [Google Scholar]
- 9.Czepak C, Albernaz KC, Vivan LM, Guimarães HO, Carvalhais T. Primeiro registro de ocorrência de Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) no Brasil. Pesquisa Agropecuária Tropical. 2013;43:110–3. [Google Scholar]
- 10.Sosa-Gómez DR, Specht A, Paula-Moraes SV, Lopes- Lima A, Yano SAC, Micheli A, et al. Timeline and geographical distribution of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae: Heliothinae) in Brazil. Revista Brasileira de Entomologia. [Google Scholar]
- 11.Zalucki MP, Furlong MJ. Forecasting Helicoverpa populations in Australia: A comparison of regression based models and a bioclimatic based modelling approach. Insect Science. 2005;12(1):45–56. [Google Scholar]
- 12.Kriticos DJ, Ota N, Hutchison WD, Beddow J, Walsh T, Tay WT, et al. The potential distribution of invading Helicoverpa armigera in North America: is it just a matter of time? PLoS One. 2015;10(3):e0119618 10.1371/journal.pone.0119618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(NAPPO) North American Plant Protection Organization. 2014. Detection of old world bollworm (Helicoverpa armigera) in Puerto Rico. North American Plant Protection Organization (NAPPO) Phytosanitary Alert System official pest report. Available: http://www.pestalert.org/oprDetail.cfm?oprID/600. Accessed 5 August 2015.
- 14.Anonymous. Old world bollworm found in US poses serious risk to cotton, other crops Southeast Farm Press; 2015:20:18. [Google Scholar]
- 15.Mastrangelo T, Paulo DF, Bergamo LW, Morais EGF, Silva M, Bezerra-Silva G, et al. Detection and Genetic Diversity of a Heliothine Invader (Lepidoptera: Noctuidae) From North and Northeast of Brazil. J Econ Entomol. 2014;107(3):970–80. [DOI] [PubMed] [Google Scholar]
- 16.Leite NA, Alves-Pereira A, Corrêa AS, Zucchi MI, Omoto C. Demographics and Genetic Variability of the New World Bollworm (Helicoverpa zea) and the Old World Bollworm (Helicoverpa armigera) in Brazil. PLoS ONE. 2014;9(11):e113286 10.1371/journal.pone.0113286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downes S, Mahon R. Successes and challenges of managing resistance in Helicoverpa armigera to Bt cotton in Australia. GM crops & food. 2012;3(3):228–34. [DOI] [PubMed] [Google Scholar]
- 18.Fathipour Y, Sedaratian A. Integrated management of Helicoverpa armigera in soybean cropping systems In: El-Shemy HA, editor. Soybean—pest resistance. Cairo: InTeOpP; 2013. pp. 231–280. [Google Scholar]
- 19.Yang Y, Li Y, Wu Y. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J Econ Entomol. 2013;106(1):375–81. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Zhang H, Lu Y, Yang Y, Wu K, Tabashnik BE, et al. Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nature biotechnology. 2015;33(2):169–74. 10.1038/nbt.3100 [DOI] [PubMed] [Google Scholar]
- 21.Barros R, Degrande PE. Evaluation of Bt-cotton as a strategic tool for the control of cotton plant-pests under field conditions. Científica (Jaboticabal). 2012;40(2):117–37. [Google Scholar]
- 22.Comissão Técnica Nacional de Biossegurança (CTNBio) [Internet]. Commercial release of genetically modified insect-resistant and herbicide-tolerant soy containing genetically modified events MON 87701 and MON 89788. In: Technical Opinion No. 2542/2010. Available: http://www.ctnbio.gov.br/index.php/content/view/15558.html. Accessed 24 November 2015.
- 23.Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger GU, et al. Assessment of the high-dose concept and level of control provided by MON 87701 x MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci. 2012;68(7):1083–91. 10.1002/ps.3271 [DOI] [PubMed] [Google Scholar]
- 24.Bernardi O, Dourado PM, Carvalho RA, Martinelli S, Berger GU, Head GP, et al. High levels of biological activity of Cry1Ac protein expressed on MON 87701 x MON 89788 soybean against Heliothis virescens (Lepidoptera: Noctuidae). 2014. April In: Pest Manag Sci [Internet]. [588–94]. Available from: <Go to ISI>://WOS:000332393300010. 10.1002/ps.3581 [DOI] [PubMed] [Google Scholar]
- 25.Albernaz KC, Merlin BL, Martinelli S, Head GP, Omoto C (2012) Baseline susceptibility to Cry1Ac insecticidal protein in Heliothis virescens (Lepidoptera: Noctuidae) populations in Brazil. J. Econ. Entomol. 106: 1819–1824. 10.1603/ec12222 [DOI] [PubMed] [Google Scholar]
- 26.Specht A, Sosa-Gomez DR, de Paula-Moraes SV, Cavaguchi Yano SA. Morphological and molecular identification of Helicoverpa armigera (Lepidoptera: Noctuidae) and expansion of its occurrence record in Brazil. Pesquisa Agropecuária Brasileira. 2013;48:689–692. [Google Scholar]
- 27.Macrae TC, Baur ME, Boethel DJ, Fitzpatrick BJ, Gao A-G, Gamundi JC, et al. Laboratory and Field Evaluations of Transgenic Soybean Exhibiting High-Dose Expression of a Synthetic Bacillus thuringiensis cry1A Gene for Control of Lepidoptera. J Econ Entomol. 2005;98(2):577–87. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Li Y, Li X, Romeis J, Wu K. Expression of Cry1Ac in transgenic Bt soybean lines and their efficiency in controlling lepidopteran pests. Pest Manag Sci. 2013;69(12):1326–33. 10.1002/ps.3508 [DOI] [PubMed] [Google Scholar]
- 29.Azambuja R, Degrande PE, Santos ROd, Souza EPd, Gomes CEC. Effect of Bt Soybean on Larvae of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). 2015;7(8). [Google Scholar]
- 30.Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol. 1998;43:701–26. [DOI] [PubMed] [Google Scholar]
- 31.Huang F, Andow DA, Buschman LL. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomologia Experimentalis et Applicata. 2011;140(1):1–16. [Google Scholar]
- 32.Dhurua S, Gujar GT. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci. 2011;67(8):898–903. 10.1002/ps.2127 [DOI] [PubMed] [Google Scholar]
- 33.Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Fresia P, dos Santos AC, et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014;64:150–8. [Google Scholar]
- 34.Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One. 2011;6(7):e22629 10.1371/journal.pone.0022629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, et al. Discovery and Characterization of Field Resistance to Bt Maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol. 2010;103(4):1031–8. [DOI] [PubMed] [Google Scholar]
- 36.van Rensburg JBJ. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. South African Journal of Plant and Soil. 2007;24(3):147–51. [Google Scholar]
- 37.Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM, Crivellari A, et al. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag Sci. 2016:n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 38.Tabashnik BE, Brevault T, Carriere Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotech. 2013;31(6):510–21. [DOI] [PubMed] [Google Scholar]
- 39.Andow DA, Alstad DN. F2 Screen for Rare Resistance Alleles. J Econ Entomol. 1998;91(3):572–8. [Google Scholar]
- 40.Robertson JL, Savin NE, Preisler HK, Russell RM. Bioassays with Arthropods, Second Edition: Taylor & Francis; 2007. [Google Scholar]
- 41.Blanco CA, Andow DA, Abel CA, Sumerford DV, Hernandez G, Lopez JD Jr, et al. Bacillus thuringiensis Cry1Ac resistance frequency in tobacco budworm (Lepidoptera: Noctuidae). J Econ Entomol. 2009;102(1):381–7. [DOI] [PubMed] [Google Scholar]
- 42.Gujar GT, Kalia V, Kumari A, Singh BP, Mittal A, Nair R, et al. Helicoverpa armigera baseline susceptibility to Bacillus thuringiensis Cry toxins and resistance management for Bt cotton in India. Journal of invertebrate pathology. 2007;95(3):214–9. [DOI] [PubMed] [Google Scholar]
- 43.Bird LJ, Akhurst RJ. Variation in susceptibility of Helicoverpa armigera (Hubner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) in Australia to two Bacillus thuringiensis toxins. Journal of invertebrate pathology. 2007;94(2):84–94. [DOI] [PubMed] [Google Scholar]
- 44.Luttrell RG, Wan L, Knighten K. Variation in Susceptibility of Noctuid (Lepidoptera) Larvae Attacking Cotton and Soybean to Purified Endotoxin Proteins and Commercial Formulations of Bacillus thuringiensis. J Econ Entomol. 1999;92(1):21–32. [Google Scholar]
- 45.Jalali SK, Mohan KS, Singh SP, Manjunath TM, Lalitha Y. Baseline-susceptibility of the old-world bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) populations from India to Bacillus thuringiensis Cry1Ac insecticidal protein. Crop Prot. 2004;23(1):53–9. [Google Scholar]
- 46.Yano SA, Specht A, Moscardi F, Carvalho RA, Dourado PM, Martinelli S, et al. High susceptibility and low resistance allele frequency of Chrysodeixis includens (Lepidoptera: Noctuidae) field populations to Cry1Ac in Brazil. Pest Manag Sci. 2015. [DOI] [PubMed] [Google Scholar]
- 47.Wu K, Guo Y, Lv N. Geographic Variation in Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis Insecticidal Protein in China. J Econ Entomol. 1999;92(2):273–8. [DOI] [PubMed] [Google Scholar]
- 48.Kranthi KR, Kranthi S, Wanjari RR. Baseline toxicity of Cry1A toxins to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in India. International Journal of Pest Management. 2001;47(2):141–5. [Google Scholar]
- 49.Brévault T, Prudent P, Vaissayre M, Carrière Y. Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry1Ac and Cry2Ab2 Insecticidal Proteins in Four Countries of the West African Cotton Belt. J Econ Entomol. 2009;102(6):2301–9. [DOI] [PubMed] [Google Scholar]
- 50.Head GP, Greenplate J. The design and implementation of insect resistance management programs for Bt crops. GM crops & food. 2012;3(3):144–53. [DOI] [PubMed] [Google Scholar]
- 51.Roush RT, Miller GL. Considerations for Design of Insecticide Resistance Monitoring Programs. J Econ Entomol. 1986;79(2):293–8. [Google Scholar]
- 52.ffrench-Constant RH, Roush RT. Resistance Detection and Documentation: The Relative Roles of Pesticidal and Biochemical Assays In: Roush RT, Tabashnik BE, editors. Pesticide Resistance in Arthropods. Boston, MA: Springer US; 1990. p. 4–38. [Google Scholar]
- 53.Halliday RW, Burnhaw KP. Choosing the Optimal Diagnostic Dose for Monitoring Insecticide Resistance. J Econ Entomol. 1990;83(4):1151–9. [Google Scholar]
- 54.Ali MI, Luttrell RG. Response Estimates for Assessing Heliothine Susceptibility to Bt Toxins. J Econ Entomol. 2009;102(5):1935–47. [DOI] [PubMed] [Google Scholar]
- 55.McPherson RM, Macrae TC. Evaluation of Transgenic Soybean Exhibiting High Expression of a Synthetic Bacillus thuringiensis cry1A Transgene for Suppressing Lepidopteran Population Densities and Crop Injury. J Econ Entomol. 2009;102(4):1640–8. [DOI] [PubMed] [Google Scholar]
- 56.Tabashnik BE, Van Rensburg JB, Carriere Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol. 2009;102(6):2011–25. [DOI] [PubMed] [Google Scholar]
- 57.Roush RT. Bt-transgenic crops: just another pretty insecticide or a chance for a new start in resistance management? Pesticide Science. 1997;51(3):328–34. [Google Scholar]
- 58.Tabashnik BE, Gould F, Carrière Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. Journal of Evolutionary Biology. 2004;17(4):904–12. [DOI] [PubMed] [Google Scholar]
- 59.Wilson DF, Flint HM, Deaton RW, Fischhoff DA, Perlak FJ, Armstrong TA, et al. Resistance of Cotton Lines Containing a Bacillus thuringiensis Toxin to Pink Bollworm (Lepidoptera: Gelechiidae) and other Insects. J Econ Entomol. 1992;85(4):1516–21. [Google Scholar]
- 60.Llewellyn DJ, Mares CL, Fitt GP. Field performance and seasonal changes in the efficacy against Helicoverpa armigera (Hübner) of transgenic cotton expressing the insecticidal protein vip3A. Agricultural and Forest Entomology. 2007;9(2):93–101. [Google Scholar]
- 61.Greenplate JT, Mullins JW, Penn SR, Dahm A, Reich BJ, Osborn JA, Rahn PR, Ruschke L, Shappley ZW. Partial Characterization of cotton plants expressing two toxin proteins from Bacillus thuringiensis: relative toxin contribution, toxin interaction, and resistance management. Journal of Applied Entomology. 2003;127 (340–347). [Google Scholar]
- 62.Wu KM, Lu YH, Feng HQ, Jiang YY, Zhao JZ. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science (New York, NY). 2008;321(5896):1676–8. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Yin W, Zhao J, Jin L, Yang Y, Wu S, et al. Early Warning of Cotton Bollworm Resistance Associated with Intensive Planting of Bt Cotton in China. PLoS ONE. 2011;6(8):e22874 10.1371/journal.pone.0022874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu K, Guo Y, Gao S. Evaluation of the natural refuge function for Helicoverpa arnigera (Lepidoptera: Noctuidae) within Bacillus thuringiensis transgenic cotton growing areas in north China. J Econ Entomol. 2002;95(4):832–7. [DOI] [PubMed] [Google Scholar]
- 65.Behere GT, Tay W, Russell DA, Heckel DG, Appleton BR, Kranthi KR, et al. Mitochondrial DNA analysis of field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) and of its relationship to H. zea. BMC Evolutionary Biology. 2007;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conte L. Economia de escala e substituição de fatores na produção de soja no Brasil. Piracicaba, SP, Brazil: Universidade de São Paulo; 2006. 115 p. Doctoral thesis [Online]. Available: http://www.teses.usp.br/teses/disponiveis/11/11132/tde‐21112006‐141552/. Accessed 23 November 2015.71. IBGE. Produção Agrícola Municipal. Quantidade produzida, valor da produção, área plantada e área colhida da lavoura temporária 2012.
- 67.Head G, Jackson RE, Adamczyk J, Bradley JR, Van Duyn J, et al. (2010) Spatial and temporal variability in host use by Helicoverpa zea as measured by analyses of stable carbon isotope ratios and gossypol residues. J Appl Ecol 47: 583–592. [Google Scholar]
- 68.Céleres. Informativo Biotecnologia, IB14.03, 16 December 2014. Uberlândia, MG, Brazil: Céleres; 2014. [Google Scholar]
- 69.Kasten P Jr, Precetti AACM, Parra JRP. Dados biológicos comparativos de Spodoptera frugiperda (J. E. Smith, 1797) em duas dietas artificiais e substrato natural. Rev Agric. 1978;53:69–78. [Google Scholar]
- 70.Sims. Monitoring strategies for early detection of Lepidoptera resistance to Bacillus thuringiensis insecticidal proteins In: TM B, editor.1996. [Google Scholar]
- 71.Huang F, Ghimire MN, Leonard BR, Wang J, Daves C, Levy R, et al. F2 screening for resistance to pyramided Bacillus thuringiensis maize in Louisiana and Mississippi populations of Diatraea saccharalis (Lepidoptera: Crambidae). Pest Manag Sci. 2011;67(10):1269–76. 10.1002/ps.2182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.