Abstract
Aim
To evaluate the effectiveness of the Austrian Disease Management Program (DMP) ‘Therapie aktiv—Diabetes im Griff’ for patients with type 2 diabetes mellitus concerning patient-relevant outcomes (mortality, myocardial infarction and stroke) and costs.
Methods
Based on routine health insurance data, we conducted a population-based retrospective cohort study using a propensity score (PS) matched control group design. The DMP-group consists of participants enrolled in the program during 2008 and 2009 (n = 7181). Out of 208.532 patients with no participation in the DMP up to 2013, PS-matched controls were selected with a matching ratio 1:3. In the PS-model, patient’s characteristics, form of antidiabetic drug therapy, several prescriptions, the number of hospital admissions and days, main discharge diagnoses and costs at baseline were included.
Results
Over a follow-up period of four years, we observed a significantly lower mortality rate in the DMP-group (9.4%) in comparison with the control group (15.9%, p<0.001). The cumulative number of hospital days and mean annual hospital costs were lower for DMP-participants resulting in significantly lower mean annual total costs, amounting to € 8226.80 per patient in the DMP-group and € 9231.10 in the control group respectively (p<0.001).
Conclusions
The evaluation shows a survival benefit and an average reduction of costs for participants in the DMP compared with the control-group. Despite we took great effort to ensure comparable groups, we cannot entirely rule out an influence by residual and unmeasured confounding due to the observational study design and the use of routine data. However, the results indicate that the disease management program implemented in Austria improves quality of care for patients with type 2 diabetes mellitus.
Introduction
In 2014, about 387 million people worldwide, or 8.3% of the adult population aged 20–79 years, are estimated to have diabetes with increasing incidence [1]. In Austria about 573,000–645,000 people suffer from diabetes mellitus, which represents 8%—9% of Austria's population [2]. Diabetes mellitus is associated with serious long-term complications such as cardiovascular disease, blindness, kidney failure, and amputation of the lower extremities, resulting in increased use of medical services, lower quality of life and reduced life expectancy [3]. Moreover, the medical care of diabetic patients leads to high healthcare costs and poses severe challenges for healthcare systems worldwide [4, 5].
To improve the quality of life for diabetic patients, to extend their life time in good health and further to reduce costs, disease management programs (DMPs) for chronic care, including different forms of care coordination and self-management support, were introduced in many countries since the early nineties [6]. In Germany, for example, primary-care based DMPs were implemented nationwide in 2002, including a DMP for type 2 diabetes mellitus [7]. In Austria, the DMP called “Therapie aktiv—Diabetes im Griff” (http://www.therapie-aktiv.at) has the objective to organize long term and high-quality care for patients with type 2 diabetes mellitus. The implementation of this program started in 2007 across most regions of Austria. The participation is voluntary and free of charge for physicians (general practitioners and internists in private practice) and patients. Physicians receive a basic training before they can work as so called “DMP-physicians”. The DMP includes the implementation of evidence-based clinical guidelines and assures that the necessary medical examinations are provided on a regular basis. Patient empowerment is an important component of the program with shared individual target agreements by the patient and the physician. Patients are offered lifestyle advice to enable them to change their diet habits and to encourage physical activity. Regular documentation including information on medical parameters, treatment, target agreements and quality of life is carried out by the DMP-physicians. Currently, about 45.000 diabetic patients are included in the program.
While it is important to monitor the impact of the program for the patients enrolled, the effectiveness of the DMP has to be evaluated compared with a control group [8]. So far, one cluster-randomized controlled trial on the effectiveness of the DMP in Austria was conducted with the primary endpoint change in HbA1c from baseline to one year after enrollment [9]. There were no significant improvements in metabolic control in the intervention group compared with the control group, but the process quality of care was improved. Similar results were observed after two years of follow-up [10]. However, this study was performed only for diabetic patients in one region (Salzburg) with a follow-up of up to two years.
Although a randomized design would be desirable for evaluation, observational study designs might be more suitable especially for long-term effects (i.e. overall mortality, cardiovascular diseases) in population-wide disease management programs frequently implemented in an operational setting. Therefore, studies with longer follow-up periods based on routine data are useful to get more insight in the effectiveness of DMPs.
The aim of this observational study was to evaluate the impact of the DMP in Austria on patient-relevant outcomes (mortality, myocardial infarction and stroke) and costs compared with patients with type 2 diabetes mellitus under routine care.
Materials and Methods
Study design and data
To evaluate the DMP we performed a retrospective cohort study using a propensity score (PS) matched control group design with baseline years 2007/2008 and follow-up until 2012/2013. The study was based on routine health insurance data in agreement with the Austrian general social insurance act (LEICON database). Patient information was anonymized and de-identified prior to analysis. In LEICON, the data of 13 major health insurance carriers in Austria are included with coverage of more than 90% of the statutory health insured persons. The identification of patients with type 2 diabetes mellitus in the LEICON database is based on the form of antidiabetic drug therapy, classified according to the Anatomical Therapeutic Chemical (ATC) code. In detail: the prescription of an oral antidiabetic drug (OAD, ATC-code: A10B), a combination therapy of OAD and insulin (ATC-codes: A10B and A10A) or insulin therapy only. For patients receiving insulin therapy only, age restriction is applied (50 years or older) to exclude patients with type 1 diabetes mellitus. Additionally, patients with type 2 diabetes mellitus and no antidiabetic drug therapy are identified by 4 or more blood glucose level measurements or two or more HbA1c measurements.
For this study, patients with type 2 diabetes mellitus had to be registered in LEICON in the baseline years throughout 2012 or deceased. The DMP-group consisted of patients who enrolled in the program between January 1, 2008 and December 31, 2009. To ensure that the patients actively participated in the DMP, they had to have at least one DMP-documentation by their physicians after enrollment. The control group consisted of diabetic patients not enrolled in the DMP before December 31, 2013 and predominantly (more than 80% visits) under treatment of non DMP-physicians. Both groups included only patients still alive on December 31, 2008, for baseline year 2007 and still alive on December 31, 2009, for baseline year 2008.
For the evaluation data concerning patient’s characteristics, prescriptions (S1 Table), number of hospital admissions and days, main diabetes-relevant admission and discharge diagnoses (S2 Table) and costs for in- and outpatient care per calendar year were provided.
Endpoints
Patient-relevant outcomes and the economic impact were considered. The primary outcome for the medical effectiveness of the DMP was overall mortality. In addition, the diabetes-specific complications myocardial infarction, stroke and stroke/non-traumatic intracranial bleedings identified using the International Classification of Diseases (ICD-10) codes (www.who.int/classifications/icd/en): I21-I22, I63 and I60-I64, respectively, were evaluated. With regard to the economic impact, total costs (including outpatient physician services costs, hospital costs, prescription costs, transportation costs) are defined as the primary outcome. Secondarily, the single cost components and the number of hospital admissions and days were investigated. Outpatient physician services, prescription and transportation costs are based on direct claims data from the health insurance carriers during the investigated time frame. These include all outpatient physician services, except dentistry service, prescriptions reimbursed by health insurance and transportation service. Hospital costs in LEICON are routinely calculated from the individual patient’s length of hospital stays combined with average costs for hospital care, this is due to the central hospital’s yearly lump-sum funding.
Statistical Analysis
Sample size considerations
Sample size considerations were performed for the patient-relevant outcomes and are based on the mortality rate of diabetic patients in Styria in 2006 and the evaluation results from Germany [11, 12]. Under the assumption of a 5-year mortality rate of 18% in the control group and a reduction of 2.7% (15%) in the DMP-group, for a ratio 1:3 3168 DMP-participants and 9599 controls would be needed to achieve a power of 90% for a two-sided chi square test with a significance level of 2.5%. For the secondary patient-relevant outcomes we assumed an annual incidence of 1% and a reduction of 25% for DMP-participants resulting in 5639 DMP-participants and 17087 controls. Based on these considerations, all patients who enrolled in the DMP in the years 2008 and 2009 were included.
Propensity score calculation and matching
The PS, defined as probability of participating in the DMP conditional on baseline covariates [13], was calculated using a multivariate logistic regression with DMP-participation as the dependent variable. As independent baseline covariates patient’s characteristics (sex, age, prescription fee (yes/no)), form of antidiabetic drug therapy (none, oral antidiabetic drugs (OAD) only, insulin only, OAD and insulin), several prescriptions (antihypertensive drugs, lipid modifying agents, psychiatric medications and analgesics) (S1 Table), the number of hospital days (none, 1–7, 8–14, 15–30, >30 days), main diabetes-relevant admission and discharge diagnoses (S2 Table) summarized into a single variable (yes, if one or more diagnoses were present, no otherwise) and total costs were included to reflect sociodemographic status and health status at baseline [7, 11, 12, 14]. Based on the estimated PS, for every DMP-participant three controls were matched to increase precision using a nearest-neighbour-matching algorithm without replacement adapted from the SAS macro from Coca-Perraillon [15]. The PS calculation and the matching was performed stratified by the baseline years 2007 and 2008 and stratified by the participating regions of Austria (Burgenland, Lower Austria, Upper Austria, Salzburg, Styria, Vorarlberg and Vienna). To assess the quality of the matching, i.e. if the covariates are balanced between the matched groups, standardized difference between the groups were calculated before and after matching [16]. A standardized difference close to zero indicates good balance of the covariate between the DMP-group and the controls.
Analysis
The primary patient-relevant outcome, mortality, was analyzed using Kaplan-Meier and a Cox-Proportional Hazard Model. To account for the matched nature of the data, a robust sandwich estimator was used [17]. An observation was censored if the patient was still alive after 4 years of follow-up. The observational periods for the DMP-participants and their matched controls are: January 1, 2009 to December 31, 2012 and January 1, 2010 to December 31, 2013 for the two baseline years 2007 and 2008, respectively. The results are presented as hazard ratio (HR) with a 95% confidence interval (CI). For cost analysis, the mean annual total costs per person over the 4 year follow-up period 2009/2010–2012/2013 are calculated and compared between the DMP-participants and controls using a GEE-Modell with gamma-distribution and log-link accounting for the matching [18]. For the GEE-Model, PROC GENMOD was used assuming an exchangeable correlation structure. Additionally, a 95% CI for the mean annual total cost differences between the groups was calculated using bootstrap-methods [19, 20]. This was done by drawing (with replacement) 10000 repeated random samples of the same size as the original sample. To account for the matching, we draw the bootstrap samples from the matched sets as recommended in [20]. Secondary outcomes (diabetes-specific complications, single cost components, the number of hospital admissions and days) were analyzed descriptively. Furthermore, sensitivity analyses were performed to investigate changes in the outcome parameters under varying inclusion/exclusion criteria. For the two primary endpoints a two-sided significance level of 2.5% was used to indicate statistical significance. Matching and statistical analysis were performed using SAS Version 9.2.
Results
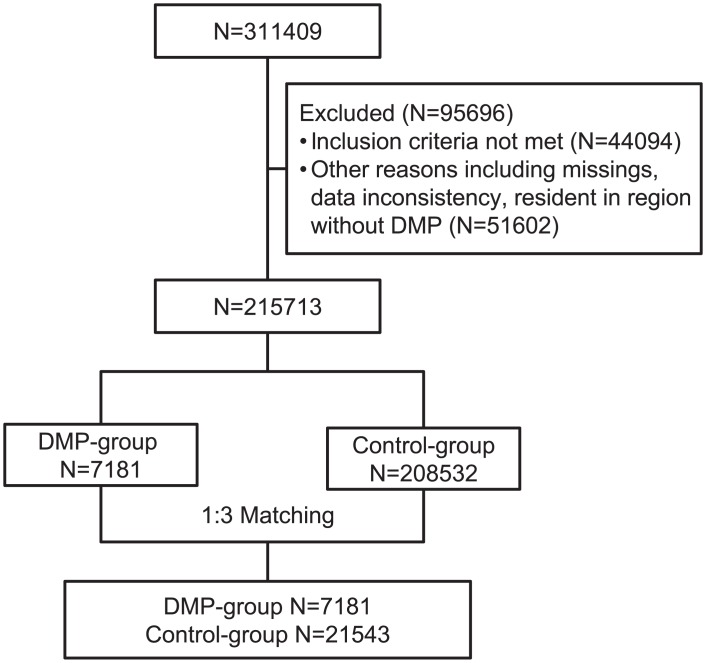
A total of 311409 patients were registered in LEICON in the baseline years 2007 and 2008 up to 2012. From those, 44094 (14.2%) patients did not meet the inclusion criteria and 51602 (16.6%) were excluded due to missing values, data inconsistency and residency in a region without DMP in the baseline years 2007 or 2008. On the remaining 215713 patients (7181 DMP-group, 208532 control group), a propensity score matching with 1:3 ratio was performed yielding 7181 DMP-participants and 21543 controls for analysis (Fig 1).
Fig 1. Flowchart of type 2 diabetic patients considered for evaluation study.
Inclusion/Exclusion criteria: DMP-group: enrollment in DMP between January 1, 2008 and December 31, 2009; at least one DMP-documentation. Control group: no enrollment in DMP before December 31, 2013; predominantly under treatment of non DMP-physicians. Both groups: patients registered in LEICON database in the baseline years throughout 2012 or deceased; still alive on December 31, 2008, (baseline year 2007) and December 31, 2009, (baseline year 2008).
Balance
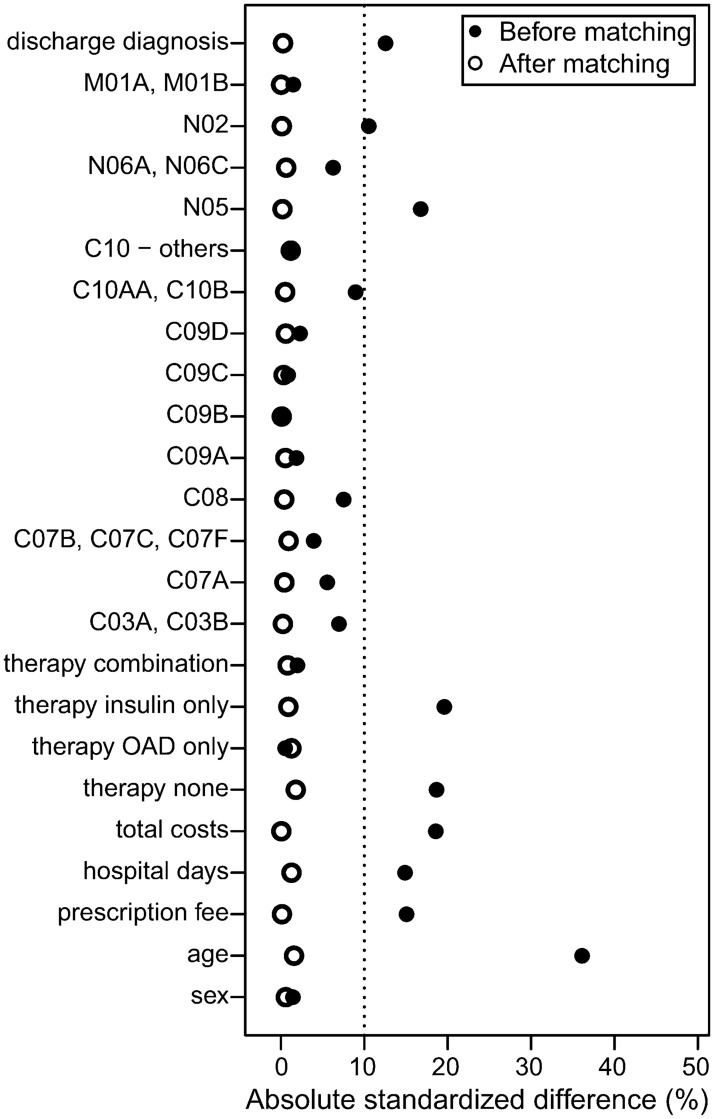
Before matching large imbalances were observed for age, the form of antidiabetic drug therapy, total costs and hospital days. After matching all measured baseline characteristics were similar between the groups (Fig 2, S3 Table). In the matched data, 50.9% of the patients were female and the mean age was 64.2 (±11.4) years. At baseline, 66.5% of the patients received oral antidiabetic drugs (OAD) only, 6.7% insulin only, 10.6% both and 15.9% diabetic patients received no antidiabetic drug therapy.
Fig 2. Absolute standardized differences (%) between DMP-participants and controls before and after matching.
Therapeutic subgroups of the Anatomical Therapeutic Chemical (ATC) codes: Analgetic medication: N02, M01A, M01B; Psychiatric medication: N05, N06A, N06C; Lipid modifying agents: C10AA, C10B, C10AB, C10AC, C10AD, C10AX; Antihypertensive drugs: C03A, C03B, C07A, C07B, C07C, C07F, C08, C09A, C09B, C09C, C09D.
Patient-relevant outcomes
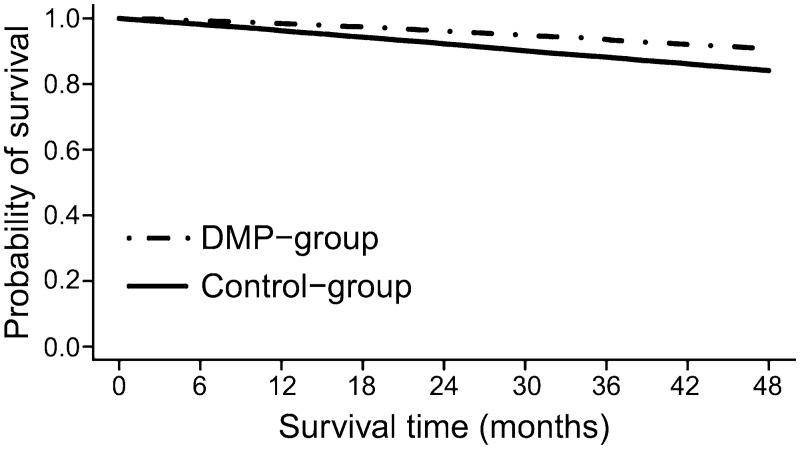
A comparison of the patient-relevant outcomes between the matched groups is presented in Table 1. Within 4 years after DMP enrollment, 9.4% (674/7181) of the patients died in the DMP-group, whereas in the control-group 15.9% (3426/21543) of the patients died. Mortality in the DMP-group was significantly reduced compared with the control-group (HR = 0.57, 95% CI: 0.52–0.61, p<0.001). In Fig 3 the Kaplan-Meier curves are shown. For the secondary outcomes, slightly lower percentages were observed for the DMP-participants (myocardial infarction: 2.0% (143/7161), stroke 2.2% (159/7161)) compared with controls (myocardial infarction: 2.3% (485/21044), stroke 2.6% (542/21044)). The percentage of at least one of the investigated diabetic-specific complications was 5.0% (359/7161) in the DMP-group and 6.1% (1279/21044) in the control-group.
Table 1. Comparison of patient-relevant outcomes between the DMP-group and the control-group for a follow-up period of 4 years.
| DMP-group N = 7181 | Control-group N = 21543 | |||
|---|---|---|---|---|
| N | % | N | % | |
| Mortality | 674 | 9.39 | 3426 | 15.90 |
| HR (95% CI) | 0.57 (0.52–0.61) | |||
| Diabetes-specific complicationsa | ||||
| Myocardial infarction (ICD: I21, I22) | 143 | 2.00 | 485 | 2.30 |
| Stroke/non-traumatic intracranial bleedings (ICD: I60-I64) | 225 | 3.14 | 828 | 3.93 |
| Stroke (ICD: I63) | 159 | 2.22 | 542 | 2.58 |
| Any complication b | 359 | 5.01 | 1279 | 6.08 |
a N = 7161 in the DMP-group and N = 21044 in the control-group due to missing values
b Included ICD: I21-I22 and/or I60-I64
Fig 3. Kaplan-Meier curves for DMP-participants and controls.
Economic impact
The average annual total costs per person over the 4-year period 2009/2010–2012/2013 amounted € 8226.80 in the DMP-group and € 9231.10 in the control-group (p<0.001). The cost difference calculated by bootstrap was € 1031.30 (95% CI: € 685.00—€ 1361.40). The mean annual cost components were similar between the groups, except for hospital costs and costs for outpatient physician services. Slightly higher outpatient physician services costs and lower hospital costs were observed in the DMP-group. The cumulative number of hospital days over 4 years follow-up tended to be lower for DMP-participants (median 16 days) compared with controls (median 18 days). There was no difference in the cumulative number of hospital admissions between the groups (median 3 in both groups) (Table 2).
Table 2. Comparison of the economic impact between the DMP-group and the control-group for a follow-up period of 4 years.
| DMP-group N = 7161 | Control-group N = 21044 | |
|---|---|---|
| Mean total costs per year | 8226.80€ | 9231.10€ |
| Outpatient physician services costs | 718.80€ | 654.40€ |
| Hospital costs | 6196.60€ | 7165.40€ |
| Prescription costs | 1243.10€ | 1296.50€ |
| Transportation costs | 68.30€ | 114.80€ |
| Hospital admissions and days | ||
| Hospital admissions and days >0, N (%) | 5201 (72.6) | 14944 (71.0) |
| Cumulative number of hospital days >0 (mean/median) | 29.5/16 | 32.5/18 |
| Cumulative number of hospital admissions >0 (mean/median) | 4.1/3 | 4.3/3 |
Discussion
In this population based cohort study, we evaluated the impact of the DMP in Austria for patients with type 2 diabetes mellitus on patient-relevant and economic outcomes in DMP-participants compared with propensity score matched controls. As primary endpoints mortality and total costs were considered. Both endpoints showed an association between participation in the DMP, and a reduction in mortality and total costs, respectively. Over a follow-up period of four years, the mortality rate in the DMP-group was 9.4% and in the control-group 15.9%. Likewise, the average annual total costs in the DMP-group were reduced on average by about € 1000.
In Germany and Austria the DMP is comparable, the main difference being the implementation of the program; nationwide implementation in Germany and decentralized in Austria [21]. In evaluation studies of the German DMP, similar results were observed which are summarized in a recent systematic review [22]. All studies refer to the first years of DMP implementation, do not go beyond the year 2008 and are very heterogeneous in terms of design, scale and considered endpoints. In summary, positive effects resulted in the evaluation studies in Germany on mortality and improved results concerning process parameters; however, a mixed picture in other investigated parameters like costs is shown. The BARMER study [12, 23] is most comparable to our study, with the exception that no patients without antidiabetic drug therapy were considered and the methodology for the selection of the control-group at both, the parameters included in the PS calculation as well as the matching method used differs in detail. Thus, the demographics slightly differ, for example, the participants in the BARMER study were on average 67 years old, in our study 64 years. After a follow-up period of 3 years, Drabik et al [12] observed a somewhat higher reduction in mortality of 7.5% (mortality rate 7.2% in the DMP-group and 14.7% in the control-group) compared with our results, i.e. reduction in mortality of 6.5% after 4 years. Diabetes-specific complications such as myocardial infarction and stroke, descriptively reported in the BARMER study, were lower in the DMP-group compared to controls [23]. After 4 years follow-up, we observed only a small difference in diabetes-specific complications between the groups (any complication in DMP-group 5.0%: controls: 6.1%). This stands in contrast to our observed substantial reduction in all-cause mortality. In other long-term evaluations of management and self-management programs for diabetic patients a decrease in mortality and cardiovascular events were observed [24, 25]. Our observed modest differences in diabetes-specific complications might be based on the fact that just the admission diagnosis and two discharge diagnoses are documented in the LEICON database and we probably missed some cases. However, in a study from Linder et al [14], no differences between the DMP-participants and controls were observed for stroke and myocardial infarction after 2 years follow-up. In Austria, Ostermann et al [26] observed an improved quality of outpatient care and lower hospitalization for DMP-participants compared with controls in 2009. This is in agreement with our observations that DMP-participants tended to have shorter hospital stays. After one year follow-up, improvements in metabolic control (although not significant), weight loss, cholesterol level and process quality were observed in Sönnichsen et al [9]. Overall, this might contribute to a lower mortality rate for DMP-participants as observed in our study.
The fewer deaths and shorter hospital stays in the DMP-group are reflected in our observed lower total costs which mostly consist of costs for inpatient hospital care. Likewise, the slightly higher outpatient physician services costs in the DMP-group are indicating that patients enrolled in the program might receive more outpatient/ambulatory health care and use more diabetes relevant health services from general practitioners, internists or ophthalmologists compared to non-participants [26]. Generally, there are only small differences in the single cost components between the groups, with the exception of hospital costs. Qualitatively similar results were observed in other studies [12, 14, 23].
For evaluation studies on the effectiveness of DMPs in other countries it must be noted that both the health systems and the DMPs are organized differently. Additionally, the studies are widely heterogeneous in terms of design, choice of outcome measure and observational period. An overview of approaches for chronic disease management and evaluations in Europe is given in Nolte et al [6]. Examples of implemented multidisciplinary management and self-management programs in non-European countries are given in [24, 25, 27, 28, 29, 30].
Frequently investigated parameters to assess the effectiveness of chronic care management programs for diabetes are intermediate clinical outcome measures such as HbA1c and systolic blood pressure. Results from recent meta-analyses generally suggest a positive impact of these programs; however, only moderate improvements in glycaemic control were observed [31, 32].
Our study has several limitations: the main limitation is that this study has no randomized design. Although our PS matching resulted in good balance, this approach adjusts only for measured confounders and further depends on the availability and quality of the routine data. The opportunities and limitations of using routine data for evaluations are discussed in [33]. In our study, the identification of patients with type 2 diabetes mellitus by ICD-10 codes was not possible, since in the outpatient sector in Austria, no standardized coding with ICD-10 is performed. However, ATC codes for prescriptions of pharmaceutical products are well documented and work has been performed to determine the reliability for predicting the ICD code from the ATC code [34]. Additionally, in 2012, the implemented algorithm in the LEICON database was tested for its accuracy to identify patients with type 2 diabetes mellitus. The results showed high sensitivity and specificity of over 80%. However, we cannot completely rule out that misclassification of type 2 diabetes mellitus in the control group is present. Furthermore, we did not have access to clinical data including HbA1c measurements or duration of diabetes which is associated with an increased risk of micro- and macrovascular complications and death [35]. However, to reduce the impact of confounding by disease severity, a large number of diabetes relevant prescriptions and discharge diagnoses were included in the PS calculation. Although, after matching no imbalances were observed in those variables, we cannot rule out that there are still differences between the groups at baseline in terms of other variables (e.g. education, health awareness), which could not be considered due to the secondary use of routine data of the Austrian social insurance institutions. Additionally, our evaluation might be influenced by spill-over effects [36], and by higher motivation of DMP-physicians. The motivation of DMP-physicians is not considered in the matching as only patient characteristics were available. To avoid treatment cross-over in the control-group, we decided to include only control patients which are predominantly under treatment of non DMP-physicians. In a sensitivity analysis without this criterion, no substantial differences in the results were observed (S4 Table). Therefore, our findings may partially be explained by a higher motivation of the patients and the physicians and a less advanced disease in DMP-participants compared to non-participants [37]. A further limitation is that indirect costs including for example additional costs for inability to work could not be considered in our analysis due to limited data availability.
Strengths of the study are the large population based cohort design with follow-up period of 4 years after program enrollment, a broad consideration of matching variables to account for selection bias and the inclusion of patients with type 2 diabetes mellitus and without antidiabetic drug therapy. Including diabetic patients based on drug prescriptions only might reduce the generalizability of the results. In our data, 15.9% (9.5% before matching) belong to diabetic patients without antidiabetic drug therapy. However, in sensitivity analyses without this group we did not observe substantial changes in the results (S5 Table). Additionally, our analysis also includes patients without continuous DMP participation to account for probably less motivated patients. Our inclusion of patients without antidiabetic drug therapy and patients with lesser program activity reflects the effectiveness of the DMP in a realistic way [8].
Conclusions
In summary, over a follow-up period of four years, we observed a significantly lower mortality rate and a reduction in total costs for the DMP-participants in comparison with the control-group. However, we cannot rule out that our findings might be influenced by confounders like disease severity, education or different health awareness among the comparison groups at baseline. Despite these limitations, our study results indicate that the DMP "Therapie aktiv" improves the care of patients with type 2 diabetes mellitus. Finally, it should also be mentioned that our results relate to the initial phase of the program. Further evaluations of the DMP are important to investigate whether the observed benefits maintain or change over time.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank DI Fritz Bruner, Mag. Helmut Nagy and Mag. Heinrich Koch from the Styrian Health Insurance Fund and Dr. Klaus Jeitler (Institute of General Practice and Evidence-based Health Services Research/Institute for Medical Informatics, Statistics and Documentation) for helpful discussion.
Data Availability
Data are owned by a third party, the LEICON database. Interested researchers may contact Ms. Grün (leicon@noegkk.at) to request the data. In order to fulfill the legal requirements to obtain that kind of data, researchers must obtain permission for a specific research question from the Main Association of Austrian Social Security. Additionally, researchers must conclude a contract with the statutory health insurance regarding data access.
Funding Statement
This study was funded by the Styrian Health Insurance Fund which is in charge for the DMP "Therapie aktiv - Diabetes im Griff". The funder provided support in the form of salaries for data extraction [MR] and for statistical analyses [RR], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of these authors is articulated in the 'author contributions' section.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas update poster, 6th edn Brussels, Belgium: International Diabetes Federation, 2014. Available: http://www.idf.org/diabetesatlas. Accessed 21 Jul 2015. [Google Scholar]
- 2.Griebler R, Geißler W, Winkler P, editors. Zivilisationskrankheit Diabetes: Ausprägungen—Lösungsansätze—Herausforderungen Österreichischer Diabetesbericht 2013. Wien: Bundesministerium für Gesundheit; 2013. [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas, 6th edn Brussels, Belgium: International Diabetes Federation, 2013. Available: http://www.idf.org/diabetesatlas. Accessed 21 July 2015. [Google Scholar]
- 4.Jönsson B, CODE-2 Advisory Board. Revealing the cost of Type II diabetes in Europe. Diabetologia. 2002;45(7):S5–12. [DOI] [PubMed] [Google Scholar]
- 5.Seuring T, Archangelidi O, Suhrcke M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics. 2015;33(8):811–31. 10.1007/s40273-015-0268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolte E, Hinrichs S. DISMEVAL. Developing and validating disease management evaluation methods for European healthcare systems. Final report. Cambridge: RAND Corporation; 2012. Available: http://www.rand.org/pubs/technical_reports/TR1226.html. Accessed 15 Dec 2014.
- 7.Fullerton B, Erler A, Pöhlmann B, Krohn R. Evaluation of disease management in Germany. In: Nolte E, Hinrichs S. DISMEVAL—Developing and validating disease management evaluation methods for European healthcare systems: Final report. Cambridge: RAND Corporation; 2012. p. 69–77. Available: http://www.rand.org/pubs/technical_reports/TR1226.html. Accessed 15 Dec 2014.
- 8.Conklin A, Nolte E. Disease Management Evaluation A Comprehensive Review of Current State of the Art. Cambridge: RAND Corporation; 2011. Available: http://www.rand.org/pubs/technical_reports/TR894.html. Accessed 15 Dec 2014. [PMC free article] [PubMed] [Google Scholar]
- 9.Sönnichsen AC, Winkler H, Flamm M, Panisch S, Kowatsch P, Klima G, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster-randomised controlled trial. BMC Fam Pract. 2010;11:86 10.1186/1471-2296-11-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flamm M, Panisch S, Winkler H, Johansson T, Weitgasser R, Sönnichsen AC. Effectiveness of the Austrian disease management programme "Therapie Aktiv" for type 2 diabetes regarding the improvement of metabolic control, risk profile and guideline adherence: 2 years of follow up. Wien Klin Wochenschr. 2012;124(17–18):639–46. [DOI] [PubMed] [Google Scholar]
- 11.Miksch A, Laux G, Ose D, Joos S, Campbell S, Riens B, et al. Is there a survival benefit within a German primary care-based disease management program? Am J Manag Care. 2010;16(1):49–54. [PubMed] [Google Scholar]
- 12.Drabik A, Büscher G, Thomas K, Graf C, Müller D, Stock S. Patients with type 2 diabetes benefit from primary care-based disease management: a propensity score matched survival time analysis. Popul Health Manag. 2012;15(4):241–7. 10.1089/pop.2011.0063 [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 14.Linder R, Ahrens S, Köppel D, Heilmann T, Verheyen F. The benefit and efficiency of the disease management program for type 2 diabetes. Dtsch Arztebl Int. 2011;108(10):155–62. 10.3238/arztebl.2011.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coca-Perraillon M. Local and global optimal propensity score matching. SAS Global Forum: Statistics and Data Analysis 2007; Paper 185–2007
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84(408):1074–8. [Google Scholar]
- 18.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465–88. [DOI] [PubMed] [Google Scholar]
- 19.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 20.Austin PC, Small DS. The use of bootstrapping when using propensity-score matching without replacement: a simulation study. Stat Med. 2014;33(24):4306–19. 10.1002/sim.6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schang L, Czypionka T, Thomson S. Strengthening care coordination: comparative analysis of reform implementation in Austria and Germany, with options for Austria. Report prepared for the Main Association of Austrian Social Security Institutions. London: LSE Health. 2013. Available: http://www.hauptverband.at/portal27/portal/hvbportal/content/contentWindow?contentid=10007.695080&action=2&viewmode=content. Accessed 9 Jun 2016.
- 22.Fuchs S, Henschke C, Blümel M, Busse R. Disease management programs for type 2 diabetes in Germany: a systematic literature review evaluating effectiveness. Dtsch Arztebl Int. 2014;111(26): 453–63. 10.3238/arztebl.2014.0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock S, Drabik A, Büscher G, Graf C, Ullrich W, Gerber A, et al. German diabetes management programs improve quality of care and curb costs. Health Aff (Millwood). 2010;29(12):2197–205. [DOI] [PubMed] [Google Scholar]
- 24.Wong CK, Wong WC, Wan YF, Chan AK, Chung KL, Chan FW, et al. Patient Empowerment Programme in primary care reduced all-cause mortality and cardiovascular diseases in patients with type 2 diabetes mellitus: a population-based propensity-matched cohort study. Diabetes Obes Metab. 2015;17(2):128–35. 10.1111/dom.12397 [DOI] [PubMed] [Google Scholar]
- 25.Jiao F, Fung CS, Wan YF, McGhee SM, Wong CK, Dai D et al. Long-term effects of the multidisciplinary risk assessment and management program for patients with diabetes mellitus (RAMP-DM): a population-based cohort study. Cardiovasc Diabetol. 2015;14:105 10.1186/s12933-015-0267-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostermann H, Hoess V, Mueller M. Efficiency of the Austrian disease management program for diabetes mellitus type 2: a historic cohort study based on health insurance provider's routine data. BMC Public Health. 2012;12:490 10.1186/1471-2458-12-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang TS, Funnell MM, Brown MB, Kurlander JE. Self-management support in "real-world" settings: an empowerment-based intervention. Patient Educ Couns. 2010;79(2):178–84. 10.1016/j.pec.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang TS, Funnell MM, Oh M. Lasting effects of a 2-year diabetes self-management support intervention: outcomes at 1-year follow-up. Prev Chronic Dis. 2012;9:E109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong CK, Wong WC, Lam CL, Wan YF, Wong WH, Chung KL et al. Effects of Patient Empowerment Programme (PEP) on clinical outcomes and health service utilization in type 2 diabetes mellitus in primary care: an observational matched cohort study. PLoS One. 2014; 9(5):e95328 10.1371/journal.pone.0095328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao FF, Fung CS, Wong CK, Wan YF, Dai D, Kwok R et al. Effects of the Multidisciplinary Risk Assessment and Management Program for Patients with Diabetes Mellitus (RAMP-DM) on biomedical outcomes, observed cardiovascular events and cardiovascular risks in primary care: a longitudinal comparative study. Cardiovasc Diabetol. 2014;13:127 10.1186/s12933-014-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimouguet C, Goff ML, Thiébaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ. 2011;183(2):E115–27. 10.1503/cmaj.091786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elissen AM, Steuten LM, Lemmens LC, Drewes HW, Lemmens KM, Meeuwissen JA, et al. Meta-analysis of the effectiveness of chronic care management for diabetes: investigating heterogeneity in outcomes. J Eval Clin Pract. 2013;19(5):753–62. 10.1111/j.1365-2753.2012.01817.x [DOI] [PubMed] [Google Scholar]
- 33.Kane R, Wellings K, Free C, Goodrich J. Uses of routine data sets in the evaluation of health promotion intervention: opportunities and limitations. Health Education. 2000;100(1):33–41. [Google Scholar]
- 34.Filzmoser P, Eisl A, Endel F, ATC—ICD: Determination of the reliability for predicting the ICD code from the ATC code. Hauptverband der Österreichischen Sozialversicherungsträger, EBM Bericht, 2009. Available: http://www.hauptverband.at/portal27/portal/hvbportal/content/contentWindow?contentid=10007.693979&action=2&viewmode=content. Accessed 9 Jun 2016.
- 35.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–74. 10.1007/s00125-014-3369-7 [DOI] [PubMed] [Google Scholar]
- 36.Laxy M, Stark R, Meisinger C, Kirchberger I, Heier M, von Scheidt W, et al. The effectiveness of German disease management programs (DMPs) in patients with type 2 diabetes mellitus and coronary heart disease: results from an observational longitudinal study. Diabetol Metab Syndr. 2015;7:77 10.1186/s13098-015-0065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schäfer I, Küver C, Gedrose B, von Leitner EC, Treszl A, Wegscheider K, et al. Selection effects may account for better outcomes of the German Disease Management Program for type 2 diabetes. BMC Health Serv Res. 2010;10:351 10.1186/1472-6963-10-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are owned by a third party, the LEICON database. Interested researchers may contact Ms. Grün (leicon@noegkk.at) to request the data. In order to fulfill the legal requirements to obtain that kind of data, researchers must obtain permission for a specific research question from the Main Association of Austrian Social Security. Additionally, researchers must conclude a contract with the statutory health insurance regarding data access.