Abstract
Cannabis use has been reported to induce long-lasting psychotic disorders and a dose-response relationship has been observed. We performed a systematic review of studies that investigate the association between the degree of cannabis consumption and psychosis and a meta-analysis to quantify the magnitude of effect. Published studies were identified through search of electronic databases, supplemented by manual searches of bibliographies. Studies were considered if they provided data on cannabis consumption prior to the onset of psychosis using a dose criterion (frequency/amount used) and reported psychosis-related outcomes. We performed random effects meta-analysis of individual data points generated with a simulation method from the summary data of the original studies. From 571 references, 18 studies fulfilled inclusion criteria for the systematic review and 10 were inserted in the meta-analysis, enrolling a total of 66 816 individuals. Higher levels of cannabis use were associated with increased risk for psychosis in all the included studies. A logistic regression model gave an OR of 3.90 (95% CI 2.84 to 5.34) for the risk of schizophrenia and other psychosis-related outcomes among the heaviest cannabis users compared to the nonusers. Current evidence shows that high levels of cannabis use increase the risk of psychotic outcomes and confirms a dose-response relationship between the level of use and the risk for psychosis. Although a causal link cannot be unequivocally established, there is sufficient evidence to justify harm reduction prevention programs.
Key words: psychotic disorders, schizophrenia, dose response, drug use, systematic review
Introduction
The United Nations Office on Drugs and Crime (UNODC) reports that 3.9% of the global adult population uses cannabis, with a total number of 180.6 million of cannabis users worldwide.1 This outweighs the number of users of all other illicit substances considered together. In some countries, cannabis is categorized as a drug of abuse and its use is strictly prohibited, while in others it is perceived as a benign, relatively harmless substance.2–6 However, cannabis use has been widely reported to induce acute psychotic experiences,7–9 to affect the severity of psychotic symptoms,10,11 and previous meta-analyses have reported a 2-fold increase in the risk to develop a psychotic disorder in cannabis users compared to nonusers.12–14
In order to establish which users are most likely to suffer negative effects, studies have focused on different characteristics of the users or the pattern of use that might determine those most at risk. A dose-response relationship between cannabis use and psychosis related outcomes, in terms of duration of use and/or frequency of use, has been reported.15–20 However, the extent of this relationship remains uncertain.
We therefore performed a systematic review of the literature investigating the association between the extent of cannabis consumption and psychosis-related outcomes and proceeded with a meta-analysis to quantify the magnitude of effect. Unlike previous meta-analyses that used only the comparison between the extreme categories (no users vs heavy users) to produce a pooled estimate of the effect, we express the increased risk of psychosis relative to a continuous variable of the level of exposure to cannabis.
Methods
Search Strategy
Search strategy was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement.21 Potential studies were identified by a comprehensive search via the electronic databases Pubmed, Embase, and PsychINFO. Terms related to a dose-response relationship, ie, “dose-response,” “daily use,” “duration,” “high frequency” or “heavy use” were combined with the terms “psychosis,” “schizophrenia” or “schizphreni*” and with “cannab*,” “cannabis”, “marijuana” or “marihuana”.
The search was limited to studies published from the inception of databases to December 31, 2013. This search yielded 571 articles that were screened for inclusion on the basis of their titles, abstracts or the whole text, if necessary. Reference lists from all included studies, and from the main published reviews of cannabis and psychosis12–14,22,23 were examined and experts were contacted to obtain additional reports.
Selection Criteria and Data Extraction
Only peer-reviewed articles were considered and no language limitations were applied in the attempt to locate all the relevant papers. Studies were identified by one of the authors (A.M.) and independently checked for inclusion by another (E.V.). Both cohort and cross-sectional studies were included in the systematic review when they met the following criteria: (1) assessment of cannabis use with a dose criterion (frequency/amount used/severity) before the onset of psychosis, and (2) psychosis-related outcomes established with validated clinical measures. Reasons for exclusion at this stage were: (1) enrolment of subjects known as suffering from mental illness before the use of cannabis or ultra-high risk subjects, (2) studies examining the comorbidity between substance use disorders and psychosis, (3) neuropsychological measures or schizoid personality traits rather than psychosis as main outcome, (4) data available on cannabis consumption not classified according to at least 3 different levels of use (ie, nonusers plus 2 or more other levels), and (5) studies investigating only the effect of cannabis use on age at onset of psychosis.
When the outcome was “age at onset,” papers were screened to check if suitable information about the degree of cannabis consumption at a fixed temporal threshold were available for both cases and controls (ie, past cannabis consumption and degree of consumption at a fixed year of life). The same procedure of screening of the whole text was performed whenever the title or the abstract of a study did not rule out the possibility of gathering suitable data on the association between the exposure and the aimed outcome. When studies reported various psychosis outcomes, we took the most severe (higher threshold in continuous scale of psychosis or maximum number of psychotic symptoms).
Two independent authors (A.M. and E.V.) extracted the following data from each eligible study: (1) the year and country in which the study had been conducted, (2) study design, (3) exposure measures, (4) psychosis related outcome, (5) total sample size divided in cases and controls by level of exposure, and (6) relative risks or odds ratios as presented in the studies.
Statistical Analysis
In each study we recorded the number of comparison groups defined by different levels of cannabis use, their sample size (number of cases and controls in each group) and the OR compared to the group of individuals who have never used cannabis; the latter was consistently used as the reference category. As some studies reported only adjusted OR using different covariates, we estimated crude OR from the number of cases and controls in each group by dividing the case/control ratio at each level of exposure to cannabis by the case/control ratio at the baseline group of no use.
Despite the different definitions of severity used in each study (frequency of use in lifetime or in specific periods of time, duration of use etc.), cannabis exposure was always presented as an ordered variable. As the underlying distribution of cannabis use for each study is unknown, to create an index of cannabis exposure, we employed an approach based on ranking the individuals by their cannabis use. Those in the sample who have never used cannabis were assigned a cannabis index of 0, while the ones who have been exposed to the risk factor can be assigned a cannabis index between 0 and 1 along a uniformly distributed scale, U(0,1). For each study, this scale was divided in bins equal to the number of cannabis exposure groups. The width of each bin was determined from the relevant ratio of the total number of individuals in each group, with the exception of the reference group (no exposure) which was set to 0. Summary data from the studies included in the meta-analysis is presented in table 1.
Table 1.
Summary Data From Studies Included in the Meta-analysis
| Study | Country | Design | Exposure/ Predictor | Specific Outcome (Diagnostic System) | Level of Exposure | Total | Controls | Cases | OR (Crude) |
|---|---|---|---|---|---|---|---|---|---|
| Tien et al7 | United States | Prospective cohort | Lifetime frequency | ≥1 psychotic symptom within 1-year FU (DSM III) | Never use | 1803 | 1437 | 366 | 1.00 |
| Moderate use | 357 | 294 | 63 | 0.84 | |||||
| Daily use | 135 | 87 | 48 | 2.17 | |||||
| Degenhardt et al33 | Australia | Cross sectional | Frequency of use at baseline | Positive in psychosis screener within 1 year (DSM IV) | No use | 5997 | 5928 | 69 | 1.00 |
| Moderate use | 246 | 238 | 8 | 2.89 | |||||
| Weekly use | 479 | 457 | 22 | 4.14 | |||||
| Arseneault et al32 | New Zealand | Prospective cohort | Duration/frequency: current use (>3 t/y) at age 15 and 18 | Schizophreniform disorder at year 26 (DSM IV) | No cannabis use at age 18 | 494 | 482 | 12 | 1.00 |
| Cannabis use y18 > 3t | 236 | 226 | 10 | 1.78 | |||||
| Cannabis use y15 > 3t | 29 | 26 | 3 | 4.63 | |||||
| Zammit et al15 | Sweden | Prospective cohort | Lifetime frequency | Inpatient admission for schizophrenia (ICD 8 and ICD 9) | 0 | 36 429 | 36 214 | 215 | 1.00 |
| 1 | 608 | 606 | 2 | 0.56 | |||||
| 2–4 | 1380 | 1372 | 8 | 0.98 | |||||
| 5–10 | 806 | 797 | 9 | 1.90 | |||||
| 11–50 | 689 | 676 | 13 | 3.24 | |||||
| >50 | 731 | 703 | 28 | 6.71 | |||||
| Henquet et al13 | Germany | Prospective cohort | Frequency of use at baseline | ≥2 psychotic symptoms (Psychosis section of CIDI—DSM IV) | None | 2117 | 1987 | 130 | 1.00 |
| <1 times/mo | 82 | 77 | 5 | 0.99 | |||||
| 3–4 times/mo | 80 | 70 | 10 | 2.18 | |||||
| 1–2 times/wk | 57 | 50 | 7 | 2.14 | |||||
| 3–4 times/wk | 33 | 25 | 8 | 4.89 | |||||
| Almost daily | 68 | 54 | 14 | 3.96 | |||||
| Wiles et al35 | England | Prospective cohort | Frequency within the last year | Self-reported psychotic symptoms (PSQ) | No cannabis within last year | 1629 | 1526 | 103 | 1.00 |
| Cannabis use within last year | 109 | 101 | 8 | 1.17 | |||||
| Cannabis dependence | 57 | 48 | 9 | 2.78 | |||||
| Miettunen et al19 | Finland | Cross sectional | Lifetime frequency | Prodromal symptoms in adolescence (PROD-screen) | Never use | 5948 | 4199 | 1749 | 1.00 |
| Once | 180 | 86 | 94 | 2.62 | |||||
| 2–4 | 111 | 51 | 60 | 2.82 | |||||
| ≥5 | 50 | 21 | 29 | 3.32 | |||||
| Regular use | 9 | 4 | 5 | 3.00 | |||||
| McGrath et al18 | Australia | Cross sectional | Duration | Any CIDI hallucinations (ICD 10) | Never use | 1272 | 1182 | 90 | 1.00 |
| ≤3 y | 497 | 449 | 48 | 1.40 | |||||
| 4–5 y | 411 | 370 | 41 | 1.46 | |||||
| ≥6 y | 322 | 268 | 54 | 2.65 | |||||
| Zammit et al34 | England | Prospective cohort | Lifetime frequency | Self-reported severe psychotic experiences (PLIKS-Q) | None | 2403 | 2209 | 194 | 1.00 |
| <5 times | 104 | 88 | 16 | 2.07 | |||||
| 5–20 | 42 | 33 | 9 | 3.11 | |||||
| 21–60 | 9 | 8 | 1 | 1.42 | |||||
| >60 | 9 | 6 | 3 | 5.69 | |||||
| GAP data, 201216,31 | England | Case-control | Frequency | First episode psychosis (ICD 10) | Never | 345 | 148 | 197 | 1.00 |
| Once or twice | 58 | 30 | 28 | 0.70 | |||||
| Few times/y | 63 | 41 | 22 | 0.40 | |||||
| Few times/mo | 73 | 39 | 34 | 0.65 | |||||
| Over once/wk | 106 | 40 | 66 | 1.24 | |||||
| Everyday | 153 | 27 | 126 | 3.51 |
Note: DSM, Diagnostic and Statistical Manual of Mental Disorders; PS, Psychosis Screener; ICD, International classification of Disease; CIDI, Composite International Diagnostic Interview; PSQ, Psychosis Screening Questionnaire; PROD-screen, Screening for prodromal symptoms; PLIKS-Q, Psychosis-like symptoms questionnaires.
To estimate the association between cannabis exposure and psychosis and to derive confidence intervals in each study, we used a simulation method we have previously developed.24 In brief, we simulate individual-level data from the summary statistics (number of cases and controls in each exposure level) based on the ranks of the uniform distribution and then fit a logistic regression model. The logarithm of the odds of psychosis related outcome as a function of the level of cannabis exposure is given by ln(p/1 − p) = a + bx, where p is the probability of developing the outcome, x the cannabis index between 0 and 1, a the intercept and b the regression coefficient. The exponential of b represents the OR of psychosis between the heaviest cannabis user (x = 1) and the nonuser (x = 0). The probability of psychosis for an individual with cannabis index value x is then given by p = e a+bx/(1 + e a+bx).
At the first iteration, each subject was randomly assigned to be case or control using a Bernoulli variable, with the probability of psychosis P(case) = nca/(nca + nco), where nca and nco are the numbers of cases and controls in each bin. Subsequent iterations were performed using parameter estimates from the previous iteration to produce a distribution of exposure and case status within each cannabis exposure bin that approximated the underlying distribution. We retained the regression coefficient b with its SE and after 1000 iterations we calculated their mean values. The method is illustrated with an example in supplementary figure and full details are presented in Vassos et al.24
Under the assumption of an equivalent distribution of cannabis use across studies, we performed meta-analysis to produce a single estimate of the effect size of cannabis as a risk factor of psychosis, weighting studies by the SE of b. Heterogeneity between samples was assessed using Cochran’s Q and I 2 statistics25 and, given the high heterogeneity observed (I 2 > 50%), estimates were combined with the Der-Simonian and Laird random-effects model.26 Data were analyzed using Stata release 10 (Stata Corp. 2007), and R v2.15 (www.r-project.org).
As studies used different designs and outcome measures, we performed the following subgroup analyses: (1) separate analysis of studies with cohort and cross-sectional design and (2) stratification by outcome measure (diagnosis of a psychotic disorder or presence of psychotic symptoms). A meta-regression with the year of the study as covariate was performed to assess the heterogeneity of the findings. Publication bias was examined with Egger’s and Begg’s tests for small study effects.27,28
Results
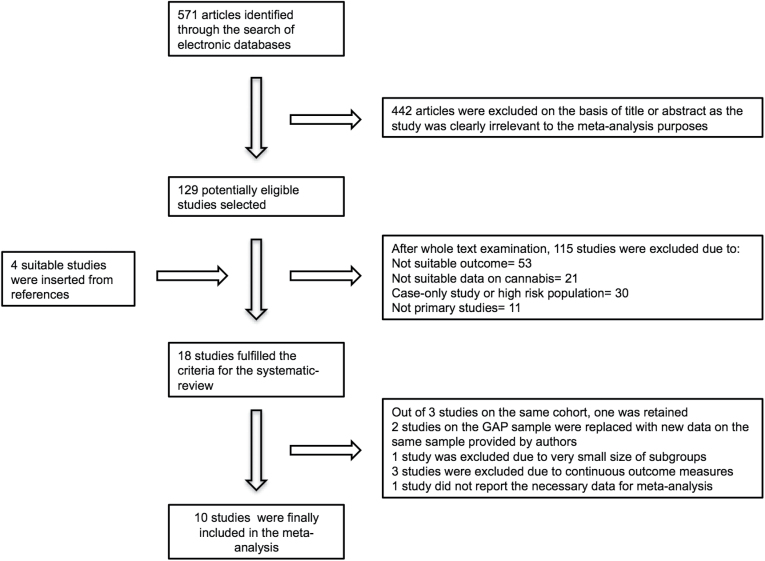
The systematic review search yielded 571 references. On the basis of their title, abstract or full text, as necessary, 557 articles were excluded as described in the flow chart (figure 1). Four additional articles cited in the selected papers were considered relevant and included in the systematic review as they fulfilled the inclusion criteria.15,20,29,30 As for the Swedish 1969 conscript cohort, 3 reports15,20,29 met our inclusion criteria, we selected one,15 based on the number of exposure groups. One study showing very high effect size based on 10 cases only30 was excluded from the meta-analysis. The London study16,31 was supplemented with all the available data from the Genetics and Psychosis (GAP) cohort.
Fig. 1.
Flow diagram of study selection process.
Diagnostic outcomes of selected studies included a diagnosis or first admission for schizophrenia,15 a diagnosis of schizophreniform disorder,32 a first contact with the clinical services for a first episode of Psychosis16 or the presence of psychotic symptoms over a certain threshold set in each study7,13,18,19,33–35 (table 1). Studies with continuous outcome measures were excluded because they report a range of heterogeneous outcomes and therefore it was inappropriate to pool them.17,36 Ten studies were finally included in the meta-analysis, enrolling a total of 66 816 individuals.
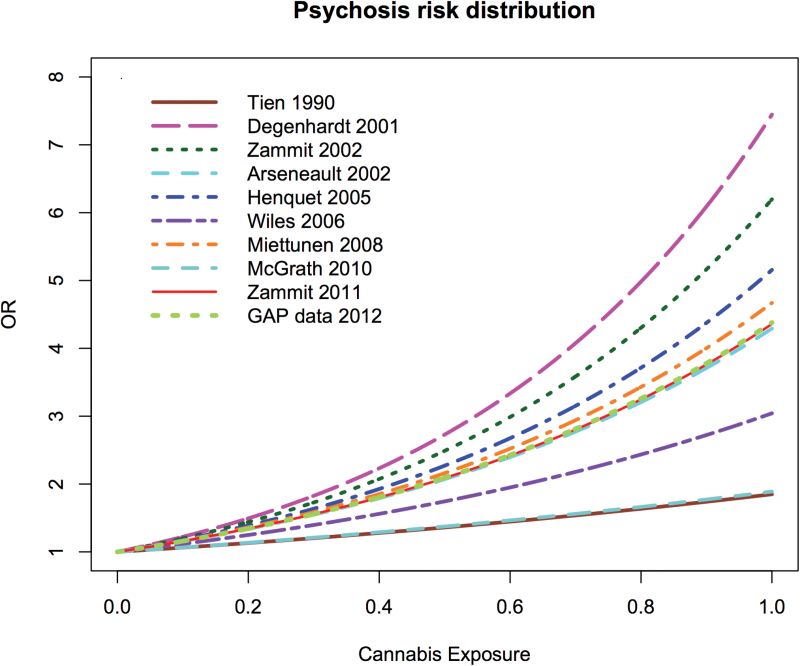
We observed a consistent increase in the risk of psychosis-related outcomes with higher levels of cannabis exposure in all the included studies (figure 2). We used a random effect model for the meta-analysis, due to the high heterogeneity of the studies (I 2 = 82%). The pooled estimate for the logistic regression coefficient b was 1.36 (95% CI: 1.04 to 1.68), corresponding to an OR of 3.90 (2.84 to 5.34) for the risk of schizophrenia and other psychosis outcomes among the most severe cannabis users compared to the nonusers. The linear expression of the risk allows the estimation of OR at different levels of exposure using the formula OR = exp(b*x), where x the rank in a scale of 0 to 1 of the median individual in the group of interest. For example, the median OR for any cannabis use is 1.97 (1.68 to 2.31) while for the top 20% group it is 3.40 (2.55 to 4.54). These estimates are comparable to previous studies that report OR for any use or severe cannabis use.
Fig. 2.
Estimated risk ratio of psychosis by level of cannabis use in original studies.
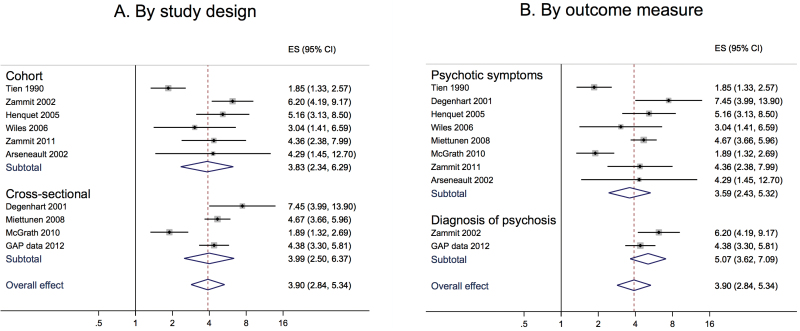
Subgroup analyses by method or outcome measure gave remarkably similar results for each category. When data were stratified by study design, the pooled OR for cross-sectional studies was 3.99 (2.50 to 6.37) and for cohort studies 3.83 (2.34 to 6.29); when stratified by outcome measure, the pooled OR for presence of psychotic symptoms was 3.59 (2.42 to 5.32) and for a diagnosis of schizophrenia or psychotic disorder was 5.07 (3.62 to 7.09; figure 3).
Fig. 3.
Forest plots including subgroup analyses of the odds ratio of psychosis in the most severe cannabis users. (A) Divided by study design (cohort and cross-sectional). (B) Divided by outcome measure (diagnosis of psychotic disorder and presence of psychotic symptoms).
Meta-regression by year of study was nonsignificant (P = .53), indicating that heterogeneity cannot be explained by the date of the study. Finally the size of the study and the SE did not correlate with the effect size as indicated by the nonsignificant publication bias tests (Egger’s P = .79, Begg’s P = .86).
Discussion
In this meta-analysis of all available published data, we confirm a positive association between the extent of cannabis use and the risk for psychosis. This association was consistent in all the individual studies included, despite differences in the effect size. The pooled analysis reported approximately a 4-fold increase in risk for the heaviest users and a 2-fold increase for the average cannabis user in comparison to nonusers. This observation remained stable irrespective of the study design (cohort or cross-sectional) or the outcome measure (broad definition of psychosis or narrow diagnosis of a psychotic disorder). Since, for the cohort studies, we excluded studies enrolling subjects already suffering from symptoms at baseline, these results are not likely to be explained by a reverse-causation mechanism.
Two types of studies investigating the association between cannabis and psychosis were identified. Those included in this meta-analysis examined the association between the level of cannabis use and categorical outcome measures (presence or absence of psychosis, individuals with psychotic symptoms exceeding a severity threshold). The second type consists of studies with continuous outcomes (eg, scores at different tests measuring psychosis). These also indicated an increase in psychotic symptomatology by the increase in the level of cannabis use, but were not pooled together as they were very heterogeneous and did not fulfil our inclusion criteria.
Considering well-established examples of the effects of drugs of abuse on health, it is well known that the health hazards depend on the pattern of use. Nobody would dispute that drinking a glass of wine everyday is less likely to be associated with serious health consequences than drinking a bottle of vodka daily. However, while the biology underlying alcohol use toxicity has been well described, the mechanism underlying cannabis associated psychosis is still largely unknown. Although extensive literature on the effects of alcohol on health and legislation on drink-driving have suggested safe doses and legal limits, there is not enough evidence to suggest a “safe dose” of cannabis. Our estimates are based on the assumption of a linear increase of risk with the increase of cannabis use.
Strengths of this study include the use of all available published data (based on 66 816 individuals), the estimation of the pooled effect size utilizing all levels of exposure, not just comparing any cannabis use with no use or limiting the comparison to the extremes (most severe use vs no use), and the expression of risk for psychosis in a linear form, allowing the estimation of relative risk of psychosis in individuals at different levels of exposure.
However, there are some important limitations to this report. First, in order to utilize all available evidence, we employed an inclusive definition of our outcome measures, examining both “soft” outcomes such as psychotic experiences and “hard” outcomes such as diagnosis or admission with a psychotic disorder and we did not have data on the time interval between regular cannabis use to illness onset. Although one would predict a greater effect of cannabis on psychotic experiences if in the original studies, the long term outcome of cannabis use was “contaminated” with reality distortion due to intoxication, we did not find significant difference in the sensitivity analysis by outcome measure; indeed, our “hard” outcomes showed a stronger association with the degree of cannabis use. This observation indicates that the risk of cannabis use is not confined to short-term effects and supports previous evidence of a continuum between psychotic experiences and schizophrenia.37 Second, we included dissimilar study designs ranging from general population cohorts to cross-sectional studies of cases and controls with retrospective measurement of cannabis use. Thus, heterogeneity was expected in both the sample characteristics and the outcomes measured. However, our sensitivity analyses, separating prospective from retrospective studies, gave remarkably similar estimates of the effect size.
Another limitation is that we could only measure the degree of exposure without taking into account the potency of cannabis or the period of use. There is previous evidence that use of high-potency cannabis as well as early onset of use16 are stronger risk factors for psychosis. More research is needed in these areas to draw safe conclusions. In addition, we pooled studies with different exposure measures, including frequency or duration of cannabis use and clinical diagnosis of use, abuse, or dependence. The rationale was that cannabis exposure was always presented as an ordered variable and in all studies we observed an increase in the risk of psychosis with increased exposure, irrespective of the method used. It is noteworthy that our estimates are based on raw (unadjusted) data; hence, the effect may partially be explained by confounders like differences in ethnicity or socioeconomic status between cannabis users and nonusers. However, studies that controlled for these effects16,19 confirmed the association.
In comparison to a previous meta-analysis by Moore and colleagues12 that estimated an odds ratio of 1.4 for any cannabis use and 2.1 for severe use, our estimates may appear high. However, we have included more recent evidence, data from all levels of exposure, unadjusted OR, and our estimates correspond to the risk of the most severe user, not the average user in the top category. Estimating the risk of the average individual in the severe use category with the linear model (eg, 3.4 for the top 20%) and reducing our estimate by an average attenuation value for fully adjusted estimates of 45%, as Moore et al12 report, would give comparable results. Our estimates are remarkably similar to a recent national epidemiologic survey that reported an adjusted for sociodemographic characteristics OR of 3.69 (2.49 to 5.47) for a diagnosis of psychosis among individuals with lifetime history of cannabis dependence.38
Although this meta-analysis shows a strong and consistent association between cannabis use and psychosis, a causal link cannot be unequivocally established. However, investigating modifiable factors with a substantial role in psychosis is useful for prevention programmes as the level of exposure to cannabis remains a very important risk marker for schizophrenia and psychosis in general. Irrespective of whether the joint effect of risk factors is better described by multiplicative39 or synergistic models40 as previously suggested, at an individual level it would seem justified to educate people at heightened risk of schizophrenia (eg, through having a family history of the disorder, or having experienced psychosis-like symptoms) of the potential additional risk of cannabis exposure.
In conclusion, our meta-analysis provides the most accurate estimate of the effect size of cannabis use as a risk factor for psychosis using all the available published data. In addition, it measures a dose-response relationship between the level of use and the risk for psychosis. Thus, for public policy, apart from prevention programmes targeting cannabis use in general, harm minimization approaches aiming at dose reduction or later onset of use are also relevant in the prevention and treatment of psychosis. For the scientific community, further research is needed to better understand the biological pathways that link cannabis use with psychosis and to establish particular patterns of cannabis use that carry the highest hazard for psychosis-related outcomes, especially in vulnerable subjects.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by a Guy’s and St Thomas Charity Grant (R080529), the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London, and the European Community’s Seventh Framework Programme under the Marie Curie Industry-Academia Partnership and Pathways, grant agreement 286213.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. United Nations Office on Drugs and Crime. World Drug Report 2013. New York, NY: United Nations Publications; 2013. [Google Scholar]
- 2. Anderson DM, Hansen B, Rees DI. Medical marijuana laws, traffic fatalities, and alcohol consumption. J Law Econ. 2013;56:333–369. [Google Scholar]
- 3. Kilmer JR, Hunt SB, Lee CM, Neighbors C. Marijuana use, risk perception, and consequences: is perceived risk congruent with reality? Addict Behav. 2007;32:3026–3033. [DOI] [PubMed] [Google Scholar]
- 4. Room R, Reuter P. How well do international drug conventions protect public health? Lancet. 2012;379:8. [DOI] [PubMed] [Google Scholar]
- 5. Korf DJ. Dutch coffee shops and trends in cannabis use. Addict Behav. 2002;27:851–866. [DOI] [PubMed] [Google Scholar]
- 6. Coombes R. Cannabis regulation: high time for change? BMJ. 2014;348:g3382. [DOI] [PubMed] [Google Scholar]
- 7. Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis. 1990;178:473–480. [PubMed] [Google Scholar]
- 8. Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. [DOI] [PubMed] [Google Scholar]
- 9. Hall W, Degenhardt L. Adverse health effects of medical cannabis use. Lancet. 2009;374:8. [DOI] [PubMed] [Google Scholar]
- 10. Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychol Med. 1986;16:515–520. [DOI] [PubMed] [Google Scholar]
- 11. Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 13. Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. [DOI] [PubMed] [Google Scholar]
- 14. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. [DOI] [PubMed] [Google Scholar]
- 15. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Brit J Psychiatry. 2009;195:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schubart CD, van Gastel WA, Breetvelt EJ, Sommer IE, Kahn RS, Boks MP. Initial age and amount of cannabis exposure are strongly associated with psychosis vulnerability in a sample of 18,000 Dutch adolescents. Schizophr Res. 2010;117:297–298. [Google Scholar]
- 18. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67:440–447. [DOI] [PubMed] [Google Scholar]
- 19. Miettunen J, Tormanen S, Murray GK, et al. Association of cannabis use with prodromal symptoms of psychosis in adolescence. Brit J Psychiatry. 2008;192:470–471. [DOI] [PubMed] [Google Scholar]
- 20. Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42:1321–1328. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–130. [PMC free article] [PubMed] [Google Scholar]
- 22. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. [DOI] [PubMed] [Google Scholar]
- 23. Gage SH, Zammit S, Hickman M. Stronger evidence is needed before accepting that cannabis plays an important role in the aetiology of schizophrenia in the population. F1000 Med Rep. 2013;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012;38:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 29. Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. [DOI] [PubMed] [Google Scholar]
- 30. van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. [DOI] [PubMed] [Google Scholar]
- 31. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt T. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Degenhardt L, Hall W. The association between psychosis and problematical drug use among Australian adults: findings from the National Survey of Mental Health and Well-Being. Psychol Med. 2001;31:10. [DOI] [PubMed] [Google Scholar]
- 34. Zammit S, Owen M, Evans J, Heron J, Lewis G. Cannabis, COMT and psychotic experiences. Br J Psychiatry. 2011;199:6. [DOI] [PubMed] [Google Scholar]
- 35. Wiles NJ, Zammit S, Bebbington P, Singleton N, Meltzer H, Lewis G. Self-reported psychotic symptoms in the general population: results from the longitudinal study of the British National Psychiatric Morbidity Survey. Br J Psychiatry. 2006;188:519–526. [DOI] [PubMed] [Google Scholar]
- 36. van Winkel R, Henquet C, Rosa A, et al. Evidence that the COMT(Val158Met) polymorphism moderates sensitivity to stress in psychosis: an experience-sampling study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:10–17. [DOI] [PubMed] [Google Scholar]
- 37. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 38. Davis GP, Compton MT, Wang S, Levin FR, Blanco C. Association between cannabis use, psychosis, and schizotypal personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Schizophr Res. 2013;151:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zammit S, Lewis G, Dalman C, Allebeck P. Examining interactions between risk factors for psychosis. Brit J Psychiatry. 2010;197:207–211. [DOI] [PubMed] [Google Scholar]
- 40. van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.