Abstract
Background and Goal:
Recent findings have provided preliminary support for the notion that basic self-disturbances (SD) are related to prodromal symptoms among nonpsychotic help-seeking adolescents. As a sizable proportion of adolescents who are at risk do not seek help, this study attempts to assess the extent to which these findings can be generalized to the entire population of adolescents who are at risk for psychosis.
Method:
The concurrent relationship between SD and prodromal symptoms was explored in a sample of 100 non-help-seeking adolescents (age 13–15) from the community. SD were assessed with the Examination of Anomalous Self-Experience (EASE); prodromal symptoms and syndromes were assessed with the Structured Interview for Prodromal Syndromes (SIPS); psychosocial functioning was assessed with the “Social and Role Global Functioning Scales”; and level of distress with the Mood and Anxiety States Questionnaire (MASQ).
Results:
SD significantly correlated with sub-clinical psychotic symptoms (r = .70, P < .0001). This correlation was significantly stronger than those of SD with mood symptoms and social functioning. Finally, SD was the single best concurrent predictor of prodromal symptoms and syndromes.
Conclusions:
These results provide preliminary support for the generalizability of the association between SD and prodromal symptoms for the entire population of adolescents who are clinically at high risk for psychosis. In addition, they further support the notion that this association is both specific and unique.
Key words: schizophrenia, psychosis, clinical high-risk, phenomenology
Introduction
Over the past 2 decades massive research has focused on detection of pre-schizophrenic or early psychotic conditions. This research is motivated by the assumption that intervention in the early stages of illness, before it becomes chronic and treatment resistant, may ameliorate long term outcomes. From an operational point of view, identification of individuals at high risk for imminent psychosis in these studies relies on a widespread acceptance of the so-called ultra-high-risk (UHR) paradigm (also referred to as “clinical high-risk” [CHR]).1–4 This paradigm defines risk by several combinations of symptomatic, functional (eg, psychosocial decline) and genetic (eg, family history of psychotic illness) criteria.
The most common symptomatic risk criteria are the so-called “attenuated” psychotic symptoms (APS), which are “sub-clinical psychotic symptoms,” compatible to their full-fledged DSM-5 counterparts. Another closely related but less common symptomatic criterion is full-fledged but brief and intermittent psychotic symptoms (BIPS). The assessment instruments most often used for such symptoms are the Structured Interview for Prodromal Syndromes (SIPS)5 and the Comprehensive Assessment of At-Risk Mental States (CAARMS).2 Another factor that amplifies the risk for psychosis is early onset of Schizotypal Personality Disorder accompanied by functional deterioration over the previous year.6,7
Although the UHR approach has documented the diagnostic validity and feasibility of prospective ascertainment of individuals at risk for psychosis7,8 and provided a platform for studies assessing benefits of early interventions,9–12 there is a wide consensus that it suffers from several conceptual and methodological problems that should be addressed in order to improve its scientific and clinical/societal utility. Most importantly, growing evidence calls the specificity of APS into question. In fact, the literature on this subject suggests that APS are quite common in a broad range of nonpsychotic psychiatric conditions.13,14 Second, as already noted by Parnas 15 and Nelson et al16, UHR predictive algorithms use increasing intensity of “positive” psychotic symptoms to predict “psychosis,” as opposed to a comprehensive psychopathological theory about the nature of psychosis. Therefore, they are rather limited in their informative value regarding the phenotypic markers of vulnerability for psychosis.
Recently, phenomenologically-oriented researchers have proposed that an empirical exploration of subtle basic self-disturbances (SD) may address the problems described above. At the heart of this proposal, which is based on clinical observations and philosophical considerations, is the hypothesis that the phenotypic core of schizophrenia spectrum disorders consists of a disturbance of the so-called minimal or core self; namely, an instability in the tacit (pre-reflective) sense of being a self-coinciding and self-present subject of experience and action.16–18 According to this hypothesis, the emergence of elaborated psychotic symptoms in schizophrenia is a quasi-compensatory response to the profound disturbances in this core sense of selfhood. However, these disturbances are not sub-threshold positive symptoms but rather anomalous transformations of the normal articulation of the structure of consciousness.18,19
Early empirical support for this notion came from clinical studies20,21 followed by systematic empirical studies.22–30 Together, these studies showed that SD aggregate specifically in the schizophrenia spectrum disorders (schizophrenia and schizotypal personality disorder), compared to other psychiatric conditions (such as bipolar psychosis), among first-time hospitalization cases27–30 and among family members of schizophrenia patients, while showing an incremental increase across the severity of the schizophrenia spectrum condition. More recently, several prospective studies showed that SD prospectively predict incidents of new cases of schizophrenia spectrum disorder that occur 5 years after first-time hospital admission for a nonpsychotic illness,23 and may provide a means of further “closing in” on individuals truly at high risk for psychotic disorders, particularly schizophrenia spectrum disorders, in a UHR population.31 Finally, 3 recent cross-sectional studies, including from our own group, have provided preliminary evidence suggesting that SD and prodromal symptoms constitute distinct but moderately related dimensions of potential risk among adolescents and young adults.32–34
However, to the best of our knowledge, there is no empirical data to date that elucidates the degree to which these findings can be generalized for the entire population of adolescents and young adults who are at risk for schizophrenia spectrum disorders. This is so because no studies of SD, to date, have included non-help-seeking (ie, self-presenting) adolescents and young adults from the community. This lack of information is particularly important in light of recent studies that suggest that some 8%–13% of young adolescents in the community meet the symptom criteria for the risk syndrome,35–37 and that a sizeable percentage of those who are at high-risk for psychosis in the community do not seek help.38,39
In this study, we attempted to take a preliminary step towards addressing this lacuna in the literature by examining the relationship between SD and prodromal symptoms among non-help-seeking adolescents from the community. More specifically, our goals were to: (1) replicate previous findings40 regarding the prevalence and nature of SD among non-help-seeking adolescents from the community, (2) examine the degree of association between SD and prodromal symptoms/syndromes, (3) assess the degree to which this association is specific to prodromal symptoms/syndromes compared to other mood and behavioral symptoms/syndromes, and (4) assess the unique contribution of SD to explanation of variance in prodromal symptoms and syndromes.
Based on theoretical considerations and preliminary empirical findings, we hypothesized that: (1) there would be a moderate to high link between SD and prodromal symptoms at baseline; (2) this link would be specific to prodromal symptoms; and (3) SD would contribute uniquely to the explanation of variance in prodromal symptoms. To the best of our knowledge, this is the first study to examine the association between SD and prodromal symptoms among young adolescents from the community (ie, who do not already suffer from a psychotic disorder and are not seeking help for other problems).
Method
Participants
Participants in the study were 100 junior high school students, aged 13 to 16 years (Mean = 13.37, S.D. = 0.46) from the general population. The recruitment process for the study sample proceeded as follows: first, the study was advertised on virtual and digital billboards in 2 major districts in Israel (Haifa and vicinity, and Tel Aviv and vicinity). Approximately 239 families responded to the ads. Next, those who agreed to hear more about the study (n = 104, 43.5%) were invited for a face-to-face interview to evaluate their eligibility for the study. The inclusion and exclusion criteria were: (1) age 13 to 16 years (this range was selected in order to allow sufficient time for follow-up before the participants are recruited to the military), (2) no known past or present psychotic episodes as determined by a clinical psychiatric diagnostic assessment, (3) no organic causes for presentation, (4) no history of severe head injury or organic brain disorder, and (5) no documented diagnosis of intellectual disability (ie, IQ below 70). Based on these criteria, one of the candidates was excluded due to a history of severe head injury. The remaining 103 adolescents and their parents signed an informed consent form, which was reviewed and approved by the University of Haifa IRB committee (ref. #043/13).
Of the 103 adolescents that signed the informed consent, 3 (2.9%) withdrew before completing the initial assessment. Hence, the final sample in the study included 100 participants. The sociodemographic and clinical characteristics of the final sample appear in table 1. The sample was representative of the adolescent population in Israel in terms of socio-economic background, religious affiliation, place of birth (immigrants vs longtime residents), and residence (urban vs rural) (based on population totals taken from the Israeli Central Bureau of Statistics). In addition, it was similar to the representative sample of 957 adolescents that participated in the Israel Survey of Mental Health among Adolescents (ISMEHA).41 Finally, the sample’s overall level of behavioral difficulties and prosocial behavior was similar to that of the representative sample of 611 adolescents that participated in the validation study of the Hebrew version of the Strengths and Difficulties Questionnaire (SDQ-H)42 (for a comparison of the 3 samples see supplementary table S1).
Table 1.
Basic Sociodemographic, Educational, and Clinical Characteristics of the Study Sample
| Entire Sample (n = 100) | Non-APS (n = 88) | APS (n = 12) | Significance Test | |
|---|---|---|---|---|
| Mean (S.D.) age (y) | 14.0 (0.9) | 14.0 (0.9) | 13.9 (0.7) | t(98) = −0.35, P = .73 |
| Gender (% male) | 37.0 | 40.0 | 16.7 | χ2(1) = 2.4, P = .11 |
| Place of birth (% Israel) | 97.0 | 96.6 | 100.0 | χ2(1) = 0.38, P = .54 |
| Religion (% Jewish) | 94.0 | 94.3 | 91.6 | χ2(1) = 0.13, P = .72 |
| Father’s place of birth (% Israel) | 66.0 | 63.6 | 83.3 | χ2(1) = 1.8, P = .18 |
| Mother’s place of birth (% Israel) | 66.0 | 63.6 | 83.3 | χ2(1) = 1.8, P = .18 |
| Mean (S.D.) father’s years of education | 14.8 (3.0) | 14.9 (3.0) | 13.8 (2.8) | t(96) = −1.2, P = .25 |
| Mean (S.D.) mother’s years of education | 15.2 (2.7) | 15.2 (2.8) | 15.2 (1.8) | t(97) = −0.8, P = .93 |
| Mean (S.D.) SDQ self report | 10.1 (5.2) | 9.7 (5.4) | 13.1 (2.6) | t(98) = 3.6, P = .001 |
| Mean (S.D.) SDQ parent report | 8.4 (5.1) | 7.9 (5.5) | 11.6 (5.0) | t(98) = 2.4, P = .02 |
| Family history of psychosis (% yes) | 8.0 | 7.9 | 8.3 | χ2(2) = 0.002, P = .96 |
| History of traumatic events (% yes) | 38.0 | 37.5 | 41.7 | χ2(2) = 0.08, P = .78 |
| Any Axis I disordera (% yes) | 27.0 | 25.0 | 41.7 | χ2(2) = 1.5, P = .22 |
| Axis I anxiety disordera (% yes) | 8.0 | 6.8 | 16.7 | χ2(2) = 1.4, P = .23 |
| Axis I mood disordera (% yes) | 7.0 | 4.5 | 25.0 | χ2(2) = 6.8, P = .009 |
| Axis I ADHDa (% yes) | 13.0 | 12.5 | 16.7 | χ2(2) = 0.16, P = .69 |
Note: APS, Attenuated Psychotic Symptoms; ADHD, Attention Deficit Hyperactivity Disorder; SDQ, Strengths and Difficulties Questionnaire.
aBased on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS).
Measures and Procedure
Adolescents and parents who consented to participate were assessed for past and present psychopathology using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-aged Children, Present and Lifetime Versions (K-SADS-PL).43 The K-SADS is a well-validated, semi-structured diagnostic interview for the assessment of Axis-1 psychiatric disorders in children and adolescents. Adolescents and parents were interviewed separately, both answering the same questions about the adolescent.
Prodromal symptoms and syndromes then were screened with the Prodromal questionnaire (PQ),44 and thoroughly assessed with the SIPS and the Scale of Prodromal Symptoms (SOPS).3,5 The PQ is a 92-item, self-report questionnaire that targets positive, negative, disorganized, and general symptoms. The SIPS is a structured diagnostic interview for prodromal syndromes.
Basic SD were assessed using the Examination of Anomalous Self-Experience questionnaire (EASE).45 The EASE is a semi-structured interview consisting of 57 items divided into 5 rational domains: (1) cognition and stream of consciousness (17 items); (2) self-awareness and presence (18 items); (3) bodily experiences (9 items); (4) demarcation/transitivism (5 items); and (5) existential reorientation (8 items). The EASE has been shown to possess excellent internal consistency25,46 and inter-rater reliability.46 The interviewers (Liza Lacoua, Adva Brenner, Shiri Frum, and Maya Rothbaum) underwent an intensive 3-day training course at the University Psychiatric Center in Hvidovre, Copenhagen, supervised by senior clinician Dr Julie Nordgrad who is a member of Parnas’ study group. The EASE items were scored both dichotomously (ie, present vs absent) and continuously (on a 0 to 5 severity scale). However, because the correlation between the 2 scoring methods was 0.97, and because almost all previous studies on SD adopted the first scoring method, for the sake of simplicity and comparability, the remaining analyses include only frequency scores.
Inter-rater reliability in the present study was established using a random sub-sample of 30 (30%) interviews. The intraclass correlation coefficient (ICC) was 0.96 at the total score level, and 0.84 to 0.95 (median = 0.91) at the 5 domains levels. The internal consistency for all 57 items across the 4 raters in the present study was 0.92 (Cronbach’s alpha).
Social and role functioning were assessed with the Hebrew version of the SDQ-H,42 and the Global Functioning: Social (GF:S) and Global Functioning: Role (GF:R) scales.47 The SDQ is a 25-item screening diagnostic instrument designed for evaluating social, emotional and behavioral function in children and adolescents aged 4–17 years. It includes 5 subscales: 4 that refer to difficulties (hyperactivity-inattention, emotional symptoms, peer-relationship problems and conduct problems), and 1 to the adolescents’ strengths (prosocial behavior). The questionnaire was answered by the adolescents and their parents. The GF:S and GF:R scales,47 are clinician-rated scales that were recently developed to assess social and role functioning among individuals at high risk for schizophrenia. Both scales generate 2 scores: level of current function and level of function over the previous year. The scales were rated by the same clinicians who administered and scored the K-SADS, SIPS and EASE.
Finally, presence and severity of depressive and anxiety symptoms were assessed with the Mood and Anxiety Symptom Questionnaire (MASQ),48 a 61-item, self-report questionnaire covering general distress anxiety, general distress depression, anxious arousal, and anhedonic depression.
Statistical Analysis
The first hypothesis, regarding a moderate to high link between SD and prodromal symptoms, was tested using Pearson correlations for continuous data and a series of t tests for dichotomous data. The second hypothesis, regarding the specificity of the association between SD and prodromal symptoms, was assessed with Pearson correlations for continuous data and a series of 2-way ANOVAs for dichotomous variables, with SIPS prodromal status and comorbid diagnoses as the independent variables. Finally, the third hypothesis regarding the unique contribution of SD to the explanation of variance in prodromal symptoms was tested using a series of multivariate linear regression analyses with stepwise selection. All analyses were performed using SAS 9.3 for Windows.
Results
Prevalence of Prodromal Symptoms/Syndromes
On the PQ, 97% of the adolescents endorsed at least one of the 46 positive symptom items. The mean number of positive items was 13.2 (S.D. = 9.3) and the median 14.0. The mean score on the positive scale of the SOPS was 2.1 (S.D. = 2.1) and the median 1.0. A full-scale SIPS interview classified 12 (12%) participants as prodromal based on presence of APS.
Prevalence and Nature of SD
Nearly all of the participants in the study reported at least 1 anomaly out of the 57 EASE items (Mean = 9.6, S.D. = 6.9). However, only 78% of the participants reported at least 1 anomaly from the smaller sub-set of 10 anomalies that were shown in previous studies to be specific for schizophrenia spectrum disorders30 (Mean = 2.1, S.D.= 2.1). The most commonly reported domain of SD was Stream of Consciousness, with 96% reporting at least 1 item out of the 17 that comprise this domain (Mean = 5.0, S.D. = 3.1). The least commonly reported domain was Transitivistic Experiences, with 24% of the participants reporting at least 1 item of the 5 that comprise this domain (Mean = 0.3, S.D. = 0.6). The Self-Awareness, Existential Reorientation, and Body Demarcation domains were reported by 79% (Mean = 2.6, S.D. = 2.5), 50% (Mean = 1.0, S.D. = 1.2), and 49% (Mean = 0.8, S.D. = 1.0) of participants, respectively.
Association Between SD and Prodromal Symptoms/Syndromes
Consistent with our first hypothesis, a series of Pearson correlations revealed significant and high correlations between the EASE frequency score and the positive scale of the SIPS (see top section of table 2). Similarly, the EASE frequency score was significantly higher among APS compared to non-APS adolescents (see top section of table 3).
Table 2.
Simple and Partial Pearson Correlations of the EASE Total Frequency Score With Prodromal Symptoms
| EASE Total Frequency Score | ||
|---|---|---|
| Simple Correlations (n = 100) | Partial Correlationsa (n = 100) | |
| r, P | r, P | |
| SIPS—Positive | .67, <.0001 | |
| SIPS—Negative | .08, .40 | −.19, .06 |
| SIPS—Disorganized | .33, .0007 | .02, 84 |
| SIPS—General | .43, <.0001 | .24, .02 |
| MASQ—Mixed | .59, <.0001 | .47, <.0001 |
| MASQ—Anxiety | .37, .0002 | .24, .02 |
| MASQ—Depression | .48, <.0001 | .41, <.0001 |
| MASQ—Arousal | .51, <.0001 | .26, .01 |
| MASQ—Anhedonia | .19, .05 | .08, .46 |
| SDQ—Self report | .42, <.0001 | .23, .02 |
| SDQ—Parent report | .10, .32 | −.10, .34 |
| GF—Social | −.03, .75 | .16, .11 |
| GF—Role | −.13, .19 | −.05, .62 |
Note: GF, Global Functioning; SIPS, Structured Interview for Prodromal Syndromes; MASQ, Mood and Anxiety Symptom Questionnaire; EASE, Examination of Anomalous Self-Experience.
aControlling for SIPS—Positive score.
Table 3.
Means and Standard Deviations of SD and Other Risk Markers Among APS vs Non-APS Adolescents
| APS (n = 12) | Non-APS (n = 88) | Significance Test t(98), P | Effect Size (Cohen’s d) | |
|---|---|---|---|---|
| EASE—Total frequency score | 18.8 (7.0) | 8.3 (5.9) | 5.6, <.0001 | 1.6 |
| MASQ—Total | 179.5 (48.2) | 141.0 (32.7) | 3.6, .0005 | 0.7 |
| SDQ—Self report | 13.1 (2.6) | 9.7 (5.4) | 3.6, .001 | 0.8 |
| SDQ—Parent report | 11.6 (5.0) | 7.9 (5.5) | 2.4, .02 | 0.7 |
| GF—Social | 7.6 (1.7) | 8.3 (1.2) | 2.0, .05 | 0.5 |
| GF—Role | 7.8 (1.3) | 8.4 (0.9) | 2.0, .05 | 0.5 |
Note: SD, self-disturbances.
Specificity of the Association Between SD and Prodromal Symptoms/Syndromes
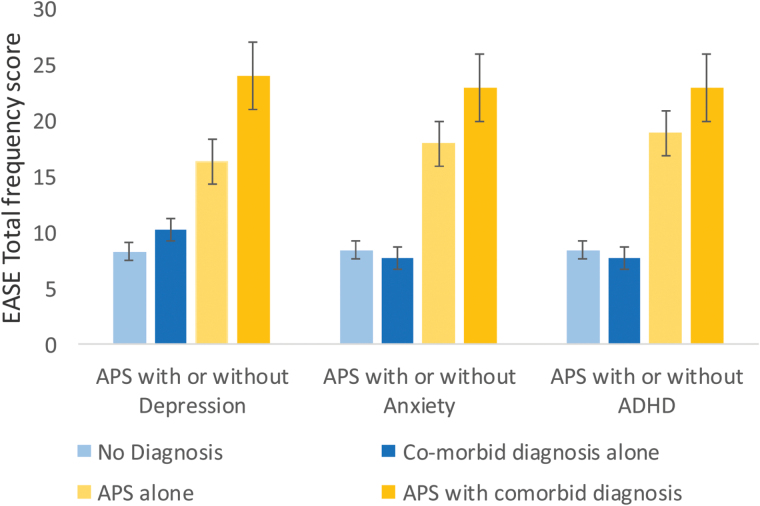
Contrary to our second hypothesis, a series of Pearson correlations revealed moderate and significant correlations between the EASE and several scales from the MASQ (Anxiety, Depression, Mixed, and Arousal) and the adolescent version of the SDQ (total difficulties) (see bottom section of table 2). Moreover, these correlations remained significant after partialing out the shared association of these variables with prodromal symptoms (see right-hand column in table 2). However, in partial support of our hypothesis, the effect size of the correlations of SD with mood symptoms and social/role functioning was significantly lower than that found with the positive scale of the SIPS (Z of the smallest difference = 1.8, P = .03). Furthermore, a series of 2-way ANOVAs with APS status and an Axis I diagnosis of Depression, Anxiety and/or Attention Deficit Hyperactivity Disorder (ADHD) as the independent variables and EASE as the dependent variable, revealed a significant main effect of APS (F (1, 96) = 32.3, P < .0001, F (1, 96) = 31.2, P < .0001, and F (1, 96) = 31.2, P < .0001, respectively), but not of Depression (F (1, 96) = 3.5, P = .06), Anxiety (F (1, 96) = 0.07, P = .80), or ADHD (F (1, 96) = 0.4, P = .54). Figure 1 presents these results visually.
Fig. 1.
Mean number of Examination of Anomalous Self-Experience (EASE) items and standard errors according to attenuated psychotic symptoms (APS) status and presence of Depression, Anxiety or ADHD.
The Unique Contribution of SD to the Explanation of Prodromal Symptoms/Syndromes
Consistent with our third hypothesis, the effect size of the relationship between APS status and the EASE total frequency score was almost twice as high as that of the relationship between APS status and the other variables (see right-hand column in table 3). Similarly, a multivariate linear regression analyses with stepwise selection revealed that SD (β = .34, t (97) = 8.99, P < .0001) is the single best variable that contributes to the explanation of sub-clinical positive symptoms, as measured by the SIPS (R 2 = .44, F (1, 97) = 76.98, P < .0001), and that global social functioning (β = −.61, t (97) = −2.85, P = .005) is the only variable that slightly improves the explanation model beyond SD (R 2 Change = .043, F (1, 97) = 8.15, P = .005). Finally, because the EASE contains several items, which have some affinity to psychotic symptoms (eg, perceptualization of inner speech and thought and transitivism), we repeated the above analyses without inclusion of these items in the EASE Total score. The above results remained practically unchanged.
Discussion
This study aimed to replicate and expand findings from previous studies that examined the relationship between SD and prodromal symptoms among help-seeking31–34 and non-help-seeking adolescents from the community. To the best of our knowledge, this is the first study to pursue this goal. In line with our hypotheses, the results of the study showed that: (1) SD are highly related to prodromal symptoms among non-help-seeking adolescents, (2) the association analyzed is considerably stronger than the association between SD and mood symptoms and social functioning, and (3) SD is the best single predictor of prodromal symptoms/syndromes. The next sections elaborate upon each of the main findings of the study, followed by a discussion of the limitations of the current study and suggested directions for future studies.
Prevalence of Prodromal Symptoms/Syndromes
Overall, the prevalence and average of prodromal symptoms in the sample used in this study were comparable to those reported for previous samples of non-help-seeking young adults that were assessed using the PQ.44,49 Similarly, the percentage of adolescents meeting the criteria for prodromal syndromes according to the SIPS (12%) was similar to that (8%–13%) found in previous samples of adolescents and young adults from the community.35–37 The closeness of the present percentage to the upper end of the range in previous studies can be explained by the younger age of participants in the present study. Support for this possibility comes from Schimmelman et al36 which found a strong effect of age on APS around age 16. Another support comes from a recent comprehensive meta-analysis,50 which suggests that psychotic-like experiences are associated with younger age. The relatively high prevalence of APS in the present study may also be explained by the voluntary nature of the sample, however, this is less likely considering the similarities in the overall degree of behavioral difficulties and prosocial behavior found between the sample in this study and the random sample of 611 adolescents that participated in the validation study of the Hebrew version of the SDQ-H.42 Regardless of these speculative explanations, the overall similar levels of prodromal symptoms and syndromes support the external validity of the present findings. They suggest that these finding can be safely generalized to other non-help-seeking adolescents or young adults from the community.
Prevalence of SD Among Adolescents in the Community
Consistent with previous studies of basic symptoms51 and SD,40 the first group of findings showed that SD are common among nonpsychotic non-help-seeking adolescents. However, as expected, their overall frequency was significantly lower than those found in previous samples of help-seeking32–34 and first-time hospitalization individuals.27,29 To the best of our knowledge, these results provide the first replicated support for the notion that disorders of the basic sense of self, shown in previous studies to be a core trait characteristic of schizophrenia spectrum disorders, do exist and can be reliably assessed among non-help-seeking adolescents from the community as well.
Association Between SD and Prodromal Symptoms/Syndromes
The second group of findings showed moderate-to-high association between SD and prodromal symptoms (table 2). Similarly, they showed moderate-to-high association between SD and prodromal (ie, APS) syndrome (table 3). These findings replicate findings from previous studies of help-seeking individuals,31–34 which suggest that SD and prodromal symptoms are 2 distinct but related markers of potential clinical vulnerability for future schizophrenia spectrum disorders.
Specificity of the Association Between SD and Prodromal Symptoms/Syndromes
The results pertaining to this question were mixed. On the one hand, contrary to our initial prediction, SD showed moderate correlations with mood (anxiety and depression) symptoms and social functioning when these symptoms were considered continuous variables. Furthermore, these correlations remained significant even after partialing out the overlap of these symptoms with prodromal symptoms. However, in line with our hypothesis, the effect size of the correlations of BDS with mood symptoms and social functioning was significantly lower than that of the correlation of SD with prodromal symptoms. Moreover, APS classification had a significant main effect on SD while a diagnosis of depression or anxiety did not, when these constructs were considered categorical variables. One likely explanation for these mixed results is that much of the association between SD and mood symptoms is due to the high correlation of mood symptoms with prodromal symptoms. Another possible explanation is that SD induce demoralization and fear. Support for this possibility comes from 2 recent studies from our group.52,53 Independently, these results and particularly the latter ones, provide a preliminary replication of previous studies which found similar pattern of results among first-time hospitalization patients.21,27–30
The Unique Contribution of SD to Explanation of Prodromal Symptoms/Syndromes
Consistent with our third hypothesis, the last group of findings showed that SD are the single best predictor of prodromal symptoms and syndromes. Moreover, they showed that other risk markers such as subjective distress and social/role functioning add very little, if anything at all, to the ability to explain prodromal symptoms beyond the results for SD. Finally, they showed that the above results remain unchanged by exclusion of EASE items that has some affinity to psychotic symptoms. The findings suggest that the association between SD and prodromal symptoms is not only specific but also that it is unique, meaning that it is not mediated or explained neither by other factors nor by measurement overlapping. These results are consistent with previous studies, including from our own group, which showed that SD and mood symptoms load on different, albeit interrelated, factors.32,33
Because the EASE contains several items, which have some affinity to psychotic symptoms (eg, perceptualization of inner speech and thought, transitivism), an important question is how much of the observed association between SD and APS can be attributed to these closely related items.
Strengths and Limitations
The most important strength of the present study lies in the large and “non-enriched” nature of its sample. Unlike previous studies that used “enriched” samples (ie, samples of distressed, help-seeking adolescents of whom many are specifically at-risk for schizophrenia-spectrum disorders), participants for this study were not selected according to initial level of distress and/or risk of future psychosis. Consequently, it supports a rather secure generalization of its findings to the entire population of adolescents who are at risk for psychosis.
Nevertheless, the study also suffers from several limitations that require caution when interpreting its results. First and most importantly, the study lacks follow-up data. This limits the ability to draw conclusions about the predictive value of SD for actual transition to psychosis as opposed to other disorders. In addition, it does not allow estimating the level of clinical risk that is associated with APS in non-help-seeking populations. This, together with the fact that there is no such follow-up data in the literature,54 explains the reason that adolescents with APS are not referred to in this study as at CHR for psychosis. Further longitudinal research with larger samples and longer follow-up intervals is needed in order to establish SD and APS as potent markers of vulnerability for psychosis in non-help-seeking populations.
A second important limitation of the study is the nonrandom, voluntary-response nature of its sample. Although the sociodemographic and clinical characteristics of the final sample were highly similar to a large sample of 957 adolescents that were recruited using probability methods,41 and although the prevalence of APS in the present sample was almost identical to that detected in a large sample of randomly selected young adolescents,36,37 it is impossible to rule out possible self-selection biases based on special interest in self-experiences (which is how the study was advertised). Further research using probability sampling is necessary in order to address this limitation.
A third important limitation of this study is its lack of blinding (ie, both SD and prodromal symptoms were assessed by the same interviewer). Although coding and scoring of the interviews were done only in retrospect, it is impossible to rule out that associations between the 2 factors are at least partially due to unintentional influences of these circumstances on the data that has or has not been collected.
A fourth limitation of the study relate to the low frequency of the main clinical outcome (ie, APS syndrome). While no doubt significant from an epidemiological point of view, an N of 12 is by no means sufficient, from a statistical power and precision point of view, to secure robust inferences about the exact magnitude of association between SD and APS.
Conclusion and Future Directions
Although further replication and confirmation is certainly necessary to address the above limitations, the present study provides preliminary support for the possibility15,16 that disturbances of self-experience exist and correlate with clinical risk for psychosis long before the acute onset of the illness. This applies not only to clinical samples, but to the general population as well. Besides the important theoretical implications that this may have for furthering our understanding of the etiology of schizophrenia-spectrum disorders, the findings provide preliminary support for the clinical potential of supplementing and refining current UHR methods for early detection using SD assessment. Finally, the study provides rational background for further prospective investigation of the longitudinal relationship between SD, prodromal symptoms, and risk for schizophrenia-spectrum conditions.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by a grant to D.K. from the Israel Science Foundation (ISF 548/09), and by a doctoral scholarship to L.L. from the University of Haifa.
Supplementary Material
Acknowledgments
The authors wish to thank Adva Brenner, Shiri Frum, Maya Rothbaum, and Aya Zalzniak for their help with data collection and coding. In addition, they wish to thank Noa Barash, Hanit Cishenevski, Elinor Kimmel, Noa Navon, Rachil Shehadi, Zvi Stoller, and Shachaf Zilberman for their help with participant recruitment and data recording. Finally, the authors wish to thank the anonymous reviewers for their valuable comments. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Yung AR, Phillips LJ, Yuen HPet al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. [DOI] [PubMed] [Google Scholar]
- 2. Yung AR, Yuen HP, McGorry PDet al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. [DOI] [PubMed] [Google Scholar]
- 3. Miller TJ, McGlashan TH, Woods SWet al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. [DOI] [PubMed] [Google Scholar]
- 4. Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. [DOI] [PubMed] [Google Scholar]
- 5. Miller TJ, McGlashan TH, Rosen JLet al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 6. Cannon TD, Cadenhead K, Cornblatt Bet al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon TD, Cornblatt B, McGorry P. The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull. 2007;33:661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woods SW, Addington J, Cadenhead KSet al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon TD. Clinical and genetic high-risk strategies in understanding vulnerability to psychosis. Schizophr Res. 2005;79:35–44. [DOI] [PubMed] [Google Scholar]
- 10. McGorry PD, Edwards J, Mihalopoulos Cet al. EPPIC: an evolving system of early detection and optimal management. Schizophr Bull. 1996;22:305–326. [DOI] [PubMed] [Google Scholar]
- 11. McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips LJ, McGorry PD, Yung ARet al. Prepsychotic phase of schizophrenia and related disorders: recent progress and future opportunities. Br J Psychiatry Suppl. 2005;48:s33–44. [DOI] [PubMed] [Google Scholar]
- 13. Yung AR, et al. Psychotic-like experiences in nonpsychotic help-seekers: associations with distress, depression, and disability. Schizophr Bull. 2006;32:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yung AR, Stanford C, Cosgrave Eet al. Testing the Ultra High Risk (prodromal) criteria for the prediction of psychosis in a clinical sample of young people. Schizophr Res. 2006;84:57–66. [DOI] [PubMed] [Google Scholar]
- 15. Parnas J. Clinical detection of schizophrenia-prone individuals: critical appraisal. Br J Psychiatry Suppl. 2005;48:s111–s112. [DOI] [PubMed] [Google Scholar]
- 16. Nelson B, Yung AR, Bechdolf Aet al. The phenomenological critique and self-disturbance: implications for ultra-high risk (“prodrome”) research. Schizophr Bull. 2008;34:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huber G, Gross G. The concept of basic symptoms in schizophrenic and schizoaffective psychoses. Recenti Prog Med. 1989;80:646–652. [PubMed] [Google Scholar]
- 18. Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44:121–134. [DOI] [PubMed] [Google Scholar]
- 19. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444. [DOI] [PubMed] [Google Scholar]
- 20. Møller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26:217–232. [DOI] [PubMed] [Google Scholar]
- 21. Parnas J, Handest P, Saebye Det al. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108:126–133. [DOI] [PubMed] [Google Scholar]
- 22. Parnas J, Bovet P. Research in psychopathology: epistemologic issues. Compr Psychiatry. 1995;36:167–181. [DOI] [PubMed] [Google Scholar]
- 23. Parnas J, Raballo A, Handest Pet al. Self-experience in the early stages of schizophrenia: 5 year follow-up in the Copenhagen Prodromal Study. World Psychiatry. 2011;10:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raballo A, Parnas J. The Silent side of the spectrum: schizotypy and the schizotaxic self. Schizophr Bull. 2011;37:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raballo A, Parnas J. Examination of anomalous self-experience: initial study of the structure of self-disorders in schizophrenia spectrum. J Nerv Ment Dis. 2012;200:577–583. [DOI] [PubMed] [Google Scholar]
- 26. Raballo A, Saebye D, Parnas J. Looking at the schizophrenia spectrum through the prism of self-disorders: an empirical study. Schizophr Bull. 2011;37:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haug E, Lien L, Raballo Aet al. Selective aggregation of self-disorders in first-treatment DSM-IV schizophrenia spectrum disorders. J Nerv Ment Dis. 2012;200:632–636. [DOI] [PubMed] [Google Scholar]
- 28. Nelson B, Thompson A, Yung AR. Not all first-episode psychosis is the same: preliminary evidence of greater basic self-disturbance in schizophrenia spectrum cases. Early Interv Psychiatry. 2013;7:200–204. [DOI] [PubMed] [Google Scholar]
- 29.Nordgaard J, Parnas J. Self-disorders and the schizophrenia spectrum: a study of 100 first hospital admissions. Schizophr Bull. 2014;40:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parnas J, Handest P, Jansson Let al. Anomalous subjective experience among first-admitted schizophrenia spectrum patients: empirical investigation. Psychopathology. 2005;38:259–267. [DOI] [PubMed] [Google Scholar]
- 31. Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull. 2012;38:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Comparelli A, Corigliano V, De Carolis Aet al. Anomalous self-experiences and their relationship with symptoms, neuro-cognition, and functioning in at -risk adolescents and young adults. Comprehensive Psychiatry. 2016;65:44–49. [DOI] [PubMed] [Google Scholar]
- 33. Koren D, Reznik N, Adres Met al. Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychol Med. 2013;43:1365–1376. [DOI] [PubMed] [Google Scholar]
- 34. Raballo A, Pappagallo E, Dell’Erba Aet al. Self-Disorders and clinical high risk for psychosis: an empirical study in help-seeking youth attending community mental health facilities [published online ahead of print January 12, 2016]. Schizophr Bull. doi:10.1093/schbul/sbv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelleher I, Murtagh A, Molloy Cet al. Identification and characterization of prodromal risk syndromes in young adolescents in the community: a population-based clinical interview study. Schizophr Bull. 2012;38:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schimmelmann BG, Michel C, Martz-Irngartinger Aet al. Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: findings from the BEAR and BEARS-kid studies. World Psychiatry. 2015;14:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultze-Lutter F, Michel C, Ruhrmann Set al. Prevalence and clinical significance of DSM-5–attenuated psychosis syndrome in adolescents and young adults in the general population: the Bern Epidemiological At-Risk (BEAR) study. Schizophr Bull. 2014;40:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poulton R, Caspi A, Moffitt TEet al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. [DOI] [PubMed] [Google Scholar]
- 39. Addington J, Epstein I, Reynolds Aet al. Early detection of psychosis: finding those at clinical high risk. Early Interv Psychiatry. 2008;2:147–153. [DOI] [PubMed] [Google Scholar]
- 40. Torbet G, Schulze D, Fiedler Aet al. Assessment of self disorders in a non-clinical population: reliability and association with schizotypy. Psychiatry Res. 2015;228:857–865. [DOI] [PubMed] [Google Scholar]
- 41. Farbstein I, Apter A, Kanaaneh Ret al. The Israel survey of mental health among adolescents: aims and methods. Isr J Psychiatry Relat Sci. 2010;47:244. [PubMed] [Google Scholar]
- 42. Mansbach-Kleinfeld I, Apter A, Farbstein Iet al. A population-based psychometric validation study of the Strengths and Difficulties Questionnaire–Hebrew version. Front Psychiatry. 2010;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaufman J, Birmaher B, Brent Det al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 44. Loewy RL, Bearden CE, Johnson JKet al. The prodromal questionnaire (PQ): preliminary validation of a self-report screening measure for prodromal and psychotic syndromes. Schizophr Res. 2005;79:117–125. [PubMed] [Google Scholar]
- 45. Parnas J, Moller P, Kircher Tet al. EASE: Examination of Anomalous Self-Experience. Psychopathology. 2005;38:236–258. [DOI] [PubMed] [Google Scholar]
- 46. Moller P, Haug E, Raballo Aet al. Examination of anomalous self-experience in first-episode psychosis: interrater reliability. Psychopathology. 2011;44:386–390. [DOI] [PubMed] [Google Scholar]
- 47. Cornblatt BA, Auther AM, Niendam Tet al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Watson D, Clark LT. The Mood and Anxiety Symptoms Questionnaire. Iowa City, IA: University of Iowa Department of Psychology; 1991. [Google Scholar]
- 49. Loewy RL, Johnson JK, Cannon TD. Self-report of attenuated psychotic experiences in a college population. Schizophr Res. 2007;93:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linscott R, Van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 51. Meng H, Schimmelmann BG, Koch Eet al. Basic symptoms in the general population and in psychotic and non-psychotic psychiatric adolescents. Schizophr Res. 2009;111:32–38. [DOI] [PubMed] [Google Scholar]
- 52. Skodlar B, Tomori M, Parnas J. Subjective experience and suicidal ideation in schizophrenia. Compr Psychiatry. 2008;49:482–488. [DOI] [PubMed] [Google Scholar]
- 53. Skodlar B, Parnas J. Self-disorder and subjective dimensions of suicidality in schizophrenia. Compr Psychiatry. 2010;51:363–366. [DOI] [PubMed] [Google Scholar]
- 54. Fusar-Poli P, Cappucciati M, Rutigliano G, et al. At risk or not at risk? A meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.