Abstract
Impairments of social cognition are well documented in patients with schizophrenia (SCZ), but the neural basis remains poorly understood. In light of evidence that suggests that the “mirror neuron system” (MNS) and the “mentalizing network” (MENT) are key substrates of intersubjectivity and joint action, it has been suggested that dysfunction of these neural networks may underlie social difficulties in SCZ patients. Additionally, MNS and MENT might be associated differently with positive vs negative symptoms, given prior social cognitive and symptom associations. We assessed resting state functional connectivity (RSFC) in meta-analytically defined MNS and MENT networks in this patient group. Magnetic resonance imaging (MRI) scans were obtained from 116 patients and 133 age-, gender- and movement-matched healthy controls (HC) at 5 different MRI sites. Network connectivity was analyzed for group differences and correlations with clinical symptoms. Results demonstrated decreased connectivity within the MNS and also the MENT in patients compared to controls. Notably, dysconnectivity of the MNS was related to symptom severity, while no such relationship was observed for the MENT. In sum, these findings demonstrate that differential patterns of dysconnectivity exist in SCZ patients, which may contribute differently to the interpersonal difficulties commonly observed in the disorder.
Key words: model-based analysis, functional connectivity, resting state fMRI, mirror neuron system, mentalizing network, schizophrenia
Introduction
Schizophrenia (SCZ) is a complex mental disorder, whose underlying neurobiology is only incompletely understood. Due to its early peak age of onset, severity of symptoms, and associated disability, SCZ is one of the costliest mental disorders in terms of human suffering and economic expenditure.1 Concerning psychopathology, SCZ is characterized by delusions and hallucinations (positive symptom dimension), but also alterations of drive and volition (negative symptom dimension), cognitive symptoms and affective dysregulation.2
Furthermore, impairments of social cognition are well documented in patients with SCZ3–5 with a general consensus that social cognition is distinct from, though related to, basic neurocognition and other clinical features (for review Green et al6) and shows unique relationships to functional outcome, above and beyond basic cognition7 thus providing an even better target for intervention than basic cognition. On the one hand, patients with SCZ are thought to “hypermentalize,” ie, attribute more meaning to their surroundings than usual as reflected in positive symptoms such as delusions and paranoia.8 On the other hand, alterations of social cognition in SCZ have been described as a “loss of natural evidence” for being in a world that is implicitly and intersubjectively shared with others, thereby leading to alienation and social withdrawal.9 The latter changes have been specifically related to the negative symptom dimension of SCZ, which is known to be highly relevant for prognosis and socioeconomic outcome.10,11
Neuroimaging has indicated the involvement of 2 distinct large-scale neural networks in social cognition: The so-called “mentalizing network” (MENT) has been shown to be activated by tasks, which require study participants to explicitly think about the mental states of others or engage in joint attention with them, but is also engaged during states of unconstrained cognition, which are known to give rise to “social” thoughts.12–15 It has furthermore been suggested that the human “mirror neuron system” (MNS) plays an important role in social cognition by providing a pre-reflexive sense of being and acting with others.16–20 Moreover, activity in this latter network has been shown to be subject to modulations induced by social interactions.21–24 In light of these studies pointing towards the importance of both the MNS and the MENT for social cognition, it can be hypothesized that the neurobiological underpinnings of social impairments in SCZ may arise from aberrant functioning of either one or both of these networks.25–28
Indeed, several recent findings have already pointed towards aberrant functional connectivity in SCZ patients between brain regions that are discussed as important network hubs of the MNS and MENT.26,29,30 Also, it has been proposed that using measures of functional dysconnectivity rather than neuroanatomical alterations, such as volume changes, could help to establish a more sensitive and clinically relevant biomarker of SCZ, one that might lend itself to early detection and therapeutic endeavors.31 Resting state functional magnetic resonance imaging (fMRI) analyses as a clinically available measure of functional connectivity could, therefore, provide a particularly powerful approach to investigate network dysfunctions in SCZ. Most importantly, in contrast to task-based neuroimaging, such analyses are less confounded by cognitive and/or motivational impairments, which are commonly observed in SCZ patient populations and often impair sufficient task performance.32,33
The reliable assessment of functional connectivity networks is, however, challenged by various methodological issues.34 Solely data-driven approaches do not allow the investigation of aberrant functional connectivity in a priori brain networks that are objectively related to the processes of interest, here, the MNS and MENT networks. Thus, to circumvent these problems we relied on meta-analytically defined network maps of the MNS and the MENT to study fMRI resting-state functional connectivity using a seed-based approach (resting state functional connectivity [RSFC]) in SCZ (MNS: Caspers et al35; MENT: Schilbach et al36; see figure 1 for illustration and table 1 for further information).
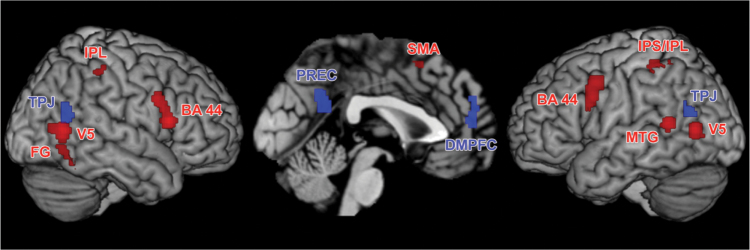
Fig. 1.
Significant results of the mirror neuron system (MNS, displayed in red) and mentalizing network (MENT; displayed in blue) meta-analyses used for the functional connectivity analysis in healthy controls and patients (taken from: Caspers et al34 & Schilbach et al35). BA 44: Broca’s area, DMPFC: dorso-medial prefrontal cortex, FG: fusiform gyrus, MTG: middle temporal gyrus, IPL: inferior parietal lobe, IPS: intraparietal sulcus, PREC: precuneus, SMA: supplementary motor area, TPJ: temporo-parietal junction, V5: extrastriate visual area.
Table 1.
“Centers of Gravity” of MNS Network (Conjunction Between “Action Observation” and “Action Imitation,” Taken From: Caspers et al34, See Also figure 1 in Red) and MENT Network (Conjunction Between “Social Cognition” and “Default Mode Network”, Taken From: Schilbach et al35, See Also figure 1 in Blue)
| Macroanatomical Location | MNI Coordinates | ||
|---|---|---|---|
| x | y | z | |
| MNS network | |||
| L IFG (BA 44) | −56 | 8 | 28 |
| R IFG (BA 44) | 58 | 16 | 10 |
| L SMA | −1 | 16 | 52 |
| L IPS/IPL | −38 | −40 | 50 |
| R IPL | 51 | −36 | 50 |
| L MTG | −54 | −50 | 10 |
| L V5 | −52 | −70 | 6 |
| R V5 | 54 | −64 | 4 |
| R FG | 44 | −54 | −20 |
| MENT network | |||
| L PreC | −6 | −54 | 24 |
| L DMPFC | −2 | 52 | 14 |
| R TPJ | 52 | −62 | 16 |
| L TPJ | −46 | −66 | 18 |
Note: pMTG, posterior middle temporal gyrus; SMA, supplementary motor area (hidden within the interhemispheric fissure); IPS, intraparietal sulcus; MTG, middle temporal gyrus; FG, fusiform gyrus; PreC, Precuneus; DMPFC, dorsomedial prefrontal cortex; TPJ, temporo-parietal junction; MENT, mentalizing network; MNS, mirror neuron system; IPL, inferior parietal lobe; V5, extrastriate visual area; BA 44, Broca’s area; MNI, Montreal Neurological Institute. All peaks are assigned to the most probable brain areas as revealed by the SPM Anatomy Toolbox.
Based on the “dysconnection hypothesis” of SCZ and in light of well-known social impairments associated with the disorder, the current study pursues the idea that functional connectivity in neural networks relevant for social cognition (MNS and MENT) could be disturbed in SCZ.30,37,38 In that way, specific points of vulnerability may be identified which may contribute to the impaired social functioning frequently reported.39 To investigate this hypothesis, we used meta-analytically defined network maps of the MNS and MENT to interrogate RSFC in a group of SCZ patients and matched controls. Furthermore, the relationship between RSFC in the MNS and MENT and clinical characteristics was tested, as symptom severity has been shown to influence social cognition40 and functional connectivity.41
Material and Methods
Meta-analytically Derived Network Models
The MNS network was based on results of a recent large-scale meta-analysis of neuroimaging studies on activation observation and action imitation (Caspers et al35; see figure 1 in red and table 1). This meta-analysis included data from 87 neuroimaging studies. The MENT network was derived from another meta-analysis of neuroimaging studies, which had focused on self-referential and social cognition (Schilbach et al36; see figure 1 in blue and table 1). This meta-analysis included data from 608 neuroimaging studies.
Participants
We investigated RSFC within the meta-analytically defined networks described above in 116 patients with SCZ (recruited at 5 sites: Aachen, Alberquerque, Göttingen, Groningen, and Utrecht) and 133 age- and gender-matched healthy controls (HC; recruited at the same 5 sites) without any record of neurological or psychiatric disorders (for group characteristics see table 2), confirmed via structured clinical interview (SCID). Diagnosis of SCZ was confirmed via clinical examination of the attending psychiatrist in accordance with the International Classification of Diseases (ICD-10) or the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV-TR). Except for 1 patient, all patients received their regular medication as prescribed by the attending psychiatrist. There was a considerable variability in the exact compounds that were used. Many patients also received combination drug therapy (see table 3 for more information). We were, thus, not able to perform sub-analyses of patients with different medication status, but rather regard differences in medication and their potential effects on functional connectivity as a nonsystematic source of variance in the patient group. In this context, it should be noted that the variability in the patient pool potentially introduced by this heterogeneous medication should make it harder for the statistical analysis to identify consistent differences between patients and controls due to the increased variance in the patient group.
Table 2.
Group Characteristics for Age and Gender
| Site | Age Mean (SD) | P-value | Males (n) | Females (n) | |
|---|---|---|---|---|---|
| Site 1 | Controls | 33.75 (12.34) | .882a | 9 | 3 |
| Patients | 34.46 (11.35) | 10 | 3 | ||
| Site 2 | Controls | 31.65 (12.29) | .838a | 11 | 9 |
| Patients | 32.39 (9.38) | 11 | 7 | ||
| Site 3 | Controls | 38.14 (14.18) | .481a | 7 | 7 |
| Patients | 34.45 (9.35) | 4 | 6 | ||
| Site 4 | Controls | 31.30 (9.12) | .898a | 21 | 6 |
| Patients | 30.96 (9.93) | 23 | 5 | ||
| Site 5 | Controls | 36.25 (12.07) | .849a | 44 | 16 |
| Patients | 36.71 (13.33) | 39 | 10 | ||
| All | Controls | 34.53 (11.91) | .853a | 92 | 41 |
| Patients | 34.25 (11.58) | 87 | 31 | ||
Note: Site 1 = Aachen, Site 2 = Groningen, Site 3 = Utrecht, Site 4 = Göttingen, Site 5 = COBRE/The MIND Research Network.
aStatistical comparison performed via t test.
Table 3.
Information on How Many of the Patients Were Medicated or Unmedicated at Time of Measurement and Mean Medication (CPZ-equivalents) per Site
| Site | Unmedicated (n) | Medicated (n) | Mean CPZ- equivalents (SD) |
|---|---|---|---|
| Aachen | 0 | 13 | 505.46 (363.7) |
| Groningen | 0 | 18 | 415.33 (322.8) |
| Göttingen | 1 | 25 | 366.83 (287.4) |
| Utrecht | 0 | 10 | 344.50 (199.3) |
| COBRE | 0 | 49 | 289.42 (240.2) |
| Total | 1 | 115 | 384.31 (282.7) |
All participants gave written informed consent to participate in the study as approved by the ethics committees of the universities of Aachen, Albuquerque, Goettingen, Utrecht, and Groningen (for information on MRI-protocols see table 4). Joint re-analysis was approved by the ethics committee of the Heinrich-Heine University Duesseldorf.
Table 4.
Information on Scanning Sites Including Scanner Type and MRI Parameters
| Site | Scanner | TR/TE (ms) | Number of Slices | Slice- Thickness (mm) | Gap (mm) | Flip Angle | Orientation | In-plane Resolution | Volumes Acquired | Measure Time (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Siemens 3T Tim Trio | 2000/28 | 34 | 3.3 | 0.3 | 77° | Axial | 3.6×3.6mm2 | 210 | 420 |
| Site 2 | Philips Achieva 3T | 2400/28 | 43 | 3 | - | 85° | Axial | 3.44×3.44mm2 | 200 | 480 |
| Site 3 | Philips Achieva 3T | 21.75/32.4a | 40 | 4 | - | 10° | Coronal | 4×4mm2 | 600 | 366 |
| Site 4 | Siemens 3T Tim Trio | 2000/30 | 33 | 3 | 0.6 | 70° | Axial | 3×3mm2 | 156 | 312 |
| Site 5 | Siemens 3T Tim Trio | 2000/29 | 32 | 3.5 | 1 | 75° | Axial | 3.75×3.75mm2 | 150 | 300 |
Note: MRI, magnetic resonance imaging; TR, repetition time; TE, echo time. Site 1 = Aachen, Site 2 = Groningen, Site 3 = Utrecht, Site 4 = Göttingen, Site 5 = Alberquerque (COBRE).
aUtrect used a PRESTO-SENSE Sequence. This scan sequence achieves full brain coverage within 609ms by combining a 3D-PRESTO pulse sequence with parallel imaging in 2 directions (8-channel SENSE headcoil).
Clinical Scales and Parameters
Symptom severity was assessed by use of the Positive and Negative Syndrome Scale (PANSS42). Moreover, we assessed duration of illness and age at onset in all patients. Additionally, chlorpromazine-equivalents (CPZ-equivalents) were estimated as described previously.43
Resting State fMRI Data: Imaging
For each participant, resting state EPI (echo-planar-imaging) images were acquired using a standard blood-oxygen-level-dependent (BOLD) contrast (please see table 4 for details on EPI sequence for each site; notably, Utrecht [site 3] used a PRESTO-SENSE sequence which typically has shorter repetition time [TR] than echo time [TE] times). Participants were instructed to lie still during the scanning session and to let their mind wander, but not to fall asleep. The latter was confirmed during a post-scan debriefing interview. While it obviously would have been advantageous to have exactly matching protocols, this unfortunately was not possible in this retrospective pooling of data. While we acknowledge differences in acquisition parameters as a weakness of our study, we would argue that this should not influence our results as we explicitly removed any potential site-effects prior to statistical inference. In this context, we would raise attention to the fact that patients and controls were closely matched within each site, not only for age and gender but also movement-matching (DVARS, FD and RMD) was performed (all P values > .3). This balanced design, therefore, allows estimating and removing any global site-associated effects that may be related to scanner and imaging parameters.
Resting State fMRI Data: Preprocessing
Prior to further processing (using SPM8, www.fil.ion.ucl.ac.uk/spm) the first 4 images were discarded allowing for magnetic field saturation. The EPI images were corrected for head movement by affine registration using a 2-pass procedure. The mean EPI image for each subject was then spatially normalized to the MNI ICBM-152 template using the “unified segmentation” approach,44 the ensuing deformation field was applied to the individual EPI volumes and the output images were smoothed by a 5-mm FWHM Gaussian kernel.
In order to reduce spurious correlations induced by motion,45 variance that could be explained by the head motion was removed from each voxel’s time series. In particular, according to published evaluations,46 we removed: (1) the 6 motion parameters derived from the image realignment, (2) their first derivative, and the respective squared terms (ie, 24 parameter regression). These corrections have been shown47,48 to increase specificity and sensitivity of the analyses and to detect valid correlation and anti-correlations during rest, which are not an artifact of the preprocessing method, but may reflect valid biological signals and can be used to robustly identify group-differences in resting-state functional connectivity.49 In turn, given recent reports of spurious effects in between group comparisons due to global signal removal,50,51 we did not employ global signal regression.
Using a matlab code from the CONN toolbox (https://www.nitrc.org/projects/conn), data was band pass filtered preserving frequencies between 0.01 and 0.08Hz52 applying a frequency-domain filter. The time course for each of the regions identified in the meta-analyses described above was extracted for each subject as the first eigenvariate of all gray-matter (identified using segmentation in SPM8) voxels located within the volumes of interest (VOI).
Resting State fMRI Data: Individual and Group Level Analyses
For each subject and network (MNS and MENT), we computed linear (Pearson) correlation coefficients between the extracted time series, which were subsequently transformed into Fisher’s Z-scores representing the functional connectivity for each connection in each subject. As part of a confound-removal procedure, any variance that could be explained by the factors “site,” “age,” and “gender,” as well as their 2-way interactions was removed from these scores to prevent site-diagnosis interactions. Group comparison between patients and controls was then performed by a non-parametric approach using 10 000 realizations of the null hypothesis (label exchangeability) in a Monte-Carlo simulation. Results were regarded as significant if they passed P < .05, following Bonferroni-correction for multiple comparisons, ie, P values were divided by the number of possible edges (MNS: 36, MENT: 6). Thus, to reach significance P values had to be below P = .0013 in the MNS and below P = .008 in the MENT.
Furthermore, relationships between RSFC and clinical characteristics (PANSS subscale scores and PANSS total scores, illness duration) were tested by means of linear correlation analyses.
Brain Volume: Image Acquisition and Analysis
To analyze possible group differences in brain volume, a high-resolution anatomical image was acquired using an MPRAGE (3-D Magnetization Prepared Rapid Gradient Echo) sequence consisting of 160 sagittal slices (TR = 1900ms, TE = 2.52ms, 1×1 × 1mm resolution, field of view (FOV) 25×25cm, slice oversampling = 18.2%, flip angle [FA] = 9°). Anatomical scans were preprocessed with VBM8 toolbox (dbm.neuro.uni-jena.de/vbm) in SPM8 using standard settings (DARTEL normalization, spatially adaptive nonlinear means denoising). Within a unified segmentation model,44 images were corrected for biasfield inhomogenities. Brain tissue was classified into gray matter, white matter, and cerebrospinal fluid (adjusted for partial volume effects, spatially normalized to the MNI template, nonlinear modulation of segmented images to adjust for the amount of expansion and contraction applied during normalization using the nonlinear only modulation function within the VBM8 toolbox). We computed the volumes for all of the network regions by integrating the (nonlinearly) modulated voxel-wise gray matter probabilities for each participant. Age was included as a nuisance variable. The (nonlinearly modulated) calculated gray matter volume represents the amount of gray matter corrected for individual brain size. We tested for group differences in gray matter volume of the network-specific regions by applying 2-sample t tests with group as factor. To evaluate the effect of gray matter volume on significant functional connectivity differences, ANCOVAs with gray matter volume of both included regions as covariate were performed. Additionally in patients, we tested for associations between clinical parameters and regional volume differences using structural covariance analyses.
Results
Functional Connectivity Differences in MNS Network
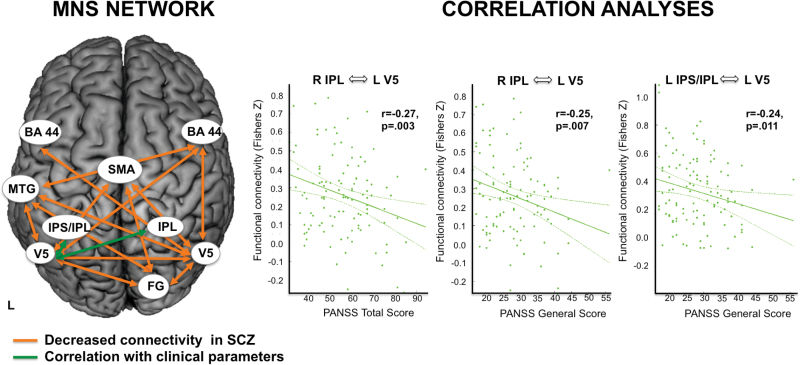
Resting-state functional connectivity differences between SCZ patients and HC were assessed between all nodes of the MNS (figure 1, table 1). Significant differences (P < .05, Bonferroni corrected for multiple comparisons across all connections) in RSFC between patients and controls were found along several edges of the MNS network model: In particular, functional connectivity of left middle temporal gyrus (MTG), supplementary motor area (SMA), right fusiform gyrus (FG), and right inferior frontal gyrus (BA44) with left and right extrastriate cortex (V5) was decreased. Furthermore, RSFC between left and right V5 did also show a decrease in patients with SCZ. Moreover, functional connectivity between right FG and left MTG, right FG and SMA, as well as left MTG and right BA44 was decreased in patients (see figure 2 and table 5). We did not find any connections showing increased RSFC in patients compared to controls.
Fig. 2.
(Left) Schematic illustration of significant resting state functional connectivity (RSFC) differences in the mirror neuron system (MNS) network depicting reduced RSFC in patients. (Right) Correlations with clinical characteristics. BA 44: Broca’s area, FG: fusiform gyrus, MTG: superior temporal gyrus, IPL: inferior parietal lobe, IPS: intraparietal sulcus, SMA: supplementary motor area, V5: extrastriate visual area.
Table 5.
Overview on Functional Connectivities in SCZ and Controls, Listing Fisher’s Z and d′ and P Values
| Connectivity | SCZ Patients Fishers Z | Controls Fishers Z | d′ | P |
|---|---|---|---|---|
| MNS | ||||
| V5 left ↔ V5 right | 0.580 | 0.833 | −0.82 | <.001 |
| V5 left ↔ FG | 0.398 | 0.504 | −0.42 | <.001 |
| V5 left ↔ IPL left | 0.433 | 0.523 | −0.37 | .005 |
| V5 left ↔ IPL right | 0.271 | 0.338 | −0.28 | .049 |
| V5 left ↔ MTG | 0.350 | 0.520 | −0.63 | <.001 |
| V5 left ↔ SMA | 0.156 | 0.275 | −0.58 | <.001 |
| V5 left ↔ BA 44 left | 0.351 | 0.428 | −0.33 | .008 |
| V5 left ↔ BA 44 right | 0.282 | 0.386 | −0.43 | <.001 |
| V5 right ↔ FG | 0.379 | 0.578 | −0.78 | <.001 |
| V5 right ↔ IPL left | 0.338 | 0.445 | −0.39 | .006 |
| V5 right ↔ IPL right | 0.270 | 0.334 | −0.27 | .054 |
| V5 right ↔ MTG | 0.338 | 0.498 | −0.60 | <.001 |
| V5 right ↔ SMA | 0.185 | 0.266 | −0.41 | <.001 |
| V5 right ↔ BA 44 left | 0.267 | 0.375 | −0.48 | <.001 |
| V5 right ↔ BA 44 right | 0.300 | 0.420 | −0.53 | <.001 |
| FG ↔ IPL left | 0.271 | 0.361 | −0.40 | .005 |
| FG ↔ IPL right | 0.228 | 0.288 | −0.26 | .105 |
| FG ↔ MTG | 0.166 | 0.349 | −0.78 | <.001 |
| FG ↔ SMA | 0.158 | 0.250 | −0.46 | <.001 |
| FG ↔ BA 44 left | 0.247 | 0.322 | −0.35 | .018 |
| FG ↔ BA 44 right | 0.259 | 0.341 | −0.36 | .018 |
| IPL left ↔ IPL right | 0.516 | 0.572 | −0.22 | .319 |
| IPL left ↔ MTG | 0.235 | 0.315 | −0.33 | .019 |
| IPL left ↔ SMA | 0.297 | 0.339 | −0.18 | .140 |
| IPL left ↔ BA 44 left | 0.499 | 0.567 | −0.28 | .046 |
| IPL left ↔ BA 44 right | 0.363 | 0.425 | −0.25 | .053 |
| IPL right ↔ MTG | 0.175 | 0.265 | −0.38 | .006 |
| IPL right ↔ SMA | 0.250 | 0.312 | −0.27 | .022 |
| IPL right ↔ BA 44 left | 0.424 | 0.490 | −0.29 | .035 |
| IPL right ↔ BA 44 right | 0.531 | 0.577 | −0.19 | .243 |
| MTG ↔ SMA | 0.226 | 0.283 | −0.29 | .030 |
| MTG ↔ BA 44 left | 0.288 | 0.361 | −0.30 | .030 |
| MTG ↔ BA 44 right | 0.221 | 0.355 | −0.58 | <.001 |
| SMA ↔ BA 44 left | 0.278 | 0.350 | −0.28 | .017 |
| SMA ↔ BA 44 right | 0.292 | 0.375 | −0.36 | .010 |
| BA 44 left ↔ BA 44 right | 0.526 | 0.615 | −0.32 | .015 |
| MENT | ||||
| PREC ↔ DMPFC | 0.501 | 0.598 | − 0.45 | .001 |
| PREC ↔ TPJ left | 0.562 | 0.556 | 0.02 | .842 |
| PREC ↔ TPJ right | 0.466 | 0.488 | −0.08 | .485 |
| DMPFC ↔ TPJ left | 0.379 | 0.417 | −0.18 | .211 |
| DMPFC ↔ TPJ right | 0.318 | 0.351 | −0.16 | .262 |
| TPJ left ↔ TPJ right | 0.526 | 0.628 | −0.39 | .009 |
Note: SCZ, schizophrenia. Significant group differences (following Bonferroni correction) are marked in bold, always indicating a decrease in functional connectivity in SCZ.
Functional Connectivity Differences in MENT Network
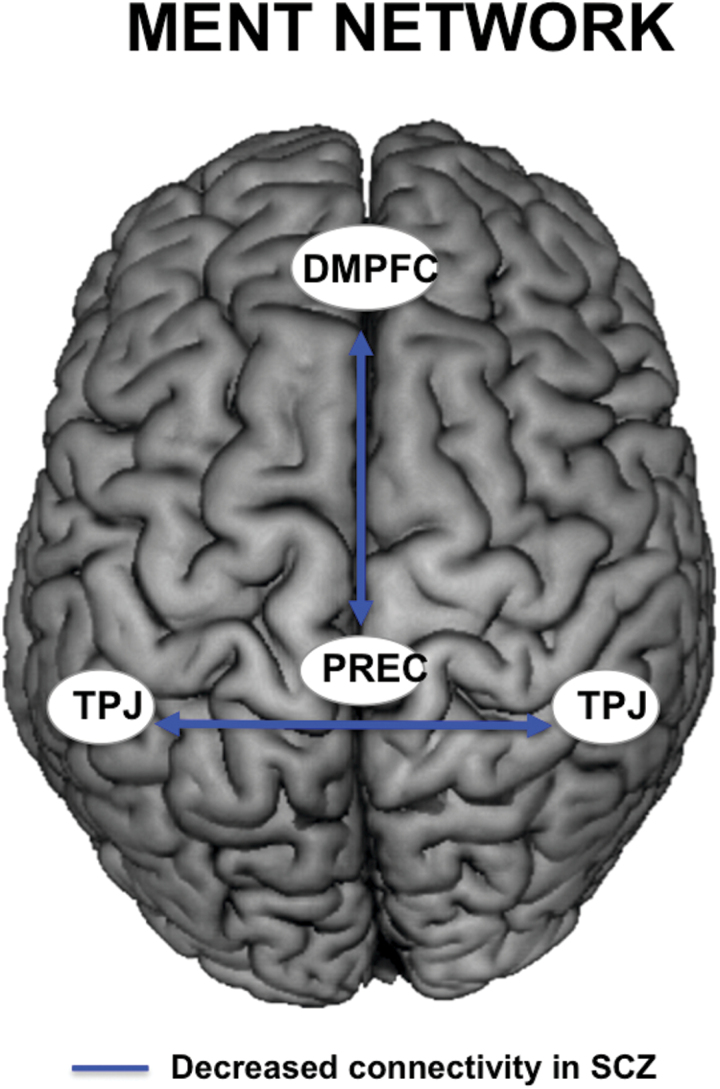
For the MENT network, significant differences in functional connectivity (P < .05, Bonferroni corrected for multiple comparisons across all connections) were observed between patients and controls, indicating decreased functional connectivity between the precuneus (PREC) and dorsomedial prefrontal cortex (DMPFC), as well as left temporo-parietal junction (TPJ) with right TPJ in the patient group (see figure 3 and table 5 for details).
Fig. 3.
Schematic illustration of significant resting state functional connectivity (RSFC) differences in the mentalizing network (MENT) network, depicting reduced RSFC in patients. TPJ: temporo-parietal junction, PREC: precuneus, DMPFC: dorsomedial prefrontal cortex.
Correlations With Clinical Characteristics
Correlational analyses further indicated statistically significant relationships between measures of functional connectivity within the MNS and clinical characteristics in the examined group of SCZ patients: With regard to overall symptom severity (PANSS total) we observed a negative relationship with RSFC of right inferior parietal lobe (IPL) and left V5 (r = −.27, P = .003; figure 2). For PANSS general, a negative relationship with RSFC between right IPL and left IPS and left V5 was observed (right IPL – left V5: r = −.25, P = .007; left IPS – left V5: r = −.24, P = .011; figure 2). No statistically significant relationships between measures of functional connectivity and clinical characteristics were observed for the MENT network. No significant correlations emerged with duration of illness or CPZ-equivalents (all Ps > .139).
Gray Matter Volumes: Group Differences
Significant group differences in gray matter volume emerged for the DMPFC (t = 4.376, P < .001), right BA44 (t = 4.439, P < .001), right IPS (t = 2.993, P = .003), as well as for left STG (t = 4.082, P < .001) correction indicating smaller volumes in patients. No other comparison survived Bonferroni correction (all Ps > .040).
Additional analyses on the significant group differences in RSFC by controlling for gray matter volume differences in these regions revealed the same pattern as described above and no significant effect of volume (DMPFC: P = .828; right BA44: P > .198; left MTG: P > .469).
Gray Matter Volumes: Correlation Analyses With Clinical Parameters and Duration of Illness
Correlation analyses revealed significant negative associations between PANSS negative and PANSS total scores and right BA44 volume (PANSS negative: r =−.239, P = .012; PANSS total: r = −.256, P = .007). No other correlation with symptom severity or CPZ-equivalents reached significance after Bonferroni correction (all Ps > .019). Furthermore, significant negative correlations with duration of illness and gray matter volume were observed (DMPFC: r = −.324, P = .003; right BA44: r = −.362, P = .001; left IPS: r = −.350, P = .001; left MTG: r = −.342, P = .002).
Discussion
Here, we assessed RSFC aberrations in SCZ patients using 2 a priori defined neural networks, which are thought to constitute the neural substrates of social cognition (MNS and MENT). Furthermore, we investigated correlations with clinical characteristics based upon the idea that RSFC aberrations in the different networks could be related to different symptom dimensions8,10 and use measures of gray matter volume to control the possible influence of volumetric group differences on RSFC. Our results demonstrated region-specific differences in the functional connectivity profiles of the MNS and the MENT network when comparing SCZ patients to HC. Moreover, connectivity within the MNS, but not the MENT, was related to clinical symptoms in the patient group. In sum, these findings demonstrate that differential patterns of functional dysconnectivity exist in SCZ, which affect both investigated networks.
Dysconnectivity of the MNS in SCZ
Visual Cortex (V5).
As a key finding our results demonstrate reduced connectivity of most MNS regions with V5 bilaterally. V5 is richly connected with cortical and subcortical structures53 and has long been known to be of particular importance for the processing of visual motion54,55 and eye-movements56 as it appears to integrate local visual motion signals into the global motion percept of complex objects. Deficits in the magnocellular visual pathway have been reported in SCZ indicating a global visual processing deficit.57,58 Specifically, velocity discrimination seems to be disturbed.59 Investigation of the neural correlates of speed and direction discrimination point to the posterior extrastriate cortex (V5), with diminished activation of this region in patients.60 Reduced connectivity of visual areas has been reported during task-performance,61,62 also affecting memory performance.63 Previous studies investigating resting-state functional connectivity frequently reported reduced connectivity of visual areas in SCZ.41,64,65
Fusiform Gyrus.
Apart from V5, we also observed dysconnectivity of the fusiform gyrus. While not usually described as a “classical” MNS area, the fusiform face and body area have been shown to be consistently involved in studies of non-hand action observation.35 Consistent with our findings, recent studies have demonstrated dysconnectivity of the fusiform gyrus in SCZ.64,66 Furthermore, a loss of gray matter volume in this brain region has also been described in SCZ,67 which—in the absence of gray matter volume loss in this region in our cohort—raises the possibility that functional connectivity alterations may actually be a precursor to brain atrophy. The observed dysconnectivity of FG with SMA in the current study, may not only be important for action observation and consequent action preparation, but also recognition of “abstract” motor behavior.68 More precisely, deficits in facial emotion recognition (for review see Kohler et al69), empathy (behavior70,71; neural activation72,73) and approach-avoidance tendencies74 are well-documented in SCZ patients. Future studies might want to directly test this assumption by linking behavioral performances with resting-state connectivity.
Middle Temporal Gyrus.
The MTG is known to be involved in biological motion,75 while BA44 is mostly responsible for the initiation and termination of simple actions.76 While we assume that the connectivity between FG and SMA might be particularly essential for face processing and consequent preparation of actions, the connectivity between MTG and BA44 might be particularly essential for the processing and imitation of simple action movements. This fits to the observed difficulties in simple hand and mouth imitation in SCZ.77 However, authors also observed a deficit in complex emotional face imitation in SCZ. Here it would be interesting to see, whether complex and simple imitation deficits are associated with different neural networks.
Taken together our findings of decreased functional connectivity within the MNS support the assumption that dynamic action processing is deficient in SCZ. The observed dysconnectivity within the MNS may play a critical role in the frequently reported impairments in imitation, simulation, learning, and execution of action in SCZ, thus basic competencies helping us to understand the actions and intentions of others and to learn new skills.
Dysconnectivity of the MENT in SCZ
For the MENT, decreased functional connectivity between the PREC and DMPFC, as well as bilateral TPJ in SCZ was evident.
Precuneus.
Activation of the PREC has frequently been linked to spatial attention, mental and motor imagery and memory-related processes. Moreover, this region also plays a particular role when humans are engaged in self-related mental representations (ie, self-agency and self-processing), as well as social cognition.36,78,79 In particular, PREC has both been associated with top-down control of attention and indirectly with sensorimotor integration thereby providing a coherent self-representation across space and time.80 Dysfunctional activation of PREC in SCZ was reported in studies assessing attributional biases81 empathy40 and executive function.82
Dorsomedial Prefrontal Cortex.
The DMPFC has also been involved in various aspects of social cognition, including “theory of mind”83,84 and moral reasoning,85 but also “non-social” semantic processing86 and autobiographical memory retrieval.84 Thus, the DMPFC may also subserve sophisticated manipulations of social information. Both PREC and DMPFC have been described as key nodes of the so-called “default mode network” (DMN87) which—consistent with our findings—has previously been shown to exhibit substantial dysconnectivity in SCZ.65,88 Interestingly, DMN dysconnectivity has also been observed in drug-naïve first-episode patients,64 but seems to be intact in persons at high-risk for psychosis.89
In the current study, we observed significantly reduced connectivity between PREC and DMPFC in SCZ compared to HC. While the PREC may be critical to direct attention to self- or other-related mental representation, the DMPFC may render these representations by adding autobiographical memory or reasoning.
Temporo-Parietal Junction.
Apart from PREC and DMPFC, the TPJ, too, has been reported to play a pivotal role in social interaction, namely being involved in perspective-taking and empathy,84,90,91 “theory of mind,”36,92 directing attention to salient events,93 and self-agency.94,95 While these roles have mainly been ascribed to the right TPJ (for a review see Decety and Lamm96), several studies indicate that the left TPJ is also vitally engaged in processing socially meaningful cues such as gaze direction and goal-directed action,97,98 affective speech comprehension,99 perception of social threat,100 orientation of attention or retrieving stimulus-driven attention,101 and temporal order judgments.102 Reduced TPJ activation has previously been reported in SCZ (for a review see Bosia et al103).
Hence, our findings of decreased functional connectivity within the MENT may contribute to the impairments in directing attention to self- or other-related mental representations and the rendering of these via adding of autobiographical memory or reasoning.
Interhemispheric Dysconnectivity
Besides reduced connectivity of bilateral TPJ, we also observed functional alterations of interhemispheric connectivity of V5. Consistent with our findings of decreased RSFC, decreased interhemispheric connectivity of amygdala, orbitofrontal cortex and temporal pole,104 occipital regions, the thalamus and cerebellum,105 language-related cortical regions, particularly Broca’s area in the prefrontal cortex,106 and the PREC, the precentral gyrus, STG, middle occipital gyrus and fusiform gyrus.64
Taken together, interhemispheric dysconnectivity seems to involve a wide range of brain regions in SCZ, ranging from frontal to cerebellar, from cortical to subcortical parts of the brain. It has been proposed that the lateralized abnormality in SCZ can be explained either by hemispheric dysfunction, or by impaired interhemispheric coupling, leading to a failure of the dominant hemisphere to over-rule nondominant homologous areas.106 More evidence for this longstanding abnormal asymmetry hypothesis in SCZ comes from tract-tracing and automated imaging studies, which have consistently demonstrated the impaired integrity of white matter (WM) fibers interconnecting the 2 hemispheres, such as the corpus callosum (CC) and anterior commissure (AC).107 Interestingly, Penades and colleagues108 have suggested that treatment success after cognitive remediation therapy in SCZ patients could be related to an increase of interhemispheric information transfer between bilateral prefrontal cortices. It remains to be tested, whether therapeutic treatment and success also improve interhemispheric connections other than prefrontal cortices.
Correlations With Clinical Characteristics
With regard to correlations between measures of functional connectivity and clinical characteristics, we expected to find a relationship between MNS-based connectivity differences in SCZ and the negative (rather than positive) symptom dimension, while MENT-based connectivity was thought to be related to the positive (rather than negative) symptom dimension. These hypotheses were based on the suggestion of “hypermentalizing,” ie, an excess of mental state attribution and assignment of social meaning, as a characteristic feature of positive symptoms observed during acute psychotic episodes,8 which could be related to neurofunctional alterations in the MENT. The more permanent and arguably more fundamental alterations of social cognition that constitute a main reason why many patients with SCZ cannot live independently, hold jobs, establish personal relationships, and manage everyday social interactions, on the other hand, are thought be closely related to the negative symptom dimension10,31,109 and were hypothesized to be related to dysfunctional connectivity of the MNS, which normally provides an implicit sense of being in world that is shared and acted upon with others.
Our correlational analyses, however, demonstrated a negative and significant relationship for functional connectivity for the connections between right IPL and left V5 and the PANSS total score, as well as PANSS general score. Moreover, measures of connectivity between left IPL and left V5 were found to correlate with PANSS general (figure 2). Evidence has accumulated that connectivity of specific parts of the parietal cortex, in particular IPS and IPL, contributes to a finely tuned interplay between stimulus-oriented and stimulus-independent cognition.110 An alteration of these connections might, therefore, lead to impairments of the ability to flexibly produce attentional shifts, which are particularly relevant for our capacity to navigate the social environment.111,112 Given that we observe these correlations in the MNS, which is known to play an important role for providing a pre-reflexive sense of being and acting with others, it is tempting to interpret such alterations as contributing to—what has become known as—a “loss of natural evidence” for being in a world that is intersubjectively shared with others in patients with SCZ.9 However, including CPZ equivalents as covariate in these correlation analyses (partial correlations) diminished the significant associations to trend level (PANSS total) or even nonsignificant findings (PANSS general). Therefore, interactions of medication and symptom severity are apparent that have to be further analyzed in future studies.
Correlations of RSFC in the MENT were neither observed for PANSS negative, positive, general symptoms nor total scores. In our view, these findings suggest that it is alterations of RSFC in the MNS (rather than the MENT), which may present an essential and possibly enduring pathophysiological feature of SCZ. Against our expectations, we did not observe significant correlations with PANSS positive/negative symptom scores or other clinical characteristics such as duration of illness. Apparently, the initially assumed dichotomy of positive symptoms being particularly associated with alterations in the MENT and negative symptoms correlating specifically with alterations in the MNS may be too simplistic. Based on our findings, rather more general worries and the totality of symptoms as assessed with the PANSS general and total scale are related to alterations in functional connectivity, here in the MNS.
Gray Matter Volume
We observed significantly reduced gray matter volume in 4 of our target regions, ie, DMPFC, right BA44, right IPS, left MTG, in patients and thus resemble recent meta-analytic findings including morphometry data from 8327 medicated SCZ patients by Haijma et al.113 Notably, these volume changes do not explain the reported RSFC findings, indicating that volume and connectivity measures are somewhat independent from each other.
Moreover, performing correlation analyses between gray matter volume and clinical parameters revealed significant negative associations with PANSS negative and total scores and right BA44 volume, ie, the higher the scores the more reduced the volume. Additionally, significant negative correlations with duration of illness and gray matter volume of DMPFC, right BA44, left IPS, and left MTG occurred. Our data thus support the notion that gray matter volume changes in SCZ are coupled to illness progress and symptom severity114 but cannot explain reduced RSFC in MNS and MENT networks. However, longitudinal studies that gather both, RSFC and brain morphometry data are necessary to clarify this issue.
Limitations
As listed in table 3, all but 1 patient were medicated in our study. Antipsychotic drugs are a common covariate in this field of research and have been shown to alter RSFC,115 as well as functional activation in SCZ.116 Hence, antipsychotic treatment could be a confounding factor and more research on duration of intake, dosage and compliance needs to be performed in future studies. In this study we focused on characterizing 2 distinct neural networks in SCZ. To do so, we restricted our analysis on an a priori selection of brain regions. Due to this approach, other neural networks and their connectivity were not analyzed. Another limitation is that we do not have behavioral measures of social cognitive abilities or social functioning73 that could further support our assumption that these dysfunctions in RSFC of MENT and MNS contribute significantly to the social impairments associated with the disorder.
Moreover, we were not able to collect information from all sites whether participants were instructed to keep their eyes open or closed. This instruction however may change connectivity patterns as has been shown previously.117 Previous resting-state fMRI studies in SCZ have mostly focused on the “default mode network” reporting mixed results, ranging from increased connectivity to expansion of the DMN, but also reduced connectivity has been reported.30 Particularly connectivity between the posterior cingulate cortex and the precuneus, 2 key hubs in the DMN, has been targeted and DMN dysfunction has been postulated to be a central neurobiological feature of the disorder.41 However, using data-driven methods (eg, ICA) makes it harder to compare findings across different patient samples, because the network architecture is detected in relationship to a given data set. In our study, we circumvent this issue by using meta-analytically defined networks in a hypothesis-driven manner to investigate functional connectivity. Regarding the significant group differences, we only observed decreased connectivity, which fits to results from seed-based RSFC analyses in SCZ (for review Yu et al30). Future studies might want to address and compare the meta-analytically derived networks with the DMN or other ICA-derived networks in order to better characterize the contribution of alterations within these networks to the current symptomatology and pathology of SCZ.
Conclusion
Taken together, our findings indicate that alterations of RSFC in the MNS and the MENT may present an important pathophysiological feature of SCZ. In particular, our data suggest that V5 and fusiform gyrus play a major role regarding functional connectivity alterations in the MNS network in SCZ. In particular, the observed neurofunctional alterations may lead to impairments of social interaction by disallowing for the rapid integration of sensory processing with the motor system (MNS) and difficulties directing attention to self- or other-related mental representation, as well as reasoning about them (MENT). Within the MNS we also observed significant correlations with general severity of symptomatology suggesting that aberrant connectivity may also be linked to dysfunctional social cognition in SCZ that is not simply linked to positive or negative symptoms but more general disease-related burden. In conclusion, our findings demonstrate that analyses of RSFC can advance our understanding of the neural bases of social cognitive dysfunctions in SCZ and point towards network-specific dysconnectivity patterns.
Based on the assumption that psychiatric disorders can be reconstructed as disorders of social cognition, attempts have been made to relate social cognitive deficits in patient populations to possible dysfunctions of the “social brain.” While there is great merit to be found in this approach, it may have underrepresented those processes that are most relevant for successful participation in real-time social interactions. Consequently, the knowledge of alterations of neurofunctional systems which are relevant for successful participation in real-life social interaction is still limited, which has impeded translational social neuroscience approaches. Future research, therefore, needs to examine how these specific disruptions of connectivity contribute to impaired social cognition and, in particular, real-life social functioning in SCZ. Moreover, further research is mandatory to investigate whether and how the network-specific dysconnectivity patterns reported here could be used to contribute to, evaluate or guide treatment regimes and their focus on either biological or social and psychotherapeutic factors.
Funding
L.S. was supported by the Volkswagen Foundation, the Deutsche Forschungsgemeinschaft (DFG) and the Max-Planck Society (MPG). B.D. was supported by JARA BRAIN and the DFG (DE 2319/2-3). S.B.E. was supported by the DFG (EI 816/4-1, LA 3071/3-1; EI 816/6-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 604102 (Human Brain Project).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 2. Eaton WW, Thara R, Federman B, Melton B, Liang KY. Structure and course of positive and negative symptoms in schizophrenia. Arch Gen Psychiatry. 1995;52:127–134. [DOI] [PubMed] [Google Scholar]
- 3. Derntl B, Habel U. Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci. 2011;261:S145–149. [DOI] [PubMed] [Google Scholar]
- 4. Billeke P, Aboitiz F. Social cognition in schizophrenia: from social stimuli processing to social engagement. Front Psychiatry. 2013;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Neurosci Rev. 2015;16:620–631. [DOI] [PubMed] [Google Scholar]
- 7. Pinkham AE. Social cognition in schizophrenia. J Clin Psychiatry. 2014;75:14–19. [DOI] [PubMed] [Google Scholar]
- 8. Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–389. [DOI] [PubMed] [Google Scholar]
- 9. Blankenburg W. The Loss of Natural Self-Evidence: A Contribution to the Study of Symptome-Poor Schizophrenias. Berlin, Germany: Parodos; 1971. [Google Scholar]
- 10. Salvatore G, Dimaggio G, Lysaker PH. An intersubjective perspective on negative symptoms of schizophrenia: implications of simulation theory. Cogn Neuropsychiatry. 2007;12:144–164. [DOI] [PubMed] [Google Scholar]
- 11. Fulford D, Niendam TA, Floyd EG, et al. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr Res. 2013; 147:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–457. [DOI] [PubMed] [Google Scholar]
- 13. Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. [DOI] [PubMed] [Google Scholar]
- 14. Schilbach L, Wilms M, Eickhoff SB, et al. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci. 2010;22:2702–2715. [DOI] [PubMed] [Google Scholar]
- 15. Schilbach L, Eickhoff SB, Cieslik EC, Kuzmanovic B, Vogeley K. Shall we do this together? Social gaze influences action control in a comparison group, but not in individuals with high-functioning autism. Autism. 2012;16:151–162. [DOI] [PubMed] [Google Scholar]
- 16. Gallese V. Motor abstraction: a neuroscientific account of how action goals and intentions are mapped and understood. Psychol Res. 2009;73:486–498. [DOI] [PubMed] [Google Scholar]
- 17. Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. [DOI] [PubMed] [Google Scholar]
- 18. Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. [DOI] [PubMed] [Google Scholar]
- 19. Newman-Norlund RD, van Schie HT, van Zuijlen AM, Bekkering H. The mirror neuron system is more active during complementary compared with imitative action. Nat Neurosci. 2007;10:817–818. [DOI] [PubMed] [Google Scholar]
- 20. Shibata H, Inui T, Ogawa K. Understanding interpersonal action coordination: an fMRI study. Exp Brain Res. 2011;211:569–579. [DOI] [PubMed] [Google Scholar]
- 21. Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol. 2007;17:1527–1531. [DOI] [PubMed] [Google Scholar]
- 22. Heyes C. Where do mirror neurons come from? Neurosci Biobehav Rev. 2010;34:575–583. [DOI] [PubMed] [Google Scholar]
- 23. Schilbach L, Wohlschlaeger AM, Kraemer NC, et al. Being with virtual others: neural correlates of social interaction. Neuropsychologia. 2006;44:718–730. [DOI] [PubMed] [Google Scholar]
- 24. Schilbach L, Timmermans B, Reddy V, et al. Toward a second-person neuroscience. Behav Brain Sci. 2013;36:393–414. [DOI] [PubMed] [Google Scholar]
- 25. Buccino G, Amore M. Mirror neurons and the understanding of behavioural symptoms in psychiatric disorders. Curr Opin Psychiatry. 2008;21:281–285. [DOI] [PubMed] [Google Scholar]
- 26. Guo S, Kendrick KM, Yu R, Wang HL, Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp. 2012;35:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehta UM, Basavaraju R, Thirthalli J, Gangadhar BN. Mirror neuron dysfunction-a neuro-marker for social cognition deficits in drug naïve schizophrenia. Schizophr Res. 2012;141:281–283. [DOI] [PubMed] [Google Scholar]
- 28. McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. 2012;201:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meda SA, Gill A, Stevens MC, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu Q, Allen EA, Sui J, Arbabshirani MR, Pearlson G, Calhoun VD. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Curr Top Med Chem. 2012;12:2415–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 32. Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. [DOI] [PubMed] [Google Scholar]
- 33. Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schilbach L, Bzdok D, Timmermans B, et al. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 39. Schilbach L. Towards a second-person neuropsychiatry. Philos Trans R Soc Lond B Biol Sci. 2016;371:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Derntl B, Finkelmeyer A, Voss B, et al. Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res. 2012;136:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 43. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 45. Bandettini PA, Bullmore E. Endogenous oscillations and networks in functional magnetic resonance imaging. Hum Brain Mapp. 2008;29:737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB. Resting state functional connectivity in patients with chronic hallucinations. PLoS One. 2012;7:e43516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeh CJ, Tseng YS, Lin YR, Tsai SY, Huang TY. Resting-state functional magnetic resonance imaging: the impact of regression analysis. J Neuroimaging. 2015;25:117–123. [DOI] [PubMed] [Google Scholar]
- 52. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 53. Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. [DOI] [PubMed] [Google Scholar]
- 54. Dubner R, Zeki SM. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971;35:528–532. [DOI] [PubMed] [Google Scholar]
- 55. Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dürsteler MR, Wurtz RH, Newsome WT. Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey. J Neurophysiol. 1987;57:1262–1287. [DOI] [PubMed] [Google Scholar]
- 57. Johnson SC, Lowery N, Kohler C, Turetsky BI. Global-local visual processing in schizophrenia: evidence for an early visual processing deficit. Biol Psychiatry. 2005;58:937–946. [DOI] [PubMed] [Google Scholar]
- 58. Martínez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb Cortex. 2012;22:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen Y, Levy DL, Sheremata S, Holzman PS. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophr Res. 2006;88:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takahashi H, Kato M, Sassa T, et al. Functional deficits in the extrastriate body area during observation of sports-related actions in schizophrenia. Schizophr Bull. 2010;36:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kang SS, Sponheim SR, Chafee MV, MacDonald AW. III Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia. 2011;49:2836–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia. Arch Gen Psychiatry. 2010;67:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bittner RA, Linden DE, Roebroeck A, et al. The when and where of working memory dysfunction in early-onset schizophrenia – a functional magnetic resonance imaging study. Cereb Cortex. 2015;25:494–506. [DOI] [PubMed] [Google Scholar]
- 64. Guo W, Yao D, Jiang J, et al. Abnormal default mode network homogeneity in first-episode, drug-naïve schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:16–20. [DOI] [PubMed] [Google Scholar]
- 65. Littow H, Huossa V, Karjalainen S, et al. Aberrant functional connectivity in the default mode and central executive networks in subjects with schizophrenia – a whole-brain resting-state ICA study. Front Psychiatry. 2015;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Argyelan M, Ikuta T, DeRosse P, et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takahashi H, Sassa T, Shibuya T, et al. Effects of sports participation on psychiatric symptoms and brain activations during sports observations in schizophrenia. Transl Psychiatry. 2012;2:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Binkofski F, Amunts K, Stephan KM, et al. Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp. 2000;11:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Derntl B, Finkelmeyer A, Toygar TK, et al. Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res. 2009;108:197–206. [DOI] [PubMed] [Google Scholar]
- 71. Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41:2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harvey PO, Zaki J, Lee J, Ochsner K, Green MF. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr Bull. 2013;39:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Smith MJ, Schroeder MP, Abram SV, et al. Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophr Bull. 2015;41:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Radke S, Pfersmann V, Derntl B. The impact of emotional faces on social motivation in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2015;265:613–622. [DOI] [PubMed] [Google Scholar]
- 75. Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. 2003;358:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koechlin E, Jubalt T. Broca’s area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. [DOI] [PubMed] [Google Scholar]
- 77. Park S, Matthews N, Gibson C. Imitation, simulation, and schizophrenia. Schizophr Bull. 2008;34:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koenigsberg HW, Fan J, Ochsner KN, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009;66:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends Cogn Sci. 2003;7:38–42. [DOI] [PubMed] [Google Scholar]
- 80. Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee TY, Hong SB, Shin NY, Kwon JS. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr Res. 2015;164:28–34. [DOI] [PubMed] [Google Scholar]
- 82. Liemburg EJ, Dlabac-De Lange JJ, Bais L, et al. Neural correlates of planning performance in patients with schizophrenia – relationship with apathy. Schizophr Res. 2015;161:367–375. [DOI] [PubMed] [Google Scholar]
- 83. Mar RA. The neural basis of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. [DOI] [PubMed] [Google Scholar]
- 84. Spreng RN, Mar RA, Kim SA. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. [DOI] [PubMed] [Google Scholar]
- 85. Bzdok D, Schilbach L, Vogeley K, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 88. Landin-Romero R, McKenna PJ, Salgado-Pineda P, et al. Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol Med. 2015;45:1315–1325. [DOI] [PubMed] [Google Scholar]
- 89. Heinze K, Reniers RL, Nelson B, et al. Discrete alterations of brain network structural covariance in individuals at ultra-high risk for psychosis. Biol Psychiatry. 2015;77:989–996. [DOI] [PubMed] [Google Scholar]
- 90. Derntl B, Finkelmeyer A, Eickhoff SB, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82 [DOI] [PubMed] [Google Scholar]
- 91. Schulte-Rüther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42:393–403. [DOI] [PubMed] [Google Scholar]
- 92. Döhnel K, Schuwerk T, Meinhardt J, Sodian B, Hajak G, Sommer M. Functional activity of the right temporo-parietal junction and of the medial prefrontal cortex associated with true and false belief reasoning. Neuroimage. 2012;60:1652–1661. [DOI] [PubMed] [Google Scholar]
- 93. Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. Eur J Neurosci. 2006;23:91–596. [DOI] [PubMed] [Google Scholar]
- 94. Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist. 2005;11:16–24. [DOI] [PubMed] [Google Scholar]
- 95. Seidel EM, Eickhoff SB, Kellermann T, et al. Who is to blame? Neural correlates of causal attribution in social situations. Soc Neurosci. 2010;5:335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. [DOI] [PubMed] [Google Scholar]
- 97. Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. [DOI] [PubMed] [Google Scholar]
- 98. Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–1695. [DOI] [PubMed] [Google Scholar]
- 99. Hervé PY, Razafimandimby A, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. Disentangling the brain networks supporting affective speech comprehension. Neuroimage. 2012;61:1255–1267. [DOI] [PubMed] [Google Scholar]
- 100. Kret ME, Denollet J, Grèzes J, de Gelder B. The role of negative affectivity and social inhibition in perceiving social threat: an fMRI study. Neuropsychologia. 2011;49:1187–1193. [DOI] [PubMed] [Google Scholar]
- 101. Ravizza SM, Hazeltine E, Ruiz S, Zhu DC. Left TPJ activity in verbal working memory: implications for storage- and sensory-specific models of short term memory. Neuroimage. 2011;55:1836–1846. [DOI] [PubMed] [Google Scholar]
- 102. Davis B, Christie J, Rorden C. Temporal order judgments activate temporal parietal junction. J Neurosci. 2009;29:3182–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bosia M, Riccaboni R, Poletti S. Neurofunctional correlates of theory of mind deficits in schizophrenia. Curr Top Med Chem. 2012;12:2284–2302. [DOI] [PubMed] [Google Scholar]
- 104. Pu W, Rolls ET, Guo S, et al. Altered functional connectivity links in neuroleptic-naïve and neuroleptic-treated patients with schizophrenia, and their relation to symptoms including volition. Neuroimage Clin. 2015;6:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hoptman MJ, Zuo XN, D’Angelo D, et al. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res. 2012;141:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M, Faragian S, Hendler T. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr Res. 2012;134:131–136. [DOI] [PubMed] [Google Scholar]
- 107. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2011;129:149–155. [DOI] [PubMed] [Google Scholar]
- 108. Penades R, Pujol N, Catalan R, et al. Brain effects of cognitive remediation therapy in schizophrenia: a structural and functional neuroimaging study. Biol Psychiatry. 2013;73:1015–1023. [DOI] [PubMed] [Google Scholar]
- 109. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146:231–237. [DOI] [PubMed] [Google Scholar]
- 110. Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schwarzkopf S, Schilbach L, Vogeley K, Timmermans B. “Making it explicit” makes a difference: evidence for a dissociation of spontaneous and intentional level 1 perspective taking in high-functioning autism. Cognition. 2014;131:345–354. [DOI] [PubMed] [Google Scholar]
- 113. Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Frascarelli M, Tognin S, Mirigliani A, et al. Medial frontal gyrus alterations in schizophrenia: relationship with duration of illness and executive function. Psychiatry Res. 2015;231:103–110. [DOI] [PubMed] [Google Scholar]
- 115. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naïve first episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 116. Abbot C, Juarez M, White T, et al. Antipsychotic dose and diminished neural modulation: a multi-site fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Song X, Zhou S, Zhang Y, Liu Y, Zhu H, Gao JH. Frequency-dependent modulation of regional synchrony in the human brain by eyes open and eyes closed resting-states. PLoS One. 2015;10:e0141507. [DOI] [PMC free article] [PubMed] [Google Scholar]