Abstract
When studying selective attention in people with schizophrenia (PSZ), a counterintuitive but replicated finding has been that PSZ display larger performance benefits than healthy control subjects (HCS) by cues that predicts the location of a target stimulus relative to non-predictive cues. Possible explanations are that PSZ hyperfocus attention in response to predictive cues, or that an inability to maintain a broad attentional window impairs performance when the cue is non-predictive. Over-recruitment of regions involved in top-down focusing of spatial attention in response to predictive cues would support the former possibility, and an inappropriate recruitment of these regions in response to non-predictive cues the latter. We probed regions of the dorsal attention network while PSZ (N = 20) and HCS (N = 20) performed a visuospatial attention task. A central cue either predicted at which of 4 peripheral locations a target signal would appear, or it gave no information about the target location. As observed previously, PSZ displayed a larger reaction time difference between predictive and non-predictive cue trials than HCS. Activity in frontoparietal and occipital regions was greater for predictive than non-predictive cues. This effect was almost identical between PSZ and HCS. There was no sign of over-recruitment when the cue was predictive, or of inappropriate recruitment when the cue was non-predictive. However, PSZ differed from HCS in their cue-dependent deactivation of the default mode network. Unexpectedly, PSZ displayed significantly greater deactivation than HCS in predictive cue trials, which may reflect a tendency to expend more processing resources when focusing attention in space.
Key words: fMRI, default network, top-down
Introduction
The widely held assumption that schizophrenia involves deficits in selective attention has been challenged by reports that people with schizophrenia (PSZ) are unimpaired at directing and limiting attention to relevant subportions of the available visual input. This has been demonstrated both when items are selected based on their physical properties and when attention is focused spatially.1–3 Particularly surprising were results from spatial attention paradigms based on Posner4 in which a cue directs attention to 1 of 2 possible target locations. Here, the reaction time (RT) benefit conferred by a cue predicting the target location, relative to a non-predictive cue, actually tended to be larger in PSZ than in healthy control subjects (HCS),5–9 suggesting greater attentional selection. Consistent with this, it has been suggested that PSZ have a narrowed “attentional spotlight” and difficulty maintaining a wide visual span.10
We followed up on these findings with a visuospatial attention paradigm, the Spatial Attentional Resource Allocation Task (SARAT), in which a central cue predicts the location of a peripheral target stimulus (figure 1A). One, 2, or all 4 possible target locations could be cued simultaneously, manipulating the degree to which attention had to be focused narrowly or distributed broadly. Both HCS and PSZ displayed step-wise faster RT with more precise cueing. However, this effect was substantially larger in PSZ than in HCS.11 Potential explanations for this finding are that (1) PSZ “hyperfocused” the location to which a predictive cue directed their attention, resulting in disproportionate RT benefits in predictive cue trials, or (2) PSZ had difficulty distributing attention broadly, resulting in greater RT costs when there was no advance information about the target location. The above study provided some evidence supporting the second explanation. For example, RT of PSZ was slower when the cue was non-predictive than when the cue predicted the wrong target location, indicating that trying to monitor all 4 locations was more deleterious to performance even than focusing attention away from the location of the upcoming target. However, other recent studies have also suggested a tendency of PSZ to hyperfocus processing resources.12–14
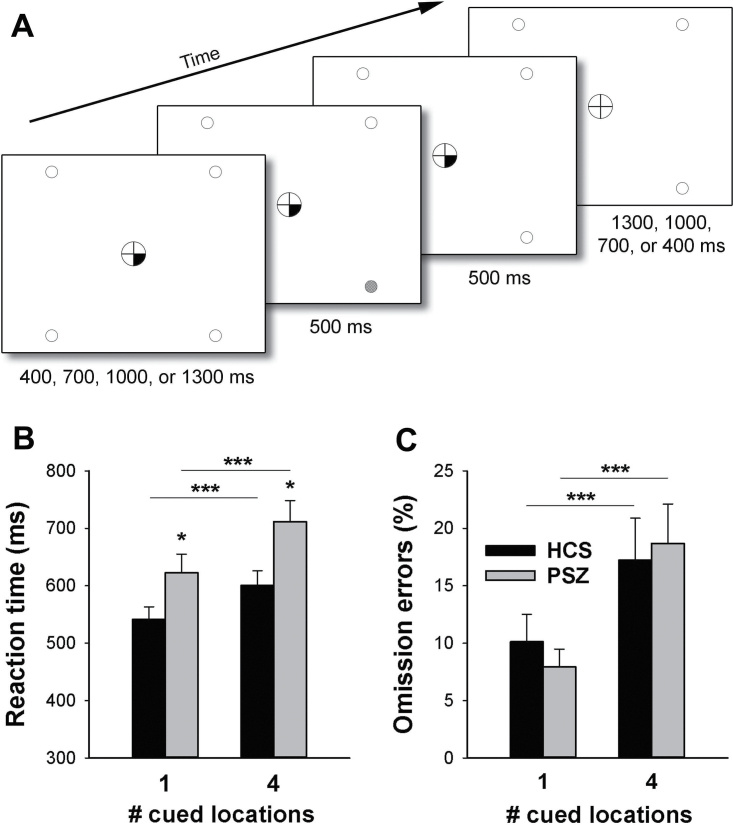
Fig. 1.
(A) Components of a Spatial Attentional Resource Allocation Task (SARAT) trial. Target onset was preceded by a central cue. Either 1 or all 4 locations could be cued. On one-third of trials, the cue was not followed by a target. (B) Average (±SEM) reaction time in people with schizophrenia (PSZ) and healthy control subjects (HCS). (C) Average (±SEM) omission errors, expressed as percentage of all trials. *P < .05, ***P < .001, in paired or independent-samples t test.
A way of shedding light onto the nature of the disproportionately large spatial cueing effect in PSZ is by studying brain activity differences relative to HCS that accompany this phenomenon. In a previous functional Magnetic Resonance Imaging (fMRI) study of the SARAT in healthy participants,15 we have shown that brain regions typically recruited by top-down attentional resource allocation,16–18 often termed the dorsal attention network,19–21 display step-wise greater activation with fewer cued target locations, ie, with a progressively narrow attentional focus. Specifically, middle and superior frontal gyri (MFG, SFG), intraparietal sulcus (IPS), superior and inferior parietal lobule (SPL, IPL), precuneus, and middle and inferior occipital gyri (MOG, IOG) displayed more activation in response to more predictive cues. A possible mechanism underlying the greater spatial cueing effect in PSZ, consistent with the hyperfocusing explanation, would be an over-recruitment of this top-down attention orienting network in response to predictive cues. A second possible mechanism would be an inappropriate recruitment of these regions when the cue does not provide information about where to focus attention. This would be consistent with an inability to spread attention widely and a serial focusing of individual target locations.
A third potential mechanism involves the default mode network (DMN), a set of brain regions that deactivates during various external processing tasks relative to an unconstrained resting state, thought to subserve stimulus-independent thought processes such as mind-wandering.22,23 The down-regulation of DMN activity is necessary for successful cognitive task performance.24–30 Accordingly, enhanced cue-induced DMN deactivation in the SARAT was associated with performance benefits in HCS.31 A range of studies reported that task-induced DMN deactivation was reduced in PSZ,32–40 which may impact task performance.
In the present study, we performed fMRI during performance of the SARAT to compare activity induced by spatially predictive and non-predictive cues between PSZ and HCS. The focus was on typical top-down attentional orienting regions and the DMN.
Methods
Participants
Twenty-four outpatients meeting Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV)41 criteria for schizophrenia (N = 23) or schizoaffective disorder (N = 1), and 22 HCS completed this study. Diagnosis was established using a best estimate approach combining information from a Structured Clinical Interview for DSM-IV (SCID) with a review of medical records. Data from 4 PSZ and 2 HCS were excluded from analyses due to excessive head motion or suspected sleeping in the scanner, resulting in N = 20 per group. Table 1 summarized demographic information of these participants. Groups did not differ in age, sex, ethnicity, or parental education, although PSZ tended to have fewer years of education. PSZ scored lower than HCS on most neuropsychological tests of cognitive functioning (table 1). Because blood pressure can affect blood oxygen-level dependent (BOLD) responses to neuronal activation,42 we compared PSZ and HCS on systolic and diastolic blood pressure taken during screening (P > .7 in each case). Smoking status also did not differ between groups (table 1).
Table 1.
Group Demographics
| PSZ (N = 20) | HCS (N = 20) | Statistic, P-value | |
|---|---|---|---|
| Age | 35.4±10.6 (range 20–53) | 37.5±13.5 (range 19–55) | t(38) = 0.53, P = .60 |
| Male: Female | 13: 7 | 12: 8 | χ2 = 0.11, P = .74 |
| Afr Am: Cauc: Other | 5: 14: 1 | 7: 12: 1 | χ2 = 0.49, P = .78 |
| Education (y) | 12.8±2.7 | 14.1±1.7 | t(38) = 1.82, P = .08 |
| Parental education (y)a | 13.4±2.9 | 14.1±2.1 | t(38) = 0.94, P = .36 |
| Estimated IQb,f | 104.4±13.9 | 114.9±8.4d | t(36) = 2.77, P < .01 |
| MCCBc,f | 38.4±13.5 | 53.1±7.7d | t(36) = 4.08, P < .001 |
| WRAT 4d,f | 98.8±14.2 | 110.1±12.5 | t(36) = 2.59, P < .02 |
| WTARe,f | 101.3±18.8 | 110.8±10.3 | t(36) = 1.90, P = .066 |
| Systolic blood pressure (mm Hg) | 126 | 125 | t(38) = 0.77, P > .7 |
| Diastolic blood pressure (mm Hg) | 76 | 75 | t(38) = 0.80, P > .7 |
| Current smokers (total) | 4 | 2 | χ2 = 0.78, P > .3 |
| FTNDg of current smokers | 4 | 4 | P = 1 |
Note: PSZ, people with schizophrenia; HCS, healthy control subjects.
aAverage over maternal and paternal education.
bBased on vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence.43
cComposite score on the MATRICS Consensus Cognitive Battery.
dWide Range Achievement Test.44
eWechsler Test of Adult Reading.45
fData missing for 2 HCS.
gFagerstrom Test for Nicotine Dependence.46
PSZ scored 33.5±7.4 (mean ± SD) on the Brief Psychiatric Rating Scale (BPRS)47 (range 21–48), 32.0±15.5 on the Scale for the Assessment of Negative Symptoms (SANS)48 (range 7–68), and 21.8±6.0 on the Level Of Functioning Scale (LOFS)49 (range 12–31). All PSZ were receiving second-generation antipsychotic medication. Thirteen PSZ additionally received antidepressant medication, 2 mood stabilizers, 5 anxiolytic, and 2 antiparkinsonian medication. Medication had not changed in the preceding 4 weeks. Drug or alcohol abuse was exclusionary. HCS were recruited via random digit dialing, word of mouth, and online advertising, and had no Axis 1 or 2 diagnoses as established by a SCID, had no self-reported family history of psychosis, and were not taking any psychotropic medication. Participants provided informed consent for a protocol approved by the Institutional Review Boards of the University of Maryland School of Medicine and the National Institute on Drug Abuse—Intramural Research Program. Before PSZ signed the consent form, the investigator, in the presence of a witness, formally evaluated basic understanding of study demands, risks, and what to do if experiencing distress or to end participation.
Procedure
During an initial training session, participants received task instructions, performed the full-length SARAT on a bench computer, and completed neuropsychological testing and questionnaires. The average number of days between this training and the MRI session was 13.6±17.2 (SD) days in HCS (range 1–80), and 11.6±9.1 days (range 2–34) in PSZ (t(38) = 0.46, P > .6). When disregarding 1 HCS who was scanned 80 days after training (resulting in 10.1±7.4 days, range 1–29), the groups were still matched (t(37) = 0.56, P > .5). Participants were instructed not to consume any caffeine on the scan day. Upon arrival, an alcohol breathalyzer reading was taken, and a urine drug test and, if female, pregnancy test were performed, all of which had to be negative.
The Spatial Attentional Resource Allocation Task (SARAT; figure 1A):
Participants fixated a central circle (3.0° diameter) containing a fixation cross and responded by button press to a target appearing at any of 4 peripheral locations marked by placeholders (1.5° diameter, centered at 12.5° eccentricity). The central circle and placeholders, black against white, remained on display throughout the task. A target consisted of 1 placeholder filling with a checkerboard of grey (20% contrast) and white squares of 3×3 pixels each. Upon detecting a target, participants pressed a button as quickly as possible.
A trial began with the presentation of a central cue. A 500-ms target was presented following a variable stimulus-onset-asynchrony of 400, 700, 1000, or 1300ms. The cue remained on display until 500ms after target offset. It consisted of either 1 or all 4 quarters of the fixation circle turning black, indicating that the target would appear in the corresponding quadrant of the display (predictive cue), or in any of the 4 quadrants (non-predictive cue). Thus, 1 cued location provided precise information about the target location, allowing for attention to be narrowly focused on the cued location, while 4 cued locations created spatial uncertainty and the need to monitor broadly.
In one-third of all trials, presented unpredictably, the cue was not followed by a target (cue-only trials). Cue-only trials were identical to the other trials, except that no target was presented during the 500-ms target interval. Because attention would be oriented in space in anticipation of a target, these trials enabled analysis of typical cue-induced activity, independent of target-induced activity. Our fMRI analyses focused on these trials.
All trials were followed by a variable intertrial interval (400–1300ms). There were eight 194-second task runs, separated by rest periods. In each run, there were 32 cue+target trials (16 each for 1 and 4 cued locations) and 16 cue-only trials (8 each for 1 and 4 cued locations), presented randomly. Temporal jitter was provided by 24 randomly interspersed 2.7-second no-event periods during which neither cue nor target stimuli were presented. Task completion took approximately 30 minutes.
During SARAT performance in the training session, eye-tracking was performed to monitor central fixation. Central fixation performance was somewhat poorer in PSZ than HCS but did not differ as a function of cue type in either group. Detailed eye-tracking methods and results are provided in the supplementary materials, including supplementary figure 1.
Magnetic Resonance Imaging
Scanning was performed on a 3 Tesla Siemens Tim Trio scanner. Whole-brain EPI images were acquired for measurement of T2*-weighted BOLD effects (4-mm oblique [30°] axial slices, 64×64 matrix, field-of-view [FOV] = 22×22cm, repetition time [TR] = 2.7s, echo time [TE] = 27ms). A sagittal T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1-mm3 voxels, TR = 1.9s, TE = 3.51ms, flip angle [FA] = 9°).
Data were processed using AFNI.50 Motion correction was performed by registering each volume to a base volume. The SARAT time series was analyzed as an event-related design by voxel-wise multiple regression. Regressors were expressed as a delta function, time-locked to the onset of each central cue, convolved with a model hemodynamic response function and its temporal derivative. The regressors of interest corresponded to cue-only trials with 1 or 4 cued locations. Nuisance regressors corresponded to cue+target trials with 1 or 4 cued locations, omission error trials, and the 6 motion parameters. For each subject, the voxel-wise average amplitude of signal change produced by cue-only trials with 1 or 4 cued locations was determined. These activation maps were re-sampled to a 1-μL resolution, converted to a standard stereotaxic coordinate system,51 and spatially blurred using a Gaussian 5-mm rms isotropic kernel.
For each subject, a composite motion index was calculated from the 6 motion correction parameters.52 These composite scores were compared between HCS and PSZ by independent-sample t test (equal variances not assumed, based on Levene’s test). There was a trend suggesting greater motion among PSZ then HCS (t(20.3) = 2.04, P = .055). Therefore, the composite motion index was entered as a covariate into second-level analyses.
Second-level analyses were performed on regions of interest (ROIs) sensitive to top-down attentional orienting, identified by voxel-wise paired t tests comparing cue-only trials with 1 and 4 cued locations. The t-test included all subjects, combining 20 HCS and 20 PSZ, to give equal weight to cue-sensitive voxels in either group and ensure that ROI selection did not favor either group. Using the 3dttest++ function in AFNI, the motion composite index was entered as a covariate. Voxel-wise P < .001 combined with a 2000-µL cluster threshold yielded overall P < .001 as determined by Monte Carlo simulation. Five contiguous clusters were identified. The largest cluster spanned both parietal hemispheres and was split along the midline. Activity was averaged within each of the resulting 6 ROIs, and analyzed by 3-factor ANOVA with Group (PSZ, HCS) as a between-subjects factor and Cue type (predictive, non-predictive) and ROI (1 through 6) as within-subject factors.
To select typical task-negative regions, but with the constraint that they were also deactivated by the present task, we created 7-mm-diameter spheres centered on peak foci of the task-negative network identified by Fox et al53 (Table 1, page 9676), and only spheres displaying at least 50% overlap with regions deactivated by the SARAT were further considered. Regions deactivated by the SARAT were identified by a t test against 0 performed on activity averaged over predictive and non-predictive cue trials (voxel-wise P < .001, 1000-µL cluster threshold). Again, the t test included all 20 HCS and 20 PSZ, giving equal weight to task-negative voxels in either group, and included the composite motion index as covariate. Seven out of 13 spheres displayed the required overlap. Activity was averaged within each of these ROIs and analyzed by 3-factor ANOVA (Group × Cue type × ROI), as for the top-down attention ROIs.
Results
Task performance during fMRI was analyzed by 2-factor ANOVA with Group (HCS, PSZ) as a between-subjects factor and Cue type (predictive, non-predictive) as a within-subject factor.
RT (figure 1B) was slower in non-predictive than in predictive cue trials (main effect of Cue type: F(1,38) = 110.6, P < .001) and slower in PSZ than in HCS (main effect of Group: F(1,38) = 5.37, P = .03). As in previous studies,11,54 the RT difference between cue types was larger in PSZ than in HCS, giving rise to a significant Group × Cue type interaction (F(1,38) = 4.57, P = .04). This held true even when RTs for each cue type were expressed as a proportion of each participant’s average RT across cue types (F(1,38) = 4.63, P = .04), thus controlling for the possibility that difference scores were larger in PSZ simply as a result of overall slower RTs.
Omission errors (figure 1C) were more numerous in non-predictive than predictive cue trials (F = 42.0, P < .001), but did not differ between PSZ and HCS (F(1,38) = 0.01, P > .9). There was no Group × Cue type interaction (F(1,38) = 1.74; P = .2).
Magnetic Resonance Imaging
Activity in the Dorsal Attention Network.
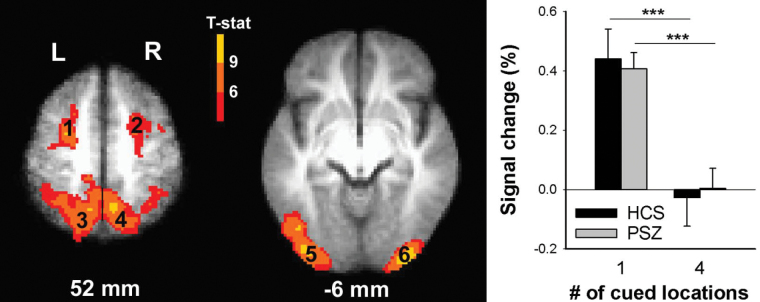
The 6 ROIs identified by contrasting predictive and non-predictive cue trials (table 2, figure 2) displayed close similarity with regions modulated by cue-induced attentional resource allocation, identified previously with this and other paradigms.15–18 Specifically, bilateral MFG and SFG; SPL, IPL and IPS; and MOG and IOG all displayed more activation in predictive cue trials than in non-predictive cue trials, consistent with involvement in top-down spatial focusing of attention. In 3-factor ANOVA (Group × Cue type × ROI), there was a significant main effect of Cue type (F(1,38) = 102.6, P < .001), but no main effect of Group (P > .9) and, importantly, no Group × Cue type interaction (F(1,38) < 1, P > .4), suggesting that PSZ and HCS did not differ in their cue-dependent engagement of these regions. Because there was no Group × Cue type × ROI interaction (P = .15), the graph in figure 2 represents the average activity over all 6 ROIs (see graphs for individual ROIs in supplementary figure 2).
Table 2.
Brain Regions Whose Activity Differed Between Predictive and Non-predictive Cue Trials
| Region | Side | Center of Mass (mm) RL, AP, IS | Brodmann Area(s) | Size (μl) | |||
|---|---|---|---|---|---|---|---|
| 1 | Middle & superior frontal gyrus | L | 25 | 1.3 | 54.5 | 6 | 5454 |
| 2 | Middle & superior frontal gyrus | R | −28.4 | −1.4 | 50.8 | 6 | 3563 |
| 3 | Intraparietal sulcus, inferior & superior parietal lobule, precuneus | L | 23.8 | 53.2 | 48.1 | 7, 40 | 26 770 |
| 4 | Intraparietal sulcus, inferior & superior parietal lobule, precuneus | R | −20.2 | 54.8 | 52.4 | 7, 40 | 13 996 |
| 5 | Middle & inferior occipital gyrus, inferior temporal gyrus | L | 36.9 | 82 | −1.5 | 18, 19, 37 | 11 878 |
| 6 | Middle & inferior occipital gyrus | R | −33.9 | 88.8 | −0.9 | 18 | 7556 |
Note: The numbering corresponds to that of figure 2. L = left; R = right.
Fig. 2.
Brain regions displaying a significant blood oxygen-level dependent (BOLD) signal difference between predictive and non-predictive cue trials across all 40 participants. Group activation maps are overlaid onto the average of all 40 anatomical scans in Talairach space. The numbering corresponds to regions of interest (ROIs) in table 2. The graph displays average (±SEM) cue-induced activity over all ROIs for each cue type in healthy controls subjects (HCS) and people with schizophrenia (PSZ). ***P < .001 in paired t test.
The weak trend towards a Group × Cue type × ROI interaction (P = .15) was followed up by 2-factor ANOVA in each ROI. Only the left occipitotemporal ROI displayed a Group × Cue type interaction (F(1,38) = 4.83, P = .03; P = .28 for right occipital; Ps > .6 for all other ROIs). This interaction was based on a smaller effect of cue type in PSZ than HCS, although both groups displayed a significant effect of cue type (t(19) > 5.7, P < .001 in each group), and activity did not differ between groups for either cue type (t(38) < 1, P > .3 in each case). To substantiate the overall absence of a group difference in the cueing effect, we tested whether effects of cue type may differ between groups in subregions of the ROIs, remaining undetected because activity was averaged over relatively large regions. To this end, we analyzed individual voxels of all ROIs by 2-factor ANOVA (Group × Cue type). Even with lenient thresholding of voxel-wise P < .05 and a 500-µL cluster threshold, the only region displaying a Group × Cue type interaction was located within the left occipitotemporal ROI, specifically in MOG/IOG (center-of-mass right/left [RL] = 35.2 mm, anterior/posterior [AP] = 85.9 mm, inferior/superior [IS] = −0.6 mm; 1781 µL). The pattern underlying the interaction was as described above for this region.
The above findings support neither an over-recruitment of the dorsal attention network in response to predictive cues in PSZ, nor an inappropriate recruitment when the cue does not provide information about where to focus attention. Thus, neither of these mechanisms appears to underlie the larger spatial cueing effect on RT in PSZ.
Task-negative ROIs.
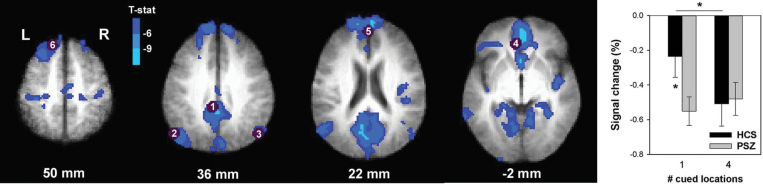
The 7 spherical ROIs (table 3, figure 3) were located in typical DMN regions,23,55,56 including rostromedial prefrontal cortex, SFG, posterior cingulate cortex, angular gyrus, and parahippocampal gyrus. Three-factor ANOVA revealed no main effect of Cue type (F(1,38) < 1, P > .3) and no main effect of Group (F(1,38) < 1, P > .3), but a significant Group × Cue type interaction was identified (F(1,38) = 4.23, P < .05). The absence of a Group × Cue type × ROI interaction (F(6,228) < 1, P > .4) indicated that the nature of the Group × Cue type interaction did not differ between ROIs. The graph in figure 3 thus represents the average activity over all task-negative ROIs. HCS displayed greater deactivation in non-predictive than predictive cue trials. PSZ presented significantly greater deactivation (“hyperdeactivation”) in predictive cue trials relative to HCS. This eliminated any difference between predictive and non-predictive cue trials in PSZ. This overall pattern could be seen in all DMN ROIs except the medial PFC (see graphs for individual ROIs in supplementary figure 3).
Table 3.
Regions of Interest (ROIs) Representing 7-mm-Diameter Spheres Centered on Peak Foci of Task-Negative ROIs Identified by Fox et al,53 Which Overlapped With Areas Deactivated by the Present Task
| Region | Side | Center of Mass (mm) RL, AP, IS | Brodmann Area(s) | |||
|---|---|---|---|---|---|---|
| 1 | Posterior cingulate gyrus | L/R | 2 | 36 | 37 | 31 |
| 2 | Lateral parietal (angular gyrus) | L | 47 | 67 | 36 | 39 |
| 3 | Lateral parietal (angular gyrus) | R | −53 | 67 | 36 | 39 |
| 4 | Medial prefrontal cortex | L/R | 3 | −39 | −2 | 32 |
| 5 | Medial prefrontal cortex | L/R | −1 | −54 | 21 | 10 |
| 6 | Superior frontal gyrus | L | 14 | −38 | 52 | 8 |
| 7 | Parahippocampal gyrus | L | 22 | 26 | −16 | 35 |
Note: The numbering corresponds to that of figure 3.
Fig. 3.
Brain regions displaying cue-induced deactivation across all 40 participants. Purple circles demarcate 7-mm spheres centered on peak foci of the task-negative network identified by Fox et al.53 Only spheres with ≥50% overlap with regions deactivated by the present task were defined as regions of interest (ROIs). The numbering corresponds to ROIs in table 3. The graph displays average (±SEM) blood oxygen-level dependent (BOLD) activity over all ROIs in healthy controls subjects (HCS) and people with schizophrenia (PSZ) for predictive and non-predictive cue trials. *P < .05 in paired or independent-samples t test.
Correlations.
To test whether DMN hyperdeactivation was associated with cognitive performance, psychiatric symptoms, or antipsychotic medication in PSZ, we correlated the average percent signal change in predictive cue trials in the DMN ROIs with the RT difference between 1 and 4 cued locations, with the MATRICS Consensus Cognitive Battery composite score, BPRS, SANS and LOFS total scores, and chlorpromazine equivalents.57 There were no significant correlations or trends.
Discussion
The present findings indicate that the greater performance effects of spatial cueing in PSZ did not result from over-recruitment of the top-down attentional orienting network when attention was focused on 1 cued location, nor was it the consequence of inappropriately recruiting these regions when attention had to be spread widely. Instead, the BOLD effects of cue type in these regions were almost identical between groups, indicating that PSZ employed the dorsal attention network in a similar fashion to HCS to orient attention in space. The only group difference in this network was that PSZ displayed a slightly smaller activation difference between predictive and non-predictive cues in the left visual cortex. This effect did not result from a clear group difference in either predictive or non-predictive cue trials. It may reflect an inefficient transmission of top-down biasing signals from frontoparietal regions to a visual effector region. However, such a mechanism could not account for the larger performance effect of cue type in PSZ seen in this and previous studies.11,54
In contrast to the remarkably normal recruitment of the dorsal attention network, cue-induced DMN deactivation in PSZ differed from HCS selectively in predictive cue trials. The difference consisted of a significantly greater deactivation in PSZ relative to HCS. Given previous reports of reduced task-induced DMN deactivation (“hypodeactivation”), this finding was unexpected; however, it may suggest a possible mechanism underlying the group difference in the performance effects of cue type. Greater processing or cognitive control demands have been shown to increase DMN deactivation,58–61 while task practice, which promotes a more automatic processing mode, reduces deactivation.62 It seems plausible, therefore, that the increased DMN deactivation in PSZ when a cue predicted the location of a target reflected greater engagement of processing resources and a more effortful processing mode. HCS may have adopted a more automatic orienting of attention in response to predictive cues, reflected by less DMN deactivation in these trials relative to non-predictive cue trials. PSZ, in contrast, may have maintained a more effortful execution of spatial orienting of attention, resulting in greater deactivation of the DMN in predictive cue trials.
The adoption of a more effortful processing mode when focusing attention in space would be consistent with hypothesis (1) above that PSZ hyperfocus in response to a spatially predictive cue. However, PSZ did not over-recruit regions typically activated during top-down spatial selective attention. Thus, any such hyperfocusing does not appear to reflect over-engagement of attentional selection mechanisms. The DMN itself subserves a range of specialized mental operations,23 and deactivation of the DMN and activation of the dorsal attention network may not always be strictly inversely related. For example, non-predictive cues generally did not activate the dorsal attention network, but induced greater DMN deactivation than predictive cues in HCS. While dorsal attention network activity was sensitive specifically to spatial orienting demands, the level of DMN deactivation may have been more reflective of a general level of attentional vigilance or effort, which would be expected to be elevated by the spatial uncertainty of non-predictive cue trials. We suggest that such non-specific effort may have been disproportionately elevated in PSZ in predictive-cue trials, leading to DMN hyperdeactivation.
It is currently unclear whether a more effortful processing mode when focusing attention in space may bear an additional neural signature outside the DMN that remained undetected in the present study. It is also unclear whether DMN hyperdeactivation and any more effortful processing gave rise to the larger performance effect of cue type seen in PSZ. This would be a plausible mechanism; we did not identify a significant association of DMN hyperdeactivation with this RT effect, but this may reflect power limitations.
The discrepancy between the hyperdeactivation we observed in PSZ and the hypodeactivation reported in several prior studies employing different cognitive task paradigms32–40 deserves some more consideration. An important factor differentiating the present study may be the low task difficulty of the SARAT, combined with the extensive practice before performing the task in the scanner. There is a possibility that the previously observed DMN hypodeactivation was secondary to an impaired ability to engage the required cognitive processes, resulting in reduced task engagement and more time off-task. Performance in the present study suggested no group difference in task engagement. More time spent off-task would result in more omission errors, but omission errors did not differ between PSZ and HCS during the MRI test session. To test whether this was a result of the pre-training, we performed post hoc analysis of performance during the training session and found significantly more omission errors in PSZ than in HCS. Thus, equal task engagement between groups during scans appears to have been achieved by the pre-training, presumably by ensuring that PSZ were fully familiarized with and competent at the task.
It is striking that in most studies reporting reduced DMN suppression, PSZ displayed worse performance than HCS. One study approximated delayed match-to-sample performance between groups by making the encoding and probe stimuli more dissimilar for PSZ.39 However, this may have made the task less effortful, enabling performance with lower task engagement and reduced DMN deactivation. Indeed, greater stimulus dissimilarity in an auditory discrimination task reduced deactivation of several DMN regions.59 Furthermore, not all studies of DMN functioning in PSZ reported hypodeactivation. There is 1 report of hyperdeactivation,63 1 of hyperdeactivation in some and hypodeactivation in other DMN regions,64 and 1 of no difference between PSZ and HCS.65 In the one study reporting hyperdeactivation,63 performance of PSZ matched that of HCS, reinforcing the possibility that difficulty performing the task contributed to findings of DMN hypodeactivation in other studies. While co-varying for task performance did not abolish DMN hypodeactivation of PSZ in one study,33 another study found that it reduced the region displaying the group difference.40 Furthermore, although DMN hypodeactivation in PSZ is sometimes observed with low-demand tasks in which performance is unimpaired,33 the phenomenon does tend to be more prominent in more difficult task conditions.35,38,40,66
The present findings suggest that PSZ are able to suppress DMN activity in response to external task signals when they are fully familiarized with, and engaged in, performing the task. This speaks against a primary deficit in suppressing internal mentation. Moreover, our results indicate that DMN hyperdeactivation can be seen under specific task conditions, perhaps reflecting more effortful and resource-consuming processing.
In summary, frontoparietal regions mediating top-down visuospatial selective attention were recruited normally in PSZ. Thus, the enhanced performance benefit of spatially predictive cues relative to cues signaling the need to spread attention broadly, reported repeatedly in PSZ,9,11,54 cannot be explained by an abnormal modulation of this network. However, a tendency to expend more processing resources when focusing attention in space may be suggested by the observed hyperdeactivation of the DMN.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by a Brain & Behavior Research Foundation Young Investigator Award to B.H. and by the Intramural Research Program of the NIH, National Institute on Drug Abuse.
Supplementary Material
Acknowledgment
The authors have declared no conflicts of interest in relation to this study.
References
- 1. Gold JM, Fuller RL, Robinson BM, Mcmahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. [DOI] [PubMed] [Google Scholar]
- 2. Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol Rev. 2009;19:294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erickson MA, Hahn B, Leonard CJ, et al. Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophr Bull. 2015;41:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. [DOI] [PubMed] [Google Scholar]
- 5. Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophr Res. 1992;7:203–209. [DOI] [PubMed] [Google Scholar]
- 6. Liotti M, Dazzi S, Umiltà C. Deficits of the automatic orienting of attention in schizophrenic patients. J Psychiatr Res. 1993;27:119–130. [DOI] [PubMed] [Google Scholar]
- 7. Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrick B, Carpenter WT., Jr Visual information-processing impairments in deficit and nondeficit schizophrenia. Am J Psychiatry. 1997;154:647–654. [DOI] [PubMed] [Google Scholar]
- 8. Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15:361–370. [DOI] [PubMed] [Google Scholar]
- 9. Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elahipanah A, Christensen BK, Reingold EM. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 2010;24:192–198. [DOI] [PubMed] [Google Scholar]
- 11. Hahn B, Robinson BM, Harvey AN, et al. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012;121:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hahn B, Hollingworth A, Robinson BM, et al. Control of working memory content in schizophrenia. Schizophr Res. 2012;134:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leonard CJ, Kaiser ST, Robinson BM, et al. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luck SJ, McClenon C, Beck VM, et al. Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32:842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 17. Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. [DOI] [PubMed] [Google Scholar]
- 19. Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci. 2010;30:12557–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weissman DH, Prado J. Heightened activity in a key region of the ventral attention network is linked to reduced activity in a key region of the dorsal attention network during unexpected shifts of covert visual spatial attention. Neuroimage. 2012;61:798–804. [DOI] [PubMed] [Google Scholar]
- 22. Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. [DOI] [PubMed] [Google Scholar]
- 23. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 24. Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- 25. Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102:15700–15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. [DOI] [PubMed] [Google Scholar]
- 27. Eichele T, Debener S, Calhoun VD, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. [DOI] [PubMed] [Google Scholar]
- 29. Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009;106:5948–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. [DOI] [PubMed] [Google Scholar]
- 33. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hasenkamp W, James GA, Boshoven W, Duncan E. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr Res. 2011;125:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider FC, Royer A, Grosselin A, et al. Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr Res. 2011;125:110–117. [DOI] [PubMed] [Google Scholar]
- 36. Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res. 2011;125:101–109. [DOI] [PubMed] [Google Scholar]
- 37. Nygard M, Eichele T, Loberg EM, et al. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fMRI study using an ICA analysis approach. Front Hum Neurosci. 2012;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dreher JC, Koch P, Kohn P, Apud J, Weinberger DR, Berman KF. Common and differential pathophysiological features accompany comparable cognitive impairments in medication-free patients with schizophrenia and in healthy aging subjects. Biol Psychiatry. 2012;71:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013;39:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madre M, Pomarol-Clotet E, McKenna P, et al. Brain functional abnormality in schizo-affective disorder: an fMRI study. Psychol Med. 2013;43:143–153. [DOI] [PubMed] [Google Scholar]
- 41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 42. Wang R, Foniok T, Wamsteeker JI, et al. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. [DOI] [PubMed] [Google Scholar]
- 43. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 44. Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT) 4. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 45. Wechsler D. Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 46. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence - A Revision of the Fagerstrom Tolerance Questionnaire. Brit J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 47. Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 48. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 49. Hawk AB, Carpenter WT, Jr, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Arch Gen Psychiatry. 1975;32:343–347. [DOI] [PubMed] [Google Scholar]
- 50. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 51. Talairaque J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- 52. Yang S, Ross TJ, Zhang Y, Stein EA, Yang Y. Head motion suppression using real-time feedback of motion information and its effects on task performance in fMRI. Neuroimage. 2005;27:153–162. [DOI] [PubMed] [Google Scholar]
- 53. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. [DOI] [PubMed] [Google Scholar]
- 56. Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. [DOI] [PubMed] [Google Scholar]
- 57. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. [DOI] [PubMed] [Google Scholar]
- 60. Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A. Cognitive and emotional modulation of brain default operation. J Cogn Neurosci. 2009;21:1065–1080. [DOI] [PubMed] [Google Scholar]
- 61. Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Front Hum Neurosci. 2010;4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harrison BJ, Yucel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91:82–86. [DOI] [PubMed] [Google Scholar]
- 64. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiat. 2007;164:450–457. [DOI] [PubMed] [Google Scholar]
- 65. Nejad AB, Ebdrup BH, Siebner HR, et al. Impaired temporoparietal deactivation with working memory load in antipsychotic-naive patients with first-episode schizophrenia. World J Biol Psychiatry. 2011;12:271–281. [DOI] [PubMed] [Google Scholar]
- 66. Pomarol-Clotet E, Salvador R, Sarro S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.