Abstract
Objectives:
Inflammation, measured by abnormal blood C-reactive protein (CRP) level, has been described in schizophrenia (SZ), being inconsistently related to impaired cognitive functions. The aim of the present study is to investigate cognitive impairment associated with abnormal CRP levels in a large multi-centric sample of community-dwelling SZ patients, using a comprehensive neuropsychological battery.
Method:
Three hundred sixty-nine community-dwelling stable SZ subjects (76.2% men, mean age 32.7 y) were included and tested with a comprehensive battery of neuropsychological tests. Abnormal CRP level was defined as >3mg/L.
Results:
Multiple factor analysis revealed that abnormal CRP levels, found in 104 patients (28.2%), were associated with impaired General Intellectual Ability and Abstract Reasoning (aOR = 0.56, 95% CI 0.35–0.90, P = .014), independently of age, sex, education level, psychotic symptomatology, treatments, and addiction comorbidities. Abnormal CRP levels were also associated with the decline of all components of working memory (respectively effect size [ES] = 0.25, P = .033; ES = 0.27, P = .04; ES = 0.33, P = .006; and ES = 0.38, P = .004) and a wide range of other impaired cognitive functions, including memory (ES = 0.26, P = .026), learning abilities (ES = 0.28, P = .035), semantic memory (ES = 0.26, P = .026), mental flexibility (ES = 0.26, P = .044), visual attention (ES = 0.23, P = .004) and speed of processing (ES = 0.23, P = .043).
Conclusion:
Our results suggest that abnormal CRP level is associated with cognitive impairment in SZ. Evaluating the effectiveness of neuroprotective anti-inflammatory strategies is needed in order to prevent cognitive impairment in SZ.
Key words: C-reactive protein (CRP), schizophrenia, cognition, cognitive impairment, inflammation
Introduction
The contribution of chronic inflammation to schizophrenia (SZ) has received considerable attention in the last decade.1,2 In clinical practice, chronic inflammation is primarily measured by an elevated blood level of C-reactive protein (CRP).3 A recent meta-analysis revealed an increased rate of abnormal CRP levels ≥5mg/L in 28% of SZ subjects compared to healthy controls,4 with abnormal CRP levels being recently found to be a risk factor for late-onset SZ.5 Chronic inflammation was also suggested to be one of the biological substrates of several major psychiatric disorders including depression6,7 and bipolar disorders.8
Cognitive symptoms are thought to have a higher impact on the outcome of SZ than any other symptoms, and are considered as a core feature of this disorder.9 Approximately 25% of people with SZ have a poor long-term outcome due to impaired cognition.10 However the association between abnormal CRP levels and impaired cognitive function in SZ is unclear. In non-SZ populations, increased CRP levels has been associated with a wide range of brain dysfunctions including age-related cognitive decline,11,12 all-cause dementia,13 and stroke.14
In SZ, only 2 studies exploring the impact of inflammation on cognition have been carried out to date. In the first study, abnormal CRP levels were associated with cognitive impairment.15 This study was well performed although the cognitive assessment comprised a 30 minutes-long battery, which provided only a brief screening of different cognitive domains associated with abnormal CRP levels. In a second study using the MATRICS battery, no association between CRP levels and cognitive decline was found.16 However, the sample size (N = 88) was small and the absence of association may be due to a lack of statistical power. Thus, given the potential impact of cognitive impairment prevention and treatment in SZ, the association between abnormal CRP levels and cognitive function deserves further explorations in this population.2
The objective of the present study was to investigate if cognitive impairment was associated with elevated CRP levels in a large multi-centric sample of community dwelling stabilized subjects with SZ, using an exhaustive neuropsychological battery.
Materials and Methods
Study Population
The FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) cohort is based on a French national network of 10 Schizophrenia Expert Centers (Bordeaux, Clermont-Ferrand, Colombes, Créteil, Grenoble, Lyon, Marseille, Montpellier, Strasbourg, Versailles), set up by a French scientific cooperation foundation, FondaMental Foundation (www.fondation-fondamental.org) and created by the French Ministry of Research in order to build a platform that links systematic clinical assessment to research.17
Inclusion Criteria
Consecutive clinically stable patients (defined by no hospitalization and no treatment changes during the 4wk before evaluation) with a DSM-IV-TR diagnosis of SZ or schizoaffective disorder were consecutively included in the study. Diagnosis was confirmed by 2 trained psychiatrists of the Schizophrenia Expert Centres network. All subjects were referred by their general practitioner or psychiatrist who subsequently received a detailed evaluation report with suggestions for personalized interventions.
Noninclusion Criteria
Patients with a history of neurological disorders (including stroke, epilepsy, and head injury) or all non-psychiatric concurrent illnesses affecting the central nervous system and inflammation (especially auto-immune illnesses, such as lupus and rheumatoid arthritis) were excluded from the present study.
Measurements
Clinical and Sociodemographic Measures.
All patients were interviewed by members of the specialized multidisciplinary team of the Expert Centre. All subjects were interviewed by 2 independent psychiatrists with the Structured Clinical Interview for Mental Disorders (SCID 1.0) to confirm the diagnosis. Information about education, onset and course of the illness, family history, somatic diseases and comorbidities were recorded. Schizophrenic symptomatology was assessed using the Positive And Negative Syndrome Scale (PANSS).18 Current depressive symptoms were evaluated using the Calgary Depression Rating Scale for Schizophrenia (CDRS).19 Manic symptoms were assessed with Young Mania Rating Scale (YMRS).20 Ongoing psychotropic treatment, current cannabis consumption and tobacco smoking were also recorded.
Neuropsychological Measures.
The National Adult Reading Test21 provides an estimate of premorbid intellectual ability. French version of the National Adult Reading Test was used in our analysis.22
Wechsler Adult Intelligence Scale—3rd Edition23 provides a measure of general intellectual function in older adolescents and adults. Seven subtest short form24 was used to estimate the Full Scale IQ (FSIQ), Verbal IQ (VIQ) and Performance IQ (PIQ), and allowed exploration of the following cognitive areas: Picture Completion (visual exploration and detail perception), Digit-Symbol Coding (visual-motor coordination, motor and mental speed), Similarities (abstract verbal reasoning), Arithmetic (mathematical problem solving), Matrix Reasoning (nonverbal abstract problem solving, inductive spatial reasoning), Digit span (attention, working memory, mental control), Information (general information acquired from culture, semantic memory). An additional subtest, Letter-Number Sequencing was administered, which along with 2 other primary subtests, Digit Span and Arithmetic, allowed the calculation of a Working Memory Index (WMI; auditory working memory and mental control).
Trail Making Test25 reflects the control of attention, visual exploration, speed and mental flexibility. The subject is asked to connect, by making pencil lines, encircled numbers randomly arranged on a page in proper order (Part A) and encircled numbers and letters in alternating order (Part B). A French version of the normative data was used.26
California Verbal Learning Test27 is designed to measure verbal learning and memory using a multiple-trial list-learning task. The examiner reads the word list and records the patient’s oral responses verbatim in the order in which they are given. Learning efficiency, strategies, interference management and learning bias are measured. A French version of the task was used in this study.28
Doors test29 is a visual recognition memory test in which participants view photographs of 12 doors for 3 seconds each. Immediately thereafter, participants are presented with 12 arrays of 4 doors each, and are asked to identify the door from the previous list. In the second part new photographs of doors are displayed, but the recognition stimuli are rather similar to the key list.
The Continuous Performance Test—Identical Pairs (CPT-IP) is a computerized measure of sustained, focused attention or vigilance. This version is a part of MCCB-Matrics Consensus Cognitive Battery and involves monitoring a series of multiple digits and responding with a button press each time that 2 stimuli in a row are identical.30
Biological Measures.
High sensitivity CRP (hs-CRP) was measured with an assay using nephelometry (Dade Behring). Abnormal CRP level was defined as >3mg/L according to the (“The Emerging Risk Factors Collaboration”; 2010). Patients with hs-CRP levels >30mg/L, which corresponds to an acute inflammation, were not included in the analyses (“The Emerging Risk Factors Collaboration”; 2010).
Ethical Concerns
The study was carried out in accordance with ethical principles for medical research involving humans (WMA, Declaration of Helsinki). The assessment protocol was approved by the relevant ethical review board (CPP-Ile de France IX, January 18, 2010). All data were collected anonymously. As this study include data coming from regular care assessments, a non-opposition form was signed by all participants.
Statistical Analysis
Sociodemographics, clinical and neuropsychological characteristics and treatments are presented using measures of means and dispersion (SD) for continuous data and frequency distribution for categorical variables. The data were examined for normal distribution with the Shapiro-Wilk test and for homogeneity of variance with the Levene test. Univariate associations between demographic, neuropsychological and clinical characteristics of patients with abnormal CRP levels were performed using the chi-square test for categorical variables. Continuous variables were analyzed with Student t-tests for normally distributed data and Mann-Whitney tests in case of non-normal distributions. Effect size-defined as the mean difference divided by the SD of the whole sample- were computed for each cognitive tests.31 According to Samsa et al,32 an effect size of at least 0.2 is considered as clinically relevant. An effect size of 0.2 is considered a small effect, 0.5 a moderate effect, and 0.8 a large effect.33
A multivariate logistic regression was performed to estimate the adjusted Odds Ratio (aOR) and its corresponding 95% CI for an association between cognition and CRP. In order to eliminate multicollinearity associated with cognitive tests, we performed a principal component analysis (PCA) with varimax rotation to reduce the large number of cognitive tests to a smaller number of uncorrelated cognitive component scores. The number of components was chosen based on the Kaiser stopping criterion (ie, all components with eigenvalues greater than 1) and the scree test. The use of component scores as the independent variables in multivariate models is considered as relevant in the case of multicollinearity.34 The cognitive scores were thus used for multiple regression analysis, associated to a set of confounding factors selected from the univariate analysis, with selection based on a threshold P-value < .20 (ie, age, years of education, illness duration). Sex and PANSS score were also included because of their sociodemographic and clinical interest. CRP levels were also analyzed in a quantitative manner using a multiple linear regression to check the robustness of our findings.
All of the tests were 2-sided. Statistical significance was defined as P < .05. Statistical analysis was performed using the SPSS version 18.0 software package (SPSS Inc). This study was a confirmatory analysis. The hypothesis was that abnormal CRP levels were associated with cognitive impairment, based on the previous preliminary data.35 No correction for multiple testing has been therefore carried out, consistently with recommendations.36
Results
A sample of 369 community-dwelling stable SZ subjects enrolled in FACE-SZ cohort was included in this study. Table 1 shows demographical, clinical and neuropsychological characteristics of the sample, as well as associations with abnormal CRP levels. The majority of the subjects in the sample (76.2%) were men and the mean age of the patients was 32.7±10 years. Overall, 104 patients (28.2%) had abnormal CRP levels. The mean age at SZ onset was 21.7±6.4 years, the mean duration of illness was 11±8.6 years and the mean PANSS total score was 70.8±18. Current tobacco smoking, current cannabis consumption, current alcohol disorder and current substance abuse disorders were reported in table 1. None of these variables was significantly associated with abnormal CRP levels (all P > .05).
Table 1.
Association Between Peripheral Inflammation (Defined by Abnormal C-Reactive Protein [CRP] Level [>3mg/L]) and Neuropsychological Impairment (N = 369)
| Whole Sample (N = 369) | Abnormal CRP | Statistic | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (N = 265) | Yes (N = 104) | |||||||||
| Sociodemographic characteristics | ||||||||||
| Sex (male) | 281 | 76.2% | 203 | 76.6% | 78 | 75.0% | Chi-square | .745 | ||
| Mean age (y) (SD) | 32.72 | 10.03 | 32.29 | 10.00 | 33.80 | 10.08 | Student t | .195 | ||
| Mean years of education (SD) | 12.37 | 2.86 | 12.49 | 2.85 | 12.08 | 2.90 | U Mann-Whitney | .130 | ||
| Clinical variables | ||||||||||
| Age at onset, mean (SD) | 21.68 | 6.44 | 21.52 | 6.12 | 22.07 | 7.21 | Student t | .476 | ||
| Age at first antipsychotic treatment, mean (SD) | 22.89 | 6.54 | 22.74 | 6.71 | 23.23 | 6.12 | Student t | .531 | ||
| Illness duration (y), mean (SD) | 11.01 | 8.61 | 10.78 | 8.60 | 11.64 | 8.64 | U Mann-Whitney | .194 | ||
| Psychotic symptomatology (PANSS total score), mean (SD) | 70.84 | 18.05 | 70.97 | 18.60 | 70.50 | 16.66 | Student t | .822 | ||
| Positive symptoms (PANSS positive score), mean (SD) | 14.69 | 5.33 | 14.73 | 5.39 | 14.60 | 5.21 | U Mann-Whitney | .939 | ||
| Negative symptoms (PANSS negative score), mean (SD) | 20.96 | 7.09 | 21.11 | 7.31 | 20.60 | 6.54 | Student t | .539 | ||
| General psychopathology (PANSS general score), mean (SD) | 35.14 | 9.82 | 35.13 | 10.10 | 35.17 | 9.14 | Student t | .972 | ||
| Depressive symptoms (CDSS score), mean (SD) | 3.97 | 4.29 | 3.97 | 4.21 | 3.97 | 4.48 | U Mann-Whitney | .807 | ||
| Manic symptoms (YMRS score), mean (SD) | 2.73 | 4.31 | 2.87 | 4.49 | 2.38 | 3.81 | U Mann-Whitney | .253 | ||
| Current alcohol disorder, mean (SD) | 16 | 4.3% | 13 | 4.9% | 3 | 2.9% | Chi-square | .391 | ||
| Current substance use disorder, mean (SD) | 4 | 1.4% | 3 | 1.4% | 1 | 1.3% | Chi-square | .924 | ||
| Current daily tobacco smoking | 192 | 53.0% | 133 | 51.4% | 59 | 53.0% | Chi-square | .308 | ||
| Current cannabis consumption disorder | 23 | 19.2% | 19 | 21.1% | 4 | 13.3% | Chi-square | .349 | ||
| Treatment | ||||||||||
| Second generation antipsychotics | 266 | 88.7% | 191 | 89.3% | 75 | 87.2% | Chi-square | .614 | ||
| First generation antipsychotics | 80 | 26.7% | 53 | 24.8% | 27 | 31.4% | Chi-square | .240 | ||
| Cholinergic drugs | 50 | 16.7% | 35 | 16.4% | 15 | 17.4% | Chi-square | .819 | ||
| Mean rangea | Whole sample (N = 369) | Abnormal CRP | ||||||||
| No (N = 265) | Yes (N = 104) | Statistic | Effect sizeb | |||||||
| Current and premorbid intellectual ability | ||||||||||
| fNART based premorbid IQ, mean (SD) | [80–119] | 104.61 | 8.45 | 105.01 | 8.37 | 103.50 | 8.63 | Student t | 0.18 | 0.166 |
| Full Scale IQ, mean (SD) | [80–119] | 86.17 | 15.38 | 87.72 | 15.64 | 82.45 | 14.15 | Student t | 0.34 | 0.004 |
| Verbal IQ, mean (SD) | [80–119] | 90.27 | 14.64 | 91.68 | 14.53 | 86.89 | 14.43 | Student t | 0.33 | 0.006 |
| Performace IQ, mean (SD) | [80–119] | 83.26 | 17.45 | 84.76 | 18.12 | 79.76 | 15.27 | Student t | 0.29 | 0.016 |
| Working memory | ||||||||||
| Digit Span (standard score), mean (SD) | [7–13] | 8.16 | 2.79 | 8.37 | 2.81 | 7.67 | 2.69 | Student t | 0.25 | 0.033 |
| Letter-Number Sequencing (standard score), mean (SD) | [7–13] | 8.03 | 2.74 | 8.23 | 2.72 | 7.49 | 2.73 | Student t | 0.27 | 0.040 |
| Arithmetic (standard score), mean (SD) | [7–13] | 7.29 | 3.08 | 7.58 | 3.16 | 6.57 | 2.78 | Student t | 0.33 | 0.006 |
| Working Memory Index (WMI), mean (SD) | [80–119] | 87.08 | 14.88 | 88.59 | 14.94 | 82.91 | 13.97 | Student t | 0.38 | 0.004 |
| Learning abilities, episodic and semantic memory | ||||||||||
| CVLT short delay free recall, mean (SD) | [9–15.5] | 9.24 | 3.51 | 9.50 | 3.34 | 8.58 | 3.85 | U Mann-Whitney | 0.26 | 0.026 |
| CVLT short delay cued recall, mean (SD) | [10.5–15.5] | 9.93 | 3.27 | 10.02 | 3.29 | 9.71 | 3.23 | U Mann-Whitney | 0.09 | 0.329 |
| CVLT long delay free recall, mean (SD) | [10–15.5] | 9.75 | 3.43 | 9.87 | 3.32 | 9.44 | 3.71 | U Mann-Whitney | 0.13 | 0.233 |
| CVLT long delay cued recall, mean (SD) | [11–15.5] | 10.02 | 3.34 | 10.12 | 3.30 | 9.76 | 3.44 | U Mann-Whitney | 0.11 | 0.249 |
| CVLT recognition, mean (SD) | [14–16] | 14.57 | 3.13 | 14.64 | 3.25 | 14.38 | 2.81 | U Mann-Whitney | 0.08 | 0.098 |
| Doors Test (A&B), mean (SD) | [16–22] | 15.30 | 4.58 | 15.67 | 4.31 | 14.39 | 5.11 | Student t | 0.28 | 0.035 |
| Information (standard score), mean (SD) | [7–13] | 8.98 | 3.26 | 9.23 | 3.23 | 8.38 | 3.25 | Student t | 0.26 | 0.026 |
| Executive functions and problem solving | ||||||||||
| Trail Making Test B (time), mean (SD) | [40–81] | 104.37 | 59.01 | 100.16 | 51.21 | 115.67 | 75.27 | U Mann-Whitney | 0.26 | 0.044 |
| Trail Making Test B (errors), mean (SD) | [ 0 ] | 0.69 | 1.24 | 0.69 | 1.32 | 0.68 | 1.01 | U Mann-Whitney | 0.01 | 0.500 |
| Matrix Reasoning (standard score), mean (SD) | [7–13] | 7.97 | 3.56 | 8.28 | 3.61 | 7.26 | 3.36 | Student t | 0.29 | 0.016 |
| Similarities (standard score), mean (SD) | [7–13] | 8.56 | 3.08 | 8.73 | 3.06 | 8.17 | 3.13 | Student t | 0.18 | 0.121 |
| Visual attention and speed of processing | ||||||||||
| Trail Making Test A (time), mean (SD) | [19–44] | 42.55 | 19.73 | 41.15 | 18.59 | 46.03 | 22.05 | U Mann-Whitney | 0.25 | 0.043 |
| Trail Making Test A (errors), mean (SD) | [ 0 ] | 0.21 | 0.52 | 0.17 | 0.52 | 0.29 | 0.52 | U Mann-Whitney | 0.23 | 0.004 |
| Digit Symbol Coding (standard score), mean (SD) | [7–13] | 5.90 | 2.88 | 6.07 | 2.97 | 5.52 | 2.63 | Student t | 0.19 | 0.108 |
| Picture Completion (standard score), mean (SD) | [7–13] | 7.56 | 3.46 | 7.75 | 3.58 | 7.12 | 3.12 | Student t | 0.18 | 0.123 |
| CPT-IP d-prime, mean (SD) | [2.25–4] | 2.31 | 0.74 | 2.34 | 0.76 | 2.20 | 0.68 | Student t | 0.19 | 0.199 |
Note: Statistically significant in bold (P < .05). PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; CPT-IP, Continuous Performance Test—Identical Pairs; CDSS, Calgary Depression rating Scale for Schizophrenia; fNART, French National Adult Reading Test; CVLT, California Verbal Learning Test.
aThe provided scores are issued from the normative data used for clinical interpretation of the neuropsychological tests. The scores provided correspond the mean age and education of the clinical sample and are therefore reported here for informative purpose only. The normal range is defined following usual conventions: Z scores ranging from −1.33 to +1.33; Percentiles ranging from 10 to 90, Standard scores ranging from 7 to 13 and IQ scores ranging from 80 to 119.
bEffect size-defined as the mean difference divided by the SD of the whole sample—were computed for each cognitive tests.32 According to Samsa et al,33 an effect size of at least 0.2 is considered as clinically relevant. An effect size of 0.2 is considered a small effect, 0.5 a moderate effect and 0.8 a large effect.34
The impaired cognitive functions associated with abnormal CRP levels are presented in table 1. The following cognitive functions were found to be significantly impaired in SZ subjects with abnormal CRP levels compared to those without abnormal CRP levels: current intellectual ability (FSIQ, effect size [ES] = 0.34, P = .004; VIQ, ES = 0.33, P = .006; PIQ, ES = 0.29, P = .016), working memory (all measures, ES = 0.25–0.38, P < .05), memory and learning abilities (short delay free verbal recall, ES = 0.26, P = .026; visual recognition, ES = 0.28, P = .035), semantic memory (Information subtest, ES = 0.26, P = .026), mental flexibility (TMT B, time, ES = 0.26, P = .044), abstract thinking (Matrix reasoning subtest, ES = 0.29, P = .016), visual attention (TMT A, errors, ES = 0.23, P = .004) and speed of processing (TMT A, time, ES = 0.25, P = .043). These results were independent of sociodemographic variables, illness duration and severity (psychotic symptomatology) in multivariate analysis.
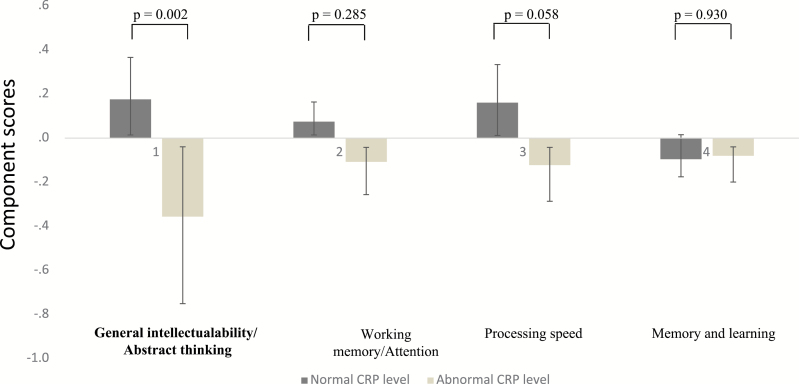
Four components emerged in the PCA. All 4 components had eigenvalues >1 and in total they accounted for 67.03 % of the variance (table 2). The content of each component was considered as relevant and meaningful by 2 experts in psychology (E.B. and G.F.) and named: “General intellectual ability/ Abstract thinking,” “Working memory/Attention,” “Processing speed,” and “Memory and learning.” In the multivariate analyses reported in table 3, the relationship between factor 1 (“General intellectual ability/ Abstract thinking”) score and CRP level remained significant after adjusting for sociodemographic and clinical variables. The component scores are presented in patients with normal and abnormal CRP levels in figure 1. These findings were confirmed using CRP in a quantitative manner. The component 1 remained significant (standardized β = −.20; P = .04).
Table 2.
Principal Component Analysis After Varimax Rotation
| Component 1, General Intellectual Ability/ Abstract Thinking | Component 2, Working Memory/ Attention | Component 3, Processing Speed | Component 4, Memory and Learning | |
|---|---|---|---|---|
| Information (standard score) | 0.876 | |||
| Full Scale IQ | 0.766 | |||
| Similarities (standard score) | 0.638 | |||
| fNART IQ | 0.632 | |||
| Picture Completion (standard score) | 0.552 | |||
| Matrix Reasoning (standard score) | 0.480 | |||
| Digit Span (standard score) | 0.772 | |||
| Letter-Number Sequencing (standard score) | 0.737 | |||
| CPT-IP mean d-prime | 0.681 | |||
| Arithmetic (standard score) | 0.620 | |||
| Trail Making Test A (time) | −0.808 | |||
| Trail Making Test B (time) | −0.761 | |||
| Digit Symbol Coding (standard score) | 0.560 | |||
| CVLT recognition | 0.812 | |||
| CVLT short delay free recall | 0.797 | |||
| Doors Test (A&B) | 0.454 |
Table 3.
Factors Associated With High CRP: Multivariate Analysis
| Factors | Adjusted Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Sex (male) | 1.213 | 0.473–3.110 | .756 |
| Component 1: “General intellectual ability / Abstract thinking” | 0.560 | 0.347–0.904 | .014 |
| Component 2: “Working memory/Attention” | 0.841 | 0.565–1.251 | .271 |
| Component 3: “Processing speed” | 0.721 | 0.471–1.104 | .227 |
| Component 4: “Memory and learning” | 1.120 | 0.763–1.644 | .516 |
| PANSS (total score) | 0.986 | 0.963–1.011 | .212 |
| Age (y) | 1.024 | 0.961–1.091 | .621 |
| Illness duration (y) | 1.002 | 0.936–1.073 | .984 |
| Years of education | 0.962 | 0.813–1.140 | .776 |
Note: Statistically significant in bold.
Fig. 1.
Cognitive impairment associated with chronic peripheral inflammation in schizophrenia (measured by C-Reactive protein [CRP] blood levels ≥3mg/L).
Discussion
The major results of this study may be summarized as follows: peripheral sub-inflammation, assessed here by abnormal CRP levels, is associated with a wide array of small to moderate cognitive impairments in a large sample of community-dwelling SZ patients, independently of SZ characteristics, sociodemographic variables, treatments, tobacco smoking and cannabis consumption. Multivariate analysis revealed that General Intellectual Ability/Abstract Reasoning was the most specifically impaired cognitive factor associated with abnormal CRP levels, being independent of age, sex, education level, psychotic symptomatology, treatments, and addiction comorbidities. Elevated CRP levels were also associated with an impairment of all components of working memory and a wide range of other impaired cognitive functions including memory and learning abilities, as well as semantic memory, mental flexibility, visual attention and speed of processing. Firstly, the data presented here indicate peripheral inflammation to be associated with a global impairment of current intellectual ability in SZ subjects. This suggests a major role for peripheral inflammation in the debilitating course of SZ, even in young patients (mean age was 32 y, with a mean illness duration of 11 y). Such data overlaps with data in older populations, where elevated CRP levels were associated with age-related cognitive decline (“inflammaging”),11,12 as well as with all-cause dementia.13
The cognitive impairment following the onset of SZ has been well documented (see recent meta-analyses).37–40 This life span neurodegenerative perspective is not new as SZ was initially termed “dementia praecox” in 1891 by the German psychiatrist Arnold Pick (1851–1924), due to the cognitive decline during the first 2 decades of the illness. From a neuroanatomical point of view, abnormal CRP is known to be associated with dysexecutive syndrome and a cerebral microstructure disintegration in frontal pathways,41,42 a crucial cortical area in SZ pathophysiology.43–45 CRP is one of the triggering molecules of peripheral inflammation that may also activate brain inflammation. A growing body of literature has further underscored the potential role of microglial activation in SZ, including an infection-triggered immune reaction.46,47 Altogether, our results combined with literature suggest that abnormal CRP may be considered as a peripheral biomarker of cognitive impairment in SZ.
As noted, the association between inflammation and cognition is not limited to SZ, however the data presented here indicate some specificity. Inconsistent results have been found in depression and bipolar disorder, as to inflammation and cognitive decline. Lower cognitive functioning in individuals with bipolar disorder has been associated with elevated levels of CRP.8 The authors found an inverse relationship between CRP level and performance on RBANS battery, which was significant on immediate memory, attention and language factors, as well as psychomotor speed. In major depression, except psychomotor speed and some executive components, most of the neuropsychological variables (verbal and visual memory, working memory, attention, language) were not associated with higher CRP levels.48,49 In summary, CRP is associated with lowered psychomotor speed in a non-specific (transnosographic) manner. But our results further suggest that CRP-associated impaired general intellectual ability, abstract reasoning, working memory, semantic memory and mental flexibility is a more diagnosis-specific aspect of peripheral inflammation in SZ. It may be hypothesized that these discrepancies may be due to several factors: genetic brain vulnerabilities, origin of chronic inflammation (infections, perivisceral fat, antidepressant consumption50), different immunological disturbances across illnesses. Further studies should determine if cognition is differently impacted according to inflammation etiological factors, and if these factors may explain the differences between SZ and bipolar disorders.
Second, peripheral inflammation was found in our study to be associated with a global impairment of all assessed working memory components. This association was already described in other non-psychiatric populations (including cancer, coronary heart disease and lupus patients, as well as middle-age healthy subjects),51–54 but not in SZ. This suggests a deleterious effect of high CRP levels on working memory across illnesses. The role of working memory is crucial to temporarily maintain and store information. This system supports thought mechanisms by providing an interface between perception, long-term memory and action.55 All working memory measures were significantly lower in patients with higher levels of CRP. Targeting CRP may thus be decisive for improving working memory in SZ subjects.
Thirdly, elevated CRP was associated with impaired verbal and visual episodic learning abilities, as well as impaired semantic memory. This is consistent with previous findings in non-SZ populations, where healthy-aging subjects with higher CRP had lower memory performance and smaller left temporal lobe volumes56 as well as impaired frontal42 and hippocampal functioning.57 The last 2 areas are essential for memory formation and its biological underpinnings that are thought to arise from long-term potentiation. CRP-associated impaired memory was also described in type II diabetes patients.58 However, no association was observed between abnormal CRP and long-term verbal recall impairment in SZ. Memory impairment related to inflammatory processes is less clear in depression. In 1 study, no association has been found between memory functions and CRP in moderately depressed elderly.59 Instead, encoding and recall were inversely associated with interleukin (IL)-6 across the groups. While the results in bipolar patients8 are comparable to those in this study, this was inconsistent with previous findings in healthy aging population.60,61 Several explanations may be suggested to explain these discrepancies, including variations in the delay of recall across studies. Also, the wide range of cognitive impairment in SZ may have erased the CRP-long-term-verbal memory association, which was found in healthy-aging subjects. It may be hypothesized that SZ subjects with normal CRP levels may have decreased verbal memory due to other causes.
Fourthly, peripheral inflammation was found here to be associated with lower performance in inductive reasoning and mental flexibility. Along with working memory and abstract thinking, these functions form a broad executive control and problem solving system that is necessary to adapt to novel contexts, as well as to initiate and regulate autonomous behaviors. Executive functions correlate with the ability to solve interpersonal problems,62 vocational functioning, daily management of personal and professional activities,63,64 and quality of life.66 Our results are consistent with those found in middle-aged healthy subjects,53 patients with diabetes,58 and healthy aging.42,66–68 In patients suffering from depression, with elevated CRP levels, there seems to be a tendency for poorer executive function as indicated on the Wisconsin card sorting test measures: number of completed categories,48 and design fluency.49
Fifthly, peripheral inflammation was associated with impaired components of visual attention and processing speed. Similarly, increased levels of hsCRP were associated with lower psychomotor speed (Trail making A, Finger Tapping test) in depressed outpatients.48,49 This association is also observed in nondepressed subjects with higher CRP levels related to arterial disease.52 A potential mediator, as discussed might be the cytokine-induced hypermetabolism in the basal ganglia leading to a psychomotor slowing.
Our major results were inconsistent with the Joseph et al study,16 and consistent with Dickerson et al.15 This is probably due to the higher mean age of SZ subjects (49 y, mean illness duration 23 y) in Joseph et al study vs 40 years (mean illness duration 19 y) and 32 years (mean illness duration 11 y), respectively in Dickerson et al study and ours. Overall, these studies indicate that CRP mostly impacts cognitive functioning in the first 2 decades following SZ onset. Future longitudinal studies are required to investigate this hypothesis.
The absence of association between abnormal CRP levels and psychotic symptoms in our sample was consistent with Dickerson et al15 results. However a recent meta-analysis suggested an association between abnormal CRP and positive symptoms, which may be due to the inclusion of untreated patients in this meta-analysis.1,69,70 In our sample, all patients were treated by antipsychotics, which possibly contributes to the non-replication of this association in the data presented here.
Contrary to Dickerson et al and Joseph et al studies, we found no sex differences. Our sex ratio (76% male) was comparable to Dickerson et al15 (70%), although not with Joseph et al16 (39%). The mean age at illness onset was also comparable (respectively 21 y here and in the Dickerson et al study, vs 26 y for Joseph et al) possibly due to the later onset in SZ female patients. Future meta-analyses should explore such sex differences to determine if CRP levels are significantly altered in SZ females compared to males.
The neurobiological underpinnings of CRP-associated cognitive impairment in SZ are not fully understood to date. Inflammation in the central nervous system is closely related to neurodegeneration. Blood CRP levels may be seen as an indirect peripheral common marker of central neuroinflammation. CRP levels are raised by the increase of pro-inflammatory cytokines, including IL-1β, TNF-α and IL-6. These pro-inflammatory cytokines have individual effects on neurogenesis. IL-1β induces focal and sustained hippocampal inflammation, resulting in severe depletion of developing neuroblasts.71 IL-1β was also found to suppress cell proliferation in the dentate gyrus.72 IL-1β generally contributes to memory formation at the physiological level. However, when IL-1β is expressed excessively, it inhibits hippocampus-mediated memory formation in a manner of an inverted U shape.73 Activated microglia-derived TNF-§ was found to enhance the death of hippocampal progenitor cells.74 IL-6 may be the most important cytokine involved in microglial activity and inflammatory responses. Overexpressed IL-6 inhibits hippocampal neurogenesis.75 All these mechanisms may be involved in CRP-associated cognitive impairment.
Elevation in CRP levels may have direct therapeutic relevance to the prevention of cognitive impairment, within a personalised approach to the management of SZ. A recent meta-analysis highlighted the clinical utility of anti-inflammatory add-on therapies in SZ, perhaps especially aspirin.76 These interventions are described as potentially more effective early in the course of SZ, reinforcing the need to develop an early prevention-focused perspective, perhaps especially in patients with elevated CRP levels.77 CRP is also associated with both increased atheroma genesis78 and increased risk of stroke.14 As the prevalence of metabolic syndrome is high in young SZ subjects,79 it may be reasonably suggested that atheroma may contribute to the mechanisms of cognitive impairment in SZ.80 Aspirin may therefore be doubly helpful, by its anti-inflammatory and anti-atheroma properties.81 Future studies should assess the pro-cognitive effects of anti-inflammatory agents that have been proposed as beneficial in aged populations.82,83 Beyond anti-inflammatory agents, an “anti-inflammatory diet” was also suggested to improve cognitive function in healthy participants and should be evaluated in SZ patients with both inflammatory disturbances and impaired cognitive functions.82–84 The impact of lifestyle hygiene factors (such as psychological stress management or physical activity) on the reduction of inflammatory response should also be taken into consideration.85,86 In summary, abnormal CRP levels in SZ may be a biomarker for cognitive dysfunction and therefore an indication for anti-inflammatory strategies as well as early cognitive remediation. Future studies are needed to investigate the effectiveness of anti-inflammatory add-on therapies for cognitive improvement in SZ patients with peripheral inflammation.
The purpose of the present study was not to determine the etiological factors of chronic peripheral inflammation in SZ. However, unraveling etiological factors may help guiding anti-inflammatory and preventive strategies for the future. For example, abnormal CRP levels have been independently associated with both antidepressant consumption and abdominal obesity in SZ, but not with tobacco or cannabis consumption.87 The lack of association between tobacco smoking or cannabis consumption in the present study is consistent with previous results.88 Future studies should determine if different etiological factors of peripheral inflammation may be associated with different cognitive outcomes.
Strengths
The present study has clear strengths, particularly the use of homogenous and exhaustive standardized diagnostic protocols and neuropsychological assessment in a large national multicentric study. Important confounding factors were taken into account in our analyses, especially sociodemographic characteristics, antipsychotic treatments, tobacco smoking and cannabis consumption. Our sample was relatively young (mean age 32 y, mean illness duration 11 y).
Limits
Due to the cross-sectional design of this study, no longitudinal data of abnormal CRP on cognitive trajectory outcome were obtained. First, an important methodological problem is the definition of our groups (normal CRP level and high CRP). Indeed, there are no generally accepted criteria. However, we have chosen the most consensual definition in the recent scientific literature, which is recognized as the cut-off point for high cardiovascular risk.89–91 Second, CRP was the sole marker of inflammation in this study. Although CRP is strongly associated with IL-6 activity, we did not directly assess any pro-inflammatory cytokines. Future studies with extensive assessment of inflammatory markers may be required. Third, we have only collected CRP at one time point, and repeated testing has been recommended to confirm elevated plasma levels89 especially as concentrations can be affected by acute infection. However, patients with acute infections were removed and were not included in this study. Finally, although the experimental protocol takes into account a large set of potentially confounding variables, additional factors might have been interesting to consider. In particularly, cognitive impairment, a core symptom of SZ, is associated with metabolic syndrome92 and more specifically with perivisceral fat.79 Alcohol consumption or substance abuse have not been described as a risk factor for peripheral inflammation in SZ to date, however this data may have been explored in the present study as a confounding factor. The purpose of the present study was not to determine the potential etiological factors associated with abnormal CRP levels, which has been done elsewhere87 and would require larger samples.
Conclusion
Altogether, our results yielded important findings. We confirmed that peripheral inflammation was associated with a general loss of intellectual abilities in SZ, which strongly impacts on functional outcomes, and is independent of psychotic symptomatology, age, sex, treatments and addiction co-morbidities. Therapeutic trials are needed to determine if anti-inflammatory add-on strategies are effective in preventing cognitive impairment in SZ, especially at the beginning of the illness.
Funding
This work was funded by Assistance Publique des Hôpitaux de Paris (AP-HP), Fondation FondaMental (RTRS Santé Mentale), by the Investissements d’Avenir program managed by the ANR under reference ANR-11-IDEX-0004-02 and ANR-10-COHO-10-01, and by Institut National de la Santé et de la Recherche Médicale (INSERM).
Acknowledgments
FACE-SCZ Group: Méja Andrianarisoaa,b, Bruno Aouizerateb,c, Fabrice Bernab,d, Olivier Blancb,e, Lore Brunela,b, Ewa Bulzackaa,b, Delphine Capdevielleb,f, Isabelle Chereau-Boudetb,e, Gabrielle Chesnoy-Servaninb,g, Jean-Marie Danionb,d, Thierry D’Amatob,g, Deloge Arnaudb,h, Claire Delormeb,i, Hélène Denizotb,e, Jean-Michel Doreyb,g, Caroline Dubertretb,j, Julien Dubreucqb,i, Catherine Fagetb,k, Cécile Fluttazb,i, Guillaume Fonda,b, Sandrine Fonteneaub,l, Franck Gabayetb,i, Elisabeth Giraud-Barob,i, Marie-Christine Hardy-Bayléb,l, Delphine Lacelleb,e, Christophe Lançonb,k, Hakim Laouamrib, Marion Leboyera,b, Tifenn Le Gloaheca,b, Yann Le Stratb,j, Pierre-Michel Llorcab,e, Jasmina Malletj,k, Emeline Metairieb,k, David Misdrahib,h, Isabelle Offerlin-Meyerb,d, Christine Passerieuxb,l, Pauline Perib,k, Sylvie Piresb,e, Céline Portalierb,j, Romain Reyb,g, Céline Romanb,i, Mathilde Sebilleaub,l, Aurélie Schandrinb,f, Franck Schurhoffa,b, Arnaud Tessierb,h, Anne-Marie Troncheb,e, Mathieu Urbachb,l, Florence Vaillantb,k, Aurélie Vehierb,g, Pierre Vidailhetb,d, Estelle Vilàb,h, Hanan Yazbekb,f, and Anna Zinetti-Bertschyb,d. Affiliations: aAP-HP, DHU Pe-PSY, Pôle de Psychiatrie et d’addictologie des Hôpitaux Universitaires H Mondor,INSERM U955, Eq 15 Psychiatrie Translationelle, Université Paris Est-Créteil, Créteil, France; bFondation Fondamental; cCentre Hospitalier Charles Perrens, F-33076 Bordeaux, France; Université de Bordeaux, Inserm, Neurocentre Magendie, Physiopathologie de la Plasticité Neuronale, U862, F-33000 Bordeaux, France; dHôpitaux Universitaires de Strasbourg, Université de Strasbourg, INSERM U1114, Fédération de Médecine Translationnelle de Strasbourg, Strasbourg, France; eCMP B, CHU, EA 7280 Faculté de Médecine, Université d’Auvergne, BP 69 63003 Clermont-Ferrand Cedex 1, France; fService Universitaire de Psychiatrie Adulte, Hôpital la Colombière, CHRU Montpellier, Université Montpellier 1, Inserm 1061, Montpellier, France; gUniversité Claude Bernard Lyon 1/Centre Hospitalier Le Vinatier Pole Est BP 300 39 - 95 bd Pinel - 69678 BRON Cedex, France; hCentre Hospitalier Charles Perrens, F-33076 Bordeaux, France; Université de Bordeaux, CNRS UMR 5287-INCIA; iCentre Référent de Réhabilitation Psychosociale, CH Alpes Isère, Grenoble, France; jAP-HP, Department of Psychiatry, Louis Mourier Hospital, Colombes, Inserm U894 Université Paris Diderot, Sorbonne Paris Cité, Faculté de médecine, France; kAssistance Publique des Hôpitaux de Marseille (AP-HM), pôle universitaire de psychiatrie, Marseille, France; lService de psychiatrie d’adulte, Centre Hospitalier de Versailles, Le Chesnay, EA 4047 HANDIReSP, UFR des Sciences de la Santé Simone Veil, Université Versailles Saint-Quentin en Yvelines, Versailles, France. We express all our thanks to the nurses, and to the patients who were included in the present study. We thank Hakim Laouamri, and his team (Stéphane Beaufort, Seif Ben Salem, Karmène Souyris, Victor Barteau, and Mohamed Laaidi) for the development of the FACE-SZ computer interface, data management, quality control, and regulatory aspects. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fernandes BS, Steiner J, Bernstein H-G, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2015;21:554–564. doi:10.1038/mp.2015.87. [DOI] [PubMed]
- 2. Fond G, d’Albis M-A, Jamain S, et al. The promise of biological markers for treatment response in first-episode psychosis: a systematic review. Schizophr Bull. 2015;41:559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:102–111. [DOI] [PubMed] [Google Scholar]
- 4. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia. Clin Schizophr Relat Psychoses. 2014;7:223–230. [PubMed] [Google Scholar]
- 5. Dickerson F. Elevated C reactive protein in adults predicts the later development of late-onset or very-late-onset schizophrenia. Evid Based Ment Health. 2015;18:e2–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strawbridge R, Arnone D, Danese A, Papadopoulos A, Vives AH, Cleare AJ. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–1543. [DOI] [PubMed] [Google Scholar]
- 7. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. [DOI] [PubMed] [Google Scholar]
- 8. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J Affect Disord. 2013;150:456–459. [DOI] [PubMed] [Google Scholar]
- 9. Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:21. doi:10.1615/CritRevNeurobiol.v14.i1.10. [PubMed] [Google Scholar]
- 10. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laurin D, Curb JD, Masaki KH, White LR, Launer LJ. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol Aging. 2009;30:1724–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancinella A, Mancinella M, Carpinteri G, et al. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Arch Gerontol Geriatr. 2009;49:185–194. [DOI] [PubMed] [Google Scholar]
- 13. Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo H-K, Yen C-J, Chang C-H, Kuo C-K, Chen J-H, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. [DOI] [PubMed] [Google Scholar]
- 15. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93:261–265. [DOI] [PubMed] [Google Scholar]
- 16. Joseph J, Depp C, Martin AS, et al. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr Res. 2015;168:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schürhoff F, Fond G, Berna F, et al. A National network of schizophrenia expert centres: an innovative tool to bridge the research-practice gap. Eur Psychiatry. 2015;30: 728–735. [DOI] [PubMed] [Google Scholar]
- 18. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 19. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. [DOI] [PubMed] [Google Scholar]
- 20. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 21. Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex J Devoted Study Nerv Syst Behav. 1978;14:234–244. [DOI] [PubMed] [Google Scholar]
- 22. Mackinnon A, Mulligan R. [The estimation of premorbid intelligence levels in French speakers]. L’Encéphale. 2005;31:31–43. [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D, Coalson DL, et Raiford SE. WAIS-IV: Wechsler adult intelligence scale. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 24. Ryan JJ, Ward LC. Validity, reliability, and standard errors of measurement for two seven-subtest short forms of the Wechsler Adult Intelligence Scale—III. Psychol Assess. 1999;11:207. [Google Scholar]
- 25. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 26. Godefroy O. Fonctions exécutives et pathologies neurologiques et psychiatriques: évaluation en pratique clinique. Groupe de Boeck. 2008. [Google Scholar]
- 27. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation. Harcourt Assessment Company; 2000. [Google Scholar]
- 28. Poitrenaud J, Deweer B, Kalafat M, Van der Linden M. Adaptation en langue française du California Verbal Learning Test. Paris Éditions Cent Psychol Appliquée. 2007. [Google Scholar]
- 29. Baddeley AD, Emslie H, Nimmo-Smith I. Doors and people: a test of visual and verbal recall and recognition. Thames Valley Test Company. 1994. [Google Scholar]
- 30. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 31. Thalheimer W, Cook S. How to calculate effect sizes from published research: a simplified methodology. Work-Learn Res. 2002;1–9. [Google Scholar]
- 32. Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures. Pharmacoeconomics. 1999;15:141–155. [DOI] [PubMed] [Google Scholar]
- 33. Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–189. [DOI] [PubMed] [Google Scholar]
- 34. Tabachnick BG, Fidell LS. Using Multivariate Statististics. Boston, MA: Allyn and Bacon Pearson Education Company; 2001. [Google Scholar]
- 35. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93:261–265. [DOI] [PubMed] [Google Scholar]
- 36. Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 37. Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr Bull. 2014;40:602–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161:102–112. [DOI] [PubMed] [Google Scholar]
- 42. Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. [DOI] [PubMed] [Google Scholar]
- 43. Fraguas D, Díaz-Caneja CM, Pina-Camacho L, Janssen J, Arango C. Progressive brain changes in children and adolescents with early-onset psychosis: a meta-analysis of longitudinal MRI studies. Schizophr Res. 2014. [DOI] [PubMed] [Google Scholar]
- 44. Tse MT, Piantadosi PT, Floresco SB. Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research. Biol Psychiatry. 2015;77:929–939. [DOI] [PubMed] [Google Scholar]
- 45. Zhou Y, Ma X, Wang D, et al. The selective impairment of resting-state functional connectivity of the lateral subregion of the frontal pole in schizophrenia. PloS One. 2015;10:e0119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis J, Moylan S, Harvey BH, Maes M, Berk M. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48:512–529. [DOI] [PubMed] [Google Scholar]
- 47. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161:102–112. [DOI] [PubMed] [Google Scholar]
- 48. Chang HH, Lee IH, Gean PW, et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav Immun. 2012;26:90–95. [DOI] [PubMed] [Google Scholar]
- 49. Krogh J, Benros ME, Jørgensen MB, Vesterager L, Elfving B, Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun. 2014;35:70–76. [DOI] [PubMed] [Google Scholar]
- 50. Fond G, Godin O, Brunel L, et al. Peripheral sub-inflammation is associated with antidepressant consumption in schizophrenia. Results from the multi-center FACE-SZ data set. J Affect Disord. 2016;191:209–215. [DOI] [PubMed] [Google Scholar]
- 51. Amidi A, Wu LM, Agerbaek M, et al. Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psychooncology. 2015;24:1174–1180. [DOI] [PubMed] [Google Scholar]
- 52. Mangiafico RA, Sarnataro F, Mangiafico M, Fiore CE. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to C-reactive protein and D-dimer levels. Age Ageing. 2006;35:60–65. [DOI] [PubMed] [Google Scholar]
- 53. Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shucard JL, Gaines JJ, Ambrus J, Shucard DW. C-reactive protein and cognitive deficits in systemic lupus erythematosus. Cogn Behav Neurol Off J Soc Behav Cogn Neurol. 2007;20:31–37. [DOI] [PubMed] [Google Scholar]
- 55. Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. [DOI] [PubMed] [Google Scholar]
- 56. Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. [DOI] [PubMed] [Google Scholar]
- 58. Akrivos J, Ravona-Springer R, Schmeidler J, et al. Glycemic control, inflammation, and cognitive function in older patients with type 2 diabetes. Int J Geriatr Psychiatry. 2015;30:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. Am J Geriatr Psychiatry. 2012;20:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Teunissen CE, Van Boxtel MPJ, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. [DOI] [PubMed] [Google Scholar]
- 62. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia. Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 63. Kessler RK, Giovannetti T, MacMullen LR. Everyday action in schizophrenia: performance patterns and underlying cognitive mechanisms. Neuropsychology. 2007;21:439. [DOI] [PubMed] [Google Scholar]
- 64. Penadés R, Catalán R, Puig O, et al. Executive function needs to be targeted to improve social functioning with Cognitive Remediation Therapy (CRT) in schizophrenia. Psychiatry Res. 2010;177:41–45. [DOI] [PubMed] [Google Scholar]
- 65. Tolman AW, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gunstad J, Bausserman L, Paul RH, et al. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. J Clin Neurosci Off J Neurosurg Soc Australas. 2006;13:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heringa SM, van den Berg E, Reijmer YD, et al. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population - the Hoorn Study. Psychoneuroendocrinology. 2014;40:108–118. [DOI] [PubMed] [Google Scholar]
- 68. Schram MT, Euser SM, de Craen AJM, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. [DOI] [PubMed] [Google Scholar]
- 69. Faugere M, Micoulaud-Franchi JA, Alessandrini M, et al. Quality of life is associated with chronic inflammation in schizophrenia: a cross-sectional study. Sci Rep. 2015;5:10793. doi:10.1038/srep10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Micoulaud-Franchi J-A, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165:94–96. [DOI] [PubMed] [Google Scholar]
- 71. Wu MD, Hein AM, Moravan MJ, Shaftel SS, Olschowka JA, O’Banion MK. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behav Immun. 2012;26:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koo JW, Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci. 2008;105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goshen I, Kreisel T, Ounallah-Saad H, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. [DOI] [PubMed] [Google Scholar]
- 74. Cacci E, Claasen J-H, Kokaia Z. Microglia-derived tumor necrosis factor-α exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. [DOI] [PubMed] [Google Scholar]
- 75. Aravena O, Pesce B, Soto L, et al. Anti-TNF therapy in patients with rheumatoid arthritis decreases Th1 and Th17 cell populations and expands IFN-γ-producing NK cell and regulatory T cell subsets. Immunobiology. 2011;216:1256–1263. [DOI] [PubMed] [Google Scholar]
- 76. Sommer IE, van Westrhenen R, Begemann MJH, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2013, sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fond G, Hamdani N, Kapczinski F, et al. Effectiveness and tolerance of anti-inflammatory drugs’ add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr Scand. 2014;129:163–179. [DOI] [PubMed] [Google Scholar]
- 78. Schaub N, Reichlin T, Meune C, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–256. [DOI] [PubMed] [Google Scholar]
- 79. Godin O, Leboyer M, Gaman A, et al. Metabolic syndrome, abdominal obesity and hyperuricemia in schizophrenia: results from the FACE-SZ cohort. Schizophr Res. 2015. [DOI] [PubMed] [Google Scholar]
- 80. Vincenzi B, Stock S, Borba CPC, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res. 2014;159:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fond G, Girerd N, Clavel F, Tamouza R, Leboyer M. Recently discovered properties of aspirin may be doubly helpful in bipolar disorders. Med Hypotheses. 2014;82:640–641. [DOI] [PubMed] [Google Scholar]
- 82. Kelley BJ, McClure LA, Unverzagt FW, et al. Regular aspirin use does not reduce risk of cognitive decline. J Am Geriatr Soc. 2015;63:390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wichmann MA, Cruickshanks KJ, Carlsson CM, et al. NSAID use and incident cognitive impairment in a population-based cohort. Alzheimer Dis Assoc Disord. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nilsson A, Tovar J, Johansson M, Radeborg K, Björck I. A diet based on multiple functional concepts improves cognitive performance in healthy subjects. Nutr Metab. 2013;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wolf SA, Melnik A, Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Spec Issue Adapt Immun Cent Nerv Syst Funct. 2011;25:971–980. [DOI] [PubMed] [Google Scholar]
- 87. Fond G, Godin O, Llorca PM, Leboyer M. Abnormal C-reactive protein (CRP) levels in schizophrenia and schizoaffective disorders. Results from the FACE-SZ dataset. Eur Psychiatry. 2015;30:S112. [Google Scholar]
- 88. Barzilay R, Lobel T, Krivoy A, Shlosberg D, Weizman A, Katz N. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8–12. [DOI] [PubMed] [Google Scholar]
- 89. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 90. Sicras-Mainar A, Rejas-Gutiérrez J, Navarro-Artieda R, Blanca-Tamayo M. C-reactive protein as a marker of cardiovascular disease in patients with a schizophrenia spectrum disorder treated in routine medical practice. Eur Psychiatry. 2013;28:161–167. [DOI] [PubMed] [Google Scholar]
- 91. Wysokinski A, Margulska A, Strzelecki D, Kloszewska I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord J Psychiatry. 2015;69:346–353. [DOI] [PubMed] [Google Scholar]
- 92. Boyer L, Richieri R, Dassa D, et al. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Res. 2013;210:381–386. [DOI] [PubMed] [Google Scholar]