Abstract
Background:
Oxidative stress and glutathione (GSH) metabolism dysregulation has been implicated in the pathophysiology of schizophrenia. GAG-trinucleotide repeat (TNR) polymorphisms in the glutamate-cysteine ligase catalytic gene (GCLC), the rate-limiting enzyme for GSH synthesis, are associated with schizophrenia. In addition, GSH may serve as a reserve pool for neuronal glutamate (Glu) through the γ-glutamyl cycle. The aim of this study is to investigate brain [GSH] and its association with GCLC polymorphism, peripheral redox indices and brain Glu.
Methods:
Magnetic resonance spectroscopy was used to measure [GSH] and [Glu] in the medial prefrontal cortex (mPFC) of 25 early-psychosis patients and 33 controls. GCLC polymorphism was genotyped, glutathione peroxidases (GPx) and glutathione reductase (GR) activities were determined in blood cells.
Results:
Significantly lower [GSHmPFC] in GCLC high-risk genotype subjects were revealed as compared to low-risk genotype subjects independent of disease status. In male subjects, [GSHmPFC] and blood GPx activities correlate positively in controls (P = .021), but negatively in patients (P = .039). In GCLC low-risk genotypes, [GlumPFC] are lower in patients, while it is not the case for high-risk genotypes.
Conclusions:
GCLC high-risk genotypes are associated with low [GSHmPFC], highlighting that GCLC polymorphisms should be considered in pathology studies of cerebral GSH. Low brain GSH levels are related to low peripheral oxidation status in controls but with high oxidation status in patients, pointing to a dysregulated GSH homeostasis in early psychosis patients. GCLC polymorphisms and disease associated correlations between brain GSH and Glu levels may allow patients stratification.
Key words: schizophrenia, glutathione, oxidative stress, MRS, GCLC, glutamate, redox, glutathione peroxidase
Introduction
Schizophrenia is a major psychiatric disorder which results from a complex interplay between genetic and environmental risk factors during neurodevelopment. There is increasing evidence suggesting that oxidative stress and dysregulation of the glutathione (GSH) metabolism play a role in the pathophysiology of the disease.1–5 GSH serves as a major cellular redox regulator and antioxidant protecting cell from damages induced by reactive oxygen species. Decreased [GSH] have been reported in cerebro-spinal fluid (−27%) of patients with chronic schizophrenia.6 A “postmortem” study also revealed lower caudate [GSH] in treated patients than in control subjects.7 Furthermore, similar reduction of [GSH] was observed in blood plasma of treated and untreated patients.8–11
Studies of cerebral [GSH] in vivo in schizophrenia are limited and exclusively achievable by using 1H magnetic resonance spectroscopy (MRS). Do et al reported a 52% reduction of [GSH] in medial prefrontal cortex of chronic schizophrenia patients at 1.5T (14 controls and 14 patients, in Do et al6; extended to 18 controls and 20 patients, unpublished results). However, 2 consecutive studies in schizophrenia patients did not reveal significant GSH alterations in the anterior cingulate cortex at 4T (9 controls and 11 patients)12 or in posterior medial frontal cortex at 3T (16 controls and 20 patients), though low [GSH] in this later region were associated with more severe negative symptoms.13 Reasons for such inconsistencies remain unclear. Differences in methodological aspects, such as magnetic field strengths, pulse sequences, number of subjects, and stage of disease may be relevant.
Whether alterations of cerebral [GSH] are already present in the early phase of psychosis (EP) remains an open question. Only 1 study has reported 22% higher [GSH] in medial temporal lobe of first episode patients using PRESS 1H MRS sequence at 3T.14 However, the mean Cramer-Rao lower-bound (CRLB) for the corresponding measurement of GSH was moderately high (~21%). Therefore, further investigations of cerebral [GSH] in EP patients including those in other brain regions are required.
One genetic factor has been reported to influence [GSH] in the periphery: a GAG trinucleotide repeat (TNR) polymorphism in the gene coding for the catalytic (GCLC) subunit of glutamate-cysteine ligase (GCL), the rate-limiting enzyme for GSH synthesis. Importantly, this polymorphism was associated with schizophrenia in 2 case-control studies15: the GCLC high-risk genotypes (ie, with 7/8, 8/8, 8/9 and 9/9 TNR) were more frequent in patients (30%) and were associated with lower GCLC protein expression, GCL activity and [GSH] in fibroblasts when challenged with oxidative stress conditions, as compared with GCLC low-risk genotypes (ie, with 7/7 and 7/9 TNR). However, the peripheral GSH levels may not reflect GSH status in the central nervous system and the association between cerebral GSH levels and GCLC polymorphism has never been investigated. The understanding of their relation may shed light on current discrepancies concerning cerebral [GSH] in psychotic patients.
Studies on the various antioxidant systems in the peripheral tissue of schizophrenia patients showed large discrepancies.1,4 Altered levels of scavenging antioxidant enzymes were reported, however with mixed results.16 Glutathione peroxidases (GPx) are important antioxidant enzymes that eliminate hydrogen and lipid peroxides by converting GSH into oxidized GSH (GSSG). Then GSSG is reduced back to GSH by glutathione reductase (GR; supplementary figure 1s). The precise quantification of blood GSH/GSSG balance in a psychiatric clinical setting is quite challenging and subjected to artefactual oxidations leading to unreliable results.16 To capture the (dys)regulation of redox status, we assessed enzymatic activities of GPx and GR, as well as their ratio (GPx/GR) in blood cells instead of GSH/GSSG ratio.
Besides its antioxidant properties, the tripeptide GSH has been proposed to constitute a reserve pool for neuronal glutamate (Glu) through the γ-glutamyl cycle.17 γ-glutamyltranspeptidase (GGT) transfers γ-glutamyl moiety of extracellular GSH to an acceptor amino acid, generating γ-glutamyl amino acid and cysteinylglycine. On the one side, the cysteinylglycine can then provide substrates to synthesize GSH; on the other side, γ-glutamyl amino acid can be metabolized to amino acid and 5-oxoproline, which can be further catalyzed by the 5-oxoprolinase to form Glu, a precursor of GSH.18,19 The inhibition of 5-oxoprolinase reduces [Glu] in cortical and hippocampal neurons, with no changes in [GSH].17 This mechanism is of high interest in the context of the glutamatergic hypothesis in schizophrenia,20 and has never been evaluated in vivo in humans.
Therefore, the aims of this study were (1) to assess [GSH] in medial prefrontal cortex of early psychosis patients relative to control subjects, (2) to test its association with GAG-TNR of GCLC genotypes and (3) with peripheral GSH redox indices (GPx, GR activities and their ratio GPx/GR) and finally, (4) to investigate the relationship between brain GSH and Glu.
Methods and Materials
Subjects
Twenty-five early psychosis patients (within the first 3 years of treatment for a psychotic disorder and having met psychosis threshold according to the Comprehensive Assessment of At Risk Mental States criteria)21 were recruited from the Treatment and Early Intervention in Psychosis Program (TIPP, University Hospital of Lausanne, Lausanne, Switzerland).22 Medication prescribed to patients followed international recommendations for early psychosis treatment,23 was recorded in the medical file and adherence to treatment was rated by clinicians. CPZ equivalents were calculated on the basis of formulas using regression with power transformation suggested by Andreasen et al24 and of maximum daily doses25 for the remaining medication (table 1).
Table 1.
Demographic Characteristics of Controls and Early Psychosis Patients
| Controls (n = 33) | Patients (n = 25) | P Value | |
|---|---|---|---|
| Age, years (mean ± SD) | 25.4±4.5 | 24.8±6.1 | n.sa |
| Sex, Male/Female | 18/15 | 18/7 | n.sb |
| Ethnicity | Caucasian | Caucasian | — |
| Education of parents, years (mean ± SD) | 14.4±3.6 | 15.1±4.6 | n.sa |
| Cigarettes, smokers/nonsmokers | 4/29 | 13/12 | .001b |
| Handedness, Right/Left/Ambidextrous | 28/5/0 | 22/2/1 | n.sc |
| Illness duration, days (mean ± SD) | — | 933±867 | — |
| CPZ equivalents, mg (mean ± SD) | — | 325±317d | — |
| Antipsychotic medications | |||
| Amisulpride | 3 | ||
| Aripiprazole | 5 | ||
| Clozapine | 1 | ||
| Olanzapine | 2 | ||
| Paliperidone | 1 | ||
| Quetiapine | 5 | ||
| Risperidone | 3 | ||
| None | 5 | ||
Note: CPZ equivalents, chlorpromazine equivalents; n.s., not significant (P > .05).
aUnpaired 2-tailed Student t test.
bChi-square.
cFisher’s exact test.
dFive patients without antipsychotic medication.
Onset of illness was defined as the time when a definite diagnosis of psychosis was reached by the presence of clear evidence of delusions, hallucinations, first rank symptoms, catatonic symptoms for at least 1 week on the basis of elements gathered by treating psychiatrist and case managers through interviews with patients, their relatives and any mental health professional who would have been involved in the care of the patient at that time. Duration of illness was defined as the time that elapsed between onset of illness and the time when MRI was performed. Diagnosis for patients was based on DSM-IV criteria and is the result of an expert consensus procedure based on information gathered over the 3 years of treatment.
Thirty-three age-, sex-matched healthy controls were recruited and assessed by the Diagnostic Interview for Genetic Studies26 with the following exclusion criteria: having major mood, psychotic, or substance-use disorder and a first-degree relative with a psychotic disorder. Neurological disorder and severe head trauma were exclusion criteria for all subjects. Demographic and clinical details of all participants are shown in table 1. All participants gave informed written consent prior to participate in this study which was approved by the ethics review board of the Lausanne University Hospital.
1H Magnetic Resonance Spectroscopy
All MRS measurements were carried out on a 3T MR scanner (Magnetom TimTrio, Siemens Healthcare) with a transverse electromagnetic (TEM 3000) head coil (MR Instruments, Inc). The magnetic field homogeneity was optimized by adjusting first- and second-order shims using FAST(EST)MAP.27,28 In vivo 1H MR spectra were acquired from a volume of interest (VOI = 20×20×25 mm3) positioned in the medial prefrontal cortex (mPFC; figure 1A) using a short-TE spin-echo full-intensity acquired localized single voxel spectroscopy technique (SPECIAL).29,30 The following scan parameters were used: echo time/repetition time (TE/TR) = 6/4000ms, acquisition bandwidth = 2kHz, number of averages = 148, vector size = 2048. Outer volume suppression (OVS)31 and water suppression with variable-pulse power and optimized relaxation delays (VAPOR)32 were applied prior to the SPECIAL localization sequence.
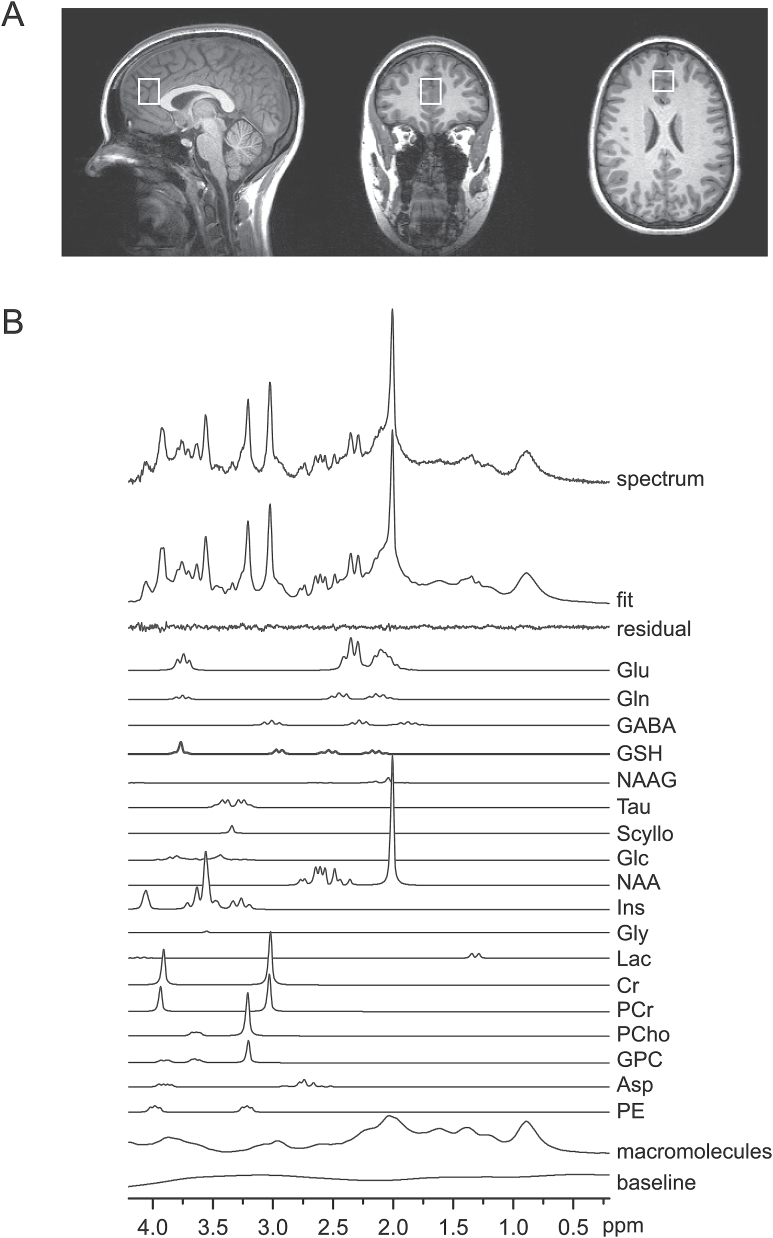
Fig. 1.
(A) T1-weighted MPRAGE images with the volume of interest (20×20×25 mm3) for magnetic resonance spectroscopy (MRS) in the medial prefrontal cortex. (B) A representative 1H MR spectrum acquired with the SPECIAL sequence at 3T (TE/TR = 6/4000ms, number of averages = 148), the corresponding LCModel spectral fit, fit residual, macromolecules, baseline and individual metabolite fits including glutathione (GSH).
In addition, to evaluate tissue composition inside the VOI, 3-dimensional T1-weighted anatomical images were obtained using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence33 with a 32-channel head array coil, TE/TR = 2.98/2300ms, inversion time = 900ms, flip angle = 9°, field of view = 240×256mm2, matrix = 240×256, slice thickness = 1.2mm.
Spectral Quantification
To obtain [GSHmPFC] and [GlumPFC], water suppressed in vivo 1H MR spectra were analyzed by LCModel (Stephen Provencher, Inc)34 as a linear combination of model spectra provided in a basis-set consisting of 20 simulated individual metabolite spectra: alanine (Ala), aspartate (Asp), phosphocreatine (PCr), creatine (Cr), γ-aminobutyric acid (GABA), glutamine (Gln), Glu, phosphorylcholine (PCho), glycerophosphorylcholine (GPC), GSH, glucose (Glc), lactate (Lac), glycine (Gly), myo-inositol (mIns), N-acetylaspartyl-glutamate (NAA), N-acetylaspartylglutamate (NAAG), ascorbate (Asc), phosphoryl-ethanolamine (PE), scyllo-inositol (Scyllo), taurine (Tau), and an experimentally measured macromolecule baseline.35 The errors of calculated concentrations obtained from the fit are shown by CRLBs. Unsuppressed water 1H MR spectra were used as an internal reference for the quantification of metabolite concentrations. The spectral range for analysis was set to 0.2–4.2ppm. Tissue composition inside the VOI was calculated based on the segmentation of 3D T1-weighted anatomical images using an in-house software.36 Water concentrations, used in LCModel analysis, were calculated based on the volume fractions of white matter (WM), grey matter (GM), and cerebrospinal fluid (CSF) assuming water concentrations of WM, GM, and CSF are 35880, 43300, and 55556mM, respectively. Metabolite concentrations were then divided by the fraction of WM and GM to correct for CSF inside the VOI, since metabolites are mainly present in WM and GM.37 The signal-to-noise ratio (SNR) was obtained using NAA peak height at 2.01ppm divided by the SD of the noise.
Genotyping of GCLC Tri-nucleotide Polymorphism
DNA was extracted from whole blood. PCR was performed according to Walsh et al38 and modified as in Gysin et al15: in a 20 μL reaction mix with GoTaq polymerase, 1.5mM MgCl2, 0.25 μM of each primer (forward: TTCTGCGGGCGGCTGAGTGTCC; reverse: ATGGCGCTTGGTTTCCTCCC), 0.2mM of dNTP and 100ng of genomic DNA. Amplification temperature were: 95° for 2 minutes followed by 32 cycles of 95° for 30 seconds, 62° for 30 seconds, 72° for 1 minute and 1 step at 72° for 5 minutes. PCR products were separated on 10% polyacrylamide gels and were visualized under UV after 15 minutes staining in GelRed solution. Classification into GCLC high- or GCLC low-risk genotype is based on the number of GAG repeats as defined in Gysin et al15 (7/8, 8/8, 8/9, 9/9 and 7/7, 7/9 respectively).
[GSHB], GPx and GR Enzymatic Activities in Blood
Blood was collected by venipuncture in Vacutainer-tubes coated with Li-heparinate (Becton Dickinson), between 7 AM and 8:30 AM under restricted activity conditions and fasting from the previous midnight. An aliquot of whole blood is sampled and frozen at −80°C until analysis of [GSHB]. [GSHB] was measured in 45 μL of whole blood using a diagnostic kit and according to manufacturer instructions (Calbiochem); [GSHB] are normalized to blood volume. Blood cells were prepared as in Gysin et al15. All manipulations were performed rapidly with cooling to avoid artefactual oxidation of thiol compounds. Essentially, GR activity39 was assessed in 8 μL of hemolyzed blood incubated in a phosphate buffer solution (100mM, pH 7.5) with EDTA (0.6mM), oxidized glutathione (2.5mM), NADPH (0.25mM). GPx activity40 was assessed in 8μL of hemolyzed blood incubated in phosphate buffer solution (100mM, pH 7.5) with EDTA(0.6mM), glutathione (2.5mM), NADPH (0.25mM), GR (0.84U/ml; Fluka) and Tert-butyl hydroperoxide (0.8mM, Fluka). Enzymatic activities were determined in triplicates as a function of the decrease in NADPH measured at 340nm and normalized to hemoglobin content.
Statistical Analysis
Differences between patients and controls in sex, smoking and handedness were assessed using Chi-square test or Fisher’s exact test accordingly. Differences in age, education of parents, fractions of WM, GM and CSF, spectral quality (SNR and linewidth) between patients and controls, [GSHmPFC] between smokers and non-smokers were evaluated by unpaired t test (2-tailed). The effects of GCLC GAG-TNR polymorphism (low-risk vs high-risk), disease (controls vs patients), sex (male vs female) and age on [GSHmPFC], [GSHB], blood GPx and GR activities were investigated using generalized linear model in Matlab (R2013b,The MathWorks, Inc). Two-tailed Mann-Whitney test was used to investigate the alterations in [GlumPFC] and the difference of [GSHmPFC] between medicated and unmedicated patients. All correlation tests were performed using the Spearman rank correlation (2-tailed) in GraphPad Prism (Version 5.04, GraphPad Software, Inc). The difference between 2 independent correlation coefficients was tested online (Calculation for the test of the difference between 2 independent correlation coefficients is available from http://quantpsy.org).
Results
There were no statistical differences in age, sex, handedness and education of parents between patients and controls indicating a good matching for these criteria (table 1). All individuals were Caucasian. Since the high prevalence of smoking in schizophrenia patients,41 we also observed more smokers in patients.
A representative short-TE 1H MR spectrum obtained from mPFC, LCModel spectral fit and individual metabolites fits are shown in figure 1B. [GSHmPFC] and [GlumPFC] were measured by short-TE 1H MRS at 3T and quantified using LCModel yielding CRLBs of 10±3% and 2.0±0.5% (mean ± SD), respectively. In addition, other 11 metabolites were quantified from LCModel fitting including Asp, Cr, PCr, Gln, Ins, NAA, total Choline (GPC+PCho) with mean CRLBs < 10% and GABA, Lac, PE, Tau with mean CRLBs < 20%. Spectral SNR and linewidth (LCModel output) were 104±16, 0.031±0.005ppm for controls and 97±12, 0.033±0.006ppm for patients, respectively. No significant differences (P > .05) in spectral quality (ie, SNR and linewidth) between groups were observed. Tissue compositions inside the MRS voxel were measured and there is no differences of WM, GM and CSF fractions between patients and controls (supplementary table 1s).
GSH Levels in mPFC
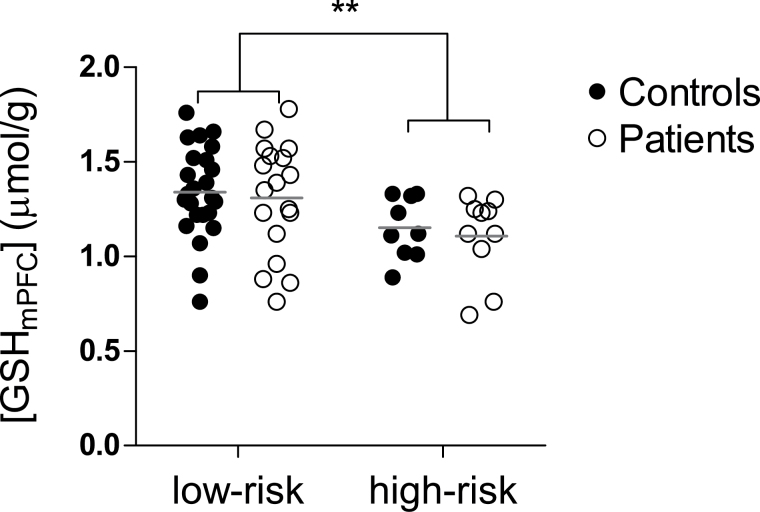
To discern [GSHmPFC] changes in early psychosis and its association with GCLC polymorphism, GCLC genotypes were included as a covariant together with age and sex to test whether [GSHmPFC] were different between 25 EP patients and 33 controls. There were significantly lower [GSHmPFC] in subjects with the GCLC high-risk genotypes (1.15±0.17 μmol/g) as compared to those with low-risk genotypes (1.34±0.25μmol/g; P = .006; figure 2), independent of the disease status (P > .05), age (P > .05) and sex (P > .05). To study the potential effect of medication, illness duration and smoking, we first correlated [GSHmPFC] with CPZ equivalents and disease duration. No significant correlations were found between them (P > .05). [GSHmPFC] were further compared between smokers and non-smokers, no significant difference was revealed (P > .05). Including smoking as one covariant showed no effect on the result and [GSHmPFC] association with GCLC polymorphism still remained significant (P = .006).
Fig. 2.
[GSHmPFC] are lower in glutamate-cysteine ligase catalytic gene (GCLC) high-risk genotypes (7/8, 8/8, 8/9 and 9/9; n = 17) than in low-risk genotypes (7/7 and 7/9; n = 41). From left to right: [GSHmPFC] in low-risk genotype controls, low-risk genotype patients, high-risk genotype controls and high-risk genotype patients, respectively. Each horizontal bar indicates the mean value of the group. **P = .006.
To decipher whether blood GSH reflects cerebral GSH, we studied the relationship between them. No correlation was found between [GSHB] and [GSHmPFC] (supplementary figure 2s) and [GSHB] were not affected by disease, age, sex, and GCLC polymorphism (supplementary table 2s).
Relationship Between [GSHmPFC] and [GlumPFC]
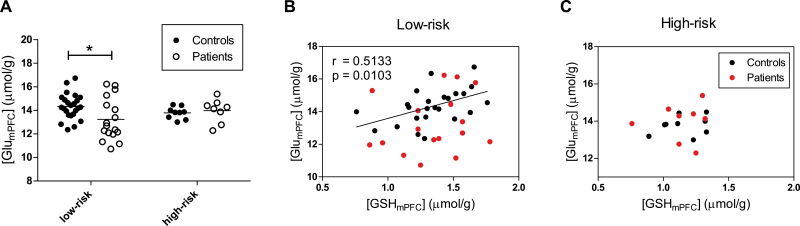
We revealed that [GSHmPFC] were affected by GCLC polymorphism and additionally GSH may serve as a reservoir for neuronal Glu,17 which is involved in the NMDA receptor hypofunction hypothesis of schizophrenia. Therefore, we evaluated [GlumPFC] between patients and controls in GCLC low-risk and high-risk group respectively and revealed a 8% reduction of [GlumPFC] in GCLC low-risk patients relative to low-risk controls (P = .01), while no difference was found between high-risk genotypes in controls and patients (figure 3A). In GCLC low-risk controls, [GlumPFC] positively correlated with [GSHmPFC] (r = .5133, P = .010; figure 3B); however this correlation was absent in low-risk genotype patients. Interestingly, correlations were observed neither in controls nor in patients with high-risk genotypes (figure 3C).
Fig. 3.
(A) [GlumPFC] in glutamate-cysteine ligase catalytic gene (GCLC) low-risk controls (n = 24), low-risk patients (n = 17), high-risk controls (n = 9) and high-risk patients (n = 8). The bars represent mean values of each data group. *P = .010. Different associations between [GSHmPFC] and [GlumPFC] in GCLC low-risk (B) and high-risk (C) subjects. In GCLC low-risk controls, [GSHmPFC] positively correlated with [GlumPFC]. The correlations between [GSHmPFC] and [GlumPFC] were absent in GCLC low-risk patients, high-risk controls and high-risk patients.
Correlation Between Brain GSH and Peripheral Redox Enzymatic Activities
To test the association between cerebral GSH and peripheral redox indices, we investigated enzymatic activities of GPx and GR in blood cells, and their correlations with [GSHmPFC]. No significant differences were observed for GPx and GR activities between patients and controls, nor between GCLC high-risk and low-risk genotype (supplementary table 2s). Female subjects showed higher GR activities than male subjects (P = .015, supplementary table 2s), which is consistent with,42 therefore, following analyses were performed separately for each sex group.
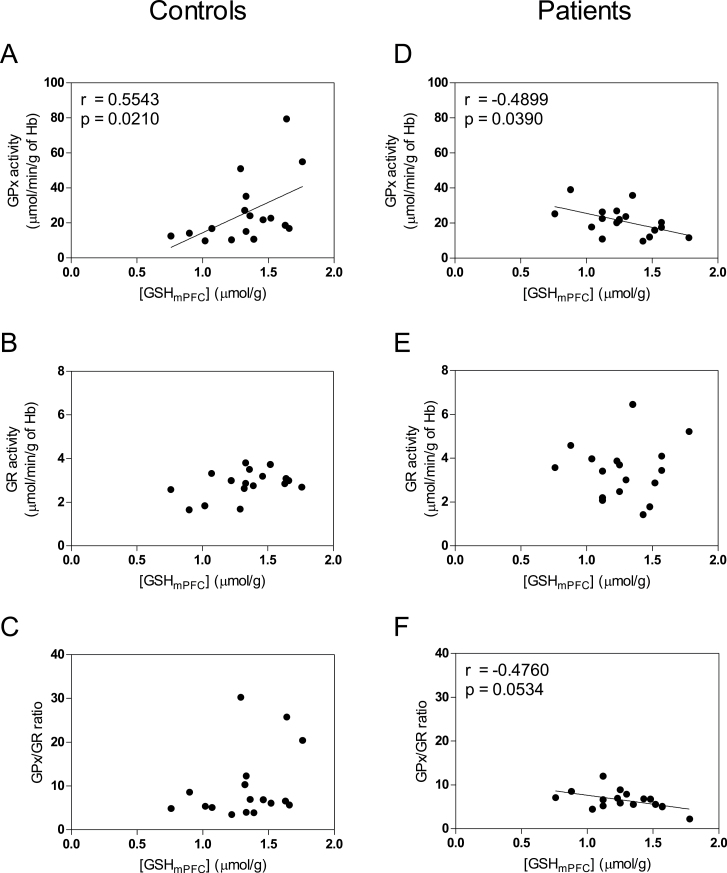
[GSHmPFC] were positively correlated with GPx activities in male controls (figure 4A; r = .5543, P = .021). In contrast, in male patients an inverse correlation was obtained between [GSHmPFC] and GPx activities (figure 4D; r = −.4899, P = .039). There is a trend of a positive correlation between GR activities and [GSHmPFC] in male controls (figure 4B; r = .3973, P = .114) but not in male patients (figure 4E; r = .0111, P = .966). The ratio of GPx/GR activity, which reflects the regulation of redox status, tends to correlate negatively with [GSHmPFC] in male patients (figure 4F; r = −.4760, P = .0534), whereas such correlation was absent in male controls (figure 4C). We further tested the significance of the difference between patients and controls for the correlation coefficients of [GSHmPFC] with GPx activities, and with GPx/GR ratio. Patients and controls demonstrated significant differences in correlation coefficients of [GSHmPFC] with GPx activities (z = 2.07, P = .002) and with GPx/GR ratio (z = 2.18, P = .029). For female controls or patients, we did not observe any significant correlations (supplementary figure 3s).
Fig. 4.
Correlations between [GSHmPFC] and blood redox enzymatic activities of glutathione peroxidases (GPx), glutathione reductase (GR) and ratio GPx/GR in male controls (n = 17) and in male patients (n = 17). [GSHmPFC] were positively correlated with GPx activities in controls (A). Conversely, in patients [GSHmPFC] were negatively correlated with GPx activities (D). There is a trend of positive correlation between GR activities and [GSHmPFC] in controls (B) but not in patients (E). The ratio of GPx/GR activity, reflecting the regulation of redox status, tends to correlate negatively with [GSHmPFC] in patients (F) not in controls (C).
Discussion
In this study, we assessed [GSHmPFC] of 25 early psychosis patients and 33 control subjects. We showed that GAG-TNR polymorphisms in GCLC, which have been previously linked to schizophrenia, are associated with [GSHmPFC] irrespectively of disease status. Additional analyses also revealed that correlation between cortical GSH levels and peripheral redox marker, ie, blood cells GPx enzymatic activities, differed in patients and controls: their correlation is positive in controls and negative in patients. Low [GSHmPFC] are associated with low peripheral oxidation status in controls but with high oxidation status in patients, suggesting a dysregulation of GSH homeostasis under oxidative conditions. Furthermore, in GCLC low-risk genotypes, [GlumPFC] are lower in patients as compared to controls, while it is not the case for GCLC high-risk genotypes. This suggests a predominant pathogenic role of glutamatergic system impairments in GCLC low-risk genotypes.
In vivo Measurement of Cerebral GSH
The in vivo measurement of GSH concentration in human brain using (short-TE) 1H MRS is challenging because of its low concentration and strong spectral overlap mainly with resonances eg, originated from glutamate and glutamine. Therefore, spectral editing schemes43–45 combined with localization sequences have been developed for the detection of GSH. Although an editing approach provides a resolved measurement of GSH, several downside aspects remain, such as signal reduction induced by editing efficiency,44 sensitive editing performance to the frequency drift of the narrow band editing pulse,46 T2 relaxation assumption for quantification and restricted number of measureable metabolites.44 As an alternative approach, short-TE MRS has been employed to detect GSH at 3T and above.12,29,47,48 The main benefit of short-TE MRS, apart from GSH measurement, is the possibility to simultaneously assess a large number of metabolites. Nevertheless, due to spectral overlap, GSH quantification relies on deconvolution methods such as LCModel.34 In the current study, we performed GSH measurement by short-TE 1H-MRS to obtain other metabolic information, eg, Glu, in parallel. A previous validation study of Terpstra et al12 compared GSH measurements using both, MEGA-PRESS and short-TE STEAM methods, at 4T and demonstrated a good agreement between these 2 methods. Recently, Wijtenburg et al48 showed an excellent reproducibility of GSH measurement using short-TE MRS at 3T. We further evaluated the accuracy and precision of GSH measurement by the short-TE MRS method at 3T showing that comparable spectral quality in terms of SNR and linewidth would allow the accurate measurement of GSH changes between groups.49 Therefore, the matched spectral quality is a prerequisite for the detection of GSH alterations. This requirement was well satisfied in this work as judged from equivalently high spectral quality of both patient and control groups.
Association of [GSHmPFC] With GCLC Polymorphism
The current study provided the original finding that GCLC high-risk genotypes are associated with low [GSHmPFC]. The functional impact of short GAG repeats on GCLC regulation is still being debated: at the molecular level, a recent study suggested that the length of the TNR modulates mRNA translation,50 but it is controversial whether longer repeats increase or decrease GCLC and subsequently GSH levels. Interestingly, Nichenametla et al suggest that the effect might be cell and tissue specific. Indeed, in vitro,50 in tumor cell lines,51 and in mononuclear blood cells,50 the 7/7 genotype (ie, GCLC low-risk) is associated with low GSH and low GCL activity as compared to 9/9 genotype (ie, GCLC high-risk). But in other systems, such as red blood cells or fibroblasts, submitted to oxidative stress the regulation is opposite: 7/7 genotype display more GSH and higher GCL activity than 9/9 genotype.15,50,52 Similarly, we now show that GCLC low-risk genotypes (7/7 and 7/9) are associated with higher [GSHmPFC] than high-risk genotypes (9/9, 8/8, 8/9, 7/8). Therefore, our study highlights which type of regulation is linked with GAG repeats in cerebral cortex. Since GCLC high-risk genotypes were more frequent in patients,15 this result may thus clarify the potential implication of GCLC high-risk genotypes in psychiatric diseases.
In addition, this polymorphism in GCLC could be a plausible reason for not revealing [GSHmPFC] changes in the patient cohort of the current study, ie, group differences depend on the proportion of the various GAG-TNR genotypes. Indeed, in contrast to the larger cohort of patients with chronic schizophrenia previously studied, in which the GCLC high-risk genotypes were more frequently observed in the disease group,15 this is not the case for the present small sample in which the percentage of high-risk controls subjects (27%) was similar to that of EP patients (31%). Importantly, this could thus be an explanation for inconsistent results observed for brain GSH levels in schizophrenia patients, ie, essentially different GAG-TNR genotypes distribution in the very small cohorts of previous studies leading to discrepancies. In this regard, it is important to note that allele frequencies vary strongly with the ethnic background and therefore not controlling for this factor may induce bias.52,53 In the context of a redox dysregulation hypothesis in schizophrenia pathophysiology,2,3 the presence of GCLC high-risk genotypes in the control population suggests the possibility of a protective factor in healthy subjects carrying these variants.
Moreover, stratification of patients based on this genetic marker may help identifying distinct pathogenic mechanisms in GCLC low-risk vs high-risk schizophrenia patients. Indeed, in GCLC low-risk individuals [GlumPFC] are lower in patients as compared to controls, while it is not the case in GCLC high-risk genotypes (figure 3A). Furthermore, a positive correlation was found between [GlumPFC] and [GSHmPFC] in GCLC low-risk controls, but not in low-risk patients (figure 3B). This contrasts with GCLC high-risk genotypes, in which the tight correlation between [GSHmPFC] and [GlumPFC] is absent both in controls and patients (figure 3C). However, the latter result was based on a small number of high-risk subjects; a relationship between brain GSH and Glu levels may emerge with a larger number of subjects. Together, these data suggest that aberrant functions of enzymes in the γ-glutamyl cycle or other glutamatergic system impairments may be critical, at least in GCLC low-risk patients. Note that Glu and GSH were both quantified using water as an internal reference, therefore, water can be a potential driving factor in the correlation between GSH and Glu. However, such effect should be general for all groups, yet we did not see a correlation eg, in low-risk patients, suggesting that such an effect is likely of limited importance.
The present study did not reveal altered [GSHmPFC] in early psychosis patients and therefore contrasts with findings in patients with chronic schizophrenia.6 Apart from the genetic factor mentioned above, other hypotheses can be mentioned (1) GSH measurement was performed under resting conditions and alterations that can be reliably measured may only appear under particular conditions, such as psychological stress, which leads to oxidative stress at cellular levels.54 Indeed, lower [GSH] in fibroblasts of GCLC high-risk subjects as compared to low-risk genotypes were only observed under oxidative stress conditions, suggesting a deficit in the induction of regulatory mechanisms or adaptive responses. (2) GSH concentration determination by MRS does not allow the discrimination of GSH in the neuronal, glial or extracellular pools.55 As [GSH] is about 10 times higher in astrocytes than in neurons, a differential neuronal regulation might be masked. (3) Early psychosis patients might have an intermediary phenotype between control subjects and patients with chronic schizophrenia. There could be a stage specificity for oxidative stress in the brain: alterations of cerebral [GSH] might be more prominent in chronic phase of the disease, while soluble superoxide dismutase-1 defects have recently been suggested to be an early phase marker.56 Importantly, our data indicates that medication and smoking are probably not biases, as we did not find a correlation between [GSHmPFC] and CPZ equivalents, and as a covariant smoking has no effects on [GSHmPFC] and the current results. Finally, there is a possibility that antipsychotics may have antioxidant effect and raise GSH to levels comparable to healthy controls. Indeed in the current study with early psychosis patients, the majority were medicated (20 out of 25) while in our previous study with chronic schizophrenia patients,6 only a minority were medicated (5 out of 14). The comparisons within studies of unmedicated to medicated patients are likely underpowered to detect a difference in brain GSH levels due to medication status. However, comparison of the 2 studies suggests that 2 key factors differed between them: illness phase (early vs chronic stage), as well as medication status. The medication effect on cerebral GSH levels thus requires further investigation.
Dysregulated GSH Redox Activity in Patients
Glutathione is critically involved in redox regulation: A balanced activity of GPx to eliminate peroxides by metabolizing GSH and of GR to recycle GSH is crucial to maintain GSH homeostasis (supplementary figure 1s). Disturbed GSH redox coupling with the antioxidant defense system was suggested in the postmortem caudate, supported by the lack of correlations between GSH and GPx or GR in patients.7 Our study indicates similar defects of redox regulation in vivo in early psychosis (figure 4). In male subjects, blood GPx activities and brain GSH levels correlate negatively in patients (figure 4D), but positively in controls (figure 4A) with a significant difference between the 2 correlation coefficients. Similarly, we observed a trend of inverse correlation between blood GPx/GR enzymatic ratio and brain GSH levels in patients (figure 4F) but not in controls (figure 4C), with a significant difference between the 2 correlation coefficients. On the other hand, blood GR activities and brain GSH levels were not correlated in both patients and controls. These correlation analyses of brain GSH levels with blood GPx and GPx/GR activities imply a proper regulation of the redox balance in controls and a disrupted redox homeostasis in patients. Specifically, low brain GSH levels are associated with low peripheral oxidation status in controls but with high oxidation status in patients, as indicated by their high blood GPx activities, pointing to a dysregulated GSH homeostasis in early psychosis patients.
Lack of Link Between Peripheral and Central GSH Levels
The lack of relationship between [GSHB] and [GSHmPFC] is consistent with the reported lack of significant transport system for GSH across the blood brain barrier.57 Moreover peripheral GSH levels measurements showed large discrepancies in the literature due to not only differences in analytical methodologies but also in testing materials (whole blood vs serum vs blood cells vs plasma), exposure to medication (drug naïve vs drug withdrawal vs medicated), stages of the disease (early psychosis vs chronic or active vs remission phase), lifestyle or dietary pattern.1 It is also important to note that blood GSH levels are susceptible to artefactual oxidation during preparation and longtime storage of samples, unless special care is taken to prevent it immediately after blood collection (such as scold conditions, thiol blocking reagents), which is difficult to guarantee in psychiatric clinical settings. To our knowledge, the present study is the first allowing a comparison between [GSHB] and [GSHmPFC], revealing that they are not correlated. This suggests that peripheral GSH levels unlikely reflects brain levels, in keeping with Wu et al58 and the reported predominant liver origin of blood GSH.59,60 Raffa et al10,11 reported decreased plasma GSH levels in drug naïve first episode patients. On the other hand, in the current study in which maximal care was taken to minimize artefactual factors during blood collection and processing, no difference was found in total blood GSH levels (blood cells plus plasma) of medicated patients in the EP vs matched controls. Indeed, GSH levels quantification in total blood was chosen instead of plasma levels as the latter are susceptible to more variability due to the interference of high intracellular GSH due to hemolysis.61 In regards to relationship to GCLC genotypes, we did not observe differences in [GSHB] between long and short repeats, which may be ascribed to the fact that we assessed the free GSH instead of both free and protein bound GSH as measured in.50 This result is consistent with our previous study in patients with chronic schizophrenia61 showing no link between blood GSH levels and GCLC genotypes (unpublished data).
Limitations
This study has some limitations. Firstly, in line with most early psychosis studies, there were much less female patients in our cohort than males, which affects the generality of our results. Therefore further studies with more female patients are required for investigation of GSH redox regulation in female cohort. Secondly, considering GCLC high-risk subjects represent only about 30% of the patient population,14 it is challenging to recruit them in sufficient number to perform comparative studies with low risk genotype patients. Lastly, medication is a common confounding factor in studies conducted in patient samples, and although we did not observe differences in GSH levels between medicated and unmedicated patients, we can’t rule out an influence of medication on GSH levels due to the small size of the sample. In addition, while the absence of a correlation between CPZ equivalents and GSH levels seem to exclude a correlation between these 2 variables, we can’t exclude an influence of medication on GSH levels at dosage below standard prescription; if a ceiling effect were to exist for this correlation at a relatively low CPZ dosage, it could very well not be observed in patients exposed to standard levels of medication. This issue needs therefore further investigation.
Conclusions
We show for the first time that GAG-TNR of GCLC gene affects [GSHmPFC], with high-risk genotypes associated with low [GSHmPFC]. These results extend to the central nervous system previous results in fibroblasts15 and in red blood cells,50 therefore highlighting that GCLC genotypes should be taken into account for the investigation of cerebral GSH levels in any pathology studies. Moreover, low [GSHmPFC] are associated to low peripheral oxidation status in controls but with high oxidation status in patients, pointing to a dysregulated GSH homeostasis in early psychosis patients and paving the way for the search of central and peripheral markers of the disease. Lastly, GCLC polymorphisms and disease associated correlations between [GlumPFC] and [GSHmPFC] may allow the stratification of patients and open new avenues to biomarker guided treatment strategies.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the Swiss National Science Foundation (320030_122419 to P.C. and K.Q.D.), National Center of Competence in Research (NCCR) “SYNAPSY—The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (n° 51AU40_125759), the foundations Avina, Damm-Etienne and Alamaya, Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations.
Supplementary Material
Acknowledgments
We are grateful for technical assistance to Hélène Moser for measurements in blood samples. We would like to thank all patients and volunteers for their enduring participation. We thank Dr Mehdi Gholam for advices on statistics. The authors declare no competing financial interests.
References
- 1. Do KQ, Bovet P, Cabungcal JH, et al. Redox Dysregulation in Schizophrenia: Genetic Susceptibility and Pathophysiological Mechanisms. In: Lajtha A, ed. Handbook of Neurochemistry and Molecular Neurobiology. 3rd ed. New York: Springer; 2009:285–311. [Google Scholar]
- 2. Do KQ, Cuenod M, Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull. 2015;41:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? [published online ahead of print July 4, 2014] Schizophr Res. doi:10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15:2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17:125–134. [DOI] [PubMed] [Google Scholar]
- 6. Do KQ, Trabesinger AH, Kirsten-Krüger M, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 7. Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altuntas I, Aksoy H, Coskun I, Cayköylü A, Akçay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med. 2000;38:1277–1281. [DOI] [PubMed] [Google Scholar]
- 9. Micó JA, Rojas-Corrales MO, Gibert-Rahola J, et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry. 2011;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–1183. [DOI] [PubMed] [Google Scholar]
- 12. Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. MAGMA. 2005;18:276–282. [DOI] [PubMed] [Google Scholar]
- 13. Matsuzawa D, Obata T, Shirayama Y, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wood SJ, Berger GE, Wellard RM, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33:354–357. [DOI] [PubMed] [Google Scholar]
- 15. Gysin R, Kraftsik R, Sandell J, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koga M, Serritella AV, Messmer MM, et al. Glutathione is a physiologic reservoir of neuronal glutamate. Biochem Biophys Res Commun. 2011;409:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanigan MH, Pitot HC. Gamma-glutamyl transpeptidase–its role in hepatocarcinogenesis. Carcinogenesis. 1985;6:165–172. [DOI] [PubMed] [Google Scholar]
- 19. Larsson A, Ristoff E, Anderson ME. Glutathione synthetase deficiency and other disorders of the γ-glutamyl cycle. In: Valle D, ed. The Online Metabolic and Molecular Bases of Inherited Disease. Chapter 96. The McGraw-Hill Companies, Inc; 1995. [Google Scholar]
- 20. Egerton A, Stone JM. The glutamate hypothesis of schizophrenia: neuroimaging and drug development. Curr Pharm Biotechnol. 2012;13:1500–1512. [DOI] [PubMed] [Google Scholar]
- 21. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. [DOI] [PubMed] [Google Scholar]
- 22. Baumann PS, Crespi S, Marion-Veyron R, et al. Treatment and early intervention in psychosis program (TIPP-Lausanne): implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry. 2013;7:322–328. [DOI] [PubMed] [Google Scholar]
- 23. Lambert M, Conus P, Lambert T, McGorry PD. Pharmacotherapy of first-episode psychosis. Expert Opin Pharmacother. 2003;4:717–750. [DOI] [PubMed] [Google Scholar]
- 24. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 26. Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. [DOI] [PubMed] [Google Scholar]
- 27. Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. [DOI] [PubMed] [Google Scholar]
- 28. Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. [DOI] [PubMed] [Google Scholar]
- 29. Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. [DOI] [PubMed] [Google Scholar]
- 30. Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56:965–970. [DOI] [PubMed] [Google Scholar]
- 31. Tkác I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–456. [DOI] [PubMed] [Google Scholar]
- 32. Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn Reson Med. 1999;41:649–656. [DOI] [PubMed] [Google Scholar]
- 33. Mugler JP, III, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990;15:152–157. [DOI] [PubMed] [Google Scholar]
- 34. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 35. Schaller B, Xin L, Cudalbu C, Gruetter R. Quantification of the neurochemical profile using simulated macromolecule resonances at 3 T. NMR Biomed. 2013;26:593–599. [DOI] [PubMed] [Google Scholar]
- 36. Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated Model-Based Bias Field Correction of MR Images of the Brain. Paper presented at: IEEE Transactions on Medical Imaging; October 1999. [DOI] [PubMed] [Google Scholar]
- 37. Provencher S. LCModel & LCMgui User’s Manual 2013. http://s-provencher.com/pages/lcm-manual.shtml. Accessed October 26, 2013.
- 38. Walsh AC, Li W, Rosen DR, Lawrence DA. Genetic mapping of GLCLC, the human gene encoding the catalytic subunit of gamma-glutamyl-cysteine synthetase, to chromosome band 6p12 and characterization of a polymorphic trinucleotide repeat within its 5’ untranslated region. Cytogenet Cell Genet. 1996;75:14–16. [DOI] [PubMed] [Google Scholar]
- 39. Long WK, Carson PE. Increased erythrocyte glutathione reductase activity in diabetes mellitus. Biochem Bioph Res Co. 1961;5:394–399. [Google Scholar]
- 40. Gunzler WA, Kremers H, Flohe L. Improved Coupled Test Procedure for Glutathione Peroxidase (Ec 1.11.1.9.) in Blood. Zeitschrift fur klinische Chemie und klinische Biochemie. 1974;12:444–448. [DOI] [PubMed] [Google Scholar]
- 41. Lohr JB, Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8:93–102. [DOI] [PubMed] [Google Scholar]
- 42. Habif S, Mutaf I, Turgan N, et al. Age and gender dependent alterations in the activities of glutathione related enzymes in healthy subjects. Clinical Biochemistry. 2001;34:667–671. [DOI] [PubMed] [Google Scholar]
- 43. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. [DOI] [PubMed] [Google Scholar]
- 44. Terpstra M, Marjanska M, Henry PG, Tkác I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med. 2006;56:1192–1199. [DOI] [PubMed] [Google Scholar]
- 45. Trabesinger AH, Weber OM, Duc CO, Boesiger P. Detection of glutathione in the human brain in vivo by means of double quantum coherence filtering. Magn Reson Med. 1999;42:283–289. [DOI] [PubMed] [Google Scholar]
- 46. Harris AD, Glaubitz B, Near J, et al. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014;72:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tkác I, Oz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wijtenburg SA, Gaston FE, Spieker EA, et al. Reproducibility of phase rotation STEAM at 3T: focus on glutathione. Magn Reson Med. 2014;72:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xin L, Gruetter R. Glutathione measurement using short-TE 1H MRS at 3T: accuracy and precision assessment. Paper presented at: The International Society for Magnetic Resonance in Medicine; 30 May–5 June 2015; Toronto, ON, Canada. [Google Scholar]
- 50. Nichenametla SN, Lazarus P, Richie JP., Jr A GAG trinucleotide-repeat polymorphism in the gene for glutathione biosynthetic enzyme, GCLC, affects gene expression through translation. FASEB J. 2011;25:2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walsh AC, Feulner JA, Reilly A. Evidence for functionally significant polymorphism of human glutamate cysteine ligase catalytic subunit: association with glutathione levels and drug resistance in the National Cancer Institute tumor cell line panel. Toxicol Sci. 2001;61:218–223. [DOI] [PubMed] [Google Scholar]
- 52. Nichenametla SN, Ellison I, Calcagnotto A, Lazarus P, Muscat JE, Richie JP., Jr Functional significance of the GAG trinucleotide-repeat polymorphism in the gene for the catalytic subunit of gamma-glutamylcysteine ligase. Free Radic Biol Med. 2008;45:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willis AS, Freeman ML, Summar SR, et al. Ethnic diversity in a critical gene responsible for glutathione synthesis. Free Radic Biol Med. 2003;34:72–76. [DOI] [PubMed] [Google Scholar]
- 54. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. [DOI] [PubMed] [Google Scholar]
- 55. Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. [DOI] [PubMed] [Google Scholar]
- 56. Coughlin JM, Ishizuka K, Kano SI, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry. 2013;18:10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. [DOI] [PubMed] [Google Scholar]
- 58. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. [DOI] [PubMed] [Google Scholar]
- 59. Bartoli GM, Sies H. Reduced and oxidized glutathione efflux from liver. FEBS Lett. 1978;86:89–91. [DOI] [PubMed] [Google Scholar]
- 60. Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis. 1998;18:313–329. [DOI] [PubMed] [Google Scholar]
- 61. Gysin R, Kraftsik R, Boulat O, et al. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2011;15:2003–2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.