Abstract
By definition, hallucinations occur only in the full waking state. Yet similarities to sleep-related experiences such as hypnagogic and hypnopompic hallucinations, dreams and parasomnias, have been noted since antiquity. These observations have prompted researchers to suggest a common aetiology for these phenomena based on the neurobiology of rapid eye movement (REM) sleep. With our recent understanding of hallucinations in different population groups and at the neurobiological, cognitive and interpersonal levels, it is now possible to draw comparisons between the 2 sets of experiences as never before. In the current article, we make detailed comparisons between sleep-related experiences and hallucinations in Parkinson’s disease, schizophrenia and eye disease, at the levels of phenomenology (content, sensory modalities involved, perceptual attributes) and of brain function (brain activations, resting-state networks, neurotransmitter action). Findings show that sleep-related experiences share considerable overlap with hallucinations at the level of subjective descriptions and underlying brain mechanisms. Key differences remain however: (1) Sleep-related perceptions are immersive and largely cut off from reality, whereas hallucinations are discrete and overlaid on veridical perceptions; and (2) Sleep-related perceptions involve only a subset of neural networks implicated in hallucinations, reflecting perceptual signals processed in a functionally and cognitively closed-loop circuit. In summary, both phenomena are non-veridical perceptions that share some phenomenological and neural similarities, but insufficient evidence exists to fully support the notion that the majority of hallucinations depend on REM processes or REM intrusions into waking consciousness.
Key words: sleep, misperception, hypnopompic and hypnagogic hallucination, nightmare, parasomnia, REM, Parkinson’s disease, schizophrenia, eye disease, consciousness
Introduction
Philosophers and scientists have long been fascinated by perceptual phenomena occurring around and during sleep, such as the hypnagogic and hypnopompic hallucinations on the borders of sleep and the dreams and parasomnias of sleep. The similarities to “daytime” hallucinations received much scrutiny over the centuries. The French researcher, Alfred Maury,1 noted a continuum of form and cause between dreams and hallucinations, and the English neurologist, John Hughlings Jackson,2 argued that strong “sensory discharges” were likely a common mechanism of dreams and hallucinations. Lhermitte3 elaborated further by designating the midbrain structures associated with peduncular hallucinations as the brain’s “dream centre.” Observations of sleep disturbances in clinical disorders associated with hallucinations (eg, schizophrenia and Lewy body disorders) also prompted suggestions of a common aetiology for both dreams and hallucinations,4,5 and the notion that hallucinations may be the results of “rapid eye movement (REM) intrusions” of visual imagery into wakefulness.5–7
Despite these suggestions of continuity at some level, a strict dichotomy between sleep-related perceptual phenomena and hallucinations is still central to definitions of hallucinations.8 The main objective of this article is to revisit the status of perceptual experiences that occur during sleep. This task will better distinguish the characteristics and properties specific to daytime hallucinations from those that are general to sleep perceptions, and draw upon information regarding underlying mechanisms.
We marshal empirical and theoretical work to address the questions:
What mental functions are active during sleep?
What are the similarities and differences in phenomenological features, and in neuroanatomical and neurophysiological mechanisms, between sleep-related perception and hallucinations? Specifically, are sleep-related perceptions closely related to hallucinations in psychosis, neurodegeneration, or eye disease?
Can hallucinations be conceptualized as REM dream intrusions? Or conversely: might night-time perceptions be reclassified as hallucinations?
This knowledge has the potential to refine our understanding of hallucinations, destigmatize hallucinations in clinical disorders, and point the way towards new treatment approaches.
Hallucinations—Definition Issues
Hallucinations are perceptual experiences that are primarily defined by their subjective reports rather than by their underlying neurobiological mechanisms. Three criteria converge in the majority of definitions.9 Hallucinations are: (1) perceptions (in the auditory, visual and/or other sensory modalities), (2) not elicited by a corresponding stimulus from the outside world, and (3) unbidden, although this latter criterion is not a necessary criterion in all definitions.
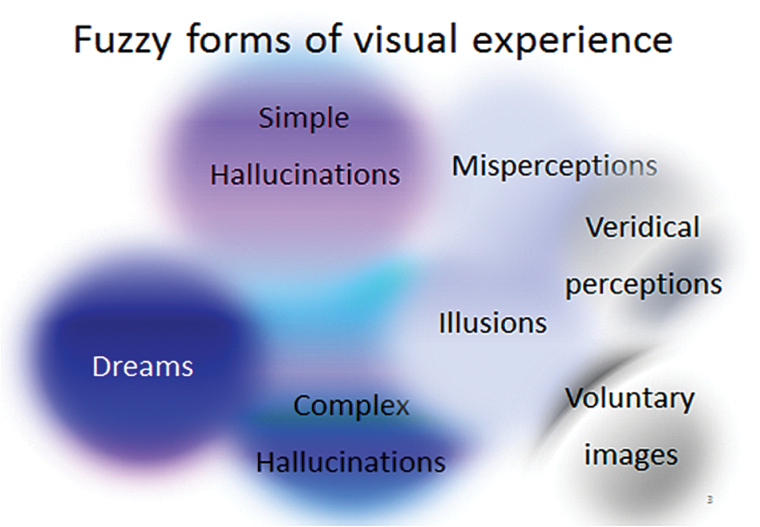
There is no “archetype” for hallucinations. Halluc inations can occur in any sensory modality with features that are markedly different between each other. Furthermore, there is no clear demarcation between sensory perceptions and misperceptions and evidence exists of a continuity between subjective perceptions (see example for visual experiences, figure 1).
Fig. 1.
Fuzzy forms of visual experience. There are no clear boundaries between perceptions. Here, sensory perceptions overlap with illusions and voluntary internal images, hallucinations, and dreams.
The auditory and visual modalities of hallucinations are the most commonly enquired about and reported, but have a varied presentation depending on the population group in which they are studied. For example, hallucinations are prominent in people with schizophrenia spectrum disorders, Parkinson’s disease (PD), and eye disease (in which they are known as Charles Bonnet Syndrome [CBS]), each showing a distinct character.
In schizophrenia, most hallucinations are auditory (“voices”) although hallucinations in other modalities also occur (Waters F, Collerton D, Jardri R, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(suppl 4):S233–S245.). Hallucinations are often mistaken for veridical perceptions and interpreted as symbolic and personally meaningful. Importantly, they are often laden with affect, and believed to originate from external (nonself) sources.10 In PD, visual hallucinations are predominant, although auditory hallucinations and hallucinations of sensed presence are common. Visual hallucinations often comprise formed complex percepts (eg, people, faces, animals, objects), or, less commonly, simple percepts (flashes, dots).11 Hallucinations in PD are usually perceived to be real and unpleasant, but not frightening.12 Finally, visual hallucinations can occur in healthy individuals with CBS. These individuals with eye disease commonly report simple hallucinations (shapes, lines, colors), but complex visual hallucinations also occur. These are often of intense color and rich in detail, and may include panoramic hallucinations. They are usually recognized as non-veridical and do not lead to intense distress.
Consciousness and the Sleeping Brain
The notion of “clear consciousness” has been decisive in some definitions of hallucinations.9 Complicating this requirement, however, is the contemporary view that consciousness represents multiple and graded mental states,13,14 including several overlapping constructs such as sensory discrimination, perceptual awareness, focused attention, introspection, and volitional actions.13,14
In sleep, many of these mental functions remain active. During REM sleep (Box 1), pontine nuclei and cholinergic neurons preserve a high level of activity,15 and, in deep NREM, transient patterns of neural activation in brainstem structures16 resemble micro-wake “fragments.”17 The brain is still attentive to internal mental events, and processes involving early sensory selection and discrimination are intact.18,19 Key differences from waking states, however, are that: (1) awareness to external stimuli is greatly reduced, (2) higher-level functions are suspended, and (3) meaningful interaction with the external world is lacking. Thus, sleep is not constituted by a mere state of brain quiescence, and the notion of “consciousness” is too broad a construct to meaningfully inform our current understanding of sleep-related perceptions.
Box 1: Sleep Essentials
Briefly, sleep comprises 2 states, NREM (N1, N2, N3) and REM, which alternate cyclically. Humans enter sleep through NREM stage 1 (N1) sleep, and NREM (N2, N3 or “deep sleep”) alternates with REM throughout the night in cycles of approximately 90 minutes. Specific hypothalamic nuclei, especially those in the preoptic area, and thalamocortical loops, play a major role in the onset and progression of NREM sleep. During REM sleep, brainstem cholinergic projections are known to play an important role in the generation of this sleep stage.
A prominent property of neural networks is their tendency to engage in oscillatory activity at multiple frequency bands. Sleep stages are defined by the specific proportion of different rhythmic electroencephalographic (EEG) activity. EEG activity is commonly subdivided in 5 major frequency bands: the delta (<4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (14–30 Hz) and gamma (>30 Hz) frequency bands. During sleep, a sigma band is also described (11–16 Hz).
N1 is comprised of marked and distributed changes including the disappearance of alpha rhythms and the progressive appearance of theta EEG activity.50 N1 corresponds with drowsiness and constitutes a transitional state, with a gradual progression to N2 and N3.52 Behavioral indicators include slow rolling eye movements, moderate decreases in muscle tone, and changes in sensory threshold and responses to external stimuli.
N2 occupies approximately half of the total sleep time.15 N2 is defined by the appearance of waxing-and-waning bursts of thalamo-cortical oscillations in the sigma frequency range, called sleep spindles, and high-amplitude waves in the delta range, called k-complexes. Muscular activity and conscious awareness of the external environment are further decreased, due in part to the gating mechanisms of spindle oscillations on sensory reactivity.
N3 is also called slow-wave-sleep (SWS), due to the abundance of high-amplitude waves in the delta range (also called slow waves).15 N3 corresponds to the deepest stage of sleep, and is defined by the presence of slow waves in more than 20% of the EEG epoch. Slow waves are cortically generated rhythms representing the alternation of neuronal hyperpolarization and depolarization phases. Muscle tone and responsiveness to the environment are further reduced. Stage N3 has important restorative functions, as slow waves play a central role in sleep homeostasis, as reflected by the boosting of slow waves after sleep deprivation. This is also the stage in which parasomnias such as night terrors, nocturnal enuresis, and sleepwalking occur.31
During REM sleep, the EEG is characterized by high-frequency waves and theta waves similar to a waking or N1 state.42,101 The most striking characteristic of this stage is the presence of rapid ocular saccades along with a sustained state of muscle atonia. In addition, the instability of the autonomic nervous system during REM sleep leads to fluctuations in heart rate and blood pressure. REM sleep is also the predominant stage during which the sleeper may experience vivid dreams.63
Phenomenology
Phenomenology of Sleep-Related Perceptions
A variety of perceptual experiences are common around and during sleep. The most common ones are hypnagogia and dreams.
Hypnagogia.
This term refers to fleeting perceptual experiences that occur during the transition from wakefulness to sleep (hypnagogic hallucinations) and from sleep to wakefulness (hypnopompic hallucinations).20 They are involuntary, spontaneous, of varying emotionality, and are found in up to 70% of the general population.21–24 Visual, auditory and tactile sensations are most commonly reported (alone or co-occurring25). Visual phenomena (86% of all hypnagogia22,26) typically consist of kaleidoscopically changing—possibly entoptic—phenomena such as geometric patterns, shapes and light flashes. Images involving animals, people and faces, and scenes also occur and are described as “lifelike,” highly detailed and colorful. Voices and other sounds (phone, doorbell, music) occur less commonly (8% to 34%22,26–28). They comprise vivid auditory impressions of words or names, people talking, and environmental or animal sounds.29 Somatic experiences also occur (25% to 44%21,22), including bodily distortions, feelings of weightlessness, flying or falling, and a sense of presence in the room.30 Hypnopompic hallucinations are usually continuations of dream sequences during the first seconds or minutes of wakefulness.
Dreams.
Dreaming occurs during a physiological sleep state which is accompanied by a continuous stream of perceptions, thoughts, and emotions.31 The dreamer typically experiences visual (100%), auditory (40%–60%), and somatic/tactile (15%–30%) percepts, and more rarely, smell, taste, pain, sexual sensations, and proprioceptive or kinesthetic sensations (1%32,33). Dreams typically feature visual images of people, faces, objects, animals, and scenes that are rich in shape and full of movement.33,34 They may have nonsensical or bizarre contents35 that are usually readily accepted by the dreamer. Dreams can also include sounds and speech such as conversations with other people or people talking about commonplace, or unrealistic, matters.34 Dreams of entire scenes are very common. Dreams can evoke a range of intense emotions,36 congruent with the dream’s content.37
A key feature of dreams is that they are immersive experiences in which the dreamer is at once actor and spectator. They may follow a narrative structure, sometimes involving a replay or combination of the day’s events, past experiences and fantastical elements. They are largely beyond the control of the sleeper (except during episodes of lucid dreaming). Finally, since the person is only aware of dream contents upon waking, the phenomenon is necessarily experienced in retrospect. Dreams are predominantly reported from REM sleep, but also happen to a lesser extent during non-REM sleep during which they may have a pseudo-quality that allows the dreamer to distance himself from what he is dreaming.
Other Sleep-Related Perceptual Phenomena.
The incubus phenomenon is a hypnopompic hallucination that refers to a group of nocturnal phenomena associated with hallucinations and sleep paralysis with a lifetime prevalence of around 30%.38 Somatic changes may also include experiences of floating, flying, and out-of-body experiences.30 The person feels awake but unable to move, perceives ominous sounds (such as approaching footsteps), feels movement in the bed, and then feels (and/or smells) a person, creature or unspecified entity climbing upon the chest, a smothering sensation, and sometimes even a physical or sexual assault.30 An intensely frightening sensation usually accompanies this experience. In contrast to simple hypnagogic experiences, these events are typically accepted as vividly real, sometimes taken as assaults by human intruders but often interpreted as occult or metaphysical events.39 In schizophrenia, these features may become incorporated into the person’s delusions and hallucinations. In contrast to dreams, incubus experiences occur in a semi-waking state and may be superimposed upon the perceptual world. Incubus experiences have been associated with mixed REM and waking EEG.40,41
“Parasomnias” are sleep disorders characterized by undesirable behaviors with hallucinatory-like experiences as a clinical feature.42,43 Primary parasomnias can be categorized by the state of sleep during which they occur, including REM sleep (REM sleep behavior disorder), NREM sleep (sleepwalking, sleep terrors, confusional arousals) and a “miscellaneous category.” Amongst the latter is narcolepsy, which is characterized by sudden sleep episodes that can occur at any time. In narcolepsy associated with cataplexy, muscle weakness and vivid hypnagogic images suddenly co-occur.44 Of relevance, the prominence of these hallucinatory-like experiences can lead to a mistaken diagnosis of schizophrenia.45
The Gradual Descent Hypothesis.
The “gradual descent hypothesis”46,47 suggests that the above phenomena constitute a meaningful class of related phenomena sharing overlapping mechanisms.20,47–50 In support, neuroanatomical and neurophysiological evidence shows that mental functions during sleep involve a reorganization of the same systems which function during the day.51 As the brain transits across the different sleep stages, progressive changes occur in oscillatory neural activation and regional flow of information.42,47 EEG frequency and amplitude also gradually change according to the depth and stage of sleep. In addition, descriptions of mental events appear to follow a continuum, starting with waking thoughts, hypnagogic hallucinations (at sleep onset), dreams, and finally hypnopompic hallucinations (with the re-emergence of wakefulness). From light sleep (N1) to NREM and REM dream periods, internal representations gradually evolve, starting with visual images only, to which somatic and auditory images are added, followed by emotional contents with a narrative structure.20,25,50,52 Moreover, the contents of sleep-related perceptions are roughly similar during REM and NREM sleep, although REM dreams are described as more vivid and intense, and involving a narrative.
Similarities and Differences in Phenomenology
Sleep-related perceptions and hallucinations arise in different contexts, the first during sleep and its transitions (ie, hypnagogia), and the second in the context of real-world perceptions. While there are limitations to direct comparisons between these phenomena, such comparisons are important so that overlapping and differential characteristics may be identified. Table 1 contrasts the key characteristics of hallucinations against those of hypnagogia and dreams. It shows that both hypnagogia and dreams meet the 3 aforementioned core criteria of hallucinations (denoted as a “yes” in all the columns of table 1 rows 1–2). Beyond these 3 criteria, interesting similarities and differences exist between these 2 sets of experiences.
Table 1.
Comparison of the Phenomenological Features of Sleep-Related Perceptions and Hallucinations in Schizophrenia Spectrum Disorders, Parkinson’s Disease, and Charles Bonnet Syndrome (Differential Features are Boldfaced)
| Dreams | Hypnagogia | Hallucinations in Schizophrenia | Hallucinations in Parkinson’s Disease | Hallucinations in Charles Bonnet Syndrome | |
|---|---|---|---|---|---|
| 1. Non-veridical perceptions (not elicited by a corresponding external stimulus) and sensory modality implicated | Yes | Yes | Yes | Yes | Yes |
| 100% Visual; 40% Auditory; 15% Somatic | 86% Visual; 8% Auditory; 44% Somatic | 30% Visual; 75% Auditory; 10%–20% Somatic | 100% Visual; 50% Auditory | 100% Visual; 5% Auditory | |
| 2. Unbidden, reduced volitional control | Yes | Yes | Yes | Yes | Yes |
| 3. Perceptual range | Immersive scenes; Panoramic scenes; | Isolated elements in a scene | Elements in a scene | Elements in a scene | Elements in a scene; Panoramic scenes |
| 4. Emotional contents | Yes | No | Yes | Yes | No |
| 5. Superimposed on veridical perceptions | No | Sometimes | Yes | Yes | Yes |
| 6. Association with memory | Poorly recalled | Poorly recalled | Well recalled | Well recalled | Well recalled |
| 7. Appraisal, significance, false beliefs | No | No | Often | Often | Seldom |
| 8. Attribution to external agent | No | No | Yes | No | No |
| 9. Daytime interference | No | No | Yes | Yes | Yes |
Phenomenological Similarities.
In hypnagogia, the contents of visual percepts (highly detailed, colorful, and at times grotesque images of people or animals) are more similar to visual hallucinations in CBS than to hallucinations in schizophrenia or PD. Also similar to CBS, the contents of hypnagogia undergo rapid transformations and rarely invoke emotions. Auditory percepts in hypnagogia are, however, more similar to schizophrenia or PD comprising words, sentences and conversational replays, together with a range of somatic percepts, although the contents are rarely as negative or anxiety-provoking as in schizophrenia.
In dream descriptions, the visual contents resemble the hallucinations of schizophrenia, PD or CBS. Complex scenes and action sequences, which are experienced as real and emotionally charged, resemble closely hallucinations in schizophrenia and PD, although the panoramic scenes bear more resemblance to those in CBS. Similarly, dreams include a broad mix of auditory, somatic and other sensations ranging from simple (eg, amorphous sounds) to complex and fully formed representations (eg, conversations with people), which are similar to hallucinations in psychosis. Other elements akin to hallucinations in acute psychosis include an acceptance of bizarre and implausible scenarios, disordered thought processes, and a lack of reality testing.53 The visual and auditory percepts of dreams thus include a summation of all daytime hallucinations.
Phenomenological Differences.
While similarities exist, there are also important differences.
Multisensory experiences (eg, simultaneous visual and somatic perceptions) are common in sleep-related perceptions, but they are rare in hallucinations, which commonly occur in a single modality, or different modalities in a sequence.
In dreams, the person is a direct participant, rather than a spectator, and experiences are almost entirely dominated by internal events.
Sleep-related perceptions appear as if they occur in the external world. This stands in contrast to hallucinations where percepts are overlaid onto veridical perceptions,54 and coexist with the ongoing flow of thoughts. Hallucinations are also experienced as separate (often foreign or nonself) percepts.
Sleep-related perceptions rarely affect or change the person’s sense of self, personal narrative, or beliefs. Most are hermetically confined to sleep states and have little impact on daytime activities. Hallucinations, by contrast, are reflected upon, appraised, and reacted to, and assimilated with internal representations. This, in turn, informs top-down expectations and responsivity to sensory cortical activation.55
Finally, dreams are often, but not invariably, forgotten when the person wakes up (“dream amnesia”), which differs prominently from hallucinations which are usually remembered well.
Altogether, at the level of contents and subjective descriptions, sleep-related perceptions show many similarities with hallucinations. However, the lack of overlap and interaction with reality, separation from the flow of thoughts, and altered recollection, are key differences that differentiate them from hallucinations.
Brain Functions and Systems
Brain Functions and Systems in Hypnagogia and Dreams
The neurobiology of sleep-related perceptions is typically examined by focusing on the sleep states in which they most frequently occur (Box 1). Thus, hypnagogia are associated with NREM Stages 1–3, and dreams with REM sleep, since 75% of all dreams are experienced during REM sleep.42,56 Other sleep-related phenomena are similarly examined in terms of REM/NREM.
Neurobiological knowledge regarding NREM and REM sleep states can be used as a basis for comparisons of commonalities and differences with hallucinations. In common to hallucinations, which include spontaneous perceptions in different sensory modalities and reduced volitional control (rows 1–3 in table 1), one might anticipate primary and secondary sensory cortices activation, and disrupted recruitment of the prefrontal cortex in sleep-related perceptions. The presence of emotional contents in some hallucinations and dreams also implicates the limbic system (row 4).
By contrast, hallucinations are superimposed onto veridical perceptions (row 5), whereas REM dreams occur in dissociated sleep states, suggesting that the 2 phenomena might differ with respect to the processing of incoming sensory information. Furthermore, hallucinations are associated with good memory recall and, in psychosis, with external attributions and negative appraisal/beliefs (rows 6–8), suggesting “whole brain” involvement and integration particularly involving temporal, parietal and frontal interactions, which might be lacking in sleep-related perceptions.
Regional Brain Activation and Neurotransmitter Action During NREM and REM Sleep.
As the brain moves from NREM to REM sleep stages, progressive changes occur in neural activation and regional blood flow. Processing of external stimuli (auditory, tactile, and somatic in nature) still occurs intermittently in NREM sleep, and is gradually extinguished during REM sleep.
During NREM, cortical responsivity to external stimuli remains high, particularly during the production of K-complex oscillatory modes.57,58 Furthermore, surges of activation occur in the brainstem and cortical areas (inferior frontal gyrus, precuneus, parahippocampal gyrus),42,59 providing a window of cortical reactivity to incoming sensory stimuli.60 This suggests that, during NREM sleep, sensory information continues to be transferred and encoded in a similar way to the brain when awake—at least at the level of primary cortices.57,58 Such cortical responsivity may be enhanced in individuals with common hypnagogia.57 As sleep progresses, reactivity to and awareness of external stimuli decrease. Perceptual activity becomes progressively autonomous.20,48–50 The brain then increasingly focuses inwards18 and becomes less constrained by input from the external environment.
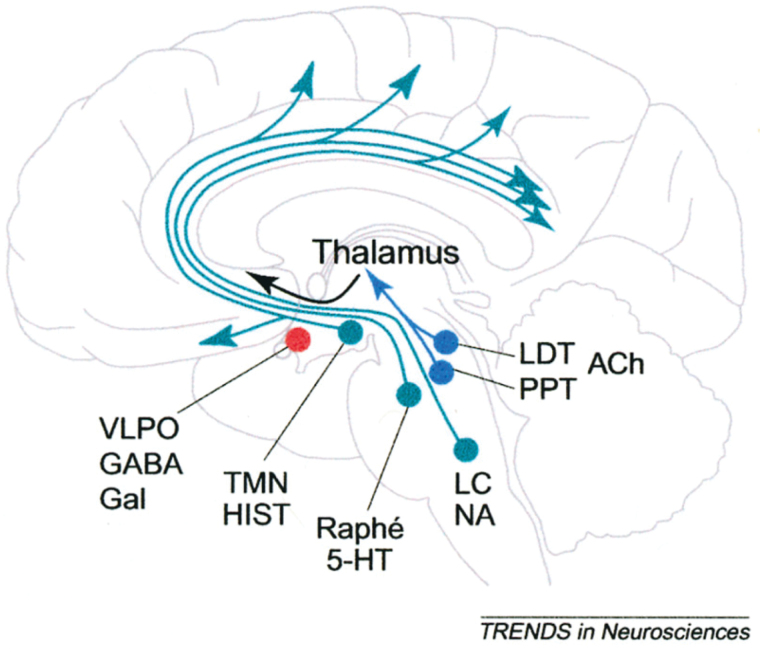
During REM sleep, neural firing is sustained and of high frequency,36 and localized to specific cortical and subcortical areas.42,52 Multiple neurotransmitters are implicated in this stage, including high levels of acetylcholine (ACh), low levels of serotonin (5HT), and elevated levels of GABA.61–63 The release of ACh by neurons in the midbrain is particularly important for creating a chain of events which includes the release of intrinsic sensory activity and lowered recruitment of prefrontal cortex (see figure 2). The sequence of steps following ACh release is as follows:
Fig. 2.
Changes in the brain during sleep modulated by acetylcholine (ACh) input.45 Brainstem neurons (localized in the pedunculopontine [PPT] and laterodorsal tegmental [LDT] nuclei of the midbrain) release ACh which prompts the activation of ascending projections to the thalamus. This is accompanied by generalized activation of limbic, parahippocampal, and other thalamic pathways. This cholinergic enhancement is followed by major glutamatergic release to basal ventrolateral preoptic area (VLPO), and it has the simultaneous effect of decreasing the recruitment of prefrontal areas and of the inferior parietal cortex.
Activation of ascending projections to the thalamus (dorsal pathways), and particularly to thalamic regions connected to the temporal and occipital cortices, acting to increase cortical signaling responsible for perceptual release.64,65 It has been speculated that such activity in the primary and secondary sensory cortices is responsible for the spontaneous release of perceptual images during sleep.61,66 In support, EEG spectral power in the alpha band shows modulation over sensory cortical areas (particularly occipital regions) which appears to correlate with visual dreams.67
Activating influences arising from the thalamus then send signals to the limbic, parahippocampal and related thalamic pathways which operate to process, transfer and modulate the above cortical activity.
Finally, inhibitory neurotransmitters such as 5HT and glutamate are released in the basal forebrain (ventral pathways). These act to “decrease” signal propagation to prefrontal areas, particularly the dorso-lateral prefrontal cortex (DLPFC).51,63 This is thought to operate as a thalamic “gate”18 by restraining signal propagation within a limited circuit involving mostly posterior regions.18,51,63 Suspended interactions between primary and association areas are also a hallmark of REM sleep.50,64,65 The apparent disengagement of prefrontal executive areas is an important finding, as it implies a lack of inhibitory influences on perceptual release, along with restriction of information processing within a limited set of neuroanatomical loops.68 Such disengagement of frontal functions has also been used to explain the dreamer’s reduced self-awareness and the bizarreness of some dream contents.51
Interaction Between Brain Networks (Resting-State Networks).
Analyses of resting-state networks (RSNs) are helpful to examine interactions between functionally integrated regions of the brain. Findings during sleep are consistent with the above findings of state-dependent reorganizations during NREM and REM, and a gradual decrease in interactions between occipital, parietal and prefrontal regions.69
In NREM sleep, much RSN connectivity is preserved: one study,60 ie, reported connectivity levels between the Default-Mode Network (DMN) and Sensory Networks similar to wakefulness, and consistent with the experience of mind wandering.60,70 Connectivity to the Dorsal Attention Network remains high during NREM, but connectivity to the Central Executive Network (CEN) is slightly reduced.60
During deep NREM sleep (stage N3 or slow-wave sleep), the DMN appears to decouple, causing desynchronization between anterior and posterior DMN hubs.70,71 A similar decoupling has been observed in Alzheimer’s Disease, and interpreted as providing an index of functional perturbation and disruptions of brain connectivity.72
During REM, the anterior and posterior DMN nodes recouple (similar to waking), although in the context of anticorrelations between unimodal sensory areas and higher-order areas (including DMN). This is in line with the concept of a “closed-loop” system where internal sensory activations are disconnected from prefrontal high-order control.68,71
Other Perceptual Phenomena During Sleep.
A brief look at perceptual phenomena and their neural correlates during specific sleep conditions is helpful to understand the mechanisms of perception during sleep. Sleep stage 3 (slow-wave sleep) is the stage in which NREM parasomnias (such as night-terrors, nocturnal enuresis and sleepwalking) occur. Sleepwalking is associated with increased brain activation in the precuneus and cerebellum, alongside decreased prefrontal cortical activation.73 This has been interpreted as reflecting a dissociative state combining mixed arousal and sleep-like patterns, while altered prefrontal activity prevents fully conscious awareness. Changes in associative visual areas during wakefulness might also predispose sleepwalkers to experience visual percepts during sleep.74
Similarities and Differences in Brain Functions and Systems
In summary, the similarities and differences in the neurobiological underpinnings of sleep-related perceptions and hallucinations are as follows:
Similarities in Brain Functions and Systems.
The thalamo-cortical circuit described in models of sleep regulation and dreams (involved in perceptual release mechanisms) is also implicated in complex hallucinations of neurodegenerative disease and psychosis66,75–77;
Increased cortical activation in primary and secondary sensory cortices is consistent with perceptual images in hallucinations and sleep-related perceptions (rows 1 and 3, table 1);
In both sleep-related phenomena and hallucinations, the thalamus is activated (potentiated by ACh in sleep), and the brain is focused inwards and selectively attentive to internal experiences19;
Limbic activity is thought to contribute to affective salience and emotive contents of both sleep-related perceptions78 and hallucinations in psychosis (row 4);
Finally, in both phenomena, a possibility exists of spontaneous “sensory discharges” arising from a process of deafferentiation. Cortical deafferentiation is produced by ascending brainstem cholinergic projections activated under conditions of reduced perceptual input, resulting in a failure of the brainstem to deactivate.79 During sleep, this would occur due to the suppression of external signals (eg, eyes closed), resulting in intrinsic sensory excitability. Deafferentiation is also a prominent model of hallucinations in disorders of sensory pathways such as eye disease, where it is thought to occur because of the loss of incoming sensory input in the visual sensory pathways. This model is more often difficult to apply to sensory compound hallucinations, which often involve multiple modalities, although a transient loss of synchrony across distant cortical networks80 is a possibility.
Differences in Brain Functions and Systems.
The pattern of interconnections between brain functional regions appears to differentiate sleep-related perceptions from hallucinations, creating a point of difference with regards to the transfer of information between functional networks.
Compromised signal propagation to the prefrontal cortex appears common to both phenomena, explaining the failure of top-down control and inhibitory influences in hallucinations (row 2, table 1),81,82 but the point of rarity may lie in the extent and severity of this disconnection.
In REM sleep, connectivity between higher-order association and prefrontal areas, and unimodal sensory areas, is entirely suspended68 and characterized by closed-loop circuits. This characteristic feature of REM sleep perhaps promotes the continuous stream of perceptions with bizarre and fantastical elements reported during dreams. With waking hallucinations, by contrast, connectivity between anterior frontal and posterior sensory regions is generally retained, although typically abnormally modulated83,84 and more precisely delineated. Similarly, connectivity of DMN with other networks may be unstable or weakly connected, but nonetheless functionally active.85 In support, some level of top-down integration is necessary in hallucinations to account for appraisals and beliefs (row 7) and daytime dysfunctions (row 9);
During hallucinations, the planum temporale is activated and correlated with the experience of a sensory signal originating in external space (row 8, table 1).86 This activation may assist in amplifying signals to acquire external qualities, and is absent during sleep.
Neurotransmitters.
Findings from neurotransmitter action studies appear less clear-cut than imaging findings. There is convergence for ACh as a key neuromodulator in sleep-related perceptions, and as a trigger for visual hallucinations in PD, dementia and delirium.19 One difference is that hallucination models posit for the role of “low” ACh levels (contrasting with “high” ACh levels in REM sleep, although ACh levels are low in NREM sleep), or at least a dysfunction in cholinergic neurotransmission. This apparent contradiction may be resolved if we consider the possibility of irregular ACh surges in PD and psychosis.80 Such irregular ACh surges would produce spontaneous (and transient) activation of the brainstem,61 perhaps explaining the episodic nature of hallucinations in contrast with the prolonged and steady narrative structure of dreams.
Serotonin and glutamate are both intimately related in both sleep-related perceptions and hallucinations. A fall in 5HT levels (and a reciprocal relationship with ACh) has consistently been implicated in hallucinations,87–91 and GABAergic neurons are overactive in psychotic disorders,92 although evidence is sparse and contradictory in Lewy body dementia and PD.93,94
GABA is also intimately related to dopamine, whose role in sleep-related perceptions is not as clear-cut as that of other neurotransmitters considered here, although a rise in cortical dopamine activity during sleep has been causally linked to emotive dreams and nightmares.63,91 Dopamine levels have a prominent role in models of disorders like schizophrenia and PD,95 including hallucinations.96
Overall, there are many similarities in neurotransmitters between sleep-related perceptions and waking hallucinations, although the balance of neurotransmitter levels appears to be different. In REM sleep, sustained and high ACh levels from the forebrain and tegmental nucleus are important, whereas low ACh levels (or possibly transient ACh release) seem to characterize hallucinations. Serotonin and glutamate are implicated in all conditions, and dopamine in the emotive contents of dreams and hallucinations.
The “REM Dream Stage” and “REM Intrusion” Hypotheses of Hallucinations
Because of the resemblance between dreams and hallucinations, and because REM sleep disorders usually co-occur with clinical conditions in which hallucinations are common (ie, schizophrenia and PD), it has been claimed that the REM dream stage and hallucinations may have common underpinnings.2,97 A related theory suggests that hallucinations represent an intrusion of REM dreams into waking life.5–7
There is great appeal in an overarching and common theory for these phenomena. Support for these theories, however, would require a demonstration that hallucinations share the same mechanisms and underpinnings as the REM dream stage. As shown above, however, REM dreams (1) do not fully capture the phenomenological complexity of hallucinations, (2) might appear to activate only a small number of functional networks, and (3) include a different balance of neurotransmitters, suggesting that REM dreaming processes are unlikely to co-occur during the waking state.
The notion of intrusions into wakefulness requires a separate explanation. Evidence in support of a similar physiological breach comes from findings of “micro-arousals” during the REM dream stage, arising from the presence of transient bursts of alpha spectral power lasting about 3 seconds.67 Cantero and Atienza98 speculated that these bursts might facilitate the connection between the dreaming brain and the external world, thus allowing the person to awake briefly in this state and become briefly aware of dream contents. Evidence is still needed however, to show that “micro-sleep” during wake periods actually produce REM imagery experienced as if awake (and with eyes open), rather than a temporary dissociation associated with a brief return to a sleep state (and eyes closed). Altogether, although an attractive explanation, the notion of REM dream intrusions requires further study.
Other evidence appears to argue against the notions of REM dream stage and REM intrusions:
Sleep disorders and hallucinations follow separate courses in clinical disorders, with sleep dysfunction preceding the onset of hallucinations and disease, sometimes up to several years,99 raising questions regarding additional mechanisms responsible for the triggering of hallucinations.
Theoretical models of hallucinations also do not clearly align with mechanisms of sleep-related phenomena. For example, some models of hallucinations focus on attempts by the brain to compensate for disrupted sensory processing81 or to make sense of intrusive mental representations unconstrained by prefrontal cortical inhibition.82 In these models, hallucinations are the secondary by-product when sensory pathways are stressed or overactive, whereby sleep-related perceptions are primary events which occur as a direct consequence of ascending cholinergic projections during sleep.
Finally models of hallucinations posit a key role of top-down mechanisms in making abnormal associations (similarly to dreams) but also in dictating attentional expectancies, and assigning personal meaning and importance to stimuli (which contrasts with sleep).82
Altogether, there is currently insufficient evidence to fully support the notion that hallucinations comprise dream intrusions.
Conclusion
In this article, we reported on the similarities and differences between sleep-related perceptions and “daytime” hallucinations. An examination of the descriptive features of sleep-related experiences shows that they meet the minimum criteria for hallucinations (non-veridical perceptions, unbidden). The brain is active during sleep and capable of producing spontaneous perceptual material for which awareness is maintained, as shown by the rich array of vivid and multisensory images reported when individuals are awoken from sleep. Indeed, if these experiences occurred during waking, aspects of them would undoubtedly be classified as hallucinatory.
Regarding the prominence of visual contents, they resemble hallucinations in peripheral eye disease (eg, CBS). However, the involvement of multiple modalities, complex and emotive contents, and a network involving primary, secondary, and subcortical structures, suggests greater similarities with hallucinations in central disorders (neurodegeneration and psychosis).
Notwithstanding these similarities, there are important phenomenological differences with hallucinations. Most sleep-related perceptions are immersive and largely cut off from reality, whereas many types of hallucination are discrete and appear as overlaid on regular sense perceptions, with the physical environment as a backdrop onto which hallucinated contents are projected. Such superimposition of real and hallucinated events renders hallucinations truly distinctive: It makes people wonder if their eyes/ears are playing tricks, and leaves them searching for an explanation.
Hallucinations may share greater underlying mechanisms with hypnagogic experiences (which more closely reflect REM intrusions into waking state than dreams) given that external stimuli continue to be processed and reacted to during NREM sleep, albeit intermittently. During the short transition between sleep and wake, cortical inhibitory control is reduced but sufficiently sporadic to allow conscious awareness. Hypnagogia may, therefore, be an intermediate phenomenon between REM dreams and hallucinations.
Neurobiological findings show that thalamocortical circuits, active during sleep as in wakefulness, serve as important modulators of internally generated percepts. This suggests that sleep-related perceptions and hallucinations may rely on overlapping processes, and that unconstrained perceptions can arise spontaneously and be linked to sensory awareness without a need for top-down control, belief systems and integration with conscious awareness.
One key point of difference between hallucinations and REM sleep-related perceptions, however, is that closed-loop circuits represent a fundamental signature of REM dreams.68 During REM sleep, spontaneous perceptual signals are immersive, processed in a subsystem which is functionally dissociated from the external environment, and barred from higher-order processing. By contrast, whole-brain networks appear necessary to achieve hallucinations, although their interconnections may be abnormally modulated.100 This allows for maximum information-based exchange between brain areas, and the production of highly complex mental experiences which are overlaid over veridical perceptions and which include negative appraisals and misattributions.
Overall, phenomenological and neurobiological findings show overlap between sleep-related perceptions and hallucinations. REM dreams, however, appear to occur in a sensory buffer and do not co-exist alongside regular perceptions and other thoughts limiting their usefulness as a model for most types of hallucinations. This finding can inform the operational definition of hallucinations, which should include the key characteristic that they appear as overlaid on veridical perceptions, therefore acting as a differential feature from REM dreams.
Funding
Wellcome Trust Grants (WT098455 and WT103817 to A-D.B.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Maury A. Le Sommeil et les Rêves (Sleep and Dreams). Paris, France: Didier; 1865. [Google Scholar]
- 2. Jackson JH, Beevor CE. On a case of epileptic attacks with an olfactory aura from a tumour in the right temporosphenoidal lobe. Lancet. 1889;1:381. [Google Scholar]
- 3. Lhermitte J. Syndrome de la calotte du pédoncule cerebral: Les troubles psycho-sensoriels dans les lésions du mésocéphale. Rev Neurol (Paris). 1922;38:1359–1365. [Google Scholar]
- 4. Gottesmann C. Find out about dreams and you will find out about insanity (Hughlings Jackson). In: Pletson JE, ed. Progress in Schizophrenia Research. New York, NY: Nova Science; 2004:23–43. [Google Scholar]
- 5. Kelly PH. Defective inhibition of dream event memory formation: a hypothesized mechanism in the onset and progression of symptoms of schizophrenia. Brain Res Bull. 1998;46:189–197. [DOI] [PubMed] [Google Scholar]
- 6. Arnulf I, Bonnet A-M, Damier P, et al. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology. 2000;55:281–288. [DOI] [PubMed] [Google Scholar]
- 7. Manni R, Mazzarello P. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology. 2001;57:1350–1351. [DOI] [PubMed] [Google Scholar]
- 8. Blom JD, Sommer IE. Auditory hallucinations: nomenclature and classification. Cogn Behav Neurol. 2010;23:55–62. [DOI] [PubMed] [Google Scholar]
- 9. Aleman A, Larøi F. Hallucinations: The Science of Idiosyncratic Perception. Washington, DC: American Psychological Association; 2008. [Google Scholar]
- 10. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26:177–189. [DOI] [PubMed] [Google Scholar]
- 11. Mosimann UP, Rowan EN, Partington CE, et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am J Geriatr Psychiatry. 2006;14:153–160. [DOI] [PubMed] [Google Scholar]
- 12. Lai S, Bruce V, Collerton D. Visual hallucinations in older people: appraisals but not content or phenomenology influence distress. Behav Cogn Psychother. 2015;1–6. [DOI] [PubMed] [Google Scholar]
- 13. Dehaene S, Changeux J-P, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trend Cogn Sci. 2006;10:204–211. [DOI] [PubMed] [Google Scholar]
- 14. Seth AK, Dienes Z, Cleeremans A, Overgaard M, Pessoa L. Measuring consciousness: relating behavioural and neurophysiological approaches. Trend Cogn Sci. 2008;12:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. [DOI] [PubMed] [Google Scholar]
- 16. Eschenko O, Magri C, Panzeri S, Sara SJ. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb Cortex. 2012;22:426–435. [DOI] [PubMed] [Google Scholar]
- 17. Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trend Neurosci. 2007;30:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braun AR, Balkin TJ, Wesensten NJ, et al. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science. 1998;279:91–95. [DOI] [PubMed] [Google Scholar]
- 19. Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trend Neurosci. 1999;22:273–280. [DOI] [PubMed] [Google Scholar]
- 20. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231–243. [DOI] [PubMed] [Google Scholar]
- 21. Ohayon MM, Priest RG, Caulet M, Guilleminault C. Hypnagogic and hypnopompic hallucinations: pathological phenomena? Brit J Psychiatry. 1996;169:459–467. [DOI] [PubMed] [Google Scholar]
- 22. Sidgwick H, Johnson A, Myers FWH, Podmore F, Sidgwick E. Report on the census of hallucinations. In: Proceedings of the Society for Psychical Research. Volume XXVI. Part X. London, UK: Kegan Paul, Trench, Trübner & Co; 1894:25–422.
- 23. McKellar P. Experience and Behaviour. Harmondsworth, UK: Penguin Books Ltd; 1968. [Google Scholar]
- 24. Barrett TR, Etheridge JB. Verbal hallucinations in normals, I: people who hear ‘voices’. Appl Cogn Psychol. 1992;6:379–387. [Google Scholar]
- 25. Mavromatis A. On shared states of consciousness and objective imagery. J Mental Imag. 1987;11:125–130. [Google Scholar]
- 26. Leaning F. An introductory study of hypnagogic phenomena. Paper presented at: Proceedings of the Society for Psychical Research; 1925. [Google Scholar]
- 27. Leroy E-B. Les Visions du Demi-Sommeil: Hallucinations Hypnagogiques. Paris, France: Librairie Félix Alcan; 1933. [Google Scholar]
- 28. Hori T, Hayashi M, Morikawa T. Topographical EEG changes and the hypnagogic experience. In: Ogilvie RD, Harsh JR, eds. Sleep Onset: Normal and Abnormal Processes. Washington, DC: American Psychological Association; 1994:237–253. [Google Scholar]
- 29. Jones SR, Fernyhough C, Larøi F. A phenomenological survey of auditory verbal hallucinations in the hypnagogic and hypnopompic states. Phenomen Cogn Sci. 2010;9:213–224. [Google Scholar]
- 30. Cheyne JA, Rueffer SD, Newby-Clark IR. Hypnagogic and hypnopompic hallucinations during sleep paralysis: neurological and cultural construction of the night-mare. Conscious Cogn. 1999;8:319–337. [DOI] [PubMed] [Google Scholar]
- 31. Stickgold R. Introduction to dreams and their pathology. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005:519–521. [Google Scholar]
- 32. Strauch I, Meier B. In Search of Dreams: Results of Experimental Dream Research. Albany, NY: State University of New York Press; 1996. [Google Scholar]
- 33. Zadra AL, Nielsen TA, Donderi DC. Prevalence of auditory, olfactory, and gustatory experiences in home dreams. Percept Mot Skills. 1998;87:819–826. [DOI] [PubMed] [Google Scholar]
- 34. Snyder F, Karacan I, Tharp VK, Jr, Scott J. Phenomenology of REM dreaming. Psychophysiology. 1968;4:375. [Google Scholar]
- 35. Dorus E, Dorus W, Rechtschaffen A. The incidence of novelty in dreams. Arch Gen Psychiatry. 1971;25:364–368. [DOI] [PubMed] [Google Scholar]
- 36. Dang-Vu TT, Schabus M, Desseilles M, Schwartz S, Maquet P. Neuroimaging of REM sleep and dreaming. In: Barrett D, McNamara P, eds. The New Science of Dreaming: Biological Aspects. Westport, CT: Praeger Publishers; 2007:95–113. [Google Scholar]
- 37. Foulkes D, Sullivan B, Kerr NH, Brown L. Appropriateness of dream feelings to dreamed situations. Cogn Emot. 1988;2:29–39. [Google Scholar]
- 38. Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev. 2011;15:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheyne JA, Pennycook G. Sleep paralysis postepisode distress: modeling potential effects of episode characteristics, general psychological distress, beliefs, and cognitive style. Clin Psychol Sci. 2013: 1-14. doi:10.1177/2167702612466656 [Google Scholar]
- 40. Takeuchi T, Miyasita A, Inugami M, Sasaki Y, Fukuda K. Laboratory-documented hallucination during sleep-onset REM period in a normal subject. Percept Mot Skills. 1994;78:979–985. [DOI] [PubMed] [Google Scholar]
- 41. Takeuchi T, Miyasita A, Sasaki Y, Inugami M, Fukuda K. Isolated sleep paralysis elicited by sleep interruption. Sleep. 1992;15:217–225. [DOI] [PubMed] [Google Scholar]
- 42. Carskadon M, Dement W. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement W, eds. Principles and Practice of Sleep Medicine. 4th ed Philadelphia, PA: Elsevier Saunders; 2005:13–23. [Google Scholar]
- 43. Vaughn BV, D’Cruz OF. Cardinal manifestations of sleep disorders. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005:594–601. [Google Scholar]
- 44. Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–204. [DOI] [PubMed] [Google Scholar]
- 45. Kishi Y, Konishi S, Koizumi S, Kudo Y, Kurosawa H, Kathol RG. Schizophrenia and narcolepsy: a review with a case report. Psychiatry Clin Neurosci. 2004;58:117–124. [DOI] [PubMed] [Google Scholar]
- 46. Fosse R, Stickgold R, Hobson JA. The mind in REM sleep: reports of emotional experience. Sleep. 2001;24:947–955. [PubMed] [Google Scholar]
- 47. Dinges DF. Are you awake? Cognitive performance and reverie during the hypnopompic state. In: Bootzin RR, Kihlstrom JF, Schacter DL, eds. Sleep and Cognition. Washington, DC: American Psychological Association; 1990:159–175. [Google Scholar]
- 48. Vogel GW, Barrowclough B, Giesler DD. Limited discriminability of REM and sleep onset reports and its psychiatric implications. Arch Gen Psychiatry. 1972;26:449–455. [DOI] [PubMed] [Google Scholar]
- 49. Vogel G, Foulkes D, Trosman H. Ego functions and dreaming during sleep onset. Arch Gen Psychiatry. 1966;14:238–248. [DOI] [PubMed] [Google Scholar]
- 50. Rowley JT, Stickgold R, Allan Hobson J. Eyelid movements and mental activity at sleep onset. Conscious Cogn. 1998;7:67–84. [DOI] [PubMed] [Google Scholar]
- 51. Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci. 2010;14:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–690. [DOI] [PubMed] [Google Scholar]
- 53. Scarone S, Manzone ML, Gambini O, et al. The dream as a model for psychosis: an experimental approach using bizarreness as a cognitive marker. Schizophr Bull. 2008;34:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blom JD. A Dictionary of Hallucinations. New York, NY: Springer; 2010. [Google Scholar]
- 55. Woodruff PW, Benson RR, Bandettini PA, et al. Modulation of auditory and visual cortex by selective attention is modality-dependent. Neuroreport. 1996;7:1909–1913. [DOI] [PubMed] [Google Scholar]
- 56. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. [DOI] [PubMed] [Google Scholar]
- 57. Dang-Vu TT, Bonjean M, Schabus M, et al. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc Natl Acad Sci. 2011;108:15438–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schabus M, Dang-Vu TT, Heib DPJ, et al. The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front Neurol. 2012;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dang-Vu TT, Schabus M, Desseilles M, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105:15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci. 2009;106:4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perry EK, Perry RH. Acetylcholine and hallucinations - disease-related compared to drug-induced alterations in human consciousness. Brain Cogn. 1995;28:240–258. [DOI] [PubMed] [Google Scholar]
- 62. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trend Neurosci. 2001;24:726–731. [DOI] [PubMed] [Google Scholar]
- 63. Pace-Schott E. The neurobiology of dreaming. In: Kryger M, Roth T, Dement W, eds. Principles and Practice of Sleep Medicine. 5th ed. Philadelphia, PA: Elsevier Saunders; 2010:563–575. [Google Scholar]
- 64. Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. [DOI] [PubMed] [Google Scholar]
- 65. Maquet P, Peters J-M, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. [DOI] [PubMed] [Google Scholar]
- 66. Collerton D, Perry E. Dreaming and hallucinations – Continuity or discontinuity? Perspectives from dementia with Lewy bodies. Conscious Cogn. 2011;20:1016–1020. [DOI] [PubMed] [Google Scholar]
- 67. Cantero JL, Atienza M, Salas RM. Spectral features of EEG alpha activity in human REM sleep: two variants with different functional roles? Sleep. 2000;23:746–750. [PubMed] [Google Scholar]
- 68. Chow HM, Horovitz SG, Carr WS, et al. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc Natl Acad Sci U S A. 2013;110:10300–10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. [DOI] [PubMed] [Google Scholar]
- 70. Sämann PG, Wehrle R, Hoehn D, et al. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cerebral Cortex. 2011;21:2082–2093. [DOI] [PubMed] [Google Scholar]
- 71. Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci. 2009;106:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mevel K, Chetelat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:535816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–485. [DOI] [PubMed] [Google Scholar]
- 74. Dang-Vu TT, Zadra A, Labelle MA, Petit D, Soucy JP, Montplaisir J. Sleep deprivation reveals altered brain perfusion patterns in somnambulism. PLoS One. 2015;10:e0133474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Santhouse AM, Howard RJ, ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain. 2000;123:2055–2064. [DOI] [PubMed] [Google Scholar]
- 76. Behrendt R-P, Young C. Hallucinations in schizophrenia, sensory impairment, and brain disease: a unifying model. Behav Brain Sci. 2004;27:771–787. [DOI] [PubMed] [Google Scholar]
- 77. Adriano F, Spoletini I, Caltagirone C, Spalletta G. Updated meta-analyses reveal thalamus volume reduction in patients with first-episode and chronic schizophrenia. Schizophr Res. 2010;123:1–14. [DOI] [PubMed] [Google Scholar]
- 78. Dang-Vu TT, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tandon R, Shipley JE, Taylor S, et al. Electroencephalographic sleep abnormalities in schizophrenia: relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry. 1992;49:185–194. [DOI] [PubMed] [Google Scholar]
- 80. Uhlhaas PJ, Haenschel C, Nikolić D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Collerton D, Mosimann UP, Archibald N. Disorders of visual perception in Parkinson’s disease and other Lewy body disorders. In: Ebmeier KP, O’Brien JT, Taylor JP, eds. Psychiatry of Parkinson’s Disease. Basel, Switzerland: Karger; 2012. [Google Scholar]
- 82. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hoffman RE, Pittman B, Constable RT, Bhagwagar Z, Hampson M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Brit J Psychiatry. 2011;198:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 85. Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex. 2013;23:1108–1117. [DOI] [PubMed] [Google Scholar]
- 86. Hunter MD, Griffiths TD, Farrow TF, et al. A neural basis for the perception of voices in external auditory space. Brain. 2003;126(Pt 1):161–169. [DOI] [PubMed] [Google Scholar]
- 87. Bowers MB. Serotonin (5HT) systems in psychotic states. Psychopharmacol Commun. 1975;1:655–662. [PubMed] [Google Scholar]
- 88. Meltzer HY. Treatment of schizophrenia and spectrum disorders: pharmacotherapy, psychosocial treatments, and neurotransmitter interactions. Biol Psychiatry. 1999;46:1321–1327. [PubMed] [Google Scholar]
- 89. Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. [DOI] [PubMed] [Google Scholar]
- 90. Benson KL, Zarcone VP. Schizophrenia. In: Kryger M, Roth T, Dement W, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, PA: W. B. Saunders; 2000:1159–1167. [Google Scholar]
- 91. Gottesmann C. Paradoxical sleep and schizophrenia have the same neurobiological support. Behav Brain Sci. 2004;27:794–795. [Google Scholar]
- 92. Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. [DOI] [PubMed] [Google Scholar]
- 93. Francis PT, Perry EK. Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Mov Disord. 2007;22:S351–S357. [DOI] [PubMed] [Google Scholar]
- 94. Wang H-F, Yu J-T, Tang S-W, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015;86:135–143. [DOI] [PubMed] [Google Scholar]
- 95. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci. 2000;97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Howes OD, Shotbolt P, Bloomfield M, et al. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gottesmann C. The dreaming sleep stage: a new neurobiological model of schizophrenia? Neuroscience. 2006;140:1105–1115. [DOI] [PubMed] [Google Scholar]
- 98. Cantero JL, Atienza M. Alpha burst activity during human REM sleep: descriptive study and functional hypotheses. Clin Neurophysiol. 2000;111:909–915. [DOI] [PubMed] [Google Scholar]
- 99. Goetz CG, Ouyang B, Negron A, Stebbins GT. Hallucinations and sleep disorders in PD: ten-year prospective longitudinal study. Neurology. 2010;75:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vaitl D, Birbaumer N, Gruzelier J, et al. Psychobiology of altered states of consciousness. Psychol Bull. 2005;131: 98–127. [DOI] [PubMed] [Google Scholar]
- 101. Maquet P, Laureys S, Peigneux P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. [DOI] [PubMed] [Google Scholar]