Abstract
In recent years, there has been increasing interest in the potential for alterations to the brain’s resting-state networks (RSNs) to explain various kinds of psychopathology. RSNs provide an intriguing new explanatory framework for hallucinations, which can occur in different modalities and population groups, but which remain poorly understood. This collaboration from the International Consortium on Hallucination Research (ICHR) reports on the evidence linking resting-state alterations to auditory hallucinations (AH) and provides a critical appraisal of the methodological approaches used in this area. In the report, we describe findings from resting connectivity fMRI in AH (in schizophrenia and nonclinical individuals) and compare them with findings from neurophysiological research, structural MRI, and research on visual hallucinations (VH). In AH, various studies show resting connectivity differences in left-hemisphere auditory and language regions, as well as atypical interaction of the default mode network and RSNs linked to cognitive control and salience. As the latter are also evident in studies of VH, this points to a domain-general mechanism for hallucinations alongside modality-specific changes to RSNs in different sensory regions. However, we also observed high methodological heterogeneity in the current literature, affecting the ability to make clear comparisons between studies. To address this, we provide some methodological recommendations and options for future research on the resting state and hallucinations.
Key words: psychosis, schizophrenia, fMRI, default mode network, perception
Introduction
Auditory hallucinations (AH) are vivid perceptions of sound that occur without corresponding external stimuli and have a strong sense of reality. AH feature in 60%–90% of schizophrenia cases, in other psychiatric and neurological conditions, and in a minority of the general population.1 While many involve voices, nonverbal AH also occur (including environmental sounds, animal noises, and music).
Despite much research on the topic, many questions remain regarding the brain mechanisms of AH.2 One unanswered question is how they can occur spontaneously from the brain’s intrinsic activity. This has been explored by studying the brain in its so-called “resting state,” ie, the spontaneous neural activity and patterns of connectivity between brain regions that are observable when participants are asked to lie still in a scanner and not engage in any particular task.
The International Consortium on Hallucination Research (ICHR) is a global network of researchers, clinicians, and people with lived experience of hallucinations that was created to facilitate multisite collaborations.3,4 This ICHR report outlines our current knowledge of the resting state in relation to AH. Although elements of this topic have been reviewed elsewhere,5,6 this report extends prior work by incorporating evidence from a range of methods and populations, including a specific comparison of auditory and visual hallucinations. This allows us to identify the most important changes to the resting state, establishing what may be specific to AH, what may be specific to a disorder (such as schizophrenia), and what may act as a general marker for unusual perceptions across various populations. A critical review of existing methodologies and potential confounds is also presented.
Here, we first outline the general characteristics of the brain’s resting state and introduce some of the most commonly studied resting-state networks (RSNs). In the following sections, we then review functional MRI findings on RSNs relating to schizophrenia and AH and compare them with (1) evidence from other investigative methods (EEG/MEG and structural MRI) and (2) resting-state research on visual hallucinations, both in schizophrenia and in other conditions such as dementia. In the final 2 sections, we evaluate existing methodological approaches, offer a model that summarizes AH findings to date, and discuss the key issues and implications for future research.
What Is Rest? Intrinsic Activity and Its Networks
Resting-state activity refers to the intrinsic patterns of brain activity that are observable in the absence of an external task.7 In fMRI, this is typically described in terms of functional connectivity: the correlations between signals in different brain regions.8 Spatially, the brain’s intrinsic activity can be divided into RSNs such as the default mode network (DMN), central executive network (CEN), salience network (SN), and sensorimotor networks.9,10 Regions involved in these RSNs (table 1) show dense functional connectivity at low frequencies (0.01–0.1 Hz) in the resting state. The DMN is often deactivated during many tasks and may be associated with self-referential or internally directed processing.9,11 It shows anticorrelated intrinsic activity to a collection of “task-positive” networks, including the CEN, SN, and sensorimotor networks.12,13 The CEN has been linked to executive functioning and cognitive control, including working memory and top-down attention.12 The SN has been associated with monitoring and selecting behaviorally relevant events for further processing.14,15 Effective goal-directed information processing may require a carefully controlled interaction between the SN and CEN, which may in turn affect processing in sensory and motor networks.12 Importantly though, intra- and internetwork connectivity is thought to constantly change over time.16,17 This generates a dynamic spatial structure to intrinsic activity, partly but not fully determined by underlying anatomy.18,19
Table 1.
Common Resting-State Networks
| Network | Regions | Studies in Healthy Population |
|---|---|---|
| Default mode network (DMN) | mPFC, precuneus, PCC, TPJ, MTL | Raichle et al9; Buckner et al11 |
| Central executive network (CEN) | dlPFC, supragenual ACC, lateral parietal cortex | Fox et al20; Seeley et al15 |
| Salience network (SN) | Right anterior insula, ventral striatum, dorsal ACC | Menon10; Goulden et al21 |
| Sensorimotor networks (including language and auditory regions) | HG, left IFG, insula, bilateral STG, inferior temporal cortex, caudate, SMA | Hampson et al22; Beckmann et al23; Lee et al24 |
Note: ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; HG, Heschl’s gyrus; IFG, inferior frontal gyrus; mPFC, medial prefrontal cortex; MTL, medial temporal lobe; PCC, posterior cingulate cortex; SMA, supplementary motor area; STG, superior temporal gyrus; TPJ, temporoparietal junction.
Fluctuations in electrophysiological oscillatory activity also provide a spatiotemporal structure to intrinsic activity: Functional connectivity can be assessed via the synchronization of neural oscillations between different brain regions and frequency bands such as theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (>30 Hz). However, the large majority of resting-state studies on AH have only used fMRI.6
The Resting State and AH: Evidence From fMRI
AH are particularly common in schizophrenia-spectrum disorders (Sz), where they occur alongside other psychotic symptoms (such as delusions), cognitive, and functional changes. Given the primacy of AH in the disorder, RSNs of participants with schizophrenia may provide important clues to those involved in AH.
Resting-State Findings in Schizophrenia
Default Mode Network.
Consistent with a cognitive profile characterized by executive dysfunction, Sz studies often show altered connectivity within the DMN and reduced anticorrelation with areas associated with the CEN, such as dorsolateral prefrontal cortex.25 Posterior sections of DMN in Sz also show greater connectivity to surrounding sensory areas (such as lateral occipital cortex), which may reflect problems with cognition and unusual experiences in the disorder.26 When DMN alterations correlate with clinical scores, they often associate with positive symptom ratings27,28 (for a review, see29).
Salience Network.
Striatal regions of the SN includes projections of the mesolimbic dopaminergic system (MDS), which are important in assigning novelty and significance to sensorimotor and mental events,30 while the anterior insula has been implicated in monitoring surprise-based prediction errors during decision-making31 (see Box 1). In terms of RSN dynamics, the SN has been suggested to assign salience by switching attention between the DMN and CEN.14 Thus, disrupted MDS activity in schizophrenia could result in atypically modulated RSNs. However, evidence of SN dysfunction specific to psychosis has been inconsistent. Two trait studies showed decreases in functional connectivity in Sz during information processing and at rest,32,33 and another 2 have linked connectivity alterations to general psychotic symptoms.34,35 Orliac et al36 showed that reduced connectivity in Sz between SN and DMN was linked to delusion but not hallucination severity. Negative findings of SN dysfunction also exist in Sz,37 although this may be due to methodological or sampling differences.
Box 1: Predictive Coding, Auditory Hallucinations, and Rest
A further challenge is how to integrate evidence of resting-state alterations with computational models of auditory hallucinations (AH). In parallel to the rising interest in resting-state networks, some researchers have advocated predicting processing approaches to understanding perception.38 Predictive coding (PC) and Bayesian models of the brain posit that perception and inference are part of a unitary process.39,40 Under PC, brain systems have a hierarchical organization of message passing that reduces coding of predictable information by minimizing prediction errors (PEs) in internal predictive models about the external environment. Each level of the hierarchy is comprised of functional units signaling feedback predictions and feedforward PEs, the latter being essential teaching signals that prompt updating of internal predictive models. Intuitively, PC suggests that the brain is not merely a passive feature detector, but an active creator of internal, predictive models of the environment, which determine both perception and inference about external stimuli. It follows that abnormalities in encoding of such internal predictive signals could result in abnormal percepts such as hallucinations.41
This framework accommodates models of dysfunctional corollary discharge in psychosis (which can be regarded as a special case of predictive coding), as well as findings of deficient mismatch negativity (MMN) in psychosis, as MMN has been considered a type of sensory PE signal.42 Ample evidence supports deficits in both corollary discharge mechanisms and MMN in schizophrenia43 with some evidence supporting their relationship to AH.44 Computational-model-based analyses of EEG and fMRI data have also suggested specific deficits in sensory PE signals in schizophrenia during auditory processing45 and in hallucinating patients with schizophrenia during speech discrimination,46 respectively. Deficient PEs have also been linked to increased activity in voice-selective regions of the auditory cortex,47 a neural phenotype previously linked to AH.46,48,49
According to PC and associative learning models more generally, PEs prompt learning by inducing changes in synaptic plasticity that remodel connection strengths encoding predictions.50 Some fMRI studies have provided evidence for connectivity changes as a function of associative learning in healthy individuals51–53 and for a relationship between learning and intrinsic connectivity.54 PC models of psychosis may therefore share a common ground with dysconnectivity views of schizophrenia, which posit that failures in synaptic plasticity (eg, NMDA-dependent plasticity and its modulation by neurotransmitters like dopamine and acetylcholine) are at the core of the disorder.55 Key questions to explore are how voice-selective changes to PE in auditory cortex relate to local functional connectivity within surrounding temporal cortex (which could be assessed using methods such as Regional Homogeneity) and long-range functional connectivity with default mode network, salience network (SN), and central executive network. It has also been suggested that the anterior insula, within the SN, is important for integrating interoceptive PEs that give rise to a sense of agency or presence56; if this were to be disrupted, it could be involved in the alterations of agency that are common in AH. Recent advances in task-based and resting-state fMRI analysis, including dynamic causal modeling,57 are a promising avenue to investigate the relationships between abnormal connectivity, predictive learning mechanisms, and unusual experiences such as AH.
Central Executive Network.
As noted above, anticorrelation between the DMN and task-oriented RSNs such as the CEN is often reduced in Sz.29 Consistent with executive dysfunction in Sz, resting-state connectivity increases and decreases have been reported in this network37,58 alongside atypical correlations with frontotemporal regions.37
Resting-State Findings Specific to AH
Given DMN, SN, and CEN alterations in Sz, AH studies have focused on these RSNs, as well as on sensory networks involving auditory and language regions. Studies have either focused solely on participants with AH,59 compared those with and without AH,60 or reported correlations with hallucination severity.61 Few non-Sz resting-state studies of AH exist, although 2 have included people in the general community who regularly experience AH (“nonclinical voice hearers”).62,63
Default Mode Network.
Various articles have posited a specific link between DMN function and AH. For instance, Northoff and Qin5 proposed that resting interactions between auditory cortex and parts of the DMN may produce a state of confusion regarding external stimulation and resting-state activity. Support was provided by Jardri et al,64 who compared hallucinations and “real rest” periods in 20 adolescents with psychosis (participants had either AH, VH, or both). Hallucinations were associated with spontaneous engagement of sensory cortex, specific to modality, alongside disengagement and weaker integrity of the DMN (as measured by its consistency over time during scanning), suggesting that unstable DMN states may be an important precursor to AH states. Further support came from Alonso-Solis et al,26 who observed atypical connectivity-specific AH between hubs of the DMN and SN.
Other studies show inconsistent DMN alterations in AH. In a Sz + AH sample, Wolf et al61 observed no difference in DMN function or correlation with symptoms: Connectivity alterations were observed in precuneus and posterior cingulate but these were specific to an executive control network and a left frontoparietal network, respectively (see below). In contrast, van Lutterveld et al63 observed increased connectivity in posterior regions of the DMN in a sample of nonclinical AH participants, which may indicate differing routes to AH-proneness in clinical and nonclinical populations.
Central Executive Network and Salience Network.
While showing no differences in DMN function, Wolf et al61 observed reduced connectivity in the posterior CEN (precuneus) and increased connectivity in anterior CEN (right middle and superior frontal gyri), with increased middle frontal gyrus connectivity correlating with AH severity. Correlations with AH were also observed within a left-lateralized frontoparietal network that the authors related to speech processing and monitoring: More severe AH associated with decreased anterior cingulate cortex (ACC) connectivity and increased left superior temporal gyrus (STG) connectivity. Correlations between hallucination severity and altered resting connectivity have also been reported for the ACC,65,66 medial prefrontal cortex (mPFC),59 and anterior insula.58
Sensorimotor Networks.
Most RSN research on AH has focused on connectivity in auditory and language regions. Oertel-Knochel et al67 examined resting connectivity between seeds identified with an auditory language task, observing largely reduced connectivity between left auditory cortex and limbic regions in Sz + AH. Similar results were reported by Shinn et al65 who observed widespread reductions in connectivity for left primary auditory cortex (PAC), and by Gavrilescu et al,68 who observed reduced interhemispheric PAC connectivity. However, recent work by Chyzhyk et al60 identified right rather than left PAC as a key discriminator of AH status in Sz patients, highlighting some inconsistency in current findings.
Based on the role of Broca’s and Wernicke’s areas in speech processing, other studies have focused on connectivity between left inferior frontal gyrus (IFG) and the posterior STG, respectively. Hoffman et al69 observed elevated connectivity within a corticostriatal loop including STG (bilaterally), left IFG, and the putamen, in a pattern specific to Sz + AH. In contrast, Sommer et al70 found reduced connectivity between left IFG and left STG in AH participants, although only in comparison with healthy controls (ie, no clinical group without AH was included). In nonclinical AH, the left STG shows elevated connectivity with right STG and right IFG62 and acts as stronger connectivity “hub” at rest.63 Language lateralization may differ between clinical and nonclinical voice hearers,71 suggesting that extrapolating across these groups may be problematic, but taken together, these results indicate that atypical resting connectivity between left STG and other areas is common in AH.
Altogether, these studies point to a complex interaction between sensory, default mode, executive, and salience networks in AH. Inconsistent findings link overall DMN activity to AH, but there is evidence of DMN instability over time correlating with hallucination occurrence.64 Associations are also evident between AH and connectivity within SN and CEN, suggesting that problems with salience processing and cognitive control could contribute to a less stable balance between RSNs involved in external, sensory-guided attention.5 In addition, studies point to altered resting connectivity in left temporal regions implicated in auditory and language processing, although these findings require replication as some results (such as PAC connectivity) appear contradictory. In this context, drawing on evidence from other research methods, modalities, and conditions involving hallucination could help to parse out specific and general RSN properties important to AH.
Evidence From Neurophysiology and Structural Connectivity
From the Resting State to AH in EEG/MEG
Compared with stimulus-driven research, few EEG/MEG studies have examined the resting state in relation to either AH specifically or positive symptoms more broadly. At rest, Lee et al72 reported greater amplitude of beta oscillations in those with Sz + AH compared with Sz, with group differences localizing to left frontoparietal regions implicated in speech and language processing (left medial frontal gyrus and inferior parietal lobule). Andreou et al73 observed generally increased resting-state gamma oscillations within a left frontotemporoparietal network in Sz participants but surprisingly reduced gamma for those with higher positive symptoms (ie, those with greater levels of hallucinations and delusions had more normalized resting gamma). The actual occurrence of AH has been associated with increased gamma-theta coupling in frontotemporal areas,74 decreased beta power in left temporal cortex,75 and increased alpha connectivity between left and right auditory cortices.76 Analysis of rapid connectivity patterns known as “microstates” has also linked AH occurrence to shortened frontoparietal network patterns linked to error monitoring.77 Taken together, these findings support the primary role of left-lateralized frontotemporal cortex during AH but highlight how resting markers are likely to encompass a wider network of frontoparietal regions.
Comparisons With Structural Connectivity
Because of the auditory-verbal nature of many AH, much research on structural connectivity has focused on integrity of the arcuate fasciculus (AF), the main white matter tract linking inferior frontal and superior temporal cortex. Consistent with RSN evidence of STG alterations, AF has generally reduced white matter integrity in Sz + AH compared with controls.78 There is also some evidence that this is specific to Sz + AH compared with Sz,79 especially for verbal AH (AVH80), while milder alterations to AF are evident in nonclinical voice hearers.81 However, as in the RSN literature, some studies have reported constrasting results, including elevated connectivity in people with Sz + AH82 or positive correlations between integrity and AH severity.83
A second tract linking STG to frontal and occipital regions (supporting a ventral rather than dorsal language pathway) is the inferior occipital-frontal fasciculus (IOFF). Two studies have reported reduced structural integrity of the left frontotemporal segment of the IOFF being specifically related to AVH.79,84 Consistent evidence linking AH and structural connectivity elsewhere is so far lacking. Two studies79,85 have reported reduced structural integrity of the corpus callosum in Sz + AH compared with Sz and healthy controls—consistent with evidence of altered interhemispheric resting connectivity in AH68—but an earlier study reported stronger connectivity in an Sz + AH group.86 There is also some evidence that integrity of the cingulum (thought to be important in DMN connectivity, eg,87) may be reduced in Sz + AH, although this has also been observed in Sz-only samples without any correlation to AH.88 No studies have specifically sought to examine structural connectivity in AH for the main RSNs discussed above. Thus, though there is emerging evidence of white matter differences beyond the AF, more evidence is needed on AH-specific white matter changes and how they may relate to resting-state pathology.
Comparisons With VH
Comparing the Resting State in Auditory and Visual Hallucinations in Schizophrenia
VH are roughly half as common as AH in schizophrenia89,90 and are more prominent in neurological conditions with known etiology (such as Lewy Body Dementia [LBD] and Charles Bonnet syndrome91) leading to them being less studied in Sz. Indeed, common instruments for assessing hallucinations in Sz either neglect the distinction between AH and VH92 or primarily focus on AH.93 Importantly, the majority of Sz who experience VH also experience AH, but not necessarily simultaneously, allowing for comparison between the 2 experiences.
Four studies have specifically compared Sz participants with AH and AH + VH. Because of its proposed role in salience, Rolland et al94 focused on connectivity of the nucleus accumbens (NAc), finding greater connectivity between NAc and bilateral insula, putamen, parahippocampal gyri, and ventral tegmental area for participants with AH + VH (compared with just AH). Meanwhile, because of its involvement in both VH95 and AH,48 Amad et al96 analyzed hippocampal connectivity in both groups, observing hyperconnectivity to mPFC and caudate in participants with AH + VH specifically. Those with AH + VH also had higher white matter connectivity between the hippocampus and visual cortex and greater hippocampal hypertrophy.
In contrast, Ford et al97 observed no differences in hippocampal connectivity between AH and AH + VH participants but reported hyperconnectivity between visual cortex and amygdala specific to those with AH + VH. In the same participants, Hare et al98 identified decreased amplitude of low frequency fluctuations (ALFF) in AH compared with AH + VH, in retrosplenial/inferior precuneus (BA29), left hippocampus, bilateral insula, thalamus, medial cingulate, and the medial temporal lobe. Overall, these findings show much overlap in AH and VH circuitry but also that multisensory elements involve further alterations in connectivity to limbic and striatal cortex.
Comparisons With VH in Other Disorders
Aberrant perceptual phenomena also occur in a range of other neuropsychiatric disorders but often manifest in the visual rather than auditory domain. They are particularly common in Parkinson’s disease (PD) and LBD.99 Popular models implicate widespread impairments in attention and perception in the manifestation of hallucinations,100 which are supported by evidence from resting-state studies that show impaired connectivity within the DMN101,102 and between attentional networks in the brain.103 In addition, task-based fMRI has shown that visual misperceptions in PD are associated with increased DMN connectivity to ventral occipitotemporal regions that process object-related visual information.104 Interestingly, the latter results have strong convergence with the finding that the visual cortices show increased resting connectivity with the amygdala in Sz with AH + VH,97 suggesting similar yet divergent mechanisms underlying the manifestation of aberrant visual perception in different disorders.
Other neuropsychiatric disorders with VH demonstrate impairments in non-DMN networks. For instance, in posttraumatic stress disorder (PTSD), hallucinations and illusions (ie, misperceptions of actual stimuli) are relatively stereotyped and are closely related to the context in which the original stress occurred (eg, a war veteran with PTSD might hallucinate an enemy combatant or misperceive a telescope as a sniper rifle). Importantly, the primary pathological impairment in PTSD is related to impaired amygdala function105 and overactivity of regions within the ventral attention network (VAN),106,107 an RSN that overlaps anatomically and functionally with the SN but also typically includes regions of temporoparietal cortex.13 Due to this overactivity, it is assumed that any stimulus that closely matches the object related to the original stressor recruits attentional systems, leading to a rapid (and incorrect) increase in top-down influence over the ventral visual cortex, essentially “priming” the brain to incorrectly interpret the incoming stimulus. Although both LBD and PTSD are phenomenologically distinct from schizophrenia, the impairments in DMN29 and VAN108 suggest potential overlap between the different conditions and provide evidence of how hallucinations in different modalities can arise from the interaction of domain-general RSNs (managing salience and attention) with modality-specific regions.
Current Methodology in Resting-State Studies
The study of RSNs and AH is an emerging field with a variety of often inconsistent findings. In the following section, we consider a number of potential confounds and recommendations for improving comparability and reliability of results.
Resting-State Design and Potential Confounds
The designs of resting-state studies on AH to date have been far from uniform (see table 2), which hampers comparison of study findings. In table 3, we offer some suggestions to improve uniformity across experiments, some of which are specific to AH research and some that are more general. Most important in AH studies are participant instructions and debriefing: Instructing participants to keep their eyes closed is associated with higher functional connectivity within auditory networks109 (which may not be desirable) and arguably increases the likelihood of participants falling asleep. We therefore suggest that future studies use an eyes-open design. Beyond this, instructing participants to do anything other than relax can lead to signal changes (eg,110) and can create demand characteristics: When participants know in advance that they will be asked about AH, this can induce attention effects which confound hallucination-related brain activity.111 A thorough debrief about unusual experiences after scanning is preferred to online monitoring.
Table 2.
Study Characteristics of Resting-State fMRI Studies Into AH
| Study | EO/EC | Scan Length (min) | RS Instructions | Presence of AH Asked? |
|---|---|---|---|---|
| Alonso-Solis et al26 | EC | 6 | Pts instructed to close eyes and remain awake | Not reported |
| Chyzhyk et al60 | EO | 10 | Stay awake, keep eyes open, and think of nothing in particular | Yes for some participants |
| Clos et al59 | EC | 6 | Lie in the scanner as still as possible with their eyes closed yet stay awake | Yes |
| Diederen et al62 | EC | 6 | Pts kept eyes closed but stayed awake | Yes |
| Gavrilescu et al68 | EC | 5 | Relax with eyes closed | Yes |
| Jardri et al64 | EC | 15 | Pts kept still in a state of wakeful rest with eyes closed | Yes |
| Manoliu et al58 | EC | 10 | Eyes closed and not to fall asleep | Pts asked about any “feelings of odd situations” during scan. |
| Oertel-Knochel et al67 | EO | 6.7 | Lie still, do not engage in any speech, think nothing specially and look at white fixation cross | Yes |
| Rotarska-Jagiela et al28 | EO | 6.7 | Lie still with eyes open fixating on a white cross presented in the center of visual field. | Not reported |
| Shinn et al65 | EO | 10 | Stay awake, keep eyes open, and think of nothing in particular | Yes for some participants |
| Sommer et al70 | EC | 6 | Pts instructed to lie in scanner as still as possible with eyes closed yet stay awake | Yes |
| Sorg et al112 | EC | 10 | Keep eyes closed and not to fall asleep. | Pts asked about any “feelings of odd situations” during scan. |
| Van Lutterveld et al63 | EC | 6 | Pts kept eyes closed but stayed awake | Yes |
| Vercammen et al66 | EC | 7.8 | Close eyes and try to “clear your mind” but not fall asleep. | No |
| Wolf et al61 | EC | 6 | Relax without falling asleep, keep eyes closed, not think about anything in particular, and move as little as possible | Yes |
Note: AH, auditory hallucinations; EC, eyes closed; EO, eyes open; Pts, participants. Hoffman et al69 extracted intermittent resting data from a symptom capture paradigm involving button pressing, and so is not included here.
Table 3.
Recommended Methods for Resting Studies of AH
| Basic demographics and pre-interview | Age, gender, sleep patterns, nicotine use, medication, hallucination phenomenology |
| Participant instructions | Eyes open, relax, keep still. Emphasize not falling asleep |
| Stimuli | Fixation cross on gray, nonbright background (if eyes open) |
| Scan length | At least 6min; over 10min preferred |
| Concurrent measures | Head movements, cardiorespiratory signal, sleep monitoring (camera or concurrent EEG) |
| Debrief | Presence of hallucinations during scanning, emotional state (eg, anxiety), other unusual experiences |
| Analysis | Seed-based (for hypothesis-testing); common seeds include IFG, STG, TPJ, ACC, hippocampus, insula. ICA-based (preferred); allows analysis of network interactions. Graph-theoretical measures (eg, path- length, betweenness centrality) |
| Common network frameworks | Triple network (DMN/CEN/SN10) or Yeo et al113 7 or 17 network solution |
Note: Abbreviations are explained in the first footnote to table 1. AH, AH, auditory hallucinations; CEN, central executive network; DMN, default mode network; ICA, independent component analysis; SN, salience network.
In general, longer scan times are preferable (increasing scan time to 13min greatly improves reliability114), and it is important to control for potential effects of head movement, cardiac rate, and respiration.115–117 Data scrubbing of bad volumes118 or matching groups for head motion are 2 ways of counteracting potential movement effects. Finally, during data analysis, use of global signal regression to account for nonneuronal artifacts should be avoided, as this can introduce spurious negative correlations.119
Resting-State Analysis
There are 2 common approaches to analyzing resting-state fMRI connectivity data: strongly and weakly model-driven. In seed-regression analyses, correlations are calculated between an a priori selected region and the rest of the brain. This is a strongly model-driven approach, as the selected seed region represents a spatial hypothesis about the brain state of interest, and is the most common approach used in resting-state AH studies. In contrast, 2 other methods have been used to characterize functional brain dynamics in a multivariate, weakly model-driven approach: independent component analysis (ICA) and graph theory. ICA is a multivariate, data-driven analysis tool of the Blind Source Separation family,120 which has been shown to be particularly well-suited for analyzing distributed networks of intrinsic brain activity.121 Unlike seed-based analysis, ICA does not require a priori definition of a seed region or ideal model of activity and provides multiple networks in a single analysis. Moreover, it has been argued that ICA has better test-retest reliability than seed-based methods122 and may be better at estimating functional statistical maps in the case of unpredictable events such as hallucinations without the need for signaling events’ occurrences online.64,123 Crucially, when participants hallucinate during a scan, ICA can be used to capture both DMN and hallucinatory fMRI activity: Jardri et al64 describe a validated procedure for doing so that uses retrospective AH reports from participants. This property of ICA makes possible the exploration of the interplay between DMN and sensory cortices over time during “rest” and “hallucination” periods (defined at the individual IC level), without disrupting intrinsic RSN behavior. Finally, graph theory mathematically describes the architecture of interregional connections (ie, edges) of multiple brain areas (ie, nodes) in relation to efficiency of information processing,124 which can be used to characterize local or global changes in network architecture. Graph theory can be considered complementary to the other functional connectivity tools, as it is often applied to output of the seed-based or ICA approaches. Its value is in allowing investigators to go beyond simple correlations between regions to examine the relative importance of paths and hubs within a network.
AH and the Resting State: Key Issues and Implications
RSNs and AH: A Synthesis of Findings
The investigation of RSNs opens up a range of opportunities for understanding how and why hallucinations occur. The majority of AH research has so far focused on intrinsic connectivity of regions associated with speech, language, and auditory processing, both in fMRI and EEG/MEG: Most consistent are findings of altered connectivity in left posterior temporal regions implicated in speech perception (which are supported by structural evidence), but there are also multiple studies implicating inferior frontal, parietal, limbic, and striatal regions.6 Such findings broadly support theories that posit disruptions to internal speech and language processes,125 although not consistently (cf.69,70). This is complemented by a growing body of evidence linking the DMN, CEN, and SN to both AH occurrence and predisposition, lending support to models of hallucinations that go beyond speech processes and emphasize other factors, such as cognitive control and attention.126 As outlined in prior ICHR collaborations,127 it is generally accepted that AH are likely to involve multiple cognitive mechanisms in their development, suggesting that a focus on multiple brain networks is required.
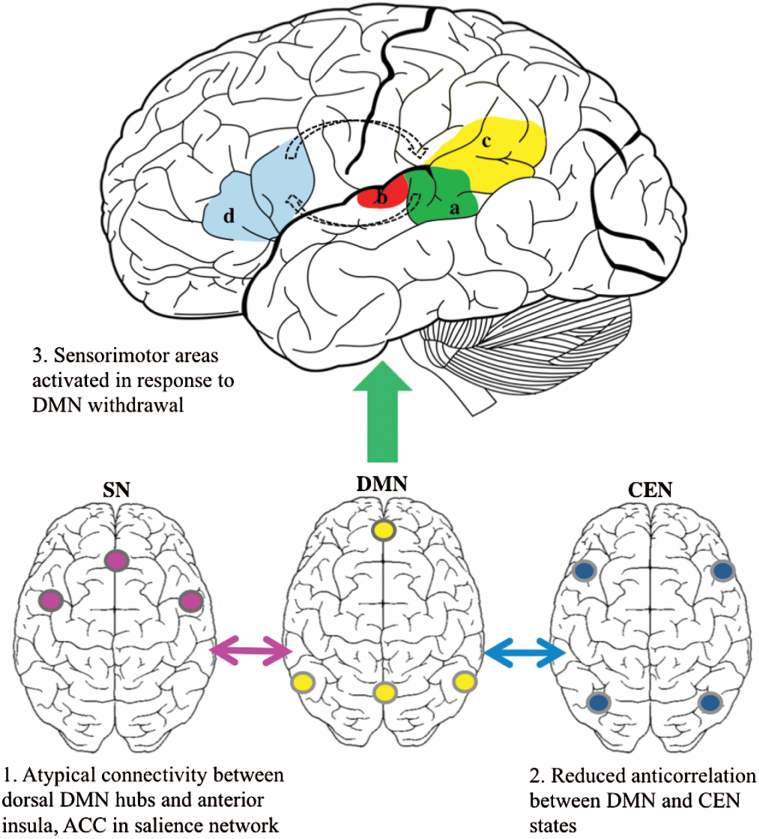
Few studies have demonstrated specific trait associations between DMN properties and AH (in comparison with evidence of general links to positive symptoms, eg,25). However, the interaction of the DMN with other RSNs26,58 and dynamic stability of the DMN64 are both implicated in hallucinatory states and traits. It is possible that atypical interactions of the DMN, SN, and CEN, when allied with altered resting connectivity in language and sensory networks, give rise to the collapse of internal states into sensory processing as described by Jardri et al64 (see figure 1). This also partly supports Northoff and Qin’s5 proposal that AH arise from elevated resting-state activity in auditory areas and irregular modulation by anterior hubs of the DMN: The evidence here does not suggest a direct link between them but implies atypical DMN-SN-CEN interaction alongside connectivity alterations to sensory cortices. However, these findings are clearly preliminary and require further testing, especially in the face of inconsistent results and methodological heterogeneity. It will be important to examine how hubs of the domain-general RSNs interact with auditory areas, and the future use of alternative seeds/ROIs or ICA will shed further light on how these other networks contribute to AH.
Fig. 1.
Initial AH studies focused on resting connectivity in auditory and language regions (upper figure), primarily identifying atypical connectivity of left posterior STG (a), PAC (b), and the TPJ area (c). Findings of atypical resting connectivity between left IFG (d) and STG are inconsistent although both areas are often implicated during AH. More recent findings implicate atypical interaction of the DMN, SN, and CEN in those prone to AH (lower figures). The combination of atypical DMN interaction with SN (1) and CEN (2) and altered resting connectivity in sensory areas could prompt the collapse of internally focused states into activation of auditory cortex (3), which is then reverberated along a frontotemporal loop. The IFG, STG, and surrounding areas are often implicated in symptom-capture studies.48 Note: ACC, anterior cingulate cortex; AH, auditory hallucination; CEN, central executive network; DMN, default mode network; IFG, inferior frontal gyrus; PAC, primary auditory cortex; SN, salience network; STG, superior temporal gyrus; TPJ, temporoparietal junction.
Comparisons with mechanisms in VH provide an important source of information. Evidence of DMN instability in both AH and VH in psychosis and disruptions to DMN, executive, and salience networks in VH in other disorders imply a similar framework for hallucination susceptibility across different modalities. However, contrasts between AH and AH + VH in schizophrenia also suggest that disruptions to limbic and striatal connectivity may distinguish trait susceptibility for these experiences,96,97 suggesting that more complex hallucinatory phenomena may require a higher base level of resting connectivity between sensory and limbic regions. Comparison with VH raises the question of whether other RSNs need to be examined in more detail in AH: eg, while a number of AH and Sz studies have focused on the SN, the VAN is relatively unstudied. The SN and VAN overlap in many ways—and the exact relationship between them is still unclear—but definitions of VAN often emphasize right frontoparietal structures (such as IFG and TPJ) rather than just insular and cingulate cortex (eg,13,128). Given that functional and structural characteristics of these regions have been related to AH occurrence and phenomenology,129,130 the VAN may prove a fruitful avenue for further investigation.
Indeed, the current focus on DMN, CEN, and SN in AH research arguably reflects only one approach to RSNs—the “triple network” model.10 Other network approaches, such as that used by Yeo et al,113 use many more functional networks in parallel. Future AH studies may choose to adopt such broader network solutions, especially to allow comparison with research on healthy cognitive function. Understanding of the DMN and other RSNs is also constantly advancing: The early discovery that the DMN is negatively correlated with another set of regions—namely those of the CEN20—was called into question by work demonstrating variability in this negative relationship over time.131 Several subsequent studies have further revealed the complex spatiotemporal patterns of activity that underlie RSNs (eg,132–134 for a review see16), while task studies show that regions distributed across the whole brain and multiple networks are often involved in “single” processes.135 In this respect, it is important to recognize that terms such as DMN or CEN are only placeholders for complex and likely cross-network processes, and researchers should be generally wary of treating them as modular, insulated entities.
Increasing Study Reliability and Aggregating Data
In moving forward with resting-state research on AH, improving the comparability and reliability of results is a clear priority. One way of doing this is following a shared “standard” protocol (such as in table 3); another, more powerful approach is to aggregate data across studies, facilitating the acquisition of a more heterogeneous and representative subject sample.136 fMRI data are typically pooled using meta-analyses of significant activations (eg,137), but such analyses cannot aggregate power across studies as they rarely report effect sizes. A more direct method for aggregating data is by combining (raw) data across centers, also referred to as mega-analysis.138 Although the instruction and fMRI protocol may differ across studies, this approach allows for the use of the same preprocessing and analysis on all data. Another option consists of multicenter studies in which the scan protocol, participant instructions, scanner, software, hardware, etc. are standardized.139 Variability between centers can be monitored through the use of acquisition of phantom data and travelling heads, ie, control subjects that are scanned at each site to estimate cross-site variability. Although recent studies indicate that scanner-related variance is low compared with between-subject variability and measurement error,140,141 it can be modeled by entering study site as a random effects factor capturing variability between study sites.142 In this vein, the authors of this article are currently planning a mega-analysis of existing resting-state data from research teams within the ICHR: To date, 7 participating centers have agreed to aggregate their data (which includes over 150 participants with AH).
Clinical Implications and Directions for Further Research
As a young field, resting-state research on AH is still at the stage of basic rather than translational science. However, the opportunity to study state and trait markers of AH using resting methods has great clinical potential: In a recent example, Mondino et al143 demonstrated changes in resting temporoparietal connectivity associated with symptom reduction in patients who had completed 10 weeks of transcranial direct current stimulation for persistent AH. A further way of making resting-state studies more clinically relevant would be to include more detailed examination of hallucinatory phenomenology and contextual factors, both in general and following scanning. While many studies have reported AH symptom correlations, this is usually a total severity score, rather than specific to different kinds of phenomenological features, and wider environmental and social variables are almost never included. Other key remaining questions, with basic and applied implications, are how resting-state fMRI findings relate to functional connectivity measured with neurophysiological methods (eg,144), evidence of changes to anatomical connectivity,78 and computational models of hallucinations, such as predictive processing approaches (see Box 1). The use of multimethod studies will go some way to address these issues in future research.
Conclusion
The spatiotemporal dynamics of the brain’s resting state have great potential to offer new insights in the study of hallucinations. Studies of AH highlight altered resting connectivity of speech and language regions, such as the left STG, although there is growing evidence of domain-general resting networks (and thus the brain’s spontaneous activity as a whole) being implicated in Sz + AH and perhaps other kinds of hallucinatory states. Studies of VH suggest that additional networks may be involved, including limbic and striatal regions, although more studies contrasting RSN in VH and AH are needed across different population groups. Alongside this, the study of RSNs is subject to a range of potential confounds and methodological differences, prompting a need to combine efforts and methods across laboratories. A more connected approach will improve comparability and reliability of studies and enhance understanding of how, in the case of AH, a signal may emerge from noise.
Funding
B.A.-D. and C.F. are supported by Wellcome Trust (WT098455); C.F. and D.S.M. by Wellcome Trust (WT103817).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Johns LC, Kompus K, Connell M, et al. Auditory verbal hallucinations in persons with and without a need for care. Schizophr Bull. 2014;40(suppl 4):255–264. doi:10.1093/schbul/sbu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen P, Modinos G, Hubl D, et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38:695–703. doi:10.1093/schbul/sbs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodward TS, Jung K, Hwang H, et al. Symptom dimensions of the psychotic symptom rating scales in psychosis: a multisite study. Schizophr Bull. 2014;40(suppl 4):S265–S274. doi:10.1093/schbul/sbu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas N, Rossell SL, Waters F. The changing face of hallucination research: the International Consortium on Hallucination Research (ICHR) 2015 meeting report. Schizophr Bull. December 16, 2015. doi:10.1093/schbul/sbv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A “resting state hypothesis” of auditory verbal hallucinations. Schizophr Res. 2011;127:202–214. doi:10.1016/j.schres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 6. Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev. 2015;55:78–87. doi:10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Northoff G. Immanuel Kant’s mind and the brain’s resting state. Trends Cogn Sci. 2012;16:356–359. doi:10.1016/j.tics.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 8. Fingelkurts AA, Fingelkurts AA, Kahkonen S. Functional connectivity in the brain - is it an elusive concept? Neurosci Biobehav Rev. 2005;28:827–836. doi:10.1016/j.neubiorev.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi:10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi:10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi:10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 12. Dosenbach NUF, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi:10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi:10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi:10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi:10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di X, Biswal BB. Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct Funct. 2015;220:37–46. doi:10.1007/s00429-013-0634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;22:144–153. doi:10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi:10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Essen DCV, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi:10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi:10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 22. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi:10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi:10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee MH, Hacker CD, Snyder AZ, et al. Clustering of resting state networks. PLoS ONE. 2012;7:e40370. doi:10.1371/journal.pone.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi:10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alonso-Solis A, Vives-Gilabert Y, Grasa E, et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. 2015;161:261–268. doi:10.1016/j.schres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 27. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi:10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 28. Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DEJ. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. doi:10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 29. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi:10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 30. Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi:10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31. Rutledge RB, Dean M, Caplin A, Glimcher PW. Testing the reward prediction error hypothesis with an axiomatic model. J Neurosci. 2010;30:13525–13536. doi:10.1523/JNEUROSCI.1747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tu P-C, Hsieh J-C, Li C-T, Bai Y-M, Su T-P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. NeuroImage. 2012;59:238–247. doi:10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 33. White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–115. doi:10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 34. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi:10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Li F, Zheng H, et al. Breakdown of the striatal-default mode network loop in schizophrenia. Schizophr Res. 2015;168:366–372. doi:10.1016/j.schres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 36. Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148:74–80. doi:10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 37. Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130:86–93. doi:10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36:181–204. doi:10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 39. Fletcher PC, Frith C. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi:10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 40. Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi:10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 41. Jardri R, Denève S. Circular inferences in schizophrenia. Brain. 2013;136:3227–3242. doi:10.1093/brain/awt257. [DOI] [PubMed] [Google Scholar]
- 42. Wacongne C, Changeux J-P, Dehaene S. A neuronal model of predictive coding accounting for the mismatch negativity. J Neurosci. 2012;32:3665–3678. doi:10.1523/JNEUROSCI.5003-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathalon DH, Ford JM. Corollary discharge dysfunction in schizophrenia: evidence for an elemental deficit. Clin EEG Neurosci. 2008;39:82–86. [DOI] [PubMed] [Google Scholar]
- 44. Fisher DJ, Labelle A, Knott VJ. Alterations of mismatch negativity (MMN) in schizophrenia patients with auditory hallucinations experiencing acute exacerbation of illness. Schizophr Res. 2012;139:237–245. doi:10.1016/j.schres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 45. Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. doi:10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- 46. Horga G, Schatz KC, Abi-Dargham A, Peterson BS. Deficits in predictive coding underlie hallucinations in schizophrenia. J Neurosci. 2014;34:8072–8082. doi:10.1523/JNEUROSCI.0200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi:10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 48. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. doi:10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 49. Horga G, Parellada E, Lomeña F, et al. Differential brain glucose metabolic patterns in antipsychotic-naïve first-episode schizophrenia with and without auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:312–321. doi:10.1503/jpn.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Friston KJ, Stephan KE. Free-energy and the brain. Synthese. 2007;159:417–458. doi:10.1007/s11229-007-9237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ouden HEM den, Daunizeau J, Roiser J, Friston KJ, Stephan KE. Striatal prediction error modulates cortical coupling. J Neurosci. 2010;30:3210–3219. doi:10.1523/JNEUROSCI.4458-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horga G, Maia TV, Marsh R, et al. Changes in corticostriatal connectivity during reinforcement learning in humans. Hum Brain Mapp. 2015;36:793–803. doi:10.1002/hbm.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wimmer GE, Daw ND, Shohamy D. Generalization of value in reinforcement learning by humans. Eur J Neurosci. 2012;35:1092–1104. doi:10.1111/j.1460-9568.2012.08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gerraty RT, Davidow JY, Wimmer GE, Kahn I, Shohamy D. Transfer of learning relates to intrinsic connectivity between hippocampus, ventromedial prefrontal cortex, and large-scale networks. J Neurosci. 2014;34:11297–11303. doi:10.1523/JNEUROSCI.0185-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi:10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seth A, Suzuki K, Critchley H. An interoceptive predictive coding model of conscious presence. Front Psychol. 2012. ;2:395. doi:10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stephan KE, Penny WD, Moran RJ, den Ouden HEM, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. NeuroImage. 2010;49:3099–3109. doi:10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40:428–437. doi:10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Clos M, Diederen KMJ, Meijering AL, Sommer IE, Eickhoff SB. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct Funct. 2014;219:581–594. doi:10.1007/s00429-013-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chyzhyk D, Graña M, Öngür D, Shinn AK. Discrimination of schizophrenia auditory hallucinators by machine learning of resting-state functional MRI. Int J Neural Syst. 2015;25:1550007. doi:10.1142/S0129065715500070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf ND, Sambataro F, Vasic N, et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:366–374. doi:10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diederen KMJ, Neggers SFW, de Weijer AD, et al. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol Med. 2013;43:1685–1696. doi:10.1017/S0033291712002541. [DOI] [PubMed] [Google Scholar]
- 63. van Lutterveld R, Diederen KMJ, Otte WM, Sommer IE. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum Brain Mapp. 2014;35:1436–1445. doi:10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex. 2013;23:1108–1117. doi:10.1093/cercor/bhs082. [DOI] [PubMed] [Google Scholar]
- 65. Shinn AK, Baker JT, Cohen BM, Öngür D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res. 2013;143:260–268. doi:10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67:912–918. doi:10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 67. Oertel-Knochel V, Knöchel C, Matura S, et al. Association between symptoms of psychosis and reduced functional connectivity of auditory cortex. Schizophr Res. 2014;160:35–42. doi:10.1016/j.schres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 68. Gavrilescu M, Rossell S, Stuart GW, et al. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med. 2010;40:1149–1158. doi:10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- 69. Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–414. doi:10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sommer IE, Clos M, Meijering AL, Diederen KMJ, Eickhoff SB. Resting state functional connectivity in patients with chronic hallucinations. PLoS ONE. 2012;7:e43516. doi:10.1371/journal.pone.0043516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Diederen KMJ, De Weijer AD, Daalman K, et al. Decreased language lateralization is characteristic of psychosis, not auditory hallucinations. Brain J Neurol. 2010;133(pt 12):3734–3744. doi:10.1093/brain/awq313. [DOI] [PubMed] [Google Scholar]
- 72. Lee S-H, Wynn JK, Green MF, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res. 2006;83:111–119. doi:10.1016/j.schres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 73. Andreou C, Nolte G, Leicht G, et al. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr Bull. 2015;41:930–939. doi:10.1093/schbul/sbu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Koutsoukos E, Angelopoulos E, Maillis A, Papadimitriou GN, Stefanis C. Indication of increased phase coupling between theta and gamma EEG rhythms associated with the experience of auditory verbal hallucinations. Neurosci Lett. 2013;534:242–245. doi:10.1016/j.neulet.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 75. Van Lutterveld R, Hillebrand A, Diederen KMJ, et al. Oscillatory cortical network involved in auditory verbal hallucinations in schizophrenia. PLoS ONE. 2012;7:e41149. doi:10.1371/journal.pone.0041149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sritharan A, Line P, Sergejew A, Silberstein R, Egan G, Copolov D. EEG coherence measures during auditory hallucinations in schizophrenia. Psychiatry Res. 2005;136:189–200. doi:10.1016/j.psychres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 77. Kindler J, Hubl D, Strik WK, Dierks T, Koenig T. Resting-state EEG in schizophrenia: auditory verbal hallucinations are related to shortening of specific microstates. Clin Neurophysiol. 2011;122:1179–1182. doi:10.1016/j.clinph.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 78. Geoffroy PA, Houenou J, Duhamel A, et al. The arcuate fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr Res. 2014;159:234–237. doi:10.1016/j.schres.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 79. Ćurčić-Blake B, Nanetti L, van der Meer L, et al. Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct Funct. 2015;220:407–418. doi:10.1007/s00429-013-0663-y. [DOI] [PubMed] [Google Scholar]
- 80. McCarthy-Jones S, Oestreich LKL, Australian Schizophrenia Research Bank , Whitford TJ. Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr Res. 2015;162:1–6. doi:10.1016/j.schres.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 81. De Weijer AD, Neggers SFW, Diederen KMS, et al. Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum Brain Mapp. 2013;34:626–634. doi:10.1002/hbm.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi:10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 83. Rotarska-Jagiela A, Oertel-Knochel V, DeMartino F, et al. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res Neuroimaging. 2009;174:9–16. doi:10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 84. Oestreich LKL, McCarthy-Jones S, Australian Schizophrenia Research Bank , Whitford TJ. Decreased integrity of the fronto-temporal fibers of the left inferior occipito-frontal fasciculus associated with auditory verbal hallucinations in schizophrenia. Brain Imaging Behav. 2015:1–10. doi:10.1007/s11682-015-9421-5. [DOI] [PubMed] [Google Scholar]
- 85. Wigand M, Kubicki M, Clemm von Hohenberg C, et al. Auditory verbal hallucinations and the interhemispheric auditory pathway in chronic schizophrenia. World J Biol Psychiatry. 2015;16:31–44. doi:10.3109/15622975.2014.948063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mulert C, Kirsch V, Whitford TJ, et al. Hearing voices: a role of interhemispheric auditory connectivity? World J Biol Psychiatry. 2012;13:153–158. doi:10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Teipel SJ, Bokde ALW, Meindl T, et al. White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage. 2010;49:2021–2032. doi:10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 88. Knöchel C, O’Dwyer L, Alves G, et al. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr Res. 2012;140:129–135. doi:10.1016/j.schres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 89. Blom JD. Hallucinations and other sensory deceptions in psychiatric disorders. In: Jardri R, Cachia A, Thomas P, Pins D, eds. The Neuroscience of Hallucinations. New York, NY: Springer; 2013:43–57. http://link.springer.com/chapter/10.1007/978-1-4614-4121-2_3 Accessed January 24, 2016. [Google Scholar]
- 90. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(suppl 4):S233–S245. doi:10.1093/schbul/sbu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Collerton D, Dudley R, Mosimann UP. Visual hallucinations. In: Blom JD, Sommer IEC, eds. Hallucinations. New York, NY: Springer; 2012:75–90. http://link.springer.com/chapter/10.1007/978-1-4614-0959-5_6 Accessed January 24, 2016. [Google Scholar]
- 92. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 93. Andreasen N. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 94. Rolland B, Amad A, Poulet E, et al. Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 2015;41:291–299. doi:10.1093/schbul/sbu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Oertel V, Rotarska-Jagiela A, van de Ven VG, Haenschel C, Maurer K, Linden DEJ. Visual hallucinations in schizophrenia investigated with functional magnetic resonance imaging. Psychiatry Res. 2007;156:269–273. doi:10.1016/j.pscychresns.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 96. Amad A, Cachia A, Gorwood P, et al. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 2014;19:184–191. doi:10.1038/mp.2012.181. [DOI] [PubMed] [Google Scholar]
- 97. Ford JM, Palzes VA, Roach BJ, et al. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr Bull. 2015;41:223–232. doi:10.1093/schbul/sbu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hare SM, Pasquerello D, Damaraju E, et al. A multi-site voxelwise analysis of the amplitude of low-frequency fluctuations in schizophrenics: exploring the effects of hallucination type. Schizophr Bull. 2015;41:S226–S226. [Google Scholar]
- 99. Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi:10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 100. Muller AJ, Shine JM, Halliday GM, Lewis SJG. Visual hallucinations in Parkinson’s disease: theoretical models. Mov Disord. 2014;29:1591–1598. doi:10.1002/mds.26004. [DOI] [PubMed] [Google Scholar]
- 101. Yao N, Shek-Kwan Chang R, Cheung C, et al. The default mode network is disrupted in Parkinson’s disease with visual hallucinations. Hum Brain Mapp. 2014;35:5658–5666. doi:10.1002/hbm.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goetz CG, Vaughan CL, Goldman JG, Stebbins GT. I finally see what you see: Parkinson’s disease visual hallucinations captured with functional neuroimaging. Mov Disord. 2014;29:115–117. doi:10.1002/mds.25554. [DOI] [PubMed] [Google Scholar]
- 103. Shine JM, Keogh R, O’Callaghan C, Muller AJ, Lewis SJG, Pearson J. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson’s disease and visual hallucinations. Proc Biol Sci. 2015;282:20142047. doi:10.1098/rspb.2014.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shine JM, Muller AJ, O’Callaghan C, Hornberger M, Halliday GM, Lewis SJ. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. Npj Park Dis. 2015;1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. El Khoury-Malhame M, Reynaud E, Soriano A, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi:10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 106. Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–266. doi:10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fani N, Jovanovic T, Ely TD, et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012;90:134–142. doi:10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. White TP, Gilleen J, Shergill SS. Dysregulated but not decreased salience network activity in schizophrenia. Front Hum Neurosci. 2013;7:65. doi:10.3389/fnhum.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Patriat R, Molloy EK, Meier TB, et al. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. NeuroImage. 2013;78:463–473. doi:10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Benjamin C, Lieberman DA, Chang M, et al. The influence of rest period instructions on the default mode network. Front Hum Neurosci. 2010;4:218. doi:10.3389/fnhum.2010.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Van Lutterveld R, Diederen K, Schutte M, Bakker R, Zandbelt B, Sommer I. Brain correlates of auditory hallucinations: stimulus detection is a potential confounder. Schizophr Res. 2013;150:319–320. doi:10.1016/j.schres.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 112. Sorg C, Manoliu A, Neufang S, et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull. 2013;39:387–395. doi:10.1093/schbul/sbr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi:10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Birn RM, Molloy EK, Patriat R, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi:10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–1548. doi:10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 116. Shmueli K, van Gelderen P, de Zwart JA, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38:306–320. doi:10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi:10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi:10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi:10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hyvärinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13:411–430. [DOI] [PubMed] [Google Scholar]
- 121. Van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DEJ. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp. 2004;22:165–178. doi:10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Foucher JR. Perspectives in brain imaging and computer-assisted technologies for the treatment of hallucinations. In: Jardri R, Cachia A, Thomas P, Pins D, eds. The Neuroscience of Hallucinations. New York, NY: Springer; 2013:529–547. http://link.springer.com/chapter/10.1007/978-1-4614-4121-2_27 Accessed January 24, 2016. [Google Scholar]
- 123. Van de Ven VG, Formisano E, Röder CH, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. NeuroImage. 2005;27:644–655. doi:10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 124. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi:10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 125. Allen P, Aleman A, Mcguire PK. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev Psychiatry. 2007;19:407–415. doi:10.1080/09540260701486498. [DOI] [PubMed] [Google Scholar]
- 126. Waters F, Badcock J, Michie P, Maybery M. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cognit Neuropsychiatry. 2006;11:65–83. doi:10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 127. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–693. doi:10.1093/schbul/sbs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weissman DH, Prado J. Heightened activity in a key region of the ventral attention network is linked to reduced activity in a key region of the dorsal attention network during unexpected shifts of covert visual spatial attention. NeuroImage. 2012;61:798–804. doi:10.1016/j.neuroimage.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 129. Sommer IE, Diederen KMJ, Blom J-D, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. doi:10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 130. Plaze M, Paillère-Martinot M-L, Penttilä J, et al. “Where do auditory hallucinations come from?”—a brain morphometry study of schizophrenia patients with inner or outer space hallucinations. Schizophr Bull. 2011;37:212–221. doi:10.1093/schbul/sbp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi:10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schaefer A, Margulies DS, Lohmann G, et al. Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front Hum Neurosci. 2014;8:195. doi:10.3389/fnhum.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Smith SM, Miller KL, Moeller S, et al. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A. 2012;109:3131–3136. doi:10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A. 2013;110:4392–4397. doi:10.1073/pnas.1216856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gonzalez-Castillo J, Hoy CW, Handwerker DA, et al. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proc Natl Acad Sci U S A. 2015;112:8762–8767. doi:10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: the FBIRN imaging consortium. Schizophr Bull. 2009;35:15–18. doi:10.1093/schbul/sbn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. [DOI] [PubMed] [Google Scholar]
- 138. Turner JA. The rise of large-scale imaging studies in psychiatry. GigaScience. 2014;3:29. doi:10.1186/2047-217X-3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Costafreda SG. Pooling FMRI data: meta-analysis, mega-analysis and multi-center studies. Front Neuroinformatics. 2009;3:33. doi:10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Costafreda SG, Brammer MJ, Vêncio RZN, et al. Multisite fMRI reproducibility of a motor task using identical MR systems. J Magn Reson Imaging. 2007;26:1122–1126. doi:10.1002/jmri.21118. [DOI] [PubMed] [Google Scholar]
- 141. Suckling J, Ohlssen D, Andrew C, et al. Components of variance in a multicentre functional MRI study and implications for calculation of statistical power. Hum Brain Mapp. 2008;29:1111–1122. doi:10.1002/hbm.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Friedman L, Glover GH, Fbirn Consortium Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage. 2006;33:471–481. doi:10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 143. Mondino M, Jardri R, Suaud-Chagny M-F, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318–326. doi:10.1093/schbul/sbv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi:10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]