Abstract
The Research Domain Criteria (RDoC) initiative was implemented to reorient the approach to mental health research from one focused on Diagnostic and Statistical Manual of Mental Disorders (DSM) nosology to one oriented to psychological constructs constrained by neurocircuitry and molecular entities. The initiative has generated significant discussion and valuable reflection on the moorings of psychiatric research. The purpose of this article is to illustrate how a basic or clinical investigator can engage RDoC to explore the neurobiological underpinnings of psychopathology and how a research question can be formulated in RDoC’s framework. We utilize a brain region with significant growing interest, the habenula, as an example for probing RDoC’s utility. Opportunities to enhance neurocircuitry-psychological construct associations and problems associated with neuronal populations that enable bidirectional circuitry influence are discussed. The exercise reveals areas for further development that could move RDoC from a promising research idea to a successfully engaged foundation for catalyzing clinically relevant discoveries.
Key words: habenula, RMTg, psychiatric diagnosis, translational, DSM, endophenotype
RDoC: A Catalyst for Stalled Progress in Psychiatric Research
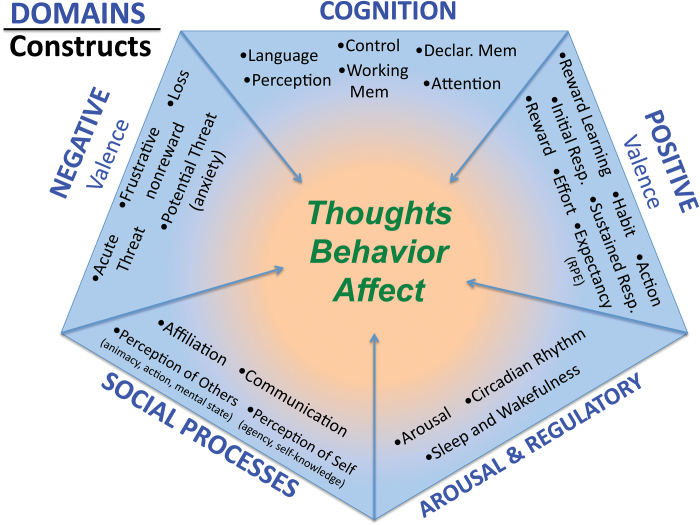
Recent efforts by National Institute of Mental Health (NIMH) to break through stalled advancements in the treatment of major psychiatric disorders have led to a reconceptualized research strategy that focuses on constructs of psychology and psychopathology delineated by specific neurocircuitry.1–3 The strategy promotes the investigation and characterization of these constructs at various levels of analysis from genes to physiology to behavior. Constructs are grouped into 5 larger domains (negative valence, positive valence, cognition, social processes, and arousal/modulation) that serve to organize closely linked psychological functions (see figure 1). For example, the negative valence domain comprises the constructs fear, anxiety, threat, loss, and frustrative nonreward. Each construct is envisioned to function along a continuum from normal to pathological. Thus, while healthy fear serves a protective function under appropriate conditions, nonspecific, inappropriate, or extreme fear could contribute to psychopathology. Importantly, the domains and constructs are uncoupled from traditional diagnostic categories.
Fig. 1.
RDoC envisions sets of psychological constructs organized into larger associated domains. Individual constructs are thought to function along a continuum from normal to abnormal. The balance of construct functionality contributes to the overall mental constitution of the individual. RDoC, Research Domain Criteria.
The development of RDoC has not been without its critics4–6 and calls for caution—“… it would indeed be unfortunate if the march to freedom from the DSM’s ‘epistemic prison’7 led merely to a padded cell in the matrix penitentiary.8 However, the shift in experimental approach is not intended to replace the current diagnostic classification systems (see specific discussions6,8–11) but rather to provide a road map for research and through this framework to “elaborate a set of psychological constructs linked to behavioral dimensions for which strong evidence exists for circuits that implement these functions, and relate the extremes of functioning along these dimensions to specified symptoms (i.e., impairment).”3
RDoC is explicitly translational and designed as a platform to engage frontline basic neuroscience, clinical neuroscience, and psychiatry. Basic science research is well positioned to investigate the molecular and neuroanatomical substrates of clinically relevant constructs, while clinical research efforts are encouraged to delineate boundaries of construct operation while remaining agnostic to psychiatric nosology. The common ground is laid out in a two-dimensional matrix (https://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml). Domains and their underlying constructs are organized in rows. Within each row, columns representing units of analysis elaborate the genetic, molecular, cellular, circuitry, physiology, and behavioral components of each construct. Importantly, the foundation and justification of each construct is based in large part upon evidence for a neural circuit or system that contributes to an attendant psychological function with demonstrated clinical relevance and validity.12
The purpose of this article is to illustrate how an investigator or clinician can engage RDoC to glimpse the current state of understanding regarding the neurobiological underpinnings of disease psychopathology and to demonstrate how a research question can be framed in the context of domains, constructs, and circuitry. Our intent is to highlight existing strengths within RDoC and to identify areas for further development that would enhance its practical utility for the scientific community.
Engaging RDoC
There are several practical objectives for which researchers and clinicians may want to engage RDoC. One may be to extract information from the matrix, for example, to answer a specific neurobiological question or to understand a clinically relevant psychopathological construct. For this purpose, simple exploration of RDoC’s matrix provides a bird’s-eye view of how aspects of human behavior are encompassed as the composite of 5 dynamically maintained domains, each animated by a cluster of associated psychological constructs (http://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdocmatrix.shtml). Further exploration reveals the psychological rails that loosely define and constrain the constructs and mechanisms that drive the constructs.
Another objective may be to use it as a standardized platform to develop and test new hypotheses and to integrate new research findings into its current domains, constructs, and units of analysis or perhaps to develop new ones. Innovative developments in methods to discover regional connections13–18 and functional relevance19–22 are rapidly advancing our understanding of circuitry dynamics and the mechanisms that drive them. Given the pace of discoveries, ensuring a viable platform to achieve this objective is of paramount importance to ensure that RDoC remains relevant as a research resource and framework for hypothesis generation and testing.
As a means of illustrating a practical engagement with RDoC, we will focus on the habenula (Hb), a region with rapidly growing implications for a broad range of psychopathology. We begin by querying RDoC using the unit of analysis: circuit as an entry point (http://www.nimh.nih.gov/research-priorities/rdoc/units/circuits/index.shtml). Selecting Hb from the list of brain regions reveals the current domain and construct associations. Selecting a specific construct reveals the information currently available related to each unit of analysis. As of this writing, the only annotation for the Hb is the positive valence domain in the construct/subconstruct “approach motivation/expectancy_reward prediction error.” Its placement here reflects a body of evidence implicating the Hb in “states that are triggered by internal or external stimuli, experiences or contexts that predict the possibility of reward” and that “the reward expectation can alter the experience of an outcome and can influence the use of resources (eg, cognitive resources)” (http://www.nimh.nih.gov/research-priorities/rdoc/constructs/expectancy-reward-prediction-error.shtml). Other brain regions involved in this construct are the amygdala, basal ganglia, dorsal anterior cingulate cortex, orbital frontal cortex, rostral medial tegmentum, substantia nigra (SN)/ventral tegmental area (VTA), and ventral striatum. Notably, a specific circuit is not defined.
Elaborating and Refining RDoC constructs
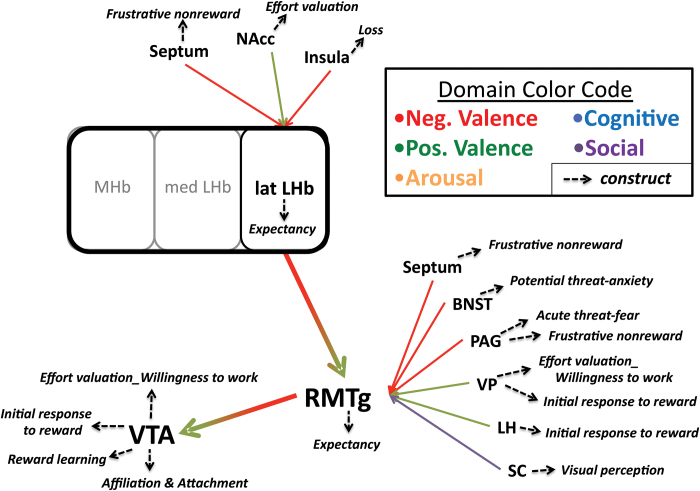
In the future, especially given the importance of neurocircuitry in delineating constructs, RDoC would ideally enable investigators to curate a list of known afferent and efferent connections derived from various levels of neuroanatomical analysis. In this manner, for example, RDoC could serve as a bioinformatics platform similar to other public and commercial genomics and connectomic platforms.23,24 Specific queries could lead to a table of known connections with annotations noting the anatomical method of determination or to searchable diagrams with coded domain and construct associations and embedded annotations containing information from all levels of analysis (see figure 2). The capacity to query upstream and downstream connections with annotated constructs and domains would be informative at several levels. For example, understanding relevant circuits could inform a query relating to psychopathology resulting from brain injury. In anatomical regions with multiple inputs and outputs, querying connected regions could inform clinical expectations and interpretations of comorbidity. Conversely, the bioinformatics approach could drive novel research questions such as, “When circuit A-X-J is activated (or inhibited) does construct Y emerge?” or “When construct Y emerges, is circuit A-X-J activated?” Nuanced questions could be considered when regions with multiple afferent and efferent connections are known. For example, “is the activation of A-X-J similar to A-X-K or B-X-J?”
Fig. 2.
Example informatics diagram based upon anatomical connections with the Hb. Clicking the region of interest (LHb) would expand afferent and efferent portions of the circuit along with associated construct annotations. Hb, habenula; BNST, bed nucleus solitarus tract; LH, lateral hypothalamus; NAcc, nucleus accumbens core; PAG, periaqueductal gray; RMTg, rostral medial tegmentum; SC, superior colliculus; VP, ventral pallidum; VTA, ventral tegmental area.
Refining and elaborating RDoC constructs will require an iterative process involving multiple levels of scientific input. The Hb is an excellent example of a structure in need of elaboration and refinement. Early studies associated the region with a number of functions including sleep, feeding, affect, stress, learning motor activity, and sex.25,26 The recent significant rise in interest was fueled by the finding that Hb activation transiently inhibited midbrain dopamine cell firing at a population level27,28 and that the unexpected omission of reward increased Hb firing rates.29–33 While these findings undoubtedly led to its inclusion within the positive valence domain, Hb activation gives rise to more than a prediction error signal. More recent studies have suggested that the Hb is involved in governing the attribution of incentive salience and in evaluating the cost and benefits associated with different actions.34–37 The Hb has also been implicated in avoidance, aversion, and anhedonia with the notion that overactivation gives rise to depressive-like behaviors.38–41 Theoretically, high levels of tonic activity within the Hb could drive sustained thoughts of “worse-than-expected” outcomes or contribute to avoidance and anhedonia, suggesting the region could play a prominent role in one or more constructs contained within the negative valence domain. For example, rumination and negative bias, both behavioral components of the construct loss, could be driven by this activity. Depressive-like somatic symptoms such as sleep disturbances and changes in psychomotor activity and appetite are likewise implicated in Hb function suggesting a potential role in constructs organized within the arousal and regulatory domain.42–44 The specific discoveries cited above have implications for the Hb’s role in specific constructs but also in the definition and implementation of circuitry as a unit of analysis within the RDoC framework.
Kozak and Cuthbert3 note that as RDoC evolves, a more satisfactory concept of circuit will be needed and that it could be considered as a “functional unit” that would account for behavioral observations. The Hb provides an excellent example of both the need and difficulty in conceptualizing a circuit in light of its unique interconnections. Recent anatomical findings place a newly characterized region, the rostromedial tegmentum (RMTg), as a critical relay in Hb communication to reward-relevant dopaminergic circuitry.45 The RMTg receives major glutamatergic input from the lateral Hb that, in turn, provides dense GABAergic innervation of the SN and VTA.46–50 The functional outcome of Hb activation is excitation of RMTg inhibitory neurons that in turn inhibit midbrain dopamine neurons. While the RMTg is strongly activated by stimuli that also activate Hb neurons, the RMTg introduces its own unique hodology into the Hb-VTA/SN pathway via regions that process fear (eg, periaqueductal gray), threat (e.g. bed nucleus solitarus tract), avoidance, and anxiety.41,45,51–53 Folding the RMTg into existing Hb circuitry adjusts our perspective and prompts different questions related to domains and constructs influenced by this structure and its connections with other brain regions. Figure 2 shows an example informatics snapshot that would enable an investigator to visualize anatomical connections and construct associations. Exploring upstream and downstream brain regions can point to previously unexplored efferents or parallel circuits that have a similar function but are not directly connected to the region of interest. This information could be the catalyst for hypothesis generation.
Central to the success of this approach is the need to consider what level of detail is required to describe a brain region to fully capture its functional role within a particular construct without also adding an overwhelming level of detail.3 For example, the distinct anatomical connections and behavioral phenotypes attributed to the medial and lateral Hb strongly argue that these regions be considered separately within the RDoC framework.54 Recent research suggests the same is true for the VTA, a structure that until recently has been considered relatively homogeneous.55 For example, while a projection from the lateral Hb to the VTA via the RMTg has been shown to mediate avoidance behavior,51,53 Hb efferents directly targeting the VTA drive approach behavior.56 Thus, the same structure, through differences in connectivity, can mediate diametrically opposed behavioral outcomes. Curating these important distinctions within a given brain region is an essential goal and major challenge confronting the RDoC platform.
That a single structure can support diametrically opposed behavioral phenotypes can in some instances be attributed to the firing properties of the constituent neurons. The autogenous electrical properties of Hb and VTA neurons allow them to exhibit spontaneous firing in the absence of synaptic input. Accordingly, their spontaneous “tonic” activity can be modulated bidirectionally. The lateral Hb is particularly well suited for bidirectional modulation, given its unique synaptic architecture that enables a single neuron to exhibit an excitatory or inhibitory response to afferent neurons that carry both glutamate (excitatory) and GABA (inhibitory) neurotransmitters.57 Thus, depending upon the dynamics of the input, opposing constructs could be engaged to produce opposite effects on behavior. As RDoC moves forward, some guiding clarification will be needed as similar regions and circuits are likely to be discovered.
What Next
RDoC is an innovative concept that could function as a catalyst to organize and synergize multidisciplinary research efforts and serve as a resource for clinician scientists and as a didactic tool for graduate and residency training.58 While RDoC seeks a paradigmatic shift in the way clinical research is conducted, it is likely to alter the trajectory of preclinical research as well. In this regard, less focus will be placed on creating and benchmarking animal models using DSM diagnostic criteria. RDoC’s reliance on phenotypes that are objective, quantifiable, and associated with specific brain circuits make it more compatible with the strengths of contemporary animal research with the added benefit of a higher order, clinically based organization.
In order to achieve its potential, RDoC will have to aggressively confront some of its shortfalls and be resourced to advance its evolution as an informatics platform. It will be important for RDoC to maintain a guided, iterative process to shape and refine constructs and units of analysis and create algorithms for adding newly discovered findings. Specific progress could be made in several areas:
RDoC would benefit from a commitment to building a research and information platform that would include a larger breadth of annotation at all levels. It has been stated that RDoC was intended as a strategic proposal rather than a content proposal, yet investigators are encouraged to refine and expand the matrix. In our opinion, RDoC cannot live in between these realities.
While leeway has be granted during its startup to encourage novel approaches, annotations for each unit of analysis should be consistent across domains and constructs—this is especially true for circuits. (For example, the substantia nigra and VTA are collectively listed as “SN/VTA,” “Substantia nigra/VTA,” “Ventral tegmental area/Sustantia Nigra,” and “VTA/SN” in different constructs. In addition, some constructs used generic reference (eg, “reward circuit”) vs regions.)
A new level of circuitry should be added. Currently, most constructs simply list brain regions in the circuit unit of analysis—this is informative at a general level, but a region does not make a circuit, and as discussed above, the growing awareness of subregion connectivity with diverse functional outcome makes this an imperative. At minimum, a 2-component pathway and ideally a 3-piece circuit would significantly enhance information and specificity.
RDoC circuits need to accommodate bivalent systems operating from a tonic baseline.
More emphasis should be given to the development of basic science research within the RDoC initiative. NIMH should provide guidance for successful application and encourage basic science research that would dovetail with the RDoC initiative.
There needs to be a method for curating content that balances the need to incorporate new findings while acknowledging the need for reproducibility. Cloud-sourcing information at various levels (basic vs clinical science and novel vs replicated) may be a means to maintain current state of the art and engender community involvement.
Whether or not RDoC succeeds will ultimately depend on the role assigned to the strategy—research guiding philosophy or engaged dynamic experimental framework—and, of course, the veracity of the assumptions.
Funding
National Institutes of Mental Health: R01MH094489 (Shepard, Elmer) and T32MH067533 (Brown).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this article.
Supplementary Material
References
- 1. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. [DOI] [PubMed] [Google Scholar]
- 2. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989. [DOI] [PubMed] [Google Scholar]
- 3. Kozak MJ, Cuthbert BN. The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology. 2016;53:286–297. [DOI] [PubMed] [Google Scholar]
- 4. Iacono WG. Achieving success with the Research Domain Criteria (RDoC): going beyond the matrix. Psychophysiology. 2016;53:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patrick CJ, Hajcak G. RDoC: translating promise into progress. Psychophysiology. 2016;53:415–424. [DOI] [PubMed] [Google Scholar]
- 6. Weinberger DR, Glick ID, Klein DF. Whither Research Domain Criteria (RDoC)?: the good, the bad, and the ugly. JAMA Psychiatry. 2015;72:1161–1162. [DOI] [PubMed] [Google Scholar]
- 7. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155–179. [DOI] [PubMed] [Google Scholar]
- 8. Lilienfeld SO, Treadway MT. Clashing diagnostic approaches: DSM-ICD versus RDoC. Annu Rev Clin Psychol. 2016;12:435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpenter WT., Jr The RDoC controversy: alternate paradigm or dominant paradigm? Am J Psychiatry. 2016;173:562–563. [DOI] [PubMed] [Google Scholar]
- 10. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. [DOI] [PubMed] [Google Scholar]
- 11. Yee CM, Javitt DC, Miller GA. Replacing DSM categorical analyses with dimensional analyses in psychiatry research: The Research Domain Criteria Initiative. JAMA Psychiatry. 2015;72:1159–1160. [DOI] [PubMed] [Google Scholar]
- 12. NIMH. Research Domain Criteria. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml. Accessed July 5, 2016. [Google Scholar]
- 13. Chen F, Tillberg PW, Boyden ES. Expansion microscopy. Science. 2015;347(6221):543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nat Methods. 2013;10:508–513. [DOI] [PubMed] [Google Scholar]
- 15. Helmstaedter M. Cellular-resolution connectomics: challenges of dense neural circuit reconstruction. Nat Methods. 2013;10:501–507. [DOI] [PubMed] [Google Scholar]
- 16. Kim CK, Yang SJ, Pichamoorthy N, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods. 2016;13:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mikula S, Denk W. High-resolution whole-brain staining for electron microscopic circuit reconstruction. Nat Methods. 2015;12:541–546. [DOI] [PubMed] [Google Scholar]
- 18. Taylor W, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55:201–207. [DOI] [PubMed] [Google Scholar]
- 19. Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. [DOI] [PubMed] [Google Scholar]
- 20. Sporns O. Making sense of brain network data. Nat Methods. 2013;10:491–493. [DOI] [PubMed] [Google Scholar]
- 21. Stephan KE, Friston KJ. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci. 2010;1:446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou IY, Liang Y-X, Chan RW, et al. Brain resting-state functional MRI connectivity: Morphological foundation and plasticity. Neuroimage. 2014;84:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Human Connectome Project. Relationship Viewer: Human Connectome Project http://www.humanconnectomeproject.org/informatics/relationship-viewer/. Accessed July 5, 2016.
- 24. Ingenuity. Ingenuity Pathway Analysis. http://www.ingenuity.com/products/ipa. Accessed July 5, 2016. [Google Scholar]
- 25. Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- 26. Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. [DOI] [PubMed] [Google Scholar]
- 27. Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salas R, Baldwin P, de Biasi M, Montague PR. BOLD responses to negative reward prediction errors in human habenula. Front Hum Neurosci. 2010;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Front Hum Neurosci. 2011;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawson RP, Seymour B, Loh E, et al. The habenula encodes negative motivational value associated with primary punishment in humans. Proc Natl Acad Sci USA. 2014;111:11858–11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bromberg-Martin ES, Matsumoto M, Hikosaka O. Distinct tonic and phasic anticipatory activity in lateral habenula and dopamine neurons. Neuron. 2010;67:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacology, Biochemistry, and Behavior. 2010;96:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hennigan K, D’Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 2015;35:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stopper CM, Floresco SB. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci. 2014;17:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B, Piriz J, Mirrione M, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christensen T, Jensen L, Bouzinova EV, Wiborg O. Molecular profiling of the lateral habenula in a rat model of depression. PLoS One. 2013;8:e80666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dillon DG, Rosso IM, Pechtel P, et al. Peril and pleasure: an RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31:233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aizawa H, Yanagihara S, Kobayashi M, et al. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J Neurosci. 2013;33:8909–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ootsuka Y, Mohammed M. Activation of the habenula complex evokes autonomic physiological responses similar to those associated with emotional stress. Physiol Rep. 2015;3:e12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yetnikoff L, Cheng AY, Lavezzi HN, Parsley KP, Zahm DS. Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: a study in rat. J Comp Neurol. 2015;523:2426–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quina LA, Tempest L, Ng L, et al. Efferent pathways of the mouse lateral habenula. J Comp Neurol. 2015;523:32–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. [DOI] [PubMed] [Google Scholar]
- 50. Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. [DOI] [PubMed] [Google Scholar]
- 51. Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jhou TC, Good CH, Rowley CS, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lammel S, Lim BK, Ran C, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Front Hum Neurosci. 2013;7:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barker DJ, Root DH, Zhang S, Morales M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J Chem Neuroanat. 2016;73:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stamatakis AM, Jennings JH, Ung RL, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Root DH, Mejias-Aponte CA, Zhang S, et al. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci. 2014;17:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Etkin A, Cuthbert B. Beyond the DSM: development of a transdiagnostic psychiatric neuroscience course. Acad Psychiatry.2014;38:145–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.