Independent evidence from rocks, fossils, and genes converge on a cohesive narrative of isthmus formation in the Pliocene.
Keywords: Evolution, ecology, land-bridge, Central America, GABI, Isthmian closure
Abstract
The formation of the Isthmus of Panama stands as one of the greatest natural events of the Cenozoic, driving profound biotic transformations on land and in the oceans. Some recent studies suggest that the Isthmus formed many millions of years earlier than the widely recognized age of approximately 3 million years ago (Ma), a result that if true would revolutionize our understanding of environmental, ecological, and evolutionary change across the Americas. To bring clarity to the question of when the Isthmus of Panama formed, we provide an exhaustive review and reanalysis of geological, paleontological, and molecular records. These independent lines of evidence converge upon a cohesive narrative of gradually emerging land and constricting seaways, with formation of the Isthmus of Panama sensu stricto around 2.8 Ma. The evidence used to support an older isthmus is inconclusive, and we caution against the uncritical acceptance of an isthmus before the Pliocene.
INTRODUCTION
One hundred and fifty years ago, the striking similarity of marine animals on either side of the Isthmus of Panama was sufficient to convince naturalists that a seaway had once flowed between the Pacific Ocean and the Caribbean Sea (fig. S1) (1, 2). Formation of the Isthmus was a pivotal event, driving global oceanic reorganization and major biotic change on land and at sea. Populations of marine organisms divided by the rising land forged separate evolutionary paths in response to new and contrasting environments, and the timing of their divergence is now used to calibrate rates of molecular evolution (3, 4). The land bridge joined North and South America, permitting interchange of previously isolated terrestrial organisms with varying levels of success (5), deeply influencing today’s continental flora and fauna (6–8).
In the 1970s, high-resolution paleoceanographic data available from deep-sea cores began to show that an isthmus, defined by the Oxford English Dictionary as a “narrow portion of land, enclosed on each side by water, and connecting two larger bodies of land,” was in place only relatively recently, around 3 million years ago (Ma) (9, 10). This date has been accepted for over 40 years (11–13) but has recently been contested by interpretations that the Isthmus formed millions of years earlier. Bacon et al. (14) proposed that there may have been an initial land bridge as early as 23 Ma and later that the “Isthmus was formed” between 10 and 6 Ma, whereas Montes et al. (15) concluded that the Central American Seaway had disappeared by 15 to 13 Ma, stating that the Isthmus of Panama had formed at that time. If true, these new interpretations would revolutionize our understanding of the timing and causal relationships among environmental, ecological, and evolutionary change in the region. Some researchers have already accepted the new dates and called for major revisions of our understanding of global paleoceanographic and climate change, an alternative explanation for the Great American Biotic Interchange, and a compilation of new rates of molecular evolution (16–22).
However, there remains considerable conflicting evidence as well as confusion within the geological and biological communities about the proposal for an “old” isthmus. To bring clarity to the topic, we combine an exhaustive review with new analyses of geological, oceanographic, molecular, and paleontological records.
GEOLOGICAL RECORDS
Formation of the Isthmus of Panama involved subduction of the Pacific-Farallon Plate beneath the Caribbean and South American plates, ultimately driving the development of a volcanic arc on the trailing edge of the Caribbean Plate. This initial Panama Arc began to form approximately 73 Ma (23) as the Caribbean Plate moved eastward, arriving at its current position by ~50 Ma. The North and South American plates continued to move westward past the Caribbean Plate after this time. In addition to their east-west (strike-slip) motion, the South American and Caribbean plates also acquired a north-south component of convergence, leading to the collision of the Panama Arc with South America. This collision drove uplift in both the Northern Andes and the Panama Block, forming the North Panama Deformed Belt and ultimately the Isthmus of Panama (24).
The Panama Arc is mostly composed of subduction-derived granitoids and associated volcanic rocks. Some early arc basement massifs appear to have been emergent since the Eocene as characterized by cooling below 200°C at that time, with continued exhumation and cooling episodes at 25 to 20 Ma, and at 12 to 6 Ma to less than 40°C (24–26). Between 25 and 23 Ma, the type of volcanic arc activity underwent a distinct change from hydrous mantle wedge–derived magmatism to localized extensional magmatism, indicating that the arc had impinged on South America (24, 27). Subsequently, the Panama Arc underwent uplift at relatively moderate but constant rates (Fig. 1). As expected, uplift rates were generally higher in the Darien Basin (table S1), closest to the initial collision with the South American Plate. By 15 Ma, continued collision led to a transition from generally deepwater biogenic to siliciclastic sedimentation (28). Between 9 and 6 Ma, rates of paleobathymetric change in sedimentary sequences reveal significant deepening across the Panama Arc (Fig. 1) (28). Eustatic sea-level rise could in part be the cause of the deepening during the 9 to 8 Ma interval (Fig. 1), but later deepening cannot be explained by sea-level change and must therefore have been due to subsidence. The deepening event is most pronounced in the Bocas del Toro and Canal basins of Panama (table S1), suggesting that the cause may have been related to local tectonic extension (24), which would explain why adjacent crystalline massifs continued to be exhumed at the same time (25, 26). At around 6 Ma, the Panama Arc began rising again (Fig. 1) and has continued to do so until the present day. Arc uplift combined with sea-level falls driven by the expansion of the Greenland ice sheet around 3 Ma (29) and the establishment of repeated Pleistocene glaciations beginning at 2.6 Ma (Fig. 1) resulted in the land bridge connecting North and South America.
Fig. 1. Uplift of the Isthmus of Panama and global sea levels over the last 20 My.
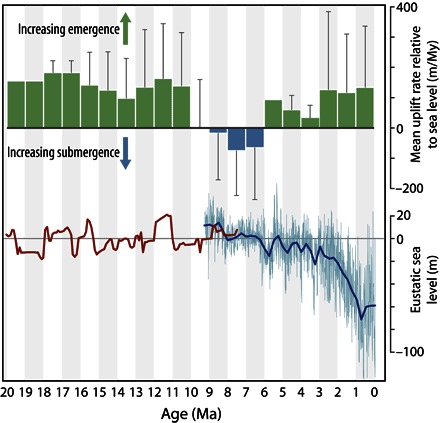
Rates of uplift are estimated from changes in the age and depth of deposition of sedimentary units across the Panama Arc (table S1) and are therefore relative to sea level. Eustatic sea-level estimates (light blue and dark red lines) from the study of Miller et al. (131). The dark blue line indicates values averaged within time bins of 250 thousand years (ky) for the 0 to 9 Ma record.
Montes et al. (15) argue that Eocene zircons found in middle Miocene river-borne sediments of Colombia’s lower Magdalena Basin have a U-Pb age fingerprint that is uniquely “Panamanian” and must therefore have arrived, by river, from the Panama Arc. Thus, they conclude that the Central American Seaway must have “disappeared” by the middle Miocene. However, several other lithologic units could have been the source of the zircons found by Montes et al. (15) in the lower Magdalena Basin (Fig. 2). More than 30 Eocene localities, some containing zircons with U-Pb ages in the exact same age range as those used by Montes et al. (15), have been identified in the Norandean region of South America (table S2). The two most likely alternative source regions are (i) a Paleocene-Eocene volcanic arc in the Central Cordillera (30) and (ii) the Sierra Nevada de Santa Marta (table S2 and Fig. 2). The true extent of Eocene zircons in the region therefore categorically negates the assertions of Montes et al. (15) that “there are no igneous bodies of that age in the northern Andes” or that the “Panama Arc and old Andean terrains are mutually exclusive geochronological domains.” It is therefore unnecessary to invoke a land connection to explain the existence of Eocene zircons in Miocene sediments in the lower Magdalena Basin.
Fig. 2. The Isthmus of Panama and northwestern Colombia with locations of some crystalline rocks (igneous and metamorphic) with reported Paleocene-Eocene radiometric ages (squares and triangles).
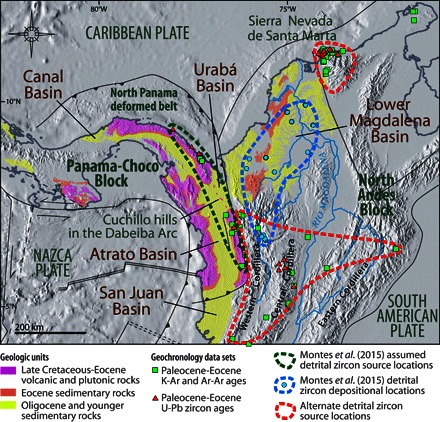
Montes et al. (15) proposed that Eocene detrital zircons in middle Miocene sediments of the Magdalena Basin (blue dots) could only be from plutonic rocks of the Panama Arc (dark green outline). However, several other rock units (for example, Western, Central, and Eastern Cordilleras plus the Sierra Nevada de Santa Marta; red outlines and table S2) exhibit radiometric ages in the same interval and therefore must also be considered as potential sources. From previous studies (24–26, 30, 31, 132).
In addition, the assumption by Montes et al. (15) that a well-established Miocene fluvial connection existed between Panama and South America disregards the sedimentary record in the Urabá–Atrato–San Juan Basin that topographically separates Panama and South America (Fig. 2). These basins contain ~2500 m of marine sediments, transitioning from deep water in the Eocene and Oligocene to shallow water in the Miocene and Pliocene, and which were overlain after 3.7 to 3.1 Ma by terrestrial sediments (31–34). Detailed surveys of the region using surface mapping, radar-derived topographic data, exploratory well logs, and seismic cross sections, as well as gravimetric and magnetic surveys, clearly show that these sediments in the Atrato Basin extend into the Urabá Basin, entirely unaffected by the Cuchillo Hills (34). The Cuchillo Hills were therefore always islands in the Atrato sea, and marine connections penetrated the Dabeiba Arc forming a marine seaway until the Pliocene (Fig. 2 and fig. S2), refuting the assumption of a complete barrier by Montes et al. (15). A 150-m rise in relative sea level would be sufficient to flood the Urabá–Atrato–San Juan Basin and the Canal Basin (fig. S2). Such a rise is easily accounted for by arc uplift and eustatic sea-level changes over the last 3 million years (My) (Fig. 1).
MARINE PALEONTOLOGICAL RECORDS
Formation of the Isthmus of Panama is recorded by changes in the chemistry, composition, and structure of sediments and fossils in deep sea and coastal rock records across the Caribbean and eastern Pacific. In the deep ocean, divergences in neodymium isotopes (Fig. 3E) (35) and benthic foraminifera δ13C (36, 37) between Caribbean and Pacific sites, as well as an abrupt increase in the Pacific carbonate compensation depth, provide strong evidence that deepwater connection was shut off between 12 and 9.2 Ma. At this time, the deepest part of the sill of the Panama Arc must have shoaled to less than 1800 m and was perhaps as shallow as 1200 m (38).
Fig. 3. Timing of divergence between Caribbean (blue) and Tropical Eastern Pacific (red) environments and ecologies in coastal, shallow, and deep waters.
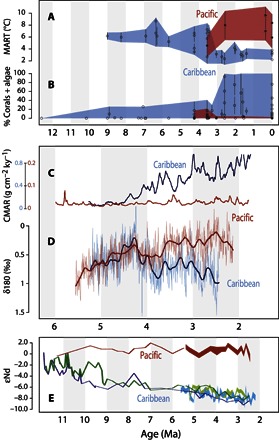
(A and B) Estimated mean annual range of temperature [MART; a proxy for strength of upwelling estimated by measuring zooid size variation in fossil bryozoan colonies (133)] (A) and the relative skeletal weight of corals and coralline algae (68, 134) (B) in replicated bulk samples from coastal shelf sediments on the Isthmus of Panama. (C and D) Rates of accumulation of carbonate (CMAR) in deep-sea sediments (C) and estimated surface water salinity (reflected in the oxygen isotope record) (D) in Caribbean and Pacific surface waters. (E) Neodymium (Nd) values from fish teeth and foraminifers in Pacific and Caribbean basins (35, 38).
Ocean circulation models suggest that constriction of interoceanic straits should have caused the Atlantic to become saltier because the trade winds transported moisture into the Pacific (39). In turn, the increase in North Atlantic salinity should have driven increased thermohaline overturning in the North Atlantic and introduced low-CO2 and low-nutrient waters into the deep Atlantic by overturning nutrient-poor surface waters (39–42). As predicted by these models, western Atlantic surface water salinity began to diverge from eastern Pacific values about 4.6 Ma and reached modern Caribbean values by about 4.2 Ma (Fig. 3D) (36, 42). Deep Caribbean δ13C increased toward modern values at the same time, in response to flooding of the Caribbean by nutrient-poor North Atlantic Deep Water (36, 37, 43). Finally, Caribbean carbonate preservation improved at 4.6 Ma, likely reflecting the replacement of corrosive Antarctic Intermediate Water by North Atlantic Deep Water as overturning increased in the northern North Atlantic (Fig. 3C) (43). The development of a large salinity contrast between the Caribbean and eastern Pacific (Fig. 3D) is a particularly strong indication that the Panama Arc was mostly emergent by 4.2 Ma (40, 44).
Nonetheless, vigorous exchange of near-surface waters between the oceans continued as demonstrated by similarities in the radiolarian (45), foraminiferal (9, 10, 46), and nannoplankton coccolithophore (47) communities of the Caribbean and Tropical eastern Pacific. Radiolarian assemblages in the Gulf of Panama and the presence of the foraminifer Neogloboquadrina pachyderma (sinistral coiling) in the Western Caribbean are distinct indicators of increased upwelling starting about 4.2 Ma that have been interpreted as shoaling of the Caribbean-Pacific sill (45, 48). The temperature gradient in the eastern Pacific thermocline also steepened between 4.2 and 3.8 Ma, consistent with increased coastal upwelling (41, 49). The Indo-Pacific planktonic foraminifer, Pulleniatina, and a suite of menardellids and other foraminifer species disappeared from the Caribbean between 3.5 and 3.0 Ma in a pattern indicative of the shoaling of the Panama Arc (9, 10, 46). Coccolithophore communities were similar between eastern Pacific sites and the southern Caribbean up until between 3.65 and 2.76 Ma and then diverged as the Panama Arc cut off surface water flow between the oceans (47). The redevelopment of similar salinity and sea surface temperatures between the Tropical Eastern Pacific and Caribbean suggests temporary breaching of the Isthmus as late as 2.45 Ma (44).
In fossil sediments in the area around the Panama Canal, coral reefs, mangroves, and deltaic sediments (50–52) demonstrate that parts of the Panama Arc were emergent since at least 30 Ma. Until around 4 Ma, there was little taxonomic or ecological difference in shelf benthic and nektonic communities between the Tropical Eastern Pacific and the Caribbean (53–56), demonstrating easy movement of water carrying larvae or adults between the oceans (57). Around 4 Ma, Caribbean-wide decline in nutrients drove a profound change in the structure of Caribbean coastal communities and environments (Fig. 3, A and B) (58–62) and the life histories of the animals that inhabited them (63–65). Oligotrophic conditions allowed reef-building corals and their associates to proliferate (Fig. 3B) (59, 66, 67). A regional extinction across the Caribbean between 4 and 2 Ma (54) was highly selective against animals suited to high planktonic productivity (60, 61, 63, 68), implicating declining nutrients due to the restriction of Pacific waters entering the Caribbean, most likely caused by the emergence of the Panama Arc and formation of the Isthmus.
MARINE MOLECULAR RECORDS
Barring some exceptional cases of migration around the southern tip of Africa (69) and transits of organisms through the Panama Canal (70), molecular divergence between eastern Pacific and western Atlantic shallow-water marine organisms provides evidence of when the last interoceanic connections were severed. To estimate dates from molecular divergence, it is necessary to (i) determine the phylogeny of a group, to identify sister species in either ocean, and (ii) estimate the rate at which their molecules have evolved. Most calibrated phylogenies of marine organisms have assumed that the Isthmus closed at 4 to 3 Ma (3), but it would be circular to use these estimated rates of molecular evolution to date Isthmus formation. In an attempt to remove this circularity, Bacon et al. (14) assumed a “universal” rate of mitochondrial DNA (mtDNA) divergence of 2% per million years. However, this is inappropriate, because rates of molecular evolution vary substantially between clades (71–74); two approaches are taken here.
In our first approach, we estimated times of separation between Atlantic and Pacific sister species from molecular phylogenies that have been calibrated by fossils at one or more nodes and are therefore independent of emergence of the Isthmus. As we were interested in the time of the most recent common ancestor of species, rather than of particular genes (which coalesce farther back in time than populations), we further restricted our choice of phylogenies to those based on a combination of mtDNA and nuclear DNA regions (see text S1 for details). Of the 38 comparisons based on fossil-calibrated phylogenies, 26 (68%) produced estimates of separation that occurred more recently than 12 Ma (Fig. 4), demonstrating that seawater passages of sufficient width and flow to convey larvae or adults between the Pacific and Caribbean continued after this date. Other comparisons indicate older splits, which may have occurred either because of gradual isthmus uplift or resulted from extinction in one ocean of one daughter species of a common ancestor originally spread in both oceans, creating the false impression that the extant remnants were actually sister species (3, 75). The four most recent divergences in our analysis are the cone snail Conus at ~4.1 Ma [95% highest posterior density (HPD), 5.80 to 2.90 Ma], the grouper Mycteroperca at 3.58 Ma (95% HPD, 5.51 to 1.90 Ma), the butterflyfish Chaetodon at ~3.4 Ma (95% HPD, 5.40 to 1.80 Ma), and the sand dollar Mellita at ~3.21 Ma (95% HPD, 3.91 to 2.51 Ma) (table S3). The interoceanic mixing necessary to produce these dates could not have occurred if the Isthmus of Panama had existed (76).
Fig. 4. Bayesian estimates of median molecular divergence times of sister taxa on either side of the Isthmus of Panama.
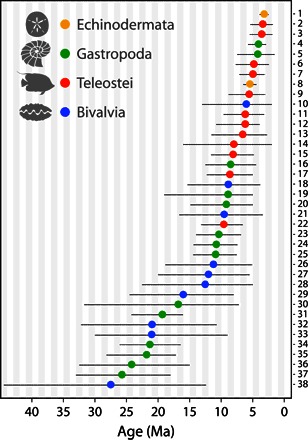
Only species for which both nuclear DNA and mtDNA data were available and for which divergence ages were calibrated by the fossil record are included. Error bars represent 95% HPDs. Numbers refer to species pairs in table S3.
For our second approach, we tabulated divergence between members of transisthmian pairs that have been shown to be sister species by molecular phylogenies, but which lack fossil calibrations, to ask whether such divergence values are consistent with an old or a recent time of separation, given the rate of mtDNA evolution estimated from our fossil-calibrated phylogenies. Of the 95 divergences without fossil calibrations (table S4), 60 show mean Kimura two-parameter distances over all sampled genes of <10% (median divergence, 4.65%). If gene flow between these most recently separated sister species pairs is assumed to have been interrupted 2.8 Ma, this value translates to a median divergence rate of 1.66% per million years over all genes and taxa. If, on the other hand, one assumes that the Panama Arc permanently blocked all genetic exchange from 23 to 13 Ma (15), the estimated median divergence rates would be 0.20 to 0.43% per million years. The median divergence rate of mtDNA evolution in transisthmian pairs with fossil-calibrated phylogenies is 1.06% per million years (table S4); thus, the rates that assume an old separation of the oceans are inconsistent with the dates derived from the fossils. It is very unlikely that evolution of mtDNA was accelerated exclusively in species that happen to have a fossil record.
Therefore, these molecular comparisons demonstrate interoceanic gene flow until approximately 3 Ma. In contrast, Bacon et al. (14) used a meta-analysis of molecular data to measure rate shifts in migration, observing concentrations of divergence “events separating marine organisms in the Atlantic and Pacific oceans at ca. 23 and 7 Ma,” then assuming that these provide evidence for early (but transient) formation of the Isthmus of Panama at these dates. Bacon et al.’s argument for the significance of the 23 Ma cluster is that there was a corresponding concentration of terrestrial divergences at ~20 Ma, implying a common geological cause. However, in addition to using an inappropriate universal rate of mtDNA divergence, a substantial number of published data sets were omitted (77), and data from marine organisms were limited to only COI with the argument that data from other genes are not as reliable (78), although most of their analyses of terrestrial organisms were based on other genes. When all of the missing marine molecular data were included, the proposed ~23 Ma marine event disappeared (Fig. 1) (77).
INTERCONTINENTAL DISPERSALS OF TERRESTRIAL ANIMALS
The earliest appearances of migrating terrestrial animals in fossiliferous deposits demonstrate the timings of successful intercontinental dispersals and help illuminate when the Isthmus of Panama formed (Fig. 5). We limit our review only to fossils with confident taxonomic placement that come from well-dated geological units. Except for a South American salt water–tolerant crocodile (79) and a monkey (80), early and middle Miocene fossils uncovered in the Panama Canal region and the Gracias Formation in Honduras have so far been almost entirely North American in their affinities (51, 81, 82). Likewise, the middle Miocene La Venta Formation in Colombia (83), the late Miocene Urumaco Formation in Venezuela (84), and the Solimões Formation in Brazil (85) contain only South American taxa. Thus, the two continents apparently exchanged few nonflying vertebrates for over 40 My.
Fig. 5. Frequency of appearances of immigrant vertebrate taxa or their oldest known descendants in opposing continents as observed in well-dated fossiliferous sediments in South and North America per million years.
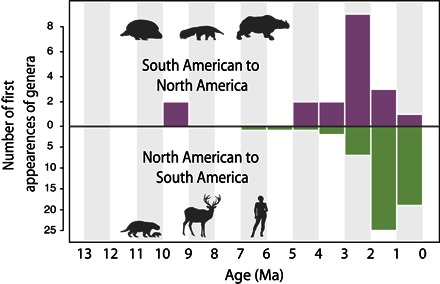
See fig. S3 for further details.
Around 9 to 8.5 Ma, the sloths Thinobadistes and Pliometanastes, derived from South American ancestors, appeared in North America (86, 87). Their success in the north, along with the arrival of the first South American edentate genera Glossotherium and Plaina in Mexico around 4.8 to 4.7 Ma (88, 89), is suggestive of their ecological, and therefore competitive, distinction from already established herbivorous mammals in North America (90, 91). The first record of North American immigrants in South America is the omnivorous endemic procyonid carnivore Cyonasua at ~7 Ma (92), followed by the endemic sigmodontine rodent Auliscomys at ~5 Ma (93), both of which are likely to represent much earlier migrations that have yet to be revealed in the fossil record. The South American flightless terror bird Titanis occurred in Texas between about 5.0 and 4.7 Ma (94). A close relative of the capybara, Neochoerus, is first recorded present in Mexico around 3.8 Ma, and Glyptotherium is also recorded in Mexico around the same time (fig. S3) (88, 89).
Some researchers have argued that dispersals of animals and plants between North and South America before 3 Ma indicate an early completion of a land bridge (14, 95). Bacon et al. (96), for example, estimated that Copernicia and Pritchardia palms dispersed between North and South America from 31 to 9 Ma, and thus concluded that the Isthmus of Panama had emerged in the Oligocene or Miocene. However, many kinds of plants and animals are known to have dispersed over ocean barriers with surprising frequency, often crossing gaps much wider than the widest plausible strait separating North and South America in the Miocene (97–99). Primates and rodents crossed the Atlantic in the Eocene (100). All the post-Eocene mammal lineages of Madagascar, including hippopotamuses, arrived from Africa (101, 102). Most of the (endemic) modern fauna of the West Indies is derived from overwater colonists (103). Salt water–intolerant amphibians crossed sea barriers numerous times (104, 105).
Rafting is the most likely method of dispersal of terrestrial organisms over water. Natural rafts of soil and vegetation that form when floods wash away parts of river banks are frequently observed far out at sea (106, 107), especially in areas receiving tropical storms, and these rafts might harbor enough food and fresh water to maintain animals for a journey of weeks or months. Rafts frequently reach very large sizes (video S1), and prevailing winds, currents, or storms can carry them relatively rapidly over long distances (101, 108). The Atlantic crossings by monkeys and rodents, which almost certainly occurred by rafting, indicate the great potential of this mechanism for long-distance dispersal.
Many of the mammals that traveled between North and South America before 3 Ma (for example, procyonids, sigmodontine, and caviomorph rodents; platyrrhine monkeys; and sloths) are members of groups that have reached islands by oceanic dispersal (101, 108–111). Hence, the early dispersal of these particular animals and not others actually argues in favor of separation of the continents. Considering how near the Panama Arc was to South America for 20 My, it is surprising that there were so few successful dispersals of terrestrial animals, especially in the light of known oceanic dispersals over much greater distances around the world. Strong ocean currents may have created a formidable barrier similar to the Indonesian throughflow (112), which accounts for the Wallace and Lydekker lines (113, 114); this interpretation is supported by paleoceanographic models of large-scale interoceanic water exchange between the Pacific and Caribbean as the Isthmus of Panama shoaled (29, 40, 115). Alternatively, dispersals may have been frequent but mostly unsuccessful because of unsuitable ecological conditions and/or stiff competition from resident incumbent faunas (13, 116–118).
The Great American Biotic Interchange (119) is characterized by a surge in successful dispersals in both directions beginning around 2.6 Ma, traditionally defined as beginning with the arrival of the South American porcupine Erethizon in North America (116), and various members of the North American families Mustelidae, Canidae, and possibly Gomphotheriidae, along with the extinct horse Hippidion, successfully colonizing South America at the same time (fig. S3) (8). This wave of successful dispersals by many large mammals is widely considered convincing evidence that animals could, at this time, have walked dry-shod across a fully formed land bridge.
In stark contrast to the available fossil record of successful dispersals, Bacon et al. (14), using molecular divergences of terrestrial plants and animals, concluded that the Isthmus of Panama formed in fits and starts ~23 to 19 Ma and then again ~8 to 5 Ma. The former date coincides roughly with initial contact of the Panama Arc with South America (24), which would likely have increased the chance of successful overwater dispersals. However, the proposed peak in divergences ~8 to 5 Ma coincides with no known geological or environmental driver; on the contrary, interoceanic seaways were growing in size at this time (Fig. 1). The disagreement could potentially result from a dearth of tropical terrestrial fossil records (120) but more likely is caused by the inappropriate use of molecular divergences between terrestrial organisms as a tool to date a land bridge. As Daza et al. (121) noted, taxa begin to diverge when they are separated; they do not diverge when they spread from one place to the other, unless that event is immediately followed by isolation. Thus, unless isthmus formation were followed by reestablishment of seaways, the early divergences discussed by Bacon et al. (14, 96) are evidence for dispersal over water, which would provide a mechanism of isolation after colonization, rather than over land.
This effect also applies to dating based on the age of crown groups now endemic to separate continents, because their stems must have been isolated to diverge. Deep divergences between such biotas, if not caused by pre-isthmus dispersal over water, could reflect the formation of two clades within one of the continents, followed by crossing by one or both clades to the other continent at any time after a land bridge formed. Subsequent extinction of one clade in one of the two places would result in two sister clades, one in North America and one in South America, the separation of which would far pre-date the time of colonization. Thus, unlike fossils, which provide definitive evidence of the minimum time of arrival, the use of divergences between North and South American terrestrial clades is an unreliable tool for dating the formation of the Isthmus of Panama.
SUMMARY
Establishing how and when the Isthmus of Panama formed is crucial for understanding the greatest “natural experiment” ever (122, 123). Our reviews and new analyses show that, before isthmus formation, the Panama Arc existed as a semi-emergent island chain through which abundant seawater flowed from the Pacific into the Caribbean since at least 30 Ma. The arc collided, initially underwater, with South America around 24 Ma and has continued to do so to this day (24). Meanwhile, the largest of the interoceanic straits were more than 1200 m deep, permitting massive interoceanic seawater exchange until these deepwater passages were extinguished by 9.2 Ma (35, 38), after more than 15 My of collision and uplift. Proximity of emergent land on the Panama Arc to South America increased the probability that terrestrial animals and plants could disperse between the continents across the seaways. Land mammal dispersals began ~20 Ma and trickled on for a further 17 My, most likely via rafting. Shallow seawater continued to be exchanged between the oceans until ~4 Ma whereupon the number of diverging marine species peaked (Fig. 4), and the Caribbean underwent a profound environmental, ecological, and evolutionary transformation (Fig. 3) resulting from significant constriction of the interoceanic seaways. Despite the near completion of a land bridge, ample interoceanic gene flow continued until at least ~3.2 Ma, suggesting that strong currents passed through the straits into the Caribbean, as suggested by models of the shoaling Panama Arc (29, 40, 115). Strong interoceanic currents could also explain why surprisingly few terrestrial animals successfully dispersed across the straits in the Miocene and Pliocene (Fig. 5) [compare with the study of Wallace (113)]. Alternatively, host continents may have been competitively or climatically unsuitable to migrants (80, 116, 117).
Formation of the isthmus sensu stricto is pinpointed by (i) the absence of further gene flow between shallow marine animal populations after ~3.2 Ma (Fig. 4), (ii) the end of surface water exchange between oceans at 2.76 Ma based on marine plankton assemblages and surface ocean salinity contrasts (Fig. 3), and (iii) an observed acceleration in the rate of dispersal of terrestrial mammals between continents sometime just before 2.7 Ma (Fig. 5 and fig. S2). These events coincide with glaciations, first of Greenland, followed by the Eurasian Arctic, Northeast Asia, and Alaska (124, 125), resulting in significant drops in sea level (Fig. 1). Uplift of the Panama Arc and falling sea level (Fig. 1) therefore worked in concert to form the Isthmus of Panama sensu stricto at this time (44). Interglacial sea-level rises may have breached the Isthmus temporarily (67), potentially permitting gene flow between the oceans. Ephemeral similarities in surface ocean salinity between the Caribbean and eastern Pacific suggest that short-lived breaching may have occurred as late as 2.45 Ma (44).
Our reviews reveal that evidence used to support an older isthmus is inconclusive. For example, zircons claimed to be uniquely Panamanian (15) could have arrived from several alternate (and less distant) sources in northwest South America. Equally, molecular divergences of terrestrial animals used to date the Isthmus (14) are a poor proxy for a land bridge, and marine molecular divergences that use universal rates (14) can be flawed. The campaign for a pre-Pliocene isthmus puzzlingly admits that seawater channels may have persisted into the Pliocene (14, 15, 78, 126), illustrating how the definition of an isthmus is far from a pedantic issue (12). In the Indonesian-Malay archipelago, for example, shallow and narrow seaways permit considerable oceanic throughflow (127) that maintains chemical balance between the Pacific and Indian oceans, controls monsoonal climate of the entire Indian Ocean region (112, 128–130), maintains liberal gene flow among a great many Indo-Pacific species, and limits intercontinental migration of terrestrial vertebrates between Asia and Australia (113). Given all the available evidence, we strongly caution against the uncritical acceptance of the old Isthmus hypothesis (16–18, 22).
Supplementary Material
Acknowledgments
We thank A. Dornburg, A. Herre, C. McMillan, D. Piperno, D. V. Vliet, E. Tonni, E. Bermingham, E. Dillon, F. Rodriguez, F. Santini, H. Lotze, I. Rubinoff, J. Hodge, J. Measey, K. O’Dea, K. Effio, M. Miller, M. Pierotti, N. Parra, O. McMillan, S. Tierney, S. Williams, and W. Schwarzhans for valuable discussion, data, and assistance. Funding: This study was supported by the Smithsonian Tropical Research Institute to A.O., J.B.C.J., N.K., and H.A.L.; the NSF (EAR 1325683) to A.O., P.G.R.-D., and E.L.G.; the National System of Investigators to A.O.; the Secretaría Nacional de Ciencia, Tecnología e Innovación (Panamá) to A.O., H.A.L., and S.E.C.; the U.S. Geological Survey to R.F.S.; and the Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina) to A.L.C., G.M.G., E.S., and L.S. Author contributions: A.O., A.G.C., R.I.E., J.B.C.J., N.K., H.A.L., R.D.N., and S.A.R.-M. designed the research. A.O., O.A., M.-P.A., W.A.B., A.F.B., A.L.C., A.G.C., L.S.C., S.E.C., M.A.C., A.d.Q., H.D.-C., R.I.E., D.W.F., S.F., G.M.G., E.L.G., J.B.C.J., K.G.J., L.D.K., N.K., E.G.L., J.S.L.-P., H.A.L., P.B.M., R.D.N., N.D.P., P.G.R.-D., S.A.R.-M., E.S., L.S., R.F.S., J.A.T., G.J.V., and M.O.W. performed the research. A.O., L.S.C., S.E.C., R.I.E., H.A.L., P.B.M., and S.A.R.-M. contributed data. A.O., L.S.C., R.I.E., S.F., and H.A.L. analyzed data. A.O., D.W.F., P.G.R.-D., S.A.R.-M., and R.F.S drafted figures. A.O., A.L.C., A.G.C., A.d.Q., R.I.E., D.W.F., H.A.L., R.D.N., S.A.R.-M., and M.O.W. wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/8/e1600883/DC1
fig. S1. The Isthmus of Panama in Tropical America.
fig. S2. The current day Isthmus of Panama submerged under 150 m of relative sea-level rise.
fig. S3. Timing of successful terrestrial dispersals between the American continents as observed in the occurrence of fossil remains in the rock record of North and South America.
video S1. Large rafts form on the Río Chagres, Gamboa, Republic of Panama, on 8 December 2010.
table S1. Estimates of rates of uplift for the Panama Arc relative to sea level, using changes in estimated median depths and median ages of sedimentary units from previous studies (28, 135–149).
table S2. Compilation of Late Paleocene–Late Eocene ages for the Colombian Andes plotted in Fig. 2.
table S3. Median and 95% HPD intervals of the time at which members of clades spanning the Isthmus of Panama were separated from each other as estimated by BEAST [1] from phylogenies calibrated by fossils at one or more nodes.
table S4. Kimura two-parameter distance between mitochondrial genes of sister clades on either side of the Isthmus of Panama.
text S1. Estimation of date of splitting from molecular divergence.
REFERENCES AND NOTES
- 1.Günther A., On the fishes of the states of Central America, founded upon specimens collected in fresh and marine waters of various parts of that country by Messrs. Salvin and Godman and Capt. J. M. Dow. Proc. Zool. Soc. Lond. 1866, 600–604 (1867). [Google Scholar]
- 2.C. Darwin, On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life (John Murray, London, ed. 5, 1869), p. 596. [PMC free article] [PubMed] [Google Scholar]
- 3.Lessios H. A., The great American schism: Divergence of marine organisms after the rise of the Central American Isthmus. Annu. Rev. Ecol. Evol. Syst. 39, 63–91 (2008). [Google Scholar]
- 4.Knowlton N., Weigt L. A., New dates and new rates for divergence across the Isthmus of Panama. Proc. Biol. Sci. 265, 2257 (1998). [Google Scholar]
- 5.G. G. Simpson, Splendid Isolation: The Curious History of South American Mammals (Yale Univ. Press, New Haven, 1980), pp. 266. [Google Scholar]
- 6.Leigh E. G., O’Dea A., Vermeij G. J., Historical biogeography of the Isthmus of Panama. Biol. Rev. Camb. Philos. Soc. 89, 148–172 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Marshall L. G., Webb S. D., Sepkoski J. J. Jr, Raup D. M., Mammalian evolution and the Great American Interchange. Science 215, 1351–1357 (1982). [DOI] [PubMed] [Google Scholar]
- 8.A. L. Cione, G. M. Gasparini, E. Soibelzon, L. H. Soibelzon, E. P. Tonni, The Great American Biotic Interchange: A South American Perspective (Springer Verlag, London, 2015), p. 97. [Google Scholar]
- 9.Keigwin L. D., Jr, Pliocene closing of the Isthmus of Panama, based on biostratigraphic evidence from nearby Pacific Ocean and Caribbean Sea cores. Geology 6, 630–634 (1978). [Google Scholar]
- 10.Saito T., Geologic significance of coiling direction in the planktonic foraminifera Pulleniatina. Geology 4, 305–309 (1976). [Google Scholar]
- 11.Jackson J. B. C., O’Dea A., Timing of the oceanographic and biological isolation of the Caribbean Sea from the Tropical Eastern Pacific Ocean. Bull. Mar. Sci. 89, 779–800 (2013). [Google Scholar]
- 12.Coates A. G., Stallard R. F., How old is the Isthmus of Panama? Bull. Mar. Sci. 89, 801–813 (2013). [Google Scholar]
- 13.Haffer J., Geologic-climatic history and zoogeographic significance of the Uraba region in northwestern Colombia. Caldasia 10, 603–636 (1970). [Google Scholar]
- 14.Bacon C. D., Silvestro D., Jaramillo C., Smith B. T., Chakrabarty P., Antonelli A., Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc. Natl. Acad. Sci. U.S.A. 112, 6110–6115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montes C., Cardona A., Jaramillo C., Pardo A., Silva J. C., Valencia V., Ayala C., Pérez-Angel L. C., Rodriguez-Parra L. A., Ramirez V., Niño H., Middle Miocene closure of the Central American Seaway. Science 348, 226–229 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Willis C. G., Davis C. C., Rethinking migration. Science 348, 766 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Hoorn C., Flantua S., An early start for the Panama land bridge. Science 348, 186–187 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Erkens R. H. J., The less-splendid isolation of the South American continent. Front. Biogeogr. 7, 89–90 (2015). [Google Scholar]
- 19.Silva-Arias G. A., González F., Tinjacá S., Chacón Sánchez M. I., Temperature niche conservatism and strong genetic structure are involved in the trans-Panamanian colonization of Matudaea (Hamamelidaceae) to Andean forests. Biochem. Syst. Ecol. 63, 98–108 (2015). [Google Scholar]
- 20.Jønsson K. A., Holt B. G., Islands contribute disproportionately high amounts of evolutionary diversity in passerine birds. Nat. Commun. 6, 8538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho S. Y. W., Tong K. J., Foster C. S. P., Ritchie A. M., Lo N., Crisp M. D., Biogeographic calibrations for the molecular clock. Biol. Lett. 11, 20150194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacon C. D., Molnar P., Antonelli A., Crawford A. J., Montes C., Vallejo-Pareja M. C., Quaternary glaciation and the Great American Biotic Interchange. Geology 44, 357–378 (2016). [Google Scholar]
- 23.Buchs D. M., Arculus R. J., Baumgartner P. O., Baumgartner-Mora C., Ulianov A., Late Cretaceous arc development on the SW margin of the Caribbean Plate: Insights from the Golfito, Costa Rica, and Azuero, Panama, complexes. Geochem. Geophys. Geosyst. 11, Q07S24 (2010). [Google Scholar]
- 24.Farris D. W., Jaramillo C., Bayona G., Restrepo-Moreno S. A., Montes C., Cardona A., Mora A., Speakman R. J., Glascock M. D., Valencia V., Fracturing of the Panamanian Isthmus during initial collision with South America. Geology 39, 1007–1010 (2011). [Google Scholar]
- 25.Montes C., Bayona G., Cardona A., Buchs D. M., Silva C. A., Morón S., Hoyos N., Ramírez D. A., Jaramillo C. A., Valencia V., Arc-continent collision and orocline formation: Closing of the Central American seaway. J. Geophys. Res. Solid Earth 117, B04105 (2012). [Google Scholar]
- 26.Montes C., Cardona A., McFadden R., Moron S. E., Silva C. A., Restrepo-Moreno S., Ramirez D. A., Hoyos N., Wilson J., Farris D., Bayona G. A., Jaramillo C. A., Valencia V., Bryan J., Flores J. A., Evidence for middle Eocene and younger land emergence in central Panama: Implications for Isthmus closure. Geol. Soc. Am. Bull. 124, 780–799 (2012). [Google Scholar]
- 27.Restrepo-Moreno S. A., Foster D. A., Stockli D. F., Parra-Sánchez L. N., Long-term erosion and exhumation of the “Altiplano Antioqueño”, Northern Andes (Colombia) from apatite (U–Th)/He thermochronology. Earth Planet. Sci. Lett. 278, 1–12 (2009). [Google Scholar]
- 28.Coates A. G., Collins L. S., Aubry M. P., Berggren W. A., The geology of the Darien, Panama, and the late Miocene-Pliocene collision of the Panama arc with northwestern South America. Geol. Soc. Am. Bull. 116, 1327–1344 (2004). [Google Scholar]
- 29.Bartoli G., Sarnthein M., Weinelt M., Erlenkeuser H., Garbe-Schönberg D., Lea D. W., Final closure of Panama and the onset of northern hemisphere glaciation. Earth Planet. Sci. Lett. 237, 33–44 (2005). [Google Scholar]
- 30.Bayona G., Cardona A., Jaramillo C., Mora A., Montes C., Valencia V., Ayala C., Montenegro O., Ibañez-Mejia M., Early Paleogene magmatism in the northern Andes: Insights on the effects of Oceanic Plateau–continent convergence. Earth Planet. Sci. Lett. 331–332, 97–111 (2012). [Google Scholar]
- 31.J. G. Tapias, A. N. Guevara, N. E. M. Ramírez, D. J. Mejía, M. T. Avella, J. S. Ospina, J. A. O. Naranjo, T. G. Narváez, H. Diederix, H. U. Peña, M. M. Penagos, Mapa Geológico de Colombia-Escala 1:2’800.000 (Instituto Colombiano de Geología y Minería INGEOMINAS -Servicio Geológico, Bogotá, 2007). [Google Scholar]
- 32.Duque-Caro H., The Chocó Block in the northwestern corner of South America: Structural, tectonostratigraphic, and paleogeographic implications. J. South Am. Earth Sci. 3, 71–84 (1990). [Google Scholar]
- 33.Duque-Caro H., Neogene stratigraphy, paleoceanography and paleobiogeography in northwest South America and the evolution of the Panama seaway. Palaeogeogr. Palaeoclimatol. Palaeoecol. 77, 203–234 (1990). [Google Scholar]
- 34.F. Garzón Varón, thesis, Universidad Nacional de Colombia, Bogotá (2012). [Google Scholar]
- 35.Newkirk D. R., Martin E. E., Circulation through the Central American Seaway during the Miocene carbonate crash. Geology 37, 87–90 (2009). [Google Scholar]
- 36.Keigwin L., Isotopic paleoceanography of the Caribbean and East Pacific: Role of Panama uplift in late Neogene time. Science 217, 350–353 (1982). [DOI] [PubMed] [Google Scholar]
- 37.Lear C. H., Rosenthal Y., Wright J. D., The closing of a seaway: Ocean water masses and global climate change. Earth Planet. Sci. Lett. 210, 425–436 (2003). [Google Scholar]
- 38.Osborne A. H., Newkirk D. R., Groeneveld J., Martin E. E., Tiedemann R., Frank M., The seawater neodymium and lead isotope record of the final stages of Central American Seaway closure. Paleoceanography 29, 715–729 (2014). [Google Scholar]
- 39.Lunt D., Valdes P. J., Haywood A., Rutt I. C., Closure of the Panama Seaway during the Pliocene: Implications for climate and Northern Hemisphere glaciation. Clim. Dyn. 30, 1–18 (2008). [Google Scholar]
- 40.Schneider B., Schmittner A., Simulating the impact of the Panamanian seaway closure on ocean circulation, marine productivity and nutrient cycling. Earth Planet. Sci. Lett. 246, 367–380 (2006). [Google Scholar]
- 41.Billups K., Ravelo A. C., Zachos J. C., Norris R. D., Link between oceanic heat transport, thermohaline circulation, and the Intertropical Convergence Zone in the early Pliocene Atlantic. Geology 27, 319–322 (1999). [Google Scholar]
- 42.Haug G. H., Tiedemann R., Zahn R., Ravelo A. C., Role of Panama uplift on oceanic freshwater balance. Geology 29, 207–210 (2001). [Google Scholar]
- 43.Haug G. H., Tiedemann R., Effect of the formation of the Isthmus of Panama on Atlantic Ocean thermohaline circulation. Nature 393, 673–676 (1998). [Google Scholar]
- 44.Groeneveld J., Hathorne E. C., Steinke S., DeBey H., Mackensen A., Tiedemann R., Glacial induced closure of the Panamanian Gateway during Marine Isotope Stages (MIS) 95–100 (~2.5 Ma). Earth Planet. Sci. Lett. 404, 296–306 (2014). [Google Scholar]
- 45.Kamikuri S.-i., Motoyama I., Nishi H., Iwai M., Evolution of Eastern Pacific Warm Pool and upwelling processes since the middle Miocene based on analysis of radiolarian assemblages: Response to Indonesian and Central American Seaways. Palaeogeogr. Palaeoclimatol. Palaeoecol. 280, 469–479 (2009). [Google Scholar]
- 46.R. D. Norris, in Proc. ODP, Sci. Results, J. Mascle, G. Lohmann, M. Moullade, Eds. (Ocean Drilling Program, College Station, TX, 1998), pp. 539–556. [Google Scholar]
- 47.Kameo K., Sato T., Biogeography of Neogene calcareous nannofossils in the Caribbean and the eastern equatorial Pacific—Floral response to the emergence of the Isthmus of Panama. Mar. Micropaleontol. 39, 201–218 (2000). [Google Scholar]
- 48.Keller G., Zenker C. E., Stone S. M., Late neogene history of the Pacific-Caribbean gateway. J. South Am. Earth Sci. 2, 73–108 (1989). [Google Scholar]
- 49.Chaisson W. P., Ravelo A. C., Pliocene development of the east-west hydrographic gradient in the equatorial Pacific. Paleoceanography 15, 497–505 (2000). [Google Scholar]
- 50.Johnson K. G., Kirby M. X., The Emperador limestone rediscovered: Early Miocene corals from the Culebra Formation, Panama. J. Paleontol. 80, 283–293 (2006). [Google Scholar]
- 51.Kirby M. X., Jones D. S., MacFadden B. J., Lower Miocene stratigraphy along the Panama Canal and its bearing on the Central American Peninsula. PLOS One 3, e2791 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budd A. F., Stemann T. A., Stewart R. H., Eocene Caribbean reef corals: A unique fauna from the Gatuncillo Formation of Panama. J. Paleontol. 66, 570–594 (1992). [Google Scholar]
- 53.Woodring W. P., The Panama land bridge as a sea barrier. Proc. Am. Philos. Soc. 110, 425–433 (1966). [Google Scholar]
- 54.Jackson J. B. C., Jung P., Coates A. G., Collins L. S., Diversity and extinction of Tropical American mollusks and emergence of the Isthmus of Panama. Science 260, 1624–1626 (1993). [DOI] [PubMed] [Google Scholar]
- 55.Aguilera O., Ramos M. I. F., Paes E. T., Costa S. A. R. F., Sánchez-Villaga M. R., The Neogene tropical America fish assemblage and the paleobiogeography of the Caribbean region. Swiss J. Paleontol. 130, 217–240 (2011). [Google Scholar]
- 56.Schwarzhans W., Aguilera O., Otoliths of the Myctophidae from the Neogene of tropical America. Palaeo Ichthyol. 13, 83–150 (2013). [Google Scholar]
- 57.Landau B., Marques Da Silva C., Vermeij G., Pacific elements in the Caribbean Neogene gastropod fauna: The source-sink model, larval development, disappearance, and faunal units. Bull. Soc. Geol. Fr. 180, 343–352 (2009). [Google Scholar]
- 58.Kirby M. X., Jackson J. B. C., Extinction of a fast-growing oyster and changing ocean circulation in Pliocene tropical America. Geology 32, 1025–1028 (2004). [Google Scholar]
- 59.Leonard-Pingel J. S., Jackson J. B. C., O’Dea A., Changes in bivalve functional and assemblage ecology in response to environmental change in the Caribbean Neogene. Paleobiology 38, 509–524 (2012). [Google Scholar]
- 60.Todd J. A., Jackson J. B. C., Johnson K. G., Fortunato H. M., Heitz A., Alvarez M., Jung P., The ecology of extinction: Molluscan feeding and faunal turnover in the Caribbean Neogene. Proc. R. Soc. Lond. 269, 571–577 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allmon W. D., Nutrients, temperature, disturbance, and evolution: A model for the late Cenozoic marine record of the western Atlantic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 166, 9–26 (2001). [Google Scholar]
- 62.J. B. C. Jackson, H. Fortunato, J. A. Todd, P. Jung, in The Neogene of the Isthmus of Panama: A Paleobiotic Survey of the Caribbean Coast, L. S. Collins, A. G. Coates, Eds. (Bulletins of American Paleontology, Ithaca, 1999), vol. 357, pp. 193–230. [Google Scholar]
- 63.O’Dea A., Jackson J., Environmental change drove macroevolution in cupuladriid bryozoans. Proc. Biol. Sci. 276, 3629–3634 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith J. T., Jackson J. B. C., Ecology of extreme faunal turnover of tropical American scallops. Paleobiology 35, 77–93 (2009). [Google Scholar]
- 65.J. Jackson, P. Jung, H. Fortunato, in Evolution and Environment in Tropical America, J. B. C. Jackson, A. F. Budd, A. G. Coates, Eds. (University of Chicago Press, Chicago, 1996), pp. 234–270. [Google Scholar]
- 66.Johnson K. G., Jackson J. B. C., Budd A. F., Caribbean reef development was independent of coral diversity over 28 million years. Science 319, 1521–1523 (2008). [DOI] [PubMed] [Google Scholar]
- 67.T. M. Cronin, H. J. Dowsett, in Evolution and Environment in Tropical America, J. B. C. Jackson, A. F. Budd, A. G. Coates, Eds. (University of Chicago Press, Chicago, 1996), pp. 76–104. [Google Scholar]
- 68.O’Dea A., Jackson J. B. C., Fortunato H., Smith J. T., D’Croz L., Johnson K. G., Todd J. A., Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl. Acad. Sci. U.S.A. 104, 5501–5506 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lessios H. A., Robertson D. R., Speciation on a round planet: Phylogeography of the goatfish genus Mulloidichthys. J. Biogeogr. 40, 2373–2384 (2013). [Google Scholar]
- 70.Roy M. S., Sponer R., Evidence of a human–mediated invasion of the tropical western Atlantic by the ’world’s most common brittlestar’. Proc. Biol. Sci. 269, 1017–1023 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hellberg M. E., No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evol. Biol. 6, 24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eytan R. I., Hellberg M. E., Nuclear and mitochondrial sequence data reveal and conceal different demographic histories and population genetic processes in Caribbean reef fishes. Evolution 64, 3380–3397 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Huang D., Meier R., Todd P. A., Chou L. M., Slow mitochondrial COI sequence evolution at the base of the Metazoan tree and its implications for DNA barcoding. J. Mol. Evol. 66, 167–174 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Dornburg A., Brandley M. C., McGowen M. R., Near T. J., Relaxed clocks and inferences of heterogeneous patterns of nucleotide substitution and divergence time estimates across whales and dolphins (Mammalia: Cetacea). Mol. Biol. Evol. 29, 721–736 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Marko P. B., Eytan R. I., Knowlton N., Do large molecular sequence divergences imply an early closure of the Isthmus of Panama? Proc. Natl. Acad. Sci. U.S.A. 112, E5766 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knowlton N., Weigt L. A., Solorzano L. A., Mills D. K., Bermingham E., Divergence in proteins, mitochondrial DNA, and reproductive compatibility across the isthmus of Panama. Science 260, 1629–1632 (1993). [DOI] [PubMed] [Google Scholar]
- 77.Lessios H. A., Appearance of an early closure of the Isthmus of Panama is the product of biased inclusion of data in the metaanalysis. Proc. Natl. Acad. Sci. U.S.A. 112, E5765 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacon C. D., Silvestro D., Jaramillo C., Smith B. T., Chakrabarty P., Antonelli A., Reply to Lessios and Marko et al.: Early and progressive migration across the Isthmus of Panama is robust to missing data and biases. Proc. Natl. Acad. Sci. U.S.A. 112, E5767–E5768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hastings A. K., Bloch J. I., Jaramillo C. A., Rincon A. F., Macfadden B. J., Systematics and biogeography of crocodylians from the Miocene of Panama. J. Vert. Paleontol. 33, 239–263 (2013). [Google Scholar]
- 80.Bloch J. I., Woodruff E. D., Wood A. R., Rincon A. F., Harrington A. R., Morgan G. S., Foster D. A., Montes C., Jaramillo C. A., Jud N. A., Jones D. S., MacFadden B. J., First North American fossil monkey and early Miocene tropical biotic interchange. Nature 533, 243–246 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Ferrusquía-Villafranca I., Chapter 13: Mexico’s middle Miocene Mammalian assemblages: An overview. Bull. Am. Mus. Nat. Hist. 279, 321–347 (2003). [Google Scholar]
- 82.MacFadden B. J., North American Miocene land mammals from Panama. J. Vert. Paleontol. 26, 720–734 (2006). [Google Scholar]
- 83.Webb S. D., A history of savanna vertebrates in the new world. II: South America and the great interchange. Annu. Rev. Ecol. Syst. 9, 393–426 (1978). [Google Scholar]
- 84.Sánchez-Villagra M. R., Aguilera O. A., Neogene vertebrates from Urumaco, Falcón State, Venezuela: Diversity and significance. J. Syst. Paleontol. 4, 213–220 (2006). [Google Scholar]
- 85.Cozzuol M. A., The Acre vertebrate fauna: Age, diversity, and geography. J. South Am. Earth Sci. 21, 185–203 (2006). [Google Scholar]
- 86.G. Morgan, in Neogene Mammals, S. Lucas, G. Mogan, J. Spielmann, D. Prothero, Eds. (Bulletin New Mexico Museum of Natural History & Science, Albuquerque, 2008), vol. 44, pp. 93–140. [Google Scholar]
- 87.Laurito C., Valerio A., Primer registro fósil de Pliometanastes sp. (Mammalia, Xenarthra, Megalonychidae) para el Mioceno Superior de Costa Rica, América Central. Una nueva pista en la comprensión del Pre-GABI. Rev. Geol. Am. Cent. 47, 95–107 (2012). [Google Scholar]
- 88.Flynn J. J., Kowallis B. J., Nuñez C., Carranza-Castañeda O., Miller W. E., Swisher C. C. III, Lindsay E., Geochronology of Hemphillian-Blancan Aged Strata, Guanajuato, Mexico, and implications for timing of the Great American Biotic Interchange. J. Geol. 113, 287–307 (2005). [Google Scholar]
- 89.Carranza-Castañeda O., Miller W. E., Late Tertiary terrestrial mammals from central Mexico and their relationship to South American immigrants. Rev. Bras. Paleontol. 7, 2249–2261 (2004). [Google Scholar]
- 90.McDonald H. G., Paleoecology of extinct xenarthrans and the Great American Biotic Interchange. Bull. Fla. Mus. Nat. Hist. 45, 313–333 (2005). [Google Scholar]
- 91.H. McDonald, V. Naples, in Evolution of Tertiary Mammals of North America, C. M. Janis, G. F. Gunnell, M. D. Uhen, Eds. (Cambridge Univ. Press, Cambridge, 2008), vol. 2, pp. 147–160. [Google Scholar]
- 92.M. A. Reguero, A. M. Candela, in Cenozoic Geology of the Central Andes of Argentina, J. A. Salfity, R. A. Marquillas, Eds. (SCS Publishers, Salta, 2011), pp. 411–426. [Google Scholar]
- 93.Pardiñas U. F. J., Tonni E. P., La procedencia estratigráfica y asignación cronológica de los más antiguos muroideos (Mammalia, Rodentia) de América del Sur. Ameghiniana 35, 473–475 (1998). [Google Scholar]
- 94.MacFadden B. J., Labs-Hochstein J., Hulbert R. C. Jr, Baskin J. A., Revised age of the late Neogene terror bird (Titanis) in North America during the Great American Interchange. Geology 35, 123–126 (2007). [Google Scholar]
- 95.Pinto-Sánchez N. R., Ibáñez R., Madriñán S., Sanjur O. I., Bermingham E., Crawford A. J., The Great American Biotic Interchange in frogs: Multiple and early colonization of Central America by the South American genus Pristimantis (Anura: Craugastoridae). Mol. Phylogen. Evol. 62, 954–972 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Bacon C. D., Mora A., Wagner W. L., Jaramillo C. A., Testing geological models of evolution of the Isthmus of Panama in a phylogenetic framework. Bot. J. Linn. Soc. 171, 287–300 (2013). [Google Scholar]
- 97.Renner S., Plant dispersal across the tropical Atlantic by wind and sea currents. Int. J. Plant Sci. 165, S23–S33 (2004). [Google Scholar]
- 98.Gillespie R. G., Baldwin B. G., Waters J. M., Fraser C. I., Nikula R., Roderick G. K., Long-distance dispersal: A framework for hypothesis testing. Trends Ecol. Evol. 27, 47–56 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Crisp M. D., Arroyo M. T. K., Cook L. G., Gandolfo M. A., Jordan G. J., McGlone M. S., Weston P. H., Westoby M., Wilf P., Linder H. P., Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Poux C., Chevret P., Huchon D., de Jong W. W., Douzery E. J. P., Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst. Biol. 55, 228–244 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Ali J. R., Huber M., Mammalian biodiversity on Madagascar controlled by ocean currents. Nature 463, 653–656 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Samonds K. E., Godfrey L. R., Ali J. R., Goodman S. M., Vences M., Sutherland M. R., Irwin M. T., Krause D. W., Imperfect isolation: Factors and filters shaping Madagascar’s extant vertebrate fauna. PLOS One 8, e62086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ricklefs R., Bermingham E., The West Indies as a laboratory of biogeography and evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2393–2413 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vences M., Vieites D. R., Glaw F., Brinkmann H., Kosuch J., Veith M., Meyer A., Multiple overseas dispersal in amphibians. Proc. Biol. Sci. 270, 2435–2442 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Measey G. J., Vences M., Drewes R. C., Chiari Y., Melo M., Bourles B., Freshwater paths across the ocean: Molecular phylogeny of the frog Ptychadena newtoni gives insights into amphibian colonization of oceanic islands. J. Biogeogr. 34, 7–20 (2007). [Google Scholar]
- 106.M. Thiel, L. Gutow, in Oceanography and Marine Biology: An Annual Review, R. N. Gibson, J. D. M. Gordon, R. J. A. Atkinson, Eds. (Taylor & Francis, Abingdon, 2005), vol. 43, pp. 279–418. [Google Scholar]
- 107.C. Van Duzer, Floating Islands: A Global Bibliography; with an Edition and Translation of G.C. Munz’s Exercitatio academica de insulis natantibus (1711) (Cantor Press, Los Altos Hills, CA, 2004), p. 204. [Google Scholar]
- 108.Houle A., The origin of platyrrhines: An evaluation of the Antarctic scenario and the floating island model. Am. J. Phys. Anthropol. 109, 541–559 (1999). [DOI] [PubMed] [Google Scholar]
- 109.MacPhee R. D. E., Singer R., Diamond M., Late Cenozoic land mammals from Grenada, Lesser Antilles island-arc. Am. Mus. Novit. 3302, 1–20 (2000). [Google Scholar]
- 110.McFadden K. W., Gompper M. E., Valenzuela D. G., Morales J. C., Evolutionary history of the critically endangered Cozumel dwarf carnivores inferred from mitochondrial DNA analyses. J. Zool. 276, 176–186 (2008). [Google Scholar]
- 111.A. de Queiroz, The Monkey’s Voyage: How Improbable Journeys Shaped the History of Life (Basic Books, New York, 2014), p. 360. [Google Scholar]
- 112.Sprintall J., Wijffels S. E., Molcard R., Jaya I., Direct estimates of the Indonesian throughflow entering the Indian Ocean: 2004–2006. J. Geophys. Res. 114, C07001 (2009). [Google Scholar]
- 113.A. R. Wallace, The origin of species and genera. Nineteenth Century 7, 93–106 (1880). [Google Scholar]
- 114.R. J. Whittaker, J. M. Fernández-Palacios, Island Biogeography: Ecology, Evolution, and Conservation (Oxford Univ. Press, New York, 2007), p. 403. [Google Scholar]
- 115.von der Heydt A., Dijkstra H. A., Flow reorganizations in the Panama seaway: A cause for the demise of Miocene corals? Geophys. Res. Lett. 32, L02609 (2005). [Google Scholar]
- 116.Woodburne M. O., The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. J. Mamm. Evol. 17, 245–264 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Webb S. D., Ecogeography and the Great American Interchange. Paleobiology 17, 266–280 (1991). [Google Scholar]
- 118.Leigh E. G. Jr, Hladik A., Hladik C. M., Jolly A., The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play? Rev. Écol. (Terre Vie) 62, 105–168 (2007). [Google Scholar]
- 119.F. G. Stehli, S. D. Webb, The Great American Biotic Interchange. Topics in Geobiology (Plenum Press, New York, 1985), p. 532. [Google Scholar]
- 120.Carrillo J. D., Forasiepi A., Jaramillo C., Sánchez-Villaga M. R., Neotropical mammal diversity and the Great American Biotic Interchange: Spatial and temporal variation in South America’s fossil record. Front. Genet. 5, 451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Daza J. M., Castoe T. A., Parkinson C. L., Using regional comparative phylogeographic data from snake lineages to infer historical processes in Middle America. Ecography 33, 343–354 (2010). [Google Scholar]
- 122.Gould S. J., A direct assault upon the citadel itself. Paleobiology 1, 125–135 (1975). [Google Scholar]
- 123.T. M. Cronin, in Ninth International Symposium on Ostracoda. Developments in Palaeontology and Stratigraphy, T. Hanai, N. Ikeya, K. Ishizaki, Eds. (Elsevier, London, 1988), vol. 11, pp. 871–889. [Google Scholar]
- 124.Tiedemann R., Sarnthein M., Shackleton N. J., Astronomic timescale for the Pliocene Atlantic δ18O and dust flux records of Ocean Drilling Program Site 659. Paleoceanography 9, 619–638 (1994). [Google Scholar]
- 125.N. Shackleton, M. Hall, D. Pate, Pliocene stable isotope stratigraphy of Site 846, in Proceedings of the Ocean Drilling Program, Scientific Results, N. G. Pisias, L. A. Mayer, T. R. Janecek, A. Palmer-Julson, T. H. van Andel, Eds. (Ocean Drilling Program, College Station, TX, 1995), vol. 138, pp. 337–355. [Google Scholar]
- 126.Sepulchre P., Arsouze T., Donnadieu Y., Dutay J.-C., Jaramillo C., Le Bras J., Martin E., Montes C., Waite A. J., Consequences of shoaling of the Central American Seaway determined from modeling Nd isotopes. Paleoceanography 29, 176–189 (2014). [Google Scholar]
- 127.Hogg N. G., Johns W. E., Western boundary currents. Rev. Geophys. 33, 1311–1334 (1995). [Google Scholar]
- 128.Wajsowicz R. C., Schneider E. K., The Indonesian throughflow’s effect on global climate determined from the COLA Coupled Climate System. J. Climate 14, 3029–3042 (2001). [Google Scholar]
- 129.W. Kuhnt, A. Holbourn, R. Hall, M. Zuvela, R. Käse, in Continent-Ocean Interactions within East Asian Marginal Seas, P. Clift, W. Kuhnt, P. Wang, D. Hayes, Eds. (American Geophysical Union, Washington, DC, 2013), pp. 299–320. [Google Scholar]
- 130.Qu T., Du Y., Sasaki H., South China Sea throughflow: A heat and freshwater conveyor. Geophys. Res. Lett. 33, L23617 (2006). [Google Scholar]
- 131.Miller K. G., Kominz M. A., Browning J. V., Wright J. D., Mountain G. S., Katz M. E., Sugarman P. J., Cramer B. S., Christie-Blick N., Pekar S. F., The Phanerozoic record of global sea-level change. Science 310, 1293–1298 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Restrepo J. J., Compilación de edades radiométricas de Colombia, departamentos Andinos hasta 1982. Bol. Cienc. Tierra 78, 201–248 (1983). [Google Scholar]
- 133.Okamura B., O’Dea A., Knowles T., Bryozoan growth and environmental reconstruction by zooid size variation. Mar. Ecol. Prog. Ser. 430, 133–146 (2011). [Google Scholar]
- 134.O’Dea A., Hoyos N., Rodríguez F., Degracia B., De Gracia C., History of upwelling in the Tropical Eastern Pacific and the paleogeography of the Isthmus of Panama. Palaeogeogr. Palaeoclimatol. Palaeoecol. 348–349, 59–66 (2012). [Google Scholar]
- 135.Cassell D. T., Sen Gupta B. K., Foraminiferal stratigraphy and paleoenvironments of the tertiary Uscari Formation, Lomon Basin, Costa Rica. J. Foraminifer. Res. 19, 52–71 (1989). [Google Scholar]
- 136.M.-P. Aubry, W. A. Berggren, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 38–40. [Google Scholar]
- 137.W. A. Berggren, D. V. Kent, C. C. Swisher III, M.-P. Aubry, in Geochronology Time Scales and Global Stratigraphic Correlation, W. A. Berggren, D. V. Kent, M.-P. Aubry, J. Hardenbol, Eds. (Society for Sedimentary Geology, Tulsa, OK, 1995), vol. 54, pp. 129–212. [Google Scholar]
- 138.Berggren W. A., Hilgen F. J., Langereis C. G., Kent D. V., Obradovich J. D., Raffi I., Raymo M. E., Shackleton N. J., Late Neogene chronology: New perspectives in high-resolution stratigraphy. Geol. Soc. Am. Bull. 107, 1272–1287 (1995). [Google Scholar]
- 139.Bybell L. M., Neogene calcareous nannofossil biostratigraphy of the Caribbean coast of Panama and Costa Rica. Bull. Am. Paleontol. 357, 41–59 (1999). [Google Scholar]
- 140.A. G. Coates, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 41–60. [Google Scholar]
- 141.A. G. Coates, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 287–298. [Google Scholar]
- 142.A. G. Coates, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 299–348. [Google Scholar]
- 143.Coates A. G., Aubry M.-P., Berggren W. A., Collins L. S., Kunk M., Early neogene history of the central American arc from Bocas del Toro, western Panama. Geol. Soc. Am. Bull. 115, 271–287 (2003). [Google Scholar]
- 144.L. S. Collins, A. G. Coates, J. B. C. Jackson, J. A. Obando, in Geologic and Tectonic Development of the Caribbean Plate Boundary in southern Central America, P. Mann, Ed. (University of Texas, Austin, 1995), vol. 295, pp. 263–290. [Google Scholar]
- 145.Collins L. S., Neogene Paleoenvironments of the Bocas del Toro Basin, Panama. J. Paleontol. 67, 699–710 (1993). [Google Scholar]
- 146.L. S. Collins, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 193–230. [Google Scholar]
- 147.L. S. Collins, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 91–108. [Google Scholar]
- 148.M. A. Cotton, in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, L. S. Collins, A. G. Coates, Eds. (Paleontological Research Institution, Ithaca, 1999), vol. 357, pp. 61–80. [Google Scholar]
- 149.J. G. Ogg, G. Ogg, F. M. Gradstein, The Concise Geologic Time Scale (Cambridge Univ. Press, Cambridge, 2008), vol. 1, p. 177. [Google Scholar]
- 150.J. Gómez-Tapias, N. Montes-Ramírez, F. A. Alcárcel-Gutiérrez, J. A. Ceballos-Hernández, Catálogo de Dataciones Radiométricas De Colombia En ArcGis 9.3 y Google Earth (Servicio Geológico Colombiano, Bogotá, 2013), vol. 33, p. 286. [Google Scholar]
- 151.Drummond A. J., Rambaut A., BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drummond A. J., Suchard M. A., Xie D., Rambaut A., Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lanfear R., Calcott B., Ho S. Y. W., Guindon S., PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012). [DOI] [PubMed] [Google Scholar]
- 154.Drummond A. J., Ho S. Y. W., Phillips M. J., Rambaut A., Relaxed phylogenetics and dating with confidence. PLOS Biol. 4, e88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frey M. A., Vermeij G. J., Molecular phylogenies and historical biogeography of a circumtropical group of gastropods (Genus: Nerita): Implications for regional diversity patterns in the marine tropics. Mol. Phylogenet. Evol. 48, 1067–1086 (2008). [DOI] [PubMed] [Google Scholar]
- 156.Sanderson M. J., R8s: Inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003). [DOI] [PubMed] [Google Scholar]
- 157.A. Rambaut, M. A. Suchard, D. Xie, A. J. Drummond, Tracer v1.6; http://beast.bio.ed.ac.uk/Tracer.
- 158.Marko P. B., Fossil calibration of molecular clocks and the divergence times of geminate species pairs separated by the Isthmus of Panama. Mol. Biol. Evol. 19, 2005–2021 (2002). [DOI] [PubMed] [Google Scholar]
- 159.Marko P. B., Moran A. L., Out of sight, out of mind: High cryptic diversity obscures the identities and histories of geminate species in the marine bivalve subgenus Acar. J. Biogeogr. 36, 1861–1880 (2009). [Google Scholar]
- 160.Nylander J. A. A., Wilgenbusch J. C., Warren D. L., Swofford D. L., AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24, 581–583 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Coppard S. E., Zigler K. S., Lessios H. A., Phylogeography of the sand dollar genus Mellita: Cryptic speciation along the coasts of the Americas. Mol. Phylogenet. Evol. 69, 1033–1042 (2013). [DOI] [PubMed] [Google Scholar]
- 162.Bellwood D. R., Klanten S., Cowman P. F., Pratchett M. S., Konow N., Van Herwerden L., Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol. 23, 335–349 (2010). [DOI] [PubMed] [Google Scholar]
- 163.Hodge J. R., van Herwerden L., Bellwood D. R., Temporal evolution of coral reef fishes: Global patterns and disparity in isolated locations. J. Biogeogr. 41, 2115–2127 (2014). [Google Scholar]
- 164.Williams S. T., Duda T. F., Did tectonic activity stimulate Oligo–Miocene speciation in the Indo-West Pacific? Evolution 62, 1618–1634 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Santini F., Sorenson L., Alfaro M. E., A new multi-locus timescale reveals the evolutionary basis of diversity patterns in triggerfishes and filefishes (Balistidae, Monacanthidae; Tetraodontiformes). Mol. Phylogenet. Evol. 69, 165–176 (2013). [DOI] [PubMed] [Google Scholar]
- 166.Frédérich B., Sorenson L., Santini F., Slater G. J., Alfaro M. E., Iterative ecological radiation and convergence during the evolutionary history of damselfishes (Pomacentridae). Am. Nat. 181, 94–113 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Herrera N. D., ter Poorten J. J., Bieler R., Mikkelsen P. M., Strong E. E., Jablonski D., Steppan S. J., Molecular phylogenetics and historical biogeography amid shifting continents in the cockles and giant clams (Bivalvia: Cardiidae). Mol. Phylogenet. Evol. 93, 94–106 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Dornburg A., Moore J., Beaulieu J. M., Eytan R. I., Near T. J., The impact of shifts in marine biodiversity hotspots on patterns of range evolution: Evidence from the Holocentridae (squirrelfishes and soldierfishes). Evolution 69, 146–161 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Santini F., Carnevale G., Sorenson L., First multi-locus timetree of seabreams and porgies (Percomorpha: Sparidae). Ital. J. Zool. 81, 55–71 (2014). [Google Scholar]
- 170.Reid D. G., Dyal P., Williams S. T., Global diversification of mangrove fauna: A molecular phylogeny of Littoraria (Gastropoda: Littorinidae). Mol. Phylogenet. Evol. 55, 185–201 (2010). [DOI] [PubMed] [Google Scholar]
- 171.Hodge J. R., Read C. I., Bellwood D. R., van Herwerden L., Evolution of sympatric species: A case study of the coral reef fish genus Pomacanthus (Pomacanthidae). J. Biogeogr. 40, 1676–1687 (2013). [Google Scholar]
- 172.Malaquias M. A. E., Reid D. G., Tethyan vicariance, relictualism and speciation: Evidence from a global molecular phylogeny of the opisthobranch genus Bulla. J. Biogeogr. 36, 1760–1777 (2009). [Google Scholar]
- 173.Duda T. F. Jr, Rolán E., Explosive radiation of Cape Verde Conus, a marine species flock. Mol. Ecol. 14, 267–272 (2005). [DOI] [PubMed] [Google Scholar]
- 174.Puillandre N., Bouchet P., Duda T. F. Jr, Kauferstein S., Kohn A. J., Olivera B. M., Watkins M., Meyer C., Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol. Phylogenet. Evol. 78, 290–303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.E. Bermingham, S. McCafferty, A. Martin, T. Kocher, C. Stepien, in Molecular Systematics of Fishes, T. Kocher, C. Stepien, Eds. (Academic Press Inc., San Diego, 1997), pp. 113–128. [Google Scholar]
- 176.Craig M. T., Hastings P. A., A molecular phylogeny of the groupers of the subfamily Epinephelinae (Serranidae) with a revised classification of the Epinephelini. Ichthyol. Res. 54, 1–17 (2007). [Google Scholar]
- 177.Quenouille B., Bermingham E., Planes S., Molecular systematics of the damselfishes (Teleostei: Pomacentridae): Bayesian phylogenetic analyses of mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 31, 66–88 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Bernardi G., Lape J., Tempo and mode of speciation in the Baja California disjunct fish species Anisotremus davidsonii. Mol. Ecol. 14, 4085–4096 (2005). [DOI] [PubMed] [Google Scholar]
- 179.Tavera J. J., Acero P. A., Balart E. F., Bernardi G., Molecular phylogeny of grunts (Teleostei, Haemulidae), with an emphasis on the ecology, evolution, and speciation history of New World species. BMC Evol. Biol. 12, 57 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sanciangco M. D., Rocha L. A., Carpenter K. E., A molecular phylogeny of the grunts (Perciformes: Haemulidae) inferred using mitochondrial and nuclear genes. Zootaxa 2966, 37–50 (2011). [Google Scholar]
- 181.Tringali M. D., Bert T. M., Seyoum S., Bermingham E., Bartolacci D., Molecular phylogenetics and ecological diversification of the transisthmian fish genus Centropomus (Perciformes: Centropomidae). Mol. Phylogenet. Evol. 13, 193–207 (1999). [DOI] [PubMed] [Google Scholar]
- 182.Gold J. R., Willis S. C., Renshaw M. A., Buentello A., Walker H. J. Jr, Puritz J. B., Hollenbeck C. M., Voelker G., Phylogenetic relationships of tropical eastern Pacific snappers (Lutjanidae) inferred from mitochondrial DNA sequences. Syst. Biodivers. 13, 596–607 (2015). [Google Scholar]
- 183.Lessios H. A., Allen G. R., Wellington G. M., Bermingham E., Genetic and morphological evidence that the Eastern Pacific damselfish Abudefduf declivifrons is distinct from A. concolor (Pomacentridae). Copeia 1995, 277–288 (1995). [Google Scholar]
- 184.Martin A. P., Naylor G. J. P., Palumbi S. R., Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature 357, 153–155 (1992). [DOI] [PubMed] [Google Scholar]
- 185.Lessios H. A., Robertson D. R., Crossing the impassable: Genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc. Biol. Sci. 273, 2201–2208 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bellwood D. R., van Herwerden L., Konow N., Evolution and biogeography of marine angelfishes (Pisces: Pomacanthidae). Mol. Phylogenet. Evol. 33, 140–155 (2004). [DOI] [PubMed] [Google Scholar]
- 187.Mccartney M. A., Acevedo J., Heredia C., Rico C., Quenoville B., Bermingham E., McMillan W. O., Genetic mosaic in a marine species flock. Mol. Ecol. 12, 2963–2973 (2003). [DOI] [PubMed] [Google Scholar]
- 188.Craig M. T., Hastings P. A., Pondella D. J. II, Speciation in the Central American Seaway: The importance of taxon sampling in the identification of trans-isthmian geminate pairs. J. Biogeogr. 31, 1085–1091 (2004). [Google Scholar]
- 189.Banford H. M., Bermingham E., Collette B. B., Molecular phylogenetics and biogeography of transisthmian and amphi-Atlantic needlefishes (Belonidae: Strongylura and Tylosurus): Perspectives on New World marine speciation. Mol. Phylogenet. Evol. 31, 833–851 (2004). [DOI] [PubMed] [Google Scholar]
- 190.Barber P. H., Bellwood D. R., Biodiversity hotspots: Evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics. Mol. Phylogenet. Evol. 35, 235–253 (2005). [DOI] [PubMed] [Google Scholar]
- 191.Teske P. R., Hamilton H., Matthee C. A., Barker N. P., Signatures of seaway closures and founder dispersal in the phylogeny of a circumglobally distributed seahorse lineage. BMC Evol. Biol. 7, 138 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rocha L. A., Lindeman K. C., Rocha C. R., Lessios H. A., Historical biogeography and speciation in the reef fish genus Haemulon (Teleostei: Haemulidae). Mol. Phylogenet. Evol. 48, 918–928 (2008). [DOI] [PubMed] [Google Scholar]
- 193.Banford H. M., Bermingham E., Collette B. B., McCafferty S. S., Phylogenetic systematics of the Scomberomorus regalis (Teleostei: Scombridae) species group: Molecules, morphology and biogeography of Spanish mackerels. Copeia 1999, 596–613 (1999). [Google Scholar]
- 194.Muss A., Robertson D. R., Stepien C. A., Wirtz P., Bowen B. W., Phylogeography of Ophioblennius: The role of ocean currents and geography in reef fish evolution. Evolution 55, 561–572 (2001). [DOI] [PubMed] [Google Scholar]
- 195.Ross Robertson D., Karg F., Leao de Moura R., Victor B. C., Bernardi G., Mechanisms of speciation and faunal enrichment in Atlantic parrotfishes. Mol. Phylogenet. Evol. 40, 795–807 (2006). [DOI] [PubMed] [Google Scholar]
- 196.Westneat M. W., Alfaro M. E., Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 36, 370–390 (2005). [DOI] [PubMed] [Google Scholar]
- 197.Streelman J. T., Alfaro M., Westneat M. W., Bellwood D. R., Karl S. A., Evolutionary history of the parrotfishes: Biogeography, ecomorphology, and comparative diversity. Evolution 56, 961–971 (2002). [DOI] [PubMed] [Google Scholar]
- 198.Rocha L. A., Robertson D. R., Rocha C. R., Van Tassell J. L., Craig M. T., Bowen B. W., Recent invasion of the tropical Atlantic by an Indo-Pacific coral reef fish. Mol. Ecol. 14, 3921–3928 (2005). [DOI] [PubMed] [Google Scholar]
- 199.Donaldson K. A., Wilson R. R. Jr, Amphi-panamic geminates of snook (Percoidei: Centropomidae) provide a calibration of the divergence rate in the mitochondrial DNA control region of fishes. Mol. Phylogenet. Evol. 13, 208–213 (1999). [DOI] [PubMed] [Google Scholar]
- 200.Lin H.-C., Hastings P. A., Evolution of a Neotropical marine fish lineage (Subfamily Chaenopsinae, Suborder Blennioidei) based on phylogenetic analysis of combined molecular and morphological data. Mol. Phylogenet. Evol. 60, 236–248 (2011). [DOI] [PubMed] [Google Scholar]
- 201.Wares J. P., Patterns of speciation inferred from mitochondrial DNA in North American Chthamalus (Cirripedia: Balanomorpha: Chthamaloidea). Mol. Phylogenet. Evol. 18, 104–116 (2001). [DOI] [PubMed] [Google Scholar]
- 202.Hiller A., Kraus H., Almon M., Werding B., The Petrolisthes galathinus complex: Species boundaries based on color pattern, morphology and molecules, and evolutionary interrelationships between this complex and other Porcellanidae (Crustacea: Decapoda: Anomura). Mol. Phylogenet. Evol. 40, 547–569 (2006). [DOI] [PubMed] [Google Scholar]
- 203.Robles R., Schubart C. D., Conde J. E., Carmona-Suárez C., Alvarez F., Villalobos J. L., Felder D. L., Molecular phylogeny of the American Callinectes Stimpson, 1860 (Brachyura: Portunidae), based on two partial mitochondrial genes. Mar. Biol. 150, 1265–1274 (2007). [Google Scholar]
- 204.Sturmbauer C., Levinton J. S., Christy J., Molecular phylogeny analysis of fiddler crabs: Test of the hypothesis of increasing behavioral complexity in evolution. Proc. Natl. Acad. Sci. U.S.A. 93, 10855–10857 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schubart C. D., Diesel R., Hedges S. B., Rapid evolution to terrestrial life in Jamaican crabs. Nature 393, 363–365 (1998). [Google Scholar]
- 206.Williams S. T., Knowlton N., Weigt L. A., Jara J. A., Evidence for three major clades within the snapping shrimp genus Alpheus inferred from nuclear and mitochondrial gene sequence data. Mol. Phylogenet. Evol. 20, 375–389 (2001). [DOI] [PubMed] [Google Scholar]
- 207.Morrison C. L., Rios R., Emmett Duffy J., Phylogenetic evidence for an ancient rapid radiation of Caribbean sponge-dwelling snapping shrimps (Synalpheus). Mol. Phylogenet. Evol. 30, 563–581 (2004). [DOI] [PubMed] [Google Scholar]
- 208.Gusmão J., Lazoski C., Monteiro F. A., Solé-Cava A. M., Cryptic species and population structuring of the Atlantic and Pacific seabob shrimp species, Xiphopenaeus kroyeri and Xiphopenaeus riveti. Mar. Biol. 149, 491–502 (2006). [Google Scholar]
- 209.Baldwin J. D., Bass A. L., Bowen B. W., Clark W. H. Jr, Molecular phylogeny and biogeography of the marine shrimp Penaeus. Mol. Phylogenet. Evol. 10, 399–407 (1998). [DOI] [PubMed] [Google Scholar]
- 210.Haye P. A., Tam Y. K., Kornfield I., Molecular phylogenetics of mole crabs (Hippidae: Emerita). J. Crustacean Biol. 22, 903–915 (2002). [Google Scholar]
- 211.Anker A., Hurt C., Knowlton N., Three transisthmian snapping shrimps (Crustacea: Decapoda: Alpheidae: Alpheus) associated with innkeeper worms (Echiura: Thalassematidae) in Panama. Zootaxa 1626, 1–23 (2007). [Google Scholar]
- 212.Harrison J. S., Evolution, biogeography, and the utility of mitochondrial 16s and COI genes in phylogenetic analysis of the crab genus Austinixa (Decapoda: Pinnotheridae). Mol. Phylogenet. Evol. 30, 743–754 (2004). [DOI] [PubMed] [Google Scholar]
- 213.Collin R., Phylogenetic relationships among calyptraeid gastropods and their implications for the biogeography of marine speciation. Syst. Biol. 52, 618–640 (2003). [DOI] [PubMed] [Google Scholar]
- 214.Latiolais J. M., Taylor M. S., Roy K., Hellberg M. E., A molecular phylogenetic analysis of strombid gastropod morphological diversity. Mol. Phylogenet. Evol. 41, 436–444 (2006). [DOI] [PubMed] [Google Scholar]
- 215.Hellberg M. E., Vacquier V. D., Rapid evolution of fertilization selectivity and lysin cDNA sequences in teguline gastropods. Mol. Biol. Evol. 16, 839–848 (1999). [DOI] [PubMed] [Google Scholar]
- 216.Meyer C. P., Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 79, 401–459 (2003). [Google Scholar]
- 217.Pierce S. K., Curtis N. E., Massey S. E., Bass A. L., Karl S. A., Finney C. M., A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sacoglossan sea slug (Gastropoda: Opisthobranchia) from the Florida Keys, USA. Molluscan Res. 26, 23–38 (2006). [Google Scholar]
- 218.Hellberg M. E., Sympatric sea shells along the sea’s shore: The geography of speciation in the marine gastropod Tegula. Evolution 52, 1311–1324 (1998). [DOI] [PubMed] [Google Scholar]
- 219.Collins T. M., Frazer K., Palmer A. R., Vermeij G. J., Brown W. M., Evolutionary history of northern hemisphere Nucella (Gastropoda, Muricidae): Molecular, morphological, ecological, and paleontological evidence. Evolution 50, 2287–2304 (1996). [DOI] [PubMed] [Google Scholar]
- 220.Lee T., Foighil D. Ó., Placing the Floridian marine genetic disjunction into a regional evolutionary context using the scorched mussel, Brachidontes exustus, species complex. Evolution 59, 2139–2158 (2005). [PubMed] [Google Scholar]
- 221.Hickerson M. J., Stahl E. A., Lessios H. A., Test for simultaneous divergence using approximate Bayesian computation. Evolution 60, 2435–2453 (2006). [PubMed] [Google Scholar]
- 222.Lessios H. A., Kessing B. D., Pearse J. S., Population structure and speciation in tropical seas: Global phylogeography of the sea urchin Diadema. Evolution 55, 955–975 (2001). [DOI] [PubMed] [Google Scholar]
- 223.Lessios H. A., Kane J., Robertson D. R., Phylogeography of the pantropical sea urchin Tripneustes: Contrasting patterns of population structure between oceans. Evolution 57, 2026–2036 (2003). [DOI] [PubMed] [Google Scholar]
- 224.Lessios H. A., Kessing B. D., Robertson D. R., Paulay G., Phylogeography of the pantropical sea urchin Eucidaris in relation to land barriers and ocean currents. Evolution 53, 806–817 (1999). [DOI] [PubMed] [Google Scholar]
- 225.McCartney M. A., Keller G., Lessios H. A., Dispersal barriers in tropical oceans and speciation in Atlantic and eastern Pacific sea urchins of the genus Echinometra. Mol. Ecol. 9, 1391–1400 (2000). [DOI] [PubMed] [Google Scholar]
- 226.Lessios H. A., Lockhart S., Collin R., Sotil G., Sanchez-Jerez P., Zigler K. S., Perez A. F., Garrido M. J., Geyer L. B., Bernardi G., Vacquier V. D., Haroun R., Kessing B. D., Phylogeography and bindin evolution in Arbacia, a sea urchin genus with an unusual distribution. Mol. Ecol. 21, 130–144 (2012). [DOI] [PubMed] [Google Scholar]
- 227.Zigler K. S., Lessios H. A., Speciation on the coasts of the new world: Phylogeography and the evolution of bindin in the sea urchin genus Lytechinus. Evolution 58, 1225–1241 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/8/e1600883/DC1
fig. S1. The Isthmus of Panama in Tropical America.
fig. S2. The current day Isthmus of Panama submerged under 150 m of relative sea-level rise.
fig. S3. Timing of successful terrestrial dispersals between the American continents as observed in the occurrence of fossil remains in the rock record of North and South America.
video S1. Large rafts form on the Río Chagres, Gamboa, Republic of Panama, on 8 December 2010.
table S1. Estimates of rates of uplift for the Panama Arc relative to sea level, using changes in estimated median depths and median ages of sedimentary units from previous studies (28, 135–149).
table S2. Compilation of Late Paleocene–Late Eocene ages for the Colombian Andes plotted in Fig. 2.
table S3. Median and 95% HPD intervals of the time at which members of clades spanning the Isthmus of Panama were separated from each other as estimated by BEAST [1] from phylogenies calibrated by fossils at one or more nodes.
table S4. Kimura two-parameter distance between mitochondrial genes of sister clades on either side of the Isthmus of Panama.
text S1. Estimation of date of splitting from molecular divergence.


