Abstract
Implementing precision medicine for complex diseases such as chronic obstructive lung disease (COPD) will require extensive use of biomarkers and an in-depth understanding of how genetic, epigenetic, and environmental variations contribute to phenotypic diversity and disease progression. A meta-analysis from two large cohorts of current and former smokers with and without COPD [SPIROMICS (N = 750); COPDGene (N = 590)] was used to identify single nucleotide polymorphisms (SNPs) associated with measurement of 88 blood proteins (protein quantitative trait loci; pQTLs). PQTLs consistently replicated between the two cohorts. Features of pQTLs were compared to previously reported expression QTLs (eQTLs). Inference of causal relations of pQTL genotypes, biomarker measurements, and four clinical COPD phenotypes (airflow obstruction, emphysema, exacerbation history, and chronic bronchitis) were explored using conditional independence tests. We identified 527 highly significant (p < 8 X 10−10) pQTLs in 38 (43%) of blood proteins tested. Most pQTL SNPs were novel with low overlap to eQTL SNPs. The pQTL SNPs explained >10% of measured variation in 13 protein biomarkers, with a single SNP (rs7041; p = 10−392) explaining 71%-75% of the measured variation in vitamin D binding protein (gene = GC). Some of these pQTLs [e.g., pQTLs for VDBP, sRAGE (gene = AGER), surfactant protein D (gene = SFTPD), and TNFRSF10C] have been previously associated with COPD phenotypes. Most pQTLs were local (cis), but distant (trans) pQTL SNPs in the ABO blood group locus were the top pQTL SNPs for five proteins. The inclusion of pQTL SNPs improved the clinical predictive value for the established association of sRAGE and emphysema, and the explanation of variance (R2) for emphysema improved from 0.3 to 0.4 when the pQTL SNP was included in the model along with clinical covariates. Causal modeling provided insight into specific pQTL-disease relationships for airflow obstruction and emphysema. In conclusion, given the frequency of highly significant local pQTLs, the large amount of variance potentially explained by pQTL, and the differences observed between pQTLs and eQTLs SNPs, we recommend that protein biomarker-disease association studies take into account the potential effect of common local SNPs and that pQTLs be integrated along with eQTLs to uncover disease mechanisms. Large-scale blood biomarker studies would also benefit from close attention to the ABO blood group.
Author Summary
Precision medicine is an emerging approach that takes into account variability in genes, gene and protein expression, environment and lifestyle. Recent advances in high-throughput genome-wide genotyping, genomics, and proteomics coupled with the creation of large, highly-phenotyped clinical cohorts now allows for integration of these molecular data sets at the individual level. Here we use genome-wide genotyping and blood measurements of 88 biomarkers in 1,340 subjects from two large NIH-supported clinical cohorts of smokers (SPIROMICS and COPDGene) to identify more than 300 novel DNA variants that influence measurement of blood protein levels (pQTLs). We find that many DNA variants explain a large portion of the variability of measured protein expression in blood. Furthermore, we show that integration of DNA variants with blood biomarker levels can improve the ability of predictive models to reflect the relationship between biomarker and disease features (e.g., emphysema) within chronic obstructive pulmonary disease (COPD).
Introduction
Implementing precision medicine will require extensive use of biomarkers and in-depth understanding of the contributions of genetic, epigenetic, and environmental variation to phenotypic diversity and disease progression. Genome-wide association studies (GWAS) linking disease phenotypes to single nucleotide polymorphic (SNP) markers have successfully identified genes and pathways involved in complex phenotypes [1, 2]. GWAS are complemented by efforts of functional studies, such as the Genotype-Tissue Expression (GTEx) program [3], which seek to identify expression quantitative trait loci (eQTLs) linking SNP markers with mRNA expression [4]. Such eQTLs can illuminate relationships between genetic variation and disease phenotypes. However, genetic variants can also affect protein levels by mechanisms not detectable by eQTL analyses by altering post-transcriptional processes involving stability, translation, secretion and/or detection of the gene product. Few studies have been focused on the impact of genetic variation on large numbers of protein biomarkers in chronic diseases. However, the recent work in Battle et al., [5] suggests that variants affecting gene expression and protein level may be distinct, so identifying the genetic features that affect protein variation [protein quantitative trait loci (pQTLs)] and gene expression for disease-relevant biomarkers will be important.
To investigate the role of genetic variation on blood biomarkers and their relationship to a chronic disease, we examined genotyping-biomarker-clinical phenotype relationships in two independent, large, well-characterized cohorts of subjects at risk for chronic obstructive lung disease (COPD): Sub-Populations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) [6] and COPDGene [7]. COPD is the third most common cause of death in developed countries [8] and has strong demographic (age, gender) and behavioral (e.g., smoking) risk factors, yet most smokers do not develop clinically important lung disease. Furthermore, COPD has several clinically important, but highly variable, phenotypes including extent and progression of airflow obstruction, loss of lung tissue (emphysema), frequent cough and sputum production (chronic bronchitis) and exacerbations. There have been many publications that have examined the relationship between blood biomarkers and these COPD phenotypes [9]. These biomarkers include both non-specific markers of inflammation (e.g., fibrinogen, C reactive protein, interleukin 6) as well as lung specific proteins (e.g., surfactant protein D, club cell 16) and other proteins [e.g., soluble receptor for advanced glycosylation endproducts (sRAGE), chemokine (C-C motif) ligand 18 (CCL18), and adiponectin]. Many of these biomarker studies have been replicated in independent cohorts and nearly all studies used antibody-based assays. The SPIROMICS and COPDGene biomarker efforts included many of these biomarkers as well as additional novel understudied biomarkers (S1 Table). Although some recent publications suggest that there may be important genetic associations for some blood protein measurements [10], there have been no studies that use multiple independent populations for large scale blood biomarkers, nor are there extensive evaluations on how the SNP-biomarker relationship influences prediction of disease phenotype. Because both SPIROMICS and COPDGene have complete genotyping data, some transcriptomic data, an identical panel of a large number of blood biomarkers, and extensive well-phenotyped clinical data, there is a unique opportunity to identify novel pQTLs and explore their influence on biomarker-disease relationships for COPD and its disease phenotypes.
Materials and Methods
Ethics statement
Written informed consent was received from all subjects. Collection and use of subject information and samples was approved at each clinical center (see S1 File) with the main approval from the IRB at National Jewish Health (HS-1883a) and the IRB at the University of North Carolina at Chapel Hill (10–0048)
Study design, COPD phenotypes, and cohorts
Study design
This study reports a meta-analysis from two large cohorts of current and former smokers with and without COPD: SPIROMICS (ClinicalTrials.gov Identifier: NCT01969344) [6] and COPDGene (ClinicalTrials.gov Identifier: NCT00608764) [7]. For the present study, we analyzed non-Hispanic white (NHW) subjects who had both genotype and biomarker data. Although both of these large studies contain subjects of multiple ethnicities, because COPDGene only has the biomarkers used in this work measured on a NHW subset, the study population for SPIROMICS was also limited to NHW subjects. The selection of subjects accommodated the meta-analysis design chosen for the present work.
COPD phenotypes
For both studies, COPD was defined by spirometric evidence of airflow obstruction [post-bronchodilator forced expiratory volume at one second (FEV1)/forced vital capacity (FVC) <0.70], with severity defined as: mild or moderate (FEV1 >50% predicted) or severe (FEV1 ≤50% predicted). Chronic bronchitis was defined as self-reported chronic cough and sputum for at least three months in each of the two years prior to baseline. Emphysema was quantified by percent of lung voxels ≤-950 Hounsfield Units (% low attenuation areas: %LAA) on the full inspiratory CT scans. Exacerbations were defined as acute worsening of respiratory symptoms requiring treatment with oral corticosteroids and/or antibiotics, emergency room visit, or hospital admission [11].
Cohort description, SPIROMICS
Written informed consent was received from all subjects. Collection and use of subject information and samples was approved at each clinical center (see http://www2.cscc.unc.edu/spiromics/site-listing and S1 File) with the main approval from the IRB at the University of North Carolina at Chapel Hill (10–0048). Subjects were recruited into SPIROMICS in four strata [never smokers (stratum 1), smokers (≥20 packs/year) without COPD (stratum 2), smokers with mild/moderate COPD (stratum 3), smokers with severe COPD (stratum 4)] ([6] and http://www.spiromics.net). The data presented represents a 2012 interim analysis of baseline blood biomarkers and SNP genotyping. For the current study, only samples available at the time that the biomarker assays were conducted were used and these represent the first recruited subset of NHW SPIROMICS subjects. DNA from an overlapping, but not identical, subset of Stratum 2, 3, and 4 subjects was genotyped, and the overlapping subject data with both biomarker and genotype data were utilized. Investigator Dataset Release 3 (INV3), representing the first 1801 enrolled subjects, was utilized for capture of the clinical and demographic variables. Blood collection procedures (EDTA plasma and serum) at the baseline visit have been described [12].
Cohort description, COPDGene
Written informed consent was received from all subjects. Collection and use of subject information and samples was approved at each clinical center (see http://www.copdgene.org/locations and S1 File) with the main approval from the IRB at National Jewish Health (HS-1883a). This multi-center study of the genetic epidemiology of COPD enrolled 10,192 NHW and African-American individuals, aged 45–80 years with ≥10 pack-year smoking history and no exacerbation for >30 days [7]. The clinical dataset Final10000_Dataset_12MAR13 was used for the analysis, which represents the complete baseline dataset. Fresh frozen plasma was collected from 1839 non-fasting subjects (1599 NHW and 240 non-Hispanic Black) using a P100 tube (BD) at five COPDGene sites [National Jewish Health (N = 916), University of Iowa (N = 670), Los Angeles Biomedical Research Institute (N = 202), Temple University (N = 36), and Baylor Medical Center (N = 15)]. A subset of 602 NHW subjects was selected for comprehensive biomarker study as described [13]. The subset was selected to include a range of COPD severities from none to severe COPD. Of the 602 subjects, 590 had genome-wide genotyping, and the overlapping subjects were utilized for this study. The COPDGene data described in this manuscript is available through dbGaP phs000179.v4.p1 as well as GEO (accession GSE42057).
Biomarker levels
114 candidate blood biomarkers (S1 Table) were initially evaluated using custom 13-panel multiplex assays (Myriad-RBM, Austin, TX). The 13-panel multiplexes were primarily selected because they contained at least one biomarker with known or putative links to COPD pathophysiology [12, 13]. Any analytes measured in addition to the pre-selected biomarkers were intended to be utilized for discovery purposes. Although reports of general assay performance are beyond the scope of the present work, details of a pilot study using the SPIROMICS samples on these assays is available that describes the coefficient of variation and reliability estimates for a majority of the analytes measured [12]. Details of the ability of the panels to detect the analyte above background [the lower limit of quantification (LLOQ)] are provided for both studies (S1 Table). Assay performance across the two cohorts was highly similar. Reproducibility of the platform was assessed for selected biomarkers (S1 Fig) using a subset of COPDGene subjects: for sRAGE using Quantikine human RAGE ELISA kit (R&D Systems, Minneapolis, MN) as previously described [14]; CRP (Roche Diagnostics, Mannheim, Germany) and fibrinogen (K-ASSAY fibrinogen test, Kamiya Biomedical Co., Seattle, WA, USA) levels were measured using immunoturbidometric assays as previously described [15]; surfactant protein D using colorimetric sandwich immunoassay method (BioVendor, Heidelberg, Germany) as previously described [16]. Additionally, serum from 63 SPIROMICS subjects who were either GG (N = 27) or TT (N = 36) at rs7041 were analyzed using a monoclonal antibody assay from R&D (Quanitkine ELISA kit) at the Clinical Research Unit Core Laboratory at Johns Hopkins. Polyclonal vitamin D binding protein measurements (ALPCO, Salem, NH) were performed in the same SPIROMICS subjects.
Genotyping
SPIROMICS
This is the first reported use of SPIROMICS genotype data derived from OmniExpress plus Exome GeneChip (Illumina; San Diego, CA). The data presented utilizes a subset of SPIROMICS samples (in database release 1; n = 1143) in which we obtained Illumina OmniExpress plus Exome GeneChip genotypes. The cell lysate for DNA extraction was prepared at the clinical sites as per the SPIROMICS protocol, shipped to the UNC Biospecimen Processing Center for DNA extraction, and then provided to the Wake Forest Genotyping Core, where the DNA was hybridized to the chips.
For the present analysis, DNA hybridization was followed by several quality control steps, which were carried out in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/) [17]. First, samples were evaluated for genetic versus reported/recorded sex, leading to removal of 5 samples due to discrepancy. Second, duplicated and/or related individuals were identified (7 pairs of related individuals were discovered with PI_HAT values > 0.1949). For these related individuals, the sample from the pair with the higher missing rate of genotype data was removed. After these clean up steps, principal component analysis (PCA) was conducted using common SNPs (N = 108,318) to identify individuals of divergent ancestry. HapMap3 populations (CEU—Utah residents with Northern and Western European ancestry from the CEPH collection; CHB—Han Chinese in Beijing, China; JPT—Japanese in Tokyo, YRI—Yoruba in Ibadan, Nigeria) were utilized in the ancestry analysis. For the cohort in the current analysis, we confirmed subject self-report as NHW by PCA. Of the genotyped samples, 856 were identified as NHW. From this subset, 761 were also evaluated in the biomarker dataset, and 11 of these subjects were dropped from the final dataset due to missing covariate values for these subjects. The final number utilized in these analyses was 750 NHW SPIROMICS subjects.
For SPIROMICS, missing genotype data rates were calculated, and SNPs with missing rate greater than 0.05 or minor allele frequency (MAF) < 0.01 were removed (2724 SNPs removed due to missing rate >0.05 and 225917 SNPs with MAF < 0.01 were removed). A Hardy Weinberg test statistic was calculated for each SNP and a test significance threshold of 0.001 was used to filter SNPs. Genotype principal components (PC’s) were then calculated after regressing out covariates site, age, gender, body mass index, smoking pack years, and current smoking status. Eigenvalues were calculated on the PCs to provide guidance for determining the number of genotype PCs to include in the final model (S2 Fig).
COPDGene
COPDGene subjects were of self-reported NHW or African-American ancestry, and genotyped using the HumanOmniExpress array (Illumina) [18]. Details on the processing of the COPDGene genotype data have been reported [18]. Briefly, genotyping was performed using the HumanOmniExpress array, and BeadStudio quality control, including reclustering on project samples was performed following Illumina guidelines. Subjects and markers with a call rate of < 95% were excluded. Population stratification exclusion and adjustment on self-reported white subjects was performed using EIGENSTRAT (EIGENSOFT Version 2.0).
Statistical analysis
General features/overview
To accommodate the meta-analysis structure, statistical analysis was conducted separately within each study cohort followed by combined p-values meta-analysis. Regression analyses with covariates and genotype principal components were used to determine association of SNPs with analyte levels (pQTLs) [17]. Linear regression was used to identify pQTLs when percent of measurable values for the analyte was above 90%; otherwise the tobit model (also called the censored regression model) [19] was used. The set of independent pQTLs per analyte were identified using forward regression. Causal relations of SNP genotype, analyte levels, and disease phenotypes (e.g., chronic bronchitis, emphysema, exacerbation history, or airflow obstruction) were inferred by a conditional dependence testing approach that has been used in previous eQTL studies. Specific details of these analyses are provided below.
Handling of samples below LLOQ
Within each study (SPIROMICS and COPDGene), for each analyte, any measured values < LLOQ were imputed as half of LLOQ. LLOQ values specific to these assay runs were provided by Myriad-RBM. Then all measured values of each analyte were normalized by normal quantile transformation, as this type of rank-based transformation can effectively remove possible bias due to outliers or skewed distributions [20]. Regression analyses were conducted to determine the association of SNPs with analyte levels using the following criteria:
No analysis was conducted on analytes that had >90% of measurements <LLOQ. This criteria removed 28 analytes from the analysis.
Linear regression was conducted on analytes in which <10% of measurements < LLOQ.
For analytes with 10–90% of measured values <LLOQ, a censored regression (tobit) model was used (implemented using the censReg package in R). Because the data had first been normal quantile transformed, the normal distribution assumption of tobit model was automatically satisfied. The truncation value of tobit model was set as the minimum value above LLOQ (normal quantile transformation) minus a small constant (10−10). When such a biomarker is used as covariate for the Conditional Dependence analysis described below, values below the LLOQ for that biomarker were set to the conditional expectation [21].
Calculating pQTLs
In SPIROMICS, the following covariates were used for pQTL mapping (either linear or tobit model): genotype PC1, biomarker PC1, sites, sex, age, BMI, smoking pack years, current smoker status (0/1). In COPDGene, the following covariates were used for pQTL mapping (either linear or tobit model): genotype PC1—PC5, sites, sex, age, BMI, smoking pack years and current smoker status (0/1). We took this approach based on an initial PC analysis of the biomarker data across subjects from both cohorts. The model for SPIROMICS, but not COPDGene, included a biomarker principal component (PC1). (S2 Fig). For COPDGene, the first biomarker principal component was highly correlated with the other covariates (sex, age, BMI, etc.). By contrast, in SPIROMICS, the first biomarker PC was not associated with any of the covariates, indicating that there was additional structure in the data that needed to be adjusted for by including biomarker PC1; subsequent PCs were not included because they were either associated with other covariates or explained only a relatively small percentage of the variability. All pQTL analysis was performed by either PLINK (v 1.9; http://pngu.mgh.harvard.edu/~purcell/plink/, for linear regression) or censReg function of R package censReg (for tobit model).
We conducted meta-analysis combining the results of SPIROMICS and COPDGene studies using Stouffer's Z-score method adjusting for direction of effect. Specifically, let Φ and Φ-1 be cumulative distribution function (CDF) and inverse CDF of standard normal distribution. Let β1 and β2 be the regression coefficients from COPDGene and SPIROMICS studies, respectively, and let p1 and p2 be the corresponding p-values from COPDGene and SPIROMICS studies, respectively. Then the combined Z-statistic and meta p-value weighted by the sample sizes of the respective study is .
where z1 = sign(β1)|Φ−1(p1/2)| and z2 = sign(β2)|Φ−1(p2/2)|. Then, the meta-analysis p-value is 2Φ(−|Z|).
Recursive conditioning
The set of independent pQTLs per analyte were identified using a forward regression approach. If K SNPs were associated with an analyte with p-values smaller than 10−8, meta-p-values were calculated for each of the K-1 SNPs conditioning on the top SNP identified from meta-analysis. The SNP with the smallest meta-p-value was considered as an independent pQTL if the p-value < 0.05/(K-1), where 0.05/(K-1) was the p-value threshold by Bonferroni correction. We applied this procedure iteratively until the smallest meta-p-value was larger than 0.05/T, where T is the number of remaining SNPs.
Effect of blood cell counts on pQTLs
We also evaluated whether the pQTLs would be significantly affected by the cellular composition of the blood. Complete cell counts were only available for the SPIROMICS cohort, so we repeated the pQTL analysis adding cell counts of neutrophil, lymphocyte, monocyte, eosinophil, basophil, red blood cells, and platelet as covariates in the models. For either all possible (SNP, analyte) pairs or only those pairs corresponding to significant pQTLs, the concordance between the pQTL p-values with and without blood cell counts as covariates were tested in SPIROMICS cohort, but not COPDGene, in which cell counts were not available.
Studying causal relations by assessing (conditional) dependence
We adopted an approach used in previous eQTL studies to infer causal relations of a trio of SNP, biomarker, and disease phenotype. We assume any associations between SNP genotype and protein levels or disease phenotypes implies a causal relation that SNP genotype alterations causes changes in protein levels or disease phenotype. This is assumption can be justified by Mendelian Randomization, which argues that the passing of DNA alleles to offspring can be considered as a randomized experiment and causal relations can be inferred from the randomized experiment. Such inference of causal relation by Mendelian Randomization is also consistent with our intuition that genetic variation causes molecular or phenotypic changes rather than vice versa. Given this assumption on the causal relation between SNP and biomarker/disease phenotypes, different models involving a SNP, a biomarker, and a disease phenotype can be distinguished because these models encode different types of conditional independence information, and thus have different likelihoods. This approach has been used in previous studies, implemented by comparing different models based on their likelihoods [22, 23]. Later more rigorous statistical arguments have been established to compare different types of causal relations by testing (conditional) dependence [24–27] or likelihood ratio test [28]. We adopted the approach of testing (conditional) dependence in our study.
We seek to classify the relations of a trio of SNP, biomarker, and disease phenotype into five categories: causal, reactive, independent, collide, and complete. Some trios may not fall into any of these categories and they are classified as other. A causal model (SNP → biomarker → disease) would suggest a SNP’s effect on disease is mediated by a biomarker, and thus conditioning on that biomarker, SNP genotype is independent with disease. A reactive model (SNP → disease → biomarker) would suggest that a SNP’s effect on a biomarker is mediated by disease, and thus conditioning on disease, SNP genotype is independent with biomarker. In an independent model (biomarker ← SNP → disease), a pQTL SNP affects biomarker and disease separately and given SNP genotype, disease is independent with biomarker. In a collide model (SNP → biomarker ← disease), the abundance of a biomarker is affected by a SNP as well as disease, and there is no direct relation between the SNP and disease; however, SNP genotype and disease are dependent with each other conditioning on the biomarker. The complete model allows all possible relations of the three variable and each of the aforementioned models can be derived from the complete model after adding certain constraints on dependence or conditional dependence relation. The “collide” relationship is well known in graphical model studies [29], however, previous eQTL studies did not explore this model because they focused only on SNPs associated with disease phenotypes.
To examine conditional dependence between a trio of SNP, biomarker, and disease phenotype, we performed a series of linear or logistic regressions with a continuous disease phenotype (emphysema or FEV1% predicted) or a binary disease phenotype (chronic bronchitis or exacerbations) as response variable, as well as additional linear regression or tobit regression with biomarker as response variable. We assessed the conditional dependence of two variables by testing the hypothesis whether a slope parameter was 0. More specifically, we obtained p-values for a particular test from both SPIROMICS and COPDGene studies and combined them using the same meta-analysis approach used to calculate pQTLs (see above). Finally, we say a slope parameter is different from 0 [i.e., (conditional) dependence] if the meta-p-value is smaller than 0.01. A specific causal relation can be inferred based on a set of conditional dependence testing results.
For our eQTL analysis, this series of regressions were also fit using the trio for SNP, haptoglobin biomarker and haptoglobin gene expression to determine the conditional relationships. In this case, the models were only fit on the 102 subjects from COPDGene having both biomarker and gene expression data.
Exploring pQTL features
pQTL features were characterized by: (1) Ensembl Variant Effect Predictor (VEP) [30]; (2) GWAS catalog [31]; and (3) comparison with gene expression QTLs (eQTLs) using subset of COPDGene blood microarrays [20, 32]. Details are provided below:
Variant effect predictor
We employed the Ensembl Variant Effect Predictor (VEP) tool to examine the consequences and locations of SNPs, using the “most severe consequence per variant” filter and genome version GRCh38.
GWAS catalog
The catalog of GWAS was obtained from NHGRI [31] containing 19,469 records (Feb 2015). For GWAS-pQTL SNP overlap, only unique entries by disease and publication were counted. Linkage disequilibrium (LD) information for the pQTL SNPs were obtained from LocusZoom [33] or HaploReg [34].
Defining relationship between pQTLs and eQTLs
Biomarkers were first mapped to gene identifiers and then to Affymetrix HGU133 plus 2 probe set symbols using Ensembl BioMart (www.ensembl.org/biomart). To examine biomarker-gene expression correlation, only the 80 biomarkers with <10% of measurements below the LLOQ were used. On average, these 80 biomarkers were encoded by genes with 2–3 Affymetrix probesets each. Overall, 199 probe sets were evaluated on n = 103 subjects with both gene expression and biomarker levels available for COPDGene. For the eQTL analysis, gene expression from all 131 NHW subjects from [32] were used with the same model as the pQTL analysis. For the 38 biomarkers with significant pQTL, 75 probesets corresponding to the genes encoding the biomarkers were used for a genome-wide eQTL analysis. The resulting eQTL were compared with the pQTL to identify if the same pQTL SNP is associated with both gene expression and protein levels for the biomarker. However, due to the loss of power with the smaller sample size for gene expression and to examine overall trends of variant effects for eQTL SNPs, we used a threshold of p-value < 10−7. This is larger than the pQTL threshold but would still correspond to the genome-wide significance threshold for local eQTL.
Results
Study subjects
Demographic and clinical characteristics of subjects from the SPIROMICS (n = 750) and COPDGene (n = 590) cohorts, including disease phenotypes, are shown (Table 1; S3 Fig). These NHW subjects were representative of NHWs in the parent cohorts (S2 Table).
Table 1. Demographics of participants by cohort at study entry*.
| SPIROMICS | COPDGene | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall | Current or former smokers without COPD | Mild or Moderate COPD | Severe COPD | Overall | Current or former smokers without COPD | Mild or Moderate COPD | Severe COPD |
| No. of participants | 750 | 206 | 367 | 177 | 590 | 242 | 150 | 198 |
| Age | 66.5 ± 7.9 | 65.1 ± 9.0 | 67.5 ± 7.4 | 66.1 ± 7.1 | 63.7 ±8.6 | 61.2 ± 8.4 | 65.1 ± 8.9 | 65.6 ± 7.8 |
| Male gender (%) | 408 (54) | 89 (43) | 222 (60) | 97 (55) | 304 (52) | 120 (50) | 75 (50) | 109 (55) |
| Current Smoker (%) | 230 (31) | 68 (33) | 123 (34) | 39 (22) | 142 (24) | 63 (26) | 46 (31) | 33 (17) |
| BMI | 27.7 ± 5.0 | 28.3 ± 5.1 | 27.7 ± 5.0 | 26.8 ± 4.8 | 28.3 ± 5.6 | 29.0 ± 5.3 | 28.8 ±5.6 | 27.1 ± 5.6 |
| Smoking pack-year | 52.6 ± 24.4 | 45.2 ± 23.1 | 55.6 ± 25.5 | 54.9 ± 21.7 | 47.5 ± 26.6 | 38.2 ± 22.6 | 51.3 ± 27.7 | 55.7 ± 27.2 |
| Emphysema (%) | 8.7 ± 10.3) | 1.9 ± 2.1 | 7.3 ± 7.4 | 19.6 ± 12.6 | 9.6 ± 11.8 | 2.3 ± 2.6 | 7.5 ±7.7 | 20.6 ±13.2 |
| FEV1% predicted | 71.0 ± 25.4 | 94.4 ± 13.7 | 75.0 ±15.8 | 35.7 ± 9.3 | 68.2 ± 29.8 | 98.1 ± 12.9 | 65.0 ±9.1 | 33.9 ± 10.3 |
| FEV1/FVC | 0.6 ±0.2 | 0.8 ±0.1 | 0.6 ±0.1 | 0.4 ±0.1 | 0.6 ± 0.2 | 0.8 ± 0.0 | 0.6 ± 0.1 | 0.4 ± 0.1 |
| Exacerbations in prior 12 mo. | 0.3 ± 0.8 | 0.2 ± 0.6 | 0.2 ± 0.7 | 0.6 ± 1.1 | 0.6 ± 1.2 | 0.2 ± 0.6 | 0.7 ± 1.2 | 1.1 ± 1.4 |
| Chronic Bronchitis (%) | 219 (29) | 46 (22) | 108 (29) | 65 (37) | 113 (19) | 26 (11) | 34 (23) | 53 (27) |
* means ± standard deviations.; BMI–body mass index; FEV1—forced expiratory volume at 1 second; FVC–forced vital capacity; Based on data retrieved August 19, 2013 (SPIROMICS Investigator Dataset Release INV3) and March 12, 2014 (COPDGene); see methods for definition of COPD severity.
Identification of SNPs associated with blood biomarkers
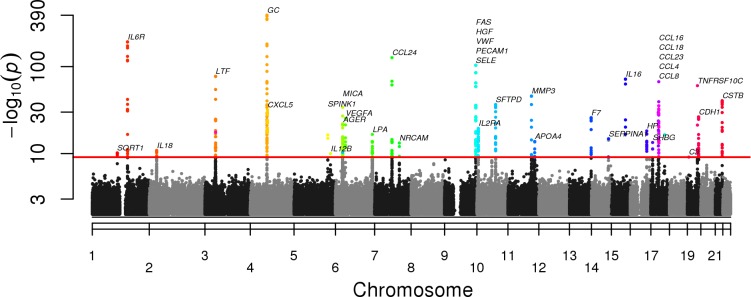
At a significance level of 8 X 10−10 we identified 290 pQTLs in the SPIROMICS cohort and 182 pQTLs in the COPDGene cohort (S3 Table). Many of the pQTLs SNPs were replicated between cohorts (Fig 1; S3 Table). Because of the similarity of the two studies in terms of sample size and subject characteristics as well as good replication of pQTLs between these two studies, we used a meta-analysis to increase power for finding pQTLs. Weighted meta-analysis identified 527 pQTL SNPs in 38 (44%) of the biomarkers (S4 Table) meeting genome-wide significance with Bonferroni correction for multiple testing of SNPs and biomarkers (P <8 X 10−10; Fig 2). The most significant independent pQTL SNP was rs7041 (P = 10−392) in GC (vitamin D binding protein—VDBP) on chromosome 4. Thirty-seven other biomarkers had significant pQTL SNPs (Table 2); corresponding Manhattan plots, Q-Q plots, and LocusZoom plots are shown for each individual analyte that had an associated pQTL (S4 Fig). Two or more independent pQTL SNPs were identified in 26 of 38 biomarkers using recursive conditioning (S5 Table).
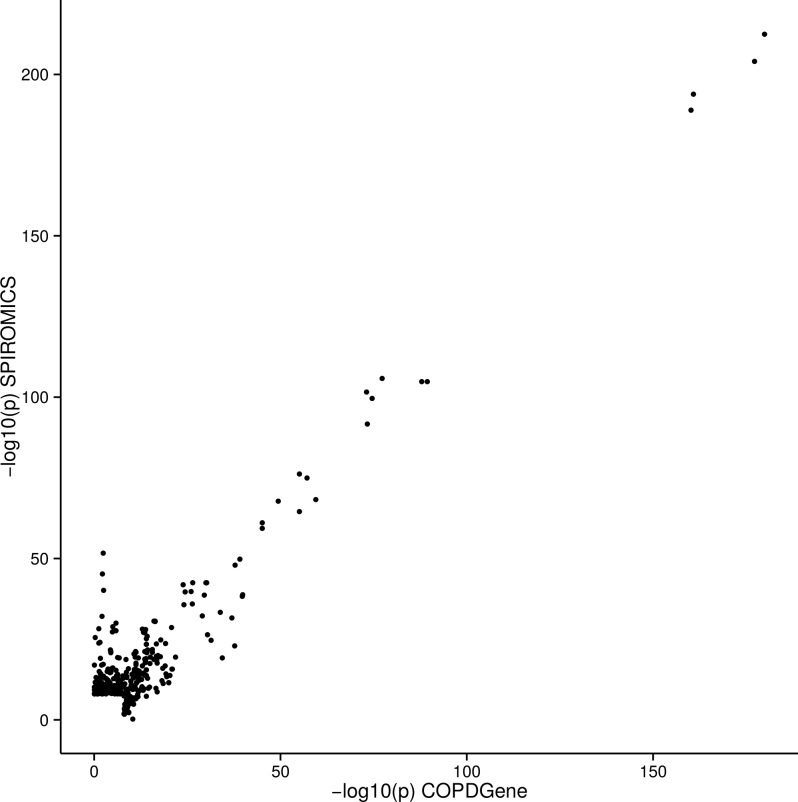
Fig 1. Comparison of–log10 p values for pQTL SNPs in SPIROMICS and COPDGene cohorts.
The–log10(P value) of each pQTL SNP is plotted on the x-axis for COPDGene and the y-axis for SPIROMICS. 164 of 182 significant pQTLs in COPDGene were replicated in SPIROMICS at a P < (0.05/182 to correct for multiple tests). 209 of 290 significant pQTLs in SPIROMICS were replicated in COPDGene at a P < (0.05/290 to correct for multiple tests). See also S3 Table.
Fig 2. Genome wide associations between single nucleotide polymorphisms (SNPs) and blood biomarkers.
Combined Manhattan plots show pQTL SNPs by chromosomal location for 38 biomarkers with at least one SNP significant at genome wide significance after adjustment for multiple testing (red line). The -10logP values are shown using results from a meta-analysis of both SPIROMICS and COPDGene SNPs. The abbreviation for the biomarker associated with the pQTL SNP can be found in S1 Table.
Table 2. Summary of pQTLs by measured biomarker.
| Analyte gene | Number of pQTLs¶ | Top pQTL SNP† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte protein name {alternative name(s)}‡ | Name | Chr | Local | Dis-tant | Multiple Independent | rs# | MAF SPIROMICS | MAF COPDGene |
P-value | Chr | Novelƪ | Function |
| Advanced glycosylation end product-specific receptor {RAGE} | AGER | 6 | 10 | rs2070600† | 0.05 | 0.04 | 7 X 10−22 | 6 | GWAS | mis | ||
| Apolipoprotein A-IV {Apo A-IV} | APOA4 | 11 | 10 | yes | rs4938353 | 0.19 | 0.18 | 4 X 10−14 | 11 | LD-GWAS | 3' | |
| Complement component 3 | C3 | 19 | 1 | rs2230203 | 0.20 | 0.18 | 1 X 10−9 | 19 | Novel | Syn | ||
| Chemokine (C-C motif) ligand 16 (pulmonary and activation-regulated) | CCL16 | 17 | 6 | yes | rs11080369 | 0.06 | 0.07 | 8 X 10−67 | 17 | Novel | NA | |
| C-C motif chemokine 18 | CCL18 | 17 | 7 | yes | rs854469 | 0.13 | 0.13 | 2 X 10−35 | 17 | Novel | NA | |
| Chemokine (C-C motif) ligand 23 {Myeloid Progenitor Inhibitory Factor 1; MPIF-1} | CCL23 | 17 | 11 | yes | rs1617208 | 0.18 | 0.19 | 5 X 10−29 | 17 | Novel | NA | |
| Chemokine (C-C motif) ligand 24 {Eotaxin-2} | CCL24 | 7 | 14 | yes | rs10755885 | 0.09 | 0.10 | 1 X 10−126 | 7 | Novel | up | |
| Chemokine (C-C motif) ligand 4 {Macrophage Inflammatory Protein-1 ß; MIP-1 ß} | CCL4 | 17 | 4 | 1 | yes | rs3213636 | 0.20 | 0.19 | 6 X 10−21 | 17 | Novel | NA |
| Chemokine (C-C motif) ligand 8 {Monocyte Chemotactic Protein 2; MCP-2} | CCL8 | 17 | 7 | yes | rs3848464 | 0.10 | 0.10 | 1 X 10−29 | 17 | Novel | inter | |
| Cadherin-1 {E-cadherin (epithelial)} | CDH1 | 16 | 29 | yes | rs516246 | 0.50 | 0.48 | 4 X 10−27 | 19 | GWAS | int | |
| Cystatin-B | CSTB | 21 | 24 | yes | rs1041456 | 0.42 | 0.43 | 9 X 10−42 | 21 | Novel | up | |
| C-X-C motif chemokine 5 {Epithelial-Derived Neutrophil-Activating Protein 78; ENA-78)} | CXCL5 | 4 | 14 | rs425535 | 0.12 | 0.12 | 1 X 10−33 | 4 | Novel | syn | ||
| Coagulation factor VII | F7 | 13 | 12 | yes | rs10665 | 0.12 | 0.13 | 1 X 10−26 | 13 | GWAS | 3' | |
| Tumor necrosis factor receptor superfamily member 6 {FASLG Receptor; CD95} | FAS | 10 | 6 | yes | rs687289 | 0.31 | 0.35 | 1 X 10−23 | 9 | GWAS | int | |
| Vitamin D-binding protein | GC | 4 | 57 | yes | rs7041† | 0.42 | 0.44 | 1 X 10−389 | 4 | GWAS | NA | |
| Hepatocyte growth factor | HGF | 7 | 15 | yes | rs687289 | 0.31 | 0.35 | 3 X 10−43 | 9 | GWAS | int | |
| Haptoglobin | HP | 16 | 11 | rs1424241 | 0.17 | 0.18 | 6 X 10−19 | 16 | Novel | int | ||
| Interleukin-12 subunit p40 {IL-12p40} | IL12B | 5 | 1 | yes | rs10045431 | 0.29 | 0.28 | 2 X 10−10 | 5 | GWAS | inter | |
| Interleukin-16 | IL16 | 15 | 7 | rs1803275 | 0.09 | 0.08 | 2 X 10−72 | 15 | Novel | syn | ||
| Interleukin-18 | IL18 | 11 | 6 | rs7577696 | 0.40 | 0.41 | 9 X 10−12 | 2 | GWAS | inter | ||
| Interleukin-23A | IL23A | 12 | 1 | yes | rs10665 | 0.12 | 0.13 | 5 X 10−10 | 13 | GWAS | 3' | |
| Interleukin-2 receptor subunit alpha | IL2RA | 10 | 26 | yes | rs12722489 | 0.14 | 0.16 | 2 X 10−20 | 10 | GWAS | int | |
| Interleukin-6 receptor subunit alpha | IL6R | 1 | 26 | yes | rs8192284 | 0.40 | 0.40 | 9 X 10−193 | 1 | GWAS | NA | |
| Apolipoprotein(a) | LPA | 6 | 19 | yes | rs9457925 | 0.02 | 0.02 | 5 X 10−18 | 6 | LD-GWAS | int | |
| Lactotransferrin | LTF | 3 | 23 | rs11707471 | 0.32 | 0.31 | 2 X 10−77 | 3 | Novel | int | ||
| MHC class I polypeptide-related sequence A | MICA | 6 | 60 | rs2256175 | 0.48 | 0.47 | 9 X 10−34 | 6 | Novel | int | ||
| Stromelysin-1 {Matrix Metalloproteinase-3; MMP-3} | MMP3 | 11 | 10 | rs645419 | 0.49 | 0.49 | 5 X 10−47 | 11 | LD-GWAS | up | ||
| Neuronal cell adhesion molecule | NRCAM | 7 | 3 | yes | rs10487851 | 0.30 | 0.30 | 8 X 10−14 | 7 | Novel | int | |
| Platelet endothelial cell adhesion molecule | PECAM1 | 17 | 1 | 18 | yes | rs507666 | 0.18 | 0.20 | 3 X 10−57 | 9 | GWAS | int |
| E-selectin | SELE | 1 | 29 | yes | rs507666 | 0.18 | 0.20 | 4 X 10−104 | 9 | GWAS | int | |
| Alpha-1-antitrypsin | SERPINA1 | 14 | 3 | yes | rs4905179 | 0.20 | 0.21 | 2 X 10−15 | 14 | LD-GWAS | inter | |
| Pulmonary surfactant-associated protein D {SP-D} | SFTPD | 10 | 22 | 1 | yes | rs2146192 | 0.11 | 0.10 | 4 X 10−37 | 10 | Novel | int |
| Sex hormone-binding globulin | SHBG | 17 | 3 | yes | rs727428 | 0.42 | 0.42 | 3 X 10−14 | 17 | GWAS | down | |
| Sortilin | SORT1 | 1 | 3 | rs7528419 | 0.22 | 0.22 | 1 X 10−10 | 1 | GWAS | 3' | ||
| Pancreatic secretory trypsin inhibitor {TATI} | SPINK1 | 5 | 2 | rs6580502 | 0.41 | 0.40 | 2 X 10−16 | 5 | Novel | int | ||
| Tumor necrosis factor receptor superfamily member 10C {TNF-Related Apoptosis-Inducing Ligand Receptor 3; TRAIL-R3)} | TNFRSF10C | 8 | 5 | rs4760 | 0.16 | 0.16 | 1 X 10−60 | 19 | Novel | mis | ||
| Vascular endothelial growth factor A | VEGFA | 6 | 6 | rs7767396 | 0.47 | 0.47 | 5 X 10−26 | 6 | LD-GWAS | inter | ||
| von Willebrand factor | VWF | 12 | 13 | rs687289 | 0.31 | 0.35 | 5 X 10−36 | 9 | GWAS | int | ||
†indicates that the analyte associated with the SNP has been associated with obstructive lung disease or emphysema (PubMed accession 23947473, 23267696 for AGER and 24857306, 21228423, 19996341 for GC).
¶Local SNPs are defined as within 1 Mb of the analyte gene; distant (trans) SNPs are denoted by red.
‡ Protein names are UniProKB/Swiss-Prot suggested names.
ƪNovel SNPs are defined as not listed in the Catalog of Published Genome-Wide Association Studies (GWAS) as of May 8, 2015, not listed in LD with any GWAS catalog SNPs (LD-GWAS), and not found on PubMed Search associated with analyte levels or disease phenotypes (see S3 Table); not all non-novel pQTL SNPs are previously linked to analyte levels (some are disease associations). Multiple Independent pQTL SNPs are listed in S4 Table. SPIROMICS/COPDGene. Chr = chromosome. Functional annotation of SNPs (variant effect predictor): up (upstream gene variant); 5’ (5’ untranslated region); syn (synonymous variant); mis (missense); spl (splice region); int (intron); exon (non-coding exon variant); mis (missense); 3’ (3’ untranslated region); up (upstream), down (downstream); inter (intergenetic).
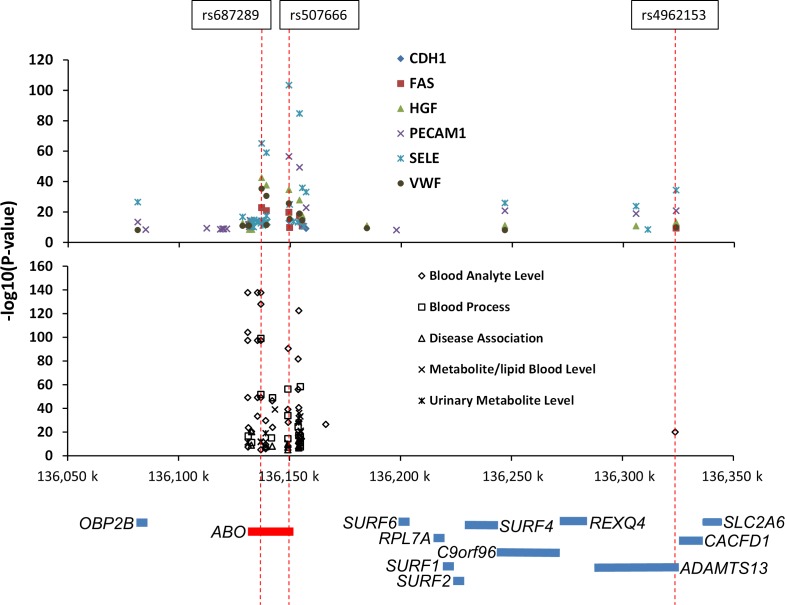
To determine whether pQTLs SNPs were local (cis) or distant (trans), we examined proximity of each SNP to its assigned biomarker gene. The majority (76%) of pQTL SNPs were local (S5 Fig; S4 Table). However, distant pQTLs were observed for eleven biomarkers, and nine biomarkers had a distant pQTL SNP as their most significant pQTL (S2 Table). Five biomarkers had their most significant pQTL SNPs (either rs687289 or rs507666) in the ABO blood group locus on chromosome 9, which encodes alpha 1-3-N-acetylgalactosaminyltransferase, a major determinant of ABO blood type. This SNP is in the same genetic region as other QTLs and disease associations reported from a wide variety of a sources, including metabolites from the urine (Fig 3). An additional region on chromosome 19 contained distant pQTLs for more than one biomarker (S4 Table). The pQTLs represented SNPs with a broad range of minor allele frequencies (MAF) with distributions of MAFs of pQTL SNPs similar to all SNPs studied (S6 Fig).
Fig 3. SNPs in ABO are the strongly associated with many blood biomarker levels as well as other non-blood analyte measurements.
Trans pQTLs in the ABO region (shown in schematic form below the plots) were common in this study (top panel) and in published studies (GWAS Catalog). The rs687289 or rs507666 SNPs in the ABO blood group locus on chromosome 9, which encodes alpha 1-3-N-acetylgalactosaminyltransferase, are a major determinant of ABO blood type. In this study, these SNPs were the strongest pQTLs for 6 blood biomarkers, all distant (trans) from their biomarker genes. Other biologic features (such as clotting time), metabolites (proteins, lipids, hormones), and urinary features have been noted to have strong association with this locus (see [10, 35–73]).
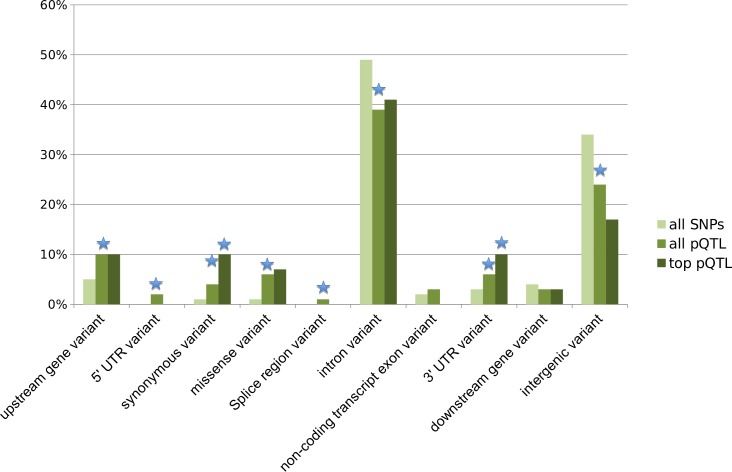
Using VEP, we found intronic SNPs to be the most represented pQTL SNP category (43%), followed by intergenic variants (22%); however, missense variants showed the most significant enrichment (P<10−12) compared to all SNPs on the genotyping platform (Fig 4). Importantly, pQTLs were robust and concordant across the two source cohorts (S4 Table; S7 Fig).
Fig 4. Consequences of pQTL SNPs.
We examined the most significant SNP for each biomarker (top pQTL) and all 590 significant pQTL SNPs (all pQTLs), compared to all 664,913 SNPs (all SNPs) used for testing with the Ensemble Variant Effect Predictor (release 78). Upstream refers to within 5 kb and downstream refers to more than 5kb distant. All pQTL SNPs were enriched for missense, synonymous, upstream and 3′ UTR variants compared to all SNPs tested on the genotyping platform, while pQTL SNPs occurred less frequently in introns and intergenic regions (binomial test p-value < 0.05 starred in blue). Most of these variant classes showed additional enrichment or reduction for the top pQTL SNPs.
Biologic significance of pQTL SNPs
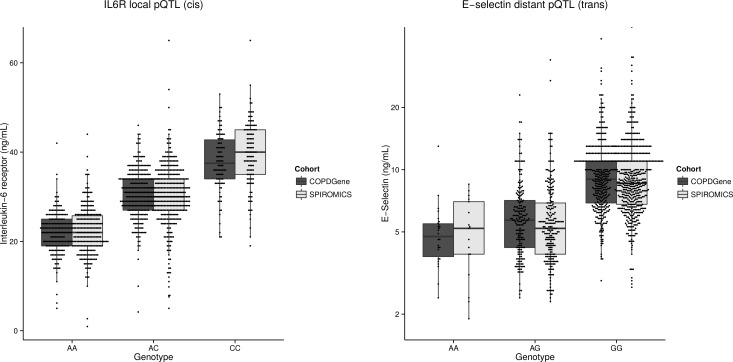
Nine biomarkers had at least 10% of their variance explained by a single pQTL SNP in both SPIROMICS and COPDGene (Fig 5). For example, a single local pQTL SNP (rs8192284 SNP in IL6R) explained 45% of variance of plasma IL6R in SPIROMICS and 50% of this variance in COPDGene, and a single distant pQTL SNP (rs507666 SNP in ABO) explained 25% of variance of blood E-selectin (SELE) in SPIROMICS and 27% of variance in COPDGene (Fig 6). In many cases, pQTL SNPs explained more variance in the quantitative biomarker than did clinical covariates.
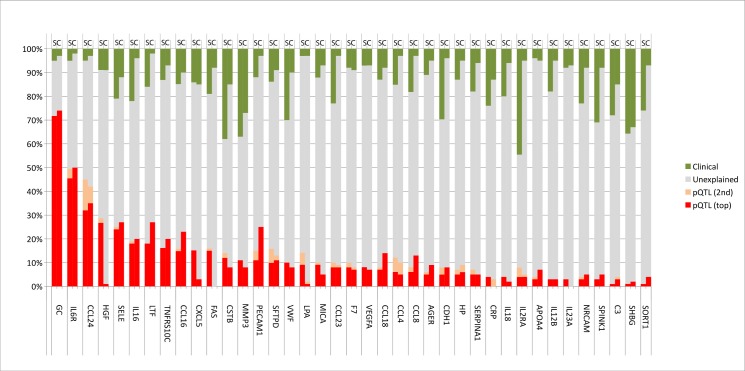
Fig 5. Blood biomarker variance explained by top two pQTLs SNPs and clinical covariates.
The percent variation for 39 blood biomarkers explained by clinical (green) top pQTL SNP (red), second top independent pQTL SNP (peach), other unknown factors (grey). Clinical factors include age, gender, body mass index, smoking status, and principal components of ancestral genetic markers as described in the methods. The analysis includes subjects from SPIROMICS (S) and COPDGene (C) cohorts. TNRF (TNF-Related Apoptosis-Inducing Ligand Receptor 3 (TRAIL-R3)); PCAM (Platelet endothelial cell adhesion molecule (PECAM-1)); SRP1 (Alpha-1-Antitrypsin (alpha-1 (AAT)); NRC (Neuronal Cell Adhesion Molecule (Nr-CAM)); SPK (Pancreatic secretory trypsin inhibitor (TATI)); SRT1 (Sortilin); other abbreviations are listed in Table 1 and S2 Table.
Fig 6. Examples of biomarker pQTL SNPs.
Plasma levels of IL6R (A) and E-selectin (B) are strongly influenced by pQTL SNPs (P = 10−193 and P = 4 X 10−104). The pQTL SNP for IL6R is on chromosome 4, which is local (cis) to IL6R, the gene coding for its protein. The pQTL SNP for E-selectin protein is on chromosome 9, which is distant (trans) from SELE (chromosome 1), the gene coding for its protein. This pQTL SNP is in the ABO locus, which encodes alpha 1-3-N-acetylgalactosaminyltransferase.
To assess the novelty of these pQTL SNPs, we cross-referenced the unique 478 pQTL SNPs we identified with SNPs associated with any published GWAS based on NHGRI GWAS catalog, including those related to COPD phenotypes or pulmonary function (n = 242). By these criteria, 90% of pQTL SNPs were novel (P < 10−34; S4 Table), even after removing SNPs in linkage disequilibrium [280 significant pQTL SNPs remained and, of those, 29 (10.4%) overlapped with at least one GWAS report (P < 10−20)].
We next evaluated whether pQTL SNPs were also eQTLs, by utilizing an overlapping dataset of peripheral blood mononuclear cell gene expression from COPDGene [32]. In this analysis, only COPDGene data were available, so results are limited to this dataset. Although there were more positive correlations between gene expression and protein levels than expected by chance (sign test P = 0.0009), the overall magnitudes of such correlations were low (S8 Fig), and there was little overlap between pQTL and eQTL SNPs (Fig 7; S6 Table). Furthermore, as previously shown, although both eQTL and pQTL SNPs were more likely to be intronic [20], among those that were not, pQTL SNPs were more likely to be in 5′ or 3′ untranslated region or to be missense SNPs, compared to eQTL SNPs (S9 Fig). Only one biomarker (haptoglobin, corresponding to gene HP) had pQTL SNPs that were also eQTL SNPs, and this is the only case where regression modeling suggested that measured biomarker levels are mediated by gene expression (S6 Table).
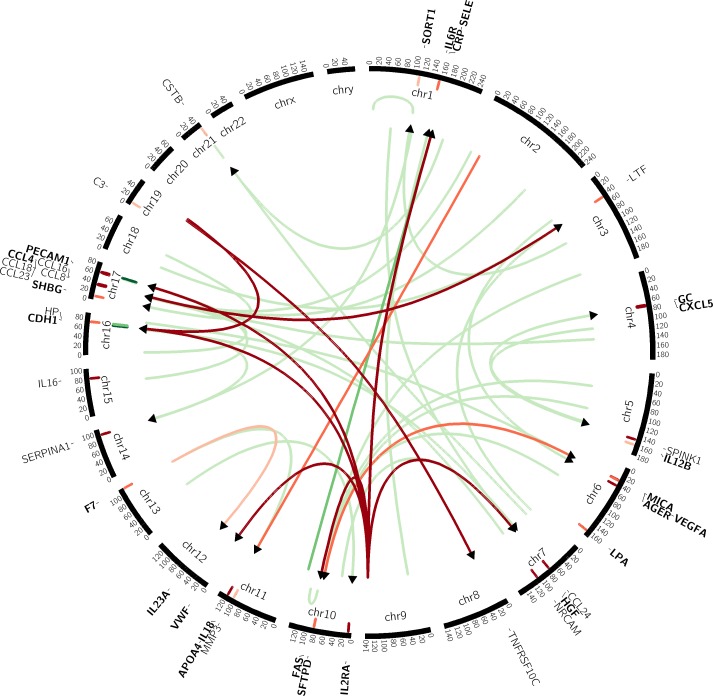
Fig 7. Circos plot showing distant (trans) pQTLs and their relationships to eQTL SNPs.
The arrows in the inner circle represent pQTL SNPs significantly associated (beginning of arrow) with biomarker (end of arrow). Biomarker abbreviations (see text for full list) are shown on the outer ring. Local (cis) pQTL SNPs are shown as hash marks adjacent to biomarker gene location. The color of represents significance of association. Red lines are associations between genes. The thinnest, darkest red lines signify associations with significance of P = 10−12, and the lines become slightly thicker and darker at the significance levels of P = 10−10 and P = 10−8. The green lines signify eQTL associations. The cutoffs for line thickness and darkness for the green lines are P = 10−7 and P = 10−6. The only eQTL association with significance P < 10−8 were local, near the HP and PECAM1 genes.
Given that QTLs may be dependent upon the cellular/tissue-specific expression [74], we examined whether the pQTLs would be significantly affected by the cellular composition of the blood by repeating the pQTL analysis adding cell counts (red blood cells, neutrophils, lymphocytes, basophils, monocytes, eosinophils, and platelets) as covariates in the models. For either all possible SNPs or only significant pQTL SNPs, the correlation between the p-values of the pQTLs with and without blood cell counts added as covariates was > 0.985, indicating that the pQTLs were not markedly dependent upon blood cell type composition (S10 Fig).
A recent report suggests that monoclonal antibodies for vitamin D binding protein may preferentially recognize a selected protein isoform [75] caused by the rs7041 pQTL, which is a missense mutation causing aspartic acid to glutamic acid change at position 432 (D432E). Therefore we used a polyclonal antibody to compare to measurements to the monoclonal assay used on the RBM platform in a subset of SPIROMICS subjects. Indeed, the measurements using the monoclonal antibody were significantly lower for the TT genotype compared to the GG genotype (P < 0.001), suggesting that measurements using the monoclonal antibody assay detected the D432E protein isoform less well compared to the polyclonal assay (S11 Fig).
The relationship between pQTL SNPs and COPD disease phenotypes
With SNPs, biomarker levels, and disease phenotypes all available for both cohorts, statistical modeling could be used to infer the relationships among these three data types employing methods previously applied to eQTL-gene expression-phenotype relationships [22–27]. We chose four clinically important COPD phenotypes [airflow obstruction (FEV1% predicted), emphysema, chronic bronchitis, and a history of exacerbations] and applied regression models adjusted for covariates and PCs [22, 26]. We categorized the relationships of all 2,108 trios of SNP, biomarker, and disease phenotype (527 pQTL SNP/biomarker pairs and four disease phenotypes) into five categories, based on (conditional) dependence testing (Fig 8 and full results supporting Fig 8, including regression coefficients, are in S7 Table). Results for biomarker associations to disease phenotype for pQTL SNPs are also provided (S8 Table).
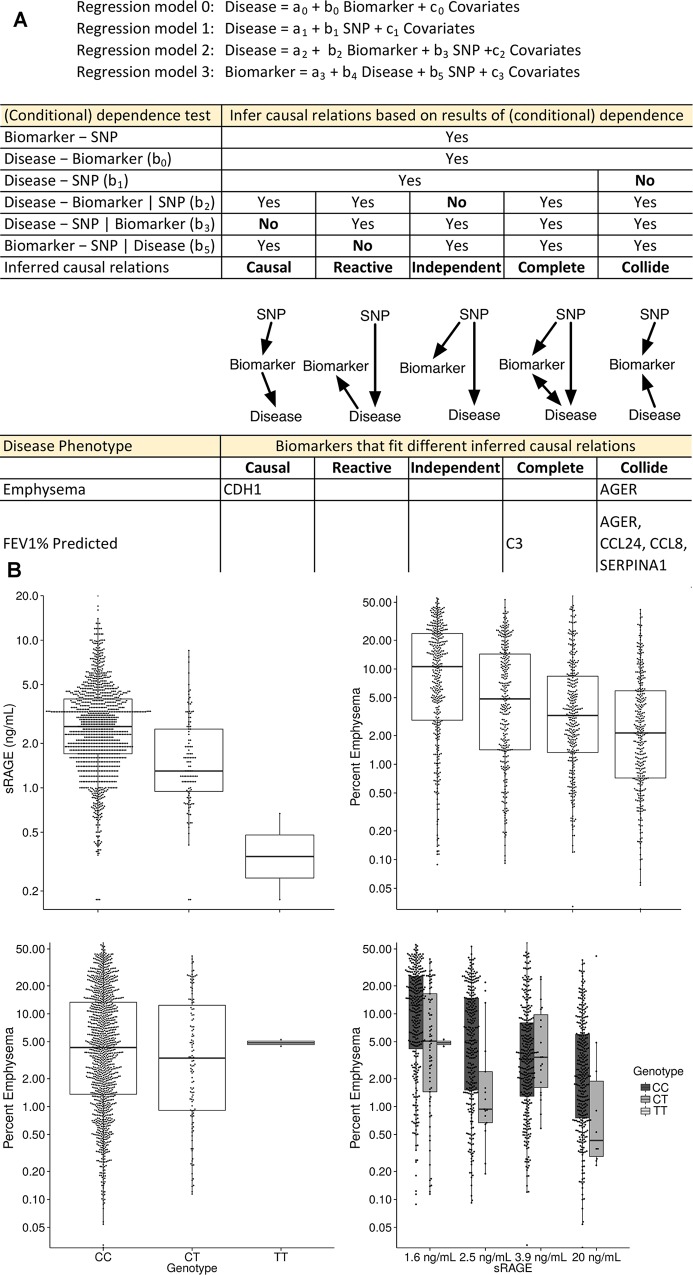
Fig 8. Clinical and biologic significance of pQTL SNPs.
(A) Biomarker pQTL SNPs were tested for association with four different COPD disease phenotypes: emphysema, airflow limitation (FEV1%), chronic bronchitis, and exacerbations using four different statistical regression models to infer the causal relations of causal, reactive, independent, complete or collide. A complete listing of pQTL SNPs disease association p-values for both cohorts can be found in S8 Table. Note that testing b2 = 0 and b4 = 0 are equivalent because in both cases, we are testing whether the disease and biomarker is conditionally dependent given SNP. Therefore, we only examined b2 in our analysis. No significant results were obtained for chronic bronchitis or exacerbations and so these two phenotypes are not shown. (B) The T allele of rs2070600 is associated with lower plasma levels of sRAGE and (C) lower plasma levels of sRAGE (shown by sRAGE quartile) are associated with more emphysema on quantitative CT scan (model 0); (D) the T allele is not clearly associated with emphysema when considering only the SNP-disease association (model 1); however, (E) the T allele is associated with less emphysema within each biomarker quartile (model 2), and the SNP fits the collide model.
Significant evidence for inferred causal, complete, or collide relationships were found for emphysema and airflow obstruction for six biomarkers, with AGER represented by the same model in both phenotypes (Fig 8). In all of these cases, the direction of the regression coefficients were the same between SPIROMICS and COPDGene (S7 Table). By contrast, no significant relationships were found for chronic bronchitis or exacerbations. In the case of the collide model, the association between pQTL SNP and disease phenotype is strengthened given the biomarker, and thus inclusion of pQTL SNP information in biomarker-disease association testing will add predictive value. An example is AGER, which is classified as the “collide” model for the phenotype of emphysema. Including both AGER levels and its top pQTL SNP improved the explanation of variance (R2) for emphysema to 40%, compared to just 30% for the biomarker alone, and 22% when only clinical covariates were used.
Discussion
In this study we identified hundreds of novel SNPs significantly associated with nearly 40% of blood biomarkers commonly used in both pulmonary and non-pulmonary clinical research. For many biomarkers, a single pQTL SNP accounted for a large percentage of measured variance. We demonstrated that pQTLs provide unique information compared to eQTLs and that inclusion of pQTL SNPs can improve explanation of variance when added to clinical covariates in statistical models, e.g., sRAGE and emphysema. Although the subjects in this study were recruited for COPD phenotypes, many of the pQTLs identified and the biomarkers studied have been associated with other diseases or traits, suggesting that the pQTL-biomarker relationships reported here are broadly relevant to human pathophysiology. Furthermore, the pQTL-biomarker-disease phenotype relationship is frequently not a simple SNP → gene expression → biomarker → disease phenotype association. These findings suggest that modeling with inclusion of measurements from multiple omics technologies may be needed to optimize precision medicine predictions.
A significant finding in this study is the number of distant pQTLs associated with the ABO locus (commonly associated with ABO blood group). PQTLs at the ABO locus were the strongest genetic association among six proteins encoded by genes on six different chromosomes. This ABO region, along with the FUT2 gene (galactoside 2-alpha-L-fucosyltransferase 2), which contained pQTLs for CDH1, was found to overlap with a growing number of previously reported QTLs for a variety of blood analytes, blood processes (such as clotting time), metabolites, lipids, and even urinary metabolites (Fig 3). The most likely explanation is these two loci affect enzymes that post-translationally modify multiple proteins leading to impaired protein function, half-life, or detection. Interestingly, older literature, prior to extensive genotyping and biomarker analysis, has reported association between ABO blood group and COPD [76] and has been associated with other diseases such as goiter [77] and hepatitis [78] in the candidate gene era. The extensive number of associations now reported at the ABO blood group from a wide variety of studies suggests that greater attention should be paid to ABO status for blood biomarker studies.
Much of the recent effort to identify genetic variants and genomic signatures associated with clinical disease has extensively used eQTLs to understand the function of loci identified in GWAS, including for COPD [4, 79–81]. We demonstrate a clear distinction between known eQTLs and pQTLs, which is consistent with previous work that compared variants associated with three different levels of gene regulation (transcription, translation and protein levels) in a study of 62 HapMap Yoruba (Ibadan, Nigeria) lymphoblastoid cell lines (LCLs) [5]. The authors used SILAC mass spectrometry to quantitate proteins and showed that only 35% of the pQTL variants overlapped with eQTLs using RNAseq. Some of the variance in protein expression was due to ribosomal occupation (ribosomal profiling); however, there were many pQTLs in which there was little variation in the mRNA or ribosomal profiling, suggesting that post-translational events may be responsible for differences in protein abundance. Similar to what we report, this is supported by the observation that the pQTLs are significantly enriched in protein coding (missense) and potential translational regulation (e.g., 3’ UTR) regions. They hypothesize this may be due to differences in protein degradation; however one cannot exclude that the peptide variants may be differentially measured with mass spectrometry, or that there may be altered biomarker stability, secretion rates, or processing/release from the cell surface. Another limitation of this study is that they only considered genetic variants within a 20-kb window around the corresponding gene; however, we found a significant number of pQTL SNPs mapped outside of this region. Another study of 441 transcription factors and signaling proteins in the Yoruban LCLs found that many pQTLs were not associated with gene expression and were also distant from the corresponding gene [82]. These studies highlight the general need to include protein expression in large-scale population variation studies such as GTEx to better understand the relationship between genome and protein in humans. Although such efforts are ongoing on a small scale (e.g. Chromosome-Centric Human Proteome Project [83]), our results imply these efforts can also be incorporated cost-effectively into large existing clinical cohorts.
These findings will be useful for GWAS and biomarker studies of other diseases. For instance, we identified novel pQTL SNPs explaining greater than 25% of variance in blood proteins such as interleukin 6 receptor, eotaxin-2, and E-selectin, which could be useful in studies of asthma and of non-pulmonary diseases. The sRAGE-emphysema example demonstrates that the application of causal modeling can provide new insights to the relationship between SNP, measured biomarker levels, and disease phenotypes. Additionally, this example demonstrates how predictive models of disease phenotype can be improved by adding pQTL information.
Furthermore, evaluating all possible statistical relationships among pQTL SNPs, biomarkers, and disease phenotypes suggests that many pQTL SNP effects may not be causally mediated directly through measured biomarkers. For instance, the minor allele rs2070600 SNP in AGER is associated with lower sRAGE in blood; COPD severity and emphysema extent have also been negatively associated with lower blood sRAGE concentrations in cross-sectional studies [13, 14]. Paradoxically, however, in large GWAS studies, the minor allele of rs2070600 is associated with reduced COPD severity and reduced emphysema [80, 81] suggesting potentially opposite effects of the SNP. Indeed, our evidence points to a “collide” relationship; however, given the previous published large scale genetic association studies have shown that rs2070600 is associated with COPD and emphysema, it is likely that this study is underpowered to distinguish between the “collide” and the “complete” model, which can be distinguished by a statistically significant association between the pQTL SNP and disease phenotype. Nevertheless, the association between pQTL SNP and disease phenotype becomes much stronger given the biomarker, which implies the collide relation. Regardless of whether rs2070600 is “collide” or “complete”, it is a missense SNP that causes a G82S amino acid change and thus illustrates the enrichment of coding SNPs in pQTL analysis. The mechanism by which rs2070600 causes disease is unknown, but the resultant amino acid substitution may block shedding of this cell surface receptor, reducing blood levels but at the same time improving sensing of damage-associated molecular pattern molecules, with a net protective effect [84]. However, once emphysema progresses, the source of sRAGE in the blood (the alveolar cells) is reduced, so that emphysema progression would be manifested by reduced sRAGE levels.
Several other relationships identified are also worth considering. For example, we identified evidence for the “collide” relationship for rs926144, an intergenic SNP in SERPINA1 (alpha-1-antitrypsin; AAT), a protein whose normal function is linked directly to the development of emphysema. Although we find strong pQTL SNPs for SERPINA1, and we see a relationship between COPD and SERPINA1 levels, we see no statistically significant evidence that pQTL SNPs associate directly with disease. This is similar to what authors of an GWAS of AAT serum levels have recently reported in this journal [85], in which they identified strong serum AAT pQTLs, but their association with lung function was driven by the rare disease variants (PiSZ and pZZ, who were excluded from SPIROMICS and COPDGene). Since SERPINA1 is produced by the liver and is well-known as marker of systemic inflammation, an established feature of COPD, this would support the finding that common SNPs may not be representative of the known disease-causing variants, which are rare, and that both non-disease causing variants and the disease itself may be associated with changes in biomarker levels.
We found that a “complete” model was suggested for the Complement Factor 3 (C3) pQTL SNP rs2230203. In a study of 111 subjects with COPD and 111 matched controls, blood C3 was noted to be lower in COPD subjects [86]. Similarly in a more recent study of 15 COPD subjects and 15 matched controls serum C3 was lower in COPD subjects [87]. Our findings confirm the relationship between C3 and COPD and emphysema and further suggest that it is partly mediated through C3 genetic variants. Although the rs2230203 variant is in the coding region of C3, it is a synonymous variant and was the only pQTL we identified for C3. The variant might affect protein levels though siRNA binding or other pre-translational mechanisms, but mechanistic studies will be necessary to confirm this.
As a final example, the “causal” relationship suggested for CDH1 (E-cadherin) for both emphysema and FEV1% predicted is also intriguing at a mechanistic level. The CDH1 pQTL SNPs are distant (trans) and are located in FUT2, which codes for a fucosyltransferase that, along with ABO, determines the expression of distinct blood group antigens. Evidence for a role of CDH1 and COPD is growing [13, 88, 89], yet the underlying mechanisms are not entirely clear. Our results suggest that future studies should focus on a direct role of CDH1 in the pathogenesis of disease.
Strengths of this study include the large number of subjects and the inclusion of validation cohorts. However, there are some limitations. Although it is one of the largest biomarker-GWAS studies reported, 1,340 subjects is still small compared to clinical GWAS studies, thus we are likely underpowered to detect some of the SNP-disease phenotype associations. Thus, we cannot say for certain, for example, that a causal or collide model might not actually be a complete model (e.g. for rs207060 in AGER with sRAGE). Second, because we identified distinct and independent pQTL SNPs for some biomarkers, there may be multiple mechanisms by which pQTL biomarkers mediate SNP-biomarker-disease phenotype interactions. Proving the validity of the causal inference models will require detailed mechanistic studies at both a genomic and proteomic level. Additionally, like nearly all biomarker assays, we used antibody based detection methods to measure biomarkers. Since antibodies recognize specific epitopes on proteins, it is possible that our pQTL may detect a specific isoforms of a protein rather than total protein. This has recently been suggested, but not proven, as an explanation for the strong genetic (racial) associations observed for vitamin D binding protein and the cis-SNP rs7041 (Asp432Glu). As we have and others have shown for vitamin D binding protein [75], assays that use polyclonal antibodies compared to the monoclonal sandwich immunoassay (R&D Systems) may overcome this limitation. Another example in the literature is a pQTL identified for TNF-alpha was not replicated when a different assay was applied to the same samples [10]. However, similar pQTLs for plasma proteins such as HP, SERPINA1, C3, APOE, and AHSG were identified using mass spectrometry [90] and for IL6R, F7, and others using aptamer-based detection [91], suggesting many pQTLs we identified were not platform specific. Thus, knowing that antibody used in biomarker measurement may preferentially detect a specific isoform of a protein does not discount its importance, particularly if the pQTL SNP has also been associated with the disease phenotype in genetic association studies, as is the case with vitamin D binding protein, sRAGE, and several other pQTL SNPs described in this study (see Table 2). Thus, investigators who conduct biomarker studies need to consider the possibility that genotype plays a role when measuring blood biomarkers.
An additional limitation of the study is using a candidate panel of 114 biomarkers that are overrepresented for inflammation and lung proteins. At the time, this was state of the art for large scale human studies; however, in the future there will be high-throughput, 1000+ biomarker panels that may be used such as SomaScan (Somalogic, Boulder, Colorado). Other limitations of this study include that it was limited to subjects over 45 years of age and only NHW subjects. Future studies should include other populations and the types of variants assessed, e.g., rare variants. Finally, due to the nature of the available data, evaluating quantitative change in biomarkers with disease progression was not conducted, but would be expected to enhance understanding of disease mechanisms in future studies.
In summary, this large scale, dual-cohort, combined GWAS and biomarker study represents a powerful approach to combine different omics data sets to better understand complex diseases such as COPD. We replicated some previously reported pQTL associations and discovered a large number of novel pQTLs, including distant pQTLs, which many studies are poorly powered to detect. Integration of pQTL genotypes with biomarker measurements will improve the precision of disease prediction for some clinically relevant phenotypes, and improve the mechanistic understanding of others, thus increasing the implementation of targeted clinical care.
Supporting Information
LLOQ = Lower Limit of Quantification. All COPDGene samples were P100 plasma. Green cells under “BIOMARKER Variable Name” represent analytes that were evaluated in this work (those not analyzed had a high % below LLOQ).
(DOCX)
(DOCX)
(XLS)
The table is sorted alphabetically by gene name and then sorted by "weighted meta-analysis P-value". Distant pQTLs are denoted by light tan shading. pQTLs determined by the tobit model are designated by an * next to the gene name. The NHGRI GWAS catalog was searched 5-8-2015; pQTLs are unique if they are not listed in the GWAS catalog (GWAS) or not in LD with any SNP in the GWAS catalog (LD-GWAS). Traits listed for GWAS catalog SNPs and PMID numbers for the appropriate references are provided. Functional annotation of SNPs (variant effect predictor): up (upstream), 5′ (5′ untranslated region), syn (synonymous variant), mis (missense), int (intron), stop (stop gained), splice (splice region variant), 3′ (3′ untranslated region), down (downstream), inter (intergenetic), nc (non-coding transcript exon). The SNPs that do not replicate in direction of effect between the two cohorts are indicated in red.
(XLSX)
The method utilized to determine these pQTLS is described in detail in the Methods (recursive conditioning). Results for both COPDGene and SPIROMICS are shown. None = all pQTLs are in strong linkage disequilibrium with the top SNP.
(DOCX)
Only those for HP (red) were also pQTLs (S4 Table). CDH1 and PECAM eQTLs are local, while pQTLs for these two analytes were distant. RNA expression levels of PECAM1 are measured by two different ProbeSetIDs in the Affymetrix arrays used for the gene expression studies. For HP, the model that best fits the evidence is listed. Causal in this case indicates that the evidence supports gene expression levels producing altered protein levels. Modeling for HP was conducted as for Fig 8 in the main manuscript using HP levels in place of disease.
(DOCX)
Specifics of the analysis are described in Fig 8 of the main text and in the methods section. Results are shown separately for the two COPD phenotypes with significant associations (percent emphysema and FEV1 percent predicted (FEVpp)]. While all pQTL SNPs were tested (results for biomarker associations shown in S8 Table), only those showing evidence for the models causal, reactive, independent, complete or collide are indicated in this table.
(XLSX)
(XLS)
A) Comparison of selected biomarker values on two different platforms by Quotient Bio Research (QBR) and Myriad Rules Based Medicine (RBM) from a selected subset of COPDGene subjects. The correlation coefficients are shown in the upper right panel and the scatterplots in the lower left panel. A histogram of the biomarker values are shown on the diagonal plots. B) Comparison of an R&D Quantikine ELISA (X axis) from serum of selected SPIROMICS subjects to the RBM (Y axis) for vitamin D binding protein. The two assays are highly concordant. See methods for details.
(DOCX)
We ran PCA on biomarker data after regressing out all the other covariates used in pQTL analyses. Based on the sizes of eigen-values shown in this plot, we choose to include the first PC into our pQTL analysis to account for unobserved confounding effects.
(DOCX)
SPIROMICS (top) and COPDGene (bottom) for (A) chronic bronchitis (0 = no; 1 = yes), (B) frequency of exacerbations in the 12 months prior to enrollment (exacerbations include respiratory events that required doctor visit, emergency room visit, hospitalization, or a change in antibiotic or steroid use), (C) FEV1% predicted, (D) percent total lung emphysema as defined by Hounsfield units -950; and (E) log transformation of percent emphysema.
(DOCX)
LocusZoom plots show a 90 kb window (center) or a 500 kb window (right). On the Manhattan plots, the red line is the significant threshold after correction for multiple comparisons. For all LocusZoom plots, the top pQTL SNP is indicated. Red boxes in the LocusZoom plots show the location of the analyte gene (not all plots show this location but local pQTLs will have a box in one or both plots; for distant pQTLs, no red box will be present in either plot). Analyte gene location is shown with red arrow in Manhattan plots.
(PDF)
Each dot represents a pQTL with P < 10−8. The x-axis denotes the location of the pQTL SNP and the y-axis denotes the location of the biomarker. The color of each dot denotes range of P-value as indicated in the legend. Dots more than 1 Mb from the identity line represent distant pQTL SNPs. Dots on the identity line represent local pQTLs. The bottom panel is useful to highlight the peak of pQTL SNPs located on chromosome 9 (ABO locus).
(DOCX)
The highly significant pQTL SNPs (right panels) represent a distribution of minor allele frequencies similar in distribution to all SNPs in the study (left panels). SPIROMICS (top panel); COPDGene (bottom panel).
(DOCX)
a)-b) For both cohorts, the percent variance explained (R2) was greater in the full model, which includes all covariates in addition to the top two independent SNP genotypes, compared to the genotype only model. The correlation (rho) between the two models was higher for COPDGene (0.92) compared to SPIROMICS (0.72). This indicates that utilized covariates are relatively more predictive of biomarker levels in SPIROMICS compared to COPDGene. c)-d) Percent variance explained correlated between COPDGene and SPIROMICS, with only genotype producing a stronger correlation (rho 0.88) compared to the full model (rho = 0.72). Thus, genotype in both cohorts have similar contributions to the percent variation in biomarker levels, while the contribution by the covariates is more variable and study dependent.
(DOCX)
For 103 subjects, both gene expression and biomarker data were available from the COPDGene cohort. In those subjects, 80 biomarkers had available gene expression data in 199 probesets (multiple probesets may be available for a gene). For these 199 biomarker-gene expression pairs, there is a significant number of positive correlations (0.007, sign test) indicating that eQTLs (based on mRNA) can effect blood biomarker levels.
(DOCX)
This figure represents an expanded version of Fig 3 in the main text.
(DOCX)
When cell counts (eosinophils, basophils, neutrophils, monocytes, platelets, and red blood cells) were included in the regression models (Tobit or Linear as described in the methods) the significance of the pQTLs (X axis) did not vary significantly from results that did not include the CBC data (Y-axis). This was true for all pQTLs (left) and for the significant pQTLs (right). CBC data was only available from SPIROMICS and so these graphs represent SPIROMICS-only p-values. The correlation = 0.9854 for all SNP biomarker pairs and >0.999 for significant pQTL biomarker pairs.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge Dr. Neil Fedarko and the Johns Hopkins Clinical Research Unit Core Laboratory for help with the vitamin D binding protein assays. The authors thank the SPIROMICS and COPDGene participants and participating physicians, investigators and staff for making this research possible. The SPIROMICS co-authors wish to acknowledge the contributions of participating individuals at the clinical sites: Carrie P Aaron, MD (Columbia University, New York, NY); Shefalee Amin, MD (University of California at Los Angeles, Los Angeles, CA); Elizabeth Ampleford, PhD (Wake Forest Medical Center, Winston-Salem, NC); Anthony F Arredondo, MD (University of California at Los Angeles, Los Angeles, CA); Nirav Bhakta, MD, PhD (University of California at San Francisco, San Francisco, CA); Surya Bhatt, MD (University of Alabama at Birmingham, Birmingham, AL); Sudheer Bolla, MD (Temple University, Philadelphia, PA); Homer A. Boushey, MD (University of California at San Francisco, San Francisco, CA); Hollins Clark, MD (Wake Forest Medical Center, Winston-Salem, NC); Christopher B Cooper, MD, PhD (University of California at Los Angeles, Los Angeles, CA); Brett Elicker, MD (University of California at San Francisco, San Francisco, CA); John Erb-Downward, PhD (University of Michigan, Ann Arbor, MI); John V. Fahey, MD, DSc (University of California at San Francisco, San Francisco, CA); Kimber L Foust, MD (University of California at Los Angeles, Los Angeles, CA); Jonathan G Goldin, MD, PhD (University of California at Los Angeles, Los Angeles, CA); Annette Hastie, PhD (Wake Forest Medical Center, Winston-Salem, NC); John Hoidal, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Gary Huffnagle, PhD (University of Michigan, Ann Arbor, MI); Carlos Iribarren, MD, MPH, PhD (Kaiser Permanente of Northern California, Oakland, CA); Jerry Krishnan, MD, PhD (Clinical Center, University of Illinois at Chicago, Chicago, IL); Stephen Lazarus, MD (University of California at San Francisco, San Francisco, CA); Xingnan Li, PhD (Wake Forest Medical Center, Winston-Salem, NC); Michael R Littner, MD (University of California at Los Angeles, Los Angeles, CA); Howard Mann, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Wendy Moore, MD (Wake Forest Medical Center, Winston-Salem, NC); Amelia A. Musto, PhD (University of Illinois at Chicago, Chicago, IL); Hrudaya Nath, MD (University of Alabama at Birmingham, Birmingham, AL); John Newell, MD (University of Iowa, Iowa City, IA); Elizabeth C Oelsner, MD, MPH (Columbia University, New York, NY); Victor Ortega, MD (Wake Forest Medical Center, Winston-Salem, NC); Robert Paine, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Tessy K Paul, MD (University of California at Los Angeles, Los Angeles, CA); Cheryl Pirrozi, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Sanjeev Raman, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Satinder Singh, MD (University of Alabama at Birmingham, Birmingham, AL); Krishna M. Sundar, MD (University of Utah Hospitals and Clinics, Salt Lake City, UT); Tisha S Wang, MD (University of California at Los Angeles, Los Angeles, CA); J Michael Wells, MD (University of Alabama at Birmingham, Birmingham, AL); Michelle R Ziedler, MD (University of California at Los Angeles, Los Angeles, CA)
Members of the SPIROMICS Research Group
The following represent the current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, PhD; Wayne H Anderson, PhD; R Graham Barr, MD, DrPH; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Elizabeth E Carretta, MPH; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; John D Newell Jr, MD; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Stephen I. Rennard, MD; Donald P Tashkin, MD; Mary Beth Scholand, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH.
Members of the COPDGene Investigators Core Units
Administrative Core. James Crapo, MD (PI), Edwin Silverman, MD, PhD (PI), Barry Make, MD, Elizabeth Regan, MD, PhD
Genetic Analysis Core. Terri Beaty, PhD, Nan Laird, PhD, Christoph Lange, PhD, Michael Cho, MD, Stephanie Santorico, PhD, John Hokanson, MPH, PhD, Dawn DeMeo, MD, MPH, Nadia Hansel, MD, MPH, Craig Hersh, MD, MPH, Peter Castaldi, MD, MSc, Merry-Lynn McDonald, PhD, Emily Wan, MD, Megan Hardin, MD, Jacqueline Hetmanski, MS, Margaret Parker, MS, Marilyn Foreman, MD, Brian Hobbs, MD, Robert Busch, MD, Adel El-Bouiez, MD, Peter Castaldi, MD, Megan Hardin, MD, Dandi Qiao, PhD, Elizabeth Regan, MD, Eitan Halper-Stromberg, Ferdouse Begum, Sungho Won, Sharon Lutz, PhD
Imaging Core. David A Lynch, MB, Harvey O Coxson, PhD, MeiLan K Han, MD, MS, MD, Eric A Hoffman, PhD, Stephen Humphries MS, Francine L Jacobson, MD, Philip F Judy, PhD, Ella A Kazerooni, MD, John D Newell, Jr., MD, Elizabeth Regan, MD, James C Ross, PhD, Raul San Jose Estepar, PhD, Berend C Stoel, PhD, Juerg Tschirren, PhD, Eva van Rikxoort, PhD, Bram van Ginneken, PhD, George Washko, MD, Carla G Wilson, MS, Mustafa Al Qaisi, MD, Teresa Gray, Alex Kluiber, Tanya Mann, Jered Sieren, Douglas Stinson, Joyce Schroeder, MD, Edwin Van Beek, MD, PhD
PFT QA Core, Salt Lake City, UT. Robert Jensen, PhD
Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO. Douglas Everett, PhD, Anna Faino, MS, Matt Strand, PhD, Carla Wilson, MS
Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO. John E. Hokanson, MPH, PhD, Gregory Kinney, MPH, PhD, Sharon Lutz, PhD, Kendra Young PhD, Katherine Pratte, MSPH, Lindsey Duca,
Members of the COPDGene Investigators–Clinical Centers
Ann Arbor VA. Jeffrey L. Curtis, MD, Carlos H. Martinez, MD, MPH, Perry G. Pernicano, MD
Baylor College of Medicine, Houston, TX. Nicola Hanania, MD, MS, Philip Alapat, MD, Venkata Bandi, MD, Mustafa Atik, MD, Aladin Boriek, PhD, Kalpatha Guntupalli, MD, Elizabeth Guy, MD, Amit Parulekar, MD, Arun Nachiappan, MD
Brigham and Women’s Hospital, Boston, MA. Dawn DeMeo, MD, MPH, Craig Hersh, MD, MPH, George Washko, MD, Francine Jacobson, MD, MPH
Columbia University, New York, NY. R. Graham Barr, MD, DrPH, Byron Thomashow, MD, John Austin, MD, Belinda D’Souza, MD, Gregory D.N. Pearson, MD, Anna Rozenshtein, MD, MPH, FACR
Duke University Medical Center, Durham, NC. Neil MacIntyre, Jr., MD, Lacey Washington, MD, H. Page McAdams, MD
Health Partners Research Foundation, Minneapolis, MN. Charlene McEvoy, MD, MPH, Joseph Tashjian, MD
Johns Hopkins University, Baltimore, MD. Robert Wise, MD, Nadia Hansel, MD, MPH, Robert Brown, MD, Karen Horton, MD, Nirupama Putcha, MD, MHS,
Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA. Richard Casaburi, PhD, MD, Alessandra Adami, PhD, Janos Porszasz, MD, PhD, Hans Fischer, MD, PhD, Matthew Budoff, MD, Harry Rossiter, PhD
Michael E. DeBakey VAMC, Houston, TX. Amir Sharafkhaneh, MD, PhD, Charlie Lan, DO
Minneapolis VA. Christine Wendt, MD, Brian Bell, MD
Morehouse School of Medicine, Atlanta, GA. Marilyn Foreman, MD, MS, Gloria Westney, MD, MS, Eugene Berkowitz, MD, PhD
National Jewish Health, Denver, CO. Russell Bowler, MD, PhD, David Lynch, MD
Reliant Medical Group, Worcester, MA. Richard Rosiello, MD, David Pace, MD
Temple University, Philadelphia, PA. Gerard Criner, MD, David Ciccolella, MD, Francis Cordova, MD, Chandra Dass, MD, Gilbert D’Alonzo, DO, Parag Desai, MD, Michael Jacobs, PharmD, Steven Kelsen, MD, PhD, Victor Kim, MD, A. James Mamary, MD, Nathaniel Marchetti, DO, Aditi Satti, MD, Kartik Shenoy, MD, Robert M. Steiner, MD, Alex Swift, MD, Irene Swift, MD, Maria Elena Vega-Sanchez, MD
University of Alabama, Birmingham, AL. Mark Dransfield, MD, William Bailey, MD, J. Michael Wells, MD, Surya Bhatt, MD, Hrudaya Nath, MD
University of California, San Diego, CA. Joe Ramsdell, MD, Paul Friedman, MD, Xavier Soler, MD, PhD, Andrew Yen, MD
University of Iowa, Iowa City, IA. Alejandro Cornellas, MD, John Newell, Jr., MD, Brad Thompson, MD
University of Michigan, Ann Arbor, MI. MeiLan Han, MD, Ella Kazerooni, MD, Carlos Martinez, MD
University of Minnesota, Minneapolis, MN. Joanne Billings, MD, Tadashi Allen, MD
University of Pittsburgh, Pittsburgh, PA. Frank Sciurba, MD, Divay Chandra, MD, MSc, Joel Weissfeld, MD, MPH, Carl Fuhrman, MD, Jessica Bon, MD
University of Texas Health Science Center at San Antonio, San Antonio, TX. Antonio Anzueto, MD, Sandra Adams, MD, Diego Maselli-Caceres, MD, Mario E. Ruiz, MD
Data Availability
The COPDGene clinical phenotype, biomarker, and genetic data are available at dbGaP phs000179.v1.p1. COPDGene microarray data are available at GEO, accession GSE42057. The SPIROMICS clinical and biomarker data are available at dbGaP phs001119.v1.p1
Funding Statement
This study was supported by grants from the NHLBI (R01 HL 09-5432, R01 HL08-9856, and R01 HL08-9897) NCRR/HIH (UL1 RR025780) for COPDGene and R01 HL12-5432 for SPIROMICS. SPIROMICS was additionally supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GSK; Grifols Therapeutics, Inc; Ikaria, Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; and Sanofi. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173–86. 10.1038/ng.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consortium GT. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60. 10.1126/science.1262110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westra HJ, Franke L. From genome to function by studying eQTLs. Biochim Biophys Acta. 2014;1842(10):1896–902. 10.1016/j.bbadis.2014.04.024 . [DOI] [PubMed] [Google Scholar]
- 5.Battle A, Khan Z, Wang SH, Mitrano A, Ford MJ, Pritchard JK, et al. Genomic variation. Impact of regulatory variation from RNA to protein. Science. 2015;347(6222):664–7. 10.1126/science.1260793 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491–4. 10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burney PG, Patel J, Newson R, Minelli C, Naghavi M. Global and regional trends in COPD mortality, 1990–2010. Eur Respir J. 2015;45(5):1239–47. 10.1183/09031936.00142414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, et al. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014;69(7):666–72. 10.1136/thoraxjnl-2013-204778 . [DOI] [PubMed] [Google Scholar]
- 10.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4(5):e1000072 10.1371/journal.pgen.1000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowler RP, Kim V, Regan E, Williams AA, Santorico SA, Make BJ, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146(4):941–50. 10.1378/chest.13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neal WK, Anderson W, Basta PV, Carretta EE, Doerschuk CM, Barr RG, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9 10.1186/1479-5876-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carolan BJ, Hughes G, Morrow J, Hersh CP, O'Neal WK, Rennard S, et al. The association of plasma biomarkers with computed tomography-assessed emphysema phenotypes. Respir Res. 2014;15:127 10.1186/s12931-014-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DT, Kim DK, Cockayne DA, Belousov A, Bitter H, Cho MH, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(8):948–57. 10.1164/rccm.201302-0247OC . [DOI] [PubMed] [Google Scholar]
- 15.Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomas DA, Silverman EK, Edwards LD, Locantore NW, Miller BE, Horstman DH, et al. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34(1):95–102. 10.1183/09031936.00156508 . [DOI] [PubMed] [Google Scholar]
- 17.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho MH, McDonald ML, Zhou X, Mattheisen M, Castaldi PJ, Hersh CP, et al. Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2(3):214–25. 10.1016/S2213-2600(14)70002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene WH. Econometric analysis 6th ed. Upper Saddle River, N.J.: Prentice Hall; 2008. xxxvii, 1177 p. p. [Google Scholar]
- 20.Wright FA, Sullivan PF, Brooks AI, Zou F, Sun W, Xia K, et al. Heritability and genomics of gene expression in peripheral blood. Nat Genet. 2014;46(5):430–7. 10.1038/ng.2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol. 2003;157(4):355–63. . [DOI] [PubMed] [Google Scholar]
- 22.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005;37(7):710–7. 10.1038/ng1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452(7186):429–35. 10.1038/nature06757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millstein J, Zhang B, Zhu J, Schadt EE. Disentangling molecular relationships with a causal inference test. BMC Genet. 2009;10:23 10.1186/1471-2156-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Tesson BM, Churchill GA, Jansen RC. Critical reasoning on causal inference in genome-wide linkage and association studies. Trends Genet. 2010;26(12):493–8. 10.1016/j.tig.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMontagne AD, Milner A, Krnjacki L, Kavanagh AM, Blakely TA, Bentley R. Employment arrangements and mental health in a cohort of working Australians: are transitions from permanent to temporary employment associated with changes in mental health? Am J Epidemiol. 2014;179(12):1467–76. 10.1093/aje/kwu093 . [DOI] [PubMed] [Google Scholar]
- 27.Chen LS, Emmert-Streib F, Storey JD. Harnessing naturally randomized transcription to infer regulatory relationships among genes. Genome Biol. 2007;8(10):R219 10.1186/gb-2007-8-10-r219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Yu T, Li KC. Detection of eQTL modules mediated by activity levels of transcription factors. Bioinformatics. 2007;23(17):2290–7. 10.1093/bioinformatics/btm327 . [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. . [PubMed] [Google Scholar]
- 30.Yourshaw M, Taylor SP, Rao AR, Martin MG, Nelson SF. Rich annotation of DNA sequencing variants by leveraging the Ensembl Variant Effect Predictor with plugins. Brief Bioinform. 2015;16(2):255–64. 10.1093/bib/bbu008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–6. 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, et al. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49(2):316–23. 10.1165/rcmb.2012-0230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–7. 10.1093/bioinformatics/btq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40(Database issue):D930–4. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41(9):986–90. 10.1038/ng.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Band G, Le QS, Jostins L, Pirinen M, Kivinen K, Jallow M, et al. Imputation-based meta-analysis of severe malaria in three African populations. PLoS Genet. 2013;9(5):e1003509 10.1371/journal.pgen.1003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbalic M, Dupuis J, Dehghan A, Bis JC, Hoogeveen RC, Schnabel RB, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863–72. 10.1093/hmg/ddq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43(11):1131–8. 10.1038/ng.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011;43(9):897–901. 10.1038/ng.898 . [DOI] [PubMed] [Google Scholar]
- 40.Chung CM, Wang RY, Chen JW, Fann CS, Leu HB, Ho HY, et al. A genome-wide association study identifies new loci for ACE activity: potential implications for response to ACE inhibitor. Pharmacogenomics J. 2010;10(6):537–44. 10.1038/tpj.2009.70 . [DOI] [PubMed] [Google Scholar]
- 41.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, et al. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS One. 2012;7(12):e51954 10.1371/journal.pone.0051954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Boer RA, Verweij N, van Veldhuisen DJ, Westra HJ, Bakker SJ, Gansevoort RT, et al. A genome-wide association study of circulating galectin-3. PLoS One. 2012;7(10):e47385 10.1371/journal.pone.0047385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desch KC, Ozel AB, Siemieniak D, Kalish Y, Shavit JA, Thornburg CD, et al. Linkage analysis identifies a locus for plasma von Willebrand factor undetected by genome-wide association. Proc Natl Acad Sci U S A. 2013;110(2):588–93. 10.1073/pnas.1219885110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dichgans M, Malik R, Konig IR, Rosand J, Clarke R, Gretarsdottir S, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke. 2014;45(1):24–36. 10.1161/STROKEAHA.113.002707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Germain M, Saut N, Greliche N, Dina C, Lambert JC, Perret C, et al. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One. 2011;6(9):e25581 10.1371/journal.pone.0025581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He M, Wu C, Xu J, Guo H, Yang H, Zhang X, et al. A genome wide association study of genetic loci that influence tumour biomarkers cancer antigen 19–9, carcinoembryonic antigen and alpha fetoprotein and their associations with cancer risk. Gut. 2014;63(1):143–51. 10.1136/gutjnl-2012-303434 . [DOI] [PubMed] [Google Scholar]
- 47.Heit JA, Armasu SM, Asmann YW, Cunningham JM, Matsumoto ME, Petterson TM, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012;10(8):1521–31. 10.1111/j.1538-7836.2012.04810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42(3):210–5. 10.1038/ng.531 . [DOI] [PubMed] [Google Scholar]
- 49.Kim YJ, Go MJ, Hu C, Hong CB, Kim YK, Lee JY, et al. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat Genet. 2011;43(10):990–5. 10.1038/ng.939 . [DOI] [PubMed] [Google Scholar]
- 50.Li J, Gui L, Wu C, He Y, Zhou L, Guo H, et al. Genome-wide association study on serum alkaline phosphatase levels in a Chinese population. BMC Genomics. 2013;14:684 10.1186/1471-2164-14-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Tang W, Huang T, Gao Y, Tan A, Yang X, et al. Genetic variations affecting serum carcinoembryonic antigen levels and status of regional lymph nodes in patients with sporadic colorectal cancer from Southern China. PLoS One. 2014;9(6):e97923 10.1371/journal.pone.0097923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naitza S, Porcu E, Steri M, Taub DD, Mulas A, Xiao X, et al. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genet. 2012;8(1):e1002480 10.1371/journal.pgen.1002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pare G, Ridker PM, Rose L, Barbalic M, Dupuis J, Dehghan A, et al. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7(4):e1001374 10.1371/journal.pgen.1001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29(11):1958–67. 10.1161/ATVBAHA.109.192971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9(2):e1003266 10.1371/journal.pgen.1003266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi L, Cornelis MC, Kraft P, Jensen M, van Dam RM, Sun Q, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19(9):1856–62. 10.1093/hmg/ddq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377(9763):383–92. 10.1016/S0140-6736(10)61996-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rueedi R, Ledda M, Nicholls AW, Salek RM, Marques-Vidal P, Morya E, et al. Genome-wide association study of metabolic traits reveals novel gene-metabolite-disease links. PLoS Genet. 2014;10(2):e1004132 10.1371/journal.pgen.1004132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. 10.1038/ng.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543–50. 10.1038/ng.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith NL, Huffman JE, Strachan DP, Huang J, Dehghan A, Trompet S, et al. Genetic predictors of fibrin D-dimer levels in healthy adults. Circulation. 2011;123(17):1864–72. 10.1161/CIRCULATIONAHA.110.009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477(7362):54–60. 10.1038/nature10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang W, Schwienbacher C, Lopez LM, Ben-Shlomo Y, Oudot-Mellakh T, Johnson AD, et al. Genetic associations for activated partial thromboplastin time and prothrombin time, their gene expression profiles, and risk of coronary artery disease. Am J Hum Genet. 2012;91(1):152–62. 10.1016/j.ajhg.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanikawa C, Urabe Y, Matsuo K, Kubo M, Takahashi A, Ito H, et al. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat Genet. 2012;44(4):430–4, S1-2. 10.1038/ng.1109 . [DOI] [PubMed] [Google Scholar]
- 65.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–13. 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teupser D, Baber R, Ceglarek U, Scholz M, Illig T, Gieger C, et al. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet. 2010;3(4):331–9. 10.1161/CIRCGENETICS.109.907873 . [DOI] [PubMed] [Google Scholar]
- 67.Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489(7416):443–6. 10.1038/nature11334 . [DOI] [PubMed] [Google Scholar]
- 68.Tregouet DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113(21):5298–303. 10.1182/blood-2008-11-190389 . [DOI] [PubMed] [Google Scholar]
- 69.van der Harst P, Zhang W, Mateo Leach I, Rendon A, Verweij N, Sehmi J, et al. Seventy-five genetic loci influencing the human red blood cell. Nature. 2012;492(7429):369–75. 10.1038/nature11677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the ABO locus: the EuroCLOT study. Ann Neurol. 2013;73(1):16–31. 10.1002/ana.23838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83(4):520–8. 10.1016/j.ajhg.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao SX, Xue LQ, Liu W, Gu ZH, Pan CM, Yang SY, et al. Robust evidence for five new Graves' disease risk loci from a staged genome-wide association analysis. Hum Mol Genet. 2013;22(16):3347–62. 10.1093/hmg/ddt183 . [DOI] [PubMed] [Google Scholar]
- 73.Zhou L, He M, Mo Z, Wu C, Yang H, Yu D, et al. A genome wide association study identifies common variants associated with lipid levels in the Chinese population. PLoS One. 2013;8(12):e82420 10.1371/journal.pone.0082420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, Attar-Cohen H, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325(5945):1246–50. 10.1126/science.1174148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-Binding Protein Concentrations Quantified by Mass Spectrometry. N Engl J Med. 2015;373(15):1480–2. 10.1056/NEJMc1502602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen BH, Ball WC Jr., Brashears S, Diamond EL, Kreiss P, Levy DA, et al. Risk factors in chronic obstructive pulmonary disease (COPD). Am J Epidemiol. 1977;105(3):223–32. . [DOI] [PubMed] [Google Scholar]
- 77.Harrison GA, Boyce AJ, Hornabrook RW, Serjeantson S, Craig WJ. Evidence for an association between ABO blood group and goitre. Hum Genet. 1976;32(3):335–7. . [DOI] [PubMed] [Google Scholar]
- 78.Padma T, Valli VV. ABO blood groups, intestinal alkaline phosphatase and haptoglobin types in patients with serum hepatitis. Hum Hered. 1988;38(6):367–71. . [DOI] [PubMed] [Google Scholar]
- 79.Obeidat M, Fishbane N, Nie Y, Chen V, Hollander Z, Tebbutt SJ, et al. The Effect of Statins on Blood Gene Expression in COPD. PLoS One. 2015;10(10):e0140022 10.1371/journal.pone.0140022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hansel NN, Pare PD, Rafaels N, Sin DD, Sandford A, Daley D, et al. Genome-Wide Association Study Identification of Novel Loci Associated with Airway Responsiveness in Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol. 2015;53(2):226–34. 10.1165/rcmb.2014-0198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castaldi PJ, Cho MH, Litonjua AA, Bakke P, Gulsvik A, Lomas DA, et al. The association of genome-wide significant spirometric loci with chronic obstructive pulmonary disease susceptibility. Am J Respir Cell Mol Biol. 2011;45(6):1147–53. 10.1165/rcmb.2011-0055OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hause RJ, Stark AL, Antao NN, Gorsic LK, Chung SH, Brown CD, et al. Identification and validation of genetic variants that influence transcription factor and cell signaling protein levels. Am J Hum Genet. 2014;95(2):194–208. 10.1016/j.ajhg.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horvatovich P, Franke L, Bischoff R. Proteomic studies related to genetic determinants of variability in protein concentrations. J Proteome Res. 2014;13(1):5–14. 10.1021/pr400765y . [DOI] [PubMed] [Google Scholar]
- 84.Yonchuk JG, Silverman EK, Bowler RP, Agusti A, Lomas DA, Miller BE, et al. Circulating Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Biomarker of Emphysema and the RAGE Axis in the Lung. Am J Respir Crit Care Med. 2015;192(7):785–92. 10.1164/rccm.201501-0137PP . [DOI] [PubMed] [Google Scholar]
- 85.Thun GA, Imboden M, Ferrarotti I, Kumar A, Obeidat M, Zorzetto M, et al. Causal and synthetic associations of variants in the SERPINA gene cluster with alpha1-antitrypsin serum levels. PLoS Genet. 2013;9(8):e1003585 10.1371/journal.pgen.1003585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller RD, Kueppers F, Offord KP. Serum concentrations of C3 and C4 of the complement system in patients with chronic obstructive pulmonary disease. J Lab Clin Med. 1980;95(2):266–71. . [PubMed] [Google Scholar]
- 87.Chauhan S, Gupta MK, Goyal A, Dasgupta DJ. Alterations in immunoglobulin & complement levels in chronic obstructive pulmonary disease. Indian J Med Res. 1990;92:241–5. . [PubMed] [Google Scholar]
- 88.Nishioka M, Venkatesan N, Dessalle K, Mogas A, Kyoh S, Lin TY, et al. Fibroblast-epithelial cell interactions drive epithelial-mesenchymal transition differently in cells from normal and COPD patients. Respir Res. 2015;16:72 10.1186/s12931-015-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milara J, Peiro T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–20. 10.1136/thoraxjnl-2012-201761 . [DOI] [PubMed] [Google Scholar]
- 90.Johansson A, Enroth S, Palmblad M, Deelder AM, Bergquist J, Gyllensten U. Identification of genetic variants influencing the human plasma proteome. Proc Natl Acad Sci U S A. 2013;110(12):4673–8. 10.1073/pnas.1217238110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lourdusamy A, Newhouse S, Lunnon K, Proitsi P, Powell J, Hodges A, et al. Identification of cis-regulatory variation influencing protein abundance levels in human plasma. Hum Mol Genet. 2012;21(16):3719–26. 10.1093/hmg/dds186 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LLOQ = Lower Limit of Quantification. All COPDGene samples were P100 plasma. Green cells under “BIOMARKER Variable Name” represent analytes that were evaluated in this work (those not analyzed had a high % below LLOQ).
(DOCX)
(DOCX)
(XLS)
The table is sorted alphabetically by gene name and then sorted by "weighted meta-analysis P-value". Distant pQTLs are denoted by light tan shading. pQTLs determined by the tobit model are designated by an * next to the gene name. The NHGRI GWAS catalog was searched 5-8-2015; pQTLs are unique if they are not listed in the GWAS catalog (GWAS) or not in LD with any SNP in the GWAS catalog (LD-GWAS). Traits listed for GWAS catalog SNPs and PMID numbers for the appropriate references are provided. Functional annotation of SNPs (variant effect predictor): up (upstream), 5′ (5′ untranslated region), syn (synonymous variant), mis (missense), int (intron), stop (stop gained), splice (splice region variant), 3′ (3′ untranslated region), down (downstream), inter (intergenetic), nc (non-coding transcript exon). The SNPs that do not replicate in direction of effect between the two cohorts are indicated in red.
(XLSX)
The method utilized to determine these pQTLS is described in detail in the Methods (recursive conditioning). Results for both COPDGene and SPIROMICS are shown. None = all pQTLs are in strong linkage disequilibrium with the top SNP.
(DOCX)
Only those for HP (red) were also pQTLs (S4 Table). CDH1 and PECAM eQTLs are local, while pQTLs for these two analytes were distant. RNA expression levels of PECAM1 are measured by two different ProbeSetIDs in the Affymetrix arrays used for the gene expression studies. For HP, the model that best fits the evidence is listed. Causal in this case indicates that the evidence supports gene expression levels producing altered protein levels. Modeling for HP was conducted as for Fig 8 in the main manuscript using HP levels in place of disease.
(DOCX)
Specifics of the analysis are described in Fig 8 of the main text and in the methods section. Results are shown separately for the two COPD phenotypes with significant associations (percent emphysema and FEV1 percent predicted (FEVpp)]. While all pQTL SNPs were tested (results for biomarker associations shown in S8 Table), only those showing evidence for the models causal, reactive, independent, complete or collide are indicated in this table.
(XLSX)
(XLS)
A) Comparison of selected biomarker values on two different platforms by Quotient Bio Research (QBR) and Myriad Rules Based Medicine (RBM) from a selected subset of COPDGene subjects. The correlation coefficients are shown in the upper right panel and the scatterplots in the lower left panel. A histogram of the biomarker values are shown on the diagonal plots. B) Comparison of an R&D Quantikine ELISA (X axis) from serum of selected SPIROMICS subjects to the RBM (Y axis) for vitamin D binding protein. The two assays are highly concordant. See methods for details.
(DOCX)
We ran PCA on biomarker data after regressing out all the other covariates used in pQTL analyses. Based on the sizes of eigen-values shown in this plot, we choose to include the first PC into our pQTL analysis to account for unobserved confounding effects.
(DOCX)
SPIROMICS (top) and COPDGene (bottom) for (A) chronic bronchitis (0 = no; 1 = yes), (B) frequency of exacerbations in the 12 months prior to enrollment (exacerbations include respiratory events that required doctor visit, emergency room visit, hospitalization, or a change in antibiotic or steroid use), (C) FEV1% predicted, (D) percent total lung emphysema as defined by Hounsfield units -950; and (E) log transformation of percent emphysema.
(DOCX)
LocusZoom plots show a 90 kb window (center) or a 500 kb window (right). On the Manhattan plots, the red line is the significant threshold after correction for multiple comparisons. For all LocusZoom plots, the top pQTL SNP is indicated. Red boxes in the LocusZoom plots show the location of the analyte gene (not all plots show this location but local pQTLs will have a box in one or both plots; for distant pQTLs, no red box will be present in either plot). Analyte gene location is shown with red arrow in Manhattan plots.
(PDF)
Each dot represents a pQTL with P < 10−8. The x-axis denotes the location of the pQTL SNP and the y-axis denotes the location of the biomarker. The color of each dot denotes range of P-value as indicated in the legend. Dots more than 1 Mb from the identity line represent distant pQTL SNPs. Dots on the identity line represent local pQTLs. The bottom panel is useful to highlight the peak of pQTL SNPs located on chromosome 9 (ABO locus).
(DOCX)
The highly significant pQTL SNPs (right panels) represent a distribution of minor allele frequencies similar in distribution to all SNPs in the study (left panels). SPIROMICS (top panel); COPDGene (bottom panel).
(DOCX)
a)-b) For both cohorts, the percent variance explained (R2) was greater in the full model, which includes all covariates in addition to the top two independent SNP genotypes, compared to the genotype only model. The correlation (rho) between the two models was higher for COPDGene (0.92) compared to SPIROMICS (0.72). This indicates that utilized covariates are relatively more predictive of biomarker levels in SPIROMICS compared to COPDGene. c)-d) Percent variance explained correlated between COPDGene and SPIROMICS, with only genotype producing a stronger correlation (rho 0.88) compared to the full model (rho = 0.72). Thus, genotype in both cohorts have similar contributions to the percent variation in biomarker levels, while the contribution by the covariates is more variable and study dependent.
(DOCX)
For 103 subjects, both gene expression and biomarker data were available from the COPDGene cohort. In those subjects, 80 biomarkers had available gene expression data in 199 probesets (multiple probesets may be available for a gene). For these 199 biomarker-gene expression pairs, there is a significant number of positive correlations (0.007, sign test) indicating that eQTLs (based on mRNA) can effect blood biomarker levels.
(DOCX)
This figure represents an expanded version of Fig 3 in the main text.
(DOCX)
When cell counts (eosinophils, basophils, neutrophils, monocytes, platelets, and red blood cells) were included in the regression models (Tobit or Linear as described in the methods) the significance of the pQTLs (X axis) did not vary significantly from results that did not include the CBC data (Y-axis). This was true for all pQTLs (left) and for the significant pQTLs (right). CBC data was only available from SPIROMICS and so these graphs represent SPIROMICS-only p-values. The correlation = 0.9854 for all SNP biomarker pairs and >0.999 for significant pQTL biomarker pairs.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The COPDGene clinical phenotype, biomarker, and genetic data are available at dbGaP phs000179.v1.p1. COPDGene microarray data are available at GEO, accession GSE42057. The SPIROMICS clinical and biomarker data are available at dbGaP phs001119.v1.p1