Abstract
Micro-organisms inhabiting animal guts benefit from a protected and nutrient-rich environment while assisting the host with digestion and nutrition. In this study we compare, for the first time, the bacterial and fungal gut communities of two species of the small desert dung beetle genus Pachysoma feeding on different diets: the detritivorous P. endroedyi and the dry-dung-feeding P. striatum. Whole-gut microbial communities from 5 individuals of each species were assessed using 454 pyrosequencing of the bacterial 16S rRNA gene and fungal ITS gene regions. The two bacterial communities were significantly different, with only 3.7% of operational taxonomic units shared, and displayed intra-specific variation. The number of bacterial phyla present within the guts of P. endroedyi and P. striatum individuals ranged from 6–11 and 4–7, respectively. Fungal phylotypes could only be detected within the gut of P. striatum. Although the role of host phylogeny in Pachysoma microbiome assembly remains unknown, evidence presented in this study suggests that host diet may be a deterministic factor.
Introduction
The microbial gut communities of a wide range of insect species have been investigated (for reviews see [1–6]). The gut environment is considered to be an unstable system, as microorganisms face secretion of digestive enzymes, physical disturbance, habitat shedding during insect moults and other physiochemical conditions that are typically unfavourable for colonisation [1, 2, 6]. However, there are significant benefits to gut colonisation, including high nutrient availability and protection from external environmental stressors [2, 7].
The relationships between host and gut microbiota range across the full spectrum of interactions; i.e., from pathogenic to obligate mutualism [1]. When beneficial to their host, insect-associated microbial communities may participate in a number of activities including degradation of recalcitrant materials such as lignocellulose [8–12], the production of nutrients and vitamins [2, 8, 12], the production of components of cohesion pheromones [13], nitrogen fixation and utilisation of nitrogenous waste products [2, 8, 12, 14], protection against parasites [2, 15], change in body colouration [16] and sterol synthesis [8, 12].
Insect gut microbiomes are known to differ between insect species, driven by variations in the gut structure, different host lifecycles, host phylogeny and diet [2, 6, 17]. The gut microbiome is also influenced within the individual insect or species, varying according to host life-stage [18–22], and/or diet [21, 23–26]. Host diet influences gut microbial communities as they adapt to dietary changes through the induction of enzymes and changes in community structure [23, 27, 28]. However, a core community may persist through major dietary changes [24, 29].
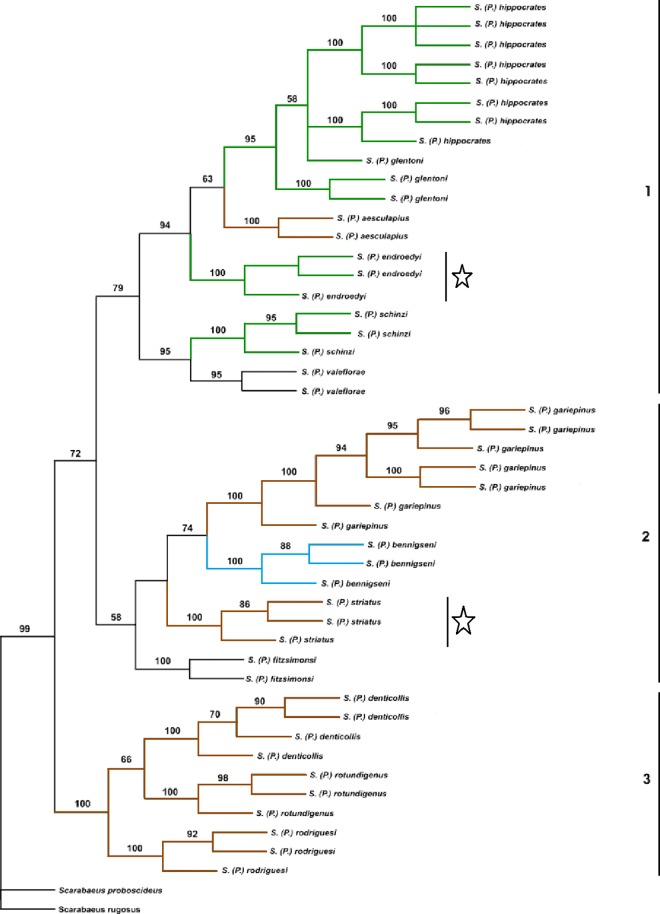
Studies on insect-microbial associations have mainly focused on termites [4, 30–34], but also on agriculturally important species such as honeybees [35, 36], and medically important insects such as mosquitoes [21, 37–40]. Little attention has been given to dung beetles, which are common and abundant insects in virtually all terrestrial environments and which facilitate nutrient cycling and bioturbation [41]. The desert dung beetle genus Pachysoma MacLeay, from the Scarabaeini tribe, of which the quintessential scarab genus, Scarabaeus is also a member, consists of 13 species endemic to the south-west African coast [42, 43]. Members of Pachysoma exhibit atypical feeding behaviour. While most adult dung beetles feed, by filtration, on minute particulate fragments in wet dung [44, 45], adult Pachysoma feed on various and varying dry food sources: plant detritus, dung pellets or both. These substrates are collected on the soil surface and masticated with specially-adapted mouthparts (Fig 1; [42, 43, 45, 46]).
Fig 1. Cytochrome oxidase I gene Parsimony tree phylogeny of 13 Pachysoma spp.
Branch colours indicate the diet of the Pachysoma spp.: dung (brown), plant detritus (green), polyphagous (blue) and unknown (no colour). This phylogentic tree was adapted, with permission, from [42] and the dietary information taken from both [43] and personal observations by Prof C. Scholtz. Two species of Scarabaeus, (S. proboscideus and S. rugosus) which is the sister-genus to Pachysoma and a typical wet-dung-feeder, were used as outgroups. Numbers to the right of the tree indicate the three Pachysoma lineages. The two species considered in this study, P. endroedyi and P. striatum, are indicated with stars (Adapted from C. Sole [42]).
Given that insect gut microorganisms are known to be involved in the degradation of recalcitrant materials such as lignocellulosic compounds [2, 6, 8, 9]), it follows that the gut microbiomes of desert insects may play a significant role in carbon-turnover in desert ecosystems. By studying the gut microbiome diversity of Pachysoma spp. feeding on different plentiful and readily-available substrates, it is possible to consider the effects of host diet and/or host phylogeny on gut microbiome assembly processes. This study was designed to characterise the gut microbial (bacterial and fungal) assemblages of coprophagous (P. striatum) [43] and detritivorous (P. endroedyi; pers. comm. C. Scholtz) members of the same genus from the same location and to potentially determine whether host diet and/or host phylogeny could be deterministic factors in Pachysoma gut microbial community assembly.
Results and Discussion
The desert beetle genus Pachysoma
The distribution of the Pachysoma species is restricted to the arid coastal regions of south-western Africa, principally because of the flightless nature of the genus [43]. The genus Pachysoma forms three distinct lineages, supporting six (lineage 1), four (lineage 2) and three (lineage 3) species, respectively [42]. Pachysoma endroedyi is located in lineage 1 and P. striatum in lineage 2 (Fig 1). The driving forces behind the formation of these three lineages are currently unknown. However, it has been noted that all members of lineage 3 have a uniform diet (Fig 1) and originate from desert areas with a consistent aridity index [42, 43], whereas both the aridity index of the desert locations from which lineage 1 and 2 members originate, and their diets, fluctuate (Fig 1).
The diet of P. striatum consists predominantly of the dry dung pellets [43, 46] of various small native mammalian herbivores and sheep. Despite observations from a decade ago stating that P. endroedyi was a polyphagous feeder [43], numerous and wide-scale recent observations suggest that P. endroedyi is a detrivore (Prof C. Scholtz, pers. comm.), the classification adopted in this study. Pachysoma species have specialised anatomical and physiological features for mastication and digestion of fragments from plant detritus and dry dung [44, 45].
The linkage between host and gut microbiome is believed to be bidirectional, in that gut microorganisms can provide nutritional assistance to the insect host [12, 47] while the host diet influences the gut microbiome assembly [21, 23–26]. However, host phylogeny may also impact gut microbiome composition [17, 48], irrespective of the diet.
Sequencing outputs and diversity indices of the bacterial 16S rRNA gene and fungal ITS region of the Pachysoma gut microbiome
The gut microbiomes of five detritivorous P. endroedyi and five coprophagous P. striatum individuals were determined by 16S rRNA gene amplicon sequencing. After removal of chimeras and singletons, 39050 bacterial and 1492 fungal reads remained, with mean read lengths of 238bp and 100bp, respectively. Only 462 bacterial reads were obtained for P. endroedyi individual 3 (Table 1), which was therefore removed from further analysis. Considerable variation in the number of bacterial sequence reads was noted between individuals, ranging from 1718 to 2817 and 3911 to 10106 for P. endroedyi and P. striatum, respectively. However, Good’s coverage (>0.97 for all samples), rarefaction and chao1 diversity indices suggested that the coverage of Pachysoma bacterial gut communities (S1A and S1B Fig) were sufficient for a valid comparison between individuals. The fungal ITS gene region could not be amplified in samples from the detritivorous species P. endroedyi, despite repeated attempts. The absence of fungi in the insect gut has previously been noted for individuals of various insect groups including Neuroptera and Coleoptera (using culture-dependent techniques: [49]). In the fungal ITS sequence datasets for P. striatum, diversity indices and rarefaction curves showed low coverage for all but P. striatum individual 2, suggesting that the fungal diversity was generally underestimated (Table 1; S1C Fig).
Table 1. Values for sequence reads, Operational Taxonomic Units (OTUs), phyla and diversity indices for bacterial and fungal gut communities of P. endroedyi and P. striatum individuals.
| Individual | Number of reads | Number of OTUs | Phyla | Singletons | Chao | Invsimpson | Shannon | Coverage | |
|---|---|---|---|---|---|---|---|---|---|
| Bacterial 16S rRNA gene | P. endroedyi 1 | 2120 | 213 | 10 | 105 | 244.61 | 19.01 | 3.88 | 0.97 |
| Bacterial 16S rRNA gene | P. endroedyi 2 | 1718 | 258 | 11 | 133 | 282.34 | 81.00 | 4.91 | 0.97 |
| Bacterial 16S rRNA gene | P. endroedyi 3 | 462 | 97 | 11 | 42 | 112.62 | 21.47 | 3.82 | 0.94 |
| Bacterial 16S rRNA gene | P. endroedyi 4 | 2175 | 271 | 6 | 177 | 287.59 | 62.44 | 4.80 | 0.98 |
| Bacterial 16S rRNA gene | P. endroedyi 5 | 2817 | 317 | 9 | 193 | 335.83 | 60.13 | 4.83 | 0.98 |
| Bacterial 16S rRNA gene | P. striatum 1 | 10106 | 157 | 4 | 84 | 174.53 | 8.77 | 2.82 | 1.00 |
| Bacterial 16S rRNA gene | P. striatum 2 | 4901 | 158 | 4 | 87 | 194.96 | 16.64 | 3.38 | 0.99 |
| Bacterial 16S rRNA gene | P. striatum 3 | 4208 | 140 | 6 | 51 | 183.05 | 8.36 | 2.88 | 0.99 |
| Bacterial 16S rRNA gene | P. striatum 4 | 3911 | 119 | 4 | 71 | 125.84 | 8.12 | 2.93 | 1.00 |
| Bacterial 16S rRNA gene | P. striatum 5 | 6620 | 172 | 5 | 100 | 201.29 | 13.36 | 3.32 | 0.99 |
| Fungal ITS gene region | P. striatum 1 | 136 | 88 | 1 | 156 | 179.50 | 96.63 | 4.29 | 0.55 |
| Fungal ITS gene region | P. striatum 2 | 939 | 202 | 2 | 602 | 222.81 | 51.93 | 4.56 | 0.94 |
| Fungal ITS gene region | P. striatum 3 | 199 | 106 | 2 | 223 | 248.38 | 88.35 | 4.40 | 0.66 |
| Fungal ITS gene region | P. striatum 4 | 107 | 70 | 2 | 153 | 157.50 | 65.94 | 4.04 | 0.53 |
| Fungal ITS gene region | P. striatum 5 | 111 | 63 | 2 | 146 | 134.75 | 52.18 | 3.88 | 0.62 |
A total of 1009 bacterial and 294 fungal OTUs were detected at an identity threshold of 97% (Table 1). Numbers ranged from 213 to 317 and 119 to 172 in the P. endroedyi and P. striatum gut samples, respectively (Table 1). These values are comparable with results obtained for termite and cockroach gut microbiomes [50]. It should be noted that the fungal ITS sequence read lengths were short (only 100bp), which could explain the poor phylogenetic resolution of P. striatum fungal gut communities [51].
In both Pachysoma spp., the number of bacterial 16S rRNA sequence reads was inversely proportional to the number of bacterial OTUs; i.e., P. striatum gut samples had a higher average number of bacterial reads (5949 ± 2550) but a lower average number of bacterial OTUs (149 ± 20) when compared to P. endroedyi (2208 ± 455 reads and 265 ± 43 OTUs, respectively). Those data suggest that the gut bacterial communities of P. striatum are composed of a relatively low number of dominant phylotypes at high abundance [25, 52, 53]. Contrastingly, the P. endroedyi gut bacterial community may include a higher bacterial diversity [25, 54]. This inverse relationship, and the higher Shannon diversity index of the P. endroedyi gut bacterial community (4.6 ± 0.5) compared with the P. striatum gut community (3.1 ± 0.3; Table 1), suggests that competition is greater in the P. striatum gut than in P. endroedyi. This difference may be a reflection of the different diets, as insects feeding on simple diets (e.g., the coprophagous diet of P. striatum) commonly have a lower gut bacterial diversity than those feeding on more complex diets (e.g., the detritivorous diet of P. endroedyi [17, 48]).
Interspecific variations in bacterial and fungal Pachysoma gut communities
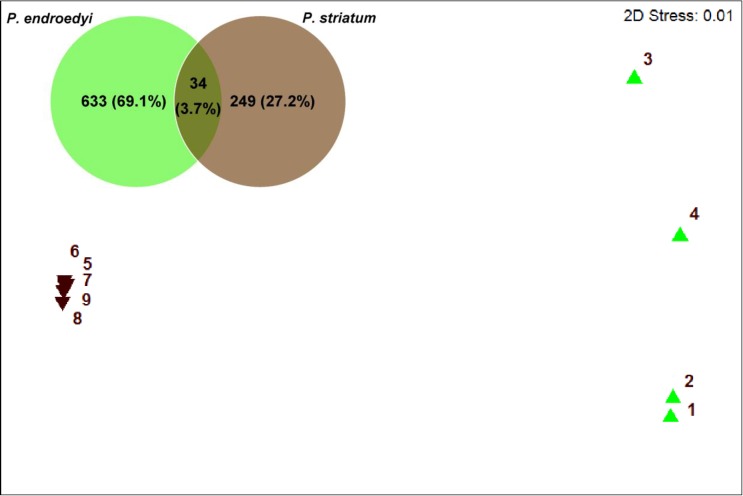
The gut bacterial communities of P. endroedyi and P. striatum were significantly different, sharing only 3.7% of bacterial OTUs (Fig 2; ANOSIM [R = 1.00, p<0.008]). Both host phylogeny and host diet could be driving forces for the observed differences [17, 55]. For example, the Hymenopteran gut microbiome has previously been shown to be influenced by host phylogeny, while the gut microbiomes of detritivorous insects (e.g., certain termites, Coleoptera and Diptera) are dictated by diet [17]. Gut bacterial communities of Drosophila spp. also appear to be impacted by host diet rather than host phylogeny [55]. In Coleoptera (the order in which Pachysoma is placed), gut bacterial communities are significantly different to those of other insect groups [17], indicating that host phylogeny is a significant driving force for gut microbiome assembly. However, within Coleoptera, significant similarities in bacterial assemblages of certain beetles with similar diets (e.g., those feeding on live arboreal tissue) have also been noted [17], which suggests that diet may also be a deterministic factor. It should, however, be noted that no coprophagous insects were included in this study [17], making a direct comparison with Pachysoma speculative.
Fig 2. nMDS ordination plot based on Bray-Curtis distance matrices of bacterial 16S rRNA gene pyrosequencing data for P. endroedyi and P. striatum individuals.
A stress value of less than 0.1 represents a high quality ordination. Pachysoma endroedyi and P. striatum are represented by green and inverted brown triangles, respectively.
It is not possible to compare the gut fungal communities of the two insect species studied, given that despite numerous attempts we were unable to PCR-amplify fungal ITS sequences from the detritivorous P. endroedyi. While we think it unlikely that fungal species are completely absent from the gut microbiome of this species, this negative result suggests that they may represent a relatively minor fraction of the total gut microbial diversity. To fully confirm this, the sample size should be increased and P. endroedyi individuals from multiple breeding populations should be investigated.
We would expect host diet to be a contributing factor in the presence (or absence) of fungi in the Pachysoma gut. For example, true yeasts (Saccharomycetes) are typically observed in the guts of litter-, plant- and wood-feeding insects [56–58], but not in those of predacious insects [49, 59].
Intraspecific variation of Pachysoma gut microbial communities
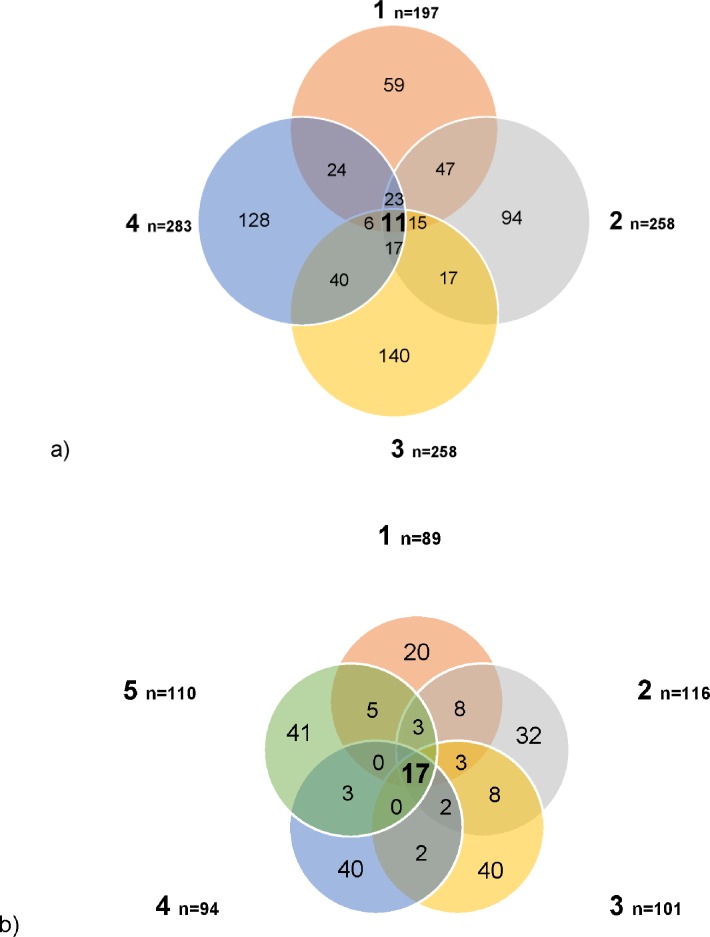
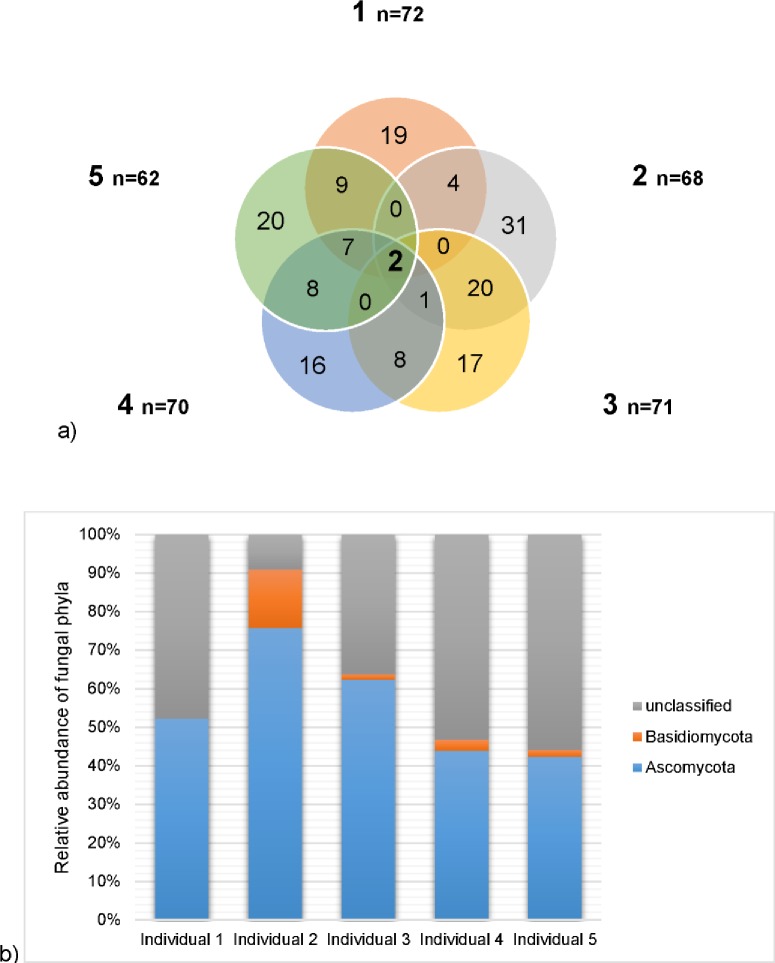
Large intraspecific differences in Pachysoma gut communities were noted, with the majority of OTUs being unique to each Pachysoma individual (Fig 3A and 3B, Fig 4A) and only 11 (1.1%) and 17 (3.3%) bacterial OTUs being shared between individuals of P. endroedyi and P. striatum, respectively. Furthermore, only two non-abundant fungal OTUs (ranging from 1.6–1.7% of the community) were shared among the five P. striatum individuals (Fig 4A). Such intraspecific differences, relating to the relative abundances and diversity of bacterial members of gut communities, are not uncommon, as has been observed for honeybees (Apis cerana and A. mellifera [52]), mosquitoes (Aedes spp., Culex spp., Anopheles spp., Mansonia spp.; [37, 38]) and the red palm weevils Rhynchophorus ferrugineus and R. vulneratus [25], among others. A recent study on the gut microbiomes of 218 different insect species from 21 orders [48] indicated that 46% of the total number of bacterial OTUs detected (n = 9301) were only observed in single individuals. The large intraspecific variation noted in Pachysoma could be influenced by the stochastic, and transient, process of microorganisms entering the gut with the food source [2] and, for P. striatum, the different amounts of feeding material contained in the guts of each individual [1]. Furthermore, it cannot be excluded that the ‘time of feeding’ prior to sampling may also have had an influence on intraspecific gut microbiome variability [1].
Fig 3.
Venn diagrams showing distribution of bacterial OTUs between (a) P. endroedyi and (b) P. striatum individuals based on the 16S rRNA gene pyrosequencing analysis. Shared OTUs are shown in bold. Numerical labels are shown for each individual.
Fig 4.
a) Venn diagram comparing the distribution of fungal OTUs between P. striatum individuals based on the ITS gene region pyrosequencing analysis and (b) relative abundance of fungal phyla in five P. striatum individuals based on ITS rRNA gene region pyrosequencing analysis at a 97% identity threshold. Shared OTUs in the Venn diagram are shown in bold with numerical labels given for each individual.
Of the shared bacterial OTUs, only one (assigned to the phylum Bacteroidetes) and eight (4 Firmicutes, 2 Actinobacteria, 1 Bacteroidetes and 1 Proteobacteria) were abundant (i.e., represented >2% reads) in the P. endroedyi and P. striatum gut samples, respectively. This distribution is strongly suggestive that the Pachysoma gut core community is very small, as has been proposed for the “minimal core” model [60]. Other studies have noted the presence of consistent core microbial communities within individuals of the same insect species (e.g., the bed bug Cimex lectularius; [61] and bumble bee Bombus terrestris [62, 63]), or across taxonomic levels (e.g., across the ant tribe Cephalotini; [64]). In the termite Reticulitermes flavipes, a substantial core bacterial microbiome (65% shared OTUs) was noted, regardless of the artificial feeding diet, suggesting that host phylogeny may play a more important role than host diet in the assembly of the gut microbiome [24]. Similar results have been noted in cockroaches [65]. However, with a minimal core microbiome in both Pachysoma spp., phylogeny appears less important than diet. Furthermore, a minimal core gut microbiome may result from negative interactions between gut microorganisms, such as antagonism or amensalism, or indicate, as for Drosophila [66], the establishment of ‘non-gut-specific’ microorganisms.
It has been suggested that a ‘functional’ rather than a ‘phylogenetic’ core microbiome may be more informative in determining the assembly of gut microbiomes [67]. In studies on humans, which typically follow the minimal core model, functional gene diversity appears to be broadly similar across individuals [67, 68]. Therefore, there may be a functional core community in each Pachysoma spp. studied, displaying shared metabolic capacities [68]; i.e., exhibiting functional redundancy. As such, it has been suggested that a comparison of functional properties of hosts feeding on different diets can guide an understanding of the functional roles of different gut microbiomes [67].
Phylogenetic diversity of bacterial and fungal Pachysoma gut communities
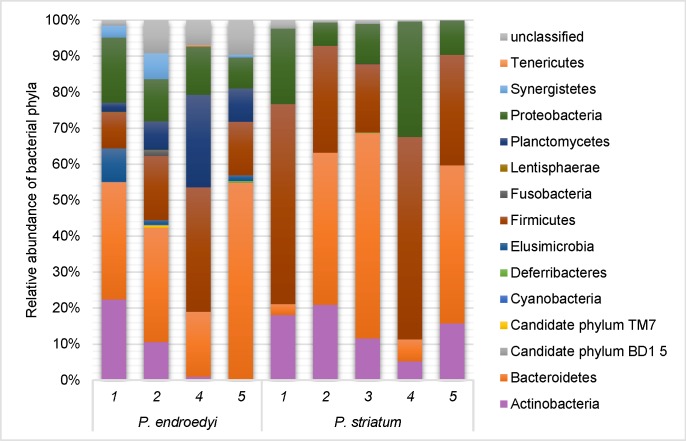
The gut bacterial diversity of P. endroedyi was higher (6–11 phyla; Fig 5) than that of P. striatum (4–7 phyla; Fig 5). The P. endroedyi gut samples were dominated by Bacteroidetes (18.0–54.8%), Firmicutes (10.0–34.6%), Proteobacteria (8.7–18.1%) and Planctomycetes (2.5–25.7%), while Actinobacteria (0.1–22.5%), Elusimocrobia (0–9.3%) and Synergistetes (0–7.3%) showed highly variable abundances (Fig 5). The remaining 7 phyla each represented less than 2% of the community and were often detected in single insects. In P. striatum, Bacteroidetes (3.0–57.1%), Firmicutes (18.9–56.2%), Proteobacteria (6.4–32.1%) and Actinobacteria (5.2–21.0%) were also dominant phyla although the relative abundances varied between individuals (Fig 5). Three minor phyla (<2% abundance) were only detected in two P. striatum individuals, namely Deferribacteres, Planctomycetes and Synergistetes (Fig 5). All the identified bacterial phyla have previously been reported in insect microbiomes, with the phyla Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria commonly abundant in insect gut samples [48, 69].
Fig 5. Comparison of interspecific differences in relative abundance of bacterial phyla in the gut of two Pachysoma spp., P. endroedyi and P. striatum, based on 16S rRNA gene pyrosequencing analysis.
The presence of specific bacterial phyla and/or their relative abundances in insect gut samples may be linked to host diet. For example, certain insects with simpler diets (e.g., feeding on pollen and nectar [52], fruit [22, 70], or sap [71]), contain gut bacterial communities which are typically dominated by heterotrophic Proteobacteria and/or Firmicutes. In contrast, Bacteroidetes (along with other phyla) were highly abundant in the gut microbiomes of insects feeding on plant materials such as wood and leaves [25, 54, 65, 72, 73]. The P. striatum gut bacterial communities did not display these patterns, suggesting that coprophagous diets may structure insect gut communities differently.
Fifteen and 11 bacterial genera were abundant (>2% relative abundance of reads) within the guts of P. endroedyi and P. striatum, respectively (Table 2). Only two of these genera were abundant in both species (Dysgonomonas and unclassified Enterobacteriaceae; Table 2). Dysgonomonas was less abundant in P. endroedyi gut samples (2.8% ± 0) than in P. striatum (26.3% ± 0.2), in which it was the most abundant genus. Dysgonomonas have been reported to be present at high abundance in the gut system of the fungus-growing termite (Macrotermes annandalei) and red palm weevil larvae (Rhynchophorus ferrugineus) [74, 75]. Two species of Dysgonomonas have previously been characterised from the gut of termites [76, 77]. Both species have been found to ferment glucose and xylan as a sole carbon source and to produce acetic acid as the major end-product [76, 77], suggesting roles in both the lignocellulosic biomass degradation pathway and in providing readily metabolisable substrates for ingestion by the host. The large difference in the abundance of this phylotype in the two Pachysoma species suggests a key nutritional role in P. striatum but not in P. endroedyi.
Table 2. Phylogenetic classification of the most abundant bacterial genera in the gut samples of P. endroedyi and P. striatum: i.e., representing >2% reads.
| Phylum | Family | Genus | P. endroedyi (%) | P. striatum (%) |
|---|---|---|---|---|
| Actinobacteria | Propionibacteriaceae | Proponiobacterium 1 | 8.2 ±0.1 | 0.1 ± 0 |
| Actinobacteria | Propionibacteriaceae | Tessaracoccus | 0 | 7.6 ± 0 |
| Actinobacteria | unclassified | unclassified | 0.1 ± 0 | 5.2 ± 0 |
| Bacteroidetes | Bacteroidaceae | Bacteroides | 7.6 ± 0 | 0 |
| Bacteroidetes | Marinilabiaceae | Uncultured 1 | 2.7 ± 0 | 0 |
| Bacteroidetes | Porphyromonadaceae 1 | Dysgonomonas | 2.8 ± 0 | 26.3 ± 0.2 |
| Bacteroidetes | Porphyromonadaceae 4 | Proteiniphilum | 0.2 ± 0 | 3.5 ± 0 |
| Bacteroidetes | Rikenellaceae | Alisitpes IV | 6.5 ± 0 | 0 |
| Bacteroidetes | Rikenellaceae | unclassified | 3.2 ± 0 | 0 |
| Bacteroidetes | unclassified | unclassified | 5.3 ± 0 | 0.1 ± 0 |
| Elusimicrobia | Endomicrobiaceae | Endomicrobium | 3.0 ± 0 | 0 |
| Firmicutes | Enterococcaceae | Vagococcus | 0.3 ± 0 | 4.9 ± 0 |
| Firmicutes | Family XI Incertae Sedis | unclassified | 0 | 10.9 ± 0.1 |
| Firmicutes | Lachnospiraceae | Uncultured 13 | 0.9 ± 0 | 2.9 ± 0 |
| Firmicutes | Lachnospiraceae | unclassified | 3.3 ± 0 | 0.7 ± 0 |
| Firmicutes | Ruminococcaceae | Termite cockroach cluster | 5.3 ± 0.1 | 0.1 ± 0 |
| Firmicutes | Ruminococcaceae | unclassified | 3.3 ± 0 | 1.4 ± 0 |
| Firmicutes | Veillonellaceae | Anaeroarcus-Anaeromusa | 0 | 11.5 ± 0.1 |
| Planctomycetes | unclassified | unclassified | 11.3 ± 0.1 | 0 |
| Proteobacteria | unclassified | unclassified | 0 | 3.1 ± 0 |
| Proteobacteria | Insect cluster | unclassified | 2.2 ± 0 | 0 |
| Proteobacteria | Enterobacteriaceae | unclassified | 4.2 ± 0.1 | 7.4 ± 0.1 |
| Proteobacteria | Enterobacteriaceae 1 | unclassified | 0 | 2.5 ± 0.1 |
| Synergistetes | Synergistaceae | Candidatus Tammella | 2.8 ± 0 | 0 |
Percentages are the average read relative abundances in each species (P. endroedyi: n = 4; P. striatum: n = 5). Colours depict the species in which the bacterial genus is abundant: P. endroedyi (green), P. striatum (brown) or both (blue). The most abundant genus of each species is shown in bold.
An unclassified Planctomycetes dominated the gut samples of P. endroedyi (11.3% ± 0.1 relative abundance of reads). To the best of our knowledge this is the first report of an insect gut microbiome dominated by Planctomycetes. Planctomycetes were only detected in a single P. striatum individual at very low abundance (0.01%). Planctomycetes have previously been detected in the guts of the termites Syntermes wheeleri and Nasutitermes spp. [58, 72], the cockroach Shelfordella lateralis [65], adult and larval beetles (Cryptocephalus spp., Prionoplus reticularis and Pachnoda spp.; [78–80]), the tree weta Hemideina thoracica [73] and the mosquito Aedes albopictus [81], but only in low abundances (<1–5% relative abundance).
Ascomycota was the most abundant fungal phylum (42.3–75.7%) in all P. striatum gut samples, which is typical for insect gut microbiomes [57, 58, 82, 83]. Basidiomycota were not ubiquitously detected, and were observed only in the gut samples of four of the five P. striatum individuals (1.8–15.2%; Fig 4B). A substantial proportion of fungal ITS sequence reads could not be classified, even at the phylum level (9.1–55.9%; Fig 4B). Unfortunately, relatively little is known about insect gut fungal diversity (compared to bacterial diversity [84]), with the majority of published studies being based on culture-dependent methods which are typically biased when compared with culture-independent methods [84, 85].
Conclusion
This is the first study to investigate the gut microbiomes of any dung beetle feeding on dry food sources and to compare those of closely related adult dung beetle species with very different diets but from the same locality. Pachysoma spp. are ecologically important in arid environments where they undoubtedly participate in nutrient cycling and bioturbation [41]. We have demonstrated that, as predicted, the gut microbiomes differed significantly between two species which feed on different substrates. However, both populations showed large intraspecific variations. Thus, to further characterise the gut microbiomes of these Pachysoma species, the number of individuals studied should be increased and populations from different sites investigated. Such experiments would make it possible to evaluate whether interspecific variation was higher than intraspecific variation within a single Pachysoma species.
We are unable to fully assess whether host phylogeny or the host diet is the dominant driver of the Pachysoma gut microbiomes. Nevertheless, we provide evidence that diet probably plays a significant role, particularly noting the fact that the gut microbiomes of the detritivorous P. endroedyi (feeding on complex food sources) have higher bacterial diversities than those of the coprophagous species (feeding on relatively simple food sources) [17, 48]. Functional gene analysis of the microbiomes of P. endroedyi and P. striatum could potentially assist in confirming the role that host diet plays in Pachysoma gut microbiome assembly [11].
Experimental procedures
Collection and storage of Pachysoma spp
Five adult individuals of P. endroedyi and of P. striatum (S2 Fig) from single breeding populations, feeding on plant detritus and dung respectively, were collected by the Scarab Research Group in September and October 2014 from coastal sandveld near Kommandokraal, Namaqualand, South Africa (S31°29'58.4" E18°12'29.2") under the Cape Nature permit number 0056-AAA008-00041. Ethical clearance is not necessary for work carried out on insects. Beetles were identified at the site. Due to their size, 99% ethanol was injected into their abdomens using sterile syringes for gut preservation [25]. Insects were then stored in 99% ethanol at -80°C, until dissection.
Gut dissection
Gut dissections were performed under a Zeiss Stemi 2000-C dissection microscope (Zeiss, Oberkochen, Germany) as previously described [86] with minor modifications. All equipment was sterilised before use with 10% bleach and 70% EtOH. The average body length of P. endreodyi ranges from 20.7–26.4mm, and the one of P. striatum ~19 mm [43]. The insects were placed in a wax-lined glass Petri dish with quarter strength autoclaved Ringer solution (0.12 g/L CaCl2, 0.105 g/L KCl, 0.05 g/L NaHCO3, 2.25 g/L NaCl; Sigma-Aldrich). The thorax and abdominal integument were removed using scissors before pinning the specimen to the wax layer in the Petri dish. Forceps were used to remove the membranes covering the internal organs. The rectum was pulled downwards, moving the gut gently out of the body cavity. The five P. endroedyi guts appeared full of diet material while the P. striatum ones were empty (n = 1), half-full (n = 1) or full (n = 3). Hindgut and midgut samples were separated and stored in 1.5ml eppendorf tubes at -20°C until DNA extraction.
Metagenomic DNA extraction
Gut-section metagenomic DNA extractions were performed using a modified version of the protocols previously described by [87, 88]. Whole-guts were weighed and crushed in liquid nitrogen using sterile mortars and pestles. For 10mg of gut, 100μl of a preheated (60°C) 2% CTAB solution (0.1M Tris HCl [pH8.0], 1.4M NaCl, 0.02M EDTA [pH8.0]) was added. The mixtures were incubated for 30min at 60°C before centrifugation for 5min at 10000rpm. The supernatant was transferred to a clean collection tube and enzymatic digestion of the gut samples was carried out with the addition of 2μl lysozyme (5mg/ml) per 100μl CTAB solution for 30min at 37°C under continuous shaking (120 rpm). 0.5μl Proteinase K (20mg/ml) per 100μl CTAB was then added [89], followed by an overnight incubation at 55°C with continuous shaking. One volume phenol:chloroform:isoamyl alcohol (25:24:1) solution was added. Tubes were inverted and centrifuged at 13000rpm at 4°C for 4min. One volume chloroform:isoamyl alcohol (24:1) solution was added to the top aqueous phase and the mixtures were inverted before centrifugation at 13000rpm at 4°C for 15min. This step was repeated until no protein contamination was observed [89]. DNA was precipitated with 3M NH4Ac [90] and ice cold 99.9% EtOH followed by overnight incubation at -20°C. Mixtures were centrifuged for 60min at 14000rpm at 4°C. The DNA pellet was washed twice with ice cold 70% EtOH and allowed to dry completely for 2 hours. The DNA pellet was resuspended in 50μl filter-sterilized nanopure H2O overnight at 4°C [90], and stored at -20°C for downstream analysis.
454 pyrosequencing of the bacterial 16S rRNA gene and fungal ITS gene region
The gut metagenomic DNA of five individuals (equal concentrations of combined hindgut and midgut-derived DNA) from each Pachysoma spp. was sent to Molecular Research (www.mrdnalab.com) for 16S rRNA gene and ITS gene region pyrosequencing using the Roche 454 GS FLX titanium platform. The primers 27F (AGRGTTTGATCMTGGCTCAG; [91]) and 338R (AGTGCTGCCTCCCGTAGGAGT; [92] were used to amplify the 16S rRNA gene region as they have a low eukaryotic coverage (27F: 0%; 338R: [93]). Fungal specific fITS9 (GAACGCAGCRAAIIGYGA; [94]) and ITS4 (TCCTCCGCTTATTGATATGC; [95]) primers were used for the amplification of the ITS gene region.
Data analysis
Raw pyrosequencing reads were filtered and analysed using MOTHUR version 1.35.1 (Accessed May 2015- January 2016; [96, 97]. In short, fasta, quality and flow files were extracted from the sff files using the sff.info command. For the bacterial 16S rRNA gene pyrosequencing reads, filtering of poor quality reads was done using the shhh.flows command allowing for reads to have one or two mismatches between the barcodes and primers respectively. Remaining sequences were quality filtered with the trim.seqs command to maximum homopolymers of 8bp and a minimum sequence length of 100bp. Sequences were aligned to the SILVA reference database (http://www.arbsilva.de/download/arb-files/) using the align.seqs command. The screen.seqs and filter.seqs commands were used to retain only overlapping sequences. Chimeras were identified and removed using the chimera.uchime command. Sequences were classified against five databases, namely the Ribosomal Database Project (RDP), SILVA, NCBI, The Dictyoptera gut microbiota reference Database (DictDb; data shown) and GreenGenes with a confidence threshold of 80%. OTUs were clustered for each individual beetle before removal of singletons using the remove.rare command. Samples were subsampled 1718 reads, i.e., the lowest number of reads across all samples.
ITS reads were analysed similarly to that of the 16S reads with minor differences as outlined previously [98]. Filtering of poor quality reads was done using the trim.seqs command allowing for reads to have one or four mismatches between the barcodes and primers respectively. Sequences were trimmed to 200bp using the chop.seqs command to ensure all sequences were the same length. Sequences were classified against the UNITE database (Version 6) with a confidence threshold of 50% and subsampled to the lowest number of OTUs across all samples (107) for statistical analyses.
Phylogenetic comparisons, of both the bacterial and fungal datasets, were done using the relative abundance of all reads in the dataset so as to ensure inclusion of rare taxa. Relative abundances (%) were calculated from the number of reads of the microbial organism(s) in question divided by the total number of reads for the particular Pachysoma individual.
Nucleotide sequences for both the bacterial and fungal datasets have been uploaded to NCBI (http://www.ncbi.nlm.nih.gov/) Short Read Archive (SRA) under the accession number SRP071915.
Statistical Analysis
Two-dimensional Non-Metric Multi-Dimensional Scaling (nMDS) plots were constructed in Primer 6 software (version 6.1.5.81 (Primer E Ltd, Plymyth, UK)) after applying square-root pre-treatment and using the Bray-Curtis coefficient [99] to build a dissimilarity matrix. Kruskal’s stress value was used to determine the efficiency of sample placement in both two- and three-dimensional nMDS plots. Significant differences in bacterial gut communities were determined using one-way global Analysis of Similarities (ANOSIM) in Primer 6 software version 6.1.5.81 (Primer E Ltd, Plymyth, UK) using 10 000 permutations [100]. A Venn plot was created using R (2.15.1) (www.rproject.org) to differentiate between unique and shared OTUs dependent on feeding strategy. Diversity indices and rarefaction curves were generated in Mothur [97]. Singletons were removed prior to analyses.
Supporting Information
Rarefactions curves showing gut microbial community richness of all Pachysoma individuals for bacterial 16S rRNA gene amplicon data of: a) P. endroedyi, b) P. striatum; and c) fungal ITS gene region amplicon data of P. striatum.
(TIF)
Photographs of P. endroedyi (a) and P. striatum (b) in their natural environment before collection (courtesy of Hennie de Klerk).
(TIF)
Acknowledgments
We would like to thank members of the Centre for Microbial Ecology and Genomics for assistance, in particular Dr S. Vikram, Dr, O. Bezuidt, Mr M. van Goethem, Dr A. Valverde and Mr. R. Johnson. We would also like to thank Hennie de Klerk for use of his Pachysoma photos.
Data Availability
Sequences for both the bacterial and fungal datasets have been uploaded to NCBI (http://www.ncbi.nlm.nih.gov/) Short Read Archive (SRA) under the accession numbers SAMN04546166--SAMN04546175.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Dillon R, Dillon V. The gut bacteria of insects: nonpathogenic interactions. Annual Reviews in Entomology. 2004;49(1):71–92. [DOI] [PubMed] [Google Scholar]
- 2.Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annual Review of Entomology. 2015;60:17–34. 10.1146/annurev-ento-010814-020822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2012;3(4):307–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brune A. Symbiotic associations between termites and prokaryotes In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes: Springer Berlin; Heidelberg; 2013. p. 545–77. [Google Scholar]
- 5.Huang SW, Zhang HY, Marshall S, Jackson TA. The scarab gut: a potential bioreactor for bio‐fuel production. Insect Science. 2010;17(3):175–83. [Google Scholar]
- 6.Engel P, Moran NA. The gut microbiota of insects–diversity in structure and function. FEMS Microbiology Reviews. 2013;37(5):699–735. 10.1111/1574-6976.12025 [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition. 2002;22(1):283–307. [DOI] [PubMed] [Google Scholar]
- 8.Douglas A. The microbial dimension in insect nutritional ecology. Functional Ecology. 2009;23(1):38–47. [Google Scholar]
- 9.Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K. Endogenous plant cell wall digestion: a key mechanism in insect evolution. Annual Review of Ecology, Evolution, and Systematics. 2012;43:45–71. [Google Scholar]
- 10.Park D, Oh H, Jeong W, Kim H, Park H, Bae KS. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. Journal of Microbiology-Seoul. 2007;45(5):394–401. [PubMed] [Google Scholar]
- 11.He S, Ivanova N, Kirton E, Allgaier M, Bergin C, Scheffrahn RH, et al. Comparative metagenomic and metatranscriptomic analysis of hindgut paunch microbiota in wood-and dung-feeding higher termites. PLoS One. 2013;8(4):e61126 10.1371/journal.pone.0061126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas AE. Microbial brokers of insect-plant interactions revisited. Journal of Chemical Ecology. 2013;39(7):952–61. 10.1007/s10886-013-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon R, Vennard C, Charnley A. A note: gut bacteria produce components of a locust cohesion pheromone. Journal of Applied Microbiology. 2002;92(4):759–63. [DOI] [PubMed] [Google Scholar]
- 14.Nardi JB, Mackie RI, Dawson JO. Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems? Journal of Insect Physiology. 2002;48(8):751–63. [DOI] [PubMed] [Google Scholar]
- 15.Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proceedings of the National Academy of Sciences. 2011;108(48):19288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, et al. Symbiotic bacterium modifies aphid body color. Science. 2010;330(6007):1102–4. 10.1126/science.1195463 [DOI] [PubMed] [Google Scholar]
- 17.Colman DR, Toolson EC, Takacs‐Vesbach CD. Do diet and taxonomy influence insect gut bacterial communities? Molecular Ecology. 2012;21(20):5124–37. 10.1111/j.1365-294X.2012.05752.x [DOI] [PubMed] [Google Scholar]
- 18.Vasanthakumar A, Handelsman J, Schloss PD, Bauer LS, Raffa KF. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environmental Entomology. 2008;37(5):1344–53. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Cordero E, Ping L, Reichwald K, Delb H, Platzer M, Boland W. Comparative evaluation of the gut microbiota associated with the below-and above-ground life stages (larvae and beetles) of the forest cockchafer, Melolontha hippocastani. PloS One. 2012;7(12):e51557 10.1371/journal.pone.0051557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diouf M, Roy V, Mora P, Frechault S, Lefebvre T, Hervé V, et al. Profiling the succession of bacterial communities throughout the life stages of a higher termite Nasutitermes arborum (Termitidae, Nasutitermitinae) using 16S rRNA gene pyrosequencing. PloS One. 2015;10(10):e0140014 10.1371/journal.pone.0140014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Gilbreath III TM, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PloS One. 2011;6(9):e24767 10.1371/journal.pone.0024767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andongma AA, Wan L, Dong Y-C, Desneux N, White JA, Niu C-Y. Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis. Scientific Reports. 2015;5 10.1038/srep09470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertino-Grimaldi D, Medeiros MN, Vieira RP, Cardoso AM, Turque AS, Silveira CB, et al. Bacterial community composition shifts in the gut of Periplaneta americana fed on different lignocellulosic materials. SpringerPlus. 2013;2(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X-F, Bakker MG, Judd TM, Reardon KF, Vivanco JM. Variations in diversity and richness of gut bacterial communities of termites (Reticulitermes flavipes) fed with grassy and woody plant substrates. Microbial Ecology. 2013;65(3):531–6. 10.1007/s00248-013-0219-y [DOI] [PubMed] [Google Scholar]
- 25.Montagna M, Chouaia B, Mazza G, Prosdocimi EM, Crotti E, Mereghetti V, et al. Effects of the diet on the microbiota of the red palm weevil (Coleoptera: Dryophthoridae). PloS One. 2015;10(1):e0117439 10.1371/journal.pone.0117439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyata R, Noda N, Tamaki H, Kinjyo K, Aoyagi H, Uchiyama H, et al. Influence of feed components on symbiotic bacterial community structure in the gut of the wood-feeding higher termite Nasutitermes takasagoensis. Bioscience, Biotechnology, and Biochemistry. 2007;71(5):1244–51. [DOI] [PubMed] [Google Scholar]
- 27.Santo Domingo J, Kaufman M, Klug M, Holben W, Harris D, Tiedje J. Influence of diet on the structure and function of the bacterial hindgut community of crickets. Molecular Ecology. 1998;7(6):761–7. [Google Scholar]
- 28.Kaufman MG, Klug MJ. The contribution of hindgut bacteria to dietary carbohydrate utilization by crickets (Orthoptera: Gryllidae). Comparative Biochemistry and Physiology Part A: Physiology. 1991;98(1):117–23. [Google Scholar]
- 29.Schauer C, Thompson C, Brune A. Pyrotag sequencing of the gut microbiota of the cockroach Shelfordella lateralis reveals a highly dynamic core but only limited effects of diet on community structure. PloS One. 2014;9(1):e85861 10.1371/journal.pone.0085861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brune A, Friedrich M. Microecology of the termite gut: structure and function on a microscale. Current Opinion in Microbiology. 2000;3(3):263–9. [DOI] [PubMed] [Google Scholar]
- 31.Husseneder C. Symbiosis in subterranean termites: a review of insights from molecular studies. Environmental Entomology. 2010;39(2):378–88. 10.1603/EN09006 [DOI] [PubMed] [Google Scholar]
- 32.Ohkuma M. Termite symbiotic systems: efficient bio-recycling of lignocellulose. Applied Microbiology and Biotechnology. 2003;61(1):1–9. [DOI] [PubMed] [Google Scholar]
- 33.Poulsen M. Towards an integrated understanding of the consequences of fungus domestication on the fungus‐growing termite gut microbiota. Environmental Microbiology. 2015;17(8):2562–72. 10.1111/1462-2920.12765 [DOI] [PubMed] [Google Scholar]
- 34.Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450(7169):560–5. [DOI] [PubMed] [Google Scholar]
- 35.Hamdi C, Balloi A, Essanaa J, Crotti E, Gonella E, Raddadi N, et al. Gut microbiome dysbiosis and honeybee health. Journal of Applied Entomology. 2011;135(7):524–33. [Google Scholar]
- 36.Moran NA. Genomics of the honey bee microbiome. Current Opinion in Insect Science. 2015;10:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osei‐Poku J, Mbogo C, Palmer W, Jiggins F. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Molecular Ecology. 2012;21(20):5138–50. 10.1111/j.1365-294X.2012.05759.x [DOI] [PubMed] [Google Scholar]
- 38.Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathogens. 2012;8(5):e1002742 10.1371/journal.ppat.1002742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Molecular Ecology. 2014;23(11):2727–39. 10.1111/mec.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proceedings of the National Academy of Sciences. 2012;109(31):12734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila M, et al. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation. 2008;141(6):1461–74. [Google Scholar]
- 42.Sole CL, Scholtz CH, Bastos AD. Phylogeography of the Namib Desert dung beetles Scarabaeus (Pachysoma) MacLeay (Coleoptera: Scarabaeidae). Journal of Biogeography. 2005;32(1):75–84. [Google Scholar]
- 43.Harrison JdG, Scholtz C, Chown S. A revision of the endemic south-western African dung beetle subgenus Scarabaeus (Pachysoma) MacLeay, including notes on other flightless Scarabaeini (Scarabaeidae: Scarabaeinae). Journal of Natural History. 2003;37(3):305–55. [Google Scholar]
- 44.Holter P, Scholtz CH. Elongated hindguts in desert‐living dung beetles (Scarabaeidae: Scarabaeinae) feeding on dry dung pellets or plant litter. Journal of Morphology. 2013;274(6):657–62. 10.1002/jmor.20123 [DOI] [PubMed] [Google Scholar]
- 45.Holter P, Scholtz CH. Re‐establishment of biting mouthparts in desert‐living dung beetles (Scarabaeidae: Scarabaeinae) feeding on plant litter—old structures reacquired or new ones evolved? Journal of Morphology. 2011;272(8):1007–16. 10.1002/jmor.10968 [DOI] [PubMed] [Google Scholar]
- 46.Scholtz C. Unique foraging behaviour in Pachysoma. Journal of Arid Environments. 1989;16(3):305–13. [Google Scholar]
- 47.Douglas AE. Lessons from studying insect symbioses. Cell Host & Microbe. 2011;10(4):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun J-H, Roh SW, Whon TW, Jung M-J, Kim M-S, Park D-S, et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Applied and Environmental Microbiology. 2014;80(17):5254–64. 10.1128/AEM.01226-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen NH, Suh S-O, Blackwell M. Five novel Candida species in insect-associated yeast clades isolated from Neuroptera and other insects. Mycologia. 2007;99(6):842–58. [DOI] [PubMed] [Google Scholar]
- 50.Sabree ZL, Moran NA. Host-specific assemblages typify gut microbial communities of related insect species. SpringerPlus. 2014;3(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim YW, Kim BK, Kim C, Jung HS, Kim B-S, Lee J-H, et al. Assessment of soil fungal communities using pyrosequencing. The Journal of Microbiology. 2010;48(3):284–9. 10.1007/s12275-010-9369-5 [DOI] [PubMed] [Google Scholar]
- 52.Ahn J-H, Hong I-P, Bok J-I, Kim B-Y, Song J, Weon H-Y. Pyrosequencing analysis of the bacterial communities in the guts of honey bees Apis cerana and Apis mellifera in Korea. Journal of Microbiology. 2012;50(5):735–45. [DOI] [PubMed] [Google Scholar]
- 53.Gauthier J- P, Outreman Y, Mieuzet L, Simon J- C. Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PloS One. 2015;10(3):e0120664 10.1371/journal.pone.0120664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boucias DG, Cai Y, Sun Y, Lietze VU, Sen R, Raychoudhury R, et al. The hindgut lumen prokaryotic microbiota of the termite Reticulitermes flavipes and its responses to dietary lignocellulose composition. Molecular Ecology. 2013;22(7):1836–53. 10.1111/mec.12230 [DOI] [PubMed] [Google Scholar]
- 55.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host–microbe model system. PLoS Genetics. 2011;7(9):e1002272 10.1371/journal.pgen.1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suh SO, Nguyen NH, Blackwell M. Yeasts isolated from plant‐associated beetles and other insects: seven novel Candida species near Candida albicans. FEMS Yeast Research. 2008;8(1):88–102. [DOI] [PubMed] [Google Scholar]
- 57.Scully ED, Geib SM, Hoover K, Tien M, Tringe SG, Barry KW, et al. Metagenomic profiling reveals lignocellulose degrading system in a microbial community associated with a wood-feeding beetle. PLoS One. 2013;8(9):e73827 10.1371/journal.pone.0073827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santana RH, Catão ECP, Lopes FAC, Constantino R, Barreto CC, Krüger RH. The gut microbiota of workers of the litter-feeding termite Syntermes wheeleri (Termitidae: Syntermitinae): archaeal, bacterial, and fungal communities. Microbial Ecology. 2015;70:545–56. 10.1007/s00248-015-0581-z [DOI] [PubMed] [Google Scholar]
- 59.Shao M-W, Lu Y-H, Miao S, Zhang Y, Chen T-T, Zhang Y-L. Diversity, bacterial symbionts and antibacterial potential of gut-associated fungi isolated from the Pantala flavescens larvae in China. PloS One. 2015;10(7):e0134542 10.1371/journal.pone.0134542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamady M, Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Research. 2009;19(7):1141–52. 10.1101/gr.085464.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meriweather M, Matthews S, Rio R, Baucom RS. A 454 survey reveals the community composition and core microbiome of the common bed bug (Cimex lectularius) across an urban landscape. PloS One. 2013;8(4):e61465 10.1371/journal.pone.0061465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Billiet A, Meeus I, Nieuwerburgh F, Deforce D, Wäckers F, Smagghe G. Colony contact contributes to the diversity of gut bacteria in bumblebees (Bombus terrestris). Insect science. 2015. [DOI] [PubMed] [Google Scholar]
- 63.Meeus I, Parmentier L, Billiet A, Maebe K, Van Nieuwerburgh F, Deforce D, et al. 16S rRNA amplicon sequencing demonstrates that indoor-reared bumblebees (Bombus terrestris) harbor a core subset of bacteria normally associated with the wild host. PloS One. 2015;10(4):e0125152 10.1371/journal.pone.0125152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson KE, Russell JA, Moreau CS, Kautz S, Sullam KE, Hu Y, et al. Highly similar microbial communities are shared among related and trophically similar ant species. Molecular Ecology. 2012;21(9):2282–96. 10.1111/j.1365-294X.2011.05464.x [DOI] [PubMed] [Google Scholar]
- 65.Schauer C, Thompson C, Brune A. Pyrotag sequencing of the gut microbiota of the cockroach Shelfordella lateralis reveals a highly dynamic core but only limited effects of diet on community structure. 2014. [DOI] [PMC free article] [PubMed]
- 66.Wong AC, Chaston JM, Douglas AE. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. The ISME Journal. 2013;7(10):1922–32. 10.1038/ismej.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karasov WH, Martínez del Rio C, Caviedes-Vidal E. Ecological physiology of diet and digestive systems. Annual Review of Physiology. 2011;73:69–93. 10.1146/annurev-physiol-012110-142152 [DOI] [PubMed] [Google Scholar]
- 68.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones RT, Sanchez LG, Fierer N. A cross-taxon analysis of insect-associated bacterial diversity. PLoS One. 2013;8(4):e61218 10.1371/journal.pone.0061218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang A, Yao Z, Zheng W, Zhang H. Bacterial communities in the gut and reproductive organs of Bactrocera minax (Diptera: Tephritidae) based on 454 pyrosequencing. PloS One. 2014;9(9):e106988 10.1371/journal.pone.0106988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandey N, Rajagopal R. Molecular characterization and diversity analysis of bacterial communities associated with Dialeurolonga malleswaramensis (Hemiptera: Aleyrodidae) adults using 16S rDNA amplicon pyrosequencing and FISH. Insect Science. 2015. 10.1111/1744-7917.12220 [DOI] [PubMed] [Google Scholar]
- 72.Köhler T, Dietrich C, Scheffrahn RH, Brune A. High-resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes spp.). Applied and Environmental Microbiology. 2012;78(13):4691–701. 10.1128/AEM.00683-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waite DW, Dsouza M, Biswas K, Ward DF, Deines P, Taylor MW. Microbial community structure in the gut of the New Zealand insect Auckland tree weta (Hemideina thoracica). Archives of Microbiology. 2015;197(4):603–12. 10.1007/s00203-015-1094-3 [DOI] [PubMed] [Google Scholar]
- 74.Zhang M, Liu N, Qian C, Wang Q, Wang Q, Long Y, et al. Phylogenetic and functional analysis of gut microbiota of a fungus-growing higher termite: Bacteroidetes from higher termites are a rich source of β-glucosidase genes. Microbial Ecology. 2014;68(2):416–25. 10.1007/s00248-014-0388-3 [DOI] [PubMed] [Google Scholar]
- 75.Tagliavia M, Messina E, Manachini B, Cappello S, Quatrini P. The gut microbiota of larvae of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae). BMC Microbiology. 2014;14(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y-j, Zhang N, Ji S-q, Lan X, Shen Y-l, Li F-l, et al. Dysgonomonas macrotermitis sp. nov., isolated from the hindgut of a fungus-growing termite. International Journal of Systematic and Evolutionary Microbiology. 2014;64(9):2956–61. [DOI] [PubMed] [Google Scholar]
- 77.Pramono AK, Sakamoto M, Iino T, Hongoh Y, Ohkuma M. Dysgonomonas termitidis sp. nov., isolated from the gut of the subterranean termite Reticulitermes speratus. International Journal of Systematic and Evolutionary Microbiology. 2015;65(2):681–5. [DOI] [PubMed] [Google Scholar]
- 78.Reid NM, Addison SL, Macdonald LJ, Lloyd-Jones G. Biodiversity of active and inactive bacteria in the gut flora of wood-feeding huhu beetle larvae (Prionoplus reticularis). Applied and Environmental Microbiology. 2011;77(19):7000–6. 10.1128/AEM.05609-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andert J, Marten A, Brandl R, Brune A. Inter-and intraspecific comparison of the bacterial assemblages in the hindgut of humivorous scarab beetle larvae (Pachnoda spp.). FEMS Microbiology Ecology. 2010;74(2):439–49. 10.1111/j.1574-6941.2010.00950.x [DOI] [PubMed] [Google Scholar]
- 80.Montagna M, Gómez‐Zurita J, Giorgi A, Epis S, Lozzia G, Bandi C. Metamicrobiomics in herbivore beetles of the genus Cryptocephalus (Chrysomelidae): toward the understanding of ecological determinants in insect symbiosis. Insect Science. 2015;22(3):340–52. 10.1111/1744-7917.12143 [DOI] [PubMed] [Google Scholar]
- 81.Zouache K, Michelland RJ, FAILLOUX AB, Grundmann GL, Mavingui P. Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Molecular Ecology. 2012;21(9):2297–309. 10.1111/j.1365-294X.2012.05526.x [DOI] [PubMed] [Google Scholar]
- 82.Suh S-O, McHugh JV, Blackwell M. Expansion of the Candida tanzawaensis yeast clade: 16 novel Candida species from basidiocarp-feeding beetles. International Journal of Systematic and Evolutionary Microbiology. 2004;54(6):2409–29. [DOI] [PubMed] [Google Scholar]
- 83.Suh S-O, McHUGH JV, Pollock DD, Blackwell M. The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research. 2005;109(03):261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibson CM, Hunter MS. Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecology Letters. 2010;13(2):223–34. 10.1111/j.1461-0248.2009.01416.x [DOI] [PubMed] [Google Scholar]
- 85.Zhang N, Suh S-O, Blackwell M. Microorganisms in the gut of beetles: evidence from molecular cloning. Journal of Invertebrate Pathology. 2003;84(3):226–33. [DOI] [PubMed] [Google Scholar]
- 86.Correa VR, Majerczak DR, Ammar E-D, Merighi M, Pratt RC, Hogenhout SA, et al. The bacterium Pantoea stewartii uses two different type III secretion systems to colonize its plant host and insect vector. Applied and Environmental Microbiology. 2012;78(17):6327–36. 10.1128/AEM.00892-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Calderón-Cortés N, Quesada M, Cano-Camacho H, Zavala-Páramo G. A simple and rapid method for DNA isolation from xylophagous insects. International Journal of Molecular Sciences. 2010;11(12):5056–64. 10.3390/ijms11125056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Z, Wang L, Zhang H. Low diversity bacterial community and the trapping activity of metabolites from cultivable bacteria species in the female reproductive system of the oriental fruit fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae). International journal of molecular sciences. 2012;13(5):6266–78. 10.3390/ijms13056266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Priya NG, Ojha A, Kajla MK, Raj A, Rajagopal R. Host plant induced variation in gut bacteria of Helicoverpa armigera. PloS one. 2012;7(1):e30768 10.1371/journal.pone.0030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lagisz M, Port G, Wolff K. A cost‐effective, simple and high‐throughput method for DNA extraction from insects. Insect Science. 2010;17(5):465–70. [Google Scholar]
- 91.Lane D. 16S/23S rRNA sequencing S E, G M, editors. Chichester: Wiley; 1991. 115–75 p. [Google Scholar]
- 92.Fierer N, Hamady M, Lauber CL, Knight R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proceedings of the National Academy of Sciences. 2008;105(46):17994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loy A, Maixner F, Wagner M, Horn M. ProbeBase—an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Research. 2007;35(suppl 1):800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ihrmark K, Bödeker I, Cruz‐Martinez K, Friberg H, Kubartova A, Schenck J, et al. New primers to amplify the fungal ITS2 region–evaluation by 454‐sequencing of artificial and natural communities. FEMS Microbiology Ecology. 2012;82(3):666–77. 10.1111/j.1574-6941.2012.01437.x [DOI] [PubMed] [Google Scholar]
- 95.Gardes M, Bruns TD. ITS primers with enhanced specificity for Basidiomycetes‐application to the identification of Mycorrhizae and rusts. Molecular Ecology. 1993;2(2):113–8. [DOI] [PubMed] [Google Scholar]
- 96.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75(23):7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PloS One. 2011;6(12):e27310 10.1371/journal.pone.0027310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bell TH, Hassan SE-D, Lauron-Moreau A, Al-Otaibi F, Hijri M, Yergeau E, et al. Linkage between bacterial and fungal rhizosphere communities in hydrocarbon-contaminated soils is related to plant phylogeny. The ISME Journal. 2014;8(2):331–43. 10.1038/ismej.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs. 1957;27(4):325–49. [Google Scholar]
- 100.CLARKE KR. Non‐parametric multivariate analyses of changes in community structure. Australian Journal of Ecology. 1993;18(1):117–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefactions curves showing gut microbial community richness of all Pachysoma individuals for bacterial 16S rRNA gene amplicon data of: a) P. endroedyi, b) P. striatum; and c) fungal ITS gene region amplicon data of P. striatum.
(TIF)
Photographs of P. endroedyi (a) and P. striatum (b) in their natural environment before collection (courtesy of Hennie de Klerk).
(TIF)
Data Availability Statement
Sequences for both the bacterial and fungal datasets have been uploaded to NCBI (http://www.ncbi.nlm.nih.gov/) Short Read Archive (SRA) under the accession numbers SAMN04546166--SAMN04546175.