Abstract
IMPORTANCE
The appropriate treatment target for systolic blood pressure (SBP) in older patients with hypertension remains uncertain.
OBJECTIVE
To evaluate the effects of intensive (<120 mm Hg) compared with standard (<140 mm Hg) SBP targets in persons aged 75 years or older with hypertension but without diabetes.
DESIGN, SETTING, AND PARTICIPANTS
A multicenter, randomized clinical trial of patients aged 75 years or older who participated in the Systolic Blood Pressure Intervention Trial (SPRINT). Recruitment began on October 20, 2010, and follow-up ended on August 20, 2015.
INTERVENTIONS
Participants were randomized to an SBP target of less than 120 mm Hg (intensive treatment group, n = 1317) or an SBP target of less than 140 mm Hg (standard treatment group, n = 1319).
MAIN OUTCOMES AND MEASURES
The primary cardiovascular disease outcome was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes. All-cause mortality was a secondary outcome.
RESULTS
Among 2636 participants (mean age, 79.9 years; 37.9% women), 2510 (95.2%) provided complete follow-up data. At a median follow-up of 3.14 years, there was a significantly lower rate of the primary composite outcome (102 events in the intensive treatment group vs 148 events in the standard treatment group; hazard ratio [HR], 0.66 [95% CI, 0.51–0.85]) and all-cause mortality (73 deaths vs 107 deaths, respectively; HR, 0.67 [95% CI, 0.49–0.91]). The overall rate of serious adverse events was not different between treatment groups (48.4% in the intensive treatment group vs 48.3% in the standard treatment group; HR, 0.99 [95% CI, 0.89–1.11]). Absolute rates of hypotension were 2.4% in the intensive treatment group vs 1.4% in the standard treatment group (HR, 1.71 [95% CI, 0.97–3.09]), 3.0% vs 2.4%, respectively, for syncope (HR, 1.23 [95% CI, 0.76–2.00]), 4.0% vs 2.7% for electrolyte abnormalities (HR, 1.51 [95% CI, 0.99–2.33]), 5.5% vs 4.0% for acute kidney injury (HR, 1.41 [95% CI, 0.98–2.04]), and 4.9% vs 5.5% for injurious falls (HR, 0.91 [95% CI, 0.65–1.29]).
CONCLUSIONS AND RELEVANCE
Among ambulatory adults aged 75 years or older, treating to an SBP target of less than 120 mm Hg compared with an SBP target of less than 140 mm Hg resulted in significantly lower rates of fatal and nonfatal major cardiovascular events and death from any cause.
In the United States, 75% of persons older than 75 years have hypertension, for whom cardiovascular disease complications are a leading cause of disability, morbidity, and mortality.1–3 Current guidelines provide inconsistent recommendations regarding the optimal systolic blood pressure (SBP) treatment target in geriatric populations.4 European guideline committees have recommended treatment initiation only above 160 mm Hg for persons aged 80 years or older.5 A recent US guideline, a report from the panel appointed to the Eighth Joint National Committee, recommended a SBP treatment target of 150 mm Hg for adults aged 60 years or older.6 However, a report from a minority of the members argued to retain the previously recommended SBP treatment goal of 140 mm Hg, highlighting the lack of consensus.7
Whether treatment targets should consider factors such as frailty or functional status is also unknown. Observational studies have noted differential associations among elevated blood pressure (BP) and cardiovascular disease, stroke, and mortality risk when analyses are stratified according to measures of functional status.8–10 A recent secondary analysis of the Systolic Hypertension in the Elderly Program showed that the benefit of antihypertensive therapy was limited to participants without a self-reported physical ability limitation.11 In contrast, analyses from the Hypertension in the Very Elderly Trial (HYVET) showed a consistent benefit with antihypertensive therapy on outcomes irrespective of frailty status.12
The Systolic Blood Pressure Intervention Trial (SPRINT) recently reported that participants assigned to an intensive SBP treatment target of less than 120 mm Hg vs the standard SBP treatment goal of less than 140 mm Hg had a 25% lower relative risk of major cardiovascular events and death, and a 27% lower relative risk of death from any cause.13 This trial was specifically funded to enhance recruitment of a prespecified subgroup of adults aged 75 years or older, and the study protocol (appears in Supplement 1) also included measures of functional status and frailty. This article details results for the prespecified subgroup of adults aged 75 years or older with hypertension.
Methods
Population
The design, eligibility, and baseline characteristics of SPRINT have been described.14 The trial protocol was approved by the institutional review board at each participating site. Study participants signed written informed consent and were required to be at increased risk for cardiovascular disease (based on a history of clinical or subclinical cardiovascular disease, chronic kidney disease [CKD], a 10-year Framingham General cardiovascular disease risk ≥15%, or age ≥75 years). A person was excluded if he or she had type 2 diabetes, a history of stroke, symptomatic heart failure within the past 6 months or reduced left ventricular ejection fraction (<35%), a clinical diagnosis of or treatment for dementia, an expected survival of less than 3 years, unintentional weight loss (>10% of body weight) during the preceding 6 months, an SBP of less than 110 mm Hg following 1 minute of standing, or resided in a nursing home.
Study Measurements
Sociodemographic data were collected at baseline, whereas both clinical and laboratory data were obtained at baseline and every 3 months. Race and ethnicity information was obtained via self-report. Blood pressure was determined using the mean of 3 properly sized automated cuff readings, taken 1 minute apart after 5 minutes of quiet rest without staff in the room. Gait speed was measured via a timed 4-m walk performed twice at the participant’s usual pace from a standing start. The use of an assistive device was permitted if typically used by the participant to walk short distances. The faster of the 2 gait speeds (measured in meters/second) was used in the analysis. Frailty status at randomization was quantified using a previously reported 37-item frailty index.15
Clinical Outcomes
A committee unaware of treatment assignment adjudicated the protocol-specified clinical outcomes. The primary cardiovascular disease outcome was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes. Secondary outcomes included all-cause mortality and the composite of the SPRINT primary outcome and all-cause mortality.
The primary renal disease outcome was assessed in participants with CKD at baseline (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2 based on the 4-variable Modification of Diet in Renal Disease equation). It was based on the composite incidence of either a decrease in eGFR of 50% or greater (confirmed by subsequent laboratory test ≥90 days later) or the development of end-stage renal disease requiring long-term dialysis or transplantation. A secondary renal disease outcome (assessed in participants without CKD at baseline) was based on incidence of a decrease in eGFR from 30% or greater at baseline to a value less than 60 mL/min/1.73 m2 (also confirmed by a subsequent test ≥90 days later).
Definition of Serious Adverse Events
Serious adverse events (SAEs) were defined as events that were fatal or life threatening, resulted in significant or persistent disability, required hospitalization or resulted in prolonged hospitalization, or medical events that the investigator judged to be a significant hazard or harm to the participant and required medical or surgical intervention to prevent any of these. The following conditions of interest were reported as adverse events if they were evaluated in an emergency department: hypotension, syncope, injurious falls, electrolyte abnormalities, and bradycardia. Episodes of acute kidney injury (or acute renal failure) were monitored if they led to hospitalization and were reported in the hospital discharge summary.
Statistical Analysis
Power to detect a 25% treatment effect for the primary outcome within the subgroup of participants aged 75 years or older was estimated assuming an enrollment of 3250. With a 2-year recruitment period, maximum follow-up of 6 years, and annual loss to follow-up of 2%, power was estimated to be 81.9%, assuming an event rate of 3.25% per year in the standard treatment group (Appendix B in Supplement 1).
Linear-mixed models with an unstructured covariance matrix, assuming independence across participants, were used to model longitudinal differences in SBP between treatment groups. Fixed effects in the model were BP at randomization and a treatment group indicator. The time to first occurrence of the primary composite outcome, all-cause mortality, primary composite outcome plus all-cause mortality, SAEs, and loss to follow-up or withdrawing consent were compared between the 2 randomized groups using Cox proportional hazards regression models with the baseline hazard function stratified by clinic site (participants were recruited at 100 clinics). Follow-up time was censored on the date of last event ascertainment on or before August 20, 2015, the date on which the National Heart, Lung, and Blood Institute director decided to stop the intervention.
Exploratory secondary analyses were conducted to examine modification of the treatment effect by frailty status and gait speed. Neither frailty status nor gait speed was a prespecified subgroup in the trial protocol. We fit separate Cox regression models for frailty status classified as fit (frailty index ≤0.10), less fit (frailty index >0.10 to ≤0.21), or frail (frailty index >0.21),16,17 and for gait speed classified as 0.8 m/s or greater (normal walker), less than 0.8 m/s (slow walker), or missing.18 Interactions between treatment group, frailty status, and gait speed were formally tested by including interaction terms within a Cox regression model (ie, using likelihood ratio tests to compare with a model that did not allow the treatment effect to vary by frailty status or gait speed). For the primary cardiovascular disease composite outcome, sensitivity analyses accounting for the competing risk of death were conducted using the subdistribution hazard model of Fine and Gray.19 All hypothesis tests were 2-sided at the 5% level of significance.
Additional analyses compared the total burden of SAEs between the randomized groups (allowing for recurrent events) using the mean cumulative count estimator (standard errors computed using bootstrap resampling).20 Hazard ratios (HRs) were computed to compare the randomized groups using the gap-time formation of the Prentice, Williams, and Peterson recurrent events regression model.21 All analyses were performed using SAS version 9.4 (SAS Institute Inc) and the R Statistical Computing Environment (http://www.r-project.org).
Results
Baseline Characteristics and Study Retention
Participants aged 75 years or older were randomized to an SBP target of less than 120 mm Hg (intensive treatment group, n = 1317) or an SBP target of less than 140 mm Hg (standard treatment group, n = 1319) (Figure 1). The treatment groups were similar for most characteristics with the exception of frailty status and aspirin use (Table 1). Overall, 815 participants (30.9%) were classified as frail and 1456 (55.2%) as less fit (Table 1). A total of 2510 (95.2%) participants provided complete follow-up data.
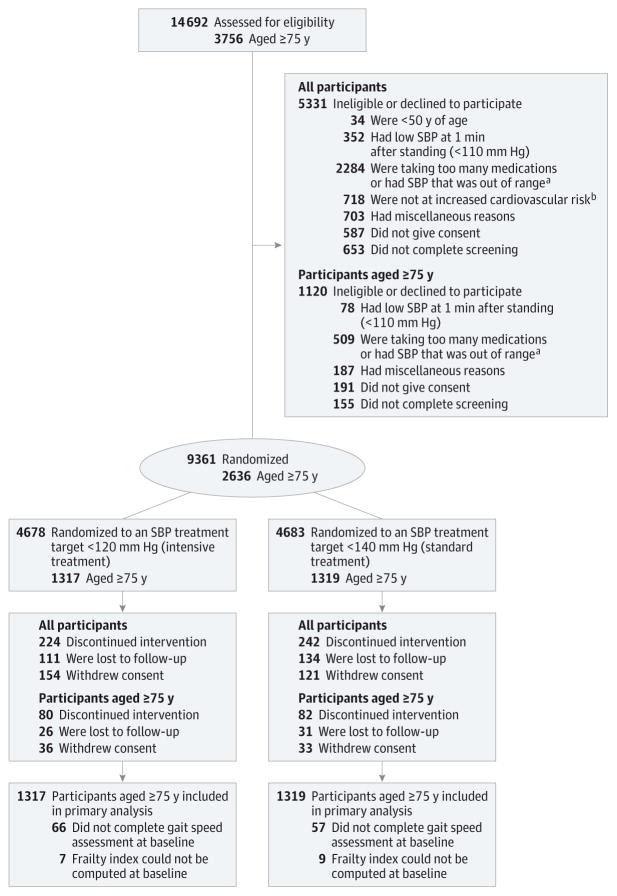
Figure 1. Eligibility, Randomization, and Follow-up for Systolic Blood Pressure (SBP) Intervention Trial (SPRINT) Participants Aged 75 Years or Older.
aSystolic blood pressure was required to be between 130 mm Hg and 180 mm Hg for participants taking 0 or 1 medication, 130 mm Hg to 170 mm Hg for participants taking 2 medications or fewer, 130 mm Hg to 160 mm Hg for participants taking 3 medications or fewer, and 130 mm Hg to 150 mm Hg for participants taking 4 medications or fewer.
bIncreased cardiovascular risk was defined as presence of 1 or more of the following: (1) clinical or subclinical cardiovascular disease other than stroke, (2) chronic kidney disease (defined as an estimated glomerular filtration rate of 20 mL/min/1.73m2 to 59 mL/min/1.73m2 based on the 4-variable Modification of Diet in Renal Disease equation and the latest laboratory value within the past 6 months), (3) Framingham risk score for 10-year cardiovascular risk of 15% or greater based on laboratory work done within the past 12 months for lipids, or (4) age of 75 years or older.
Table 1.
Baseline Characteristics of Participants Aged 75 Years or Older
| Intensive Treatment (n = 1317) | Standard Treatment (n = 1319) | |
|---|---|---|
| Female sex | 499 (37.9) | 501 (38.0) |
| Age, mean (SD), y | 79.8 (3.9) | 79.9 (4.1) |
| Race/ethnicity, No. (%) | ||
| White | 977 (74.2) | 987 (74.8) |
| Black | 225 (17.1) | 226 (17.1) |
| Hispanic | 89 (6.8) | 85 (6.4) |
| Other | 26 (2.0) | 21 (1.6) |
| Seated blood pressure, mean (SD), mm Hg | ||
| Systolic | 141.6 (15.7) | 141.6 (15.8) |
| Diastolic | 71.5 (11.0) | 70.9 (11.0) |
| Orthostatic hypotension, No. (%) | 127 (9.6) | 124 (9.4) |
| Serum creatinine, median (IQR), mg/dL | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) |
| Estimated GFRa | ||
| Mean (SD), mL/min/1.73 m2 | 63.4 (18.2) | 63.3 (18.3) |
| Level <60 mL/min/1.73 m2, No. (%) | 584 (44.3) | 577 (43.7) |
| Level <45 mL/min/1.73 m2, No. (%) | 207 (15.7) | 212 (16.1) |
| Urinary albumin to creatinine ratio, median (IQR), mg/g | 13.0 (7.2–31.6) | 13.4 (7.2–33.4) |
| History of cardiovascular disease, No. (%) | 338 (25.7) | 309 (23.4) |
| Total cholesterol, mean (SD), mg/dL | 181.4 (39.0) | 181.8 (38.7) |
| Fasting HDL cholesterol, mean (SD), mg/dL | 55.9 (15.1) | 55.7 (14.9) |
| Fasting total triglycerides, median (IQR), mg/dL | 96.0 (71.0–130.0) | 99.0 (72.0–134.5) |
| Fasting plasma glucose, mean (SD), mg/dL | 97.9 (12.1) | 98.2 (11.6) |
| Statin use, No. (%) | 682 (51.8) | 697 (52.8) |
| Aspirin use, No. (%) | 820 (62.3) | 765 (58.0) |
| 10-y Framingham cardiovascular disease risk, median (IQR), % | 24.2 (16.8–32.8) | 25.0 (17.0–33.4) |
| Body mass index, mean (SD)b | 27.8 (4.9) | 27.7 (4.6) |
| No. of antihypertensive agents taking at baseline visit, mean (SD) | 1.9 (1.0) | 1.9 (1.0) |
| Gait speed | ||
| Median (IQR), m/s | 0.90 (0.77–1.05) | 0.92 (0.77–1.06) |
| Speed <0.8 m/s, No. (%) | 371 (28.2) | 369 (28.0) |
| Frailty index, median (IQR)c | 0.18 (0.13–0.23) | 0.17 (0.12–0.22) |
| Frailty status, No. (%) | ||
| Fit (frailty index ≤0.10) | 159 (12.1) | 190 (14.4) |
| Less fit (frailty index >0.10 to ≤0.21) | 711 (54.0) | 745 (56.5) |
| Frail (frailty index >0.21) | 440 (33.4) | 375 (28.4) |
| Montreal Cognitive Assessment score, median (IQR)d | 22.0 (19.0–25.0) | 22.0 (19.0–25.0) |
Abbreviations: GFR, glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range. SI conversion factors: To convert HDL and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and glucose to mmol/L, multiply by 0.0555.
Based on the 4-variable Modification of Diet in Renal Disease equation.
Calculated as weight in kilograms divided by height in meters squared.
Scores range from 0 to 1, with higher values indicating greater frailty.
Scores range from 0 to 30, with higher scores denoting better cognitive function.
In the intensive treatment group, 440 participants (33.4%) were classified as frail compared with 375 participants (28.4%) in the standard treatment group. A total of 740 participants (28.1%) were classified as slow walkers (<0.8m/s). There was no baseline treatment group difference in the proportion of participants classified as slow walkers or in performance on the Montreal Cognitive Assessment screening test.22
Even though participants who were less fit, frail, or with reduced gait speed exhibited higher rates of loss to follow-up or withdrawal of consent, there were no significant differences between the treatment groups for frailty or low gait speed (eTable 1 in Supplement 2). The frequency at which participants discontinued the intervention but continued follow-up was 6.2% in the intensive treatment group vs 6.4% in the standard treatment group (P = .87).
Blood Pressure Levels
Throughout follow-up, the mean SBP in the intensive treatment group was 123.4 mm Hg, and it was 134.8 mm Hg in the standard treatment group. The between-group difference in mean SBP was 11.4 mm Hg (95% CI, 10.8–11.9 mm Hg), which is a smaller relative difference than the mean SBP of 14.8 mm Hg observed in the trial overall (Table 2). Mean diastolic BPs during follow-up were 62.0 mm Hg in the intensive treatment group and 67.2 mm Hg in the standard treatment group.
Table 2.
Least-Square Means for Postrandomization Blood Pressure Achieved by Treatment Group
| Intensive Treatment
|
Standard Treatment
|
Difference Between Groups, Mean (95% CI)a | P Value for Interactionb | |||
|---|---|---|---|---|---|---|
| No. | Mean (95% CI) | No. | Mean (95% CI) | |||
| Systolic blood pressure | ||||||
|
| ||||||
| Overall, mm Hg | 1317 | 123.4 (123.0–123.9)c | 1319 | 134.8 (134.3–135.2)c | 11.4 (10.8–11.9) | |
|
| ||||||
| Frailty statusd | ||||||
|
| ||||||
| Fit | 159 | 121.4 (120.3–122.5) | 190 | 134.9 (133.9–135.9) | 13.5 (12.0–15.0) | .01 |
|
| ||||||
| Less fit | 711 | 123.3 (122.8–123.9) | 745 | 134.7 (134.1–135.2) | 11.3 (10.6–12.1) | |
|
| ||||||
| Frail | 440 | 124.3 (123.5–125.0) | 375 | 135.0 (134.2–135.8) | 10.8 (9.7–11.8) | |
|
| ||||||
| Gait speed | ||||||
|
| ||||||
| Speed ≥0.8 m/s | 880 | 123.3 (122.8–123.8) | 893 | 134.6 (134.0–135.1) | 11.3 (10.6–11.9) | .67 |
|
| ||||||
| Speed <0.8 m/s | 371 | 123.8 (123.0–124.6) | 369 | 135.2 (134.4–136.0) | 11.4 (10.4–12.5) | |
|
| ||||||
| Missing | 66 | 123.5 (121.7–125.2) | 57 | 136.0 (134.0–137.9) | 12.5 (9.9–15.1) | |
|
| ||||||
| Diastolic blood pressure | ||||||
|
| ||||||
| Overall, mm Hg | 1317 | 62.0 (61.7–62.3)c | 1319 | 67.2 (66.8–67.5)c | 5.2 (4.7–5.6) | |
|
| ||||||
| Frailty statusd | ||||||
|
| ||||||
| Fit | 159 | 61.9 (61.1–62.8) | 190 | 67.4 (66.7–68.2) | 5.5 (4.3–6.6) | .07 |
|
| ||||||
| Less fit | 711 | 62.1 (61.7–62.6) | 745 | 67.6 (67.2–68.0) | 5.4 (4.9–6.0) | |
|
| ||||||
| Frail | 440 | 61.8 (61.3–62.3) | 375 | 66.2 (65.6–66.8) | 4.4 (3.6–5.1) | |
|
| ||||||
| Gait speed | ||||||
|
| ||||||
| Speed ≥0.8 m/s | 880 | 62.0 (61.6–62.3) | 893 | 67.2 (66.9–67.6) | 5.3 (4.8–5.8) | .08 |
|
| ||||||
| Speed <0.8 m/s | 371 | 62.3 (61.7–62.8) | 369 | 66.8 (66.2–67.4) | 4.6 (3.8–5.4) | |
|
| ||||||
| Missing | 66 | 61.4 (60.1–62.7) | 57 | 68.2 (66.7–69.6) | 6.8 (4.8–8.8) | |
P < .001 for all mean differences.
From amixed model.
Least-square means for blood pressure estimated from mixed model conditioned on baseline blood pressure.
Frailty status classified using 37-item frailty index (FI): fit (FI ≤0.10), less fit (FI >0.10 to ≤0.21), or frail (FI >0.21).
On average, participants in the intensive treatment group required 1 more medication to reach the achieved lower BP (eTable 2 and eFigure 1 in Supplement 2). Within the intensive treatment group, mean SBP during follow-up was higher for participants classified as less fit or frail compared with those considered fit. Differences in mean SBP by treatment group differed by frailty status (P = .01), with frail participants exhibiting smaller inter treatment group differences (10.8 mm Hg) compared with less fit participants (11.3 mm Hg) and fit participants (13.5 mm Hg). Treatment group differences in SBP were similar across subgroups defined by gait speed.
Clinical Outcomes
A primary composite outcome event was observed for 102 participants (2.59% per year) in the intensive treatment group and for 148 participants (3.85% per year) in the standard treatment group (HR, 0.66 [95% CI, 0.51–0.85]; Table 3). Results were similar for all-cause mortality (there were 73 deaths in the intensive treatment group and 107 deaths in the standard treatment group; HR, 0.67 [95% CI, 0.49–0.91]). Inference for the primary outcome was unchanged when non–cardiovascular disease death was treated as a competing risk (HR, 0.66 [95% CI, 0.52–0.85]). At 3.14 years, the number needed to treat (NNT) estimate for the primary outcome was 27 (95% CI, 19–61) and for all-cause mortality it was 41 (95% CI, 27–145).
Table 3.
Incidence of Cardiovascular, Renal, and Mortality Outcomes by Treatment Group
| Intensive Treatment
|
Standard Treatment
|
HR (95% CI)b | P Value | |||
|---|---|---|---|---|---|---|
| No. With Outcome Events (n = 1317)a | % (95% CI) With Outcome Events/y | No. With Outcome Events (n = 1319)a | % (95% CI) With Outcome Events/y | |||
| All participants | ||||||
|
| ||||||
| Cardiovascular disease primary outcomec | 102 | 2.59 (2.13–3.14) | 148 | 3.85 (3.28–4.53) | 0.66 (0.51–0.85) | .001 |
|
| ||||||
| Myocardial infarction (MI)d | 37 | 0.92 (0.67–1.27) | 53 | 1.34 (1.02–1.75) | 0.69 (0.45–1.05) | .09 |
|
| ||||||
| ACS not resulting in MId | 17 | 0.42 (0.26–0.68) | 17 | 0.42 (0.26–0.68) | 1.03 (0.52–2.04) | .94 |
|
| ||||||
| Stroked | 27 | 0.67 (0.46–0.97) | 34 | 0.85 (0.61–1.19) | 0.72 (0.43–1.21) | .22 |
|
| ||||||
| Heart failured | 35 | 0.86 (0.62–1.20) | 56 | 1.41 (1.09–1.83) | 0.62 (0.40–0.95) | .03 |
|
| ||||||
| Cardiovascular disease deathd | 18 | 0.44 (0.28–0.70) | 29 | 0.72 (0.50–1.03) | 0.60 (0.33–1.09) | .09 |
|
| ||||||
| Nonfatal MI | 37 | 0.92 (0.67–1.27) | 53 | 1.34 (1.02–1.75) | 0.69 (0.45–1.05) | .09 |
|
| ||||||
| Nonfatal stroke | 25 | 0.62 (0.42–0.91) | 33 | 0.83 (0.59–1.16) | 0.68 (0.40–1.15) | .15 |
|
| ||||||
| Nonfatal heart failure | 35 | 0.86 (0.62–1.20) | 55 | 1.39 (1.06–1.81) | 0.63 (0.40–0.96) | .03 |
|
| ||||||
| All-cause mortality | 73 | 1.78 (1.41–2.24) | 107 | 2.63 (2.17–3.18) | 0.67 (0.49–0.91) | .009 |
|
| ||||||
| Primary outcome plus all-cause mortality | 144 | 3.64 (3.09–4.29) | 205 | 5.31 (4.63–6.09) | 0.68 (0.54–0.84) | <.001 |
|
| ||||||
| CKD | ||||||
|
| ||||||
| Primary CKD outcomee | 7/584 | 0.38 (0.18–0.81) | 4/577 | 0.23 (0.08–0.60) | 1.68 (0.49–6.59) | .42 |
|
| ||||||
| Incident albuminuriaf | 26/196 | 4.43 (3.02–6.51) | 28/177 | 5.56 (3.84–8.06) | 0.96 (0.53–1.75) | .90 |
|
| ||||||
| Non-CKD | ||||||
|
| ||||||
| Secondary CKD outcomeg | 37/726 | 1.70 (1.23–2.35) | 13/732 | 0.58 (0.34–1.01) | 3.14 (1.66–6.37) | <.001 |
|
| ||||||
| Incident albuminuriaf | 29/303 | 3.31 (2.30–4.76) | 42/304 | 4.84 (3.58–6.55) | 0.80 (0.46–1.35) | .40 |
Abbreviations: ACS, acute coronary syndrome; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IQR, interquartile range.
The total No. of participants is provided if it is different from treatment group total.
Intensive treatment group vs standard treatment group.
Includes nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes. Median follow-up time for the intensive treatment group was 3.16 years (IQR, 2.63–3.70 years), with 3938.2 person-years of follow-up. In the standard treatment group, median follow-up time was 3.12 years (IQR, 2.67–3.67 years), with 3841.0 person-years of follow-up.
These rows do not sum to the cardiovascular disease primary outcome. Only the first event contributes to the primary outcome, whereas participants with multiple events could contribute to each component outcome.
Includes a 50% reduction in eGFR (measured twice at least 90 days apart), dialysis, or a kidney transplant.
Only applies to participants with urinary albumin to creatinine ratio of less than 10 mg/g at baseline, and required a doubling of the urinary albumin to creatinine ratio from less than 10 mg/g to 10 mg/g or greater (measured twice at least 90 days apart).
Includes a 30% reduction in eGFR (measured twice at least 90 days apart) to an eGFR of less than 60 mL/min/1.73m2, dialysis, or a kidney transplant.
Because the treatment effect estimate was not statistically significant for cardiovascular disease death, the NNT estimate (using the abbreviations of Altman23) was an NNTBenefit of 116 (NNTHarm of 544 to ∞ to NNTBenefit of 68). In participants without CKD at the time of randomization, more participants in the intensive treatment group compared with the standard treatment group experienced the secondary CKD outcome (a 30% decrease in eGFR from baseline to an eGFR <60 mL/min/1.73 m2 [1.70% vs 0.58% per year, respectively]; HR, 3.14 [95% CI, 1.66–6.37]). There were no significant treatment group differences in the primary renal outcome in those with baseline CKD; however, power to detect differences was limited due to low numbers of events.
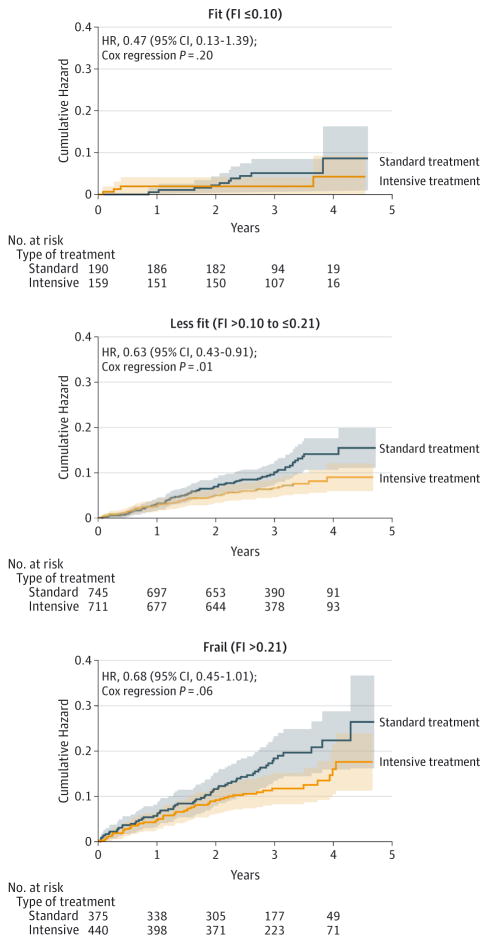
Exploratory Subgroup Analyses
Results stratified by baseline frailty status showed higher event rates with increasing frailty in both treatment groups (Table 4 and Figure 2). However, within each frailty stratum, absolute event rates were lower for the intensive treatment group (P = .84 for interaction). Results were similar when participants were stratified by gait speed (P = .85 for interaction), with the HRs in favor of the intensive treatment group in each gait speed stratum (eFigure 2 in Supplement 2).
Table 4.
Incidence of Cardiovascular and Mortality Outcomes by Frailty Status and Gait Speed
| Intensive Treatment | Standard Treatment | HR (95% CI)a | P Value | P Value for Interaction | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No./Total With Outcome Events | % (95% CI) With Outcome Events/y | No./Total With Outcome Events | % (95% CI) With Outcome Events/y | |||||
| Frailty statusb | ||||||||
| <hr/> | ||||||||
| Primary outcomec | Fit | 4/159 | 0.80 (0.30–2.12) | 10/190 | 1.72 (0.93–3.20) | 0.47 (0.13–1.39)d | .20 | .84 |
|
| ||||||||
| Less fit | 48/711 | 2.23 (1.68–2.97) | 77/745 | 3.51 (2.81–4.39) | 0.63 (0.43–0.91) | .01 | ||
|
| ||||||||
| Frail | 50/440 | 3.90 (2.96–5.15) | 61/375 | 5.80 (4.52–7.46) | 0.68 (0.45–1.01) | .06 | ||
|
| ||||||||
| All-cause mortality | Fit | 5/159 | 0.98 (0.41–2.36) | 6/190 | 1.01 (0.45–2.24) | 0.95 (0.27–3.15)d | .93 | .52 |
|
| ||||||||
| Less fit | 26/711 | 1.16 (0.79–1.71) | 52/745 | 2.24 (1.71–2.95) | 0.48 (0.29–0.78) | .003 | ||
|
| ||||||||
| Frail | 40/440 | 2.95 (2.17–4.03) | 49/375 | 4.28 (3.24–5.67) | 0.64 (0.41–1.01) | .05 | ||
|
| ||||||||
| Primary outcome plus all-cause mortalityc | Fit | 8/159 | 1.59 (0.80–3.19) | 13/190 | 2.24 (1.30–3.86) | 0.71 (0.28–1.69)d | .45 | .88 |
|
| ||||||||
| Less fit | 65/711 | 3.01 (2.36–3.84) | 108/745 | 4.90 (4.05–5.91) | 0.60 (0.44–0.83) | .002 | ||
|
| ||||||||
| Frail | 69/440 | 5.37 (4.24–6.80) | 84/375 | 7.95 (6.42–9.85) | 0.67 (0.48–0.95) | .02 | ||
|
| ||||||||
| Gait speed | ||||||||
|
| ||||||||
| Primary outcomec | Speed ≥0.8 m/s | 59/880 | 2.22 (1.72–2.87) | 86/893 | 3.24 (2.63–4.01) | 0.67 (0.47–0.94) | .02 | .85 |
|
| ||||||||
| Speed <0.8 m/s | 34/371 | 3.15 (2.25–4.41) | 54/369 | 5.22 (4.00–6.81) | 0.63 (0.40–0.99) | .05 | ||
|
| ||||||||
| Missing | 9/66 | 4.40 (2.29–8.46) | 8/57 | 5.13 (2.57–10.27) | 0.86 (0.33–2.29)d | .75 | ||
|
| ||||||||
| All-cause mortality | Speed ≥0.8 m/s | 40/880 | 1.45 (1.07–1.98) | 60/893 | 2.16 (1.67–2.78) | 0.65 (0.43–0.98) | .04 | .68 |
|
| ||||||||
| Speed <0.8 m/s | 29/371 | 2.56 (1.78–3.68) | 40/369 | 3.57 (2.62–4.86) | 0.75 (0.44–1.26) | .28 | ||
|
| ||||||||
| Missing | 4/66 | 1.85 (0.69–4.93) | 7/57 | 4.19 (2.00–8.80) | 0.44 (0.12–1.47)d | .20 | ||
|
| ||||||||
| Primary outcome plus all-cause mortalityc | Speed ≥0.8 m/s | 82/880 | 3.08 (2.48–3.83) | 119/893 | 4.48 (3.74–5.36) | 0.67 (0.50–0.89) | .006 | .91 |
|
| ||||||||
| Speed <0.8 m/s | 51/371 | 4.70 (3.57–6.18) | 73/369 | 7.00 (5.56–8.80) | 0.69 (0.46–1.01) | .06 | ||
|
| ||||||||
| Missing | 11/66 | 5.37 (2.97–9.70) | 13/57 | 8.30 (4.82–14.30) | 0.64 (0.28–1.44)d | .28 | ||
Abbreviation: HR, hazard ratio.
Intensive treatment group vs standard treatment group from Cox proportional hazards regression model with baseline hazard stratified by clinic site.
Classified using a 37-item frailty index (FI): fit (FI ≤0.10), less fit (FI >0.10 to ≤0.21), or frail (FI >0.21).
Primary outcome includes nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes.
Due to small sample size, HR estimated from Cox model assuming common baseline hazard across clinic site.
Figure 2. Kaplan-Meier Curves for the Primary Cardiovascular Disease Outcome in Systolic Blood Pressure Intervention Trial (SPRINT) in Participants Aged 75 Years or Older by Baseline Frailty Status.
Tinted regions indicate 95% confidence intervals; FI, 37-item frailty index; HR, hazard ratio. The primary cardiovascular disease outcome was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in a myocardial infarction, nonfatal stroke, nonfatal acute decompensated heart failure, and death from cardiovascular causes.
Serious Adverse Events
Detailed information regarding SAEs appears in eTable 3 and eTable 4 in Supplement 2. Data on SAEs in participants older than 75 years have been previously reported (Table S613). In the intensive treatment group, SAEs occurred in 637 participants (48.4%) compared with 637 participants (48.3%) in the standard treatment group (HR, 0.99 [95% CI, 0.89–1.11]; P = .90). The absolute rate of SAEs was higher but was not statistically significantly different in the intensive treatment group for hypotension (2.4% vs 1.4% in the standard treatment group; HR, 1.71 [95% CI, 0.97–3.09]), syncope (3.0% vs 2.4%, respectively; HR, 1.23 [95% CI, 0.76–2.00]), electrolyte abnormalities (4.0% vs 2.7%; HR, 1.51 [95% CI, 0.99–2.33]), and acute kidney injury or renal failure (5.5% vs 4.0%; HR, 1.41 [95% CI, 0.98–2.04]). However, the absolute rate of injurious falls was lower but was not statistically significantly different in the intensive treatment group (4.9% vs 5.5% in the standard treatment group; HR, 0.91 [95% CI, 0.65–1.29]).
There was no statistically significant difference in the rate of orthostatic hypotension assessed during a clinic visit between the treatment groups (21.0% in the intensive treatment group vs 21.8 %in the standard treatment group; HR, 0.90 [95% CI, 0.76–1.07]); however, the absolute rate of orthostatic hypotension in combination with a report of dizziness was higher but was not statistically significantly different in the intensive treatment group (1.9% vs 1.3% in the standard treatment group; HR, 1.44 [95% CI, 0.77–2.73]). Even though the SAE rates were higher with greater frailty or slower walking speed, these rates were not statistically different by treatment group when stratified by frailty status or gait speed.
Discussion
These results extend and detail the main SPRINT study findings in community-dwelling persons aged 75 years or older, demonstrating that a treatment goal for SBP of less than 120 mm Hg reduced incident cardiovascular disease by 33% (from 3.85% to 2.59% per year) and total mortality by 32%(from 2.63% to 1.78% per year).13 Translating these findings into numbers needed to treat suggests that a strategy of intensive BP control for 3.14 years would be expected to prevent 1 primary outcome event for every 27 persons treated and 1 death from any cause for every 41 persons treated. These estimates are lower than those from the overall results of the trial due to the higher event rate in persons aged 75 years or older. In addition, exploratory analysis suggested that the benefit of intensive BP control was consistent among persons in this age range who were frail or had reduced gait speed.
The overall SAE rate was comparable by treatment group, including among the most frail participants. There were no differences in the number of participants experiencing injurious falls or in the prevalence of orthostatic hypotension measured at study visits. These results complement results from other trials demonstrating improved BP control reduces risk for orthostatic hypotension and has no effect on risk for injurious falls.24–26 The numbers of participants aged 75 years or older who dropped out of the study, were lost to follow-up, or decided to discontinue the intervention but continued with outcome assessment were low and did not differ by treatment group.
There are several limitations to these results from SPRINT involving participants aged 75 years or older. Even though the trial was designed to enhance recruitment of a prespecified sub-group of adults aged 75 years or older, randomization in SPRINT was not stratified by categories of age. In addition, the trial did not enroll older adults residing in nursing homes, persons with type 2 diabetes or prevalent stroke (because of concurrent BP lowering trials),27,28 and individuals with symptomatic heart failure due to protocol differences required to maintain BP control in this condition. Therefore, the results reported in this study among persons aged 75 years or older do not provide evidence regarding treatment targets in these populations. Individuals with these conditions also represent a subset of older persons at increased risk for falls.
No other chronic conditions were excluded from this trial, and the frailty index applied in this study combined with the assessment of gait speed contribute to assessing possible effect modification by comorbidity and functional status. In exploratory analyses, there was no evidence of heterogeneity for the cardiovascular benefit of intensive BP management by frailty or gait speed. However, these analyses should be interpreted cautiously. The analyses were not prespecified in the trial protocol and were possibly under powered because SPRINT was designed to consider only the ability to detect a treatment effect in participants aged 75 years or older as a whole.
Despite excluding some chronic conditions, 30.9% of participants aged 75 years or older in this trial were categorized as frail at baseline, and the distribution of frailty status parallels that estimated for ambulatory, community living populations of similar age.15 In addition, the proportion of US adults aged 75 years or older who have hypertension and meet the study entry criteria has been estimated to represent 64% of that population using the 2007–2012 National Health and Nutrition Surveys (approximately 5.8 million individuals).29 Therefore, participants aged 75 years or older in this trial are representative of a sizeable fraction of adults in this age group with hypertension.
There are several important comparisons to make with HYVET,30 which randomized 3845 patients aged 80 years or older within Europe and Asia (mean age, 83 years [3 years older than SPRINT]; mean entry SBP, 173 mm Hg [31 mm Hg higher than SPRINT]) to either therapy with indapamide, with or without the angiotensin-converting enzyme inhibitor perindopril, or placebo with an SBP treatment goal of less than 150 mm Hg. The 2-year between-group SBP difference was 15 mm Hg (the active treatment group achieved a mean SBP of 143 mm Hg, slightly higher than the SPRINT baseline SBP). Similar to SPRINT, HYVET was terminated early (at a median follow-up time of 1.8 years) due to significant reductions in the incidence rate of total mortality. A retrospective analysis of the HYVET population conducted to determine its frailty status identified that (1) the cohort’s frailty status was similar to that of community living populations of similar age and (2) the treatment benefits were similar even in the most frail participants.12 Taken together, current results from SPRINT also reinforce and extend HYVET’s conclusions that risk reductions in cardiovascular disease events and mortality from high BP treatment are evident regardless of frailty status.
Among all participants aged 75 years or older, the SAEs related to acute kidney injury occurred more frequently in the intensive treatment group (72 participants [5.5%] vs 53 participants [4.0%] in the standard treatment group). The differences in adverse renal outcomes may be related to a reversible intrarenal hemodynamic effect of the reduction in BP and more frequent use of diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers in the intensive treatment group.31,32 Although there is no evidence of permanent kidney injury associated with the lower BP goal, the possibility of long-term adverse renal outcomes cannot be excluded and requires longer-term follow-up.
Considering the high prevalence of hypertension among older persons, patients and their physicians may be inclined to underestimate the burden of hypertension or the benefits of lowering BP, resulting in under treatment. On average, the benefits that resulted from intensive therapy required treatment with 1 additional antihypertensive drug and additional early visits for dose titration and monitoring. Future analyses of SPRINT data may be helpful to better define the burden, costs, and benefits of intensive BP control. However, the present results have substantial implications for the future of intensive BP therapy in older adults because of this condition’s high prevalence, the high absolute risk for cardiovascular disease complications from elevated BP, and the devastating consequences of such events on the independent function of older people.3,29,33,34
Conclusions
Among ambulatory adults aged 75 years or older, treating to an SBP target of less than 120 mm Hg compared with an SBP target of less than 140 mm Hg resulted in significantly lower rates of fatal and nonfatal major cardiovascular events and death from any cause.
Supplementary Material
Acknowledgments
Funding/Support: The SPRINT study was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke) under contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Azilsartan and chlorthalidone (combined with azilsartan) were provided by Takeda Pharmaceuticals International Inc. Additional support was provided by grants P30-AG21332 and R01-HL10741 from the Wake Forest Claude Pepper Older Americans Independence Center; through the following National Center for Advancing Translational Sciences clinical and translational science awards: UL1TR000439 (awarded to Case Western Reserve University); UL1RR025755 (Ohio State University); UL1RR024134 and UL1TR000003 (University of Pennsylvania); UL1RR025771 (Boston University); UL1TR000093 (Stanford University); UL1RR025752, UL1TR000073, and UL1TR001064 (Tufts University); UL1TR000050 (University of Illinois); UL1TR000005 (University of Pittsburgh); 9U54TR000017-06 (University of Texas Southwestern Medical Center); UL1TR000105-05 (University of Utah); UL1 TR000445 (Vanderbilt University); UL1TR000075 (George Washington University); UL1 TR000002 (University of California, Davis); UL1 TR000064 (University of Florida); and UL1TR000433 (University of Michigan); and by National Institute of General Medical Sciences, Centers of Biomedical Research Excellence award NIGMS P30GM103337 (awarded to Tulane University).
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01206062
Role of the Funder/Sponsor: The SPRINT steering committee was responsible for the design and conduct of the study, including the collection and management of the data. Scientists at the National Institutes of Health as a group and the principal investigator of the Veterans Affairs clinical network had 1 vote on the steering committee of the trial. There were 7 voting members of the steering committee. The National Institutes of Health, the US Department of Veterans Affairs, and the US government had no role in analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US government.
Previous Presentation: Presented in part at the Gerontological Society of America annual meeting; November 21, 2015; Orlando, Florida.
Additional Contributions: We thank Sarah Hutchens and Pamela Nance, BA (both with the Wake Forest School of Medicine), for their assistance in preparation of the manuscript, for which neither received any compensation.
Author Contributions: Dr Pajewski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Williamson, Supiano, Applegate, Berlowitz, Chertow, Fine, Kitzman, Launer, Rodriguez, Shorr, Sink, Wadley, Whelton, Wright, Pajewski.
Acquisition, analysis, or interpretation of data: Williamson, Supiano, Applegate, Berlowitz, Campbell, Chertow, Fine, Haley, Hawfield, Ix, Kostis, Krousel-Wood, Oparil, Rodriguez, Roumie, Shorr, Sink, Whelton, Whittle, Woolard, Wright, Pajewski.
Drafting of the manuscript: Williamson, Supiano, Applegate, Shorr, Wright, Pajewski.
Critical revision of the manuscript for important intellectual content: Williamson, Supiano, Applegate, Berlowitz, Campbell, Chertow, Fine, Haley, Hawfield, Ix, Kitzman, Kostis, Krousel-Wood, Launer, Oparil, Rodriguez, Roumie, Sink, Wadley, Whelton, Whittle, Woolard, Wright, Pajewski.
Statistical analysis: Pajewski.
Obtained funding: Williamson, Chertow, Fine, Kitzman, Oparil, Wright.
Administrative, technical, or material support: Williamson, Campbell, Ix, Kitzman, Kostis, Oparil, Roumie, Whelton, Woolard, Wright.
Study supervision: Williamson, Applegate, Berlowitz, Fine, Kitzman, Oparil, Wadley, Whelton, Wright.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Williamson reported receiving nonfinancial support from Takeda Pharmaceuticals and Arbor Pharmaceuticals during the conduct of the study. Dr Kitzman reported receiving personal fees from Merck, Forest Labs, and Abbvie; personal fees and other from Gilead and Relypsa; and grants from Novartis outside the submitted work. Dr Oparil reported receiving personal fees from Forest Laboratories Inc; grants, personal fees, and nonfinancial support from Medtronic; personal fees from Amgen (Onyx is subsidiary); grants and personal fees from AstraZeneca and Bayer Healthcare Pharmaceuticals Inc; personal fees from Boehringer-Ingelheim and GlaxoSmithKline; grants from Merck and Co; and serving as co-chair for the Eighth Joint National Committee. No other disclosures were reported.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. JAMA. 1997;277(9):728–734. [PubMed] [Google Scholar]
- 3.den Ouden MEM, Schuurmans MJ, Mueller-Schotte S, Bots ML, van der Schouw Y. Do subclinical vascular abnormalities precede impaired physical ability and ADL disability? Exp Gerontol. 2014;58:1–7. doi: 10.1016/j.exger.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Kjeldsen S, Feldman RD, Lisheng L, et al. Updated national and international hypertension guidelines: a review of current recommendations. Drugs. 2014;74(17):2033–2051. doi: 10.1007/s40265-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 6.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 7.Wright JT, Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160(7):499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 8.Windham BG, Griswold ME, Lirette S, et al. Effects of age and functional status on the relationship of systolic blood pressure with mortality in mid and late life: the ARIC Study [published online September 25, 2015] J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJM, Westendorp RGJ. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke. 2013;44(1):15–20. doi: 10.1161/STROKEAHA.112.663062. [DOI] [PubMed] [Google Scholar]
- 10.Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014;64(3):472–480. doi: 10.1161/HYPERTENSIONAHA.114.03831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlesworth CJ, Peralta CA, Odden MC. Functional status and antihypertensive therapy in older adults: a new perspective on old data [published online November 4, 2015] Am J Hypertens. doi: 10.1093/ajh/hpv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78. doi: 10.1186/s12916-015-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JT, Jr, Williamson JD, Whelton PK, et al. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosius WT, Sink KM, Foy CG, et al. SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pajewski NM, Williamson JD, Applegate WB, et al. SPRINT Study Research Group. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71(5):649–655. doi: 10.1093/gerona/glv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24(9):10–17. [PubMed] [Google Scholar]
- 17.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60(3):464–470. doi: 10.1016/j.archger.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A propotional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532–540. doi: 10.1093/aje/kwu289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentice R, Williams B, Peterson A. On the regression analysis of multivariate failure time data. Biometrika. 1981;68(2):373–379. [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ooi WL, Barrett S, Hossain M, Kelley-Gagnon M, Lipsitz LA. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA. 1997;277(16):1299–1304. [PubMed] [Google Scholar]
- 25.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59(3):383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29(12):1599–1606. doi: 10.1007/s11606-014-2961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benavente OR, Coffey CS, Conwit R, et al. SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial [published correction appears in Lancet. 2013;382(9891):506] Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the US adult population. J Am Coll Cardiol. 2016;67(5):463–472. doi: 10.1016/j.jacc.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 31.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160(5):685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 32.Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997;51(3):793–797. doi: 10.1038/ki.1997.111. [DOI] [PubMed] [Google Scholar]
- 33.Ettinger WH, Jr, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J CHS Collaborative Research Group. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 1994;42(10):1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 34.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128(21):2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.