Abstract
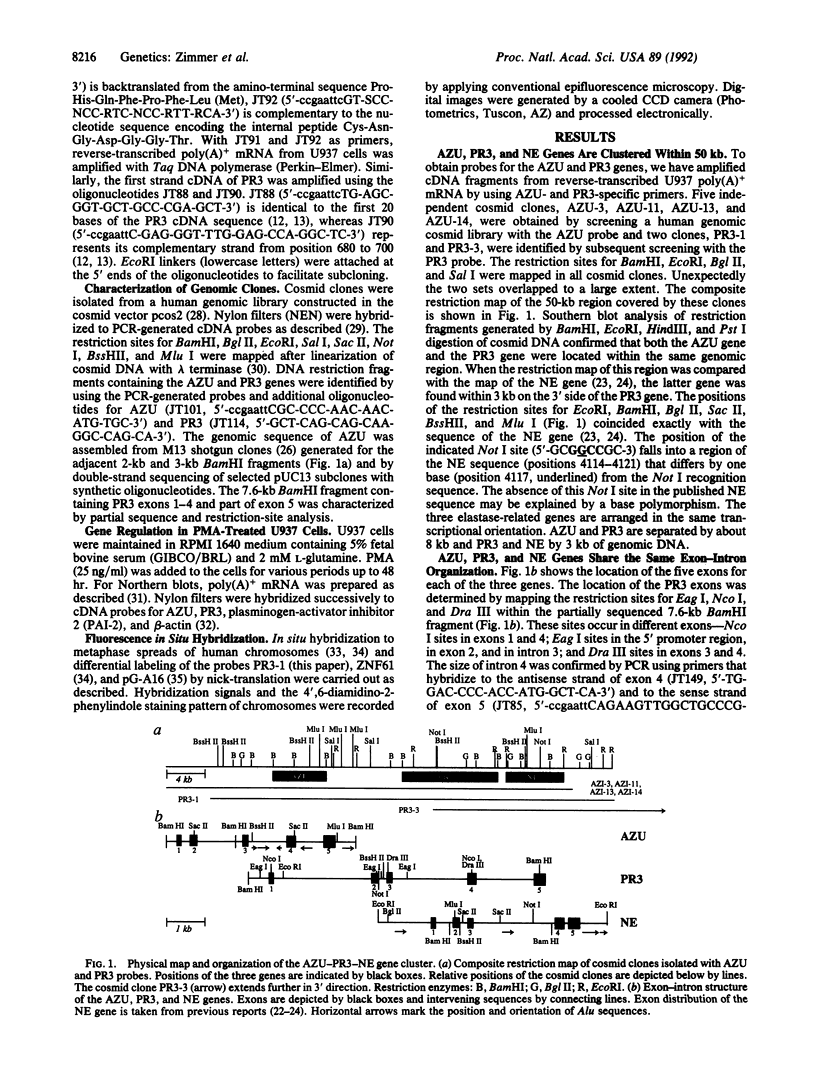
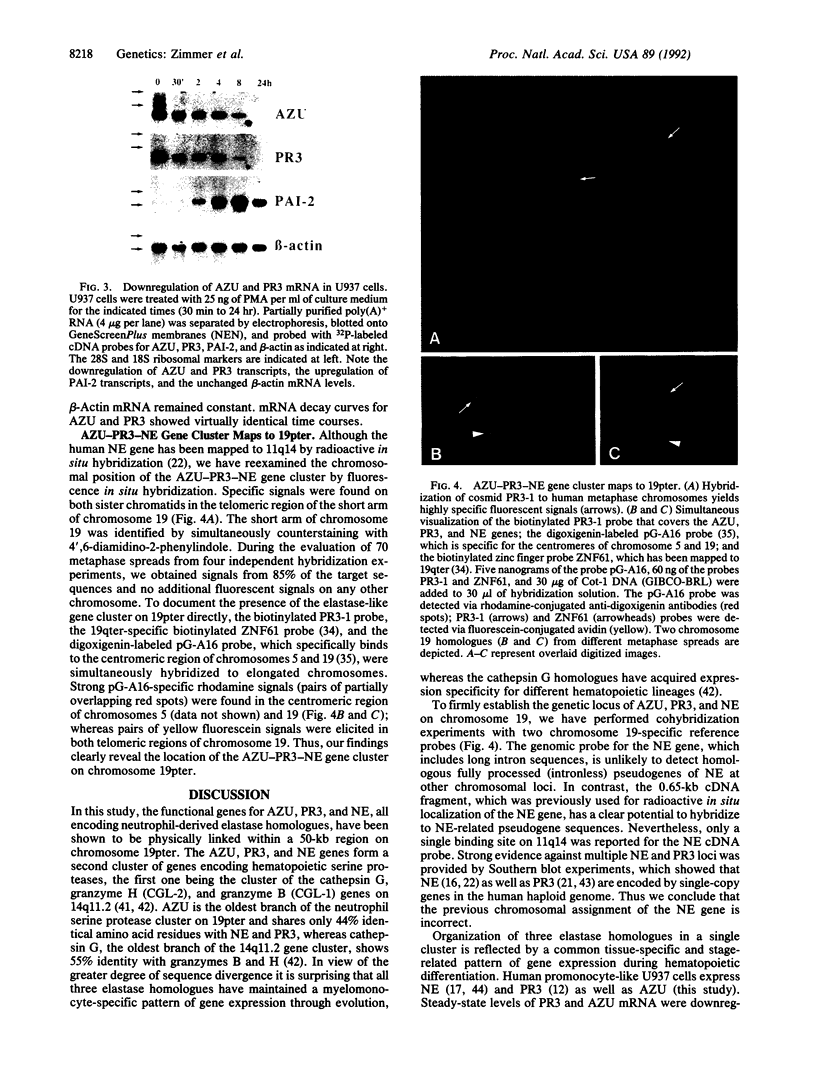
The human neutrophil and monocyte-derived serine protease homologues neutrophil elastase (NE), proteinase 3 (PR3), and azurocidin (AZU) are involved in a variety of immune defense reactions. NE and PR3 assist in the destruction of phagocytosed microorganisms, cleave the important connective-tissue protein elastin, and generate chemotactic activities by forming alpha 1-proteinase inhibitor complexes and elastin peptides. AZU is cytotoxic to certain microorganisms and chemotactic for monocytes. All three proteins are produced and packaged into azurophil granules in large quantities during neutrophil differentiation. We have isolated several cosmid clones each of which contains the functional genes for AZU, PR3, and NE in this order. The PR3 gene is separated by 8 kilobases from the 3' end of the AZU gene and by 3 kilobases from the 5' end of the NE gene. We report a physical map of the gene cluster, its location on chromosome 19pter, and the exon-intron organization of the AZU and PR3 genes. Our fluorescence in situ hybridization studies disprove the previous chromosomal assignment of the human NE gene to 11q14. The five exons of AZU and PR3 are organized like those of NE and other granule-associated serine proteases of hematopoietic cells. NE, PR3, and AZU are coordinately downregulated in the premonocytic cell line U937 during induced terminal differentiation. The cluster-like physical organization of these genes and concerted regulation during hematopoietic differentiation suggests that they are located in a developmentally activated chromatin domain promoting high-level, cell-specific expression in the monocyte-myelocyte lineage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. P., Melchior M., Campanelli D., Nathan C., Gabay J. E. Complementary DNA sequence of human neutrophil azurocidin, an antibiotic with extensive homology to serine proteases. Biochem Biophys Res Commun. 1991 Jun 14;177(2):688–695. doi: 10.1016/0006-291x(91)91843-2. [DOI] [PubMed] [Google Scholar]
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Bories D., Raynal M. C., Solomon D. H., Darzynkiewicz Z., Cayre Y. E. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989 Dec 22;59(6):959–968. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- Brendel V., Dohlman J., Blaisdell B. E., Karlin S. Very long charge runs in systemic lupus erythematosus-associated autoantigens. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1536–1540. doi: 10.1073/pnas.88.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli D., Melchior M., Fu Y., Nakata M., Shuman H., Nathan C., Gabay J. E. Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J Exp Med. 1990 Dec 1;172(6):1709–1715. doi: 10.1084/jem.172.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G. The alpha 1-antitrypsin gene and its deficiency states. Trends Genet. 1989 Dec;5(12):411–417. doi: 10.1016/0168-9525(89)90200-x. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley D., Salvesen G., Travis J. Molecular cloning of human neutrophil elastase. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):3–7. [PubMed] [Google Scholar]
- Farley D., Travis J., Salvesen G. The human neutrophil elastase gene. Analysis of the nucleotide sequence reveals three distinct classes of repetitive DNA. Biol Chem Hoppe Seyler. 1989 Jul;370(7):737–744. doi: 10.1515/bchm3.1989.370.2.737. [DOI] [PubMed] [Google Scholar]
- Fouret P., du Bois R. M., Bernaudin J. F., Takahashi H., Ferrans V. J., Crystal R. G. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989 Mar 1;169(3):833–845. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad P., Jenne D., Tschopp J., Clément M. V., Mathieu-Mahul D., Sasportes M. Structure and evolutionary origin of the human granzyme H gene. Int Immunol. 1991 Jan;3(1):57–66. doi: 10.1093/intimm/3.1.57. [DOI] [PubMed] [Google Scholar]
- Hanson R. D., Connolly N. L., Burnett D., Campbell E. J., Senior R. M., Ley T. J. Developmental regulation of the human cathepsin G gene in myelomonocytic cells. J Biol Chem. 1990 Jan 25;265(3):1524–1530. [PubMed] [Google Scholar]
- Hanson R. D., Hohn P. A., Popescu N. C., Ley T. J. A cluster of hematopoietic serine protease genes is found on the same chromosomal band as the human alpha/delta T-cell receptor locus. Proc Natl Acad Sci U S A. 1990 Feb;87(3):960–963. doi: 10.1073/pnas.87.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Flemington E., Kieff E., Deininger P. Repeat arrays in cellular DNA related to the Epstein-Barr virus IR3 repeat. Mol Cell Biol. 1985 Mar;5(3):457–465. doi: 10.1128/mcb.5.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn P. A., Popescu N. C., Hanson R. D., Salvesen G., Ley T. J. Genomic organization and chromosomal localization of the human cathepsin G gene. J Biol Chem. 1989 Aug 15;264(23):13412–13419. [PubMed] [Google Scholar]
- Hulsebos T., Schonk D., van Dalen I., Coerwinkel-Driessen M., Schepens J., Ropers H. H., Wieringa B. Isolation and characterization of alphoid DNA sequences specific for the pericentric regions of chromosomes 4, 5, 9, and 19. Cytogenet Cell Genet. 1988;47(3):144–148. doi: 10.1159/000132533. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J., Lüdemann J., Utecht B., Gross W. L. Wegener's autoantigen decoded. Nature. 1990 Aug 9;346(6284):520–520. doi: 10.1038/346520a0. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Zimmer M., Garcia-Sanz J. A., Tschopp J., Lichter P. Genomic organization and subchromosomal in situ localization of the murine granzyme F, a serine protease expressed in CD8+ T cells. J Immunol. 1991 Aug 1;147(3):1045–1052. [PubMed] [Google Scholar]
- Jennette J. C., Charles L. A., Falk R. J. Antineutrophil cytoplasmic autoantibodies: disease associations, molecular biology, and pathophysiology. Int Rev Exp Pathol. 1991;32:193–221. doi: 10.1016/b978-0-12-364932-4.50009-5. [DOI] [PubMed] [Google Scholar]
- Kallenberg C. G., Tervaert J. W., van der Woude F. J., Goldschmeding R., von dem Borne A. E., Weening J. J. Autoimmunity to lysosomal enzymes: new clues to vasculitis and glomerulonephritis? Immunol Today. 1991 Feb;12(2):61–64. doi: 10.1016/0167-5699(91)90159-q. [DOI] [PubMed] [Google Scholar]
- Kao R. C., Wehner N. G., Skubitz K. M., Gray B. H., Hoidal J. R. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988 Dec;82(6):1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbaye C., Musette P., Cayre Y. E. Wegener autoantigen and myeloblastin are encoded by a single mRNA. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9253–9256. doi: 10.1073/pnas.88.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990 Dec 1;76(11):2169–2181. [PubMed] [Google Scholar]
- Leiden J. M. Transcriptional regulation during T-cell development: the alpha TCR gene as a molecular model. Immunol Today. 1992 Jan;13(1):22–30. doi: 10.1016/0167-5699(92)90200-q. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Lüdemann J., Utecht B., Gross W. L. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990 Jan 1;171(1):357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. G., Sukiennicki T., Pereira H. A., Spitznagel J. K., Guerra M. E., Larrick J. W. Cloning of the cDNA for the serine protease homolog CAP37/azurocidin, a microbicidal and chemotactic protein from human granulocytes. J Immunol. 1991 Nov 1;147(9):3210–3214. [PubMed] [Google Scholar]
- Mueller S. G., Paterson A. J., Kudlow J. E. Transforming growth factor alpha in arterioles: cell surface processing of its precursor by elastases. Mol Cell Biol. 1990 Sep;10(9):4596–4602. doi: 10.1128/mcb.10.9.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Okano K., Aoki Y., Shimizu H., Naruto M. Nucleotide sequence of human bone marrow serine protease (medullasin) gene. Nucleic Acids Res. 1987 Nov 25;15(22):9601–9602. doi: 10.1093/nar/15.22.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. A., Shafer W. M., Pohl J., Martin L. E., Spitznagel J. K. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990 May;85(5):1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl J., Pereira H. A., Martin N. M., Spitznagel J. K. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990 Oct 15;272(1-2):200–204. doi: 10.1016/0014-5793(90)80484-z. [DOI] [PubMed] [Google Scholar]
- Porteu F., Brockhaus M., Wallach D., Engelmann H., Nathan C. F. Human neutrophil elastase releases a ligand-binding fragment from the 75-kDa tumor necrosis factor (TNF) receptor. Comparison with the proteolytic activity responsible for shedding of TNF receptors from stimulated neutrophils. J Biol Chem. 1991 Oct 5;266(28):18846–18853. [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Murialdo H., Delius H., Chai J. H., Poustka A., Frischauf A., Lehrach H. Analysis of cosmids using linearization by phage lambda terminase. Gene. 1985;40(2-3):259–266. doi: 10.1016/0378-1119(85)90048-4. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Wehner N. G., Marshall B. C., Gray W. R., Gray B. H., Hoidal J. R. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991 May 25;266(15):9540–9548. [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Schleuning W. D., Medcalf R. L., Hession C., Rothenbühler R., Shaw A., Kruithof E. K. Plasminogen activator inhibitor 2: regulation of gene transcription during phorbol ester-mediated differentiation of U-937 human histiocytic lymphoma cells. Mol Cell Biol. 1987 Dec;7(12):4564–4567. doi: 10.1128/mcb.7.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K. Antibiotic proteins of human neutrophils. J Clin Invest. 1990 Nov;86(5):1381–1386. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Basset P., Crystal R. G. Myelomonocytic cell lineage expression of the neutrophil elastase gene. J Biol Chem. 1988 Feb 15;263(5):2543–2547. [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Yoshimura K., Quick C. D., States D. J., Holmes M. D., Whang-Peng J., Knutsen T., Crystal R. G. Structure of the human neutrophil elastase gene. J Biol Chem. 1988 Oct 15;263(29):14739–14747. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Connolly N. L., Senior R. M. 12-o-Tetradecanoyl-phorbol-13-acetate-differentiated U937 cells express a macrophage-like profile of neutral proteinases. High levels of secreted collagenase and collagenase inhibitor accompany low levels of intracellular elastase and cathepsin G. J Clin Invest. 1986 May;77(5):1675–1681. doi: 10.1172/JCI112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. G., Snable J. L., Griffith J. E., Scott R. W. Characterization of two azurphil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990 Feb 5;265(4):2038–2041. [PubMed] [Google Scholar]





