Abstract
Purpose of review
This review will examine recent advances in our understanding of atopic dermatitis (AD) and how these mechanisms provide a framework for new approaches to the management of this common skin disease.
Recent findings
The mechanisms by which epithelial skin barrier and immune responses contribute to the complex clinical phenotypes found in AD are being elucidated. AD often precedes food allergy because reduced skin barrier function allows environmental food allergens to penetrate the skin leading to systemic allergen sensitization. There is increasing evidence that AD is a systemic disease. New treatments are focused on intervention in polarized immune responses leading to allergic diseases. This includes antagonism of IL-4 and IL-13 effects. Prevention strategies involve maintaining normal skin barrier function with emollients to prevent allergens and microbes from penetrating the skin.
Summary
Recent work on the pathogenesis of AD has important implications for its clinical management including the development of effective barrier creams and biologicals targeting specific polarized immune pathways resulting in skin inflammation.
Keywords: Atopic dermatitis, eczema, skin barrier, immune response, staphylococcus
INTRODUCTION
Atopic dermatitis (AD) is the most common inflammatory skin disease in the general population [1•]. Recent studies have documented that AD has a major emotional and financial impact on patients and their families thereby causing significant effects on quality of life [2•–4]. Behavioral difficulties, poor school performance and depression are also common [5]. Strong evidence supports the concept that genetic variation of skin barrier genes as well as innate and adaptive immune responses genes increases the risk of AD [6••]. The prevalence of AD, however, varies according to geographic locations even in genetically homogenous populations. This suggests that environmental factors also play a critical role in clinical expression of this genetically complex disease.
The current review will examine recent evidence that epidermal skin barrier dysfunction plays a key role in driving the atopic march. Mechanisms underlying skin barrier dysfunction include genetic mutations as well as lack of terminal keratinocyte differentiation due to activation of certain immune pathways. Environmental factors including the microbiome, UV light exposure, pollution and allergens can also modulate the skin epithelium [7–9]. Different combinations of these multiple factors may account for heterogeneity in age of AD onset, propensity to bacterial and viral infections, differences in severity of skin disease and the natural course of illness. A multi-pronged approach to the prevention and treatment of AD is important for the control and progression of the atopic march.
AD is a Systemic Disease
AD often precedes the onset of food allergy, asthma and allergic rhinitis, i.e. the atopic march [10••]. Given this multi-organ involvement is associated with circulating levels of IgE and activated immune cells with a variety of tissue homing capabilities, AD and other allergic diseases should be considered to be part of a systemic disease. Interestingly, several recent large cohort studies suggest that AD is associated with other systemic inflammatory diseases such as inflammatory bowel disease, type 1 diabetes mellitus, cardiovascular disease, and increased heart attacks [11•, 12].
Ewald et al [13] carried out a meta-analysis combining previously reported AD datasets to define a robust disease profile, termed meta-analysis derived AD (MADAD) transcriptome. This transcriptome identified atherosclerosis signaling and lipid metabolism pathways as being significantly associated with AD. These genes have been associated with coronary heart disease and carotid artery atherosclerosis, consistent with previous findings of increased vascular inflammation in AD. Taken together, recent studies support the concept that AD is a systemic disease.
Skin Barrier Dysfunction in AD
The epidermis provides an important physical and permeability barrier to prevent the penetration of allergens and microbes through the skin. This is achieved in healthy skin by terminal differentiation of keratinocytes into a cornified envelope of keratin filaments aggregated by filaggrin and other structural proteins that are crosslinked by transglutaminases. A variety of lipids, including ceramides, surround the aggregated keratin filaments [14]. An optimal matrix of epidermal lipids and structural proteins are required to create an impermeable skin barrier. Tight junctions between keratinocytes such as claudins are required to prevent water loss and modifies responses to allergens such as mold [15].
Epithelial barrier dysfunction is a central abnormality that drives AD pathobiology. This includes filaggrin deficiency, the lack of certain epidermal lipids and fatty acids as well as tight junction abnormalities. Of the greater than 50 genes implicated in AD pathogenesis, null mutations in the filaggrin gene (FLG) or reduced FLG gene copy number are by far the most compelling in terms of consistent replication and biologic plausibility [16]. FLG loss of function mutations are strongly associated with early onset, more persistent severe skin disease, allergen sensitization and propensity to eczema herpeticum [17,18]. Filaggrin deficiency is associated with reduced skin hydration and enhanced allergen penetration through the skin. Filaggrin breakdown products have been reported to determine corneocyte conformation and natural moisturizing factor levels [19].
The clinical relevance of filaggrin deficiency in the development of food allergy has been demonstrated by Brough et al [20] who found that, in patients from the United Kingdom, the probability of peanut allergen sensitization correlated linearly with environmental peanut exposure in AD children with loss of function FLG mutations. Interestingly, the same group did not find an association of peanut allergy with FLG mutations in the United States. They did find, however that severe AD (associated with immune mediated skin barrier dysfunction) was associated with environmental peanut exposure [21••]. Consistent with these findings, a recent study found that filaggrin gene mutations, without concomitant AD in adults, are not associated with food and aeroallergen sensitization [22]. This suggests that mechanisms other than filaggrin gene mutations can alter skin barrier function and enhance food allergy.
Interestingly, transcriptome profiling of non-lesional skin from AD patients with normal filaggrin genes found dysregulation of genes involved in lipid metabolism [13, 23]. These include genes encoding proteins essential to the de novo synthesis of ceramides that play a critical role in maintaining the epidermal permeability barrier, and proteins involved in the elongation of long chain fatty acids that are essential for prevention of transepidermal water loss. Lipid abnormalities may therefore contribute to defective barrier function in AD independent of FLG gene mutations.
Immune pathway activation
Since filaggrin mutations are found in only the minority of AD patients, immune responses are likely critical in driving the skin barrier dysfunction in the majority of AD [24••,25]. The early immune response in AD skin is predominantly type 2 mediated with multiple cell types including keratinocytes, Th2 cells, mast cells and innate lymphoid cells producing TSLP, IL-4, IL-13, IL-25 and IL-33 [1]. Type 2 responses dominate throughout the course of skin disease even when there is appearance of other cytokines. The importance of Type 2 responses in humans has been recently demonstrated by the remarkable improvement in skin disease when patients with severe AD are treated with a humanized antibody that blocks the action of IL-4 and IL-13 [26–28]. Dendritic cell derived IL-23 also plays an important role in driving differentiation of Th17 and Th22 cells which produce IL-22 that inhibit keratinocyte differentiation. In addition to T cells, mast cells have been found to be major IL-22 producers in patients with AD reinforcing the importance of neutralizing key effector cytokines due to the redundancy of cell types producing similar cytokines [29]. Indeed, recent case reports suggest anti-IL23 can improve severe AD [30].
It is well established that Type 2 immune cytokines, such as IL-4 and IL-13, can profoundly inhibit terminal differentiation of keratinocytes and therefore the expression of genes required for skin barrier function (e.g. filaggrin, loricrin, involucrin). Type 2 cytokines have also been found to suppress production of epidermal lipids and reduce the function of tight junctions in experimental systems. Transcriptomic analyses have demonstrated a strong negative correlation between expression of Type 2 cytokines and epidermal lipids [13]. In contrast, Type 1 cytokines, such as IFNγ, have been shown to induce ceramide synthesis and protect against microbial toxins [31]. These studies support the key role that immune pathways play in modulating skin barrier function.
AD patients have recently been found to have high systemic immune activation with increased polar differentiation of effector and memory T cells particularly in those T cells homing to the skin [32,33]. Importantly there is heterogeneity in AD immune responses stratified by age, race and severity of disease [34]. The Asian form of AD has increased Th17 polarization [35]. Young children with pediatric AD shows only a Th2/Th1 cell imbalance in their skin homing T cells, whereas adults express higher numbers of Th22 cells in their skin homing T cells [36]. Severe AD is characterized by selective expansion of circulating Th2 and Th22 cells, but not Th17, cells within the skin-homing T-cell population [37]. These considerations will be critical as we develop Precision Medicine approaches involving the use of biologics that block particular polarized immune pathways.
Microbiome
Patients with AD are prone to bacterial and viral skin infections [38]. The importance of Staphylococcus aureus infections in driving disease severity is supported by the observation that exacerbations of AD are associated with increased abundance of S. aureus and reduced diversity of the bacterial flora [39]. Furthermore, antibiotic therapy that reduce S. aureus colonization in experimental models lead to improvement in AD skin inflammation [40]. The risk of frequent antibiotic use, however, is that it may contribute to Methicillin Resistant S. Aureus (MRSA) colonization and infection [41].
A subset of patients are prone to disseminated viral skin infections, e.g. eczema herpeticum (EH) or eczema vaccinatum. In experimental animal models, type 2 immune responses mediated by TSLP and IL-33 promote skin inflammation and vaccinia virus replication [42]. This is consistent with previous studies demonstrating patients with EH have very high serum IgE levels and eosinophilia. Gene transcriptome studies of peripheral blood cells from AD patients prone to EH have also revealed a defect in their interferon signaling pathways [43, 44]. Further studies are needed to look directly at their skin host response to viral infections.
Prevention of AD and food allergy
In the absence of a cure for AD, there has been increasing interest in developing approaches to prevent the occurrence of AD. This will likely require optimization of diet, skin care and control of the skin microbiome [45, 46]. Given the overwhelming literature demonstrating that impaired skin barrier leads to AD and food allergy, there have been studies to determine whether the use of skin emollients from birth will prevent AD. The concept underlying this approach is illustrated in Figure 1 (left panel), i.e. skin barrier dysfunction leads to enhanced penetration of the skin by environmental allergens and microbes thereby increasing skin inflammation. The use of effective skin emollients would prevent absorption of AD triggers through the skin and prevent skin inflammation (Figure 1, right panel). Indeed, studies have found that use of skin emollients from birth can prevent AD in than 50% of infants born to high risk parents [47,48].
Figure 1.
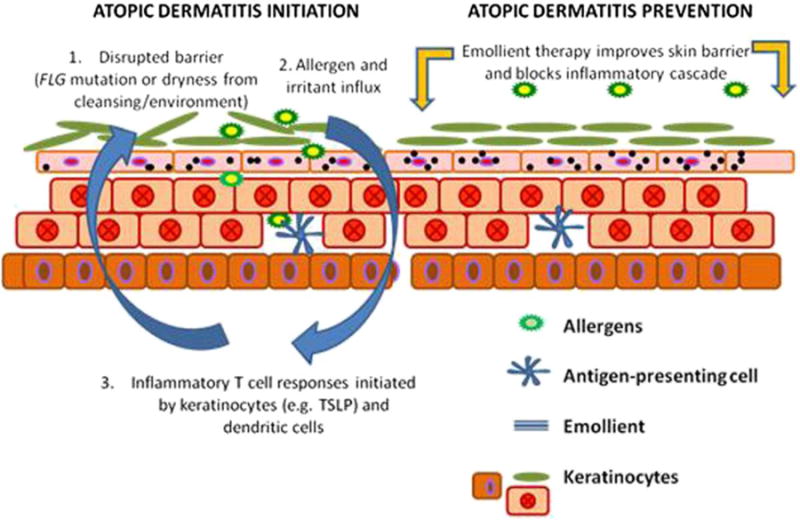
Atopic dermatitis initiation and prevention.
Left panel illustrates how skin barrier dysfunction may lead to skin inflammation. Right panel suggests skin barrier protection may prevent AD development. From reference 47 with permission from publisher.
Complete prevention of AD will likely need more targeted combined approaches to patients at high risk for AD. This will require the development of physiologic measures and biomarkers that predict occurrence of AD. Interestingly several groups have now found that measurement of transepidermal water loss as early as 2 days and 2 months of life can predict the subsequent development of AD regardless of FLG mutations [49•, 50•]. More recently, a birth cohort study, measuring protein content in skin tape strips from early infancy found that the epidermal TSLP could be found in the skin prior to the development of AD [51•• and Figure 2). These studies provide hope that in infants at high risk for AD, early measures including proactive anti-inflammatory therapy could be introduced prior to onset of AD to effectively prevent loss of skin barrier and allergen sensitization.
Figure 2.
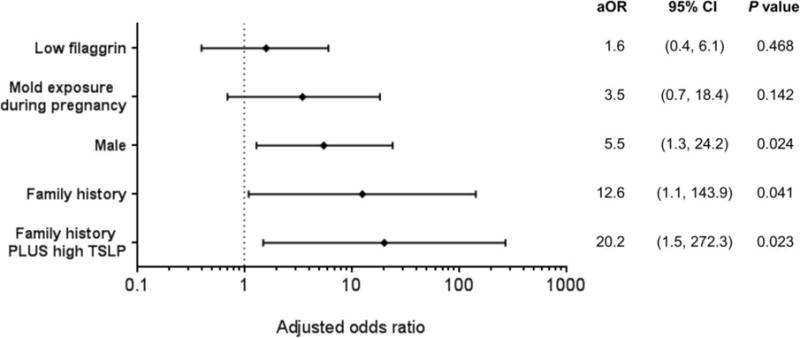
Multivariable analysis for factors influencing AD development by age 24 months.
When TSLP expression was dichotomized into low or high levels according to its median value and added to family history, the predictive value for AD development was further increased in subjects with family history and high TSLP expression than in those with family history alone. From reference 51 with permission from publisher.
Aside from genetic and immunologic factors that may alter skin barrier function, microbes can also play an important role. S. aureus produces a number of virulence factors such as lipases and proteases which directly breakdown the skin barrier. In experimental animals, superantigens from S. aureus have also been shown to augment Th2 responses and subvert the activity of T regulatory cells known to promote peanut allergy. A review of 718 children with AD, seen at National Jewish Health in Denver, revealed a strong association between S. aureus colonization and peanut allergy [52••]. This raises the possibility that eliminating early colonization with S. aureus could abort skin barrier dysfunction leading to peanut allergy.
Management of AD
A multidisciplinary approach is needed for optimal management of AD and its comorbidities. These include strategies which improve the skin barrier including bleach baths, proactive therapy with topical corticosteroids or calcineurin inhibitors to control skin inflammation and strategies to address sleep disturbance and behavioral problems that often accompany AD [53–57]. Testing may also be required to rule out environmental triggers, food allergy or sensitivities to skin products the patient is using [58]. Insomnia and daytime fatigue is common in patients with AD. Difficulty falling asleep may be due in part to delayed production of melatonin in the pineal gland. A recent study suggests melatonin supplementation can be beneficial [59•].
In patients who fail first line therapy, phototherapy or systemic immunosuppressive drugs are potential alternatives but they are often expensive and accompanied by significant adverse events. Additional research is needed to develop effective therapies targeting polarized immune pathways that are not controlled by current anti-inflammatory medications is essential [60]. Clinical trials with these selective agents such as IL-4/IL-13, IL-17, IL-22, IL-23, IL-31, IL-33 antagonists and Janus kinase inhibitors will teach us their relative importance in AD pathobiology while providing a new dimension of therapy that may be beneficial to AD who are refractory to current therapies. In concert, it will be important to develop validated biomarkers that identifies immune pathways leading to clinical disease.
Clinical Implications and Conclusions
AD have a significant negative impact on quality of life including gmental health, sleep and productivity at school and work. AD represents a common clinical phenotype driven by complex pathways leading to individual differences in response to therapy. Its natural history results from the interplay between environmental exposures, underlying genetic variation, abnormal skin epithelial barrier, and their innate/adaptive immune response. This heterogeneity includes onset of AD, severity and duration of disease.
Optimal management requires strategies which improve the skin barrier, proactive therapy to control skin inflammation and patient education. Additional research is needed to develop effective therapies directed at polarized immune pathways that are not controlled by current anti-inflammatory medications are essential. To target specific mechanisms, it will be important to develop validated biomarkers that will identify clinically relevant immune pathways leading to AD.
KEYPOINTS.
AD is the first step in the atopic march leading to food allergy, asthma and allergic rhinitis.
AD is associated with systemic inflammation and may be a risk factor for autoimmune and inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease and vascular inflammation.
Successful treatment of AD requires a multi-pronged approach eliminating AD triggers, improving skin barrier function, and proactive anti-inflammatory therapy.
Predictive markers of AD include increased transepidermal water loss and expression of epidermal TSLP prior to onset of skin disease.
Identification of polarized immune pathways are leading the way to new biologic therapies for patients refractory to conventional approaches.
Acknowledgments
The authors wish to acknowledge The Edelstein Family Foundation for their generous support of this work. We also thank JoAnn Ferguson for her administrative support of the manuscript preparation.
FINANCIAL SUPPORT AND SPONSORSHIP
This research was also supported in part by Colorado Clinical and Translational Sciences Institute (CCTSI), and in part by Colorado Grant UL1 RR025780 from NCRR/NIH and UL1 TR000154 from NIH/NCATS.
Footnotes
CONFLICT OF INTEREST
The author has served as a consultant to Regeneron Pharmaceuticals and Sanofi; he also has a grant from MedImmune.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Leung DYM, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–79. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284–90. doi: 10.1016/j.jpeds.2015.10.077. This study reports on the financial and emotional impact of atopic dermatitis on children and their families. A greater financial burden of AD is associated with greater emotional impact of disease. [DOI] [PubMed] [Google Scholar]
- 3.Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016 Jan 27; doi: 10.1007/s40257-015-0171-3. [DOI] [PubMed] [Google Scholar]
- 4.Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–8. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Yu SH, Silverberg JI. Association between atopic dermatitis and depression in US adults. J Invest Dermatol. 2015;135(12):3183–6. doi: 10.1038/jid.2015.337. [DOI] [PubMed] [Google Scholar]
- 6••.Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47(12):1449–56. doi: 10.1038/ng.3424. This study identified ten new risk genetic loci, bringing the total number of known AD risk loci to 31. Notably, the new loci include candidate genes with roles in the regulation of innate host defenses and T cell function, underscoring the important contribution of immune mechanisms to AD pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim EH, Kim S, Lee JH, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS One. 2015;10(3):e0119501. doi: 10.1371/journal.pone.0119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol. 2015;136(5):1163–9. doi: 10.1016/j.jaci.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Werfel T, Heratizadeh A, Niebuhr M, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136(1):96–103. doi: 10.1016/j.jaci.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 10••.Tsakok T, Marrs T, Mohsin M, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.10.049. This review confirms a strong and dose-dependent association between AD, food sensitization, and food allergy. Severe chronic AD is particularly associated with food allergy. There is also evidence that AD precedes the development of food sensitization and allergy, in keeping with a causal relationship. This has important implications for prevention and treatment. [DOI] [PubMed] [Google Scholar]
- 11•.Schmitt J, Schwarz K, Baurecht H, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137(1):130–6. doi: 10.1016/j.jaci.2015.06.029. This study finds that AD is a risk factor for the development of rheumatoid arthritis and inflammatory bowel disease. This excess comorbidity cannot be attributed to major known IBD and RA genetic risk factors and suggests that AD is a potential risk factor for inflammatory comorbidities. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70(10):1300–8. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 13.Ewald DA, Malajian D, Krueger JG, et al. Meta-analysis derived atopic dermatitis (MADAD) transcriptome defines a robust AD signature highlighting the involvement of atherosclerosis and lipid metabolism pathways. BMC Medical Genomics. 2015;8:60. doi: 10.1186/s12920-015-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Smeden J, Bouwstra JA. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016;49:8–26. doi: 10.1159/000441540. [DOI] [PubMed] [Google Scholar]
- 15.Yu HS, Kang MJ, Kwon JW, et al. Claudin-1 polymorphism modifies the effect of mold exposure on the development of atopic dermatitis and production of IgE. J Allergy Clin Immunol. 2015;135(3):827–30. doi: 10.1016/j.jaci.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Li K, Seok J, Park KY, et al. Copy-number variation of the filaggrin in Korean patients with atopic dermatitis: what really matters - “number” or “variation”? Br J Dermatol. 2015 doi: 10.1111/bjd.14287. [DOI] [PubMed] [Google Scholar]
- 17.Szegedi A. Filaggrin mutations in early- and late-onset atopic dermatitis. Br J Dermatol. 2015;172(2):320–1. doi: 10.1111/bjd.13534. [DOI] [PubMed] [Google Scholar]
- 18.De Marchi F, Piacentini GL, Piazza M, et al. Correlation of skin barrier impairment in atopic dermatitis with aeroallergen sensitization. Allergy Asthma Proc. 2015;36(6):e127–33. doi: 10.2500/aap.2015.36.3872. [DOI] [PubMed] [Google Scholar]
- 19.Riethmuller C, McAleer MA, Koppes SA, et al. Filaggrin breakdown products determine corneocyte conformation in patients with atopic dermatitis. J Allergy Clin Immunol. 2015;136(6):1573–80. doi: 10.1016/j.jaci.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134:867–75. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Brough HA, Liu AH, Sicherer S, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135(1):164–70. doi: 10.1016/j.jaci.2014.10.007. In this study of patients with AD, an exposure-response relationship was found between peanut protein levels in household dust and peanut skin prick test (SPT) sensitization and likely allergy. The effect of environmental peanut exposure on peanut SPT sensitization was augmented in children with a history of AD and even greater in children with severe AD. This study suggests exposure to peanut antigen in dust through an impaired skin barrier in AD is a plausible route for peanut SPT sensitization and peanut allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyssen JP, Tang L, Husemoen LL, et al. Filaggrin gene mutations are not associated with food and aeroallergen sensitization without concomitant atopic dermatitis in adults. J Allergy Clin Immunol. 2015;135(5):1375–8. doi: 10.1016/j.jaci.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Cole C, Kroboth K, Schurch NJ, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;34:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Esaki H, Ewald DA, Ungar B, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol. 2015;135(1):153–63. doi: 10.1016/j.jaci.2014.10.037. This study identified novel immune and barrier genes in AD, including the IL-34 cytokine and claudins 4 and 8, as well as the key AD genes, IL22, TSLP, CCL22, and CCL26. They were also able to localize individual transcripts as primarily epidermal (defensin, beta 4A [DEFB4A]) or dermal (IL22, cytotoxic T-lymphocyte antigen 4 [CTLA4], and CCR7). This is the first report that establishes robust epidermal and dermal genomic signatures of lesional and nonlesional AD skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh D, Ding L, Sivaprasad U, et al. Multiple transcriptome data analysis reveals biologically relevant atopic dermatitis signature genes and pathways. PLoS One. 2015;10(12):e0144316. doi: 10.1371/journal.pone.0144316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043–58. doi: 10.2217/imt.15.69. [DOI] [PubMed] [Google Scholar]
- 27.Tsianakas A, Stander S. Dupilumab: a milestone in the treatment of atopic dermatitis. Lancet. 2016;387(10013):4–5. doi: 10.1016/S0140-6736(15)00389-X. [DOI] [PubMed] [Google Scholar]
- 28.Buzney CD, Gottlieb AB, Rosmarin D. Asthma and atopic dermatitis: a review of targeted inhibition of interleukin-4 and interleukin-13 as therapy for atopic disease. J Drugs Dermatol. 2016;15(2):165–71. [PubMed] [Google Scholar]
- 29.Mashiko S, Bouguermouh S, Rubio M, et al. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136(2):351–9. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Shroff A, Emma Guttman-Yassky. Successful use of ustekinumab therapy in refractory severe atopic dermatitis. J Am Acad Dermatol Case Reports. 2015;1:25–26. doi: 10.1016/j.jdcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brauweiler AM, Goleva E, Leung DYM. IFN-g protects from staphylococcal alpha toxin induced keratinocyte death through apolipoprotein L1. J Invest Dermatol. 2015;136(3):658–64. doi: 10.1016/j.jid.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136(1):208–11. doi: 10.1016/j.jaci.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Tatsuno K, Fujiyama T, Yamaguchi H, et al. TSLP directly interacts with skin-homing Th2 cells highly expressing its receptor to enhance IL-4 production in atopic dermatitis. J Invest Dermatol. 2015;135(12):3017–24. doi: 10.1038/jid.2015.318. [DOI] [PubMed] [Google Scholar]
- 34.Leung DYM. Atopic dermatitis: age and race do matter! J Allergy Clin Immunol. 2015;136:1265–7. doi: 10.1016/j.jaci.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–64. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Czarnowicki T, Esaki H, Gonzalez J, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136(4):941–51. doi: 10.1016/j.jaci.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136(1):104–15. doi: 10.1016/j.jaci.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15(11):65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- 39.Tauber M, Balica S, Hsu CY, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Glatz M, Horiuchi K, et al. Dysbiosis and staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015;42(4):756–66. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaptini C, Quinn S, Marshman G. Methicillin-resistant Staphylococcus aureus in children with atopic dermatitis from 1999 to 2014: A longitudinal study. Australas J Dermatol. 2015 doi: 10.1111/ajd.12371. [DOI] [PubMed] [Google Scholar]
- 42.Oyoshi MK, Venturelli N, Geha RS. Thymic stromal lymphopoietin and IL-33 promote skin inflammation and vaccinia virus replication in a mouse model of atopic dermatitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao L, Bin L, Rafaels NM, et al. Targeted deep sequencing identifies rare loss-of-function variants in IFNGR1 for risk of atopic dermatitis complicated by eczema herpeticum. J Allergy Clin Immunol. 2015;136:1591–1600. doi: 10.1016/j.jaci.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brar K, Leung DY. Recent considerations in the use of recombinant interferon gamma for biological therapy of atopic dermatitis. Expert Opin Biol Ther. 2016;16(4):507–14. doi: 10.1517/14712598.2016.1135898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Donovan SM, OBH J, Murray DM, et al. Neonatal adiposity increases the risk of atopic dermatitis during the first year of life. J Allergy Clin Immunol. 2016;137(1):108–17. doi: 10.1016/j.jaci.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 46.Lynde CW, Andriessen A, Bertucci V, et al. The skin microbiome in atopic dermatitis and its relationship to emollients. J Cutan Med Surg. 2016;20(1):21–8. doi: 10.1177/1203475415605498. [DOI] [PubMed] [Google Scholar]
- 47.Simpson EL, Chalmers JR, Williams HC. Emollient enhancement of skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–23. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horimukai K, Morita K, Narita M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 2014;134:824–830. doi: 10.1016/j.jaci.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 49•.Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135(4):930–5. doi: 10.1016/j.jaci.2014.12.013. The study of northern Europeans suggests serial measurements of transepidermal water loss during infancy may predict AD later in childhood. This may allow physicians to intervene with proactive therapy to prevent clinical AD. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50•.Horimukai K, Morita K, Narita M, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol Int. 2016;65(1):103–8. doi: 10.1016/j.alit.2015.09.004. An important replication in Asians demonstrating that transepidermal water loss can predict later development of AD in a population where filaggrin mutations are rare. [DOI] [PubMed] [Google Scholar]
- 51••.Kim J, Kim BE, Lee J, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1306. This study did not find that transepimderal water loss was predictive of AD later in childhood. However epidermal TSLP in symptom-free infants was predictive of AD. [DOI] [PubMed] [Google Scholar]
- 52••.Jones AL, Curran-Everett D, Leung DYM. Food allergy is associated with S. aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.01.010. This study suggests S. aureus colonization may be associated with the occurrence of peanut allergy in children with AD. It raises the possibility that the microbiome plays a key role in the pathogenesis not only of AD but also food allergy. [DOI] [PubMed] [Google Scholar]
- 53.Shi VY, Foolad N, Ornelas JN, et al. Comparing the Effect of Bleach and Water Baths on Skin Barrier Function in Atopic Dermatitis: A Split-Body Randomized Controlled Trial. Br J Dermatol. 2016 doi: 10.1111/bjd.14483. [DOI] [PubMed] [Google Scholar]
- 54.Weidinger S, Baurecht H, Schmitt J. A critical appraisal of the PETITE study report: topical corticosteroids are safe and effective in the long-term treatment of infantile atopic ermatitis. Pediatrics. 2015;136(5):e1485. doi: 10.1542/peds.2015-2785A. [DOI] [PubMed] [Google Scholar]
- 55.Eichenfield DZ, Eichenfield LF. Pimecrolimus is safe and effective in treating atopic dermatitis. J Pediatr. 2015;167(5):1169–72. doi: 10.1016/j.jpeds.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 56.Cury Martins J, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015;7:CD009864. doi: 10.1002/14651858.CD009864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishbein AB, Vitaterna O, Haugh IM, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol. 2015;136(5):1170–7. doi: 10.1016/j.jaci.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 58.Radhakrishna N, Prickett S, Phan T, et al. Anaphylaxis to oats after cutaneous sensitization by oatmeal in skin products used for the treatment of atopic dermatitis. J Allergy Clin Immunol Pract. 2016;4(1):152–3. doi: 10.1016/j.jaip.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 59•.Chang YS, Lin MH, Lee JH, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170(1):35–42. doi: 10.1001/jamapediatrics.2015.3092. Sleep disturbance is a common problem in patients with AD. The finding that melatonin supplementation may be useful in promoting sleep in children with AD provides a welcome approach to this common problem. [DOI] [PubMed] [Google Scholar]
- 60.Suarez-Farinas M, Ungar B, Correa da Rosa J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol. 2015;135(5):1218–27. doi: 10.1016/j.jaci.2015.03.003. [DOI] [PubMed] [Google Scholar]


