Abstract
Purpose
Capecitabine 1,000 mg/m2 bid × 14 days every 21 days (14/21) has been reported to have similar efficacy but more favorable toxicity profile than the approved dosage of 1,250 mg/m2. However, a dose-toxicity relationship of capecitabine in breast cancer patients has not been fully elucidated. We performed a systematic review and meta-analysis to compare a safety profile between capecitabine starting dose of 1,000 and 1,250 mg/m2 bid.
Methods
Studies were identified using PubMed, ASCO and San Antonio Breast Cancer Symposium abstract databases through December 2015. Eligible trials included phase II/ III trials of capecitabine monotherapy at 1,000 or 1,250 mg/m2 bid (14/21) for breast cancer patients that reported adequate safety data for all (Grade 1-4) or high (Grade 3-4) grade hand foot syndrome (HFS), diarrhea, fatigue, nausea, vomiting, stomatitis, neutropenia, thrombocytopenia, or anemia, as well as dose reductions, treatment discontinuation or treatment-related deaths. The summary incidence was calculated using random- effects models.
Results
A total of 4,833 patients from 34 trials were included. 1,218 and 3,615 patients were treated with capecitabine 1,000 and 1,250 mg/m2 bid, respectively. A significantly lower incidence of dose reduction (15.9 vs. 39.0%; P = 0.007), high-grade HFS (12.0 vs. 19.0%; P = 0.01), diarrhea (5.3 vs. 9.1%; P = 0.01), and neutropenia (1.8 vs. 7.3%; P < 0.01) and all-grade neutropenia (5.8 vs. 25.4%; P = 0.01) was seen in capecitabine 1,000 mg/m2 compared to 1,250 mg/m2.
Conclusions
Capecitabine monotherapy at 1,000 mg/m2 bid (14/21) has a clinically meaningful and significantly better toxicity profile compared to 1,250 mg/m2 bid (14/21).
Keywords: Capecitabine, Lower dose, Meta-analysis, Standard dose, Systematic review, Toxicity profile
Introduction
Capecitabine is an oral pro-drug that is converted to 5-fluorouracil (5-FU) via a three-step enzymatic process, the final step of which is mediated by thymidine phosphorylase. Given this enzyme is over expressed in tumor compared with normal tissue, 5-FU is preferentially generated within the tumor tissue, conferring relatively selective cytotoxicity to the tumor [1]. Capecitabine is one of the most active agents in metastatic breast cancer (MBC). The FDA has approved capecitabine monotherapy 1,250 mg/m2 twice daily (bid) on days 1–14 followed by a 7-day rest period (14/21) for MBC that is resistant to both paclitaxel and anthracyclines [2]. It has also been extensively studied in both pretreated and previously untreated MBC patients and demonstrated efficacy in response rate and progression-free survival (PFS) [3,4] .However, 26%–65% of patients had their dose reduced by at least 20% in these trials [5,6]. The main treatment-limiting toxicities at this dosage were hand-and-foot syndrome (HFS; also called palmer-plantar erythrodysesthesia) and diarrhea. Based on this experience, a number of investigators have evaluated capecitabine at a lower starting dose (1,000 mg/m2 bid) and demonstrated similar efficacy to the approved dose and a more favorable side effect profile with an incidence of dose reduction, ranging from 16 to 34% in phase II trials [7,8]. However, there has been a substantial variation in the incidence of toxicities among clinical trials and there has been no systematic attempt to synthesize these data in order to define the overall risk of toxicities induced by the lower and standard dose capecitabine. Therefore, we conducted a systematic review and meta-analysis of available clinical trials to compare a safety profile between capecitabine starting dose of 1,000 and 1, 250 mg/m2 bid in breast cancer patients.
Methods
Data source
This analysis was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [9]. We conducted an independent review of PubMed from January 1966 to December 2015. Searches were performed by using the keywords “capecitabine” and “breast cancer” and were limited to clinical trials. We searched abstracts and virtual meeting presentations utilizing the same search terms from the American society of clinical oncology (ASCO) conference and San Antonio breast cancer symposiums (SABCS) through December 2015 to identify relevant studies. An independent search of the web of science, Embase, and Cochrane electronic databases was also performed to ensure that no additional studies were overlooked. In cases of duplicate publications, only the most complete, recent, and updated report of the study was included.
Study selection
Clinical trials that met the following criteria were included: (1) phase II and III trials of capecitabine monotherapy at 1,000 or 1,250 mg/m2 bid on day 1 through 14 every 3 weeks for breast cancer patients; and (2) reporting events or event rate and sample size for any all (grade 1-4) or high (grade 3-4) grade adverse events (AEs), individual, all or high grade AEs, dose reductions, treatment discontinuation or treatment-related deaths. We assessed nine individual AEs which were commonly reported in clinical trials. They included hand foot syndrome (HFS), diarrhea, fatigue, nausea, vomiting, stomatitis, neutropenia, anemia and thrombocytopenia. We did not include trials of capecitabine combined with other agents. Independent reviewers (TFN and MS) screened reports that included the key terms by their titles and abstracts for relevance. Then, full texts of the relevant articles were retrieved to assess eligibility. The references of relevant reports were also reviewed manually.
Data extraction
Two investigators (TFN and MS) independently performed data extraction. The following information was recorded for each study: first author's name, year of publication, trial phase, age, disease stage, treatment setting, capecitabine dose, number of patients available for analysis, number of cycles of capecitabine, CTCAE version, and number of the following adverse events: any, all, or high grade AEs, individual, all or high grade AEs (HFS, diarrhea, fatigue, nausea, vomiting, stomatitis, neutropenia, anemia and thrombocytopenia), dose reductions, treatment discontinuation and treatment-related deaths. Any discrepancies between reviewers were resolved by consensus. The number of patients evaluable for toxicity was utilized as the number analyzed for each trial, unless this was not indicated in the publication, in which case, the number of patients enrolled was utilized. In selected clinical trials, the adverse events were recorded according to the CTCAE.
Statistical analysis
The principal summary measures were incidence and corresponding 95 % confidence intervals (CIs) of the following AEs: any, all, or high grade AEs, individual, all or high grade AEs (HFS, diarrhea, fatigue, nausea, vomiting, stomatitis, neutropenia, thrombocytopenia, and anemia), dose reductions, treatment discontinuation and treatment-related deaths. The proportion of patients with those adverse outcomes and 95 % CIs were derived from each trial. Statistical heterogeneity in results between trials included in the meta-analysis was examined using Cochrane's Q statistic, and inconsistency was quantified with I2 statistic [100 % × (Q - df)/Q], which estimates the percentage of total variation across studies due to heterogeneity rather than chance [10]. The assumption of homogeneity was considered invalid for P values less than 0.10. We used a random-effects model to produce a pooled overall estimate for incidence of the adverse outcomes. Differences in the incidences between the two groups were assessed using Q statistics. We evaluated publication bias using funnel plots and with the Begg and Egger tests [11,12]. A two tailed P value of less than 0.05 was considered statistically significant. Statistical analyses were performed by using the comprehensive meta-analysis program (Version 2, Biostat, Englewood, NJ, USA).
Results
Search results and population characteristics
Our search strategy yielded 391 potentially relevant publications. 360 citations were excluded. This large proportion of studies that had to be excluded from analyses consisted of reviews, observational studies, non-capecitabine trials, non-breast cancer trials and trials of capecitabine combined with other therapy This selection process and reasons for study exclusion are shown in a flow diagram (Fig. 1). We found additional reports identified through manual review of the references of included publications and ASCO and SABCS abstract database [7,13,14]. Thus, a total of 34 trials with 4,833 patients were considered eligible for the meta-analysis, including 12 phase III trials and 22 phase II trials. 30 trials were in the locally advanced or MBC setting, two trials were in the neoadjuvant setting, and two trials were in the adjuvant setting. 1,218 patients from 13 trials and 3,615 patients from 23 trials were treated with capecitabine starting dose at 1,000 and 1,250 mg/m2 bid, respectively. There were two trials which modified the capecitabine starting dose from 1,250 to 1,000 mg/m2 bid. These trials reported toxicity outcomes according to the capecitabine starting dose [15,16]. We did not include a trial which administered capecitabine 1,250 mg/m2 bid in patients ≤ 65 years and 1,000 mg/m2 bid in patients > 65 years and did not report toxicity outcomes separately for the two dosing groups [17]. There were differences in the number of trials included for each endpoint because the study endpoints of interest were not consistently reported in all trials.
Figure 1. Flow diagram; selection process for the trials.
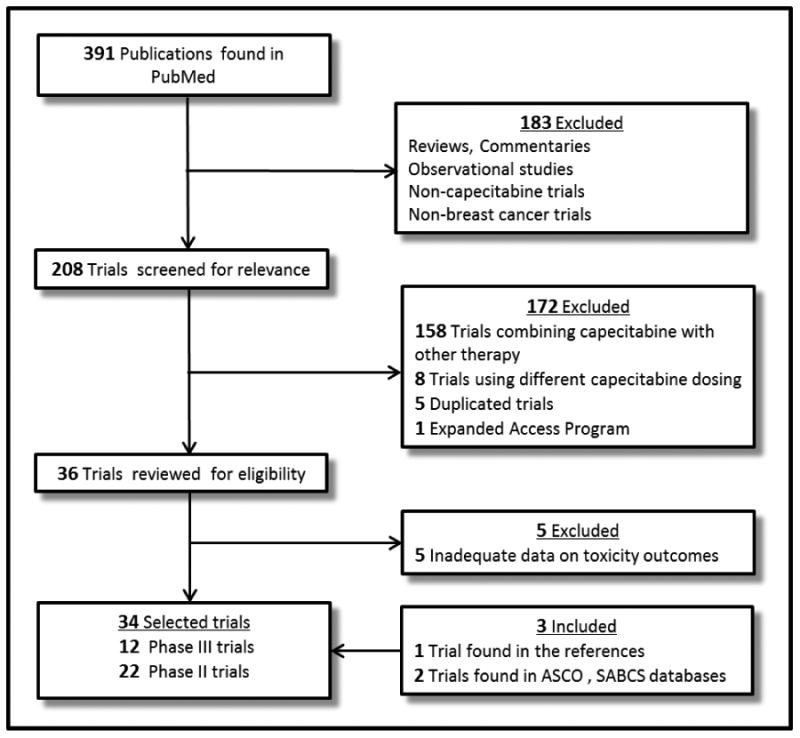
Abbreviations: ASCO, American Society of Clinical Oncology; SABCS, San Antonio Breast Cancer Symposium
Comparison of toxicity profiles (Toxicity profile)
Toxicity data for high grade HFS were available for 33 trials. The incidence of high grade HFS in patients receiving capecitabine 1,000 mg/m2 bid and 1,250 mg/m2 bid was respectively 12.0% (95% CI, 9.0-15.7%) and 19.0% (95% CI, 15.2-23.4%) by using the random-effects model (Table 2). The test for heterogeneity was significant in the lower (Q = 17.5; P = 0.06; I2 = 42.9) and approved (Q = 149.0; P < 0.001; I2 = 85.9) dose groups. There was a significant decrease in the incidence of high grade HFS with the use of the lower dose compared with the standard dose (P = 0.01). High grade diarrhea developed less frequently in patients treated with the lower dose of capecitabine than those treated with the approved dose (5.3 vs. 9.1%; P = 0.011). There were fewer all-grade neutropenia events among patients treated with capecitabine 1,000 mg/m2 bid (5.8%) than among patients treated with 1,250 mg/m2 bid (25.4%). A lower incidence of high-grade neutropenia was also observed in capecitabine 1,000 mg/m2 bid group (1.8%) in comparison with 1,250 mg/m2 bid group (7.3%). Patients treated with capecitabine 1,000 mg/m2 bid required a dose reduction for toxicity less frequently than those treated with 1,250 mg/m2 bid (P = 0.007). The incidence of dose reduction with the lower and approved dose capecitabine was respectively 15.9% (95% CI, 7.5-30.5%) and 39.0% (95% CI, 32.4-45.9%). There was also a trend toward lower incidence of high-grade vomiting (P = 0.05) and high-grade anemia (P = 0.08) in the capecitabine 1,000 mg/m2 bid group compared with the 1,250 mg/m2 bid group. There was no significant difference in the incidence of treatment discontinuation and treatment-related deaths between the two groups.
Table 2. Incidence of toxicities, including 95% CI from the random effects model and number of trials in each group.
| Capecitabine 1,000 mg/m2 bid | Capecitabine 1,250 mg/m2 bid | ||||||
|---|---|---|---|---|---|---|---|
| No. of trials | Incidence % | 95% CI | No. of trials | Incidence % | 95% CI | P value | |
| Non-hematological AEs | |||||||
| All-grade HFS | 8 | 52.8 | 40.4-64.9 | 20 | 52.4 | 46.4-58.4 | 0.951 |
| High-grade HFS | 11 | 12.0 | 9.0-15.7 | 22 | 19.0 | 15.2-23.4 | 0.010 |
| All-grade Diarrhea | 8 | 32.7 | 22.1-45.4 | 18 | 36.7 | 31.6-42.1 | 0.551 |
| High-grade Diarrhea | 9 | 5.3 | 3.9-7.0 | 20 | 9.1 | 6.7-12.3 | 0.011 |
| All-grade Fatigue | 7 | 35.9 | 22.4-52.0 | 17 | 25.8 | 21.7-30.3 | 0.183 |
| High-grade Fatigue | 10 | 5.1 | 3.1-8.2 | 18 | 4.5 | 2.7-7.6 | 0.743 |
| All-grade Nausea | 7 | 35.8 | 22.8-51.3 | 18 | 38.1 | 32.2-44.3 | 0.784 |
| High-grade Nausea | 8 | 2.9 | 1.7-4.8 | 18 | 3.7 | 1.9-7.3 | 0.541 |
| All-grade Vomiting | 6 | 17.9 | 9.8-30.3 | 17 | 23.0 | 19.4-27.0 | 0.393 |
| High-grade Vomiting | 7 | 1.8 | 1.0-3.1 | 18 | 3.8 | 2.3-6.4 | 0.050 |
| All-grade Stomatitis | 6 | 18.6 | 12.3-27.0 | 15 | 15.3 | 11.5-20.1 | 0.437 |
| High-grade Stomatitis | 8 | 1.9 | 1.1-3.2 | 13 | 2.2 | 1.3-3.7 | 0.659 |
| Hematological AEs | |||||||
| All-grade Neutropenia | 5 | 5.8 | 1.8-17.5 | 10 | 25.4 | 17.3-35.7 | 0.011 |
| High-grade Neutropenia | 9 | 1.8 | 1.1-2.9 | 18 | 7.3 | 5.8-9.1 | < 0.001 |
| All-grade Anemia | 3 | 28.5 | 12.6-52.6 | 11 | 29.3 | 15.5-48.4 | 0.954 |
| High-grade Anemia | 5 | 1.9 | 1.0-3.5 | 15 | 3.4 | 2.7-4.2 | 0.084 |
| All-grade Thrombocytopenia | 4 | 8.5 | 2.2-27.9 | 10 | 13.9 | 8.0-22.8 | 0.491 |
| High-grade Thrombocytopenia | 6 | 2.4 | 1.0-5.3 | 16 | 2.6 | 2.0-3.4 | 0.806 |
| Any all-grade AEs | 2 | 81.1 | 2.4-99.9 | 6 | 92.7 | 87.8-95.7 | 0.681 |
| Any high-grade AEs | 6 | 27.8 | 19.9-37.4 | 7 | 40.4 | 28.4-53.7 | 0.109 |
| Dose Reductions | 5 | 15.9 | 7.5-30.5 | 14 | 39.0 | 32.4-45.9 | 0.007 |
| Treatment Discontinuation | 9 | 11.6 | 8.8-15.3 | 18 | 10.6 | 8.5-13.2 | 0.603 |
| Treatment-related Deaths | 13 | 1.6 | 1.0-2.5 | 21 | 1.3 | 0.8-2.1 | 0.269 |
Abbreviations: AEs, adverse events; HFS, hand foot syndrome
Publication bias
We found no evidence of publication bias for incidence of any all- and high-grade AEs, all- and high grade HFS, fatigue, vomiting, and stomatitis and all-grade diarrhea, nausea and anemia and dose reductions, treatment discontinuation and treatment-related deaths. The Egger test suggested some evidence of publication bias (P < 0.05) for incidence of all- and high-grade neutropenia and thrombocytopenia and high-grade diarrhea, nausea, and anemia. However, the Begg tests showed no evidence of bias for the incidence of these outcomes (P > 0.05). This difference in the results obtained from the two methods may be due to a greater statistical power of the Egger test [44].
Discussion
A goal of the current analysis was to systematically assess the overall risk of toxicities associated with the lower and standard dose capecitabine. Our meta-analysis of 34 clinical trials demonstrated a dose-toxicity relationship of capecitabine in breast cancer patients. A significantly lower incidence of dose reductions, high grade HFS, diarrhea, neutropenia and all grade neutropenia was observed in capecitabine starting dose of 1,000 mg/m2 bid compared to 1,250 mg/m2 bid. These findings reinforce the results of the previous retrospective study performed by Hennessy et al. at M. D. Anderson Cancer Center [45]. In their study, 106 patients receiving capecitabine monotherapy who were evaluable for toxicity were grouped according to the starting dose of capecitabine: A= 1250 ± 5% mg/m2 bid (n = 51); B = 1125 ± 5% mg/m2 bid. (n = 16); C ≤ 1000 +5% mg/m2 bid (n = 39). Although no statistical comparison was performed, the incidence of dose reduction (28 vs. 41%), high grade HFS (20 vs. 33%), and diarrhea (3 vs. 13%) was numerically lower in the group C compared with group A. Overall it showed a trend to a better tolerability with the lower dose of capecitabine. In addition to the milder toxicity profile, clinical trials have shown that a lower starting capecitabine dose is comparable in efficacy to the approved dose despite the lack of a randomized trial comparing the two approaches. Randomized phase II/III clinical trials of first-line capecitabine (1,250 mg/m2 bid (14/21)) in MBC patients showed a median time to progression (TTP) of 4.1-7.1 months and a median OS of 19.6-29.4 months [19, 46]. Findings from phase III trials of first-line capecitabine (1,000 mg/m2 bid (14/21)) in MBC patients are similar to the results of the approved dose capecitabine. The median PFS and TTP were in the range of 5.7–6.0 months and the median OS was within the range of 21.2–24.0 months [34,35]. Because of the substantial heterogeneity in the trial settings including treatment intent (curative vs. palliative) and line of therapy, we did not perform a meta-analysis of the trials to compare the efficacy of capecitabine starting dose of 1,250 to 1,000 mg/m2 bid.
These data coupled with clinical experience and the palliative goal of treatment in MBC have led many clinicians to start their MBC patients on a lower starting dose of capecitabine than the 1,000 mg/m2 bid dose, whether as first-line treatment or later in the course of therapy. Ambros and colleagues commonly prescribe capecitabine in their MBC population at a fixed dose of 1000mg bid for 14 of every 21 days within their single large breast-specific oncology group [47]. Given the lack of published data on this dose, they retrospectively analyzed data from 86 patients treated with this regimen (CAPE-L) regardless of the number of prior therapy lines. The median starting dose was 633.5mg/m2 (range: 303.4–965.3) bid, roughly 50% of the FDA approved dose. They compared outcomes in this population to a historical control group based on literature review of 12 studies incorporating the approved dose and schedule of capecitabine. Overall response rate and median TTP was similar between the CAPE-L and the standard dose cohorts (24.3 vs. 24 %), and (7 months, 95 % CI 5.5-8.5 vs. 5.1 months, 95 % CI 4.5-5.7, respectively). Median OS was longer in the CAPE-L cohort (24 months, 95 % CI 16.8-31.2) versus (12.1 months, 95 % CI 9.6-14.4), however, this was attributed by the investigators to the higher percentage of patients in the CAPE-L group receiving capecitabine as first-line chemotherapy and harboring endocrine positive disease. They observed a lower incidence of grade 3–4 HFS (5.8 vs. 11.4 %) and diarrhea (4.7 vs. 10.2 %) with CAPE-L compared to the historical control group. Bertelsen et al. also performed a retrospective analysis of 84 patients treated with a low dosage of capecitabine monotherapy as their first, second, or third line of chemotherapy for metastatic or unresectable locally advanced breast cancer [48]. Eighty-six percent of the patients received a flat dosage of 1000 mg bid and the median starting dosage was 565mg/m2 bid, with a range of 305 to 1057 mg/m2. The median PFS for patients with measurable disease was 4.1 months (95 % CI 2.9-5.7) which was similar to the median PFS values 4.2-4.4 months for capecitabine monotherapy reported in the randomized trials with similar eligibility criteria [30,31]. Although the authors did not report detailed toxicity outcomes, they stated that the low dose of capecitabine was well tolerated and only 2 patients discontinued capecitabine due to toxicity. In addition to the approach of lowering the starting capecitabine dose, alternative schedules have been investigated to improve treatment tolerability. Continuous metronomic capecitabine monotherapy has been tested in two phase II trials in patients with advanced breast cancer. Capecitabine was given continuously at 666 mg/m2 bid in Harvey's trial and at fixed dose 1500 mg once a day in Fedele's trial [49,50]. Overall response rate and median TTP/PFS was 36% and 3.1 months in Harvey's trial and 24% and 7 months in Fedele's trial, respectively. The most common grade 3-4 AE was hand foot syndrome (5-17%) and in general these regimens were well tolerated. In Japan, an intermittent 4-week schedule (828 mg/m2 bid, days 1–21 every 28 days) has been studied in phase II trials of advanced breast cancer [51-53]. In the trials, overall response rate and median TTP/PFS was 18%-46% and 5.1-7.2 months, respectively. These results are comparable to those with the standard 3-weekly intermittent schedule. An incidence of hand–foot syndrome (15-18%) was similar to the 3-weekly regimen, but high grade diarrhea was seen in only one patient in these trials. Notably, a 7-days-on, 7-days-off (7/7) regimen of capecitabine was developed by Traina et al. based on the Norton–Simon mathematical model [54]. In their phase I trial, the most frequently grade 2-3 AEs were hand-foot syndrome (29%), leukopenia/neutropenia (24%), and fatigue (19%). The maximum-tolerated doses of capecitabine was 2,000 mg bid. Although capecitabine monotherapy on this 7/7 schedule has not been evaluated in larger clinical trials, this dosing schedule is commonly used in the U.S. because of its good tolerability seen in daily practice.
As treatment for metastatic breast cancer is palliative, minimizing toxicity and loss of function associated with treatment is of major importance. Improvement in chemotherapy tolerability without compromising efficacy is of particular importance in older adults with MBC. Elderly cancer patients are known to be at higher risk of chemotherapy-related adverse events [55,56]. In a pooled analysis of five phase II/III trials of capecitabine 1,250 mg/m2/day bid (14/21), an incidence of treatment discontinuation due to toxicity was higher in women >65 years (24.4%) compared with younger women (13.0-15.0%) [3]. Additionally, treatment related mortality was observed in a phase 2 study of capecitabine monotherapy in the older (≥ 65 years) adults with MBC [15]. In this trial two of 30 patients treated with capecitabine starting dose of 1250mg/m2 bid and one of 43 patients treated with a lower starting dose (1000 mg/m2) died due to toxicities. Given these findings, a prospective study of low dose capecitabine monotherapy (e.g. fixed-dose 1,000 mg bid, 2 weeks on and 1 week off) in this population may be indicated in order to not only better assess the survival outcomes but also toxicity and outcomes particularly important for the elderly such as the maintenance of independent physical and social function, and quality of life.
Our study had several limitations. First, significant heterogeneity was observed in the incidence analyses although we included only phase II and III trials of breast cancer patients. This may be related to the differences in treatment intent, line of therapy, prior treatments and sample size. We conducted all analyses using the random-effects model to take into account the between-study variation. Second, this is a meta-analysis at study level; therefore variables at the patient level were not incorporated into the analysis. Thus we could not establish risk factors associated with the development of toxicities. Third, as with any meta-analysis, the results described here are affected by the limitations of individual clinical trials that were selected for this meta-analysis.
In conclusion, our meta-analysis showed that capecitabine monotherapy at 1,000 mg/m2 bid for 14 days every 21 days has a clinically meaningful and significantly better toxicity profile in patients with breast cancer compared to the approved dose of capecitabine at 1,250 mg/m2 bid. Given our finding and the efficacy results from the clinical trials, capecitabine monotherapy at 1,000 mg/m2 bid for 14 days every 21 days is a reasonable standard of care. Prospective studies are warranted to determine whether further lower doses of capecitabine or other doses and schedules can achieve improvement in tolerability without compromising efficacy in patients with advanced breast cancer.
Table 1. Characteristics of the trials included in the meta-analysis.
| Author, Year | Phase | Age (years) median (range) | Disease stage | Treatment setting | Capecitabine dose | No. of patients for analysis | Number of cycles median (range) | CTCAE version |
|---|---|---|---|---|---|---|---|---|
| Blum, 199918 | II | 56 (26-78) | Metastatic | 2nd or 3rd line | 1,255 mg/m2 bid | 162 | NR | 1 |
| Oshaughnessy, 200119 | II RCT | 69 (54-83) | Locally advanced/ Metastatic | 1st line | 1,255 mg/m2 bid | 61 | 4 | 1 |
| Blum, 200120 | II | 53 (29–77) | Locally advanced/ Metastatic | 2nd or later line | 1,255 mg/m2 bid | 74 | NR | 1 |
| Jakob, 200221 | II | 46 (35-60) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 14 | 5 (1–19) | 1 |
| Talbot, 200222 | II RCT | 52 (33-67) | Locally advanced/ Metastatic | 1st, 2nd or 3rd line | 1,255 mg/m2 bid | 22 | NR | NR |
| Reichardt, 200323 | II | 56 (32–77) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 136 | 4 (1–33) | NR |
| Fumoleau, 200424 | II | 54 (30–80) | Locally advanced/ Metastatic | 2nd or later line | 1,250 mg/m2 bid | 126 | 6 (1–15) | NR |
| Wist, 200425 | II | 55 (35-74) | Locally advanced/ Metastatic | 2nd or later line | 1,250 mg/m2 bid | 48 | NR | 1 |
| Lee, 200426 | II | 48 (31–66) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 38 | 6 (1–15) | 2 |
| Miller, 20056 | III | 52 (30-77) | Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 215 | NR | 2 |
| Bajetta, 200515 | II | 73 (65-89) | Metastatic | 1st or 2nd line | 1,250 mg/m2 bid | 30 | 6 (1-8) | NR |
| 1,000 mg/m2 bid | 43 | 6 (1-8) | ||||||
| El-Helw, 20057 | II | 48 (20–73) | Metastatic | 1st, 2nd or later line | 1,000 mg/m2 bid | 57 | 4 (1-44) | NR |
| Cameron, 200827 | II | 51 (28–83) | Locally advanced/ Metastatic | 2nd or later line | 1,250 mg/m2 bid | 191 | NR | 3 |
| Rossi, 200716 | II | 62 (38-87) | Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 7 | 9 (1-35) | NR |
| 1,000 mg/m2 bid | 30 | |||||||
| von Minckwitz, 200928 | III | 59 (33-82) | Locally advanced/ Metastatic | 1st or 2nd line | 1,250 mg/m2 bid | 78 | 6 (1-42) | 2 |
| Muss, 200929 | III | >=65 | Stage I to IIIB | Adjuvant | 1,000 mg/m2 bid | 299 | 6 | 3 |
| Hortobagyi, 201030 | III | 52 (25–79) | Locally advanced/ Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 368 | 4 (1-33) | 3 |
| Sparano, 201031 | III | 53 (24-81) | Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 603 | 5 (1-50) | 3 |
| Kaufmann, 201032 | II | 65 (37–90) | Metastatic | 1st line | 1,000 mg/m2 bid | 161 | 7 | 2 |
| Clemons, 201033 | II RCT | 54 (31-74) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 43 | 3 (1-19) | 3 |
| Robert, 201134 | III | 57 (23-88) | Metastatic | 1st line | 1,000 mg/m2 bid | 201 | 9 | NR |
| Pallis, 20125 | III | 60 (34–82) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 74 | 6 (1–15) | 3 |
| Stockler, 201135 | III | 62 | Locally advanced/ Metastatic | 1st line | 1,000 mg/m2 bid | 107 | 12 | 2 |
| Baselga, 20128 | II RCT | 54 | Locally advanced/ Metastatic | 1st or 2nd line | 1,000 mg/m2 bid | 112 | 7 | 3 |
| Huang, 201236 | II | 50 (26–71) | Metastatic | 1st, 2nd or later line | 1,000 mg/m2 bid | 64 | 4 (1–20) | 3 |
| Goldstein, 201313 | II RCT | 57 (31-80) | Metastatic | 1st line | 1,000 mg/m2 bid | 66 | 7 | NR |
| Crown, 201337 | III | 54 (31-77) | Metastatic | 2nd or later line | 1,250 mg/m2 bid | 215 | 6 (1-47) | 3 |
| Arowolo, 201338 | II | 50 (32–70) | Locally advanced | Neoadjuvant | 1,000 mg/m2 bid | 16 | (3-8) | 2 |
| Tolaney, 201439 | II | 53 (29–71) | Early stage operable | Neoadjuvant | 1,000 mg/m2 bid | 24 | 4 | NR |
| Mita, 201440 | II RCT | 54 (39-69) | Locally advanced/ Metastatic | 2nd line | 1,250 mg/m2 bid | 14 | NR | 3 |
| Smorenburg, 201441 | III | 75 (65–86) | Metastatic | 1st line | 1,000 mg/m2 bid | 38 | 7 (1–8) | 3 |
| Kaufman, 201542 | III | 53 (26-80) | Locally advanced/ Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 546 | 5 (1-61) | 3 |
| Martín, 201543 | II RCT | 61 (34–87) | Metastatic | 1st, 2nd or later line | 1,250 mg/m2 bid | 95 | NR | NR |
| Lee, 201514 | III | 48 | Stage I to IIIB | Adjuvant | 1,250 mg/m2 bid | 455 | NR | NR |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; NR, not reported; RCT, Randomized Controlled Trial
Acknowledgments
Funding: None.
Role of the funding source: None
Footnotes
Conflicts of interest: Dr. Muss has held consultant/advisory role for Pfizer and HarborPath. The remaining authors state that they have no conflict on interests.
Authors' contributions: TFN involved in study concept and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript. MS involved in acquisition of data and critical revision of the manuscript. HBM participated in the elaboration of the research design and made important contributions in revising the manuscript.
References
- 1.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274–81. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Capecitabine (Xeloda - Genentech) package insert. http://www.gene.com/download/pdf/xeloda_prescribing.pdf.
- 3.Blum JL, Barrios CH, Feldman N, Verma S, McKenna EF, Lee LF, et al. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast cancer research and treatment. 2012;136(3):777–88. doi: 10.1007/s10549-012-2288-x. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy JA, Kaufmann M, Siedentopf F, Dalivoust P, Debled M, Robert NJ, et al. Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer. The oncologist. 2012;17(4):476–84. doi: 10.1634/theoncologist.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallis AG, Boukovinas I, Ardavanis A, Varthalitis I, Malamos N, Georgoulias V, et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(5):1164–9. doi: 10.1093/annonc/mdr405. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(4):792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 7.El-Helw L, Coleman RE. Reduced dose capecitabine is an effective and well-tolerated treatment in patients with metastatic breast cancer. Breast. 2005;14(5):368–74. doi: 10.1016/j.breast.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(13):1484–91. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein LJ, Oliveira CT, Heinrich B, Stemmer SM, Mala C, Kastner S, et al. A randomized double-blind phase II study of the combination of oral WX-671 plus capecitabine versus capecitabine monotherapy in first-line HER2-negative metastatic breast cancer (MBC) J Clin Oncol. 2013;31 (suppl; abstr 508) [Google Scholar]
- 14.Lee SJ, Toi M, Lee ES, Ohtani S, Im YH, Im SA, Park BW, et al. A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive disease after neoadjuvant chemotherapy (CREATE-X, JBCRG-04) San Antonio Breast Cancer Symposium; 2015. Abstract S1-07. [Google Scholar]
- 15.Bajetta E, Procopio G, Celio L, Gattinoni L, Della Torre S, Mariani L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(10):2155–61. doi: 10.1200/JCO.2005.02.167. [DOI] [PubMed] [Google Scholar]
- 16.Rossi D, Alessandroni P, Catalano V, Giordani P, Fedeli SL, Fedeli A, et al. Safety profile and activity of lower capecitabine dose in patients with metastatic breast cancer. Clinical breast cancer. 2007;7(11):857–60. doi: 10.3816/CBC.2007.n.050. [DOI] [PubMed] [Google Scholar]
- 17.Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast cancer research and treatment. 2010;121(1):121–31. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759–68. doi: 10.1002/1097-0142(20011001)92:7<1759::aid-cncr1691>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2001;12(9):1247–54. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 20.Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(2):485–93. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 21.Jakob A, Bokemeyer C, Knop S, Schupp M, Mayer F, Kanz L. Capecitabine in patients with breast cancer relapsing after high-dose chemotherapy plus autologous peripheral stem cell transplantation--a phase II study. Anticancer Drugs. 2002;13(4):405–10. doi: 10.1097/00001813-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Talbot DC, Moiseyenko V, Van Belle S, O'Reilly SM, Alba Conejo E, Ackland S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. British journal of cancer. 2002;86(9):1367–72. doi: 10.1038/sj.bjc.6600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichardt P, Von Minckwitz G, Thuss-Patience PC, Jonat W, Kolbl H, Janicke F, et al. Multicenter phase II study of oral capecitabine (Xeloda(″)) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003;14(8):1227–33. doi: 10.1093/annonc/mdg346. [DOI] [PubMed] [Google Scholar]
- 24.Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40(4):536–42. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Wist EA, Sommer HH, Ostenstad B, Risberg T, Bremnes Y, Mjaaland I. Oral capecitabine in anthracycline- and taxane-pretreated advanced/metastatic breast cancer. Acta Oncol. 2004;43(2):186–9. doi: 10.1080/02841860310023165. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Lee J, Park J, Park SH, Lee KE, Lee SI, et al. Capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Med Oncol. 2004;21(3):223–31. doi: 10.1385/MO:21:3:223. [DOI] [PubMed] [Google Scholar]
- 27.Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast cancer research and treatment. 2008;112(3):533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 28.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 29.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. The New England journal of medicine. 2009;360(20):2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hortobagyi GN, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, et al. Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes. Breast cancer research and treatment. 2010;122(2):409–18. doi: 10.1007/s10549-010-0901-4. [DOI] [PubMed] [Google Scholar]
- 31.Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(20):3256–63. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann M, Maass N, Costa SD, Schneeweiss A, Loibl S, Sutterlin MW, et al. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur J Cancer. 2010;46(18):3184–91. doi: 10.1016/j.ejca.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Clemons M, Joy AA, Abdulnabi R, Kotliar M, Lynch J, Jordaan JP, et al. Phase II, double-blind, randomized trial of capecitabine plus enzastaurin versus capecitabine plus placebo in patients with metastatic or recurrent breast cancer after prior anthracycline and taxane therapy. Breast cancer research and treatment. 2010;124(1):177–86. doi: 10.1007/s10549-010-1152-0. [DOI] [PubMed] [Google Scholar]
- 34.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(10):1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 35.Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(34):4498–504. doi: 10.1200/JCO.2010.33.9101. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Jiang Z, Wang T, Zhang S, Bian L, Cao Y, et al. Single-agent capecitabine maintenance therapy after response to capecitabine-based combination chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2012;23(7):718–23. doi: 10.1097/CAD.0b013e328351802e. [DOI] [PubMed] [Google Scholar]
- 37.Crown JP, Dieras V, Staroslawska E, Yardley DA, Bachelot T, Davidson N, et al. Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(23):2870–8. doi: 10.1200/JCO.2012.43.3391. [DOI] [PubMed] [Google Scholar]
- 38.Arowolo OA, Njiaju UO, Ogundiran TO, Abidoye O, Lawal OO, Obajimi M, et al. Neo-adjuvant capecitabine chemotherapy in women with newly diagnosed locally advanced breast cancer in a resource-poor setting (Nigeria): efficacy and safety in a phase II feasibility study. Breast J. 2013;19(5):470–7. doi: 10.1111/tbj.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolaney SM, Jeong J, Guo H, Brock J, Morganstern D, Come SE, et al. A phase II study of preoperative capecitabine in women with operable hormone receptor positive breast cancer. Cancer Med. 2014;3(2):293–9. doi: 10.1002/cam4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clinical breast cancer. 2014;14(3):169–76. doi: 10.1016/j.clbc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Smorenburg CH, de Groot SM, van Leeuwen-Stok AE, Hamaker ME, Wymenga AN, de Graaf H, et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(3):599–605. doi: 10.1093/annonc/mdt588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(6):594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin M, Martinez N, Ramos M, Calvo L, Lluch A, Zamora P, et al. Standard versus continuous administration of capecitabine in metastatic breast cancer (GEICAM/2009-05): a randomized, noninferiority phase II trial with a pharmacogenetic analysis. The oncologist. 2015;20(2):111–2. doi: 10.1634/theoncologist.2014-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53(11):1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy BT, Gauthier AM, Michaud LB, Hortobagyi G, Valero V. Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at M. D. Anderson Cancer Center and a review of capecitabine toxicity in the literature. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16(8):1289–96. doi: 10.1093/annonc/mdi253. [DOI] [PubMed] [Google Scholar]
- 46.Jäger E, Al-Batran S, Saupe S, Schmidt R, Kreienberg LM, Otremba BJ, et al. A randomized phase III study evaluating pegylated liposomal doxorubicin (PLD) versus capecitabine (CAP) as first-line therapy for metastatic breast cancer (MBC): Results of the PELICAN study. J Clin Oncol. 2010;28(15 suppl):1022. [Google Scholar]
- 47.Ambros T, Zeichner SB, Zaravinos J, Montero AJ, Ahn E, Aruna M, et al. A retrospective study evaluating a fixed low dose capecitabine monotherapy in women with HER-2 negative metastatic breast cancer. Breast cancer research and treatment. 2014;146(1):7–14. doi: 10.1007/s10549-014-3003-x. [DOI] [PubMed] [Google Scholar]
- 48.Bertelsen C, Ji L, Garcia A, Russell C, Spicer D, Sposto R, et al. Efficacy of Very-Low-Dose Capecitabine in Metastatic Breast Cancer. American Journal of Hematology / Oncology. 2014;11(2):20–30. [Google Scholar]
- 49.Harvey VJ, Sharples KJ, Isaacs RJ, Jameson MB, Jeffery GM, McLaren BR, et al. A randomized phase II study comparing capecitabine alone with capecitabine and oral cyclophosphamide in patients with advanced breast cancer-cyclox II. Ann Oncol. 2013;24(7):1828–34. doi: 10.1093/annonc/mdt065. [DOI] [PubMed] [Google Scholar]
- 50.Fedele P, Marino A, Orlando L, Schiavone P, Nacci A, Sponziello F, et al. Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer. Eur J Cancer. 2012;48(1):24–9. doi: 10.1016/j.ejca.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 51.Saeki T, Kimura T, Toi M, Taguchi T. A pilot phase II study of capecitabine in advanced or recurrent breast cancer. Breast Cancer. 2006;13(1):49–57. doi: 10.2325/jbcs.13.49. [DOI] [PubMed] [Google Scholar]
- 52.Kusama M, Nomizu T, Aogi K, Yoshimoto M, Horikoshi N, Tabei T, et al. Phase II study of 4-weekly capecitabine monotherapy in advanced/metastatic breast cancer. Breast Cancer. 2010;17(4):233–40. doi: 10.1007/s12282-009-0137-5. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi T, Nakayama T, Masuda N, Yoshidome K, Akagi K, Nishida Y, et al. Study of low-dose capecitabine monotherapy for metastatic breast cancer. Chemotherapy. 2010;56(2):166–70. doi: 10.1159/000313531. [DOI] [PubMed] [Google Scholar]
- 54.Traina TA, Theodoulou M, Feigin K, Patil S, Tan KL, Edwards C, et al. Phase I study of a novel capecitabine schedule based on the Norton-Simon mathematical model in patients with metastatic breast cancer. J Clin Oncol. 2008;26(11):1797–802. doi: 10.1200/JCO.2007.13.8388. [DOI] [PubMed] [Google Scholar]
- 55.Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(24):3699–704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 56.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(25):3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]


