Abstract
Background and study aims: Endoscopic resection is one treatment option for residual or locally recurrent esophageal cancer after definitive chemoradiotherapy or radiotherapy alone. However, little is known about the clinical benefit of salvage endoscopic resection for these lesions. Therefore, the effectiveness and prognostic factors of salvage endoscopic resection were investigated.
Patients and methods: A total of 37 patients with esophageal squamous cell carcinoma (SCC) who underwent salvage endoscopic resection after definitive chemoradiotherapy or radiotherapy alone were reviewed. The method of salvage endoscopic resection was endoscopic mucosal resection using a cap (EMR-C), strip biopsy, or endoscopic submucosal dissection. The effectiveness and prognostic factors of salvage endoscopic resection were retrospectively analyzed.
Results: A total of 37 patients with 49 lesions underwent salvage endoscopic resection. Baseline clinical stages were I in 23 patients, II in 3 patients, III in 9 patients, and IV in 2 patients. The number of locoregional recurrences and residual lesions were 35 and 14, respectively. The curative en bloc resection rate was 53.1 % (26/49). The total incidence of complications was 18.9 % (7/37); all were successfully managed conservatively. The 3-year and 5-year overall survival rates were 72.9 % and 53.3 %, respectively, with a median follow-up period of 54 months. Baseline clinical T1 – 2 and N0 were significant factors for good prognosis in terms of overall survival on univariate analysis.
Conclusions: Salvage endoscopic resection, especially EMR-C, is a safe and feasible procedure to control residual or recurrent superficial esophageal SCC after definitive chemoradiotherapy or radiotherapy alone. The present results showed that baseline clinical T1 – 2 and N0 before chemoradiotherapy or radiotherapy were significant prognostic factors.
Introduction
Recently, definitive chemoradiotherapy has become one of the treatment options for esophageal squamous cell carcinoma (SCC) 1 2 3. Definitive radiotherapy alone has also been one of the treatment options for mucosal esophageal SCC 4 5. However, local recurrence after chemoradiotherapy or radiotherapy remains a major problem. Some reports have shown the effectiveness of salvage esophagectomy after definitive chemoradiotherapy as additional treatment 6 7 8 9 10 11 12. However, salvage esophagectomy is reported to have higher mortality and complication rates than radical esophagectomy with or without neoadjuvant therapy 9 10 13.
Endoscopic treatment is a minimally invasive procedure. Recently, endoscopic resection has been considered to be one of the curative options for residual or recurrent esophageal SCC after definitive chemoradiotherapy and radiotherapy when it is localized to the superficial layer. However, little is known about the usefulness, indications, and prognostic factors of endoscopic resection for residual or recurrent tumor after chemoradiotherapy or radiotherapy 14 15 16 17 18. The aim of this study was to investigate the effectiveness and prognosis of salvage endoscopic resection with a larger number of patients than previous reports.
Patients and methods
Patients
A database of all patients with esophageal SCC at the Aichi Cancer Center Hospital, Aichi, Japan from January 2000 to May 2010 was retrospectively analyzed. A total of 544 patients with esophageal SCC received definitive chemoradiotherapy or radiotherapy.
The definitive chemoradiotherapy or radiotherapy consisted of at least 50-Gy irradiation, regardless of concurrent chemotherapy. Most chemotherapeutic regimens comprised two cycles of continuous infusion of 5-fluorouracil and cisplatin or nedaplatin with concurrent radiation (data not shown). A patient with renal dysfunction was treated with low-dose docetaxel. Locoregional recurrence was defined as a cancer relapse at the primary site, and metachronous SCC was defined as a cancer relapse at a site away from the primary site more than 6 months after the initial treatment.
Staging and follow-up
Pretreatment staging of the esophageal cancers was determined using the tumor-node-metastasis (TNM) classification of the International Union Against Cancer, 7th edition (2009). Staging involved endoscopy with iodine staining, esophagography, and contrast-enhanced neck-to-abdomen computed tomography (CT). Lymph node metastasis was defined as more than 10 mm in diameter on CT. Complete response was defined as no tumor at follow-up endoscopy with biopsy and neck-to-abdomen CT 3 to 6 weeks after completion of initial treatment. After the confirmation of complete response, follow-up endoscopy with iodine staining was scheduled every 3 months for the first year, every 4 months for the next year, and every 6 months thereafter. Neck-to-abdomen CT was performed to detect lymph node or distant metastases every 3 months for the first year, every 6 months for the next 2 years, and annually thereafter.
Complete follow-up information until death or September 2014 was available for all patients.
The effectiveness of salvage endoscopic resection was retrospectively analyzed. Written, informed consent was obtained from all patients. This study was approved by the Institutional Review Board at Aichi Cancer Center Hospital (2014-1-095) and was carried out in accordance with the Declaration of Helsinki.
Endoscopic resection
Salvage endoscopic resection was defined as endoscopic resection for a recurrent or residual lesion at the primary site after definitive chemoradiotherapy or radiotherapy. It was based on the methods of endoscopic mucosal resection using a cap (EMR-C), strip biopsy, or endoscopic submucosal dissection (ESD). The indication for salvage endoscopic resection was histologically proven SCC by biopsy, endoscopically diagnosed depth of epithelium to two-thirds layer of the submucosa, and the lesion involving less than two-thirds of the esophageal circumference. Lesions that showed the shape of a submucosal tumor or were ulcerative were excluded. They were diagnosed as invading the muscularis propria or more. White light imaging and iodine staining were performed for diagnosis of locoregional and residual lesions. Endoscopic ultrasound (EUS) had not been performed as a staging modality for these lesions because of diagnostic difficulty. There were no distant and/or lymph node metastases on CT. All endoscopic resection treatments were performed with the patients under intravenous sedation with midazolam (Astellas Pharma Co., Tokyo, Japan) and pethidine (Mitsubishi Tanabe Pharma Co., Osaka, Japan).
Endoscopic mucosal resection (EMR)
EMR was performed by the EMR-C method and the strip biopsy method 19. For EMR-C, a forward-viewing endoscope (GIF-Q240 or GIF-Q260J; Olympus Medical Systems, Tokyo, Japan) with a plastic cap (MH-594 or MH-595; Olympus) on its tip was introduced. Saline solution was injected into the submucosa beneath the lesion with an injection needle. A crescent-moon-shaped snare (SD-221L-25; Olympus) was opened within the plastic cap, and the lesion was aspirated into the cap. The snare was then closed, and a forced coagulation current was applied to resect the lesion (Fig. 1). For the strip biopsy method, a double-channel endoscope (GIF-2T240; Olympus) was required. After saline solution injection into the submucosa, a snare and grasping forceps were each inserted through a channel. The forceps were then passed through the opened snare, and the snare was closed lightly around them. An area near the lesion was grasped with the forceps to evaluate the lesion, the snare was opened, the lesion was strangulated, and the tumor was then resected by applying an electrosurgical current. After resection, iodine staining was performed to check for a residual lesion. If a residual lesion was found, additional piecemeal resection or argon plasma coagulation (APC) (ICC-200; Erbe Elektromedizin Ltd, Tübingen, Germany) was performed.
Fig. 1.
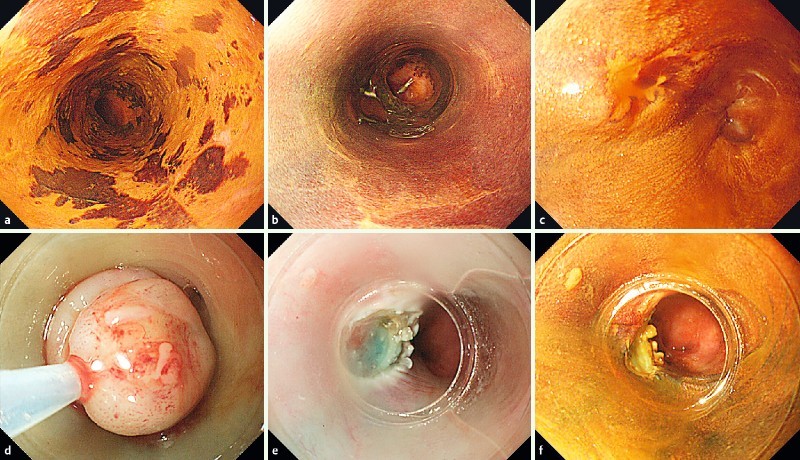
Salvage endoscopic mucosal resection using a cap (EMR-C) for locoregional recurrence after chemoradiotherapy. a Endoscopy after iodine staining shows a circumferential, slightly depressed lesion in the lower thoracic esophagus. b Complete response is achieved after chemoradiotherapy. c Twenty-four months after chemoradiotherapy, locoregional recurrence occurs on the same site. d The lesion aspirated into the cap is strangulated by a snare. e The lesion is resected. f Iodaine staining is performed to check for a residual lesion.
Endoscopic submucosal dissection (ESD)
ESD was started from August 2008 for residual or recurrent esophageal SCC after chemoradiotherapy 20. The indication for ESD was a lesion more than 10 mm in diameter on endoscopy. ESD was performed using a Flush-Knife® (DK2618JN20, DK2618JN10; Fujifilm Co., Ltd., Tokyo, Japan) or a ball tip bipolar needle-knife (B-Knife®) (BSJB15B, Zeon Medical Co., Ltd., Tokyo, Japan). The choice of the specific modality was decided by the endoscopist who performed the procedure. A forward-viewing endoscope (GIF-Q260J; Olympus) with a transparent attachment (D-201-11804; Olympus) on its tip was introduced.
Histologic evaluation and assessment of therapeutic efficacy
Resected specimens cut into 2-mm-thick slices were examined histologically according to the guidelines for the diagnosis and treatment of esophageal cancer by the Japan Esophageal Society 21. R0 resection was defined as en bloc resection with tumor-free horizontal margins. R1 resection was defined as a positive resection margin for tumor cells. Curative resection was defined as a tumor that was resected en bloc with a tumor-invasion depth of epithelium to submucosa, with no lymphovascular involvement and tumor-free margins.
Complications
Bleeding related to the procedure was defined as bleeding that required postoperative endoscopic hemostasis, such as endoscopic clipping or thermocoagulation. Perforation was diagnosed as mediastinal connective tissue that was observed during the procedure. A postoperative stricture was defined as a stricture that required endoscopic balloon dilation because of symptoms.
Statistical analysis
Overall survival was calculated from the date of first salvage endoscopic resection to the occurrence of death or to the date of last follow-up. Overall survival included deaths from any cause. For statistical analysis, Mann–Whitney’s U test was used. Actual survival was calculated by the Kaplan–Meier method. A Cox proportional hazards regression model was used for univariate analysis of the simultaneous effects of prognostic factors. Therefore, the measure of association in this study was the hazard ratio along with the 95 % confidence interval (95 %CI). In all analyses, a P value < 0.05 was accepted as significant. All statistical analyses were performed using the SPSS statistical software package 11.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Patients
Of the 544 patients, 294 achieved a complete response, and 82 of the 294 patients thereafter developed locoregional or metachronous SCC without lymph node or distant metastatic recurrence until March 2011 (Fig. 2). Of the 82 patients with local recurrence, 52 had locoregional recurrence, and 30 had metachronous SCC. Patients with endoscopically diagnosed superficial type, for which endoscopic resection could be indicated, included 37 with locoregional recurrence. Of these 37 patients, 27 underwent salvage endoscopic resection. On the other hand, 250 of 544 patients did not achieve a complete response, of whom 11 patients remained with a localized residual primary lesion in the shape of the superficial type without metastatic findings (Fig. 2); 10 of them underwent salvage endoscopic resection. Three patients with local superficial recurrence and one patient with superficial residual tumor underwent APC because of their underlying disease (severe cardiac disease and severe cirrhosis among others). Thirty-seven patients (36 males, one female) underwent salvage endoscopic resection because of locoregional recurrence or residual disease (Fig. 2). Pretreatment patient characteristics are shown in Table 1. Their median age was 66 years (range 50 – 84 years). The middle thoracic area was the most frequent primary site. The number of patients with clinical stages I, II, III, and IV was 23, 3, 9, and 2, respectively. The details of chemoradiotherapy and radiotherapy are shown in Table 2. Twenty-eight patients were post-chemoradiotherapy, while nine were post-radiotherapy alone. All patients who underwent radiotherapy alone were clinical T1. Complete response was achieved in 71 % (20/28) in the chemoradiotherapy group and 77 % (7/9) in the radiotherapy group. The median time from the first day of radiation therapy to confirming local relapse by endoscopy for these 27 patients who achieved a complete response was 11.3 months (range 4.4 – 74.9 months).
Fig. 2.

Representative flow chart of the patient sample assessed in this study. CRT, chemoradiotherapy; ER, endoscopic resection; RT, radiotherapy; APC, argon plasma coagulation; CTx, chemotherapy; BSC, best supportive care.
Table 1. Patients’ baseline characteristics before chemoradiotherapy or radiotherapy (n = 37).
| Median age (range), years | 66 (50 – 84) |
| Sex | |
| Male | 36 |
| Female | 1 |
| HNC | |
| Negative | 25 |
| Positive | 12 |
| Location | |
| Cervical | 1 |
| Upper thoracic | 3 |
| Middle thoracic | 17 |
| Lower thoracic | 12 |
| Upper-middle thoracic | 1 |
| Middle-lower thoracic | 1 |
| Cervical-lower thoracic | 1 |
| Upper thoracic + lower thoracic | 1 |
| Primary tumor | |
| cT1 | 28 |
| cT2 | 1 |
| cT3 | 3 |
| cT4 | 5 |
| Regional lymph nodes | |
| cN0 | 24 |
| cN1 | 9 |
| cN2 | 4 |
| cN3 | 0 |
| Distant metastasis | |
| cM0 | 35 |
| cM1 | 2 |
| Clinical stage | |
| cStage I | 23 |
| cStage II | 3 |
| cStage III | 9 |
| cStage IV | 2 |
HNC, previous or recently discovered concurrent head and neck cancers.
Table 2. Initial treatment before salvage endoscopic resection (n = 37).
| Chemoradiotherapy (n = 28) | Radiotherapy (n = 9) | |
| Radiotherapy (Gy) | ||
| Median | 60 | 60 |
| Range | 50.4 – 64 | 60 – 66 |
| Chemotherapy | ||
| 5FU + cisplatin | 20 | |
| 5FU + nedaplatin | 5 | |
| Cisplatin | 1 | |
| 5FU | 1 | |
| Docetaxel | 1 | |
| Complete response | 20 | 7 |
| Partial response | 8 | 2 |
5FU, 5-fluorouracil.
Lesions
The lesions’ characteristics for salvage endoscopic resection are summarized in Table 3. Six patients were found to have two lesions, and two patients were found to have four lesions concurrently. Thus, 37 patients with 49 lesions underwent salvage endoscopic resection. The lower thoracic area was the most frequent region. The majority of lesions were 0-IIc type and mucosal depth on endoscopy, with 35 locoregional recurrences and 14 residual lesions.
Table 3. Tumor characteristics of salvage endoscopic resection (49 lesions in 37 patients).
| Tumor status | |
| Residual | 14 |
| Recurrent | 35 |
| Tumor location | |
| Cervical | 2 |
| Upper thoracic | 4 |
| Middle thoracic | 20 |
| Lower thoracic | 23 |
| Macroscopic type | |
| 0-IIc | 45 |
| 0-Is | 4 |
| Depth with endoscopic findings | |
| Mucosal | 39 |
| Submucosal | 10 |
0-IIc, slightly depressed type; 0-Is, sessile (broad-based) type.
Results of endoscopic resection
The endoscopic resection results are shown in Table 4. Among the 49 lesions, the EMR-C method was performed for 44 cases, the strip biopsy method was performed for two, and the remaining three cases underwent ESD. The selection depended on the skill of the investigator and the period. Before February 2008, all salvage endoscopic resection procedures were performed by the EMR method. After that, the ESD method was used for large lesions. Forty lesions (81.6 %) were histologically confirmed to be mucosal lesions, seven lesions (14.3 %) showed submucosal invasion, and two lesions were unknown due to the burning effect. Curative resection was obtained for 26 lesions (53.1 %). The curative resection rate with ESD was 100 % (3/3).
Table 4. Clinical results of salvage endoscopic resection (49 lesions in 37 patients).
| Method of endoscopic resection | |
| EMR-C | 44 |
| Strip biopsy | 2 |
| ESD | 3 |
| Resection type | |
| En bloc resection | 40 |
| Piecemeal resection | 9 |
| Adverse events | |
| Postoperative bleeding | 1 |
| Perforation | 1 |
| Pneumonia | 1 |
| Stricture | 4 |
| Histological evaluation | |
| R0 resection | 29 |
| R1 resection | 11 |
| Unknown (piecemeal, burned) | 9 |
| Curative resection rate | |
| Curative resection | 26 |
| Non-curative resection | 23 |
| Median tumor size (range), mm | 11 (3 – 35) |
| Depth of histological invasion | |
| EP-LPM | 37 |
| MM | 3 |
| SM1 | 2 |
| SM2 or more | 5 |
| Unknown (burned) | 2 |
EMR-C, endoscopic mucosal resection using a cap; ESD, endoscopic submucosal dissection; R0, tumor-free margins with en bloc resection; R1, tumor-positive margins with en bloc resection; EP, epithelium; LPM, lamina propria mucosae; MM, muscularis mucosae; SM, submucosal layer.
Complications of endoscopic resection
The overall complication rate was 18.9 % (7/37). Four patients developed postoperative strictures, all of whom were successfully treated by endoscopic balloon dilation. One case of postoperative bleeding occurred and was successfully treated by endoscopic coagulation therapy. One perforation with mediastinal emphysema occurred. The patient recovered well, with no oral ingestion for 5 days and antibiotic administration for 7 days. One case of aspiration pneumonia occurred and was successfully treated by intravenous administration of antibiotics. After EMR-C/strip biopsy/ESD, postoperative bleeding occurred in 1/0/0, perforation occurred in 1/0/0, aspiration pneumonia occurred in 0/0/1, and postoperative stricture occurred in 3/0/1, respectively. There were no treatment-related deaths with salvage endoscopic resection.
Follow-up data and survival
The median follow-up period of all 37 patients was 54 months (range, 3.2 – 116.1 months). All patients were followed-up for at least 3 years or until death.
The clinical course of all lesions after salvage endoscopic resection is summarized in Fig. 3. During the follow-up period, 15 patients had no recurrence. However, eight of them died of other diseases; five of the eight died of head and neck cancer. Local recurrence was observed in 14 patients after salvage endoscopic resection, of whom 11 patients were successfully treated by additional endoscopic resection. Two patients underwent esophagectomy as an additional treatment. Lymph node and/or distant metastatic recurrences were found in eight patients after salvage endoscopic resection. The prognosis after metastatic recurrence was dismal. Most patients died within 1 year. During the follow-up period, 16 patients were alive, all of whom were disease-free, and 21 patients died; 11 patients died from progression of esophageal cancer, and the others died from head and neck cancer, liver cirrhosis, and other causes.
Fig. 3.
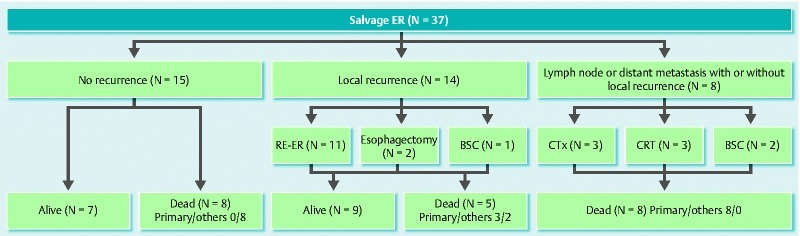
Clinical course of patients after salvage endoscopic resection (n = 37). Primary, died of primary esophageal cancer; others, died of other disease. CRT, chemoradiotherapy; ER, endoscopic resection; CTx, chemotherapy; BSC, best supportive care.
The 3-year and 5-year overall survival rates were 72.9 % and 53.3 %, respectively (Fig. 4).
Fig. 4.
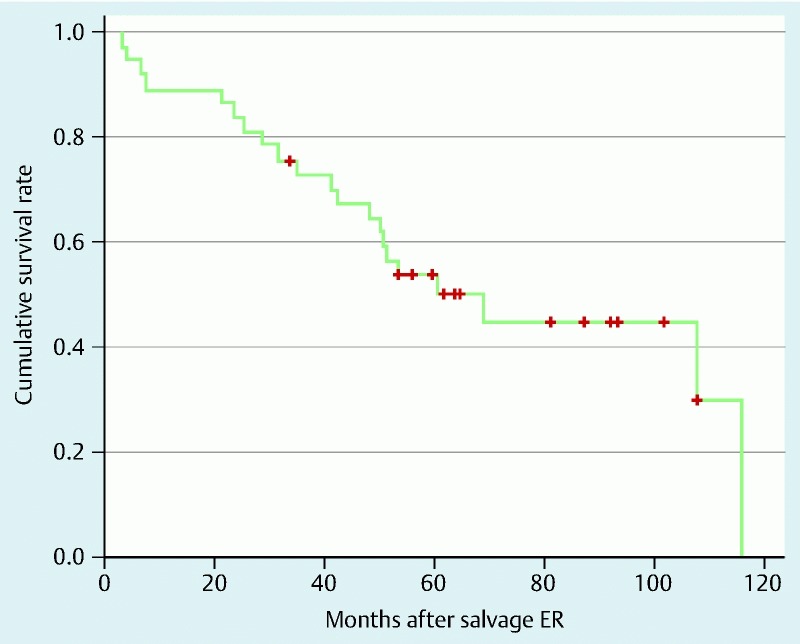
Overall survival curve for the 37 patients who underwent salvage endoscopic resection.
Univariate analysis was performed to identify independent predictors of overall survival for all patients. On both analyses, baseline clinical stage T1 – 2 and N0 were significant factors for a good prognosis compared with T3 – 4 and N1 – 3 (Table 5). Recurrent or residual tumor, tumor location, and curative resection or not with salvage endoscopic resection were also not significant in terms of overall survival.
Table 5. Univariate analysis of long-term survival after salvage endoscopic resection (n = 37).
| Characteristics | Patients (n) | HR | 95 %CI | P value |
| Age, years | 0.766 | 0.272 – 2.155 | 0.614 | |
| < 70 | 28 | |||
| ≥ 70 | 9 | |||
| Sex | 21.651 | 0.001 – 508836.525 | 0.549 | |
| Male | 36 | |||
| Female | 1 | |||
| PS | 0.441 | 0.169 – 1.154 | 0.095 | |
| 0 | 26 | |||
| 1/2 | 11 | |||
| HNC | 1.341 | 0.509 – 3.535 | 0.553 | |
| Negative | 25 | |||
| Positive | 12 | |||
| Baseline cT stage | 0.182 | 0.072 – 0.458 | < 0.001 | |
| cT1/2 | 29 | |||
| cT3/4 | 8 | |||
| Baseline cN stage | 0.151 | 0.057 – 0.396 | < 0.001 | |
| cN0 | 24 | |||
| cN1 – 3 | 13 | |||
| Baseline cM stage | 0.688 | 0.091 – 5.203 | 0.717 | |
| cM0 | 35 | |||
| cM1 | 2 | |||
| Recurrent or residual | 0.928 | 0.336 – 2.562 | 0.885 | |
| Recurrent | 27 | |||
| Residual | 10 | |||
| Resection pattern | 1.025 | 0.423 – 2.484 | 0.956 | |
| Curative | 20 | |||
| Non-curative | 17 |
HR, hazard ratio; CI, confidence interval; PS, performance status; HNC, previous or recently discovered concurrent head and neck cancers.
Discussion
In the present study, two important things about endoscopic resection were demonstrated in esophageal SCC after definitive chemoradiotherapy or radiotherapy alone. First, salvage endoscopic resection, especially EMR-C, was a safe and minimally invasive procedure to control locally recurrent or residual lesions and to preserve the esophagus itself. Second, baseline clinical stages T1 – 2 and N0 were significantly associated with a good prognosis.
First, salvage endoscopic resection, especially EMR-C, was safe and helpful to control local recurrent or residual lesions. The overall complication rate was 18.9 % (7/37), which not low, but four of the seven cases were stenoses. The occurrence of complications might be influenced not only by endoscopic resection, but also by initial radiotherapy or chemoradiotherapy. The adverse event rate of salvage EMR was almost equivalent to that of ordinary EMR (mainly using EMR-C) without chemoradiotherapy or radiotherapy 22. Postoperative stricture, postoperative bleeding, and perforation occurred in 8.8 % (3/34), 2.9 % (1/34), and 2.9 % (1/34) of cases in this salvage EMR group excluding three ESD patients and 7.8 % (14/179), 1.7 % (3/179), and 1.1 % (2/179) of cases in the ordinary EMR group, respectively. The safety, overall survival, and cause-specific survival of this study could be regarded as acceptable.
Second, the present study also showed that baseline clinical stage T1 – 2 and N0 were significantly associated with a good prognosis on univariate analysis. During the follow-up period, 16 patients were alive (Fig. 3). The survival rates for T1 – 2 and T3 – 4 were 55.2 % (16/29) and 0 % (0/8), respectively. The survival rates for N0 and N1 – 3 were 100 % (16/16) and 0 % (0/21), respectively. From this result, initial T1 – 2 and N0 appear to be a good indication for salvage endoscopic resection. There has been a report that the prognosis of baseline clinical stage T1 – 2 was better than that of T3 – 4 before salvage photodynamic therapy 23. However, no report has analyzed the prognostic factors of salvage endoscopic resection. To the best of our knowledge, this is the first study to report them. On the other hand, salvage endoscopic resection for baseline clinical T3 – 4 patients was completed mainly using EMR-C. The curative resection rate for these lesions was 37.5 % (3/8). Aspirating the lesion into the cap might be effective for these fibrotic lesions. All T3 – 4 patients died after salvage endoscopic resection during the follow-up period. However, the median survival rate was 24.7 months (range, 3.2 – 69.3 months). Furthermore, 37.5 % (3/8) of T3 – 4 patients survived more than 3 years after salvage endoscopic resection. These results show that salvage endoscopic resection might not be appropriate for T3 – 4 patients, although it seems meaningful for these patients.
The present results indicated that whether curative resection was achieved with salvage endoscopic resection was not related to overall survival. After salvage endoscopic resection, there were 14 cases of local re-recurrence, and 11 of these 14 patients underwent additional salvage endoscopic resection; of the three other patients, two of them underwent esophagectomy when diagnosed as clinical T2, and the other one was given best supportive care because of underlying renal disease. Six of 11 lesions were locoregional recurrent or residual lesions after salvage endoscopic resection. Pathological findings at re-salvage endoscopic resection indicated that one was a curative resection, but five were not. Although it may be difficult to completely resect locally recurrent or residual lesions by endoscopic resection, performing additional salvage endoscopic resection might improve these patients’ overall survival. Furthermore, most of these patients’ conditions were not perfect because of disease progression and adverse events of chemoradiotherapy. For this reason, salvage endoscopic resection and additional salvage endoscopic resection seem to make a great deal of sense to decrease tumor volume, maintain good condition, preserve the esophagus, and improve the outcome. We might consider it as palliative treatment.
The present study showed that salvage endoscopic resection was a safe procedure compared with some reports of salvage surgery 6 7 8 9 10 11 12. However, we should pay attention to the indications for salvage endoscopic resection. It is important to know that salvage endoscopic resection is not indicated for all locoregional or residual lesions. It is known that not all recurrent and residual lesions originate from the mucosa. In some cases, cancer cells are left in the deep layer of the esophageal wall after radiotherapy. Especially when the baseline clinical stage is T2 or more, recurrent or residual lesions might exist mainly in the submucosal or deeper layer. It has been reported that these lesions showed the shape of a submucosal tumor in many cases 24. Furthermore, most esophageal intramural metastases are also submucosal in shape. Therefore, in cases of lesions like a submucosal tumor, we have to consider photodynamic therapy or surgery as a salvage treatment procedure. Salvage photodynamic therapy has the possibility to cure deeper lesions than salvage endoscopic resection, and it is less invasive than salvage surgery 23 25 26. However, it has the drawbacks of high rates of severe stenosis, perforation, and phototoxicity, and pathological evaluation is not possible. Salvage surgery can also cure deeper lesions than salvage endoscopic resection and with pathological evaluation, especially in cases of R0 resection (no residual tumor) 6 7 8 9 10 11 12. However, it has the drawbacks of high adverse events, mortality, and morbidity rates. We believe that choosing the appropriate salvage treatment leads to a good prognosis for these patients.
In surveillance of patients after chemoradiotherapy, we also have to pay attention to changes of the esophagus. The layer structures of the esophageal wall are destroyed after chemoradiotherapy. For this reason, endoscopic ultrasound may not be useful to evaluate tumor depth 27 28 29. There may also be difficulty with narrow-band imaging in evaluating lesions for radiation-induced mucosal damage. We think that it is necessary to evaluate them comprehensively with both endoscopic and CT findings. Close surveillance is also important to detect residual or recurrent lesions in the early stage.
The present study has several limitations. First, this study was retrospective, from a single institution, and the sample size was small. Additionally, there were some biases. One possible bias was the unequal population at the baseline clinical stage. Another possible bias was diagnosis of regional lymph node metastases. Previous reports noted that EUS is more sensitive for the detection of regional lymph node metastases of esophageal cancer than CT 30. By the addition of EUS, the diagnostic power for staging before chemoradiotherapy or radiotherapy will increase. It can also be useful for restaging to exclude synchronous lymph node metastases when we consider salvage endoscopic resection for residual or recurrent lesions. Recently, the usefulness of positron emission tomography combined with CT (PET/CT) for evaluating lymph node metastases of esophageal cancer has been reported. These reports showed that PET/CT has a significantly higher positive predictive value than CT alone 31 32. Karashima et al. noted that specificity is also higher than CT alone 31. These results indicate that both EUS and PET/CT should be performed to evaluate lymph node metastases for staging before chemoradiotherapy or radiotherapy and for determining whether salvage endoscopic resection is indicated. Another possible bias was that the salvage endoscopic resection method was affected by the timing of treatment. For the endoscopic treatment of esophageal cancers in our hospital, EMR-C was mainly performed in an earlier period, whereas ESD was started for large lesions from 2008. Yamashita et al. reported that, for lesions 11 mm in diameter or larger, ESD was superior to EMR-C in efficacy, as assessed by achieving en bloc resection with tumor-free margins 33. The European Society of Gastrointestinal Endoscopy Guideline also recommends EMR for lesions smaller than 10 mm 34. The present results also showed that all three cases of salvage ESD were en bloc resections. All of them achieved curative resection histologically, which was superior to EMR (50 %, 23/46). Three reports showed the outcomes of salvage ESD 16 17 18. The en bloc resection rate and the curative resection rate were 91.6 – 100 % and 25 – 68.4 %, respectively. All reports showed that perforation and treatment-related death did not occur. These results indicate that ESD will be the main procedure for salvage endoscopic resection for EMR in the future. Therefore, when the procedure was performed should have a minimal impact on the results. In addition, data on effectiveness, safety, and long-term outcome for salvage ESD are still lacking in this study. However, it is difficult to plan a study of additional treatments for this patient group. This population is expected to be small. For this reason, the present outcome is clinically quite important to improve the prognosis of these patients.
In conclusion, the results of this study show that salvage endoscopic resection, especially EMR-C, is a safe and effective treatment to control recurrent or residual superficial esophageal SCC after definitive chemoradiotherapy or radiotherapy alone. Salvage endoscopic resection may also provide a survival benefit for certain patients, especially those with baseline clinical stage T1 – 2 and N0.
Footnotes
Competing interests: None
References
- 1.Kato K, Muro K, Minashi K. et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Sato A, Fukuda H. et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708) Jpn J Clin Oncol. 2009;39:638–643. doi: 10.1093/jjco/hyp069. [DOI] [PubMed] [Google Scholar]
- 3.Ishida K, Ando N, Yamamoto S. et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516) Jpn J Clin Oncol. 2004;34:615–619. doi: 10.1093/jjco/hyh107. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa H, Sakurai H, Tamaki Y. et al. Radiation therapy alone for stage I (UICC T1N0M0) squamous cell carcinoma of the esophagus: indications for surgery or combined chemoradiotherapy. J Gastroenterol Hepatol. 2006;21:1290–1296. doi: 10.1111/j.1440-1746.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- 5.Sai H, Mitsumori M, Araki N. et al. Long-term results of definitive radiotherapy for stage I esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;62:1339–1344. doi: 10.1016/j.ijrobp.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 6.Swisher S G, Wynn P, Putnam J B. et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123:175–183. doi: 10.1067/mtc.2002.119070. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Hayashi K, Ota M. et al. Salvage esophagectomy after definitive chemotherapy and radiotherapy for advanced esophageal cancer. Am J Surg. 2004;188:261–266. doi: 10.1016/j.amjsurg.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Tachimori Y. Role of salvage esophagectomy after definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg. 2009;57:71–78. doi: 10.1007/s11748-008-0337-5. [DOI] [PubMed] [Google Scholar]
- 9.Chao Y K, Chan S C, Chang H K. et al. Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol. 2009;35:289–294. doi: 10.1016/j.ejso.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Miyata H, Yamasaki M, Takiguchi S. et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 2009;100:442–446. doi: 10.1002/jso.21353. [DOI] [PubMed] [Google Scholar]
- 11.Gardner-Thorpe J, Hardwick R H, Dwerryhouse S J. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg. 2007;94:1059–1066. doi: 10.1002/bjs.5865. [DOI] [PubMed] [Google Scholar]
- 12.Tomimaru Y, Yano M, Takachi K. et al. Factors affecting the prognosis of patients with esophageal cancer undergoing salvage surgery after definitive chemoradiotherapy. J Surg Oncol. 2006;93:422–428. doi: 10.1002/jso.20475. [DOI] [PubMed] [Google Scholar]
- 13.Saeki H, Morita M, Tsuda Y. et al. Multimodal treatment strategy for clinical T3 thoracic esophageal cancer. Ann Surg Oncol. 2013;20:4267–4273. doi: 10.1245/s10434-013-3192-2. [DOI] [PubMed] [Google Scholar]
- 14.Hattori S, Muto M, Ohtsu A. et al. EMR as salvage treatment for patients with locoregional failure of definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc. 2003;58:65–70. doi: 10.1067/mge.2003.306. [DOI] [PubMed] [Google Scholar]
- 15.Yano T, Muto M, Hattori S. et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008;40:717–721. doi: 10.1055/s-2008-1077480. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Takisawa H, Suzuki H. et al. Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2008;67:355–359. doi: 10.1016/j.gie.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi M, Kobayashi M, Hashimoto S. et al. Salvage endoscopic submucosal dissection in patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Scand J Gastroenterol. 2013;48:1095–1101. doi: 10.3109/00365521.2013.822092. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi S, Jin M, Matsuhashi T. et al. Salvage endoscopic submucosal dissection for the esophagus-localized recurrence of esophageal squamous cell cancer after definitive chemoradiotherapy. Gastrointest Endosc. 2014;79:348–353. doi: 10.1016/j.gie.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Inoue H, Endo M, Takeshita K. et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC) Surg Endosc. 1992;6:264–265. doi: 10.1007/BF02498820. [DOI] [PubMed] [Google Scholar]
- 20.Fujishiro M, Yahagi N, Kakushima N. et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Kuwano H, Nishimura Y, Oyama T. et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai H, Niwa Y, Tajika M. et al. Is endoscopic mucosal resection acceptable for Stage 0 or IA esophageal squamous cell carcinoma? Nagoya Med J. 2012;52:185–197. [Google Scholar]
- 23.Yano T, Muto M, Minashi K. et al. Long-term results of salvage photodynamic therapy for patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2011;43:657–663. doi: 10.1055/s-0030-1256373. [DOI] [PubMed] [Google Scholar]
- 24.Tu C H, Muto M, Horimatsu T. et al. Submucosal tumor appearance is a useful endoscopic predictor of early primary-site recurrence after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Dis Esophagus. 2011;24:274–278. doi: 10.1111/j.1442-2050.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 25.Yano T, Muto M, Minashi K. et al. Photodynamic therapy as salvage treatment for local failures after definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc. 2005;62:31–36. doi: 10.1016/s0016-5107(05)00545-6. [DOI] [PubMed] [Google Scholar]
- 26.Wolfsen H C, Hemminger L L. Salvage photodynamic therapy for persistent esophageal cancer after chemoradiation therapy. Photodiagnosis Photodyn Ther. 2006;3:11–14. doi: 10.1016/S1572-1000(06)00002-0. [DOI] [PubMed] [Google Scholar]
- 27.Griffin J M Reed C E Denlinger C E Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer Ann Thorac Surg 2012931855–1859.; discussion 1860 [DOI] [PubMed] [Google Scholar]
- 28.Kalha I, Kaw M, Fukami N. et al. The accuracy of endoscopic ultrasound for restaging esophageal carcinoma after chemoradiation therapy. Cancer. 2004;101:940–947. doi: 10.1002/cncr.20429. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Watson D I, Lally C. et al. Endoscopic ultrasound for preoperative staging of esophageal carcinoma. Surg Endosc. 2005;19:1618–1621. doi: 10.1007/s00464-005-0250-2. [DOI] [PubMed] [Google Scholar]
- 30.van Vliet E P, Heijenbrok-Kal M H, Hunink M G. et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karashima R, Watanabe M, Imamura Y. et al. Advantages of FDG-PET/CT over CT alone in the preoperative assessment of lymph node metastasis in patients with esophageal cancer. Surg Today. 2015;45:471–477. doi: 10.1007/s00595-014-0965-6. [DOI] [PubMed] [Google Scholar]
- 32.Okada M, Murakami T, Kumano S. et al. Integrated FDG-PET/CT compared with intravenous contrast-enhanced CT for evaluation of metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. Ann Nucl Med. 2009;23:73–80. doi: 10.1007/s12149-008-0209-1. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Zeniya A, Ishii H. et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc. 2011;25:2541–2546. doi: 10.1007/s00464-011-1584-6. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T. et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]


