Abstract
The central nervous system (CNS) is a finely tuned organ that participates in nearly every aspect of our day-to-day function. Neurons lie at the core of this functional unit and maintain an active dialogue with one another as well as their fellow CNS residents (e.g. astrocytes, oligodendrocytes, microglia). Because of this complex dialogue, it is essential that the CNS milieu be tightly regulated in order to permit uninterrupted and efficient neural chemistry. This is accomplished in part by anatomical barriers that segregate vascular components from the cerebral spinal fluid (CSF) and brain parenchyma. These barriers impede entry of noxious materials and enable the CNS to maintain requisite protein and ionic balances for constant electrochemical signaling. Under homeostatic conditions, the CNS is protected by the presence of specialized endothelium/epithelium, the blood brain barrier (BBB), and the blood-CSF barrier. However, following CNS infection these protective barriers can be comprised, sometimes resulting in severe neurological complications triggered by an imbalance or blockage of neural chemistry. In some instances, these disruptions are severe enough to be fatal. This review focuses on a selection of microbes (both viruses and parasites) that compromise vascular barriers and induce neurological complications upon gaining access to the CNS. Emphasis is placed on CNS diseases that result from a pathogenic interplay between host immune defenses and the invading microbe.
Keywords: LCMV, brain, vasculature, blood vessel, blood brain barrier, malaria, Theiler’s virus, Ebola virus, Lassa fever virus, Dengue virus, hemorrhagic fevers, ventricles, cerebral spinal fluid, neutrophils, monocytes, T cells
Introduction
The central nervous system (CNS) is extraordinarily well vascularized, which enables a symphony of neural chemistry to perform continually without interruption. The complexity of the CNS necessitates this degree of vascularization, and as would be expected following a span of coevolution, an element of neurovascular coupling has become inherent in the CNS. It is now accepted that neural activity in distinct anatomical brain regions can influence blood flow to that region (Filosa and Blanco 2007; Iadecola and Nedergaard 2007). CNS cellular residents are all within close proximity of blood vessels, which enables them to communicate with vascular components and dictate their supply needs. A careful balance of nutrient input and efflux is essential to the seamless maintenance of complex chemistry. Despite their juxtaposition to CNS vasculature, neural cells are not simply fed a direct stream of materials emanating from the blood. In contrast to peripheral tissues, the CNS has added layers that protect its underlying cellular constituents from unwanted harm and imbalances. For example, most peripheral blood vessels are fenestrated, meaning that the endothelial cells which comprise these vessels are not tightly sealed. CNS blood vessels are for the most part non-fenestrated and do not allow materials to pass unchecked between endothelial junctions. Non-fenestrated vessels alone might seem like enough to ensure the safety of CNS residents; however, evolution has advanced a step further. Blood vessels that traverse CNS parenchyma are further encased in a second layer of astrocytic foot processes. It is the combination of non-fenestrated endothelium and astrocytic foot processes that gives rise to what is commonly referred to as the blood brain barrier (BBB). The CNS is also bathed in its own fluid (known as cerebral spinal fluid (CSF)) and contains the machinery to produce it (choroid plexus) as well as defined spaces to control its flow and distribution (the ventricular system). It is essential for CNS function that the distinct composition of the CSF be maintained, which is why the CNS is also fitted with a blood-CSF barrier system.
Despite exposure to environmental challenges, the CNS barrier systems are remarkably efficient at maintaining steady state neural activity. However, given the degree of vascularization and amount of anatomical specialization devoted to keeping vascular materials spatially segregated, one might predict that the CNS would be prone to disruption or injury following events that compromise barrier systems or impede vascular flow. This is indeed the case, as there are many examples of human diseases linked to CNS vascular and barrier events. One rather extreme example is an acute cerebrovascular attack (or stroke) during which disrupted cerebral blood flow causes a loss in brain function (Donnan et al. 2008). Epileptic seizures can also stem from a breach in BBB integrity (Fabene et al. 2008). While there are many diseases linked to CNS vasculature, this review will focus only on those induced by microbial challenges. Numerous microbes can access the CNS, and some of these can reap havoc upon the BBB and blood-CSF barrier either directly or indirectly. Of particular interest are pathogens that trigger our very own immune system to damage barrier structures and induce neurological dysfunction. The CNS and immune systems are heavily intertwined, and a microbe-induced dialogue between the two can sometimes prove fatal. This review is not meant to be an all inclusive summation of every pathogen that induces vascular (or barrier) pathology within the CNS, but rather a critical look at a select group of pathogens and the mechanisms that underlie the CNS vascular pathology they induce. A side-by-side comparison of distinct microbial challenges that give rise to CNS vascular breaches should facilitate identification of pathological commonalities amenable to therapeutic intervention.
Barriers to the brain
Anatomy of the brain
Based on the anatomical location of the brain, several different types of barriers exist to limit the movement of vascular components into the CNS. The brain can simplistically be broken down into three different regions: (1) the meninges, (2) the parenchyma, and (3) the ventricular system. Each compartment possesses unique anatomical features and presents different challenges from the standpoint of thwarting vascular leakage. The outermost region of the CNS, the meninges, is a series of membranes (the dura mater, arachnoid and pia mater) that cover the brain and spinal cord. Between the arachnoid and pia mater membranes is the subarachnoid space. This region is bathed in CSF and contains blood vessels that traverse through the meninges. Due to the lack of glial cells within this region, a barrier between the blood and CSF is presumably maintained though the unique qualities of brain endothelial cells that comprise meningeal vasculature. Beneath the meninges lies the brain parenchyma, which contains neurons and glial cells. It is within this region that the traditional multilayered blood brain barrier exists (Bechmann et al. 2007). This barrier relies again on the unique features of brain endothelium as well as a secondary layer made up of astrocytic foot processes. Lastly, the CNS ventricular system is a network of spaces through which CSF flows. In fact, the ventricular system contains the heart of CSF production—a structure referred to as the choroid plexus. Unlike the rest of the CNS, vasculature within this region is fenestrated, similar to peripheral vessels, and the maintenance of a blood-CSF barrier is generated by epithelial cells of the choroid plexus rather than the vascular endothelium (Engelhardt and Sorokin 2009).
The blood brain barrier
For over a century, the concept of a physical barrier between the blood and the brain has existed. Early experiments in 1885 by Paul Ehrlich demonstrated that administration of an intravital dye resulted in the labeling of peripheral tissues with little staining of the brain (Bechmann et al. 2007; Engelhardt and Sorokin 2009). Lewandowski then showed that direct application of drugs into the CSF increased sensitivity to lethality at lower doses, suggesting the existence of a CNS barrier (Bechmann et al. 2007; Engelhardt and Sorokin 2009). Likewise, a study in 1913 by Goldman demonstrated that injection of intravital dyes directly into the CSF resulted in labeling of the CNS (Bechmann et al. 2007; Engelhardt and Sorokin 2009). It was later revealed through electron microscopy that there was a physical barrier located at the inter-endothelial junctions of the brain vasculature which prevented the diffusion of a horseradish peroxidase tracer from the blood into the neuropil (Reese and Karnovsky 1967), thus defining endothelium as an important gatekeeper to the CNS. Collectively, these seminal studies gave rise to the concept now known as the BBB.
The BBB is comprised of an intricate series of layers formed by endothelial cells, basement membranes and astrocytic endfeet. Specialized endothelial cells form the first barrier to the free diffusion of solutes, ions and macromolecules into the brain parenchyma. In contrast to peripheral vessels, brain endothelium lack intracellular fenestrations, have few micropinocytotic vesicles (Reese and Karnovsky 1967) and have an abundance of junctional proteins that essentially seal the inter-cellular endothelial gaps (Forster 2008; Stamatovic et al. 2008). The endothelial cells also provide an acellular layer of protection through the deposition of a basement membrane, which includes laminin α4 and α5 chains (Sixt et al. 2001; Wu et al. 2009). Laminin α5, in particular, can block lymphocyte extravasation, indicating a protective role in neuroinflammation (Wu et al. 2009). Smooth muscle cells and pericytes are embedded in the outer vascular basement membrane in arterioles and venules. In capillary regions, where smooth muscle cells are absent, pericytes can control constriction of the microvasculature resulting in altered blood flow (Yemisci et al. 2009). Another layer of protection is formed by the parenchymal basement membrane found at the astrocytic endfeet (Alcolado et al. 1988; Engelhardt and Sorokin 2009; Zhang et al. 1990). The composition of this matrix is unique from the vascular basement membrane and contains laminin α2 and laminin α1 (Engelhardt and Sorokin 2009; Sixt et al. 2001). As a result, infiltrates that extravasate through the endothelial basement membrane may become “trapped” in the perivascular space, unless enzymes, such as matrix metalloproteinase (MMP)-2 and MMP-9, are available to disrupt this alternative set of basement membrane molecules (Agrawal et al. 2006; Bechmann et al. 2007). Finally, the last major cellular component of the BBB is comprised of astrocytic endfeet that physically occlude the movement of cells. Astrocytic endfeet together with the parenchymal basement membrane is referred to as the glial limitans (Bechmann et al. 2007). The anatomical features of the BBB are slightly altered between the pre- and post capillary vessels versus the capillaries themselves.
Endothelial cell-cell contact
Maintenance of a normal neuronal function relies on the ability to maintain differential solute concentrations between the CSF and the blood plasma (Abbott et al. 2009). To generate a sufficient barrier to the free diffusion of ions, macromolecules and cells into the brain parenchyma, the intercellular spaces between CNS vascular endothelial cells must be tightly regulated. This is accomplished though the formation of junctional complexes, including tight junctions (TJ) and adheren junctions (AJ), that provide a physical barrier at the inter-endothelial junctions. Tight junctions impede paracellular movement of ions, solutes and macromolecules and have a high electrical resistance of ~1,800 Ωcm2 (Butt et al. 1990), whereas adheren junctions provide structural support to initiate and maintain intercellular contact (Abbott et al. 2009; Gonzalez-Mariscal et al. 2003; Stamatovic et al. 2008; Wolburg and Lippoldt 2002). In both TJ and AJ, transmembrane proteins allow for intercellular interactions and associate with cytoplasmic plaque proteins that link the transmembrane proteins with signaling cascades and the actin cytoskeleton (Forster 2008; Gonzalez-Mariscal et al. 2003; Stamatovic et al. 2008; Wolburg and Lippoldt 2002).
TJ contain three major types of transmembrane proteins: occludin, claudin and junction-associated adhesion molecules (JAMs). Occludin was the first identified transmembrane protein of the TJ complex (Ando-Akatsuka et al. 1996; Furuse et al. 1993). Structurally, it contains four transmembrane domains, two extracellular loops, and two cytoplasmic tails. The C-terminal intracellular portion of occludin interacts with numerous cytoplasmic proteins including zonula occludins (ZO-1,-2,-3), PKC, and F-actin (Andreeva et al. 2006; Furuse et al. 1994; Haskins et al. 1998; Itoh et al. 2001; Lai et al. 2005; Peng et al. 2003; Stamatovic et al. 2008). Although occludin is found in the TJ complex, genetic deletion revealed that it is not necessary for the formation of functional tight junctions (Saitou et al. 1998; Saitou et al. 2000). Like occludin, claudins are not necessary for tight junction formation; however, claudin proteins play a critical role in barrier function (Nitta et al. 2003; Stamatovic et al. 2008). Claudins are structurally similar to occludin and contain four transmembrane domains with cytoplasmic N and C termini (Gonzalez-Mariscal et al. 2003; Stamatovic et al. 2008). The C terminus associates with numerous cytoplasmic plaque proteins including MUPP1, ZO-1,-2,-3 and PATJ (Hamazaki et al. 2002; Itoh et al. 1999; Roh et al. 2002). In the brain endothelium, expression of several claudins, including claudins-5, 12 and 3, have been detected (Morita et al. 1999; Nitta et al. 2003; Wolburg and Lippoldt 2002; Wolburg et al. 2003). Importantly, deletion of claudin 5 resulted in a “loosening” of the BBB allowing for passage of small molecules (<800D), but not larger molecules, (Matter and Balda 2003; Nitta et al. 2003) despite the formation of tight junctions. Therefore, control of free diffusion and size selectivity may be dictated by the composition of claudins expressed in the endothelium. Finally, JAM proteins are immunoglobulin family proteins that have been shown to interact with occludin and cytoplasmic proteins, such as ZO-1, cingulin and Par3 (D’Atri and Citi 2002; Itoh et al. 2001; Liu et al. 2000; Stamatovic et al. 2008). Relatively little is known about the contribution of JAM proteins to the diffusion barrier. However, JAM proteins have been implicated in leukocyte transmigration through the intercellular endothelial space, making them critical players during states of neuroinflammation (Bradfield et al. 2007; Chavakis et al. 2004; Johnson-Leger et al. 2002; Ostermann et al. 2005; Ostermann et al. 2002). Together these three families of transmembrane proteins form the tight junctional complex which surrounds the apical side of the endothelium, limits endothelial permeability, and creates the first inter-endothelial cell barrier to leukocyte extravasation into the brain.
Adheren junctions initiate (Weis and Nelson 2006) and maintain endothelial cell-cell adhesion through the interactions of Ca2+-dependent cadherins. Endothelial cells express both vascular endothelial (VE)-cadherin and neuronal (N)-cadherin, although only VE-cadherin expression is restricted to the endothelium (Lampugnani et al. 1997). Deficiency in VE-cadherin results in endothelial detachment from neighboring cells and basement membrane components, ultimately resulting in embryonic lethality due to severe vascular defects (Carmeliet et al. 1999; Gory-Faure et al. 1999). Additionally, deletion of the VE-cadherin associated phosphatase, VE-protein tyrosine phosphatase (VE-PTP), results in altered vascular development (Baumer et al. 2006; Dominguez et al. 2007), indicating that alterations in the phosphorylation state of VE-cadherin can critically impact endothelial adhesion. Cytoplasmic plaque proteins associated with AJ include, α-catenin, β-catenin, plakoglobin (also known as γ-catenin) and p120 (Nyqvist et al. 2008; Stamatovic et al. 2008; Weis and Nelson 2006). Cadherins bind directly to β-catenin and plakoglobin, which subsequently bind to α-catenin (Aberle et al. 1994; Weis and Nelson 2006; Yamada et al. 2005). It is thought that increases in cadherin clustering results in elevated local concentrations of α-catenin, which then dimerize, bind to actin filaments and inhibit the Arp2/3 complex resulting in decreased actin branching and induction of filament bundling (Weis et al. 2004; Yamada et al. 2005). AJ interactions may therefore dictate endothelial cell-cell adhesion through the ability to modify cytoskeletal organization. Interestingly, it was also recently shown that VE-cadherin interactions can upregulate claudin-5 expression, indicating that AJ formation can have a direct impact on tight junction complexes as well (Taddei et al. 2008). Therefore, AJ can alter barrier function indirectly by regulation of TJ proteins and directly by affecting cell adhesion through cytoskeletal rearrangments.
Microbial challenges to CNS vascular integrity
Cerebral malaria
Malaria is one of the more serious infectious diseases, with estimates of over 300 million cases worldwide and over a million deaths each year. Malaria is caused by protozoan parasites, of the genus Plasmodium, that are transmitted from infected female Anopheles mosquitoes to human hosts. After inoculation, sporozoites rapidly traffic to the liver and reside within hepatocytes in a benign manner. Following a week of replication, merozoites are released into the bloodstream and infect erythrocytes (Tuteja 2007), at which point clinical manifestations begin to emerge. Microvascular sequestration of parasitized red blood cells (PRBCs) and ensuing immune responses contribute to the disease process. Of the four major species of Plasmodium (Plasmodium ovale, Plasmodium vivax, Plasmodium malariae and Plasmodium falciparum) that infect humans, P. falciparum is associated with the most severe disease and can result in cerebral malaria (Schofield and Grau 2005). Because P. falciparum does not infect murine hosts, different Plasmodium species, including Plasmodium chabaudi, Plasmodium yoelii, Plasmodium vinckei and Plasmodium berghei are used to study anti-malaria responses (Stevenson and Riley 2004). Plasmodium berghei ANKA, in particular, is used as the model species for fatal murine cerebral malaria. In both human and murine hosts, cerebral malaria can result in BBB breakdown, an event that can potentially alter neuronal function.
During cerebral malaria, the endothelial barrier is often compromised by interactions with PRBCs as well as innate and adaptive immune responses. The culmination of these events results in vascular breakdown and mortality. One of the early steps in cerebral malaria is the sequestration of PRBCs in brain microvasculature. P. falciparum parasitized RBCs express erythrocyte membrane protein 1 (EMP1) that can adhere to a variety of endothelial surface receptors, including intercellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1), CD31, CD36, endothelial cell selectin (E-selectin), hyaluronic acid, chondroiton sulfate and thrombospondin (Craig and Scherf 2001; Ho et al. 2000; Schofield and Grau 2005). In human endothelial lines, adherence of P. falciparum PRBCs results in Rho kinase signal transduction and apoptosis of endothelial cells (Pino et al. 2003a, b; Taoufiq et al. 2008). Moreover, ICAM-1 ligation of endothelium was shown to cause stress fiber formation through cytoskeletal rearrangements (Etienne-Manneville et al. 2000), providing another potential mechanism by which PRBC adherence alters vascular permeability. Emergence of merozoites from PRBCs and release of parasitic products, including glycosylphosphatidylinositol (GPI), induces expression of ICAM-1, VCAM-1 and E-selectin on vascular endothelium (Schofield et al. 1996) and increases macrophage proinflammatory cytokine production (Schofield and Hackett 1993) further priming the endothelium. GPI also results in expression of inducible nitric oxide synthase (iNOS) in endothelial cells and macrophages (Tachado et al. 1996), increasing nitric oxide production (NO). Cytotoxic peroxynitrite (ONOO−) (Pacher et al. 2007) can then be formed by NO interactions with super oxide anion O2− resulting in cell death. In fact, reduction of oxidative stress through blockade of O2− activity decreases endothelial apoptosis during PRBC cytoadherence (Pino et al. 2003a, b). Therefore, PRBC and its bioactive products amplify the response and utilize multiple pathways to begin the process of vascular breakdown during cerebral malaria.
Although PRBC sequestration is necessary, it is not known to be sufficient for cerebral malaria induction. Examination of both humans and mice revealed that platelets and leukocytes are also retained within the microvasculature of the brain (Biswas et al. 2007; Combes et al. 2006; Curfs et al. 1993; Faille et al. 2009; Grau et al. 2003; Hunt and Grau 2003; Ma et al. 1996; Patnaik et al. 1994; Schofield and Grau 2005). Platelet activation has a multitude of effects including IL-1-mediated endothelial ICAM-1 upregulation (Hawrylowicz et al. 1991), altered endothelial integrity (Combes et al. 2006; Wassmer et al. 2006a, b) and increased PRBC cytoadherence via platelet microparticles (Combes et al. 2006; Faille et al. 2009). Recently, platelets were also shown to release platelet factor 4 (PF4 or CXCL4) during cerebral malaria, resulting in T cell recruitment and Tumor necrosis factor alpha (TNF-α) release by macrophages (Srivastava et al. 2008), which are also sequestered in the brain microvasculature in close proximity to activated platelets (Hunt and Grau 2003). During cerebral malaria, both TNF-α and CCL2 (MCP-1) (Hanum et al. 2003) are expressed in the brain. These macrophage associated proteins have the capacity to increase endothelial permeability by altering junctional protein expression and could therefore contribute to BBB breakdown (Angelini et al. 2006; Brett et al. 1989; Dimitrijevic et al. 2006; Gertzberg et al. 2007; Goldblum et al. 1989; Martinez-Estrada et al. 2005; McKenzie and Ridley 2007; Ozaki et al. 1999; Stamatovic et al. 2006; Stamatovic et al. 2003; Stamatovic et al. 2009; Stamatovic et al. 2005; Stolpen et al. 1986).
In murine models of cerebral malaria, several other innate cells including neutrophils, NK, NKT and δγ T cells have been implicated in the disease process through their ability to produce interferon gamma (IFN-γ) or through the induction of a Th1 environment (Chen et al. 2000; Hansen et al. 2007; Hansen et al. 2005; Hensmann and Kwiatkowski 2001). NK cell IFN-γ production results in increased CXCR3 expression on T cells (Hansen et al. 2007) allowing for recruitment into the CNS towards endothelial expressed chemokines including CXCL9, CXCL10 (IP-10) and CXCL11. Resistance to cerebral malaria in animals deficient in CXCR3 or CXCL10 is associated with decreased T cell infiltrates in the brain (Campanella et al. 2008; Miu et al. 2008; Nie et al. 2009) and strain susceptibility correlated with CXCR3 expression (Van den Steen et al. 2008). Likewise, proinflammatory cytokines, including IFN-γ and LT-α, are involved in leukocyte recruitment and blockade or genetic deletions that inhibit these responses also impede the development of cerebral malaria (Amani et al. 2000; Belnoue et al. 2008; Engwerda et al. 2002; Grau et al. 1989; Lucas et al. 1997; Piguet et al. 2002).
Studies in animal models have revealed that induction of cerebral malaria is ultimately dependent on the presence of an adaptive immune response (Finley et al. 1982). Both CD4+ and CD8+ T cells are thought to be involved in the disease process (Schofield and Grau 2005); however, early but not late depletion of CD4+ T cells decreases disease incidence, suggesting that CD4+ responses may only be critical during the priming phase of the response (Belnoue et al. 2002). Sequestration of CNS CD8+ T cells results in induction of vascular permeability and cerebral malaria in a perforin dependent manner (Nitcheu et al. 2003; Potter et al. 1999), which appears to be intimately linked to disease. Although similar levels of IFN-γ RNA levels were found in perforin−/− CD8+ cells (Nitcheu et al. 2003), recent studies in another pathogen model revealed that perforin−/− CD8+ cells have decreased IFN-γ at the protein level (Storm et al. 2006). Since IFN-γ can alter endothelial permeability through decreased occludin expression (Oshima et al. 2001), reduced BBB breakdown observed in perforin-deficient mice might reflect a combined deficiency in IFN-γ production as well as perforin-mediated cytotoxicity. Nevertheless, it is clear that cerebral malaria is a serious CNS disorder resulting from the pathogenic convergence of high jacked red blood cells as well as a vigorous anti-parasite immune response mounted by the infected host.
Viral hemorrhagic fevers
Viral hemorrhagic fevers (VHF) cause global, multi-organ vascular alterations during the course of infection. A variety of viral families can induce hemorrhagic fevers including filoviridae (Ebola, Marburg), arenaviridae (Lassa), flaviviridae (Dengue) and bunyaviridae (Rift valley fever) (Aleksandrowicz et al. 2008; Chen and Cosgriff 2000). Filoviruses, the prototypic inducers of VHF, are enveloped negative single stranded RNA viruses that can reach lengths of 800–1,400 nm and include two genera: Marburgvirus and Ebolavirus. The latter can be further subdivided into Zaire, Sudan, Ivory Coast or Reston Ebolavirus species (Aleksandrowicz et al. 2008; Feldmann et al. 2003). Initial manifestations of filovirus infections include fever, nausea, abdominal pain, vomiting and headache with progression into the terminal stage 7–16 days post-infection. Symptoms at the terminal stage include coma, shock, hemorrhage and vascular permeability (Bwaka et al. 1999; Colebunders and Borchert 2000; Zampieri et al. 2007). Both Zaire and Sudan Ebolavirus species are highly pathogenic with mortality rates reaching 50–88% of infected individuals (Chen and Cosgriff 2000; Hensley and Geisbert 2005), and currently there is no available treatment for infection.
Ebolavirus encodes proteins involved in viral replication, target binding and blockade of immune responses. Specifically, genes encoding nucleoprotein, glycoprotein (GP), viral polymerase and virion protein 35 (VP35), VP40, VP30 and VP24 are found within the Ebola genome (Feldmann et al. 2003). Numerous surface proteins, including Tyro3 family members, integrins, C-type lectin members DC-SIGN and hMGL, folate receptors and TREM-1 have been implicated in enhancing filovirus cellular entry, some of which bind directly to the Ebolavirus GP (Feldmann et al. 1996; Mohamadzadeh et al. 2006; Shimojima et al. 2006; Simmons et al. 2003; Takada et al. 2004). These receptors aid in the targeting of DCs, macrophages, monocytes and neutrophils during filovirus infection (Bosio et al. 2003; Feldmann et al. 1996; Geisbert et al. 2003; Mohamadzadeh et al. 2007; Mohamadzadeh et al. 2006; Ryabchikova et al. 1999; Stroher et al. 2001), which results in both immunosuppression and pathogenesis. Early in the response, Ebolavirus VP24 and VP35 proteins block interferon α/β production in infected cells, effectively crippling the generation of an anti-viral cellular environment (Cardenas et al. 2006; Gupta et al. 2001; Jin et al. 2009; Prins et al. 2009; Reid et al. 2007). Additionally, dendritic cell function is critically impaired after filovirus infection, as evidenced by diminished production of IL-12, IL-1β, TNF-α, IFN-α and IFN-β, decreased surface expression of CD40, CD80, CD86 and MHC II and a reduced capacity to stimulate allogenic Tcells (Bosio et al. 2003; Jin et al. 2009; Mahanty et al. 2003; Mohamadzadeh 2009). Consequently, the inability to effectively prime the adaptive response in conjunction with intravascular apoptosis of B cells, CD4+ cells and CD8+ T cells (Baize et al. 1999; Bradfute et al. 2007; Geisbert et al. 2003; Gupta et al. 2007; Mohamadzadeh et al. 2007; Reed et al. 2004; Sanchez et al. 2004) results in uncontrolled viremia and death.
While filovirus infection impairs anti-viral responses, it also orchestrates the induction of a proinflammatory cytokine storm. Both Ebola and Marburg viral infection of macrophages or monocytes elicit the production of TNF-α, IL-6, IL-8 and gro-α (Stroher et al. 2001) within hours of infection. Additionally, fatal cases of human Ebolavirus infection have been associated with increased serum levels of IFN-γ and TNF-α. (Villinger et al. 1999). Recently, TLR4 ligation by the Ebolavirus GP has been implicated in the production of proinflammatory cytokines from monocytic cell lines, suggesting that TLR4 acts as a sensor for filovirus infection. In addition to proinflammatory cytokine production, infected macrophages show increased production of chemokines such as CCL5, CCL3 and CCL2 (Gupta et al. 2001), allowing for elevated trafficking of other myelomonocytic cells to sites of infection. TREM-1 triggering of neutrophils also contributes to the proinflammatory milieu through the production of cytokines IL-1, IL-6, TNF-α and chemokines CCL3 and CCL2 (Mohamadzadeh et al. 2006). Therefore, filoviral infection can cause increased vascular permeability indirectly through the induction of myelomonocytic cytokine and chemokine production. In accordance with this notion, supernatants from Marburg infected macrophage/monocytes resulted in enhanced vascular permeability (Bockeler et al. 2007; Feldmann et al. 1996) via TNF-α release. Exposure to IFN-γ, TNF-α or TNF-α plus H202, resulted in decreased VE-cadherin and plakoglobin organization, interendothelial gap formation, decreased transendothelial electrical resistance and increased water permeability (Bockeler et al. 2007; Feldmann et al. 1996). Therefore, cytokine release is a critical event that can influence endothelial barrier function.
Filoviruses also have the means to directly disrupt vascular permeability by infecting endothelial cells (Geisbert et al. 2003; Yang et al. 1998). Binding of Ebola virus glycoprotein to 293T and endothelial cell lines diminishes cellular adherence (Chan et al. 2000; Simmons et al. 2002) and in some cases has been proposed to cause endothelial damage (Yang et al. 1998). Ebola virus VP40 in conjunction with GP1 and GP2 also induces expression of ICAM-1 and VCAM-1, increases actin stress fiber formation and decreases endothelial barrier function (Wahl-Jensen et al. 2005). Adhesion molecule expression may further amplify the disease process by enhancing recruitment of infected myelomonocytic cells to the vasculature, allowing for coordination of both direct and indirect mechanisms of vascular permeability at sites of infection.
Theiler’s murine encephalomyelitis virus
Multiple sclerosis (MS) is a chronic demyelinating CNS disease that is associated with local inflammation. In order to gain insights on the contribution of the immune response during MS, murine models including experimental autoimmune encepholmyelitis and Theiler’s murine encephalomyelitis virus (TMEV) infection have been utilized to recapitulate important aspects of disease. TMEV is a single stranded, positive RNA virus of the picornaviridae family (Oleszak et al. 2004; Ozden et al. 1986; Pevear et al. 1987). Several strains of TMEV, including GDVII, FA, BeAn and DA, exist and induce either acute encephalitis (GDVII, FA) or a late chronic demyelinating disease (BeAn and DA) (Lipton 1980). After intracerebral inoculation of TMEV, the virus infects numerous cellular targets including macrophage/monocytes, astrocytes, microglia, endothelial cells and oligodendrocytes (Clatch et al. 1990; Qi and Dal Canto 1996; Zheng et al. 2001; Zurbriggen and Fujinami 1988). During both early (days 3–12) and late stages of disease (days 30–40) leukocytic infiltrates, including CD4+ and CD8+ T cells, macrophage/monocytes and B cells, are observed in the CNS (Oleszak et al. 2004). Clinical manifestations of disease (i.e. hind limb paralysis, ataxia, etc.) occur during the late phase as a result of extensive demyelination and axonal loss (Oleszak et al. 2004).
Recently, modulation of TMEV-specific anti-viral immunity through peptide vaccination was shown to result in a novel model of CNS vascular permeability (Johnson et al. 2005; Johnson et al. 2007). Since general TMEV pathogenesis has been reviewed thoroughly elsewhere (Brahic et al. 2005; Kim et al. 2005; Oleszak et al. 2004), we will instead focus on the peptide-induced model of TMEV fatality due to its hallmark feature of BBB breakdown. Infection of TMEV-resistant mice (e.g. C57BL/6) with the Daniel’s strain of the virus results in the generation and CNS recruitment of CD8+ T cells, the majority (~70%) of which are specific to a single viral epitope (H-2Db VP2121-130) (Johnson et al. 2005). Notably, intravenous administration of the VP2121-130 peptide on day 7 post-infection at the peak of the CTL response results in a fatal syndrome 24 hours later that is dependent on perforin expression and H-2Db VP2121-130 restricted CD8+ T cells (Johnson et al. 2005). This acute fatal disease process is accompanied by extensive CNS vascular leakage, microhemorrhages, demyelination, glial cell activation and paralysis (Johnson et al. 2005; Johnson et al. 2007; Pirko et al. 2008; Suidan et al. 2008). In the absence of perforin, VP2121-130 peptide administration failed to induce both glial activation and vascular leakage, indicating that perforin expression is critical for the disease process.
Vascular leakage during this fatal syndrome is also associated with transient alterations in the tight junction proteins, claudin-5 and occludin, with large decreases in the latter protein occurring at 4 and 12 h post-peptide administration (Suidan et al. 2008). Decreased TJ expression and increased vascular permeability precede caspase-3 activation observed in isolated blood vessels, suggesting that junctional rearrangement, rather than cell death, mediates BBB breakdown. Overall, these studies nicely demonstrate the dangers associated with over-stimulating a CTL response directed against a CNS virus, and further research in this model should enhance our mechanistic understanding of how CD8+ T cells can facilitate rapid BBB breakdown.
Lymphocytic choriomeningitis virus
Development of viral meningitis represents a unique challenge to CNS vasculature because inflammation occurs within the meningeal space where blood vessels lack the multi-layered protection of the BBB. Lymphocytic choriomeningitis virus (LCMV) is a negative strand RNA virus of the arenaviridae family that is a natural murine and human pathogen (Lledo et al. 2003). Depending on the strain, dose and route of infection, LCMV initiates diverse responses and outcomes including viral clearance, viral persistence, and immune-mediated fatality. Intracerebral LCMV infection results in the development of acute fatal meningitis 6–7 days post-infection that is associated with severe convulsive seizures—a hallmark feature of the disorder (Camenga et al. 1977; Kang and McGavern 2008; Walker et al. 1977). LCMV was shown to infect specialized epithelial cells (e.g. ependyma and choroid plexus) as well as meningeal cells (Fig. 1a) (Cole et al. 1971; Doherty and Zinkernagel 1974; Kim et al. 2009; Schwendemann et al. 1983), but very few cells within the brain parenchyma. Virus-specific CD8+ T cells are essential for disease induction and are massively recruited into the CNS on day 6 post-infection (Fung-Leung et al. 1991), at which point increased vascular CNS permeability and seizure induction become apparent (Camenga et al. 1977; Kim et al. 2009; Marker et al. 1984). During meningitis, primed virus-specific CTL express both LFA-1 and VLA-4 which enable them to interact with ICAM-1 and VCAM-1 expressed on activated CNS endothelium (Andersson et al. 1994; Andersson et al. 1995; Christensen et al. 1995; Kang and McGavern 2008; Marker et al. 1995; Nansen et al. 2000). Increased expression of chemokines such as CCL2, CCL3, CCL4, CCL5 and CXCL10 mRNA are coordinately upregulated in the brain on day 6 post-infection when significant leukocytic infiltration is observed (Asensio and Campbell 1997; Kim et al. 2009). At this time point, the CNS of LCMV-infected mice are massively infiltrated by monocytes, dendritic cells, and neutrophils in addition to CD8+ T cells. This immune infiltration, which is mobilized to ward off the invading virus, results in a fatal neurological disorder—the disease for which LCMV is named.
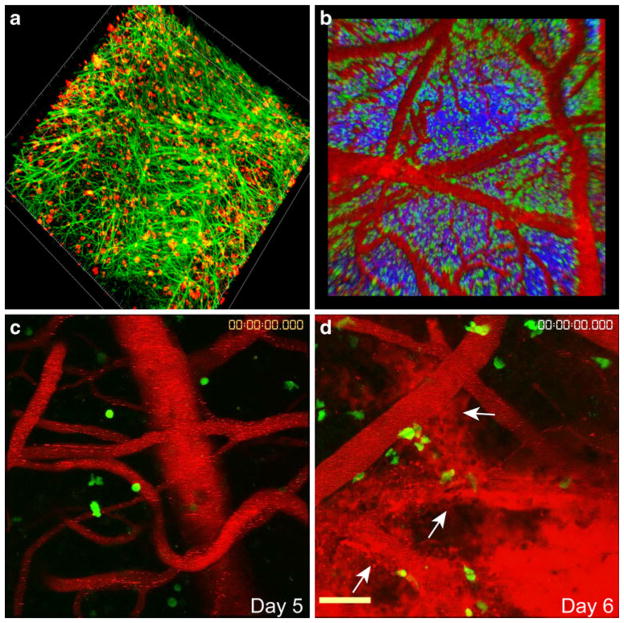
Fig. 1.
Distribution of virus and anti-viral CTL during LCMV-induced meningitis. a A representative 3D reconstruction from a two-photon z-stack depicts widespread LCMV infection (red) of the meningeal stromal network (green) at day 6 post-infection. b At this time point, virus-specific CTL (green) can be found throughout the meningeal space and are often aggregated along meningeal blood vessels (red). Blood vessels were visualized by injecting quantum dots (red) intravenously before performing two photon imaging experiments. Second harmonic signal corresponding to skull bone is shown in blue for anatomical purposes. c–d Snapshots of 3D time lapses illustrate the position of antiviral CTL (green) and the integrity of meningeal blood vessels (red) at day 5 (asymptomatic) (c) and day 6 (symptomatic) (d) post-LCMV infection. Note that the quantum dots are contained within meningeal blood vessels at day 5, but leak heavily into the subarachnoid space at day 6. White arrows denote injured blood vessels
Although CD8+ T cells are required for LCMV-induced meningitis, it was unclear how CTL effector mechanism(s) contributed to disease pathogenesis or whether secondarily recruited innate immune cells (e.g. monocytes and neutrophils) were responsible for any of the CNS injury observed in this model. We, therefore, initiated studies to elucidate the role of CTL during meningitis and to identify the mechanism(s) by which CD8+ T cells induced vascular breakdown in the meninges. Intracerebral infection of mice with single deficiencies in major CTL effector pathways (i.e. Fas, Fas ligand, perforin, IFN-γ receptor, TNF-α, and degranulation) revealed the curious observation that none of these pathways alone were responsible for fatal convulsive seizures and death (Kang and McGavern 2008; Kim et al. 2009; Nansen et al. 1998; Storm et al. 2006; Zajac et al. 2003), raising the possibility that this classic CTL-dependent disorder might rely in part on other immune cell subsets. To gain novel insights into the disease process, we illuminated the surface of LCMV-infected brains using intravital two-photon microscopy (TPM) (Figs. 1, 2). This exciting approach vastly improved our understanding of the disorder by allowing us to watch immune cell subsets in real time as they injured the virus-infected brain. We began our studies by monitoring the CNS-infiltrating virus-specific CTL known to be required for disease induction (Fig. 1b–d). This was accomplished by seeding adult mice with naïve green fluorescent protein (GFP)-tagged T cell receptor transgenic (TCR-tg) CD8+ T cells specific to the LCMV glycoprotein (DbGP33-41) (referred to as GFP+ P14 cells). Visualization of GFP+ P14 cells on the brain surface of symptomatic mice at day 6 post-infection revealed that these cells localized primarily to the meningeal space, which was in accordance with published confocal data (McGavern et al. 2002). TPM further revealed that GFP+ P14 cells were highly dynamic (moving at average speeds of 3.4 μm/min) and often associated with meningeal vasculature (Fig. 1b). The majority of virus-specific CTL exhibited a motile behavior, suggesting that few stable synapses were formed during the process of meningitis. Interestingly, we also observed that the activities of virus-specific CTL at the peak of disease did not appear to be directly linked to massive breaches in vascular integrity, which were visualized by monitoring quantum dot efflux from meningeal blood vessels into the subarachnoid space using TPM (Fig. 1c, d). Massive vascular leakage coincided temporally with the onset of convulsive seizures and could induce fatalities in two ways. First, breakdown of meningeal vasculature, which comprises a component of the blood-CSF barrier, causes fluid to build up within the subarachnoid space. This can give rise to a dangerous condition wherein fluid accumulates to an intolerable level within the confines of the skull resulting in herniation and death. Evidence for this mode of fatality is supported by a recent study that used MRI to demonstrate that edema was only detected in the brains of LCMV-infected mice at the terminal stage of disease (Matullo et al. 2009). This study further demonstrated that death was associated with unilateral papillary dilation (indicative of uncal herniation) and breakdown of the ventricular system. Simultaneous breakdown of meningeal blood vessels and ventricular epithelium is likely a fatal combination that stresses the ventricular system beyond its capacity.
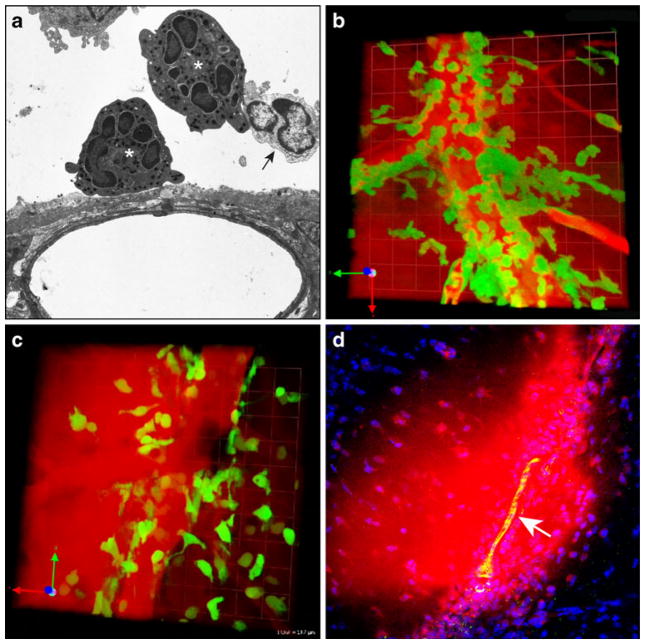
Fig. 2.
Influx of innate immune cells contributes to meningeal vascular injury. a An electron micrograph captured using brain tissue from a mouse at day 6 post-infection reveals the presence of neutrophils (white asterisks) and monocytes/macrophages (black arrow) near a meningeal blood vessel. b A snapshot from a 3D time lapse of a day 6 infected LysM-GFP mouse shows neutrophil (green) extravasation from a meningeal blood vessel and the associated leak of vascular material (red). Note that the quantum dots (red) are no longer found exclusively within the meningeal blood vessel. c In LysM-GFP mice depleted of neutrophils, the remaining cells (presumably monocytes/macrophages) (green) localized perivascularly and were also associated with quantum dot leakage (red) from meningeal blood vessels. d A representative 2D confocal image shows evidence of BBB breakdown. In day 6 mice Evans blue (red) was found within the brain parenchyma. A meningeal blood vessel is shown in green (white arrow). Note that Evans blue is not contained to the space adjacent to the meningeal blood vessel but has spread into the parenchyma. Cell nuclei are shown in blue for anatomical purposes
Another factor contributing to disease severity during LCMV-induced meningitis is disruption of the BBB itself. The anatomical positioning of immunopathology in LCMV infected brains is largely dictated by viral tropism. Following intracerebral inoculation, LCMV replicates primarily in meningeal stromal cells (Fig. 1a) (Kim et al. 2009) as well as specialized epithelial cells residing in the ventricular system and the choroid plexus. This explains the profound breakdown of the blood-CSF barriers. However, several groups have reported that the BBB is also disrupted during LCMV-induced meningitis (Kim et al. 2009; Marker et al. 1984; Matullo et al. 2009), and it is known that breakdown of the BBB alone can result in seizures (Marchi et al. 2007). Therefore, while BBB breakdown might not be the sole factor responsible for fatalities in this model (Matullo et al. 2009), this event likely contributes to neurological complications such as seizures and might in fact enhance the probability of a fatal outcome.
In light of our TPM data demonstrating infrequent stable CTL contact formation, it was not surprising that CTL effector pathways were not responsible for rapid onset fatal convulsive seizures in this model (Kim et al. 2009). We, therefore, investigated whether virus-specific CD8+ T cells could breach the meningeal blood-CSF barrier through an alternative mechanism. Previous studies demonstrated that CD4+ T cells (Dixon et al. 1987), B cells (Johnson et al. 1978) and NK cells (Allan and Doherty 1986) were not required for the disease process, and, consequently, were unlikely mediators of vascular injury. However, a role for CNS-infiltrating monocytes and neutrophils had not been evaluated. Kinetic analyses revealed a massive influx of myelomonocytic cells (i.e. monocytes and neutrophils) that coincided with the arrival of CTL on day 6 post-infection (Fig. 2a) (Kim et al. 2009). Additionally, it was demonstrated that CNS-infiltrating virus-specific CTL produced at least three chemokines (CCL3, 4, and 5) that can recruit myelomonocytic cells, which suggested that CTL directly participated in myelomonocytic cell influx into the CNS. This supposition was supported by data showing that late CD8+ T cell depletion reduced myelomonocytic cell recruitment into the CNS.
To determine the contribution of myelomonocytic cells to disease pathogenesis, we visualized these cells by TPM in infected lysozyme M-GFP (LysM-GFP) mice (Faust et al. 2000) (Fig. 2b, c). Interestingly, it was first noted in LysM-GFP mice that a population of myelomonocytic cells (later determined to be neutrophils) synchronously extravasated from meningeal blood vessels at the peak of LCMV meningitis (day 6), which resulted in severe and sustained vascular leakage (Fig. 2b) (Kim et al. 2009). Depletion of neutrophils in LysM-GFP mice further revealed that the remaining cells (presumably monocytes/macrophages) localized to perivascular regions and were associated with transient alterations in the vascular integrity (Fig. 2c). These data suggest that neutrophils and monocytes share the ability to mediate vascular injury and compromise the blood-CSF barrier during LCMV-induced meningitis. Neutrophils are known to induce edema following influx into peripheral tissues (DiStasi and Ley 2009); however, this edema is better tolerated there because the spaces are less confined and not as prone to compression injury. Rapid induction of edema in the brain can be fatal because the skull is an inflexible barrier. During LCMV meningitis, TPM has revealed an important contribution of myelomonocytic cells to the disruption of the blood-CSF barrier and the fatal accumulation of fluid (or edema) within the subarachnoid space. Importantly, this leakage is not just confined to the meninges. Confocal analyses of intravenously injected Evans blue revealed the presence of the dye several layers down into the brain parenchyma (Fig. 2d). This indicates disruption of the BBB and defines a novel mechanism by which meningeal inflammation can alter the activity (or integrity) of cells residing in the brain parenchyma (Ransohoff 2009).
In conclusion, LCMV meningitis remains as a classic CTL-dependent disorder. The data supporting a requirement for CTL in this model are irrefutable. However, our recent findings, revealed in part by illuminating the brain surface with TPM, offer an interesting twist on the mechanism underlying this classic disorder. During LCMV-induced meningitis, the contribution of CTL effector mechanisms to disease pathogenesis is likely outweighed by their ability to rapidly recruit innate immune cells, which then induce fatal vascular injury. Pathogenic links between T cells and innate immune cells undoubtedly exist in other inflammatory situations such as autoimmunity (Carlson et al. 2008), obesity (Nishimura et al. 2009), and peripheral infections (Muller et al. 2009). Presently, the exact mechanism by which myelomonocytic cells injure CNS bloods vessels during LCMV meningitis is unknown; however, there is a wealth of knowledge (see recent review (DiStasi and Ley 2009)) showing how neutrophils adhere to endothelial cells and induce vascular breakdown in other inflammatory contexts (Atherton and Born 1972; Florey and Grant 1961; Hammerschmidt et al. 1981; Hoover et al. 1978; Humphrey 1955; Issekutz 1981; Marchesi and Florey 1960). For example, neutrophil-endothelial cell interactions can alter adheren junctions and tight junctions through decreased association of VE-cadherin with β-catenin and plakoglobin as well as diminished expression occludin and ZO-1 (Bolton et al. 1998; Del Maschio et al. 1996). Neutrophil release of heparin binding protein (HBP/azurocidin) was also shown to induce endothelial signaling and subsequent actin cytoskeletal rearrangements that are thought to generate inter-endothelial gaps (Gautam et al. 2000; Gautam et al. 2001). Additionally, neutrophils can elicit endothelial cytotoxicity through the combined release of elastase and H2O2 (Lentsch and Ward 2000). Less is known about the potential mechanisms underlying monocyte/macrophage induced vascular leakage. It is conceivable that release of proinflammatory cytokines and chemokines (e.g. CCL2) by monocytes/macrophages alters endothelial barrier function (Angelini et al. 2006; Brett et al. 1989; Dimitrijevic et al. 2006; Gertzberg et al. 2007; Goldblum et al. 1989; Martinez-Estrada et al. 2005; McKenzie and Ridley 2007; Ozaki et al. 1999; Stamatovic et al. 2006; Stamatovic et al. 2003; Stamatovic et al. 2009; Stamatovic et al. 2005; Stolpen et al. 1986). Further research is required to determine whether established mechanisms or novel mediators are used by monocytes/macrophages to induce vascular breakdown. It will also be important to identify the relative contributions and precise mechanisms used by both neutrophils and monocytes/macrophages to injure CNS vasculature during LCMV meningitis.
Concluding Remarks
The CNS is a fragile compartment that is relatively intolerant of abrupt changes associated with infection and immune cell invasion. While some pathogens that access the CNS may go unnoticed, host immune surveillance is usually adept enough to quickly detect invading microbes despite anatomical isolation of the CNS behind the BBB and blood-CSF barriers. Immune cells have the capacity to purge a persistent viral infection from the CNS without causing significant tissue injury. A case in point is the immunotherapeutic clearance of a persistent LCMV infection. Adoptive transfer of memory T cells into mice persistently infected from birth with LCMV results in CNS viral clearance over a three month period with no overt signs of neurological dysfunction (Lauterbach et al. 2006; Oldstone et al. 1986). This outcome demonstrates that severe vascular injury, seizures, and death are not an inevitable consequence of T cell-mediated clearance of LCMV from the CNS. It is possible for the immune system to purge a pathogen from the CNS in a minimally injurious manner. Thus, it is important to delineate the factors that give rise to pathogenesis in the CNS following infection.
It clear, and perhaps not surprising, that the virulence of the invading infectious agent plays a big part in pathogenicity. This is best exemplified by the viruses that induce hemorrhagic fevers. Pathogens that are not well equilibrated with their host and replicate rapidly are likely to “shock” the immune system, disabling certain compartments while massively stimulating others. Viral hemorrhagic fevers are usually associated with immediate induction of cytokine release by innate immune cells. This can overwhelm the host giving rise to a variety of systemic complications including vascular leakage. Cerebral malaria is another good example of how the pathogen itself can contribute significantly to a negative outcome in the CNS. Microvascular sequestration of parasitized red blood cells directs the resultant immune response toward CNS blood vessels. Because both the parasitized red bloods cells and immune cells have the potential to mediate vascular injury, this is an equation for disaster. The same is true for LCMV-induced meningitis. Interestingly, LCMV alone does not induce any cytopathology in the CNS; however, when injected intracerebrally it replicates primarily in cells comprising the meninges and blood-CSF barrier. This pattern of replication directs a massive immune attack toward a system that serves has a protective barrier from the periphery. Breaches in this protective barrier can be tolerated in small doses without detriment to the CNS, but a large coordinated immune attack pushes the system beyond its capacity, resulting in severe neurological complications, and, in the case of LCMV meningitis, death. There are certainly commonalities in the pathways that result in CNS vasculature injury following infection, which include the release of proinflammatory cytokines, chemokines, and other soluble mediators. Leukocyte extravasation can also significantly alter inter-endothelial junctional integrity resulting in CNS vascular breakdown. Understanding precisely how CNS endothelial cells are compromised during microbial pathogenesis is essential for developing appropriate therapeutics to prevent extensive CNS vascular breakdown and subsequent neuronal dysfunction during disease.
Acknowledgments
This work was supported by National Institutes of Health intramural program. S.S.K. is supported by a National Research Service Award (NS061447-01). We would like to thank Drs. Jiyun Kim and Michael Dustin at New York University for providing the images shown in Figs. 1b–d and 2b–c as well as Dr. Bernd Zinselmeyer for the image shown in Fig. 1a.
Contributor Information
Silvia S. Kang, National Institute of Neurological Disorders and Stroke, The National Institutes of Health, 10 Center Drive, Bethesda, MD 20892, USA
Dorian B. McGavern, Email: mcgavernd@mail.nih.gov, National Institute of Neurological Disorders and Stroke, The National Institutes of Health, 10 Center Drive, Bethesda, MD 20892, USA
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2009;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolado R, Weller RO, Parrish EP, Garrod D. The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol. 1988;14:1–17. doi: 10.1111/j.1365-2990.1988.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Aleksandrowicz P, Wolf K, Falzarano D, Feldmann H, Seebach J, Schnittler H. Viral haemorrhagic fever and vascular alterations. Hamostaseologie. 2008;28:77–84. [PubMed] [Google Scholar]
- Allan JE, Doherty PC. Natural killer cells contribute to inflammation but do not appear to be essential for the induction of clinical lymphocytic choriomeningitis. Scand J Immunol. 1986;24:153–162. doi: 10.1111/j.1365-3083.1986.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Amani V, Vigario AM, Belnoue E, Marussig M, Fonseca L, Mazier D, Renia L. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur J Immunol. 2000;30:1646–1655. doi: 10.1002/1521-4141(200006)30:6<1646::AID-IMMU1646>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]
- Andersson EC, Christensen JP, Scheynius A, Marker O, Thomsen AR. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand J Immunol. 1995;42:110–118. doi: 10.1111/j.1365-3083.1995.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol. 1996;133:43–47. doi: 10.1083/jcb.133.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva AY, Piontek J, Blasig IE, Utepbergenov DI. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int J Biochem Cell Biol. 2006;38:222–233. doi: 10.1016/j.biocel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–L1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol. 1997;71:7832–7840. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton A, Born GV. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972;222:447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, Snounou G, Renia L. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–6375. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- Belnoue E, Potter SM, Rosa DS, Mauduit M, Gruner AC, Kayibanda M, Mitchell AJ, Hunt NH, Renia L. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 2008;30:544–553. doi: 10.1111/j.1365-3024.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- Biswas AK, Hafiz A, Banerjee B, Kim KS, Datta K, Chitnis CE. Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PLoS Pathog. 2007;3:1271–1280. doi: 10.1371/journal.ppat.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockeler M, Stroher U, Seebach J, Afanasieva T, Suttorp N, Feldmann H, Schnittler HJ. Breakdown of paraendothelial barrier function during Marburg virus infection is associated with early tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1. J Infect Dis. 2007;196(Suppl 2):S337–S346. doi: 10.1086/520606. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW. Lymphocyte death in a mouse model of Ebola virus infection. J Infect Dis. 2007;196(Suppl 2):S296–S304. doi: 10.1086/520602. [DOI] [PubMed] [Google Scholar]
- Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Annu Rev Microbiol. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989;169:1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, Katwiki KR, Kibadi K, Kipasa MA, Kuvula KJ, Mapanda BB, Massamba M, Mupapa KD, Muyembe-Tamfum JJ, Ndaberey E, Peters CJ, Rollin PE, Van den Enden E. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- Camenga DL, Walker DH, Murphy FA. Anticonvulsant prolongation of survival in adult murine lymphocytic choriomeningitis. I. Drug treatment and virologic studies. J Neuropathol Exp Neurol. 1977;36:9–20. doi: 10.1097/00005072-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci USA. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Chan SY, Ma MC, Goldsmith MA. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Gen Virol. 2000;81:2155–2159. doi: 10.1099/0022-1317-81-9-2155. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- Chen JP, Cosgriff TM. Hemorrhagic fever virus-induced changes in hemostasis and vascular biology. Blood Coagul Fibrinolysis. 2000;11:461–483. doi: 10.1097/00001721-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–133. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JP, Andersson EC, Scheynius A, Marker O, Thomsen AR. Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infection. J Immunol. 1995;154:5293–5301. [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Cole GA, Gilden DH, Monjan AA, Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971;30:1831–1841. [PubMed] [Google Scholar]
- Colebunders R, Borchert M. Ebola haemorrhagic fever–a review. J Infect. 2000;40:16–20. doi: 10.1053/jinf.1999.0603. [DOI] [PubMed] [Google Scholar]
- Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int J Parasitol. 2006;36:541–546. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Craig A, Scherf A. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol Biochem Parasitol. 2001;115:129–143. doi: 10.1016/s0166-6851(01)00275-4. [DOI] [PubMed] [Google Scholar]
- Curfs JH, Hermsen CC, Kremsner P, Neifer S, Meuwissen JH, Van Rooyen N, Eling WM. Tumour necrosis factor-alpha and macrophages in Plasmodium berghei-induced cerebral malaria. Parasitology. 1993;107(Pt 2):125–134. doi: 10.1017/s0031182000067226. [DOI] [PubMed] [Google Scholar]
- D’Atri F, Citi S. Molecular complexity of vertebrate tight junctions (Review) Mol Membr Biol. 2002;19:103–112. doi: 10.1080/09687680210129236. [DOI] [PubMed] [Google Scholar]
- Del Maschio A, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol. 1996;135:497–510. doi: 10.1083/jcb.135.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JE, Allan JE, Doherty PC. The acute inflammatory process in murine lymphocytic choriomeningitis is dependent on Lyt-2+ immune T cells. Cell Immunol. 1987;107:8–14. doi: 10.1016/0008-8749(87)90260-7. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Zinkernagel RM. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, Noguera-Troise I, Murphy AJ, Valenzuela DM, Davis S, Thurston G, Yancopoulos GD, Gale NW. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci USA. 2007;104:3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–1377. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faille D, Combes V, Mitchell AJ, Fontaine A, Juhan-Vague I, Alessi MC, Chimini G, Fusai T, Grau GE. Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. FASEB J. 2009;23:3449–3458. doi: 10.1096/fj.09-135822. [DOI] [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3:677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol. 2007;92:641–646. doi: 10.1113/expphysiol.2006.036368. [DOI] [PubMed] [Google Scholar]
- Finley RW, Mackey LJ, Lambert PH. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J Immunol. 1982;129:2213–2218. [PubMed] [Google Scholar]
- Florey HW, Grant LH. Leucocyte migration from small blood vessels stimulated with ultraviolet light: an electron-microscope study. J Pathol Bacteriol. 1961;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung WP, Kundig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta(2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med. 2000;191:1829–1839. doi: 10.1084/jem.191.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertzberg N, Gurnani T, Neumann P, Forbes AK, Jean-Louis N, Johnson A. Tumor necrosis factor-alpha causes barrier dysfunction mediated by tyrosine198 and tyrosine218 in beta-actin. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1219–L1229. doi: 10.1152/ajplung.00083.2007. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Hennig B, Jay M, Yoneda K, McClain CJ. Tumor necrosis factor alpha-induced pulmonary vascular endothelial injury. Infect Immun. 1989;57:1218–1226. doi: 10.1128/iai.57.4.1218-1226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Grau GE, Heremans H, Piguet PF, Pointaire P, Lambert PH, Billiau A, Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- Gupta M, Mahanty S, Ahmed R, Rollin PE. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology. 2001;284:20–25. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology. 2007;364:45–54. doi: 10.1016/j.virol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt DE, Harris PD, Wayland JH, Craddock PR, Jacob HS. Complement-induced granulocyte aggregation in vivo. Am J Pathol. 1981;102:146–150. [PMC free article] [PubMed] [Google Scholar]
- Hansen DS, Evans KJ, D’Ombrain MC, Bernard NJ, Sexton AC, Buckingham L, Scalzo AA, Schofield L. The natural killer complex regulates severe malarial pathogenesis and influences acquired immune responses to Plasmodium berghei ANKA. Infect Immun. 2005;73:2288–2297. doi: 10.1128/IAI.73.4.2288-2297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DS, Bernard NJ, Nie CQ, Schofield L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol. 2007;178:5779–5788. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
- Hanum PS, Hayano M, Kojima S. Cytokine and chemokine responses in a cerebral malaria-susceptible or -resistant strain of mice to Plasmodium berghei ANKA infection: early chemokine expression in the brain. Int Immunol. 2003;15:633–640. doi: 10.1093/intimm/dxg065. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 1991;174:785–790. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley LE, Geisbert TW. The contribution of the endothelium to the development of coagulation disorders that characterize Ebola hemorrhagic fever in primates. Thromb Haemost. 2005;94:254–261. doi: 10.1160/TH05-03-0153. [DOI] [PubMed] [Google Scholar]
- Hensmann M, Kwiatkowski D. Cellular basis of early cytokine response to Plasmodium falciparum. Infect Immun. 2001;69:2364–2371. doi: 10.1128/IAI.69.4.2364-2371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Hickey MJ, Murray AG, Andonegui G, Kubes P. Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J Exp Med. 2000;192:1205–1211. doi: 10.1084/jem.192.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover RL, Briggs RT, Karnovsky MJ. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978;14:423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Humphrey JH. The mechanism of Arthus reactions. I. The role of polymorphonuclear leucocytes and other factors in reversed passive Arthus reactions in rabbits. Br J Exp Pathol. 1955;36:268–282. [PMC free article] [PubMed] [Google Scholar]
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Issekutz AC. Vascular responses during acute neutrophilic inflammation. Their relationship to in vivo neutrophil emigration. Lab Invest. 1981;45:435–441. [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, Ganesh B, He B. The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and Lipopolysaccharide. J Gen Virol. 2009;91:352–361. doi: 10.1099/vir.0.017343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ED, Monjan AA, Morse HC., 3rd Lack of B-cell participation in acute lymphocyte choriomeningitis disease of the central nervous system. Cell Immunol. 1978;36:143–150. doi: 10.1016/0008-8749(78)90257-5. [DOI] [PubMed] [Google Scholar]
- Johnson-Leger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Mendez-Fernandez Y, Moyer AM, Sloma CR, Pirko I, Block MS, Rodriguez M, Pease LR. Antigen-specific CD8 + T cells mediate a peptide-induced fatal syndrome. J Immunol. 2005;174:6854–6862. doi: 10.4049/jimmunol.174.11.6854. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Suidan GL, McDole J, Pirko I. The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology? Int Rev Neurobiol. 2007;79:73–97. doi: 10.1016/S0074-7742(07)79004-9. [DOI] [PubMed] [Google Scholar]
- Kang SS, McGavern DB. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci. 2008;13:4529–4543. doi: 10.2741/3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Palma JP, Kwon D, Fuller AC. Innate immune response induced by Theiler’s murine encephalomyelitis virus infection. Immunol Res. 2005;31:1–12. doi: 10.1385/IR:31:1:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev. 2005;50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110(Pt 17):2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203:1963–1975. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Persistent Theiler’s murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980;46:169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- Lledo L, Gegundez MI, Saz JV, Bahamontes N, Beltran M. Lymphocytic choriomeningitis virus infection in a province of Spain: analysis of sera from the general population and wild rodents. J Med Virol. 2003;70:273–275. doi: 10.1002/jmv.10389. [DOI] [PubMed] [Google Scholar]
- Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso-Hinard C, De Kesel T, Buurman WA, Moore MW, Dayer JM, Fiers W, Bluethmann H, Grau GE. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- Ma N, Hunt NH, Madigan MC, Chan-Ling T. Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. Am J Pathol. 1996;149:1745–1762. [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- Marchesi VT, Florey HW. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker O, Nielsen MH, Diemer NH. The permeability of the blood-brain barrier in mice suffering from fatal lymphocytic choriomeningitis virus infection. Acta Neuropathol. 1984;63:229–239. doi: 10.1007/BF00685249. [DOI] [PubMed] [Google Scholar]
- Marker O, Scheynius A, Christensen JP, Thomsen AR. Virus-activated T cells regulate expression of adhesion molecules on endothelial cells in sites of infection. J Neuroimmunol. 1995;62:35–42. doi: 10.1016/0165-5728(95)00099-n. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Manzi L, Tonetti P, Dejana E, Bazzoni G. Opposite effects of tumor necrosis factor and soluble fibronectin on junctional adhesion molecule-A in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1081–L1088. doi: 10.1152/ajplung.00289.2004. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Holey barrier: claudins and the regulation of brain endothelial permeability. J Cell Biol. 2003;161:459–460. doi: 10.1083/jcb.200304039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matullo CM, O’Regan KJ, Hensley H, Curtis M, Rall GF. Lymphocytic Choriomeningitis Virus-induced mortality in mice is triggered by edema and brain herniation. J Virol. 2009;84:312–320. doi: 10.1128/JVI.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- Miu J, Mitchell AJ, Muller M, Carter SL, Manders PM, McQuillan JA, Saunders BM, Ball HJ, Lu B, Campbell IL, Hunt NH. Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol. 2008;180:1217–1230. doi: 10.4049/jimmunol.180.2.1217. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M. Potential factors induced by filoviruses that lead to immune supression. Curr Mol Med. 2009;9:174–185. doi: 10.2174/156652409787581628. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Coberley SS, Olinger GG, Kalina WV, Ruthel G, Fuller CL, Swenson DL, Pratt WD, Kuhns DB, Schmaljohn AL. Activation of triggering receptor expressed on myeloid cells-1 on human neutrophils by Marburg and Ebola viruses. J Virol. 2006;80:7235–7244. doi: 10.1128/JVI.00543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol. 2007;7:556–567. doi: 10.1038/nri2098. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Nansen A, Christensen JP, Ropke C, Marker O, Scheynius A, Thomsen AR. Role of interferon-gamma in the pathogenesis of LCMV-induced meningitis: unimpaired leucocyte recruitment, but deficient macrophage activation in interferon-gamma knock-out mice. J Neuroimmunol. 1998;86:202–212. doi: 10.1016/s0165-5728(98)00055-1. [DOI] [PubMed] [Google Scholar]
- Nansen A, Marker O, Bartholdy C, Thomsen AR. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur J Immunol. 2000;30:1797–1806. doi: 10.1002/1521-4141(200007)30:7<1797::AID-IMMU1797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, Heath WR, Engwerda CR, Hickey MJ, Schofield L, Hansen DS. IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog. 2009;5:e1000369. doi: 10.1371/journal.ppat.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, Mazier D, Combadiere B. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol. 2003;170:2221–2228. doi: 10.4049/jimmunol.170.4.2221. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyqvist D, Giampietro C, Dejana E. Deciphering the functional role of endothelial junctions by using in vivo models. EMBO Rep. 2008;9:742–747. doi: 10.1038/embor.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Blount P, Southern PJ, Lampert PW. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Fraemohs L, Baltus T, Schober A, Lietz M, Zernecke A, Liehn EA, Weber C. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–735. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- Ozden S, Tangy F, Chamorro M, Brahic M. Theiler’s virus genome is closely related to that of encephalomyocarditis virus, the prototype cardiovirus. J Virol. 1986;60:1163–1165. doi: 10.1128/jvi.60.3.1163-1165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik JK, Das BS, Mishra SK, Mohanty S, Satpathy SK, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- Peng BH, Lee JC, Campbell GA. In vitro protein complex formation with cytoskeleton-anchoring domain of occludin identified by limited proteolysis. J Biol Chem. 2003;278:49644–49651. doi: 10.1074/jbc.M302782200. [DOI] [PubMed] [Google Scholar]
- Pevear DC, Calenoff M, Rozhon E, Lipton HL. Analysis of the complete nucleotide sequence of the picornavirus Theiler’s murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987;61:1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Kan CD, Vesin C. Role of the tumor necrosis factor receptor 2 (TNFR2) in cerebral malaria in mice. Lab Invest. 2002;82:1155–1166. doi: 10.1097/01.lab.0000028822.94883.8a. [DOI] [PubMed] [Google Scholar]
- Pino P, Vouldoukis I, Dugas N, Hassani-Loppion G, Dugas B, Mazier D. Redox-dependent apoptosis in human endothelial cells after adhesion of Plasmodium falciparum-infected erythrocytes. Ann N Y Acad Sci. 2003a;1010:582–586. doi: 10.1196/annals.1299.109. [DOI] [PubMed] [Google Scholar]
- Pino P, Vouldoukis I, Kolb JP, Mahmoudi N, Desportes-Livage I, Bricaire F, Danis M, Dugas B, Mazier D. Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis. 2003b;187:1283–1290. doi: 10.1086/373992. [DOI] [PubMed] [Google Scholar]
- Pirko I, Suidan GL, Rodriguez M, Johnson AJ. Acute hemorrhagic demyelination in a murine model of multiple sclerosis. J Neuroinflammation. 2008;5:31. doi: 10.1186/1742-2094-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S, Chaudhri G, Hansen A, Hunt NH. Fas and perforin contribute to the pathogenesis of murine cerebral malaria. Redox Rep. 1999;4:333–335. doi: 10.1179/135100099101535070. [DOI] [PubMed] [Google Scholar]
- Prins KC, Cardenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Dal Canto MC. Effect of Theiler’s murine encephalomyelitis virus and cytokines on cultured oligodendrocytes and astrocytes. J Neurosci Res. 1996;45:364–374. doi: 10.1002/(SICI)1097-4547(19960815)45:4<364::AID-JNR5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Immunology: barrier to electrical storms. Nature. 2009;457:155–156. doi: 10.1038/457155a. [DOI] [PubMed] [Google Scholar]
- Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW. Depletion of peripheral blood T lymphocytes and NK cells during the course of Ebola hemorrhagic fever in cynomolgus macaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol. 2007;81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem. 2002;277:27501–27509. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- Ryabchikova EI, Kolesnikova LV, Luchko SV. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, Rollin PE. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- Schofield L, Novakovic S, Gerold P, Schwarz RT, McConville MJ, Tachado SD. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- Schwendemann G, Lohler J, Lehmann-Grube F. Evidence for cytotoxic T-lymphocyte-target cell interaction in brains of mice infected intracerebrally with lymphocytic choriomeningitis virus. Acta Neuropathol. 1983;61:183–195. doi: 10.1007/BF00691984. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J Virol. 2002;76:2518–2528. doi: 10.1128/jvi.76.5.2518-2528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, Doms RW, Bates P, Pohlmann S. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, Shirk EM, Sun H, Kowalska MA, Fox-Talbot K, Sullivan D, Zavala F, Morrell CN. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem. 2006;281:8379–8388. doi: 10.1074/jbc.M513122200. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284:19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol. 2004;4:169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- Stolpen AH, Guinan EC, Fiers W, Pober JS. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986;123:16–24. [PMC free article] [PubMed] [Google Scholar]
- Storm P, Bartholdy C, Sorensen MR, Christensen JP, Thomsen AR. Perforin-deficient CD8+ T cells mediate fatal lymphocytic choriomeningitis despite impaired cytokine production. J Virol. 2006;80:1222–1230. doi: 10.1128/JVI.80.3.1222-1230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J Virol. 2001;75:11025–11033. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, McDole JR, Chen Y, Pirko I, Johnson AJ. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PLoS ONE. 2008;3:e3037. doi: 10.1371/journal.pone.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachado SD, Gerold P, McConville MJ, Baldwin T, Quilici D, Schwarz RT, Schofield L. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol. 1996;156:1897–1907. [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Takada A, Fujioka K, Tsuiji M, Morikawa A, Higashi N, Ebihara H, Kobasa D, Feldmann H, Irimura T, Kawaoka Y. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J Virol. 2004;78:2943–2947. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufiq Z, Gay F, Balvanyos J, Ciceron L, Tefit M, Lechat P, Mazier D. Rho kinase inhibition in severe malaria: thwarting parasite-induced collateral damage to endothelia. J Infect Dis. 2008;197:1062–1073. doi: 10.1086/528988. [DOI] [PubMed] [Google Scholar]
- Tuteja R. Malaria - an overview. FEBS J. 2007;274:4670–4679. doi: 10.1111/j.1742-4658.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Deroost K, Van Aelst I, Geurts N, Martens E, Struyf S, Nie CQ, Hansen DS, Matthys P, Van Damme J, Opdenakker G. CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-gamma-induced chemokines. Eur J Immunol. 2008;38:1082–1095. doi: 10.1002/eji.200737906. [DOI] [PubMed] [Google Scholar]
- Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- Wahl-Jensen VM, Afanasieva TA, Seebach J, Stroher U, Feldmann H, Schnittler HJ. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J Virol. 2005;79:10442–10450. doi: 10.1128/JVI.79.16.10442-10450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, Camenga DL, Whitfield S, Murphy FA. Anticonvulsant prolongation of survival in adult murine lymphocytic choriomeningitis. II. Ultrastructural observations of pathogenetic events. J Neuropathol Exp Neurol. 1977;36:21–40. doi: 10.1097/00005072-197701000-00004. [DOI] [PubMed] [Google Scholar]
- Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006a;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmer SC, de Souza JB, Frere C, Candal FJ, Juhan-Vague I, Grau GE. TGF-beta1 released from activated platelets can induce TNF-stimulated human brain endothelium apoptosis: a new mechanism for microvascular lesion during cerebral malaria. J Immunol. 2006b;176:1180–1184. doi: 10.4049/jimmunol.176.2.1180. [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113:885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P, Robenek H, Tryggvason K, Song J, Korpos E, Loser K, Beissert S, Georges-Labouesse E, Sorokin LM. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med. 2009;15:519–527. doi: 10.1038/nm.1957. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Dye JM, Quinn DG. Control of lymphocytic choriomeningitis virus infection in granzyme B deficient mice. Virology. 2003;305:1–9. doi: 10.1006/viro.2002.1754. [DOI] [PubMed] [Google Scholar]
- Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat Immunol. 2007;8:1159–1164. doi: 10.1038/ni1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Calenoff MA, Dal Canto MC. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler’s murine encephalomyelitis virus infection leading to demyelination. J Neuroimmunol. 2001;118:256–267. doi: 10.1016/s0165-5728(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A, Fujinami RS. Theiler’s virus infection in nude mice: viral RNA in vascular endothelial cells. J Virol. 1988;62:3589–3596. doi: 10.1128/jvi.62.10.3589-3596.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]