Abstract
The central nervous system (CNS) is a remarkably complex structure that utilizes electrochemical signaling to coordinate activities throughout the entire body. Because the nervous system contains nonreplicative cells, it is postulated that, through evolutionary pressures, this compartment has acquired specialized mechanisms to limit damage. One potential source of damage comes from our immune system, which has the capacity to survey the CNS and periphery for the presence of foreign material. The immune system is equipped with numerous effector mechanisms and can greatly alter the homeostasis and function of the CNS. Degeneration, autoimmunity, and pathogen infection can all result in acute, and sometimes chronic, inflammation within the CNS. Understanding the specialized functionality of innate and adaptive immune cells within the CNS is critical to the design of more efficacious treatments to mitigate CNS inflammatory conditions. Much of our knowledge of CNS-immune interactions stems from seminal studies that have used static and dynamic imaging approaches to visualize inflammatory cells responding to different CNS conditions. This review will focus on how imaging techniques have elevated our understanding of CNS inflammation as well as the exciting prospects that lie ahead as we begin to pursue investigation of the inflamed CNS in real time.
1 Introduction
The CNS is the critical processing center that controls a broad range of functions from breathing to higher thought, making induction of immune responses within this tissue potentially dangerous. Because neurons are held in a postmitotic state and do not replicate readily, injury to this critical population by immune cells can damage neuronal connections that are essential for life. Generally, limited numbers of leukocytes are allowed to gain access into the CNS due to the blood brain barrier (BBB) and blood cerebral spinal fluid barrier, providing one means of protection. However, when a pathogen invades the CNS or an autoimmune response is mounted within this organ, recruited leukocytes can now encounter a variety of cells that are normally anatomically sequestered from the periphery (e.g., astrocytes, microglia, oligodendrocytes, stromal cells, and neurons). It is of great importance that we obtain a more in depth understanding of how CNS immunity is induced, and about the consequences of leukocyte interactions with CNS residents during infection, autoimmunity, and degenerative disease. Importantly, the actual consequences of CNS immunity can sometimes be obscured in systems reliant on tissue culture, because of the intricate and highly complex associations normally found between glial cells and neurons in vivo. Moreover, the location of the immune response within the CNS (e.g., the meninges versus the brain parenchyma) can also dictate the type of immune cells recruited and the resident CNS populations that are perturbed. Visualization of immunity in the CNS provides critical information that simply cannot be obtained from in vitro studies alone. In this review, we will focus primarily on studies that have used imaging techniques to advance our understanding of CNS immunity. Novel insights into CNS autoimmunity, degenerative diseases, and responses to protein and infectious agents have all been obtained using imaging approaches, and the recent use of multiphoton microscopy to probe CNS immunity is now providing our first glimpse of immune cell dynamics in the nervous system. On the horizon lies a wealth of visual knowledge to be uncovered as we probe the CNS with greater precision and resolution in real time. This review will cover the ground we have trod thus far using imaging approaches and some exciting new prospects that await our discovery in the future.
2 Static Imagery: Lessons Learned from Nondynamic Imaging Approaches
2.1 Initiation of Immune Responses to CNS-Derived Antigens
The induction of adaptive immune responses is often reliant on antigen delivery into the secondary lymphoid organs for priming. Accordingly, the efflux of CNS interstitial fluid and cerebral spinal fluid (CSF) from the CNS into the periphery provides a means for delivering brain-derived antigens predominantly into the cervical lymph nodes (Bradbury and Cole 1980; Bradbury et al. 1981; Bradbury and Westrop 1983; Szentistvanyi et al. 1984; Weller et al. 1996). CT scans monitoring radiolabeled tracers and gross analysis of India ink distribution showed that drainage from the CNS to the periphery occurs rapidly, within minutes to hours (Hunter et al. 1995; Kida et al. 1993). Visualization of CSF-injected India ink drainage by microscopy revealed the passage of CSF through the cribriform plate and into the nasal lymphatics as one major pathway for communication between the CNS and periphery (Kida et al. 1993, 1995). In fact, intracerebral injection and tracking of green fluorescent protein (GFP)-labeled T cells showed that movement through the cribriform plate into the nasal mucosa is a common pathway by which both antigen and T cells gain access to the cervical lymph nodes from the CNS (Goldmann et al. 2006). It is thought that peripheral sampling of CNS-derived antigens through this and other pathways (Cserr et al. 1992) is critical in the initiation of immune responses to pathogens that gain access to the CNS, and anatomical sequestration from these drainage pathways (Stevenson et al. 1997) may be a way for CNS pathogens to avoid detection by the immune system.
To gain additional insights into the role of antigen-presenting cells (APCs) in the initiation of peripheral immune responses to CNS antigens, confocal microscopy was recently employed in a murine system in which fluorescently-tagged ovalbumin (OVA) was used as a model antigen. Intracerebral injection of BODIPY-conjugated OVA confirmed that the rapid efflux of brain-derived antigens into cervical lymph nodes occurs within hours of injection (Ling et al. 2003). Furthermore, using antigen-loaded GFP-tagged dendritic cells (DCs), it was revealed that DCs migrate from the CNS into the cervical lymph nodes (Karman et al. 2004a, b), indicating that antigen can arrive by cellular transport as well as by free drainage through the cerebral extracellular fluid. Initiation of peripheral T cell responses triggered by brain-derived antigens (Ling et al. 2003; Walter and Albert 2007) was subsequently followed by the recruitment of activated T cells back to the regions of the brain where parenchymal antigen retention and uptake was detected (Ling et al. 2003, 2006). T cell homing to CNS tissue was dependent on CNS-derived DCs (Karman et al. 2004b), supporting the emerging idea that tissue DCs can dictate the trafficking patterns of T cells (Mora et al. 2005; Mora and von Andrian 2006). Consistent with this idea are colocalization studies demonstrating that CNS DCs process brain-derived OVA antigen (Karman et al. 2004b) and data from organotypic brain slices, showing that CD11c+ antigen-loaded cells in the CNS continue to stimulate T cell proliferation (Ling et al. 2008). Thus, CNS dendritic cells play an important role in antigen delivery to the periphery, subsequent T cell homing into the CNS, and continued T cell stimulation at the site of antigen retention and presentation. Future imaging studies focused on the dynamics of T cell–DC engagement within the CNS are required to provide novel insights into the consequences of these interactions within the CNS.
2.2 CTL Engagement of Virus-Infected CNS Targets
Generation of an effective antigen-specific adaptive immune response is essential for the clearance of pathogens. CD8+ T cells, in particular, are important for mediating antiviral immunity in the periphery. Similarly, CD8+ T cells can also dictate CNS immune responses to pathogens, resulting in viral eradication or, in some cases, immunopathology (Chua et al. 2004; McGavern et al. 2002b; Shrestha and Diamond 2007; Shrestha et al. 2006). Primed CD8+ cytotoxic T cells (CTL) can purge pathogens from the CNS using cytolytic (e.g., perforin) and/or noncytolytic (e.g., IFNγ) pathways. Both CTL activation and target recognition are reliant on the intercellular communication between T cells and APCs (or virally infected target cells), respectively. For CD8+ T cells, critical interactions between the T cell receptor (TCR) and major histocompatibility complex (MHC) I molecules displaying antigens is thought to occur through the anatomical structure referred to as the immunological synapse (Davis et al. 2007; Grakoui et al. 1999; Huse et al. 2006; Monks et al. 1998; Stinchcombe et al. 2006). The hallmark of the synapse is the formation of supramolecular activation clusters (SMAC) with enrichment of TCR molecules within the central SMAC (c-SMAC) and adhesion molecules, including LFA-1, surrounding the peripheral area to form the peripheral SMAC (p-SMAC) (Monks et al. 1998). The concept of the immunological synapse has emerged from extensive in vitro data (Davis et al. 2007 ; Dustin 2008 ; Grakoui et al. 1999 ; Huppa and Davis 2003; Monks et al. 1998). However, more recently, viral infection models were used to identify the in vivo correlate of the immune synapse between CTL and infected targets residing in the CNS (Barcia et al. 2006; McGavern et al. 2002a). Static imaging studies of the virally-infected CNS have yielded insights into the molecular anatomy of the in vivo immune synapse and demonstrated that this structure resembles the synapse observed in vitro.
In one study, lymphocytic choriomeningitis virus (LCMV), a natural pathogen for human and murine hosts (Lledo et al. 2003; Roebroek et al. 1994; Schanen et al. 1998), was used to examine the interactions of CTL responses to viral infection within the CNS. LCMV, a prototypic member of the Arenaviridae family, is a noncytopathic virus and; therefore, any pathology that develops after infection is driven by the subsequent immune response, rather than by the virus itself. Intracerebral (i.c.) infection of adult immunocompetent mice with LCMV leads to acute, fatal choriomeningitis within 6–8 days (Kang and McGavern 2008; McGavern et al. 2002b). Following i.c. inoculation, LCMV localizes to the meninges, ependyma, and choroid plexus within the CNS (McGavern and Truong 2004 ; Mims 1960 ; Schwendemann et al. 1983). CD8+ T cells are critical for LCMV-induced meningitis (Fung-Leung et al. 1991; Kang and McGavern 2008) and have been detected in the cerebral spinal fluid (CSF) of infected mice (Ceredig et al. 1987; Zinkernagel and Doherty 1973). Although bulk CD8+ T cells were observed by electron microscopy in the meninges of LCMV-infected mice (Schwendemann et al. 1983), the nature of CTL interactions during fatal meningitis were not defined. B6 mice infected with LCMV mount a robust, polyclonal T cell response directed against the glycoprotein (GP) and nucleoprotein (NP) of LCMV, and most of the MHC I and II restricted epitopes have been identified (Gairin et al. 1995; Gallimore et al. 1998; van der Most et al. 1998). This information facilitated the generation of MHC tetramers that can be used to identify LCMV-specific CD8+ T cells either flow cytometrically or on tissue sections by in situ tetramer staining (McGavern et al. 2002a ; Skinner et al. 2000 ; Skinner and Haase 2005). CD8+ T cell TCR transgenic (tg) mice (referred to as P14 mice) specific to the DbGP33–41 epitope have also been generated and provide another powerful tool for tracking LCMV-specific CTL responses (Pircher et al. 1989).
Using in situ tetramer staining and confocal microscopy, virus-specific CTL were observed in close proximity to infected target cells residing in the meninges of symptomatic mice infected i.c. with LCMV (McGavern et al. 2002a). Analysis of MHC I tetramer distribution in situ also revealed polarization of the TCR toward infected target cells suggestive of immunological synapse formation. To generate a more robust system to track LCMV-specific CTL, naive TCR-tg P14 cells genetically tagged with GFP were transferred into mice prior to infection. Coronal brain reconstructions obtained from infected mice at day 6 postinfection demonstrated that GFP+ LCMV-specific CTL localized precisely to the regions of viral infection (McGavern et al. 2002a). To examine the molecular anatomy of the CTL interactions, high-resolution confocal microscopy studies of P14 cells were performed. These studies revealed the redistribution of adhesion (LFA-1) and signaling (Lck) molecules toward the CTL-target cell interface, once again suggestive of immune synapse formation. Visual evidence of perforin deposition onto LCMV-infected cells was consistent with the idea that CTL were actively using lytic effector mechanisms to purge virus from at least some of the CNS targets (McGavern et al. 2002a). Importantly, P14 CTL were found in close juxtaposition of multiple target cells, enforcing the concept that one mechanism for efficient viral clearance is through simultaneous engagement of infected cells. A subsequent in vitro study has elaborated on this concept by demonstrating the ability of CTL to interact with multiple target cells of different antigenic potential (Wiedemann et al. 2006). Time-lapse confocal microscopy revealed double polarization of T cell lytic granules when simultaneously engaged with different target cells. Interestingly, TCR clustering and Ca2+ signaling was still observed even after target cell lysis. These data suggest that T cells can benefit from continued engagement with annihilated target cells, while making new contacts with subsequent target cells.
In vivo immune synapse formation was formally defined in 3D using a model reliant upon a nonreplicating adenovirus infection (Barcia et al. 2006, 2008). In this model, infection with thymidine kinase (TK)-tagged adenovirus predominantly targeted astrocytes within the CNS. Although both CD4+ and CD8+ T cells were required for clearance of the virus after immunization, CD4+ cells were sequestered in the perivascular compartment whereas CD8+ T cells localized to the parenchyma. Confocal studies visualizing adenovirus and CD8+ T cells demonstrated close interactions between infected targets and CTL. Within the CD8+ T cells, signaling molecules like ZAP-70 and phospho-lck polarized towards the interface with the adenovirus-infected targets. 3D reconstructions of the interface revealed central clustering of TCR molecules surrounded by a peripheral ring of LFA-1 (Barcia et al. 2006). This was consistent with molecular anatomy of synapses defined in vitro (Monks et al. 1998). However, further analysis of astrocyte viral clearance in the CNS revealed the presence of mature CTL synapses (defined by formation of the c-SMAC and p-SMAC) as well as CTL interactions that occurred in the absence of adhesion molecule and TCR polarization (Barcia et al. 2008). Previously, directionally distinct pathways for cellular secretion of cytokines were defined in vitro with some cytokines, such as TNFα, being released multidirectionally, whereas others, such as IFNγ and IL-2, were preferentially secreted at the synapse (Huse et al. 2006). Consistent with synaptic IFNγ release, static imagery confirmed that IFNγ secretion occurred at the interface of CTL–astrocyte contacts (Barcia et al. 2008). Interestingly, IFNγ secretion was observed with and without mature immunological synapse formation. IFNγ+ polarization without mature synapse formation was common in T cells associated with astrocytic processes, whereas more mature IFNγ+ synapses were observed when T cells were engaged with astrocytic cell bodies (Barcia et al. 2008). These results suggest that T cell interactions with astrocytes can occur through distinct types of synapses that depend on the location at which contact is made; however, definitive proof of this supposition would require real time imaging, as the static imaging approach used in these studies did not permit examination of synapse dynamics and transformation over time. Nevertheless, these studies did provide definitive data demonstrating the formation of mature immunological synapses in vivo during the process of CNS viral clearance. Importantly, the localization of signaling molecules and the presence of polarized IFNγ at the CTL–target cell interface suggests that synapse formation can sometimes have a functional outcome. It remains to be determined whether a mature synapse is required for T cell effector functions in vivo.
2.3 CTL-Associated Neuronal Damage
Neurological deficits have been associated with generation of CD8+ T cell responses. Because most neurons are held in a postmitotic state (Herrup and Yang 2007), these cells do not readily renew, allowing for induction of irreparable pathology through CTL targeting. Following infection with Theiler’s murine encephalomyelitis virus (TMEV), demyelination and axonal damage is accompanied by neurological deficits, which are associated with the presence of CD8+ T cells (Murray et al. 1998a, b; Rivera-Quinones et al. 1998). This suggests that recruitment of CTL to the virally-infected CNS can lead to significant neuronal damage. CTL–neuronal interactions have also been implicated in human disease. Rasmussen’s encephalitis is a progressive disorder characterized by recurrent seizures (Rasmussen et al. 1958) and diminished neurological function. The etiology of this disease is unknown, although viral infection (Iannetti et al. 1991; Power et al. 1990; Vinters et al. 1993) and a humoral response directed against the glutamate receptor 3 (Levite et al. 1999; Rogers et al. 1994; Twyman et al. 1995; Whitney and McNamara 2000) have been suggested as possible explanations for the disease.
Another possible explanation for pathogenesis in Rasmussen’s patients was set forth by a recent microscopy study (Bien et al. 2002). Immunohistochemical examination of tissue specimens from Rasmussen’s encephalitis patients revealed the presence of lymphocytic infiltrates that were predominantly CD8+. Around 7% of the CD8+ T cells were in close apposition to neurons, and some of these cells were found to have granzyme B particles polarized to the contact surface. The presence of apoptotic neurons also suggested that CTL were specifically targeting these cells during disease (Bien et al. 2002). These findings support the possibility that CTL in Rasmussen’s patients can directly engage neurons and induce lysis via a granzyme-mediated pathway. Further studies are required to determine whether these CTL are targeting a viral or self-antigen.
It is thought that one mechanism by which neurons protect themselves from CTL activity is to reduce surface MHC I expression (Joly et al. 1991; Joly and Oldstone 1992). However, elevated levels of MHC I have been observed during disease (Herrup and Yang 2007; Neumann et al. 2002) and under conditions of inflammation and electrical impairment (Neumann et al. 1995, 1997; Rensing-Ehl et al. 1996). MHC I expression is also thought to participate in neuronal function and repair (Boulanger and Shatz 2004; Huh et al. 2000; Oliveira et al. 2004). To examine the impact of elevated neuronal MHC I expression in vivo, transgenic mice were generated to express H-2Db under the neuron-specific enolase promoter (NSE-Db mice) (Rall et al. 1995). Neurons extracted from NSE-Db mice were directly targeted by CTL in vitro, and transfer of CTL into NSE-Db mice persistently infected with LCMV resulted in enhanced infiltration into the CNS, BBB breakdown, and neurological deficits. These findings indicate that low neuronal expression of MHC I is at least one mechanism that protects neurons from CTL-mediated injury.
While it is incredibly difficult to detect neuronal MHC I under steady state or inflammatory conditions, recent studies suggest that MHC I expression on neurons can direct a CTL-mediated disease process (Sanchez-Ruiz et al. 2008). Molecular mimicry between a pathogen and a self-antigen is a powerful means to induce tissue pathology and disease. Neuronal expression of OVA followed by intracerebral infection with Listeria monocytogenes-secreting OVA results in an atactic–paretic neurological syndrome during which CD8+ T cells are recruited to MHC I+ OVA+ neurons, suggestive of cognate interactions between the two cell populations (Sanchez-Ruiz et al. 2008). In vitro studies have also supported the idea that CTL can target and injure MHC I expressing neurons. Quantification of Annexin V+ apoptotic neurons by microscopy revealed that CTL targeting of hippocampal neurons was dependent on neuronal upregulation of MHC I and the presence of antigen (Medana et al. 2000). Cytoskeletal injury, as evidenced by disruptions in β-tubulin III organization, occurred primarily in the neurites but not in the neuronal cell body. In fact, it was observed that CTL-mediated neurite cleavage could occur within 25 min of contact initiation and was peptide–MHC I-dependent (Medana et al. 2001b). Similar findings were observed in sympathetic neuronal cultures (Manning et al. 1987). Collectively, these in vivo and in vitro microscopic studies indicate that under certain conditions CTL can target neurons in a MHC I-dependent manner and that neuronal projections appear to be more susceptible to injury than the soma.
CTL can injure neurons using some of the same cytopathic effector mechanisms employed in peripheral tissues. In vitro visualization of CTL-induced neuronal intra-cellular Ca2+ flux ([Ca2+]i), used to assess neuronal injury, revealed delayed kinetics for [Ca2+]i flux on the order of hours rather than minutes. This timing was consistent with Fas- rather than perforin-mediated neuronal injury (Medana et al. 2000). However, direct application of purified cytotoxic granules to neurons can induce rapid neuronal [Ca2+]i flux characteristic of perforin/granzyme mediated death, and perforin mediated [Ca2+]i flux was observed in FasL deficient neurons because of their inability to inhibit CTL degranulation via engagement of the FasL/Fas pathway (Medana et al. 2001a). Granule mediated lysis of MHC I+ cerebellar granule neurons was also observed in vitro (Rensing-Ehl et al. 1996). Therefore, at least in vitro neurons can be damaged by both the perforin/granzyme and FasL/Fas pathways.
Perforin/granzyme-mediated mechanisms of neuronal damage have been implicated in the TMEV model of CNS demyelination and in Rasmussen’s encephalitis patients, suggesting that this cytopathic pathway might also be important in injuring neurons in vivo (Bien et al. 2002; Murray et al. 1998a). While the majority of published studies suggest that engagement of cognate peptide–MHC I complexes are required for CTL to engage and injure neurons, an in vitro study has shown that human fetal neurons can be injured by T cells in a contact-dependent, but peptide–MHC I-independent manner (Giuliani et al. 2003). While this is an interesting possibility, it is not clear whether this mechanism is relevant for immune interactions with mature neurons. Presently, most in vitro and in vivo studies suggest that injury of neurons by CTL is a peptide–MHC I-dependent process.
Direct engagement of neurons is a very straightforward means for CTL to injure the CNS and is reliant on the ability of neurons to present at least some peptide–MHC complexes. However, an alternative route to neuronal injury could occur indirectly through CTL targeting of the glial cells that support neurons. In vitro, CTL targeting of astrocytes was shown to occur in a MHC I-dependent manner (Cabarrocas et al. 2003). Visualization of [Ca2+]i flux in astrocytes exposed to CTL damage revealed that perforin was the primary pathway responsible for CTL-mediated injury of astrocytes (Medana et al. 2001a, 2000) followed by the Fas/FasL pathway if the former mechanism was impaired (Medana et al. 2001a). Confocal studies on tissue samples from Rasmussen’s encephalitis patients have also captured images of granzyme B polarization towards astrocytes, suggesting that CTL targeting of astrocyte also occurs in vivo (Bauer et al. 2007).
In certain regions of the brain, astrocyte targeting can directly impact neuronal health. This was shown in a model where transferred CTL targeted astrocytes with transgenic expression of the β-gal antigen. Because antigen expression was restricted to astrocytes and not neurons, the bystander killing of neurons observed in the cerebellum was a result of CTL–astrocyte targeting (McPherson et al. 2006). While this phenomenon was observed in the cerebellum, there was little evidence of neuronal death with β-gal expression in retinal astrocytes (McPherson et al. 2006). Therefore, destruction of antigen-expressing astrocytes can occur in the absence of bystander damage. Accordingly, in the white matter of the brain, transgenic hemag-glutinin (HA) expression in astrocytes resulted in specific elimination of these cells, without bystander effects, upon transfer of antigen-specific CTL (Cabarrocas et al. 2003). Therefore, impairment of astrocyte support can indirectly result in neuronal injury, but may be depend on the types of neurons located in the anatomical site that is targeted within the CNS.
2.4 Autoimmunity in the CNS (Experimental Autoimmune Encephalomyelitis)
Multiple sclerosis (MS) is a complex inflammatory disease of the CNS. Pathologically, MS patients have demyelination and axonal/neuronal damage in the CNS. Although the cause of MS remains unknown, MS is generally thought to be caused by autoimmune targeting of CNS components, including adaptive immune responses to myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP) (Bernard and de Rosbo 1991; Gold et al. 2006; Kerlero de Rosbo et al. 1993; Lassmann et al. 2007; Siffrin et al. 2007; Sun et al. 1991; Wucherpfennig et al. 1990). Several animal models of experimental autoimmune encephalomyelitis (EAE) have been utilized to study pathological mechanisms associated with CNS autoimmunity. EAE can be induced through several strategies, including immunization with myelin proteins or peptides (e.g., PLP139–151, MOG35–55) in conjunction with adjuvants and pertussis toxin, or, alternatively, through adoptive transfer of myelin-reactive T cells. Recently, a spontaneous model of EAE-like disease was generated using double transgenic mice that have MOG reactivity in both the T and B cell compartments (Krishnamoorthy et al. 2006). Depending on the model used, EAE can vary in: (1) chronicity (monophasic versus relapsing–remitting), (2) pathology (amount of demyelination and axonal/neuronal damage), and (3) components of the adaptive response involved (CD4+ T cells, CD8+ T cells, and B cells) (Gold et al. 2006; Siffrin et al. 2007). Therefore, investigators should proceed cautiously when attempting to link their findings observed in one model to the pathogenesis of human MS.
Myelin-specific CD4+ T cells are typically thought to be involved in the induction of EAE. Autoradiographic analysis of [14C] thymidine-labeled MBP-primed cells after transfer demonstrated homing of these cells into the CNS prior to and during EAE. Using this technique, the majority of cells appeared to be restricted to the perivascular space with little parenchymal infiltration (Cross et al. 1990). Chronological localization of [14C] thymidine-labeled MBP T cells showed that myelin-specific T cells were localized in newer lesions (Cross et al. 1993). Additionally, parenchymal detection of unlabeled infiltrates coincided with clinical symptom kinetics, implicating a role for immune-mediated pathology during disease. More recently, immunohistochemical analysis of T cells in the CNS after MOG35–55 immunization revealed infiltration in the choroid plexus, meninges, and subventricular zones during early phases of disease followed later by additional T cell accumulation around the perivascular regions (Brown and Sawchenko 2007). This suggests that there are two phases of T cell entry during EAE: an early recruitment through the CSF and choroid plexus, followed by a secondary wave of vascular recruitment.
Retroviral transduction of a GFP vector into MBP-specific CD4+ T cells has been utilized to further monitor phenotypes and migratory patterns from the periphery into the CNS during monophasic adoptive EAE progression (Flugel et al. 1999). Initially, in vitro activated MBP-specific GFP+ cells trafficked primarily to the parathymic lymph nodes, followed by entry into the blood and spleen by 60 h post transfer. Recruitment into the CNS occurred after 60 h post transfer and was associated with upregulated chemokine receptor expression on splenic MBP-specific GFP+ T cells and depletion of the cells from peripheral sites, including the spleen, lymph nodes, and blood. The kinetics of splenic entry and egress of MBP-specific GFP+ T cells were also confirmed with epifluorescence microscopy (Flugel et al. 2001). Confocal analysis of MBP-specific cells within the spinal cord revealed localization to the meninges, perivascular space, and parenchyma, whereas CD8+ T cells and B cells were mostly confined to the meningeal space. Recruitment of myelin-specific CD4+ T cells into the perivascular space and parenchyma was also observed after transfer of GFP-labeled MOG-specific T cells (Yura et al. 2001), suggesting that this distribution is common to myelin-reactive cells of different specificities.
Once in the CNS, myelin-specific T cells reactivate and upregulate OX-40, IL-2R, and cytokine expression (Flugel et al. 2001). Similar to MBP-specific GFP+ CD4+ T cells, macrophages and reactive microglia (labeled with anti ED1 antibodies) localized to the meninges and parenchyma during EAE (Flugel et al. 2001). Parenchymal entry of macrophages has been associated with myelin-specific CD4+ T cell reactivation in the CNS and subsequent disease severity. Tracking of GFP-labeled autoreactive effector T cells with high or low pathogenic potential showed equal capacities to enter the parenchyma, indicating that CNS entry is not a limiting factor for inducing maximal disease. However, only highly encephalitogenic CD4+ T cells reactivated in the CNS, induced MCP-1 and MIP-1α production, and resulted in ED1+ macrophage infiltration into the parenchyma (Kawakami et al. 2004).
Immunohistochemical and immunofluorescent staining for CD3+ T cells in the CNS of CXCR3−/− mice revealed an altered localization, with a more diffuse parenchymal distribution of both T cells and activated microglia/macrophages (Muller et al. 2007). These data imply that CXCR3 is important in the perivascular retention of T cells during EAE that prevents extensive dispersion throughout the white matter. Because T cells contribute to the secondary recruitment of pathogenic macrophages into the CNS, disruption of T cell localization through blockade of chemotactic signals has the potential to greatly alter the course of disease. By tracking myelin-specific T cells through imaging and flow cytometric methods, many novel insights into EAE disease pathogenesis have been obtained, including the pathogenic link between CD4+ T cells and the secondary recruitment of innate immune cells. With the application of intravital imaging approaches, it now should be possible to further refine the exact contribution of T cells and innate immune cells to disease onset and progression, and to evaluate the efficacy of therapeutics (e.g., antichemotactic agents) in real time.
CD4+ T cells can be subdivided into distinct subsets based on cytokine profiles (Kaiko et al. 2008), and the type of helper cell (e.g., Th1, Th2, or Th17) generated can have a significant impact on subsequent disease progression. Previously, IFNγ-producing, Th1-polarized CD4+ T cells were thought to be essential for EAE. This was based on disease induction following transfer of Th1 polarized cells, IFNγ expression found in EAE lesions, and IFNγ production by T cells isolated from the CNS (Kuchroo et al. 2002). However, mice deficient in molecules associated with the Th1 pathway, including IL-12p35, IL-12Rβ2, IFNγ, IFNγR, or STAT1, had normal or increased disease severity making the role for Th1 cells in EAE controversial (Becher et al. 2002; Bettelli et al. 2004; Cua et al. 2003; Ferber et al. 1996; Gran et al. 2002; Willenborg et al. 1996; Zhang et al. 2003). In recent years, IL-23 was linked with CNS inflammation during EAE. It was observed that EAE could be induced in IL-12 p35−/−, but not IL-12 p40−/− (which also acts a subunit of IL-23; Oppmann et al., 2000) or IL-23 p19−/− mice (Cua et al. 2003). IL-23 has been associated with Th17 polarized cells (Bettelli et al. 2007; Langrish et al. 2005; Volpe et al. 2008), defined by IL-17 production, and provides an explanation for the induction of EAE in the absence of Th1 related genes. Similar to what was initially observed with IFNγ, IL-17+ myelin-specific T cells detected in the CNS by ELISPOT are associated with disease (Hofstetter et al. 2005, 2007). IL-17 is now thought to play a significant role in EAE due to the ability of: (1) IL-23 cultured IL-17 producing cells to induce EAE upon transfer (Langrish et al. 2005), (2) attenuated disease in IL-17−/− mice (Komiyama et al. 2006) or in wild type PLP immunized SJL mice depleted of IL-17 (Langrish et al. 2005) or IL-23 (Chen et al. 2006), and (3) lack of disease in RORα−/−RORγ−/− mice which lack Th17 cells (Yang et al. 2008). Increased IL-17 expression in MS lesions observed with fluorescent confocal microscopy (Kebir et al. 2007) and gene arrays (Lock et al. 2002) suggest that Th17 cells are also prevalent in human disease.
In vitro, both IL-17 and IL-22 were shown to increase the permeability of brain-derived endothelial cells and reduce the levels of tight junction proteins (Kebir et al. 2007). Electron microscopy revealed the existence of intimate associations with MBP-specific T cells and CNS endothelial cells during extravasation (Raine et al. 1990), creating an opportunity for T cells to release cytokines in close proximity to endothelium. Importantly, confocal microscopy of tissues from MS, but not control, patients demonstrated an increase in IL-17R and IL-22R on CNS endothelium (Kebir et al. 2007), suggesting that these cytokines play a potential role in permeabilization of the BBB during MS. These data have all led to a paradigm shift in the EAE field which suggests that Th17 rather than Th1 polarized T cells are more important for disease pathogenesis; however, IL-12 and Th1 cells may still play a significant role in autoimmune disease and thus their contribution should not be completely ignored. For example, it was recently shown that IL-12 and IL-23 polarized T cells induce different types of EAE that varied in the recruitment of macrophages versus neutrophils (Kroenke et al. 2008). Histological analyses hematoxylin- and eosin-stained CNS sections also revealed that infiltrates localized differently. Transfer of IL-12 polarized cells resulted in meningeal and subpial white matter leukocyte infiltration, whereas IL-23 polarized cells infiltrated more deeply into the CNS parenchyma. Further visualization studies to define (1) the specific distribution of the injected T cells and other cellular subsets, (2) the infiltrating or CNS resident cells that are engaged in situ, and (3) the pathogenic outcome (e.g., apoptosis) of such interactions need to be conducted to define the divergence between IL-12 versus IL-23 pathology. These appear to be two different pathways that lead to indistinguishable clinical disease.
Epitope spreading is a critical process for the induction of relapsing forms of EAE. This phenomenon is characterized by the generation of reactivity to epitopes other than the epitope involved in disease initiation and has been observed during both MS and EAE (Lehmann et al. 1992; McRae et al. 1995; Tuohy et al. 1999; Yu et al. 1996). In the SJL mouse model of EAE, evidence for both intra- and intermolecular epitope spreading exists (McRae et al. 1995). Following PLP 139–151 immunization, spreading to other noncross-reactive PLP epitopes (intramolecular spreading) was observed. PLP reactivity was also observed in mice that received MBP-specific CD4+ T cells, which is an example of intermolecular spreading. Diversification of antigens recognized during the course of EAE contributes significantly to disease recurrence. Evidence supporting this concept includes the association between the timing of epitope spreading and relapse, the ability to transfer disease into naive mice using CD4+ T cells that recognize secondary determinants, and the blockade of relapses by tolerance induction to novel epitopes that only emerge during the epitope spreading phase (Vanderlugt and Miller 2002; Yu et al. 1996). Immunization of SJL mice with PLP 178–191 was followed by immunoreactivity to the PLP 139–151 epitope, which was detected by CFSE dilution of transferred naive PLP 139–151-specific5B6CD4+TCR transgenic cells (McMahonetal. 2005). Interestingly, the diversification of peptide specificity was initiated in the CNS compartment prior to any detectable epitope spreading in the periphery. Isolation of potential APCs localized in the CNS during inflammation, including macrophages, microglia. and DCs, revealed that all three had the capacity to stimulate proliferation in the presence of exogenous antigen. However, only DCs were able to induce naive T cell proliferation with endogenously-derived antigen (McMahonetal. 2005). Visualization of DCs in close proximity to CD4+ T cells and the presence of myelin droplets in CNS DCs (detected by confocal imaging) substantiated the idea that naive myelin-reactive CD4+ T cells were stimulated by DCs in the CNS during EAE (McMahon et al. 2005). DC myelin uptake and juxtaposition to T cells was also noted by immunofluorescence and immunohistology performed on tissues from MS patients, suggesting that DC presentation of myelin antigens to T cells is likely to be relevant in human autoimmune disease (Serafini et al. 2006).
Characterization of CNS DCs during EAE revealed the presence of myeloid, plasmacytoid, and CD8+ DCs (Bailey et al. 2007). Static imagery of the brain and spinal cord demonstrated accumulation of myeloid DCs (mDCs) in areas of inflammation and demyelination where PLP was also detected. In contrast, plasmacytoid DCs were distributed sparsely in uninflamed regions of the CNS. Confocal analyses also revealed close juxtaposition of mDCs with CD4+ TCR transgenic cells specific for PLP 139–151, which become activated during the epitope spreading that follows disease induction with PLP 178–191 (Bailey et al. 2007). Myeloid DCs were shown to induce proliferation of PLP 139–151-reactive CD4+ T cells and cytokine profiles reflective of Th17 lineage cells. Additionally, mDCs isolated from the CNS expressed TGFβ, IL-6 and IL-23 (Bailey et al. 2007), cytokines known to aid in Th17 development (McGeachy et al. 2007), further supporting the role of these DCs in polarizing myelin-reactive CD4+ T cells toward the Th17 lineage. Collectively, these data suggest that the processing of damaged CNS tissue and subsequent presentation by local APCs results in the generation of myelin-reactive Th17 T cells directed against other self-determinants, which then contribute to EAE relapses.
3 Noninvasive Imaging: MRI, mPET, and Bioluminescence
Recently, new technologies for in vivo biological research, some which are commonly used for human clinical applications, have been harnessed to monitor alterations that occur over time in animal models of disease. These applications include magnetic resonance imaging (MRI), bioluminescence, and micropositron emission tomography (PET). The major advantage of these systems is the ability to temporally monitor anatomical or biological changes within the same animal in a noninvasive manner. Additionally, the spatial resolution of structures is on the order of millimeters to tens of microns (Pautler 2004; Poeppel and Krause 2008), and both MRI and PET have no real depth limitations (Nair-Gill et al. 2008). These techniques were recently used by immunologists to achieve a better spatiotemporal understanding of tumor growth and immune cell or antibody localization during disease progression. However, it should be noted that we are only at the inception of applying these techniques to basic immunological research, and while we can visualize some aspects of immunity, the ability to examine fine interactions and dynamics of individual cells is currently not feasible due to the limitations in resolution.
MRI is based on the idea that charged particles rotate and have a small magnetic charge associated with their movement. The basic concepts are reviewed in Pautler (2004) and Pirko et al. (2005) and will be only briefly introduced here. MRI is commonly based on the usage of hydrogen (1H) signals, which has a positively charged proton. Generally, the protons will rotate in random directions, thus canceling out any net movement or magnetic charge. However, when an external magnetic field (B0) is applied, these spins align themselves with this field. Due to the fairly uniform directional rotation created by the B0, a net magnetic vector (NMV) or moment is created. Application of a radio frequency perpendicular to the B0 can displace the protons from the axis in which it was aligned with B0, generating a new NMV that is at an angle to B0. Subsequent release of the radio frequency allows for the protons, and thus the NMV, to realign themselves with the B0. During this process, energy is emitted and exchanged as the nuclei return to alignment with B0. Because different tissues have different rates of recovery to the position B0, these discrepancies in energy can be measured and used to resolve anatomical structures. Contrast agents, such as paramagnetic complexes, have the ability to alter the process of B0 realignment, generating an alteration in the intensity of signals.
MRI has been used to detect immune cells that were labeled in vitro prior to injection in vivo (Weissleder et al. 1997; Yeh et al. 1993, 1995). More recently, MRI was utilized to track immune cells in two murine models of CNS demyelination: MBP-induced EAE and infection with Theiler’s murine encephalomyelitis virus (TMEV) (Pirko et al. 2003, 2004). A novel in vivo leukocyte-labeling technique was used in these studies for MRI visualizations. Specifically, antibodies that recognize leukocytic antigens (e.g., CD4 and CD8) were conjugated to magnetic beads (referred to as superparamagnetic antibodies) and then injected into animals prior to MRI analyses. In both systems, these antibodies were successfully used to detect infiltration of leukocytes into the CNS (Pirko et al. 2003, 2004). Therefore, MRI can be used to track in vivo labeled cells within the same host over many time points, affording an opportunity to capture noninvasive snapshots of the topography of immune cells in the CNS as a disease progresses. Novel approaches are currently under development to improve the sensitivity of MRI (Zabow et al. 2008); however, current MRI studies of CNS immunity are still confined to yielding information about cells at the population level rather than individual cellular dynamics.
Alternatives to in vivo imaging by MRI include bioluminescence or PET. Both depend on the emission of photons that are subsequently detected by the apparatus. In bioluminescence, a reporter gene expressing a light-emitting enzyme, such as luciferase, is expressed in the cells or tissue of interest (Welsh and Kay 2005). Prior to imaging, the substrate is injected into the animal, and this enzymatic reaction results in the release of photons that are subsequently detected by the machine (Negrin and Contag 2006). Similarly, tracking of immune responses by PET also involves expression of PET probes in the cells of interest, either by direct labeling or expression of protein targets for the PET probe. Positrons emitted from the PET isotope-labeled probe interact with tissue electrons and form photons, which can be detected using a microPET machine (Nair-Gill et al. 2008). Quantification of these reporters in both bioluminescence and PET allow for comparisons of the relative amounts of signal accumulated at given time points in controls versus experimental groups. Additionally, using PET it has been suggested that the level of signal intensity correlates with the number of labeled cells present within a region of interest (Su et al. 2004). Application of bioluminescence and PET for tracking leukocytes has mostly been limited to examination of peripheral immunity, although CD4+ T cell trafficking during EAE has been examined using a bioluminescence approach (Costa et al. 2001). Like MRI, these techniques have only recently been applied to tracking leukocyte responses, and in general provide an even lower spatial resolution than MRI (Nair-Gill et al. 2008). Another important aspect to consider when utilizing either PET or bioluminescence is that there is a limitation to the sensitivity of these assays. A certain signal threshold must be achieved prior to detection. Overall, these noninvasive techniques provide an excellent means to examine the general positioning of immune cells in the CNS over time, but further technological advances are required to refine spatial resolution.
4 Dynamic Imaging by Two-Photon Laser Scanning Microscopy
4.1 Introduction/Technical Advances
Neuroscientists have pioneered the usage of multiphoton-based microscopic imaging to assess biological occurrences in living tissue and animals (Denk et al. 1990; Kerr and Denk 2008). Although in vitro studies using live cells and microscopy on fixed tissue have yielded important observations, these studies are limited due to removal of cells from their natural environment and the inability to observe dynamic processes, respectively. These technical limitations have been overcome with the inception of two-photon laser scanning microscopy (TPLSM). Based on the principle that simultaneous absorption of multiple low energy photons can combine to reach a high excitation state (equivalent to what is achieved with a single high energy photon), multiphoton-based microscopy has revolutionized the field of in vivo imaging. TPLSM is advantageous for tissue microscopy because usage of low energy photons allows for an increased depth penetration and decreased photoxicity, while the imaging field is a focal point, which circumvents the background issues associated with conventional confocal microscopy (Helmchen and Denk 2005; Rocheleau and Piston 2003).
Another advance that has made in vivo imaging with TPLSM more feasible is the expression of fluorescent proteins in the cells of interest. Neuroscientists have taken advantage of GFP (Stearns 1995), and other spectral variations, that can be expressed transgenically in mice. For example, thy1 driven expression of XFP (e.g., yellow fluorescent protein, GFP, etc.) labels the entire neuron, which facilitates imaging of everything from axons to dendritic spines (Feng et al. 2000). Recently, immunologists have also begun to utilize TPLSM (Bajenoff and Germain 2007) after labeling immune cells of interest ex vivo and then transferring them in vivo to be imaged. A variety of transgenic mice have also been generated that express fluorescent proteins under lineage-specific promoters, which facilitates the tracking of innate and adaptive immune cells. Although initial studies using TPLSM to image the CNS were conducted in the early 1990s (Denk et al. 1994, 1990; Kerr and Denk 2008), the majority of in vivo immunological imaging studies conducted to date have focused on the periphery rather than the CNS. Because neuroscientists have paved the way for in vivo microscopy in the CNS, we will first briefly review some of the advances made in the field of neuroscience using TPLSM prior to discussing the pioneering studies that have examined CNS immunity.
4.1.1 Neuronal Dendritic Spines
The capacity of the brain to react and rearrange neuronal circuits during development and following experience-driven or environmental changes has been a topic of great interest. To assess the malleability of these connections, researchers have evaluated the stability of dendritic spines (which are protrusions from the dendritic shaft of neurons) as a measure of neuronal plasticity. These structures function as the postsynaptic sites of axons and increase the density of synapses between dendrites and axons (Sorra and Harris 2000). Recently, TPLSM has yielded new insights into the dynamics of spine formation and elimination, the overall stability of the structures, and alterations occurring during experience-dependent manipulations of synaptic plasticity (Alvarez and Sabatini 2007; Pan and Gan 2008).
In vivo imaging of dendritic spine dynamics in normal tissue has demonstrated developmental alterations in neuronal plasticity associated with age. Initial TPLSM studies on dendritic spine stability of apical portions of layer 5 cortical neurons using transgenic mice that express fluorescent proteins in neuronal subsets (Feng et al. 2000) revealed that spines are stable over a period of weeks to months (Grutzendler et al. 2002; Trachtenberg et al. 2002). However, the percentage of stable dendritic spines in young adolescent mice (1–2 months of age) was variable between studies with approximately 50–55% stability for ≥ 8 days in the somatosensory cortex (Holtmaat et al. 2005 ; Trachtenberg et al. 2002) and ~73–82% stability over a 1-month or 2-week (Grutzendler et al. 2002) imaging observation period, respectively, in the visual cortex. An increase in overall dendritic spine stability was observed as mice develop and transition into adulthood with approximately 70% stability over 8 days in the somatosensory cortex (Holtmaat et al. 2005) and ~96% over 1 month in the visual cortex (Grutzendler et al. 2002) of ≥4-month-old mice.
Decreased spine elimination contributes to the increased stability of dendritic spines that occurs over time. TPLSM imaging of fluorescent neurons in mice of different ages through thinned skull preparations showed ~17–23% spine elimination over a 1-month period in young mice (1-month-old) that was significantly higher than the ~10% elimination observed in 2-month-old mice, and the ~5–6% elimination observation in mice >4 months of age (Zuo et al. 2005a, b). While spine elimination rates decrease over time, spine formation appears to remain stable (Zuo et al. 2005a, b). TPLSM combined with electron microscopy has shown that generation of new stable spines precedes and results in synaptogenesis, creating new functional neuronal connections (Knott et al. 2006; Nagerl et al. 2007). The rate of spine formation, ~5%, is lower than spine elimination in young mice resulting in a reduction of overall dendritic spines. These studies have shown that as neural networks mature and the number of spines is reduced, the rates of elimination and formation become more equivalent and thus contribute to the long-term stability of dendritic spines with age.
Although there is a general consensus that dendritic spine stability increases over time, the percentage of spines that reach this state is inconsistent between studies. Variability in spine stability may depend on the brain region examined. Differences in dendritic spine motility have been observed in various sensory cortical areas, with lower spine motility observed in neurons located in the visual cortex compared to auditory and somatosensory regions (Majewska et al. 2006), suggesting that localization can impact dendritic spine dynamics. However, the more likely explanation for the disparity between degrees of stability is a technical one based on the marked alterations that occur when the skull is opened to generate a viewing window for in vivo imaging. An elegant study by Xu et al. has clearly demonstrated that open skull craniotomy windows result in significantly higher spine turnover in the barrel cortex (~34%) when compared to a thinned skull surgical approach (~6%), which results in less injury to the surface of the brain (Xu et al. 2007). Increased microglial and astrocyte reactivity was observed in tissue sections obtained from mice that underwent craniotomies, but not thinned skull preparations, demonstrating that increased glial activation is associated with more invasive surgeries (Xu et al. 2007). Therefore, the type of cranial window used in vivo imaging can greatly impact the behavior of CNS resident cells and must be carefully considered when assessing CNS intravital imaging data.
4.1.2 Microglial Activation
Microglia are CNS-resident innate immune cells that function to monitor and respond to the CNS in both quiescent and pathological states (Garden and Moller 2006; Hanisch and Kettenmann 2007). Previously, the behavior of microglia during normal physiological conditions could not be assessed due to the disruptive nature of the tissue removal process which activated these cells. However, recent studies using TPLSM have provided novel insights into “resting” microglial surveillance of the CNS. Transcranial visualization through a thinned skull of resident microglial-expressing eGFP driven by the fractalkine receptor (CX3CR1) locus (Jung et al. 2000) revealed a homogeneous tissue distribution with approximately 50–60 μm separating individual cells (Nimmerjahn et al. 2005). Time-lapse microscopy revealed that the small somata of microglial generally remained stationary whereas the numerous thin and ramified processes extending from the cell body were highly motile, with continuous random extensions and retractions occurring at similar velocities of ~ 1.5 μm min−1. Due to balanced rates of branch generation and elimination, the overall number of processes remained stable. Filipodia like microglial protrusions, which typically terminated in bulbous ends, were also observed; however, after initial extension, the protrusions were shown to stall for several minutes prior to retraction, suggestive of removal of debris in the healthy brain. Microglia in CX3CR1GFP/+ mice were also shown by TPLSM to contact neurons, astrocytes, and blood vessels, demonstrating the high connectivity of microglia with other CNS-resident cells (Nimmerjahn et al. 2005). Based on calculations of protrusion motility rates and the estimated volume of the extracellular space, resting microglia were found to survey the entirety of the brain parenchyma once every few hours.
Microglial dynamics have also been assessed using TPLSM during normal brain development in zebrafish with GFP-tagged microglia (Peri and Nusslein-Volhard 2008). Like mammalian microglia, zebrafish microglia branches underwent extensions and retractions under normal conditions. Apoptotic annexin V+ DsRed-labeled neurons were phagocytosed by microglia, indicating that microglial neuronal degradation is an essential step in clearance of neuronal debris during CNS development. Further analysis using knockdown studies and TPLSM demonstrated that in vivo microglial phagosome to lysosome fusion was dependent on the v-ATPase proton pump, in particular the a1 subunit of the V0 section of this protein (Peri and Nusslein-Volhard 2008), further defining critical elements involved in digestion after microglial engulfment. Thus, under quiescent conditions, “resting” microglia are highly dynamic cells in close contact with other CNS-resident cells and continually monitor the CNS parenchyma and eliminate potentially harmful debris.
In addition to dynamic surveillance of the resting brain, microglia are poised to react quickly to CNS injury and contain damaged tissue. TPLSM time-lapse imaging during acute brain trauma induced by two-photon laser ablation or mechanical injury in the parenchyma of CX3CR1GFP/+ mice revealed rapid microglial responses with directed extension of thickened processes towards the injury site that occurred within minutes (Davalos et al. 2005; Kim and Dustin 2006; Nimmerjahn et al. 2005). During this initial phase, minutes to hours postinjury, it was noted that repositioning of microglial processes, but not the somata, to within 90 μm of the microlesion is used to surround and contain the damaged tissue (Davalos et al. 2005; Kim and Dustin 2006; Nimmerjahn et al. 2005). Bulbous termini (Davalos et al. 2005) and inclusions within the microglia, averaging around 4.6 μm in size (Nimmerjahn et al. 2005), suggest that microglial were actively engulfing and degrading tissue debris. Later during injury responses (1–3 days after laser ablation), the somata of activated, ameboid-shaped microglia also converge onto the injury site (Kim and Dustin 2006).
Time-lapse imaging has demonstrated that destruction of an ATP gradient or inhibition of P2Y G protein-coupled metabotropic purinergic receptors, which respond to ATP, is sufficient to impede the reorientation of microglial processes towards the microlesion (Davalos et al. 2005). A potential receptor for this ATP response, the P2Y12 receptor, was shown to be expressed highly on ramified resting microglia and was decreased hours after activation and conversion of microglia to an ameboid state (Haynes et al. 2006). Microglia from P2Y12−/− CX3CR1GFP/+ mice have a significantly decreased ability to redirect processes minutes but not hours after laser ablation (Haynes et al. 2006). Therefore, P2Y12 responses are most likely involved in early but not late reorientation of microglial processes after CNS trauma.
After injury, astrocytes, visualized using TPLSM in transgenic mice that express GFP under the control of the glial fibrillary acid protein promoter, polarize toward the lesion in an ATP- and Ca2+-dependent manner. Blockade of connexin hemichannels, which disrupts astrocyte ATP release, prevents redirection of both astrocytes and microglial towards the injury site (Davalos et al. 2005; Kim and Dustin 2006). Because astrocyte responses precede microglial responses (Kim and Dustin 2006), and astrocyte connexin hemichannels are essential for microglial polarization, ATP-induced ATP release from astrocytes after injury is a likely means by which astrocytes and microglia communicate and coordinate their injury responses in the brain parenchyma.
4.1.3 Alzheimer’s Disease
Alzheimer’s disease (AD), the most common type of dementia, is characterized by the presence of beta amyloid (Aβ) plaques. Amyloid precursor protein (APP), a transmembrane protein expressed by neurons, is cleaved by β-secretase and γ-secretase to form the Aβ polypeptide that is the source of amyloid found in senile plaques (Gotz and Ittner 2008 ; Kang et al. 1987). The γ-secretase dictates the length of the subsequent Aβ polypeptide, and altered APP metabolism due to mutations in the presenilin components of the γ-secretase has been linked with disease (Scheuner et al. 1996). Cleavage of APP into the longer Aβ42 peptide results in the generation of extracellular toxic oligomers that can subsequently aggregate to form plaques (Gotz and Ittner 2008). One of the predominant theories for AD etiology is the amyloid hypothesis, which dictates that imbalanced Aβ production and accumulation initiates and drives pathology (Hardy et al. 1998; Hardy and Selkoe 2002). According to this hypothesis, other hallmark features of AD including neurofibrillary tangles, characterized by intracellular aggregates of the protein tau (Ballatore et al. 2007), are subsequent manifestations of neuronal abnormalities induced by Aβ dysregulation. In recent years, the toxicity of amyloid β oligomers (rather than fibrillar amyloid plaque deposits) has also been investigated as the factor responsible for disease pathogenesis, providing a modified view of the amyloid hypothesis in which soluble mediators become important (Castellani et al. 2008; Klein et al. 2001).
Recently, a series of TPLSM studies were conducted to assess the development of amyloid plaques and their role in the dynamics of neuronal damage in models of AD. Longitudinal in vivo imaging of APPswe/PS1d9xYFP mice (B6C3-YFP) (Jankowsky et al. 2001), which have neuronal expression of human APP, presenilin 1, and YFP, revealed that amyloid plaques (marked by methoxy-X04 labeling; Klunk et al. 2002) could form rapidly (Meyer-Luehmann et al. 2008). In order to trace occurrences within the same exact region of the brain over time, vascular and neuronal landmarks, that were distant from affected sites, were used to pinpoint the same location. Daily images of these mice revealed that plaques could be detected in a region previously devoid of plaques 24 h earlier (Meyer-Luehmann et al. 2008). These data indicate that spontaneous plaque formation is a remarkably rapid process that can occur within days rather than slowly over the course of months to years as was previously surmised. Importantly, sequential imaging of mice with fluorescently-tagged neurons (using B6C3-YFP transgenic mice) and microglia (using PDAPP+/− × CX3CR1 GFP/+ transgenic mice) demonstrated that both neurite dystrophy and microglial activation occurred after plaque formation (Meyer-Luehmann et al. 2008) and are, therefore, reactionary events rather than initiators of Aβ deposition.
In vivo imaging of established plaques and fluorescent protein-labeled neurons showed significant abnormalities in neurites and dendritic spines in close proximity to the amyloid plaques (D’Amore et al. 2003; Spires-Jones et al. 2007; Spires et al. 2005; Tsai et al. 2004). Although there is a consensus that a “halo” of toxicity surrounds the plaque, the maximal distance of neuronal alterations from the perimeter of the fibrillar deposit was about 15–50 μm (D’Amore et al. 2003; Spires et al. 2005; Tsai et al. 2004). Neuronal dystrophy, disrupted neurite trajectories, and decreased dendritic spine densities were all observed in close proximity to established plaques. Significant axonal swelling, indicative of neuronal dystrophy, was detected in 18% of hippocampal axons located near plaques (Tsai et al. 2004). Disappearance of up to ~36% of neurites was noted over a 4- to 5-week observation period (Tsai et al. 2004); however, others have suggested that neurites are not eliminated rather their trajectories near a plaque are significantly altered giving the appearance of disruption (Spires et al. 2005). Additional acquisition of the z-plane during TPLSM has revealed that neurites curve around plaques rather than penetrate through them (D’Amore et al. 2003; Spires et al. 2005). Dendritic spine densities within 15 μm of the deposit was ~38% lower than regions distal to this point, and in comparison to control animals, there was a ~54% decrease overall (Spires et al. 2005). Temporal in vivo imaging TPLSM studies revealed that reduction in the overall number of dendritic spines was due to an increase in the rate of spine elimination that outcompete the slower kinetics of spine formation (Spires-Jones et al. 2007; Tsai et al. 2004). This results in an overall decrease of spine stability in regions near the plaque. Since dendritic spines serve as postsynaptic sites for neurons, significant loss of these structures may attenuate neuronal function during AD.
The mediators that give rise to the zone of toxicity surrounding amyloid plaques are currently unknown, but the distance from the plaque suggests a role for diffusible substances, such as Aβ peptides. Another possibility is reactive oxygen species. TPLSM in vivo imaging of oxidation, using fluorescent reporter compounds, demonstrated that reactive oxygen species are associated with thioflavine S positive amyloid plaques, providing one potential mechanism for plaque toxicity (McLellan et al. 2003). The overall loss of dendritic spine stability, significant axonal dystrophy, and altered neurite projections all reflect the types of cellular alterations that may contribute to neuronal dysfunction. Further TPLSM imaging studies are required to more precisely define the toxic mediators that give rise neuronal pathology and subsequent dysfunction.
4.2 Immunity and Autoimmunity in CNS
4.2.1 Dynamics of CNS Autoimmunity
Although many aspects of EAE have been elucidated using flow cytometry, confocal microscopy, and assessment of neurological dysfunction, these methodologies cannot provide any insights into the in vivo behavior of immune cells as they contribute to autoimmune disease in the CNS. Recently, TPLSM was used to examine immune cell dynamics for the first time during EAE. To determine the motility of encephalitogenic T cells in vivo, MBP-specific CD4+ T cells expressing GFP (TMBP–GFP) were transferred into hosts to induce EAE. In vivo imaging of spinal cord explants revealed that TMBP–GFP cells fell into two distinct categories of motility: 65% of cells were highly motile, with velocities averaging 6 μm min−1, and the other 35% were stationary. In contrast, GFP+ OVA-specific T cells cotransferred with unlabeled MBP-specific T cells showed little confinement with the vast majority (~95%) exhibiting a highly motile phenotype (Kawakami et al. 2005). Confocal microscopy demonstrated the presence of synapse-like structures (marked by TCR/LFA-1 polarization) on TMBP–GFP cells, suggesting a role for cognate peptide–MHC II interactions in the CNS. Consistent with this idea, MHC II blockade resulted in a significant decrease in the percentage of stationary TMBP–GFP cells measured by TPLSM (Kawakami et al. 2005). Similarly, when TPLSM was used to examine a MOG model of EAE, T cell interactions with MHC II+ cells were observed. In this study, a significant portion of cells were found to be stationary (~94%) during the peak of disease (Elyaman et al. 2008). The discrepancy in the number of arrested T cells observed in the two models of EAE suggests motility is influenced by the amount of antigen available. Infusion of soluble MBP during acute disease resulted in a significant increase in the percentage of stationary TMBP–GFP cells, with the majority of cells arresting in the parenchyma within hours of MBP administration (Odoardi et al. 2007). This suggests that additional peptide availability generated in the MOG model by immunization may partially account for the high percentages of stationary cells.
Regardless of antigen availability, MHC II+ cells appear to play a role in both models of EAE. In the MBP model, CD45hi and CD45lo MHC II+ cells were shown to process antigen, presumably allowing for T cell reactivation in the CNS to occur on infiltrating cells or microglia, respectively (Odoardi et al. 2007). Additionally, in the MOG model, meningeal CD11b+ or CD11c+ cells were able to stimulate CD4+ T cells in vitro, confirming the stimulatory potential of these APCs (Elyaman et al. 2008). Altogether, these data clearly demonstrate that interactions between encephalitogenic T cells and MHC II+ APCs occur in the CNS, which impact T cell motility and behavior within this compartment. Peptide–MHC II stimulation of encephalitogenic CD4+ T cells within EAE lesions likely results in the reactivation, proliferation, chemokine production, and cytokine release, all culminating in the exacerbation of disease.
Further study of immune cell dynamics and EAE disease pathogenesis using TPLSM will yield additional novel insights into CNS autoimmunity. In the context of EAE, examination of 400-μm vibratome brain sections by TPLSM has facilitated the acquisition of an initial glimpse into the dynamics of T cell–neuronal interactions in a living tissue. Previously, in vitro cell culture studies have shown that T cells preferentially interact with neurites and can induce neuronal Ca2+ flux (Medana et al. 2000, 2001a, b). Consistent with these data, CMTMR-labeled T cell lines applied to brain slices penetrated into the tissue and mostly contacted neurites rather than the neuronal cell body. T cell interactions with neurons also resulted in intracellular neuronal Ca2+ oscillations that were presumed to be indicative of cell death. These results suggest that the in vitro studies on T cell–neuronal targeting reflect what is observed in ex vivo tissue preparations (Nitsch et al. 2004). However, continued examination of this and other aspects of autoimmune disease pathogenesis will need to be further validated in vivo using thinned skull surgical preparations (Xu et al. 2007) or comparable noninjurious in vivo approaches. Given the impact that a surgical preparation can have on the dynamics of cells under examination (Xu et al. 2007), it is important whenever possible to study biological processes in their natural environment. Rapid advances in noninvasive (or minimally invasive) imaging techniques should make this possible.
4.2.2 Antiviral Immunity and Pathogenesis
Understanding the dynamics of CNS antipathogen immunity and the subsequent outcomes of these responses is a fundamental step in the development of more efficacious therapeutics to treat infectious diseases of the CNS. Real time observation of CNS viral infections is particularly challenging due to the size and complexity of the CNS as well as limitations in intravital imaging approaches. Because TPLSM combined with the thinned skull surgical preparation provides minimally invasive means to examine CNS dynamics under natural conditions, we recently set out to capture the first glimpses of a virus-induced disease occurring on the surface of the brain (or meninges). Intracerebral inoculation of adult mice with LCMV induces acute meningitis that results in fatal convulsive seizures (Kang and McGavern 2008). Virus-specific CTL are known to be absolutely required for disease (McGavern and Truong 2004), but the mechanism by which they do so is unknown. To gain novel insights into the pathogenesis of virus-induced meningitis, we recently used TPLSM to first examine the dynamics of GFP-tagged virus-specific CTL in symptomatic mice at day 6 postinfection – a time point when all infected mice present with convulsive seizures (Camenga et al. 1977; Walker et al. 1977). To generate a population of traceable virus-specific CTL to study, we transferred naive DbGP33–41 CD8+ TCR transgenic T cells (P14 GFP+) intravenously into mice 1 day prior to intracerebral inoculation. This ensured that the naive precursors had the opportunity to become primed with the endogenous repertoire, transition into effector T cells, and migrate into the CNS (and other peripheral tissues) only after receiving the host-derived signals to do so.
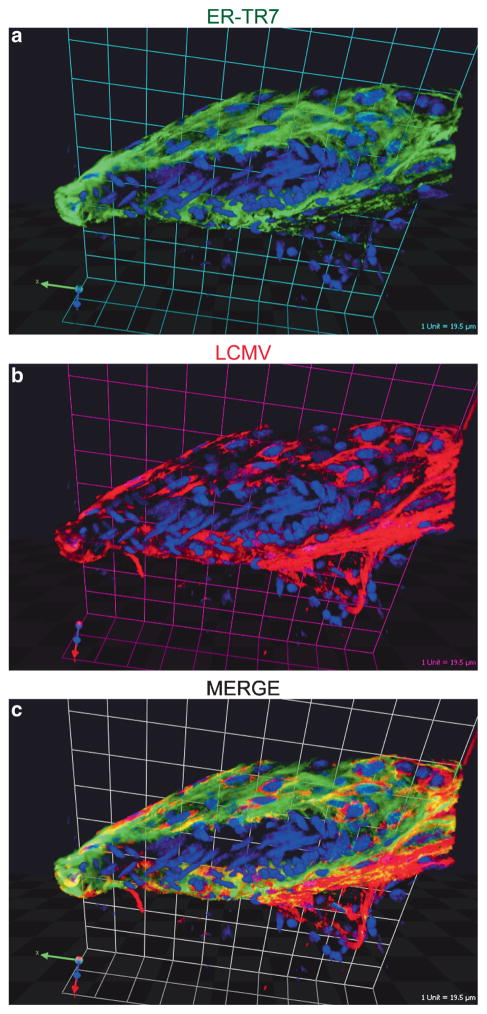
TPSLM imaging through a thinned skull viewing window at day 5 postinfection (a time point when all mice were completely asymptomatic) revealed very few CTL surveying the infected meninges and that the meningeal blood vessels were intact (Kim et al. 2008). Meningeal blood vessels were visualized by injecting quantum dots into the blood supply 10 min prior to imaging. In stark contrast to day 5 postinfection, symptomatic mice at day 6 postinfection had a massive number of highly motile CTL throughout the meninges that infrequently penetrated the brain parenchyma. 3D renderings of P14 GFP+ cells, blood vessels, and skull showed that virus-specific CTL tended to aggregate along meningeal blood vessels, which coincides with the pattern of LCMV infection in the meninges. Meningeal ER-TR7+ stromal cells were found to be the main cell population infected by LCMV on the surface of the brain (Fig. 1).
Fig. 1.
Localization of LCMV in the meninges following intracerebral inoculation. Sagittal brain vibratome sections obtained from day 6 LCMV-infected mice were stained with antibodies directed against ER-TR7 (green) and LCMV (red). ER-TR7 is a stromal cell marker that labels fibroblast-like cells. A representative 3D reconstruction of a meningeal blood vessel cross section is shown to illustrate the degree to which LCMV infects fibroblast-like cells that completely surround meningeal blood vessels. (Grid scale = 19.5 μm). Cell nuclei are shown in blue
When the dynamics of P14 CTL were quantified in the LCMV-infected meninges, surprisingly, a highly motile pattern of activity was observed (Kim et al. 2008). Meningeal CTL had an average velocity of 3.4 μm min−1, and only a minority of CTL arrested for very long, suggesting that few cells within the CNS form stable synapses during disease pathogenesis. However, direct injection of anti-MHC I antibody into the subarachnoid space revealed that most CTL interacted with MHC I during a 30-min observation period, even if only for a short time. The properties of the LCMV-infected target cell in the meninges (i.e., ER-TR7+ stromal cells) could explain paucity of CTL arrest. Stromal cells may provide strong chemotactic signals that override synapse-forming stop signals (Bromley et al. 2000), which is a possibility that is currently under investigation.
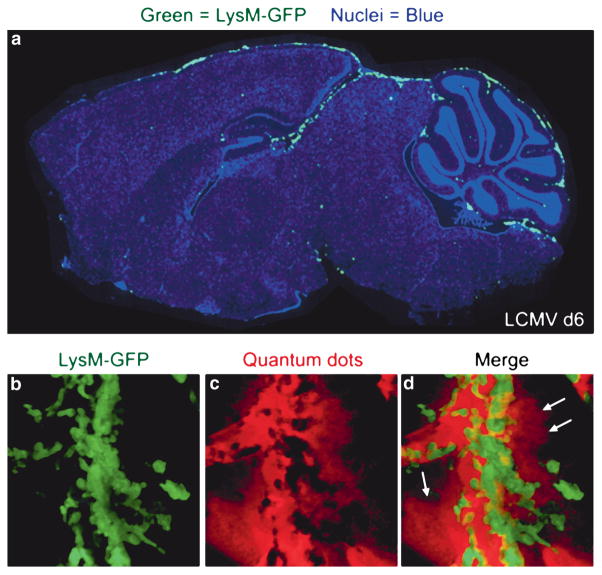
Although CTL are required for LCMV-induced meningitis, the low frequency of stable P14 CTL synapses and the inability of single deficiencies in major CTL effector mechanisms to prevent mortality (Kang and McGavern 2008; Kim et al. 2008; Nansen et al. 1998; Storm et al. 2006; Zajac et al. 2003) suggested an alternative role for CTL in pathogenesis. Analysis of other cellular infiltrates over time revealed a coordinated influx of myelomonocytic cells (i.e., monocytes and neutrophils) only on day 6 postinfection when CTL were present within the CNS (Kim et al. 2008), and these cells localized to the lining of the brain (Fig. 2). Interestingly, TPSLM imaging of myelomonocytic cells in symptomatic mice using lysozyme M-GFP (LysM-GFP) mice (Faust et al. 2000) revealed a synchronized extravasation of myelomonocytic cells from meningeal blood vessels, which resulted in a severe and sustained injury to the vessel walls (Fig. 2). Myelomonocytic cell extravasation correlated positively with sustained leakage of quantum dots from meningeal blood vessels, suggesting that these cells (not CTL) were directly responsible for the onset of fatal convulsive seizures. However, when neutrophils were depleted from mice, no alteration in disease kinetics or the onset of fatal seizures was observed. When TPSLM was again performed in LysM-GFP mice depleted of neutrophils, extravascular GFP+ cells (presumably monocytes/macrophages) were found to be associated with transient bursts of vascular leakage. Thus, in the absence of neutrophils, monocytes retained the capacity to fatally injure meningeal blood vessels. Only when neutrophils and monocytes were depleted simultaneously was survival extended and acute onset convulsive seizures prevented. These data suggest that neutrophils and monocytes share the capacity to cause vascular leakage during virus-induced meningitis and that neutrophils injure blood vessels through the process of synchronized extravasation, whereas monocytes position themselves extravascularly and mediate vascular damage through another mechanism, possibly adhesion (Ancuta et al. 2004) or chemokine release (Stamatovic et al. 2005).
Fig. 2.
Injury to meningeal blood vessels mediated by myelomonocytic cells. (a) Myelomonocytic cells (i.e., neutrophils and monocytes) were visualized by infecting lysozyme M-GFP transgenic mice intracerebrally with LCMV. The distribution of LysM-GFP+ myelomonocytic cells (green) on a sagittal brain reconstruction is shown for a representative symptomatic mouse at day 6 postinfection. Note that LysM-GFP+ cells localize primarily to the meninges and ependyma in LCMV-infected mice. Cell nuclei are shown in blue. (b–d) A representative 3D reconstruction of two-photon z-stack depicts severe injury to a meningeal blood vessel in a symptomatic LysM-GFP mouse at day 6 postinfection. Quantum dots (red) were injected intravenously to visualize vascular integrity. As LysM-GFP+ myelomonocytic cells (green) extravasate from meningeal blood vessels, they cause severe vascular leakage. Note the loss in vascular integrity and the leakage of quantum dots into the surrounding meningeal space (denoted with white arrows)
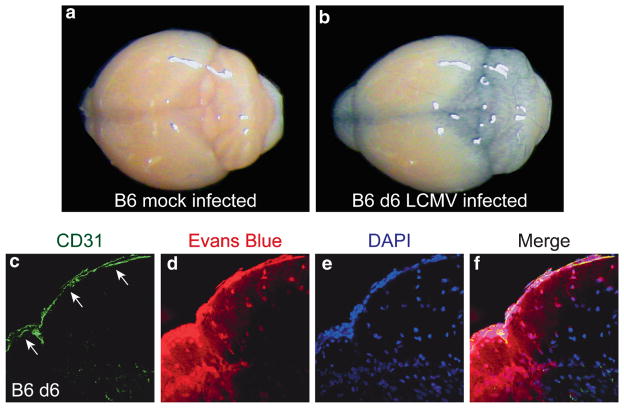
A role for seizure in mediating rapid onset death in this model is supported by a similar extension in survival observed following anticonvulsant treatment (Walker et al. 1977). It is likely that, once seizure-induced death is averted, infected mice ultimately succumb to another pathogenic mechanism possibly mediated by CTL (Camenga et al. 1977; Walker et al. 1977). To understand precisely how a meningeal disorder could trigger fatal convulsive seizures, it was important to quantify and visualize the degree to which vascular integrity was compromised in LCMV-infected mice. BBB breakdown alone was linked previously to seizure induction in a different model (Marchi et al. 2007). In symptomatic LCMV-infected mice, Evans blue injected intravenously was found leaking from meningeal blood vessels sometimes hundreds of microns down into the brain parenchyma (Fig. 3). Moreover, when quantified, only the simultaneous depletion of both monocytes and neutrophils resulted in reduced Evans blue leakage. These data suggest that injury to meningeal blood vessels mediated by an overly robust antiviral immune response has the potential to induce fatal convulsive seizures by causing blood-derived material to leak into the brain parenchyma and perturb neuronal homeostasis.
Fig. 3.
Leakage of Evans blue into the brain parenchyma of day 6 LCMV-infected mice. (a–b) To further examine vascular integrity during LCMV-induced meningitis, Evans blue dye was injected intravenously into mice. A representative brain is shown for B6 mock infected (a) and a day 6 LCMV-infected mouse (b). Note the heavy leakage of Evans blue dye (blue coloration) into the brain of the day 6 infected but not the mock-infected mouse. (c–f) The distribution of Evans blue (red) in relation to CD31+ meningeal blood vessels (green; white arrows) was examined by confocal microscopy in the brains of mock and LCMV-infected mice at day 6. Minimal to no Evans blue signal was detected in the brains of mock-infected control mice (data not shown). In contrast, severe Evans blue leakage from meningeal blood vessels into brain parenchyma was observed in day 6 LCMV-infected mice. This is indicative of blood brain barrier breakdown
It will be important to understand why CD8+ T cells recruit myelomonocytic cells to a site of viral infection. CD4+ Th17 cells recruit neutrophils in the context of extracellular bacterial infection and gut homeostasis (Weaver et al. 2007). It is unclear why CD8+ T cells would recruit myelomonocytic cells during an acute antiviral response. We observed that LCMV-specific CTL directly produced CCL-3, 4, and 5 (chemokines important in the recruitment of myelomonocytic cells), and late depletion of CD8+ T cells prevented disease onset and significantly reduced the influx of myelomonocytic cells (Kim et al. 2008). It is possible that myelomonocytic cell recruitment is part of a postresponse tissue repair process, but when CD8+ T cells are unable to engage in efficient cytotoxic synapses with ER-TR7+ stromal cells in the meninges, this repair response may itself become proinflammatory and neurotoxic. Genetic defects in selected CTL effector mechanisms result in hemophagocytic syndromes in which myelomonocytic cells engage in widespread and fatal tissue destruction (Menasche et al. 2005). Perhaps ineffective CTL-mediated killing is naturally linked to an aggressive innate response as seen in LCMV-induced meningitis. This may be why loss of additional effector mechanisms by CD8+ T cells in our genetic analyses had no impact on disease (Kim et al. 2008). In any case, recognizing the complementary pathogenic functions of neutrophils and monocytes is critical for devising therapeutic approaches in CD8+ T cell-mediated pathology.
5 Concluding Remarks
To date, visualization of CNS immunity has mostly been limited to static imagery. These studies have yielded novel insights into areas such as CNS peripheral antigen delivery, anatomical localization of leukocyte responses in the CNS, and formation of functionally relevant structures such as the immunological synapse. However, these studies have their own limitations, the most important of which is the inability to examine cellular dynamics in the living tissue. By following the methodological footprints of neuroscientists who have conducted ground-breaking research in the nervous system using intravital imaging approaches, immunologists are now poised to redefine our understanding of CNS immune responses in real time during health and disease. Recent advances in our ability to visualize CNS immunity open innumerable opportunities to study how immune cells influence CNS residents (e.g., glial cells and neurons), how pathogens are handled when they occupy different CNS niches, and how the immune system participates in pathology and repair when the CNS is faced with autoimmunity, infection, and degenerative diseases, etc. Real time imaging enables investigators to gain highly novel insights into CNS processes that could not otherwise be obtained using alternative approaches. With dynamic information in hand, existing hypotheses must often be reformulated to account for actuality. TPLSM and other noninvasive imaging approaches have the potential to provide the most comprehensive understanding to date of two highly evolved and extremely complex mammalian systems: the immune and central nervous systems. This enhanced understanding of CNS immunity should facilitate our ability to more readily purge infectious agents and thwart autoimmune and degenerative disease processes.
Acknowledgments
This work was supported by National Institutes of Health grants AI070967-01 and AI075298-01, a grant from The Burroughs Wellcome Fund, and a grant from The Ray Thomas Edwards Foundation (all to D.B.M.) S.S.K. is presently supported by a National Research Service Award (NS061447-01). We would like to thank Drs. Jiyun Kim and Michael Dustin at New York University for providing the image shown in Fig. 2b–d.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Moses A, Gabuzda D. Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology. 2004;209:11–20. doi: 10.1016/j.imbio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Germain RN. Seeing is believing: a focus on the contribution of microscopic imaging to our understanding of immune system function. Eur J Immunol. 2007;37(Suppl 1):S18–S33. doi: 10.1002/eji.200737663. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Wawrowsky K, Barrett RJ, Liu C, Castro MG, Lowenstein PR. In vivo polarization of IFN-gamma at Kupfer and non-Kupfer immunological synapses during the clearance of virally infected brain cells. J Immunol. 2008;180:1344–1352. doi: 10.4049/jimmunol.180.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, Bien CG. Astrocytes are a specific immunological target in Rasmussen’s encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard CC, de Rosbo NK. Immunopathological recognition of autoantigens in multiple sclerosis. Acta Neurol (Napoli) 1991;13:171–178. [PubMed] [Google Scholar]
- Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, Schramm J, Elger CE, Lassmann H. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Cole DF. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol. 1980;299:353–365. doi: 10.1113/jphysiol.1980.sp013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol. 1981;240:F329–F336. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol. 2000;165:15–19. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- Cabarrocas J, Bauer J, Piaggio E, Liblau R, Lassmann H. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur J Immunol. 2003;33:1174–1182. doi: 10.1002/eji.200323492. [DOI] [PubMed] [Google Scholar]
- Camenga DL, Walker DH, Murphy FA. Anticonvulsant prolongation of survival in adult murine lymphocytic choriomeningitis. I. Drug treatment and virologic studies. J Neuropathol Exp Neurol. 1977;36:9–20. doi: 10.1097/00005072-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R, Allan JE, Tabi Z, Lynch F, Doherty PC. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987;165:1539–1551. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua MM, MacNamara KC, San Mateo L, Shen H, Weiss SR. Effects of an epitope-specific CD8+ T-cell response on murine coronavirus central nervous system disease: protection from virus replication and antigen spread and selection of epitope escape mutants. J Virol. 2004;78:1150–1159. doi: 10.1128/JVI.78.3.1150-1159.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GL, Sandora MR, Nakajima A, Nguyen EV, Taylor-Edwards C, Slavin AJ, Contag CH, Fathman CG, Benson JM. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–2387. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- Cross AH, Cannella B, Brosnan CF, Raine CS. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990;63:162–170. [PubMed] [Google Scholar]
- Cross AH, O’Mara T, Raine CS. Chronologic localization of myelin-reactive cells in the lesions of relapsing EAE: implications for the study of multiple sclerosis. Neurology. 1993;43:1028–1033. doi: 10.1212/wnl.43.5.1028. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- D’Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, Hyman BT. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Denk W, Delaney KR, Gelperin A, Kleinfeld D, Strowbridge BW, Tank DW, Yuste R. Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy. J Neurosci Methods. 1994;54:151–162. doi: 10.1016/0165-0270(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- Elyaman W, Kivisakk P, Reddy J, Chitnis T, Raddassi K, Imitola J, Bradshaw E, Kuchroo VK, Yagita H, Sayegh MH, Khoury SJ. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am J Pathol. 2008;173:411–422. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Flugel A, Willem M, Berkowicz T, Wekerle H. Gene transfer into CD4+ T lymphocytes: green fluorescent protein-engineered, encephalitogenic T cells illuminate brain autoimmune responses. Nat Med. 1999;5:843–847. doi: 10.1038/10567. [DOI] [PubMed] [Google Scholar]
- Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Fung-Leung WP, Kundig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gairin JE, Mazarguil H, Hudrisier D, Oldstone MB. Optimal lymphocytic choriomeningitis virus sequences restricted by H-2Db major histocompatibility complex class I molecules and presented to cytotoxic T lymphocytes. J Virol. 1995;69:2297–2305. doi: 10.1128/jvi.69.4.2297-2305.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A, Hombach J, Dumrese T, Rammensee HG, Zinkernagel RM, Hengartner H. A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells. Eur J Immunol. 1998;28:3301–3311. doi: 10.1002/(SICI)1521-4141(199810)28:10<3301::AID-IMMU3301>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–379. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Targoni OS, Karulin AY, Forsthuber TG, Tary-Lehmann M, Lehmann PV. Does the frequency and avidity spectrum of the neuroantigen-specific T cells in the blood mirror the autoimmune process in the central nervous system of mice undergoing experimental allergic encephalomyelitis? J Immunol. 2005;174:4598–4605. doi: 10.4049/jimmunol.174.8.4598. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Toyka KV, Tary-Lehmann M, Lehmann PV. Kinetics and organ distribution of IL-17-producing CD4 cells in proteolipid protein 139–151 peptide-induced experimental autoimmune encephalomyelitis of SJL mice. J Immunol. 2007;178:1372–1378. doi: 10.4049/jimmunol.178.3.1372. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JV, Batchelder KF, Lo EH, Wolf GL. Imaging techniques for in vivo quantitation of extracranial lymphatic drainage of the brain. Neuropathol Appl Neurobiol. 1995;21:185–188. doi: 10.1111/j.1365-2990.1995.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- Iannetti P, Nigro G, Imperato C. Cytomegalovirus encephalitis and ganciclovir. Lancet. 1991;337:373. doi: 10.1016/0140-6736(91)91013-k. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- Joly E, Oldstone MB. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, McGavern DB. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci. 2008;13:4529–4543. doi: 10.2741/3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004a;92:107–115. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004b;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Lassmann S, Li Z, Odoardi F, Ritter T, Ziemssen T, Klinkert WE, Ellwart JW, Bradl M, Krivacic K, Lassmann H, Ransohoff RM, Volk HD, Wekerle H, Linington C, Flugel A. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Milo R, Lees MB, Burger D, Bernard CC, Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92:2602–2608. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Denk W. Imaging in vivo: watching the brain in action. Nat Rev Neurosci. 2008;9:195–205. doi: 10.1038/nrn2338. [DOI] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kida S, Weller RO, Zhang ET, Phillips MJ, Iannotti F. Anatomical pathways for lymphatic drainage of the brain and their pathological significance. Neuropathol Appl Neurobiol. 1995;21:181–184. doi: 10.1111/j.1365-2990.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Kim JV, Dustin ML. Innate response to focal necrotic injury inside the blood-brain barrier. J Immunol. 2006;177:5269–5277. doi: 10.4049/jimmunol.177.8.5269. [DOI] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Abeta plaques in living transgenic mice with multi-photon microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Levite M, Fleidervish IA, Schwarz A, Pelled D, Futerman AH. Autoantibodies to the glutamate receptor kill neurons via activation of the receptor ion channel. J Autoimmun. 1999;13:61–72. doi: 10.1006/jaut.1999.0301. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. 2006;26:731–741. doi: 10.1523/JNEUROSCI.3502-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Verbny YI, Banks MI, Sandor M, Fabry Z. In situ activation of antigen-specific CD8(+) T cells in the presence of antigen in organotypic brain slices. J Immunol. 2008;180:8393–8399. doi: 10.4049/jimmunol.180.12.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo L, Gegundez MI, Saz JV, Bahamontes N, Beltran M. Lymphocytic choriomeningitis virus infection in a province of Spain: analysis of sera from the general population and wild rodents. J Med Virol. 2003;70:273–275. doi: 10.1002/jmv.10389. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PT, Johnson EM, Jr, Wilcox CL, Palmatier MA, Russell JH. MHC-specific cytotoxic T lymphocyte killing of dissociated sympathetic neuronal cultures. Am J Pathol. 1987;128:395–409. [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002a;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Homann D, Oldstone MB. T cells in the central nervous system: the delicate balance between viral clearance and disease. J Infect Dis. 2002b;186(Suppl 2):S145–S151. doi: 10.1086/344264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Truong P. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J Immunol. 2004;173:4779–4790. doi: 10.4049/jimmunol.173.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- McPherson SW, Heuss ND, Roehrich H, Gregerson DS. Bystander killing of neurons by cytotoxic T cells specific for a glial antigen. Glia. 2006;53:457–466. doi: 10.1002/glia.20298. [DOI] [PubMed] [Google Scholar]
- McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Gallimore A, Oxenius A, Martinic MM, Wekerle H, Neumann H. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur J Immunol. 2000;30:3623–3633. doi: 10.1002/1521-4141(200012)30:12<3623::AID-IMMU3623>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Medana I, Li Z, Flugel A, Tschopp J, Wekerle H, Neumann H. Fas ligand (CD95L) protects neurons against perforin-mediated T lymphocyte cytotoxicity. J Immunol. 2001a;167:674–681. doi: 10.4049/jimmunol.167.2.674. [DOI] [PubMed] [Google Scholar]
- Medana I, Martinic MA, Wekerle H, Neumann H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol. 2001b;159:809–815. doi: 10.1016/S0002-9440(10)61755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche G, Feldmann J, Fischer A, de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev. 2005;203:165–179. doi: 10.1111/j.0105-2896.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, Campbell IL. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin X, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998a;18:7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998b;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Kostinger G, Anderson JC, Martin KA, Bonhoeffer T. Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J Neurosci. 2007;27:8149–8156. doi: 10.1523/JNEUROSCI.0511-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Gill ED, Shu CJ, Radu CG, Witte ON. Non-invasive imaging of adaptive immunity using positron emission tomography. Immunol Rev. 2008;221:214–228. doi: 10.1111/j.1600-065X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Nansen A, Christensen JP, Ropke C, Marker O, Scheynius A, Thomsen AR. Role of interferon-gamma in the pathogenesis of LCMV-induced meningitis: unimpaired leucocyte recruitment, but deficient macrophage activation in interferon-gamma knock-out mice. J Neuroimmunol. 1998;86:202–212. doi: 10.1016/s0165-5728(98)00055-1. [DOI] [PubMed] [Google Scholar]
- Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Pohl EE, Smorodchenko A, Infante-Duarte C, Aktas O, Zipp F. Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J Neurosci. 2004;24:2458–2464. doi: 10.1523/JNEUROSCI.4703-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoardi F, Kawakami N, Klinkert WE, Wekerle H, Flugel A. Blood-borne soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease. Proc Natl Acad Sci USA. 2007;104:18625–18630. doi: 10.1073/pnas.0705033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Thams S, Lidman O, Piehl F, Hokfelt T, Karre K, Linda H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci USA. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Pan F, Gan WB. Two-photon imaging of dendritic spine development in the mouse cortex. Dev Neurobiol. 2008;68:771–778. doi: 10.1002/dneu.20630. [DOI] [PubMed] [Google Scholar]
- Pautler RG. Mouse MRI: concepts and applications in physiology. Physiology (Bethesda) 2004;19:168–175. doi: 10.1152/physiol.00016.2004. [DOI] [PubMed] [Google Scholar]
- Peri F, Nusslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Pirko I, Ciric B, Johnson AJ, Gamez J, Rodriguez M, Macura S. Magnetic resonance imaging of immune cells in inflammation of central nervous system. Croat Med J. 2003;44:463–468. [PubMed] [Google Scholar]
- Pirko I, Johnson A, Ciric B, Gamez J, Macura SI, Pease LR, Rodriguez M. In vivo magnetic resonance imaging of immune cells in the central nervous system with superparamagnetic antibodies. FASEB J. 2004;18:179–182. doi: 10.1096/fj.02-1124fje. [DOI] [PubMed] [Google Scholar]
- Pirko I, Fricke ST, Johnson AJ, Rodriguez M, Macura SI. Magnetic resonance imaging, microscopy, and spectroscopy of the central nervous system in experimental animals. NeuroRx. 2005;2:250–264. doi: 10.1602/neurorx.2.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel TD, Krause BJ. Functional imaging of memory processes in humans: positron emission tomography and functional magnetic resonance imaging. Methods. 2008;44:315–328. doi: 10.1016/j.ymeth.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Power C, Poland SD, Blume WT, Girvin JP, Rice GP. Cytomegalovirus and Rasmussen’s encephalitis. Lancet. 1990;336:1282–1284. doi: 10.1016/0140-6736(90)92965-k. [DOI] [PubMed] [Google Scholar]
- Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/wnl.8.6.435. [DOI] [PubMed] [Google Scholar]
- Rensing-Ehl A, Malipiero U, Irmler M, Tschopp J, Constam D, Fontana A. Neurons induced to express major histocompatibility complex class I antigen are killed via the perforin and not the Fas (APO-1/CD95) pathway. Eur J Immunol. 1996;26:2271–2274. doi: 10.1002/eji.1830260945. [DOI] [PubMed] [Google Scholar]
- Rivera-Quinones C, McGavern D, Schmelzer JD, Hunter SF, Low PA, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–193. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau JV, Piston DW. Two-photon excitation microscopy for the study of living cells and tissues. Curr Protoc Cell Biol. 2003;Chap 4(Unit 4.11) doi: 10.1002/0471143030.cb0411s20. [DOI] [PubMed] [Google Scholar]
- Roebroek RM, Postma BH, Dijkstra UJ. Aseptic meningitis caused by the lymphocytic choriomeningitis virus. Clin Neurol Neurosurg. 1994;96:178–180. doi: 10.1016/0303-8467(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, Hughes TE, Heinemann SF, McNamara JO. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science. 1994;265:648–651. doi: 10.1126/science.8036512. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz M, Wilden L, Muller W, Stenzel W, Brunn A, Miletic H, Schluter D, Deckert M. Molecular mimicry between neurons and an intracerebral pathogen induces a CD8 T cell-mediated autoimmune disease. J Immunol. 2008;180:8421–8433. doi: 10.4049/jimmunol.180.12.8421. [DOI] [PubMed] [Google Scholar]
- Schanen A, Gallou G, Hincky JM, Saron MF. A rash, circulating anticoagulant, then meningitis. Lancet. 1998;351:1856. doi: 10.1016/S0140-6736(98)02207-7. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schwendemann G, Lohler J, Lehmann-Grube F. Evidence for cytotoxic T-lymphocyte-target cell interaction in brains of mice infected intracerebrally with lymphocytic choriomeningitis virus. Acta Neuropathol. 1983;61:183–195. doi: 10.1007/BF00691984. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol. 2007;81:11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffrin V, Brandt AU, Herz J, Zipp F. New insights into adaptive immunity in chronic neuroinflammation. Adv Immunol. 2007;96:1–40. doi: 10.1016/S0065-2776(07)96001-0. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Haase AT. In situ staining using MHC class I tetramers. Curr Protoc Immunol. 2005;Chap 17(Unit 17.4) doi: 10.1002/0471142735.im1704s64. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stearns T. Green fluorescent protein. The green revolution. Curr Biol. 1995;5:262–264. doi: 10.1016/s0960-9822(95)00056-x. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Storm P, Bartholdy C, Sorensen MR, Christensen JP, Thomsen AR. Perforin-deficient CD8+ T cells mediate fatal lymphocytic choriomeningitis despite impaired cytokine production. J Virol. 2006;80:1222–1230. doi: 10.1128/JVI.80.3.1222-1230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Forbes A, Gambhir SS, Braun J. Quantitation of cell number by a positron emission tomography reporter gene strategy. Mol Imaging Biol. 2004;6:139–148. doi: 10.1016/j.mibio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Sun JB, Olsson T, Wang WZ, Xiao BG, Kostulas V, Fredrikson S, Ekre HP, Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991;21:1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246:F835–F844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Tuohy VK, Yu M, Yin L, Kawczak JA, Kinkel RP. Spontaneous regression of primary autoreactivity during chronic progression of experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 1999;189:1033–1042. doi: 10.1084/jem.189.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Gahring LC, Spiess J, Rogers SW. Glutamate receptor antibodies activate a subset of receptors and reveal an agonist binding site. Neuron. 1995;14:755–762. doi: 10.1016/0896-6273(95)90219-8. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Murali-Krishna K, Whitton JL, Oseroff C, Alexander J, Southwood S, Sidney J, Chesnut RW, Sette A, Ahmed R. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology. 1998;240:158–167. doi: 10.1006/viro.1997.8934. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Wang R, Wiley CA. Herpesviruses in chronic encephalitis associated with intractable childhood epilepsy. Hum Pathol. 1993;24:871–879. doi: 10.1016/0046-8177(93)90137-6. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Walker DH, Camenga DL, Whitfield S, Murphy FA. Anticonvulsant prolongation of survival in adult murine lymphocytic choriomeningitis. II. Ultrastructural observations of pathogenetic events. J Neuropathol Exp Neurol. 1977;36:21–40. doi: 10.1097/00005072-197701000-00004. [DOI] [PubMed] [Google Scholar]
- Walter L, Albert ML. Cutting edge: cross-presented intracranial antigen primes CD8+ T cells. J Immunol. 2007;178:6038–6042. doi: 10.4049/jimmunol.178.10.6038. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Cheng HC, Bogdanova A, Bogdanov A., Jr Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging. 1997;7:258–263. doi: 10.1002/jmri.1880070140. [DOI] [PubMed] [Google Scholar]
- Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6:275–288. doi: 10.1111/j.1750-3639.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Kay SA. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005;16:73–78. doi: 10.1016/j.copbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Whitney KD, McNamara JO. GluR3 autoantibodies destroy neural cells in a complement-dependent manner modulated by complement regulatory proteins. J Neurosci. 2000;20:7307–7316. doi: 10.1523/JNEUROSCI.20-19-07307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci U S A. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Wucherpfennig KW, Ota K, Endo N, Seidman JG, Rosenzweig A, Weiner HL, Hafler DA. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990;248:1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TC, Zhang W, Ildstad ST, Ho C. Intracellular labeling of T-cells with superparamagnetic contrast agents. Magn Reson Med. 1993;30:617–625. doi: 10.1002/mrm.1910300513. [DOI] [PubMed] [Google Scholar]
- Yeh TC, Zhang W, Ildstad ST, Ho C. In vivo dynamic MRI tracking of rat T-cells labeled with superparamagnetic iron-oxide particles. Magn Reson Med. 1995;33:200–208. doi: 10.1002/mrm.1910330209. [DOI] [PubMed] [Google Scholar]
- Yu M, Johnson JM, Tuohy VK. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura M, Takahashi I, Serada M, Koshio T, Nakagami K, Yuki Y, Kiyono H. Role of MOG-stimulated Th1 type “light up” (GFP+) CD4+ T cells for the development of experimental autoimmune encephalomyelitis (EAE) J Autoimmun. 2001;17:17–25. doi: 10.1006/jaut.2001.0520. [DOI] [PubMed] [Google Scholar]
- Zabow G, Dodd S, Moreland J, Koretsky A. Micro-engineered local field control for high-sensitivity multispectral MRI. Nature. 2008;453:1058–1063. doi: 10.1038/nature07048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Dye JM, Quinn DG. Control of lymphocytic choriomeningitis virus infection in granzyme B deficient mice. Virology. 2003;305:1–9. doi: 10.1006/viro.2002.1754. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Cytotoxic thymus-derived lymphocytes in cerebrospinal fluid of mice with lymphocytic choriomeningitis. J Exp Med. 1973;138:1266–1269. doi: 10.1084/jem.138.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005a;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005b;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]