Abstract
Background
It has been well-documented that the level of serum/plasma free triiodothyronine (fT3) falls rapidly following brain death or during certain surgical procedures, e.g., heart surgery carried out on cardiopulmonary bypass. The level in patients following cardiopulmonary bypass usually recovers within 2 days.
Methods
We have measured serum fT3 in healthy naïve baboons (n=31), healthy naïve monkeys (n=5), and after pig-to-baboon heterotopic heart xenotransplantation (xenoTx) (Group1, n=9), orthotopic liver xenoTx (Group2, n=10), artery patch xenoTx (Group3, n=9), and in monkey-to-monkey heterotopic heart alloTx (Group4, n=5).
Results
The mean level of fT3 in healthy naïve baboons was 3.1±0.9pg/mL and in healthy naïve monkeys was 2.6±0.3pg/mL. Following pig heart, liver, and artery patch xenoTx and monkey heart alloTx, there was an immediate rapid fall in fT3 level. Recovery of fT3 was more rapid in Groups 3 and 4 than in Groups 1 and 2. In Group1, within 4 days fT3 had recovered, but only to the lower limit of normal range, where it remained throughout follow-up (for up to 42 days). In Group2, no recovery was seen during the 7 days of follow-up. In immunosuppressed baboons with pig patch grafts that received IL-6R blockade (n=2), the fT3 tended to rise higher than in those that received no IL-6R blockade (n=6).
Conclusions
Following operative procedures, there is a dramatic fall in serum fT3 levels. The persistent low level of fT3 after pig heart and liver xenoTx may be associated with a continuing inflammatory state. We suggest consideration should be given to the replacement of T3 therapy to maintain normal fT3 levels, particularly in nonhuman primates undergoing orthotopic pig heart or liver xenoTx.
Keywords: Euthyroid sick syndrome; Inflammation; Nonhuman primates; Pigs, genetically-engineered; Thyroid hormone; Xenotransplantation
INTRODUCTION
In acute or chronic stress conditions (medical, surgical, or traumatic), a rapid decline in serum/plasma thyroid hormone levels (free triiodothyronine [fT3] and free levothyroxine [fT4]) is recognized as the euthyroid sick syndrome (ESS) or low-T3 syndrome (1-3). In critically ill patients, the magnitude of reduction in fT3/fT4 has been shown to correlate with patient mortality (2). In these patients, fT4 is mainly converted to reverse T3 (rT3), and so the level of fT3 falls. The higher the rT3/fT3 ratio, the greater is the patient mortality.
The ESS is also seen in brain-dead potential organ donors (4) and patients on cardiopulmonary bypass (5-7). The ESS is often associated with depressed myocardial function, sometimes referred to as the ‘stunned myocardium’.
Thyroid hormones play an important role in mitochondrial function (generation of high-energy phosphates and prevention of tissue lactic acidosis) and intracellular homeostasis of calcium (8,9). The effect of T3/T4 therapy has been studied under conditions in which the ESS and myocardial functional depression have been documented. For example, in studies of brain-dead potential organ donors, T3 replacement therapy was associated with significant improvement in myocardial energy stores and hemodynamic status. There was an increased percentage of organs recovered from brain-dead donors treated with T3 hormone, which was also followed by excellent organ function in the recipients (8,10,11).
Following the administration of T3 hormone to brain-dead donors, adenosine phosphate and creatine phosphate recovered to normal levels, lactate was reduced, and glycogen levels improved. These changes were associated with improved cardiac output and stroke volume (8,12,13). In patients undergoing cardiac surgery on cardiopulmonary bypass, myocardial dysfunction rapidly improved following thyroid replacement therapy, allowing all forms of myocardial support to be discontinued or significantly reduced (7,14).
The primary aim of the present study was to measure fT3 levels in baboon recipients of pig heart, liver, or artery patch xenografts to determine whether the operative procedure and/or the inflammatory state that occurs after xenotransplantation (xenoTx) are reflected in low fT3 levels. These data would provide guidance on whether T3 replacement therapy might be indicated after pig organ xenotransplantation, particularly after orthotopic pig heart xenoTx.
MATERIALS AND METHODS
Animals
Baboons (Papio species, n=31; Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK) weighing 6-10kg and of various AB blood groups were sources of blood and recipients of pig hearts or artery patch grafts.
Genetically-engineered pigs (Revivicor, Blacksburg, VA), weighing 7-30kg, all of non-A(O) blood group, served as sources of hearts, livers, or carotid artery patch grafts (Table1) (15-19). Four baboons received heterotopic hearts from α1,3-galactosyltransferase gene-knockout (GTKO) pigs expressing two human complement-regulatory proteins (CD46/CD55), and five from GTKO/CD46 pigs expressing one or three human coagulation-regulatory proteins (GTKO/CD46/thrombomodulin [TBM] [n=3] or GTKO/CD46/CD55/TBM/ endothelial cell protein C receptor [EPCR] /CD39 [n=2]) (18,20) (Table1). Baboons (n=10) also received orthotopic liver grafts from GTKO or GTKO/CD46 pigs (21). Carotid artery patches were taken from GTKO/CD46 pigs or GTKO/CD46 pigs expressing a mutant human MHC class II transactivator (CIITA) (17,20,22) (Table1).
Table 1.
Experimental groups
| GROUP | PIG | MAINTENANCE IMMUNOSUPPRESSIVE THERAPY |
FOLLOW-UP (Days) |
|---|---|---|---|
| 1.HEART (Heterotopic) XENOTX | |||
| (n=9) | GTKO/hCRP+/−hCoagRP | CTLA4-Ig+/−anti-CD40mAb- or anti-CD154mAb-based (18) |
15-130 |
|
| |||
| 2.LIVER (Orthotopic) XENOTX | |||
| (n=10) | GTKO+/−CD46 | ATG or cyclophosphamide/tacrolimus /mycophenolate mofetil/corticosteroids (21) |
<7 |
|
| |||
| 3.ARTERY PATCH XENOTX | |||
| (n=8) | GTKO/hCRP+/−CIITA | CTLA4-Ig+/−anti-CD40mAb- or anti-CD40mAb-based (17) |
28-84 |
| (n=1) | GTKO/hCRP | None | 49 |
|
| |||
| 4.HEART (Heterotopic) ALLOTX | |||
| (n=5) | ATG/tacrolimus/rapamycin (n=4) | ||
| Alemtuzumab/mycophenolate mofetil/ rapamycin (23) (n=1) |
14 | ||
GTKO/hCRP = α1,3-galactosyltransferase gene-knockout pigs transgenic for at least one human complement-regulatory protein (CD46 and/or CD55).
GTKO/hCRP/hCoagRP = α1,3-galactosyltransferase gene-knockout pigs transgenic for one human complement-regulatory protein (CD46) and at least one human coagulation-regulatory protein (e.g., thrombomodulin).
CIITA = mutant human MHC class II transactivator knock-down.
Anti-CD40 (2C10R4) and anti-CD154 mAb were kindly provided by Dr. Keith Reimann through the NIH NHP Reagent Resource.
Cynomolgus monkeys (Macaca fascicularis, n=5, Alpha Genesis, Yemassee, SC), weighing 3-5kg, were recipients of ABO-compatible heterotopic heart allografts, as previously reported (23) (Table1).
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Heart, liver, or artery patch transplantation
Anesthesia, intravascular line placement in baboons and monkeys, and pig-to-baboon heterotopic heart (24,25), orthotopic liver (21), and artery patch (26) xenoTx, and monkey alloTx (23) have been described previously.
Immunosuppressive and anti-inflammatory therapy
For the heart or artery patch xenoTx experiments, immunosuppressive therapy was based on costimulation blockade using either (i) anti-CD154mAb or (ii) CTLA4-Ig +/− anti-CD40mAb (2C10R4) or (iii) anti-CD40mAb (17-19,21) (Table1). Two immunosuppressed baboons with pig artery patch grafts also received IL-6R blockade (tocilizumab, [10mg/kg] Actemra, Genentech, South San Francisco, CA). In the liver xenoTx (21), immunosuppressive therapy was based on induction with ATG or cyclophosphamide (+ tacrolimus/ mycophenolate mofetil/ corticosteroids). In heart alloTx (23) experiments, immunosuppressive therapy was based on ATG (+ tacrolimus / rapamycin) or alemtuzumab (+ mycophenolate mofetil / rapamycin).
Measurement of serum free triiodothyronine (fT3)
Serum samples were obtained from all recipients before and from some at intervals after Tx. The blood level of fT3 was measured by Antech Diagnostics (Southaven, MS). A total of 191 serum samples were collected from 31 baboons and 5 monkeys. The blood level of rT3 was measured by Quest Diagnostics, Pittsburgh, PA. Only 10 sera (from 5 baboons – heart 2, liver 1, artery patch 2) were available in sufficient quantity for rT3 measurement.
Statistical analysis
Statistical analysis was performed using social sciences software GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data are presented as mean and standard deviation (SD) for all variables. Significance of the difference between two groups was determined by paired Student t test or Mann-Whitney test. A p value of <0.05 was considered statistically significant.
RESULTS
Clinical course and graft survival
Baboons with pig heart xenoTx (Group1) were euthanized for either graft failure (n=3) or for complications of immunosuppressive therapy (n=5) or indwelling vascular catheters (n=1) between 2 and 18 weeks post-Tx (Table1), but serum fT3 levels were only measured during the first 6 weeks. Baboons with pig liver grafts (Group2) were euthanized or died from complications of thrombocytopenia within 7 days (21). Baboons with pig carotid artery patch xenoTx (Group3) were electively euthanized 28, 49, or 84 days post-Tx. Monkeys with heart alloTx (Group4) were euthanized after graft failure between 1 and 3 months post-Tx, but fT3 levels were only measured during the first 14 days (23).
Free triiodothyronine (fT3)
The levels of fT3 in healthy naïve baboons (n=31) ranged from 2.2-4.0pg/mL (mean 3.1pg/mL) and in healthy naïve monkeys (n=5) from 2.3-2.9pg/mL (mean 2.6pg/mL).
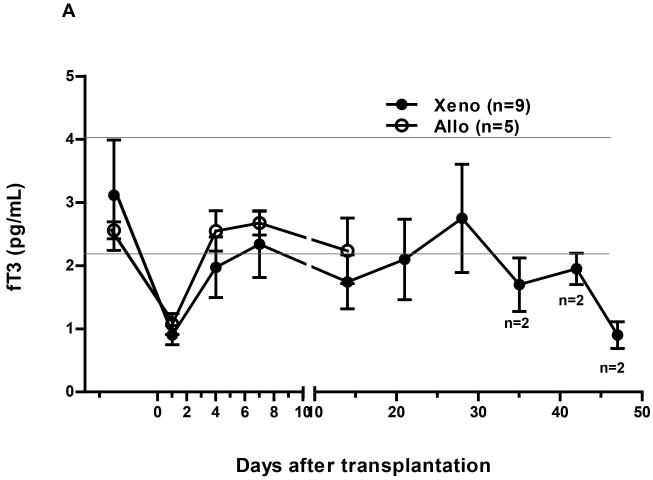
In Group1, following pig heterotopic heart xenoTx there was a rapid fall of fT3 within 4h to 0.9pg/mL, a reduction of 66% from pre-Tx (p<0.01) (Figure1A). This level remained unchanged (0.9pg/mL) at 24h. The fT3 level recovered to just below the normal range (approximately 2.0pg/mL) within 4 days, but tended to fall when either a systemic infectious complication was suspected or histopathological features of a thrombotic microangiopathy occurred in the graft.
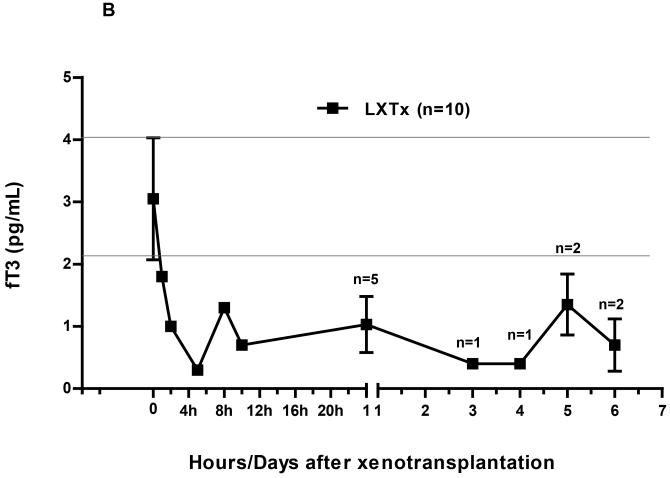
Figure 1.
Changes in fT3 levels in nonhuman primate recipients following (A) heterotopic heart grafts (xeno or allo) or (B) orthotopic liver xenotransplantation (Tx).
(A) In both xeno- and allo-heart recipients, there was a rapid fall of fT3 level to 0.9pg/mL (in xenoTx) and 1.1pg/mL (in alloTx), respectively, within 24 hours (p<0.01; p<0.05, respectively). Within 4 days after Tx, the level of fT3 had recovered after heart xenoTx to just below the lower limit of normal range, but to a higher level after alloTx. Reductions in the level of fT3 were observed in baboons that were suspected of developed a systemic infection, or had histopathological features of a thrombotic microangiopathy.
(B) After liver xenoTx, there was a rapid fall of fT3 to 0.3pg/mL within 4hours with little recovery during the 7 days of follow-up. The horizontal lines indicate the normal range of fT3 in healthy naïve baboons.
In Group2, following orthotopic pig liver xenoTx there was a rapid fall of fT3 within 4h to 0.3pg/mL, a reduction of 90% from pre-Tx (Figure1B). There was no recovery during the 7 days of follow-up.
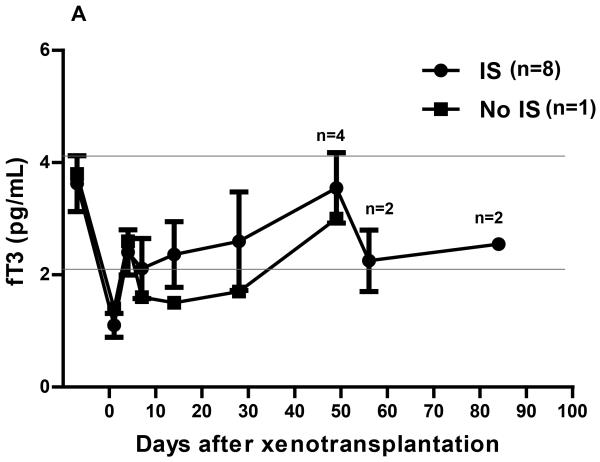
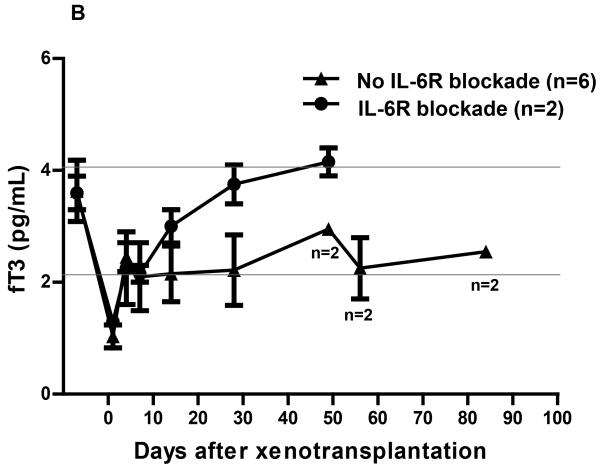
In Group3, following pig artery patch xenoTx, the fT3 fell to 1.1pg/mL at 24h, a reduction of 69% from preTx (p<0.01), recovering to 2.4pg/mL by day 4, and remaining in the normal or near-normal range thereafter (Figure2A). In one baboon that did not receive any immunosuppressive therapy, the fT3 level tended to remain rather lower during the first month than in the 8 baboons that received immunosuppressive therapy (Figure2A). After the first two weeks, the fT3 level in two immunosuppressed baboons that received IL-6R blockade tended to be higher than in the baboons that did not receive IL-6R blockade, though, in view of the small numbers, the statistical significance was not calculated (Figure2B). After the initial fall, the mean fT3 level in the baboon recipients of pig artery patch grafts tended to be higher than in the baboon recipients of pig hearts (p<0.05).
Figure 2.
Changes of fT3 levels in baboon recipients following (A) pig artery patch (with or without immunosuppressive therapy [IS]), or (B) pig artery patch xenotransplantation (with or without receiving IL-6R blockade).
(A) In immunosuppressed baboon recipients of pig artery patch grafts, mean fT3 level markedly decreased from 3.6pg/mL pre-Tx to 1.1pg/mL (p<0.01) within 24h. Within 4 days, it had recovered to within the normal range (mean 2.4pg/mL) where it remained through follow-up. In the one baboon that received no immunosuppressive therapy, the fT3 level tended to remain rather lower during follow-up (though not significantly different).
(B) A rapid fall and recovery of fT3 was observed after pig artery patch Tx in immunosuppressed baboons, but the fT3 levels recovered more rapidly in baboons receiving IL-6R blockade. When IL-6R blockade was not administered, the recovery of fT3 tended to be slower. The horizontal lines indicate the normal range of fT3 in healthy naïve baboons.
In Group4, the mean pre-Tx level of fT3 in monkeys was 2.6pg/mL, with a range from 2.3 to 2.9pg/mL (Figure1A). Following cardiac alloTx, the level fell to 1.1pg/mL at 24h, a reduction of 60% from pre-Tx (p<0.05), recovering to 2.6pg/mL by day 4, where it remained for the 14 days of follow-up.
Reverse T3 (rT3)
“Sera from 5 baboons were available for testing pre-transplant and one day post-transplant. The mean rT3 level pre-transplant was 13ng/dL (median 14ng/dL), but by post-transplant day 1 had significantly risen to a mean of 43ng/dL (median 27ng/dL) (p<0.01).”
DISCUSSION
The cause of the rapid fall in fT3 (resulting in the ESS) in brain-dead potential organ donors and after cardiopulmonary bypass is uncertain, but is associated with a generalized inhibition of mitochondrial function, which results in the loss of myocardial energy stores and diminished cardiac function (8). Deterioration of cardiac function after brain death or after open heart surgery can be reversed by the administration of T3 or T4 (2,7,8,11,14). This results in rapid replenishment of myocardial energy stores and improvement in cardiac output (8).
The ESS is frequently associated with the presence of high levels of plasma and/or tissue catecholamines (27), but may also be associated with an inflammatory response, although its cause may be multifactorial (2). We have shown that systemic inflammation in xenograft recipients (SIXR) (26,28) is associated with failure of an organ xenograft.
In the present studies, the fT3 level in healthy naïve baboons varied between 2.2 and 4.0pg/mL (mean 3.1pg/mL). In healthy naïve monkeys, the fT3 level was similar (mean 2.6pg.mL) to that of healthy naïve baboons. In all four groups, following xeno- or alloTx, there was a rapid decline of fT3 within hours, presumably a response to the stress of the procedure. Following pig heart xenoTx (Group1), fT3 recovered slowly and only to the low-normal range, where it remained throughout follow-up (42 days). It was also observed that there was a reduction in fT3 whenever a systemic infection was suspected or when thrombotic microangiopathy was developing in the graft, suggesting that monitoring of fT3 might prove to be a non-specific indicator of an inflammatory state associated with an infectious complication or graft failure, though further observations are required in this respect. In monkeys with heart alloTx (Group4), recovery of fT3 to normal levels was rather better sustained.
Following pig orthotopic liver xenoT (Group2), the reduction in fT3 was rather greater, and there was no significant recovery during the 7 days of follow-up (during which period a profound thrombocytopenia persisted). In immunosuppressed baboon recipients of pig artery patch xenoTx (Group3), the fT3 level recovered to a higher level than in the baboons with heart or liver grafts, though it took slightly longer to recover in the one baboon that did not receive immunosuppressive therapy. The addition of IL-6R blockade also appeared to be associated with a quicker recovery of fT3 levels and a higher sustained level.
Although the number of serum samples we were able to test was small, there was a reciprocal rise in rT3 when fT3 fell, as anticipated from previous studies of ESS by others (reviewed in 13).
We have also monitored serum fT3 levels in one baboon undergoing life-supporting pig kidney xenoTx. The pattern of events was similar to that in the baboons after heart xenoTx. The fT3 level rapidly fell from a pre-Tx level of 3.0pg/mL to 1.2pg/mL within 24h (a reduction of 60%) and remained low at 4 days (0.9pg/mL) (not shown). By 7 days, however, it had recovered to the lower limit of the normal range. We have also observed a significant fall in fT3 levels in baboons with major immunological or infectious complications, suggesting that changes in fT3 could be a useful marker for serious complications, e.g., impending graft failure.
Our experiments suggest that (i) pig heart, liver, kidney, or artery patch xenoTx is associated with a significant reduction in fT3, which (by correlating this observation with previous studies) might result in reduced myocardial energy stores and hemodynamic status; (ii) following heart Tx recovery of fT3 occurs within 4 days, but the level in baboons with heart xenografts remains lower when compared to monkeys with heart allografts or baboons with pig artery patch grafts; (iii) recovery of fT3 to the normal range did not occur in baboons with orthotopic pig liver grafts (during the 7 days of follow-up); (iv) the persisting lower levels of fT3 following heart or liver xenoTx may be associated with a continuing inflammatory state associated with the presence of the pig graft (2,26,28).
In our previous studies, we demonstrated that C-reactive protein (CRP) levels rose immediately after pig artery patch or heart xenoTx in baboons, and that the high level was sustained throughout the period of follow-up (26). This suggests that, even after pig artery patch xenoTx, inflammation was induced throughout the post-Tx course (with follow-up for up to 84 days). The present study indicates that the rise in CRP is associated with a sustained reduction in fT3, which we suggest is also associated with the inflammatory state.
Among multiple markers of inflammation, tumor necrosis factor-alpha (TNF-α) may participate in the mediation of the ESS. For example, the infusion of TNF-α in humans results in a decrease in serum T3 and T4 levels (29). The infusion of interleukin-1 (IL-1) (30), interleukin-6 (IL-6) (31,32), or interferon-alpha (IFN-α) (33) produces a similar effect. As both TNF-α and IL-1 can induce the release of IL-6, this suggests that IL-6 may be the mediator of cytokine-induced changes in thyroid hormone levels (34).
Furthermore, a relationship between low T3 and inflammation has been reported (35-37). Lee et al demonstrated that increased levels of serum IL-6 and TNF-α negatively correlated with thyroid hormone concentrations in transplant recipients, suggesting a role for these cytokines in ESS (35). Lubrano et al. reported that inflammatory markers, such as IL-6, TNF-α, and CRP, correlated inversely with fT3 in patients with advanced heart failure (37). In the present study, the mean levels of IL-6 continued to be elevated in the pig artery patch recipients (not shown), and correlated with CRP levels. Therefore, following xenoTx, the persisting low fT3 may be associated with an ongoing inflammatory state.
There have been reports of a high early mortality after pig orthotopic heart xenoTx in baboons, particularly within the first 48h (38; McGregor C, and Mohiuddin M, personal communications). This high early mortality may be associated with the fall in fT3 (and depletion of myocardial energy stores that perhaps are not fully replaced as the fT3 level remains low for a considerable length of time). We suggest consideration should be given to T3 replacement therapy. Whether the administration of T3 or T4 during the post-xenoTx period will help suppressing the inflammatory state remains uncertain, but there is evidence in alloTx that T3 can have this effect (2).
In summary, a rapid decline of fT3 is seen after allo- or xeno-Tx operative procedures and a low level may be sustained in the presence of a pig heart or liver xenograft. This may be associated with a generalized inhibition of mitochondrial function, resulting in loss of energy stores, and diminished organ function. The prolonged low fT3 level after xenoTx may be related to an ongoing inflammatory state. Therefore, we suggest the use of thyroid hormonal replacement therapy (T3) to any nonhuman primate with the ESS and/or a ‘stunned myocardium’. This would include baboons with orthotopic pig heart xenoTx. T3 therapy would enable normal levels of fT3 to be maintained, and may allow a rapid recovery of myocardial energy stores and cardiac function. Furthermore, thyroid hormonal replacement therapy may also help in suppressing the inflammatory state that persists in the presence of a pig xenograft.
Acknowledgements
Burcin Ekser, MD, PhD, was a recipient of NIH NIAID T32 AI 074490 postdoctoral fellowship. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and # PO1 HL107152 by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Baboon Research Resources, which is supported by the Office of the Director, NIH, under Award Number P40OD010431 and P40OD010988. We would like to express our gratitude to Dr. Keith Reimann for providing us with anti-CD40 and with anti-CD154 mAb for the NHP Reagent Resource (contract HHSN272200900037C).
Abbreviations
- CRP
C-reactive protein
- ESS
euthyroid sick syndrome
- fT3
free triiodothyronine
- GTKO
α1,3-galactosyltransferase gene-knockout
- IL-6
interleukin-6
- rT3
reverse triiodothyronine
- TNF-α
tumor necrosis factor-alpha
- Tx
transplantation
Footnotes
Conflict of interest
DA is an employee of Revivicor, Blacksburg, VA, USA. None of the other authors reports a conflict of interest.
References
- 1.WARNER MH, BECKETT GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 2.NOVITZKY D, COOPER DK. Thyroid hormone and the stunned myocardium. J Endocrinol. 2014;223:R1–8. doi: 10.1530/JOE-14-0389. Review. [DOI] [PubMed] [Google Scholar]
- 3.IWASE H, EKSER B, SATYANANDA V, EZZELARAB M, COOPER DK. Plasma free triiodothyronine (fT3) levels in baboons undergoing pig organ transplantation: relevance to early recovery of organ function. Xenotransplantation. 2014;2:582–583. doi: 10.1111/xen.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NOVITZKY D, WICOMB WN, COOPER DK, et al. Electrocardiographic, hemodynamic and endocrine changes occurring during experimental brain death in the Chacma baboon. J Heart Transplant. 1984;4:63–69. [Google Scholar]
- 5.BREMNER WF, TAYLOR KM, BAIRD S, et al. Hypothalamo–pituitary–thyroid axis function during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1978;75:392–399. [PubMed] [Google Scholar]
- 6.ROBUSCHI G, MEDICI D, FESANI F, et al. Cardiopulmonary bypass: a low T4 and T3 syndrome with blunted thyrotropin (TSH) response to thyrotropin-releasing hormone (TRH) Horm Res. 1986;23:151–158. doi: 10.1159/000180311. [DOI] [PubMed] [Google Scholar]
- 7.NOVITZKY D, COOPER DK, SWANEPOEL A. Inotropic effect of triiodothyronine (T3) in low cardiac output following cardioplegic arrest and cardiopulmonary bypass: an initial experience in patients undergoing open heart surgery. Eur J Cardiothorac Surg. 1989;3:140–145. doi: 10.1016/1010-7940(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 8.NOVITZKY D, COOPER DK, MORRELL D, ISAACS S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation. 1988;45:32–36. doi: 10.1097/00007890-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 9.STRAUB RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. 2014;16:203. doi: 10.1186/ar4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NOVITZKY D, COOPER DK, REICHART B. Value of triiodothyronine (T3) therapy to brain-dead potential organ donors. J Heart Transplant. 1986;5:486–487. [PubMed] [Google Scholar]
- 11.NOVITZKY D, COOPER DK, REICHART B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987;43:852–854. [PubMed] [Google Scholar]
- 12.WICOMB WN, COOPER DK, NOVITZKY D. Impairment of renal slice function following brain death, with reversibility of injury by hormonal therapy. Transplantation. 1986;41:29–33. doi: 10.1097/00007890-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 13.NOVITZKY D, COOPER DKC. In: The Brain-Dead Organ Donor: Pathophysiology and Management. D D Novitzky, Cooper DKC., editors. Springer; New York: 2013. [Google Scholar]
- 14.NOVITZKY D, COOPER DK, BARTON CI, et al. Triiodothyronine as an inotropic agent after open heart surgery. J Thorac Cardiovasc Surg. 1989;98:972–977. [PubMed] [Google Scholar]
- 15.PHELPS CJ, KOIKE C, VAUGHT TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science (New York, NY) 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AYARES D, PHELPS C, VAUGHT TD, et al. Genetic engineering of source pigs for xenotransplantation: progress and prospects. Xenotransplantation. 2013;20:361. (Abstract #408) [Google Scholar]
- 17.IWASE H, EKSER B, SATYANANDA V, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IWASE H, EKSER B, SATYANANDA V, et al. Pig-to-baboon heterotopic heart transplantation - exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IWASE H, LIU H, WIJKSTROM M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IWASE H, EKSER B, ZHOU H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EKSER B, LONG C, ECHEVERRI GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 22.HARA H, WITT W, CROSSLEY T, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MARCO MR, DONS EM, VAN DER WINDT DJ, et al. Post-transplant repopulation of naïve and memory T cells in blood and lymphoid tissue after alemtuzumab-mediated depletion in heart-transplanted cynomolgus monkeys. Transpl Immunol. 2013;29:88–98. doi: 10.1016/j.trim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EZZELARAB BM, GARACIA B, AZIMZADEH A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COOPER DK, YE Y, NIEKRASZ M. Heart transplantation in primates. In: Cramer DVPL, Makowka L, editors. Handbook of Animal Models in Transplantation Research. CRC Press; Boca Raton: 1994. p. 173. [Google Scholar]
- 26.EZZELARAB MB, EKSER B, ECHEVERRI G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MEBIS L, PALETTA D, DEBAVEYE Y, et al. Expression of thyroid hormone transporters during critical illness. Eur J Endocrinol. 2009;161:243–250. doi: 10.1530/EJE-09-0290. [DOI] [PubMed] [Google Scholar]
- 28.EZZELARAB MB, EKSER B, AZIMZADEH A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VAN DER POLL T, ROMIJN JA, WIERSINGA WM, SAUERWEIN HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. 1990;71:1567–1572. doi: 10.1210/jcem-71-6-1567. [DOI] [PubMed] [Google Scholar]
- 30.HERMUS RM, SWEEP CG, VAN DER MEER MJ, et al. Continuous infusion of interleukin-1b induces a nonthyroidal illness syndrome in the rat. Endocrinology. 1992;131:2139–2146. doi: 10.1210/endo.131.5.1425414. [DOI] [PubMed] [Google Scholar]
- 31.BARTALENA L, BROGIONI S, GRASSO L, VELLUZZI F, MARTINO E. Relationship of the increased serum interleukin-6 concentration to changes of thyroid function in nonthyroidal illness. J Endocrinol Invest. 1994;17:269–274. doi: 10.1007/BF03348974. [DOI] [PubMed] [Google Scholar]
- 32.STOUTHARD JM, VAN DER POLL T, ENDERT E, et al. Effects of acute and chronic interleukin-6 administration on thyroid hormone metabolism in humans. J Clin Endocrinol Metab. 1994;79:1342–1346. doi: 10.1210/jcem.79.5.7962327. [DOI] [PubMed] [Google Scholar]
- 33.CORSSMIT EP, HEYLIGENBERG R, ENDERT E, SAUERWEIN HP, ROMIJN JA. Acute effects of interferon-a administration on thyroid hormone metabolism in healthy men. J Clin Endocrinol Metab. 1995;80:3140–3144. doi: 10.1210/jcem.80.11.7593416. [DOI] [PubMed] [Google Scholar]
- 34.ZHANG YH, LIN JX, VILCEK J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990;10:3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LEE WY, KANG MI, OH KW, et al. Relationship between circulating cytokine levels and thyroid function following bone marrow transplantation. Bone Marrow Transplantation. 2004;33:93–98. doi: 10.1038/sj.bmt.1704304. [DOI] [PubMed] [Google Scholar]
- 36.PARASKEVAIDIS IA, PARISSIS JT, TH KREMASTINOSD. Anti-inflammatory and anti-apoptotic effects of levosimendan in decompensated heart failure: a novel mechanism of drug-induced improvement in contractile performance of the failing heart. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:243–247. doi: 10.2174/1568016054368232. [DOI] [PubMed] [Google Scholar]
- 37.LUBRANO V, PINGITORE A, CARPI A, IERVASI G. Relationship between triiodothyronine and proinflammatory cytokines in chronic heart failure. Biomed Pharmacother. 2010;64:165–169. doi: 10.1016/j.biopha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 38.BYRNE GW, MCGREGOR CG. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17:148–154. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]