Abstract
We introduce a strategy for preclinical research wherein promising targets for analgesia are tested in rodent and subsequently validated in human sensory neurons. Here, we evaluate group II metabotropic glutamate receptors, the activation of which is efficacious in rodent models of pain. Immunohistochemical analysis showed positive immunoreactivity for mGlu2 in rodent dorsal root ganglia (DRG), peripheral fibers in skin, and central labeling in spinal dorsal horn. We also found mGlu2-positive immunoreactivity in human neonatal and adult DRG. RNA-seq analysis of mouse and human DRG revealed a comparative expression profile between species for group II mGluRs and for opioid receptors. In rodent sensory neurons under basal conditions, activation of group II mGluRs with a selective group II agonist produced no changes to membrane excitability. However, membrane hyperexcitability in sensory neurons exposed to the inflammatory mediator prostaglandin E2 (PGE2) was prevented by (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC). In human sensory neurons from donors without history of chronic pain, we show that PGE2 produced hyperexcitability that was similarly blocked by group II mGluR activation. These results reveal a mechanism for peripheral analgesia likely shared by mouse and human and demonstrate a translational research strategy to improve preclinical validation of novel analgesics using cultured human sensory neurons.
Introduction
Concern has grown over the frequency with which promising analgesic candidates fail to show efficacy in clinical trials, yet reasons for the lack of success have been difficult to establish [32; 51]. One obstacle limiting translation may be the often erroneous assumption that results from animal models accurately predict drug efficacy in humans. Because of the inherent challenges in obtaining viable neural tissues from humans, testing species differences has not been considered feasible. However, we recently reported progress in utilizing uninjured dorsal root ganglia (DRG) from adult human donors without chronic pain to characterize the physiological properties of individual human sensory neurons [14]. Here, we functionally examine a potential analgesic at the level of the sensory neuron in a rodent model and validate its efficacy in human sensory neurons.
Group II metabotropic glutamate receptors (mGlu2 and mGlu3) are seven transmembrane domain G protein-coupled receptors that canonically activate the Gi signaling pathway [21]. In several rodent models of inflammatory and neuropathic pain, pharmacological activation of group II mGluRs reduced nocifensive behaviors [33; 35; 43; 54]. Immunoreactivity for group II mGluRs has been observed in rodent DRG neurons [4; 5], and cutaneous administration of a group II mGluR agonist suppressed capsaicin-evoked activity in nociceptors, indicating a peripheral mechanism of action [3; 16; 53]. Interestingly, pharmacological inhibition of peripheral group II mGluRs prolonged hyperalgesia and nociceptor activity, suggesting that group II mGluRs act endogenously to reverse hypersensitivity [6; 54].
Several animal studies have taken advantage of the endogenous anti-hyperalgesic action of group II mGluRs by inducing therapeutic transcriptional upregulation of group II mGluRs in DRG [8; 12; 56; 57]. Oral administration of the dietary supplement and group II mGluR epigenetic modulator acetyl-L-carnitine produced analgesic effects in humans with diabetic or HIV-related peripheral neuropathies [11; 27]. Additionally, enhanced endogenous activation of group II mGluRs with oral N-acetyl cysteine reduced nocifensive behaviors in mice [1], and laser-evoked pain ratings in humans [46].
Direct, pharmacological activation of group II mGluRs has been explored in clinical trials and a satisfactory safety profile has been established for these drugs [39]. However, clinical trials have yet to examine the effects of direct group II mGluR activation for pain relief. Here, we examine the expression of group II mGluRs in the peripheral nervous system of mouse and human. We then determine the effect of direct activation of group II mGluRs on membrane excitability from sensory neurons isolated from mouse and human DRG in a series of parallel experiments.
Materials and Methods
Animals
All experiments were conducted in accordance with the National Institute of Health guidelines and received the approval of the Animal Care and Use Committee of Washington University School of Medicine. 8–12 week old littermate mice (C57BL/6, Jackson lab) were housed on a 12 hour light-dark cycle and allowed ad libitum access to food and water. Knockout mice (mGlu2 −/−) were generously provided by Eli Lilly for immunohistochemistry.
Immunohistochemistry
For immunohistochemistry, mice were deeply anesthetized (Ketamine-Xylazine-Acepromazine) and perfused with ice cold saline solution followed by 4% paraformaldehyde. Glaborous skin from the hindpaw was dissected prior to perfusion and immersion-fixed in Zamboni’s fixative for 4–6 hours, rinsed in PBS and then cryoprotected in 30% sucrose. DRG and spinal cord were removed and stored in 30% sucrose and then embedded in cutting medium and sectioned at 30 microns on a cryostat. Sections were collected on slides. Antibodies were: anti-mGlu2 (1:200–400, Sigma, St. Louis, SAB4501318), anti-βIII tubulin (1:1,000; Covance, Princeton, NJ), anti-CGRP (1:400, AbD Serotec), Alexa-fluor 555 or 488 (1:400, Invitrogen). Isolectin B4-conjugated to Alexa-fluor 568 (1:400, Invitrogen) was used to identify versican-positive neurons. For human tissues, DRG were post-fixed in 4% PFA then stored in 30% sucrose until sectioned. Auto-fluorescence from endogenous lipofuscin prevented clear labeling with fluorescent antibodies, therefore diaminobenzidine horseradish peroxidase staining was used (Vector Labs, Burlingame, CA.).
RNA-seq analysis
Human L2 DRG from 3 female tissue donors free of pain-related disease and ranging in age from 40 – 50 years of age were obtained through AnaBios, Inc. (San Diego, CA). Poly-A+ RNA was sequenced from a 75bp paired-end library on an Illumina sequencer by ActiveMotif (Carlsbad, CA). Results were integratively analyzed with publicly available human fetal spinal cord from the ENCODE project [13] (Replicate 1 (male): ENCFF001RNA, ENCFF001RNB, Replicate 2 (female): ENCFF001RNC, ENCFF001RND) RNA-seq data. Publicly available RNA-seq data was obtained for mouse adult, female DRG (strain C57BL/6: GEO datasets GSM1150934, GSM1150935) [22] and adult, female spinal cord (strain C57Bl/6J: GEO datasets GSM1103369, GSM1103370) [7]. Paired-end datasets were converted to single-end datasets by merging read libraries. At least 2 biological replicates for each tissue type thus obtained were mapped to the reference genome and transcriptome, using the Tophat-Cufflinks pipeline [45]. Quantification of RNA abundance was performed using the Cuffdiff tool in the Tophat-Cufflinks toolkit, using the "classic" normalization mode [45]. Abundances are reported for each gene in Reads per Kilobase per Million Mapped Reads (RPKMs). Reference genomes and transcriptomes used for human and mouse RNA-seq mapping were NCBI hg19 + Gencode v14, and NCBI mm10 + Gencode vM4 respectively [24]. Data from RNA-seq experiments will be made available at the publicly accessible database of Genotypes and Phenotypes: http://www.ncbi.nlm.nih.gov/gap.
Cell culture
Wild type C57/B6 mice were sacrificed by decapitation and the DRG removed and incubated at (37°C, 5% CO2) in 3 mL Ca2+/Mg2+-free Hank’s buffered saline solution containing 10 mM HEPES for 20 minutes with 45U papain (Worthington, Lakewood, NJ) and then for 20 minutes with collagenase (1.5 mg/ml, Sigma). DRG were triturated then passed through a 40μm filter and the dissociated cells plated on poly-D-lysine and collagen coated glass coverslips. Cells were cultured overnight in Neurobasal A media supplemented with B27, 100U/mL penicillin/streptomycin, 2 mM Glutamax, and 5% fetal bovine serum (Gibco). Experiments were performed within 24 hours of plating.
Dorsal root ganglia from consented US donors were acquired by AnaBios, Inc. (San Diego, CA.) or through Mid-America Transplant Services (MTS, Saint Louis, MO.) and prepared as described previously [14]. Detailed protocols for the preparation and experimental use of human sensory neurons are available [47]. Briefly, the DRGs were dissected to remove connective tissue and fat. The ganglia were enzymatically digested and mechanically dissociated, and then cells were seeded on glass coverslips coated with poly-D-lysine. Cells were maintained in culture at 37°C with 5% CO2 in DMEM F-12 (Lonza; Allendale, NJ) supplemented with 10% horse serum (Thermo Fisher Scientific; Rockford, IL), 2 mM glutamine, 25 ng/mL hNGF (Cell Signaling Technology; Danvers, MA), 25 ng/mL GDNF (Peprotech; Rocky Hill, NJ) and Penicillin/Streptomycin (Thermo Fisher Scientific). Human DRG neurons were incubated in culture for three days before recording.
Electrophysiology
Neurons from mouse and human were tested in an external recording solution consisting of (in mM): 145 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 7 Glucose, and 10 HEPES, adjusted to pH 7.4 with NaOH and 305 mOsm with sucrose. Borosilicate, filamented glass electrodes with 2–5 MΩ resistance (Warner Instruments, Hamden, CT) contained internal solution (in mM): 130 K-gluconate, 5 KCl, 5 NaCl, 3 Mg-ATP, 0.3 EGTA, 10 HEPES, adjusted to pH 7.3 with KOH and 294 mOsm with sucrose. After gigaseal and break-in, neurons were given a series of protocols to determine membrane excitability. For acute bath application, drugs (PGE2, Sigma, St. Louis; (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC), Tocris) were diluted in external solution. For the incubation protocol, PGE2 or PGE2 plus APDC were added to the media 30 minutes prior to recordings which were performed in external solution containing the same concentrations of drug. Neurons were recorded within 30 minutes of placement in the recording chamber. Input resistance was calculated as ΔV/ΔI (using a 30–200 pA negative current injection). Rheobase was established from the 1 second step current pulse at which the first action potential was triggered. For threshold, action potentials were evoked using a series of increasing 1 second ramp current injections. For latency, the first action potential was measured from a 1 second, 150pA ramp. The first action potential of a train was used to determine threshold, defined as the voltage at which the first derivative of the membrane potential increased by 10 V/s. DRG neurons were viewed using an Olympus BX-50 epifluorescence microscope. Data were collected with a HEKA EPC 10 amplifier, digitized at 20 kHz, and recorded on a PC running Patchmaster software (v2x-71). Series resistance was kept below 10 MΩ in all recordings and only cells with a diameter of 32 µm (mouse) or 60 µm (human) or less were studied.
Statistics
Electrophysiology results are presented as mean ± SEM. Data were analyzed offline with Igor Pro (WaveMetrics; Portland, OR) using custom-written macros. Data organization and statistical analysis were performed using Microsoft Excel and Prism 6 (GraphPad; La Jolla, CA). Analysis of multiple groups was by ANOVA and pairwise comparisons by paired or unpaired t-tests as appropriate. Significance was taken at p<0.05.
Results
Expression of mGlu2 in the PNS
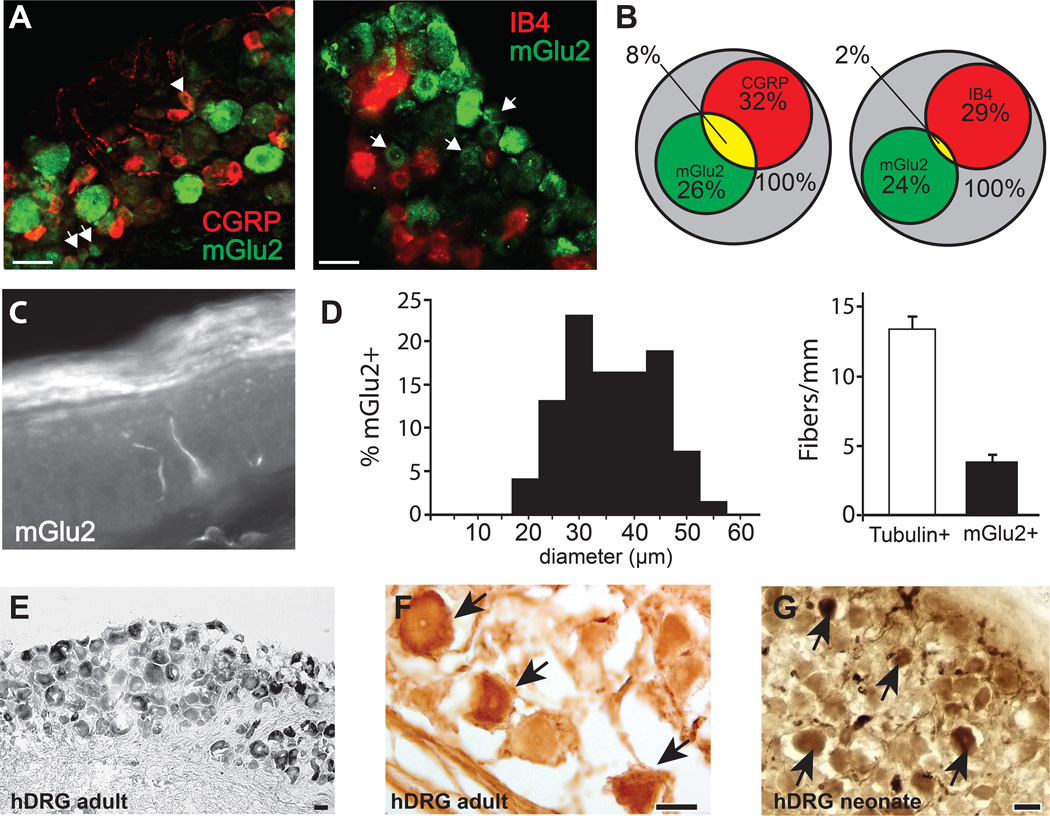
Somata within the mouse DRG and epidermal fiber terminals immunoreactive for an mGlu2-specific antibody were identified (Fig 1). One-quarter of all DRG neurons were clearly immunostained with anti-mGlu2. Of mGlu2+ neurons, 27.8 ± 2.3% were also positive for CGRP, a marker for small-diameter peptidergic sensory neurons and 6.3 ± 4.5% of mGlu2+ neurons co-expressed IB4, a marker for non-peptidergic sensory neurons (Fig 1A, B). Also, mGlu2-immunoreactivity was observed in 23.2 ± 5.1% of the CGRP population, and 5.8 ± 3.7% of the IB4 population of DRG neurons. Immunoreactivity for mGlu2 was also identified in terminal fibers within the footpad epidermis (Fig 1C) and mGlu2+ fibers accounted for 29% of the total fibers identified with the pan-neuronal marker β3-tubulin (Fig 1D). Neurons positive for mGlu2 immunofluorescence possessed diameters ranging from 18 to 56 microns with a mean of 34.3 ± 0.7µm, and 39.8% of mGlu2+ neurons were ≤ 30 µm (Fig 1D). To determine whether mGlu2 was also present in human primary afferent neurons, fixed human DRG were sectioned and stained using the same mGlu2 antibody. DRG sections from both adult and neonatal human showed positive immunoreactivity for mGlu2 in both small and larger somata, indicating persistent expression throughout human life (Fig 1E–G).
Figure 1. Anatomical localization of mGlu2 in mouse and human sensory neurons.
A) Anti-mGlu2 immunoreactivity in mouse DRG and co-expression with CGRP (arrowheads) and IB4. B) Venn diagram showing the overlap and percentages of mGlu2+, CGRP+, and IB4+ somata in the DRG (n=367–389 total DRG neurons from 4 DRG from 3 animals). C) mGlu2-IR fibers extend into the epidermis of the mouse footpad. D) Size distribution of mGlu2-IR somata (34.3 ± 8.5, mean ± SD). mGlu2+ fibers accounted for 29% of total (tubulin-positive) fibers in the epidermis. Data from 3 animals, one DRG or footpad quantified per animal. E–G) Human adult and neonatal DRG neurons immunoreactive for mGlu2 stained with diaminobenzidine. All scale bars =50μm.
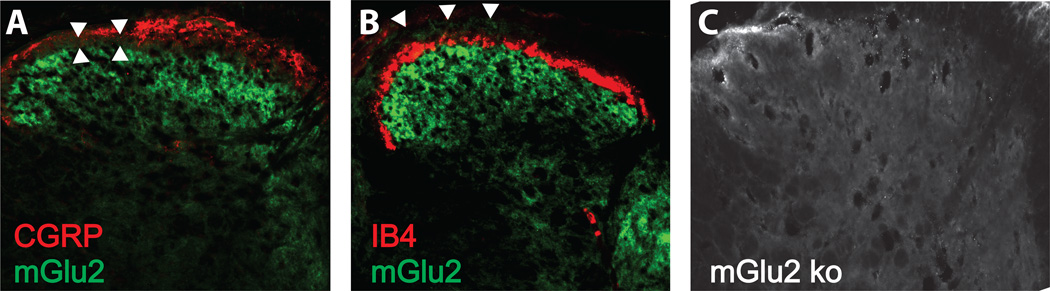
We further observed mGlu2-specific immunoreactivity most densely in laminae III and IV of the mouse spinal cord dorsal horn (Fig 2). Interestingly, little overlap was observed with expression of CGRP-positive fibers in lamina I (Fig 2A) or with IB4-postive fibers in lamina II (Fig 2B), suggesting selective targeting to the deeper dorsal horn by the central processes of mGlu2+ sensory neurons. We confirmed that spinal cord tissue from mGlu2 knockout mice did not exhibit mGlu2-immunoreactivity within the dorsal horn (Fig 2C).
Figure 2. Anatomical localization of mGlu2 in mouse spinal cord.
A) Expression of mGlu2-immunoreactivity in laminae III and IV shows little overlap with CGRP in mouse dorsal horn (arrowheads). B) Expression of mGlu2-immunoreactivity shows little overlap with IB4. C) Absence of mGlu2-immunoreactivity in mGlu2 knockout mouse.
RNA-seq for group II mGluRs
Next, we quantified the gene transcripts for mGlu2 (GRM2) and mGlu3 (GRM3) by examining the expression of GRM2 and GRM3 from RNA-seq data acquired from the DRG of 3 human donors and compared this to the abundance of RNA from public RNA-seq data obtained from reference mouse and human RNA-seq databases. Both human and mouse DRG expressed GRM2 and GRM3. GRM2 exhibited more than double the reads of GRM3 in mouse DRG, but this difference was less prevalent in human (Table 1). We observed that human DRG possessed fewer GRM2 and GRM3 reads overall compared with mouse. To determine the potential relevance of these quantities for human analgesia we examined the abundance of the gene products for Mu (OPRM1) and Delta (OPRD1) opioid receptors, both of which are functionally significant in the clinical control of pain and could serve as anchor points [44; 49]. Reads from these opioid G-protein-coupled receptors indicated expression at similar levels to the group II mGluRs, suggesting the quantities of GRM2 and GRM3 could be sufficient for analgesic effects. Additional analyses of transcripts from spinal cord are reported in Table 1.
Table 1.
RNA-seq of group II mGluRs and opioid receptors in mouse and human.
| GENE | hDRG 1 | hDRG 2 | hDRG 3 | hDRG (X̄ ± SEM) | hSC | mDRG | mSC |
|---|---|---|---|---|---|---|---|
| GRM2 | 0.14 | 0.44 | 0.14 | 0.24 ± 0.1 | 0.61 | 1.11 | 1.09 |
| GRM3 | 0.08 | 0.27 | 0.24 | 0.20 ± 0.06 | 17.68 | 0.44 | 7.34 |
| OPRM1 | 0.11 | 0.51 | 0.55 | 0.39 ± 0.14 | 7.16 | 1.83 | 0.98 |
| OPRD1 | 0.03 | 0.6 | 0.14 | 0.26 ± 0.17 | 0.39 | 2.70 | 2.10 |
Numbers are shown in reads/kilobase/million mapped reads (RPKMs) for each of the tissues listed. Human DRG samples are L2 level from 3 female donors. Human spinal cord (hSC) is from pooled fetal samples. Mouse DRG and SC are from adult female samples on C57Bl/6J background. GRM2 → mGlu2; GRM3 → mGlu3; OPRM1 → μ opioid receptor; OPRD1 → δ opioid receptor.
Block of peripheral sensitization with group II mGluRs
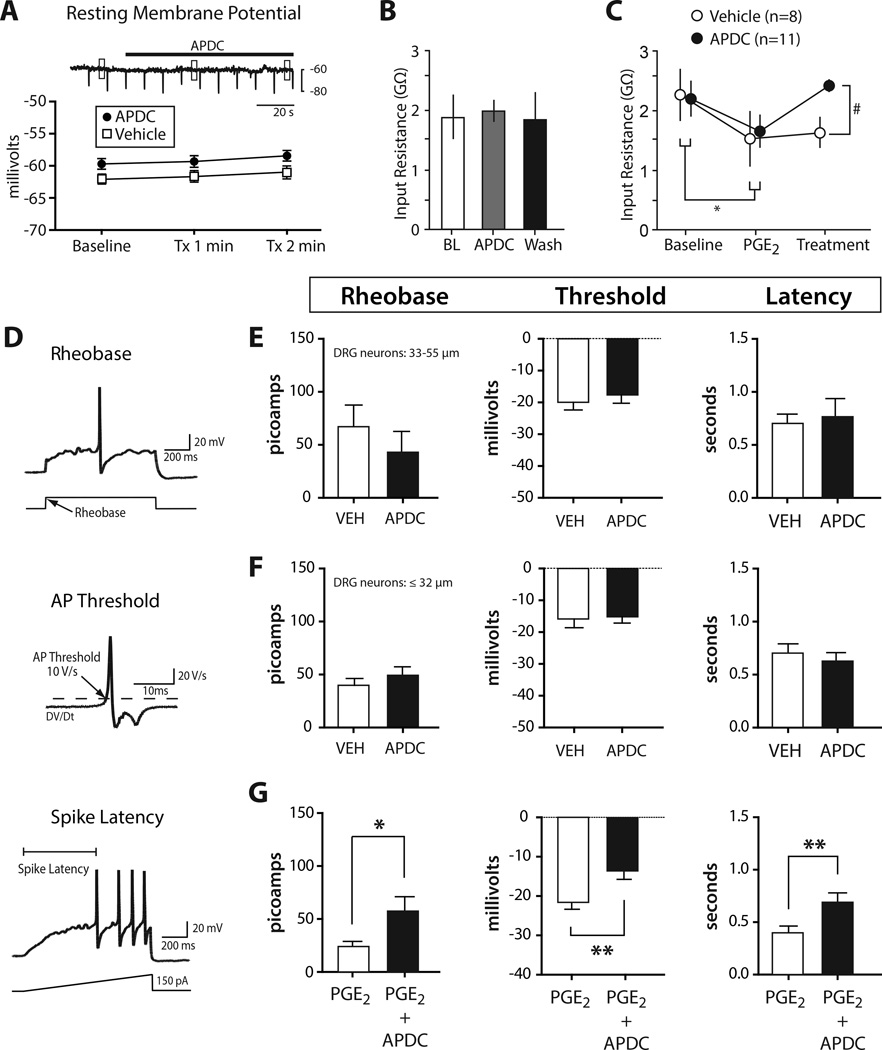
The presence of both mRNA and protein for group II mGluRs in mouse and human DRG prompted us to investigate whether activation of group II mGluRs could modulate membrane excitability and block peripheral sensitization, a mechanism for hyperalgesia. In dissociated primary sensory neurons from mouse, activation of group II mGluRs with the agonist APDC under basal conditions produced no changes in membrane resting potential (Fig 3A) or input resistance (Fig 3B). In contrast, bath application of PGE2, an inflammatory mediator that produces hyperexcitability of sensory neurons in part by increasing calcium and tetrodotoxin-resistant sodium currents [23; 28], reduced input resistance. This effect was reversed by APDC (Fig 3C).
Figure 3. Activation of group II mGluRs blocks PGE2 mediated hyperexcitability in mouse.
A) APDC (1 µM) did not change resting membrane potential. Boxes over trace show time points used to calculate means. Nor was input resistance changed by APDC (n=8). Tx = treatment with APDC or vehicle (B). C) Input resistance was significantly reduced by application of PGE2 (1 µM) and the addition of APDC returned input resistance to baseline (2RM-ANOVA, Tukey post-test; within *p<0.05, between #p<0.05; n=8–11). D) Schematic for measuring rheobase, threshold, and latency. E, F) Excitability was unchanged by APDC compared to vehicle in medium-large DRG neurons (>32 µm) and in small DRG neurons (≤32 µm); n=8–11. G) Compared with PGE2 alone, co-incubation with APDC resulted in significantly higher rheobase, threshold, and latency in DRG neurons ≤32 µm (unpaired t-test *p<0.05; **p<0.01; n=13–15).
To further examine the effects of group II mGluR activation on membrane excitability we quantified the rheobase, action potential threshold, and initial action potential latency (Fig 3D). The addition of APDC produced no changes to excitability compared to vehicle treated sensory neurons in both subsets of small or large diameter sensory neurons (Fig 3E, F). On the other hand, sensory neurons ≤32 µm diameter co-incubated with PGE2 and APDC exhibited significantly reduced membrane excitability compared to neurons incubated with PGE2 alone (Fig 2G). PGE2-alone produced long-lasting, ongoing discharge in only 6% of mouse neurons. These results indicate that activation of group II mGluRs prevents the sensitization of rodent sensory neurons by the inflammatory mediator PGE2, but has no effect on membrane excitability under basal (non-inflammatory) conditions.
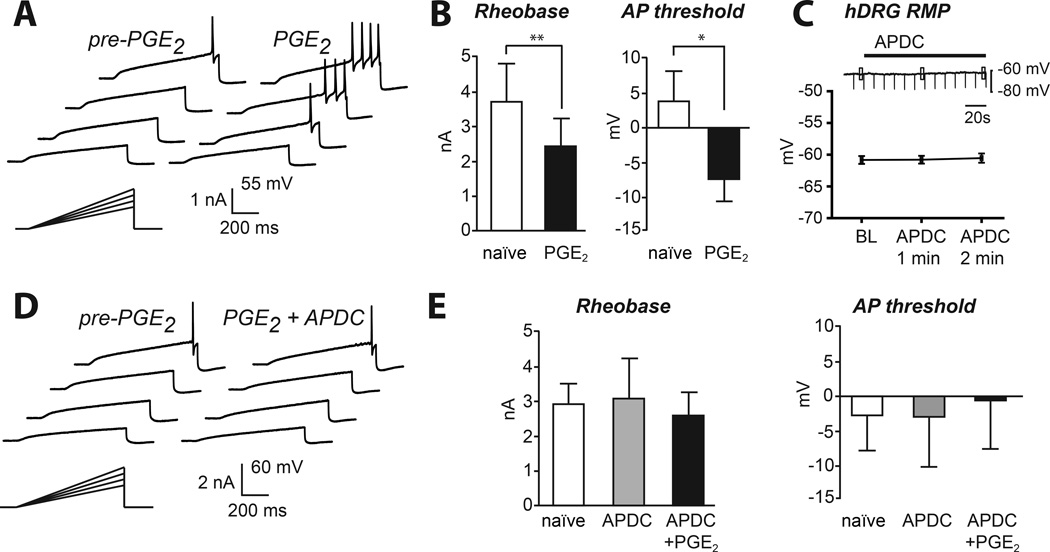
The question of whether the analgesic effect of group II mGluRs translates to humans depends critically on whether the receptor operates via similar mechanisms in human sensory neurons as it does in mouse. To test this question we extracted intact human DRG from donors without a history of chronic pain and used whole-cell patch-clamp techniques to measure neuronal excitability in vitro. We first tested whether PGE2 could directly sensitize human DRG neurons (Fig 4A). Indeed, PGE2 lowered rheobase and hyperpolarized action potential threshold (Fig 4B). Some human cells produced a transient discharge to PGE2, but we detected no long-lasting, ongoing discharge. To determine whether the anti-hyperalgesic effects of group II mGluRs previously demonstrated in mouse are mechanistically similar in human sensory neurons, APDC was bath applied to hDRG neurons. Similar to mouse sensory neurons, the resting membrane potential of naïve human DRG neurons was unaffected by activation of group II mGluRs with APDC (Fig 4C). Nor did application of APDC alter input resistance. In contrast, the presence of APDC prevented PGE2-induced hyperexcitability indicated by unchanged rheobase and action potential threshold compared to the naïve state (Fig 4D, E). Thus, activation of group II mGluRs in human sensory neurons blocks hyperexcitability but did not change basal membrane excitability.
Figure 4. Block of PGE2-induced sensitization by group II mGluRs in human sensory neurons.
A) Example of sensitization of an hDRG neuron with PGE2. B) PGE2-induced sensitization led to decreased rheobase and hyperpolarized action potential threshold of hDRG neurons (paired t-test **p<0.01, *p<0.05; n=11). C) Application of APDC to human sensory neurons produced no changes to resting membrane potential or input resistance in vitro (before APDC: 383 ± 75.6 MΩ; 2 min after APDC: 385 ± 76.1 MΩ; p=0.78; mean ± SEM, n=10). D) PGE2-induced sensitization was blocked by APDC (1 µM). E) APDC alone did not alter membrane excitability but prevented PGE2-induced sensitization (n=9).
Discussion
Prostaglandins and other inflammatory mediators increase the activity of peripheral fibers and generate pain, itch, and hyperalgesia in humans and can produce a transient discharge in human DRG neurons [14; 34; 41]. Here we show that PGE2 alters membrane excitability in cultured human sensory neurons from donors without chronic pain by lowering rheobase and the action potential threshold. Furthermore, we show that the group II mGluR agonist APDC prevents the hyperexcitability induced by exposure to PGE2 in mouse and confirm this observation in human sensory neurons. The human mGlu2 receptor shares >90% sequence homology with rodents suggesting evolutionary pressure for its maintenance, but also the possibility for important functional differences between species [18]. The present results demonstrate unprecedented preclinical functional and translational validation of a candidate analgesic target in human. To extend these observations, we established that immunoreactivity for the mGlu2 receptor is present in the peripheral nervous system and that group II mGluR gene transcripts are expressed at functionally significant levels in both mouse and human. Interestingly, the activation of group II mGluRs under non-inflammatory conditions produced no detectable changes to membrane excitability in either species, suggesting that group II mGluRs selectively counter hyperexcitability without modulating basal nociceptor excitability.
Previous work demonstrated that application of APDC into the rodent skin reduced the duration and magnitude of inflammatory pain responses, indicating a peripheral mechanism for the analgesic action [16; 54]. Group II mGluR activation also prevented PGE2-induced potentiation of capsaicin-evoked calcium responses [53], and reduced capsaicin-evoked nociceptive fiber activity, suggesting that the anti-nociceptive effect may be related to inhibition of the heat- and capsaicin-sensitive ion channel TRPV1 [3; 6]. However, it was not established whether the analgesic effects of group II mGluRs were linked specifically to reducing TRPV1-evoked activity, or whether a broader effect on membrane excitability exists. Indeed, while group II mGluR agonists display analgesic efficacy in thermal tests of nociceptive behavior as would be expected from a TRPV1-dependent mechanism, studies have also shown analgesic effects on neuropathic and formalin-induced pain models thought to be TRPV1-independent [43; 57]. Here we show that APDC raised the rheobase and threshold for firing action potentials in PGE2-exposed sensory neurons, indicating that group II mGluRs reduce overall membrane excitability under inflammatory conditions, thus supporting an alternative mechanism for group II mGluR analgesia. Further evidence for the conclusion that group II mGluRs control sensory neuron membrane excitability was observed when forskolin-induced enhancement of TTX-sensitive sodium currents was blocked by APDC [55].
Group II mGluR immunoreactivity was identified in previous studies mostly in small diameter sensory neurons, many of which bound the non-peptidergic marker IB4, and which projected centrally to outer lamina II-IV in the dorsal horn [4; 5]. These experiments were unable to differentiate between the mGlu2 and mGlu3 receptor subtypes, and because Grm3 mRNA was previously not found in rodent DRG neurons [36], the mGlu2 receptor subtype was thought to be solely responsible for the mGluR2/3 immunoreactivity and peripherally mediated analgesia. The present results show mGlu2-only immunoreactivity in mouse primary afferent fibers, as well as in mouse and human DRG somata. We found mGlu2 expression in a heterologous population of mouse DRG neurons including small diameter nociceptors marked with CGRP or IB4, although few cells co-labeled with the latter. Immunoreactivity for mGlu2 was also detected in larger neurons in both mouse and human, raising the question of whether group II agonists contribute to analgesia in part by acting on larger neurons, many of which are likely A-beta mechanoreceptors. Mechanoreceptors are thought to contribute to both neuropathic and inflammatory pain and can undergo phenotypic switching after injury facilitating nociceptive signal transduction [15]. Although the mechanisms of sensitization and nociception in large diameter neurons require further study, mGlu2 could play an inhibitory role in suppressing e.g., injury-induced ectopic activity. Differences between the present results and previous studies [4; 5] may be attributed to altered mGlu2 localization between rat and mouse, the distinct patterns of IB4 staining between these species [40], and the use of a non-selective mglu2/3 antibody in previous work.
Through quantitative RNA-seq, we show here that expression of transcripts for GRM2 and GRM3 are present in the DRG of both mouse and human. The abundance of GRM3 was comparatively low in mouse DRG, perhaps explaining the lack of detection in rodent using earlier techniques, but its presence may nevertheless contribute to the suppression of PGE2-induced hyperexcitability by APDC that we detected in physiological experiments. Interestingly, the expression levels of GRM2 and GRM3 transcript appeared equivalent in human DRG, introducing the idea of potentially targeting mGluR3 for analgesia in humans. Our strategy for RNA-seq utilized whole DRG containing a heterogeneous population of neurons, satellite glia, and possibly non-neuronal cells from vasculature or meninges in close proximity to the ganglia. It is important to develop the techniques that will allow future human work to determine the precise localization of GRM2 and GRM3 and other transcripts at the single-neuron level.
Group II mGluR expression is epigenetically modulated, involving upregulation by NFκB transcription factors or by injury, and blocking the degradation of these transcription factors to increase GRM2 has been identified as a possible analgesic therapy [8; 9; 37]. This dynamically regulated expression also suggests that group II mGluR levels in neurons processed for culture could differ from those preserved for immunohistochemistry. Sensory neurons below the threshold for immunohistochemical identification may be capable of upregulating group II mGluRs under certain conditions allowing agonists or positive allosteric modulators a broader ability to contribute to physiological and behavioral responses.
Group II mGluRs are well-positioned to regulate nociception at the first stage of sensory transduction, including within the visceral system where mGlu2 was found in the nodose ganglia and the evoked discharge of visceral afferents could be inhibited by APDC [2; 38]. The anti-nociceptive effect appears to be limited to inflammatory or potential injury states because activation of group II mGluRs in the naive state did not alter nociceptor excitability. An analogous result was observed when nociceptor fiber discharge during a brief heat stimulus was not attenuated by APDC, but the discharge generated by the longer-lasting algogen capsaicin was reduced [6]. The release of glutamate into the skin by keratinocytes [19] or reflex activation of the peripheral terminals of primary afferent fibers [25; 30] under injury or inflammatory conditions could produce endogenous activation of group II mGluRs leading to negative feedback and inhibition of the ongoing response. This schema is consistent with the presynaptic inhibitory effect of group II mGluRs reported in other parts of the nervous system [42], with the receptive peripheral ending from these pseudo-unipolar axons functioning analogously to a presynaptic terminal. Group II mGluRs likely also act presynaptically at the central terminal reducing neurotransmission from primary afferents at the first synapse in the dorsal horn [20].
Direct agonists for group II mGluRs have been efficacious for relief of pain in animal studies [26], but have only been tested in human clinical trials for non-pain related indications [17; 39]. It remains unclear whether direct, ligand-activated group II mGluRs likewise reduces pain. Although group II mGluR agonists have not been tested for pain relief in humans, dietary supplements that modulate mGlu2 expression have shown some analgesic benefits [10; 27]. A recent human study showed that N-acetyl-cysteine reduced laser-evoked pain ratings likely through a group II mGluR-dependent mechanism, while leaving thermal threshold detection unchanged [46]. In addition to direct agonists, enhanced endogenous activation by recently derived positive allosteric modulators can be used to control mGlu2 signaling [48].
A few recent studies have begun to investigate the physiology of viable human DRG neurons and have highlighted some similarities as well as some potentially important differences in how human DRG neurons respond to stimuli previously tested only in rodents [14; 29; 31; 52]. However, our study is the first to show that inflammation-induced sensitization of human nociceptors, a neural correlate of hyperalgesia, can be blocked by a candidate analgesic in vitro. The present data demonstrate that a mechanism of group II mGluR-induced analgesia identified in rodent sensory neurons translates mechanistically to human sensory neurons. Group II mGluR agonists and positive allosteric modulators have already demonstrated acceptable safety profiles in clinical trials [50] making them attractive candidates for the development of a peripherally acting, activity-dependent analgesic.
Acknowledgments
This work was supported by the NIH, R01NS042595 (RWG), F32NS076324 (SD), R01NS065926 (TP), and TR32GM108539 (BAC), and a Future Leaders in Pain Research Grant from the American Pain Society (SD).
We thank Mid-America Transplant Services and the donor families who made this research possible. We thank Andrew Torck for developing the RNA-seq analysis pipeline. We thank Eli Lilly for generously providing the mGlu2 knockout tissue for use in these studies.
Dr. Andrea Ghetti is a paid employee of AnaBios Corporation. AnaBios Corporation provided a subset of the human DRGs used in these studies.
Footnotes
All other authors declare no competing financial interests or conflicts of interest.
References
- 1.Bernabucci M, Notartomaso S, Zappulla C, Fazio F, Cannella M, Motolese M, Battaglia G, Bruno V, Gradini R, Nicoletti F. N-Acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors. Molecular pain. 2012;8:77. doi: 10.1186/1744-8069-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackshaw LA, Page AJ, Young RL. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Frontiers in neuroscience. 2011;5:40. doi: 10.3389/fnins.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain research. 2009;1248:86–95. doi: 10.1016/j.brainres.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501(5):780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- 5.Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105(4):957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 6.Carlton SM, Zhou S, Govea R, Du J. Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(36):12727–12737. doi: 10.1523/JNEUROSCI.6558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Deng S, Lu H, Zheng Y, Yang G, Kim D, Cao Q, Wu JQ. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: a resource for understanding the pathology at the systems level. PLoS One. 2013;8(8):e72567. doi: 10.1371/journal.pone.0072567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiechio S, Caricasole A, Barletta E, Storto M, Catania MV, Copani A, Vertechy M, Nicolai R, Calvani M, Melchiorri D, Nicoletti F. L-Acetylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol Pharmacol. 2002;61(5):989–996. doi: 10.1124/mol.61.5.989. [DOI] [PubMed] [Google Scholar]
- 9.Chiechio S, Copani A, De Petris L, Morales ME, Nicoletti F, Gereau RW. Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: a possible mechanism for the analgesic effect of L-acetylcarnitine. Molecular pain. 2006;2:20. doi: 10.1186/1744-8069-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiechio S, Copani A, Gereau RW, Nicoletti F. Acetyl-L-carnitine in neuropathic pain: experimental data. CNS Drugs. 2007;21(Suppl 1):31–38. doi: 10.2165/00023210-200721001-00005. discussion 45-36. [DOI] [PubMed] [Google Scholar]
- 11.Chiechio S, Copani A, Zammataro M, Battaglia G, Gereau RW, Nicoletti F. Transcriptional regulation of type-2 metabotropic glutamate receptors: an epigenetic path to novel treatments for chronic pain. Trends Pharmacol Sci. 2010;31(4):153–160. doi: 10.1016/j.tips.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Molecular pharmacology. 2009;75(5):1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 13.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau RW. Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain. 2014;155(9):1861–1870. doi: 10.1016/j.pain.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Experimental brain research. 2009;196(1):115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 16.Du J, Zhou S, Carlton SM. Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states. Neuroscience. 2008;154(2):754–766. doi: 10.1016/j.neuroscience.2008.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(7):1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- 18.Flor PJ, Lindauer K, Puttner I, Ruegg D, Lukic S, Knopfel T, Kuhn R. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 2. Eur J Neurosci. 1995;7(4):622–629. doi: 10.1111/j.1460-9568.1995.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 19.Genever PG, Maxfield SJ, Kennovin GD, Maltman J, Bowgen CJ, Raxworthy MJ, Skerry TM. Evidence for a novel glutamate-mediated signaling pathway in keratinocytes. J Invest Dermatol. 1999;112(3):337–342. doi: 10.1046/j.1523-1747.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100(2):393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 21.Gereau RW, Swanson G. The glutamate receptors. Totowa, N.J.: Humana Press; 2008. [Google Scholar]
- 22.Gerhold KA, Pellegrino M, Tsunozaki M, Morita T, Leitch DB, Tsuruda PR, Brem RB, Catania KC, Bautista DM. The star-nosed mole reveals clues to the molecular basis of mammalian touch. PLoS One. 2013;8(1):e55001. doi: 10.1371/journal.pone.0055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996;93(3):1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci. 2009;109(2):233–241. doi: 10.1254/jphs.08262fp. [DOI] [PubMed] [Google Scholar]
- 26.Kolber BJ. mGluRs head to toe in pain. Progress in molecular biology and translational science. 2015;131:281–324. doi: 10.1016/bs.pmbts.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Li Q, Li Y, Li L, Tian H, Sun X. Acetyl-L-carnitine in the treatment of peripheral neuropathic pain: a systematic review and meta-analysis of randomized controlled trials. PloS one. 2015;10(3):e0119479. doi: 10.1371/journal.pone.0119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118(1):69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Zhang Z, Cheng Z, Zhang J, Xu S, Liu H, Jia H, Jin Y. Spinal Heme Oxygenase-1 (HO-1) Exerts Antinociceptive Effects Against Neuropathic Pain in a Mouse Model of L5 Spinal Nerve Ligation. Pain medicine. 2015 doi: 10.1111/pme.12906. [DOI] [PubMed] [Google Scholar]
- 30.Miller KE, Hoffman EM, Sutharshan M, Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011;130(3):283–309. doi: 10.1016/j.pharmthera.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo X, Zhang J, Fan Y, Svensson P, Wang K. Thermal and mechanical quantitative sensory testing in chinese patients with burning mouth syndrome - a probable neuropathic pain condition? The journal of headache and pain. 2015;16(1):84. doi: 10.1186/s10194-015-0565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 33.Montana MC, Gereau RW. Metabotropic glutamate receptors as targets for analgesia: antagonism, activation, and allosteric modulation. Curr Pharm Biotechnol. 2011;12(10):1681–1688. doi: 10.2174/138920111798357438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namer B, Schick M, Kleggetveit IP, Orstavik K, Schmidt R, Jorum E, Torebjork E, Handwerker H, Schmelz M. Differential sensitization of silent nociceptors to low pH stimulation by prostaglandin E2 in human volunteers. European journal of pain. 2015;19(2):159–166. doi: 10.1002/ejp.532. [DOI] [PubMed] [Google Scholar]
- 35.Neugebauer V, Carlton SM. Peripheral metabotropic glutamate receptors as drug targets for pain relief. Expert Opin Ther Targets. 2002;6(3):349–361. doi: 10.1517/14728222.6.3.349. [DOI] [PubMed] [Google Scholar]
- 36.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335(2):252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 37.Osikowicz M, Skup M, Mika J, Makuch W, Czarkowska-Bauch J, Przewlocka B. Glial inhibitors influence the mRNA and protein levels of mGlu2/3, 5 and 7 receptors and potentiate the analgesic effects of their ligands in a mouse model of neuropathic pain. Pain. 2009;147(1–3):175–186. doi: 10.1016/j.pain.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128(2):402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 39.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 40.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. The journal of pain : official journal of the American Pain Society. 2007;8(3):263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. Journal of neurophysiology. 2003;89(5):2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 42.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. The Journal of pharmacology and experimental therapeutics. 2001;299(1):12–20. [PubMed] [Google Scholar]
- 43.Simmons RM, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73(2):419–427. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- 44.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60(1):90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truini A, Piroso S, Pasquale E, Notartomaso S, Di Stefano G, Lattanzi R, Battaglia G, Nicoletti F, Cruccu G. N-acetyl-cysteine, a drug that enhances the endogenous activation of group-II metabotropic glutamate receptors, inhibits nociceptive transmission in humans. Molecular pain. 2015;11(1):14. doi: 10.1186/s12990-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valtcheva M, Copits B, Davidson S, Sheahan T, Pullen M, McCall J, Dikranian K, Gereau RW. Surgical extraction of doral root ganglia and preparation of primary cultures for functional studies of sensory neurons. Nature protocols. 2016 doi: 10.1038/nprot.2016.111. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker AG, Conn PJ. Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics. Curr Opin Pharmacol. 2015;20:40–45. doi: 10.1016/j.coph.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107(29):13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wieronska JM, Zorn SH, Doller D, Pilc A. Metabotropic glutamate receptors as targets for new antipsychotic drugs: Historical perspective and critical comparative assessment. Pharmacology & therapeutics. 2015 doi: 10.1016/j.pharmthera.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16(11):1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 52.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21(11):1326–1331. doi: 10.1038/nm.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6388–6393. doi: 10.1523/JNEUROSCI.22-15-06388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain. 2003;106(3):411–417. doi: 10.1016/j.pain.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, Gereau RW. Group II metabotropic glutamate receptors inhibit cAMP-dependent protein kinase-mediated enhancemednt of tetrodotoxin-resistant sodium currents in mouse dorsal root ganglion neurons. Neuroscience letters. 2004;357(3):159–162. doi: 10.1016/j.neulet.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 56.Zammataro M, Chiechio S, Montana MC, Traficante A, Copani A, Nicoletti F, Gereau RW. mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Molecular pain. 2011;7:6. doi: 10.1186/1744-8069-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zammataro M, Sortino MA, Parenti C, Gereau RW, Chiechio S. HDAC and HAT inhibitors differently affect analgesia mediated by group II metabotropic glutamate receptors. Molecular pain. 2014;10:68. doi: 10.1186/1744-8069-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]