Abstract
Objectives
To determine if there is a compression of morbidity in a sample of Ashkenazi Jewish centenarians, similar to what has been reported in other cohorts with exceptional longevity.
Design
Case control study.
Setting
Longevity Genes Project (LGP) and New England Centenarian Study (NECS).
Participants
439 LGP (mean age: 97.8 ± 2.8) and 1,498 NECS (mean age: 101.4 ± 4.0) participants compared to their respective younger referent cohorts of 696 LGP and 302 NECS controls, respectively.
Measurements
Self and proxy reports of age of onset of cancer, cardiovascular disease, diabetes mellitus, hypertension, osteoporosis, and stroke.
Results
Long-lived individuals from both LGP and NECS compared to their respective younger referent groups delay the age of onset of cancer, cardiovascular disease, diabetes mellitus, hypertension, and osteoporosis. The relative risk of overall morbidity is 0.12 in NECS males and 0.20 in NECS females compared to the younger NECS referents and 0.18 in LGP males and 0.24 in LGP females compared to younger male and female LGP referents. The age at which 20% of each of the centenarian groups experienced specific diseases was significantly delayed by between 18 and 24 years relative to the referent groups, when stratified by sex.
Conclusion
The similar extension of health-span and compression of morbidity seen in both the NECS and LGP centenarian samples further validates the utility of these rare individuals for the study of factors that delay or prevent a broad spectrum of diseases otherwise associated with mortality and disability.
Keywords: Compression of morbidity, longevity, centenarians, health span
INTRODUCTION
Life expectancy has steadily increased in the US, with a 2011 life expectancy of 78.7 years.1 A recent Institute of Medicine report suggests that though life expectancy has increased, health span has not and longer life expectancy has been associated with greater rates of disability.2 An analysis of US data concluded that overall prevalence of disease, the length of life with disease, and loss of mobility have increased between 1998 and 2008.3 On the other hand, there are individuals who survive long beyond average life expectancy and spend a relatively short period of their lives with age-related diseases or disability4, 5. This is most evident among those who survive near the limit of human life span6, findings that are consistent with James Fries’ compression of morbidity hypothesis7. These findings contradict the notion that the older people get, the sicker they get and the more resources they require. In fact, Medicare spending on patients during the last year of life has been noted to decrease as age at death increases8 and total end-of-life expenditures are lowest for the oldest enrollees.9
Various distinct cohorts of individuals with exceptionally long lifespans are being studied around the world and in the US and have provided insight into the interactions of morbidity, mortality and survival to older ages.10–12 The Long Life Family Study (LLFS) and the New England Centenarian Study (NECS) recently found that the overall disease-free survival of these long-living individuals was increased compared to their referent cohorts.13 Here, we sought to assess compression of morbidity among Longevity Genes Project (LGP) participants relative to the NECS findings. While the NECS includes individuals of diverse ethnic backgrounds and has a particular focus on centenarians age 105 and above, LGP focuses on a younger sample of centenarians of a founder population, Ashkenazi Jews. We set out to determine if individuals with exceptional longevity from both NECS and LGP share similar delays in disease onset and compression of morbidity. If both cohorts display similar delays in morbidity then this study will confirm that results from one population of centenarians can be generalized to other populations and that younger centenarians, who are more prevalent, can be useful for longevity studies.
METHODS
Participants
LGP
Ashkenazi Jewish individuals, age 95 and older, termed centenarians, although the group included both centenarians and near-centenarians, were recruited from the northeastern United States for the LGP at the Albert Einstein College of Medicine (Einstein) between 1998 to present. They were recruited by word of mouth and through advertisements in Jewish aging centers and other multi-senior settings. Participants were required to be living independently at age 95 as a reflection of good health, although at the time of recruitment, at ages older than 95, they could be at any level of dependency. Birth certificates or dates of birth as stated on passports were used to verify participants’ ages.
A younger referent group was comprised of Ashkenazi Jewish individuals without parental history of longevity, who were also enrolled in aging studies at Einstein. Lack of parental history of longevity was defined as neither parent of the referent surviving to age 95 or beyond. The group included spouses of the offspring of centenarians from LGP, as well as individuals from families without exceptional longevity from the LonGenity cohort. Informed consent was obtained from the participants or their proxies if the participant lacked decisional capacity. The study was approved by the Institutional Review Board (IRB) at Einstein.
NECS
The NECS began in 1994 as a population-based study of all centenarians living within eight towns in the Boston area.14 In 2000, the study expanded enrollment to include centenarians from primarily North America and to a limited degree England, Ireland, Australia and New Zealand. Potential participants were identified from state voter registries, responses to nursing home and senior center mailings, news items appearing in print and on the internet, and enrollment inquiries made directly to the NECS. The only exclusion criterion for the NECS has been the inability to validate age. Birth certificates and other government issued documentation, such as state and local birth registries, passports, or naturalization documents, were used to validate birth dates for 78% of the participants where such documentation was available. For the remaining 22% of the participants, we used U.S. census data from the late 1800s and early 1900s, which noted the participant’s age at the time of the census15–17.
The NECS also enrolled referent participants who were individuals without evident familial predisposition for exceptional longevity. This referent group was composed of spouses of offspring of centenarians and participants of the same birth cohort as the offspring but where at least one parent died at age 73 years, which is the average life expectancy for the 1900 birth cohort.
Participants or their legally assigned representative (if the subject did not have decisional capacity) underwent informed consent, and the study was overseen up until 2001 by Beth Israel Deaconess Medical Center’s Institutional Review Board (Boston, MA) and thereafter by the Boston University Medical Campus IRB.
Measures
In both LGP and NECS, socio-demographic, vital status, and medical history data were collected via mailed questionnaires, telephone calls, or in-person visits. Medical history questionnaires in both studies asked similar questions. Next-of-kin/proxy assisted when necessary. In the NECS, we compared subject’s (or their proxy’s) responses to the medical history questionnaire with their medical records for a random subsample of 25 subjects and for the diseases included in the analysis reported here the agreement was 100%.5 Ages of onset for the following diseases were ascertained: cancers not including skin cancers, cardiovascular disease (CVD), defined as angina pectoris, cardiac arrhythmias, congestive heart failure, and/or myocardial infarction; hypertension (HTN), defined as being on an antihypertensive medication or being told by their physician that they have HTN; osteoporosis, defined as a having a history of osteoporosis or a history of hip, wrist or spine fracture at age 50 or older; stroke; and diabetes mellitus. The disease categories were harmonized between the two studies so that for the purpose of this analysis each disease was defined the same way in both LGP and NECS. The selected diseases were among the top 10 leading causes for death in people age 65 years and older according to the United States Centers for Disease Control (CDC).18 In LGP centenarians and referents, data on congestive heart failure was not elicited, and in NESC referents, data on angina was not elicited. Due to the small number of participants with diabetes mellitus in the studies, this disease was excluded from the disease-specific analyses due to potentially inaccurate estimates. Dementia was not included because of the low sensitivity in determining the presence of this disease either in the medical record or when participants or their proxies are asked. Overall morbidity was defined as one or more of the diseases listed above, including diabetes mellitus.
Data were collected from NECS participants at the time of enrollment and annually thereafter. In order to conform to the LGP data, only the disease-related data collected at enrollment was used in the disease-related analyses. Further, we included in the NECS data only for those centenarians who were at least age 95 at enrollment to better harmonize the two studies. Given the differences in the ages of NECS and LGP participants, referents and centenarians were not directly compared between the two studies. Rather, centenarians and referents were compared within their respective studies.
Statistical Methods
Participants’ phenotypic characteristics are displayed by median, range, and frequencies. Disease-free survival stratified by sex and sample cohort is described by Kaplan–Meier curves. Age at enrollment was used for censoring because follow-up disease prevalence data were not collected in LGP. Missing data were assumed to be missing at random and were ignored for purposes of these analyses. Significant differences in hazard rates among groups and sex were tested using Bayesian parametric survival analysis with Weibull regression.19 This approach tailors the Cox proportional hazard regression to model an accelerated hazard for increasing ages and offers a simple multivariate parametric approach for the estimation of risk and quantiles of specific survival. Comparisons between LGP centenarians and referents and NECS centenarians and referents according to sex were summarized by hazard ratios (HRs), Table 3, and by the gains of each of disease-free survival in years, which are derived from the HRs, Table 2. The percentiles of survival for each disease were chosen to approximate the reported prevalence for each disease amongst older adults. Specifically, 20% prevalence for cancer for ages 65–80 was based on CDC Wonder20 (year 2009); 25% prevalence CVD for ages 65 and older was based on MMWR21; 25% prevalence hypertension was based on reference22. Because of the low prevalence of osteoporosis and stroke in the LGP study participants at enrollment, 25% prevalence of osteoporosis and 10% prevalence of stroke were used.
Table 3.
Gain in Years of Disease-Free Survival of LGP and NECS Subjects
| Disease | Males | Females | NECS.C Females vs. Males | LGP.C Females vs. Males | NECS.R Females vs. Males | LGP.R Females vs. Males | ||
|---|---|---|---|---|---|---|---|---|
| NECS.C vs. NECS.R | LGP.C vs. LGP.R | NECS.C vs. NECS.R | LGP.C vs. LGP.R | |||||
| Cancer (p = 0.20) | 30.0 (26.5, 33.4) | 18.5 (13.4, 23.7) | 25.3 (20.3, 29.4) | 22.0 (18.5, 25.4) | 1.4 (−1.2, 4.0) | −0.2 (−5.2, 4.7) | 6.2 (1.4, 11.4) | −3.6 (−7.4, 0.0) |
| CVD (p = 0.25) | 23.5 (19.8, 27.0) | 17.6 (12.9, 22.5) | 17.9 (12.3, 22.6) | 12.1 (7.1, 16.7) | 1.9 (−0.7, 4.4) | 6.2 (1.2, 10.9) | 7.5 (2.1, 13.5) | 11.7 (7.1, 16.6) |
| HTN (p = 0.25) | 34.9 (31.6, 38.1) | 26.5 (21.4, 31.7) | 29.1 (25.9, 32.1) | 16.7 (13.2, 20.2) | −5.1 (−7.9, −2.4) | −7.3 (−12.9, −2.0) | 0.6 (−2.9, 4.2) | 2.5 (−0.6, 5.5) |
| Osteoporosis (p = 0.25) | 15.5 (8.8, 21.2) | 6.7 (1.2, 12.3) | 21.6 (18.5, 24.5) | 12.3 (8.0, 16.5) | −11.7 (−14.5, −9.1) | −7.6 (−12.3 −2.9) | −17.9 (−24.5, −12.1) | −13.2 (−18.2, −7.9) |
| Stroke (p = 0.10) | 22.3 (17.1, 26.7) | 6.9 (−0.5, 14.4) | 19.4 (12.1, 24.6) | 2.2 (−3.6, 9.0) | −0.3 (−3.0, 2.3) | −2.9 (−8.0, 2.4) | 2.6 (−4.3, 10.7) | 1.8 (−7.1, 9.9) |
| Morbidity (p = 0.20) | 24.2 (21.8, 26.5) | 21.3 (17.7, 25.2) | 18.4 (16.3, 20.4) | 18.0 (15.7, 20.4) | −4.6 (−6.5, −2.7) | −2.5 (−6.7, 1.4) | 1.2 (−1.3, 3.7) | 0.8 (−1.2, 2.7) |
Years delay and 95% credible intervals (in parenthesis) to have a proportion p of the group affected with disease. The delays were estimated using Weibull regression and the formula where λ and ν are the parameters of the hazard function h(age) = λν(ageν−1) (see Supplementary Material for details). The proportion p used for each disease is reported in column 1. Columns report gain in years of disease-free survival in male centenarians from the NECS relative to male referents (NECS.C vs. NECS.R); in male centenarians from the LGP relative to male referents (LGP.C vs. LGP.R); female centenarians from the NECS relative to male referents (NECS.C vs. NECS.R); in female centenarians from the LGP relative to male referents (LGP.C vs. LGP.R);in female centenarians from the NECS relative to male centenarians from NECS; in female centenarians from the LGP relative to male centenarians from LGP; in female referents from the NECS relative to male referents from NECS; and in female referents from the LGP relative to male referents from LGP. NECS = New England Centenarian Study; LGP = Longevity Genes Project.
Table 2.
Sex-Specific Hazard Ratios of Prevalent Age-Related Diseases in LGP and NECS Centenarians Compared to Referents
| Disease | Males | Females | NECS.C Females vs. Males | LGP.C Females vs. Males | NECS.R Females vs. Males | LGP.R Females vs. Males | ||
|---|---|---|---|---|---|---|---|---|
| NECS.C vs. NECS.R | LGP.C vs. LGP.R | NECS.C vs. NECS.R | LGP.C vs. LGP.R | |||||
| Cancer | 0.08 (0.06, 0.11) | 0.23 (0.16, 0.34) | 0.13 (0.09, 0.20) | 0.17 (0.13, 0.22) | 0.91 (0.76, 1.09) | 1.03 (0.71, 1.45) | 1.41 (1.00, 1.95) | 0.56 (0.33, 0.87) |
| CVD | 0.14 (0.10, 0.19) | 0.24 (0.16, 0.34) | 0.25 (0.16, 0.39) | 0.42 (0.29, 0.60) | 0.88 (0.72, 1.05) | 0.66 (0.46, 0.92) | 0.38 (0.25, 0.55) | 0.51 (0.29, 0.82) |
| HTN | 0.10 (0.08, 0.12) | 0.18 (0.13, 0.24) | 0.13 (0.10, 0.17) | 0.33 (0.26, 0.41) | 1.33 (1.14, 1.54) | 1.54 (1.12, 2.07) | 0.83 (0.66, 1.04) | 0.96 (0.70, 1.28) |
| Osteoporosis | 0.33 (0.20, 0.54) | 0.65 (0.44, 0.92) | 0.15 (0.11, 0.20) | 0.40 (0.29, 0.55) | 2.34 (1.93, 2.81) | 1.69 (1.22, 2.28) | 2.73 (1.81, 3.87) | 5.21 (3.00, 8.57) |
| Stroke | 0.09 (0.05, 0.16) | 0.54 (0.23, 1.05) | 0.13 (0.06, 0.28) | 0.83 (0.40, 1.43) | 1.04 (0.80, 1.34) | 1.37 (0.79, 2.17) | 0.93 (0.36, 2.07) | 0.79 (0.28, 1.70) |
| Morbidity | 0.12 (.010, 0.15) | 0.18 (0.14, 0.24) | 0.20 (0.16, 0.24) | 0.24 (0.19, 0.28) | 1.41 (1.22, 1.61) | 1.20 (0.91, 1.57) | 0.93 (0.77, 1.12) | 0.89 (0.68, 1.14) |
Hazard ratios and 95% credible intervals (in parenthesis) estimated using Weibull regression. Diseases are: CVD, cardiovascular disease; HTN, hypertension; morbidity, one or more of the following: cancer, CVD, HTN, osteoporosis, diabetes, or stroke. Groups are NECS.C, NECS centenarians; NECS.R, NECS referents; LGP.C, LGP centenarians; and LGP.R, LGP referents.
NECS = New England Centenarian Study; LGP = Longevity Genes Project.
The estimates of hazard-rates, risk, and quantiles of specific survival were computed using Markov Chain Monte Carlo methods and uninformative priors were assumed for all parameters (essentially, all parameter values were assumed to be, a priori, equally likely). At least 11,000 simulated values were used to estimate parameters and produced summaries of model fit. Credible intervals were estimated using the 2.5 and 97.5 percentiles from the samples generated from the posterior distribution. Statistical significance of HRs was based on 95% credible intervals. The survival distributions estimated using Weibull regression were plotted together with the Kaplan–Meier curves to evaluate adequacy of model fitting by visual inspection (see Supplementary Material). All analyses were conducted in Open Bugs 3.2.3 and R 3.1.1.
RESULTS
Table 1 shows summary statistics of the 4 comparison groups included in the analysis and the prevalences of age-related diseases. The analysis included 439 long-lived participants of the LGP (74 % female) with an age range of 95–110 years and 696 referent participants (51% female) aged 53–93 years. NECS participants consisted of 1,498 long-lived individuals (76% female) with an age range of 95–119 years. The younger referent group consisted of 302 participants (46.0% female), aged 49–89 years.
Table 1.
Characteristics of the Subjects in the Four Groups
| NECS
|
LGP
|
|||
|---|---|---|---|---|
| Referents | Centenarians | Referents | Centenarians | |
| Number of Participants | 302 | 1,498 | 696 | 439 |
|
| ||||
| Males | 163 (54%) | 367 (24%) | 339 (49%) | 112 (26%) |
|
| ||||
| Females | 139 (46%) | 1,131(76%) | 357 (51%) | 327 (74%) |
|
| ||||
| Age at Enrollmenta | 72 (49 – 89) | 101 (95 – 119) | 74 (53 – 93) | 97 (95 – 110) |
|
| ||||
| Males | 73 (57 – 89) | 100 (95 – 113) | 74 (55 – 92) | 97 (95 – 106) |
|
| ||||
| Females | 70 (49 – 85) | 101 (95 – 119) | 73 (53 – 93) | 97 (95 – 110) |
|
| ||||
| Number (%) of Cancer Cases | 111 (37%) | 266 (18%) | 141 (20%) | 78 (18%) |
|
| ||||
| Number (%) of Cardiovascular Cases | 101 (35%) | 615 (43%) | 116 (19%) | 120 (27%) |
|
| ||||
| Number (%) of Diabetes Mellitus Cases | 52 (17%) | 97 (6%) | 32 (5%) | 22 (5%) |
|
| ||||
| Number (%) of Hypertension Cases | 186 (62%) | 570 (41%) | 296 (46%) | 143 (41%) |
|
| ||||
| Number (%) of Osteoporosis Cases | 90 (31%) | 589 (47%) | 61 (9%) | 99 (22%) |
|
| ||||
| Number (%) of Stroke Cases | 36 (12%) | 261 (18%) | 10 (2%) | 42 (10%) |
Median age (range) in years.
NECS = New England Centenarian Study; LGP = Longevity Genes Project.
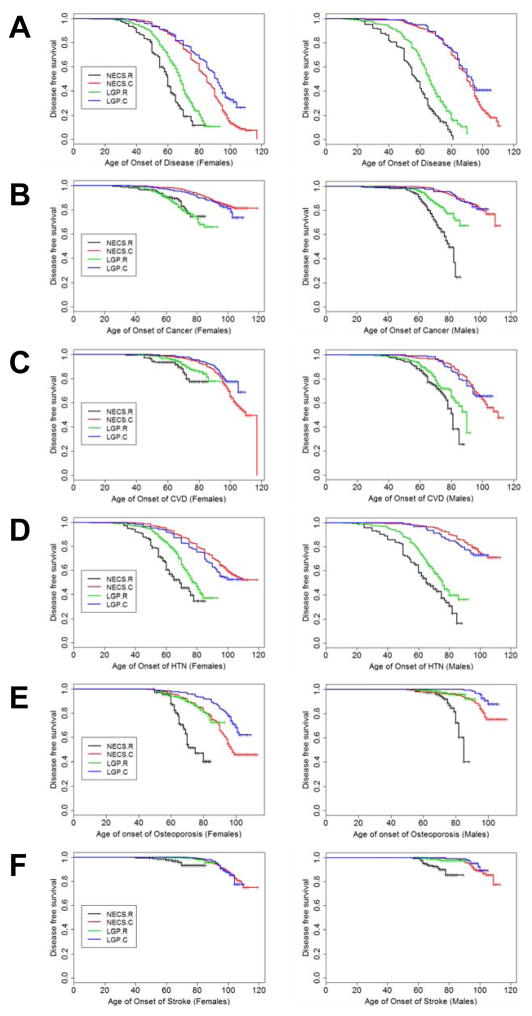
Figures 1A–F show sex and disease-specific Kaplan–Meier curves for survival free of each of five age-related diseases and overall morbidity-free survival in the four groups. Table 2 shows the HRs estimated by Bayesian–Weibull regression. Sex had a significant effect on age of onset of disease (Table 3; Figure 2). We next summarize the results for overall morbidity and by disease.
Figure 1.
Figure 1A–F. Kaplan–Meier curves of survival free of morbidity (Figure 1A), cancer (Figure 1B), cardiovascular disease (CVD) (Figure 1C), hypertension (HTN) (Figure 1D), osteoporosis (Figure 1E), and stroke (Figure 1F) in NECS referents (NECS.R, black line), LGP referents (LGP.R, green line), LGP centenarians (LGP.C, blue line), and NECS centenarians (NECS.C, red line).
Morbidity was defined as one or more of the following: cancer, CVD, diabetes, HTN, osteoporosis or stroke. CVD definition included angina pectoris, cardiac arrhythmias, congestive heart failure, and/or myocardial infarction. Osteoporosis age of onset was based on the earliest diagnosis or reported hip, wrist, and/or vertebral fracture at age 50 and older. NECS = New England Centenarian Study; LGP = Longevity Genes Project. Left panels: females; right panels: males.
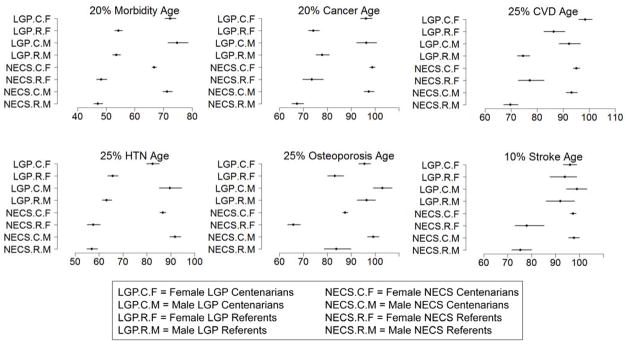
Figure 2. Estimates and 95% credible intervals of the age at which p% of subjects in the various groups had onset of disease.
The inset describes the groups’ labels. Rationale for the choice of percentages p is in methods. CVD = cardiovascular disease; HTN = hypertension; NECS = New England Centenarian Study; LGP = Longevity Genes Project.
Overall Morbidity
In the NECS, the risk of morbidity for centenarian females was approximately 1.41 times higher than the risk for morbidity for centenarian males (HR: 1.41, 95% CI: 1.22–1.61). However, there were no significant differences in risk by sex in the other groups. The risk of morbidity was significantly lower in each of the centenarian groups compared to the corresponding referent groups, with rates of risk for centenarians between 0.12 and 0.24 times that of the risk for morbidity of the corresponding referent groups. The age at which 20% of each of the centenarian groups experienced morbidity was significantly delayed by between 18 and 24 years relative to the referent groups.
Cancer
Female LGP referents had decreased risk of cancer compared to male LGP referents (HR: 0.56, 95% CI: 0.33–0.87), Table 2, while the corresponding NECS referents did not show a significant difference by sex. Sex did not modify the risk for cancer in the centenarian groups. There were significant differences between centenarians and referents in each of the two studies. The HR for cancer of male NECS centenarians was 0.08 compared to male NECS referents (95% CI: 0.06–0.11) and 0.13 for female NECS centenarians compared to female NECS referents (95% CI: 0.09–0.20). The HRs for cancer of the respective comparisons for the LGP groups were 0.23 (95% CI: 0.16–0.34) for males and 0.17 (95% CI: 0.13–0.22) for females. Figure 2 shows that by the age of approximately 67 years in males and 74 years in females, 20% of NECS referents had a cancer event. The age at which the same percentage of LGP male referents had a cancer event was approximately 78 years while it was 74 for females. The age of cancer incidence was delayed to approximately 96 years in both male and female LGP centenarians and 97 and 99 years for NECS male and female centenarians, respectively, with the differences between the centenarians and the referents being significant, Table 2. Table 3 shows the gain of years of cancer-free survival. In LGP centenarians, the estimated age at which 20% of subjects had a cancer event was delayed by approximately 19 and 22 years compared to LGP referents for males and females, respectively. The similar comparison in the NECS study showed a delay of approximately 30 and 25 years, respectively.
Cardiovascular Disease
In both the NECS and LGP, female referents had lower risk of CVD compared to male referents, Table 2, (NECS HR: 0.38, 95% CI: 0.25–0.55; LGP HR: 0.51, 95% CI: 0.29–0.82). There was a sex difference amongst LGP centenarians as well (HR: 0.66, 95% CI: 0.46–0.92), though it was not significant in NECS centenarians. All groups displayed significant differences between centenarians and referents (HR: 0.14, 95% CI: 0.10–0.19 for NECS males; HR: 0.25, 95% CI: 0.16–0.39 for NECS females; HR: 0.24, 95% CI: 0.16–0.34 for LGP males; and HR: 0.42; 95% CI: 0.29–0.60 for LGP females). Figure 2 shows that 25% of the LGP male centenarians had a CVD event by approximately age 92 contrasted with approximately 75 years for LGP male referents. For the LGP females, the approximate ages were 98 and 86 years, respectively. These age differences were found to be even greater for the NECS groups. In the LGP sample, female centenarians showed a delayed onset of CVD of 6 years, on average, compared to their male counterparts.
Hypertension
Both the NECS and LGP centenarians demonstrated an increased risk of hypertension in females compared with males (NECS HR: 1.33, 95% CI: 1.14–1.54; LGP HR: 1.54, 95% CI: 1.12–2.07). The centenarian groups all had significantly lower risks for hypertension when compared to their corresponding referent groups (male NECS HR: 0.10, 95% CI: 0.08–0.12, female NECS HR: 0.13, 95% CI: 0.10–0.17, male LGP HR: 0.18, 95% CI: 0.13–0.24, and female LGP HR: 0.33, 95% CI: 0.26–0.41). LGP female centenarians delayed the age at which 25% subjects had hypertension by about 17 years versus their female LGP referents, while for males, the difference was 27 years. The reduced risk for hypertension in NECS centenarians compared to NECS referents translated into approximately 35 years and 29 years for males and females, respectively. There were significant delays for males in both the NECS and LGP centenarian groups of approximately 5 and 7 years, respectively, as compared to their female counterparts.
Osteoporosis
Osteoporosis was more prevalent and occurred at statistically significant earlier ages in females than males of all four groups (Table 3; Figure 2). Moreover, there were significantly lower risks for osteoporosis in both sexes in each study for the centenarians compared to referents: NECS males HR: 0.33 (95% CI: 0.20–0.54), LGP males HR: 0.65 (95% CI: 0.44–0.92), NECS females HR: 0.15 (95% CI: 0.11–0.20), and LGP females HR: 0.40 (95% CI: 0.29–0.55), Table 2. LGP female centenarians significantly delayed the age at which 25% of the subjects had the disease by approximately 12 years compared to female referents, while the difference between LGP males was 7 years. Across all groups, males significantly delayed the age at which 25% of the subjects had osteoporosis compared to females by 8 and 13 years for LGP centenarians and referents and 12 and 18 years for NECS centenarians and referents, respectively (Table 3).
Stroke
Given the small prevalence of stroke in the LGP study (Table 1), many of the HRs among the LGP group were not significant, nor were the delays of onset of stroke for 10% of the participants. However, there was a statistically significant reduced risk for stroke for NECS centenarians versus NECS referents (Females: HR: 0.13, 95% CI 0.06–0.28; Males: HR 0.09, 95% CI: 0.05–0.16). Moreover, there was a statistically significant delay in the onset of stroke for 10% of NECS centenarians versus referents, of approximately 19 years in females and 22 years in males.
DISCUSSION
The results of this study demonstrate that centenarians in both the NECS and LGP cohorts markedly and similarly compress morbidity and delay the onset of major age-related diseases, as well as overall morbidity, compared to younger referent groups. This work validates previous findings that there is a relative compression of morbidity at oldest ages and suggests that this observation may be generalizable to ethnically varied longevity cohorts. These findings support the premise that centenarians are an ideal sample for the study of protective factors that promote healthy aging.
When looking at sex-specific differences in disease-free survival, the female NECS and LGP centenarians demonstrated a greater hazard for the prevalence of hypertension compared to males. This may be because hypertensive males have a higher mortality compared to hypertensive females,23, 24 thus leaving behind a selected cohort of male survivors with a low prevalence of hypertension. In contrast, female LGP centenarians were noted to have increased disease-free survival compared to male centenarians for CVD. The fact that this gender-effect was not noted in the NECS centenarians for CVD may be due to a survival bias where men with significant age-related morbidity die at a younger age, selecting for healthier male centenarians, particularly at the extremes of old age, and the NECS male centenarians were older than the LGP male centenarians. This is consistent with our previous findings that the older the age group, the greater the degree of compression of morbidity.6 Similar reasoning may explain why female NECS centenarians demonstrated an increased risk of overall morbidity compared to male NECS centenarians. However, sampling variability may have also played a role. For osteoporosis, males across all of the groups displayed a significantly lower hazard in the prevalence of disease compared to females. This is consistent with earlier onset of osteoporosis seen in women due to rapid decrease in bone mass from loss of estrogen post-menopause. However, this result may also be biased because men are screened less frequently for osteoporosis than women and thus may remain undiagnosed.
The LGP study previously demonstrated that their centenarians did not practice healthier habits throughout their lives compared to their contemporaries, suggesting that environment was not an important differentiating factor for these individuals’ enhanced life and health spans.25 Half of the subjects were overweight or obese, less than half performed moderate exercise, and 60% of the men and 30% of the women were heavy smokers for a significant part of their lives. Additionally, the LGP study performed whole genome sequencing of 44 centenarians, finding that each had an average of 5 mutations that should have resulted in a disease but none of them manifested those diseases during their life time.26 Similarly, the NECS performed a Genome-wide Association Study (GWAS) with 801 centenarians and found that they had just as many single nucleotide polymorphisms (SNPs) associated with diseases as are encountered in the general population.27 The LLFS’s GWAS also led to the same conclusion.28 If particularly healthy behaviors and a relative lack of disease-associated variants are not primarily responsible for the prolonged health spans that are observed in centenarians, then one needs to consider the presence of longevity-associated genetic variants, among other factors, that counter the effects of disease-associated variants and mechanisms that cause aging. Indeed, genomic discoveries made in centenarians delineate several mechanisms underlying exceptional longevity and healthy aging.27, 29, 30 Many of the longevity genes discovered in the NECS genetic signatures study have been validated in other ethnically diverse centenarian cohorts, including the LGP.31
Several limitations to this analysis are evident. First, the enrollment criteria for both centenarians and referents somewhat differ between the two studies. However, evaluated separately with the same methods, both studies showed the same pattern of results. Both study samples have a healthy volunteer effect, either because LGP requires participants to be independently living at the age of 95 and/or because both studies are less likely to enroll participants who are highly debilitated and less inclined to participate in any study. Thus, our findings are specific to centenarians who are not in the clinically significant disease phase. Another limitation of the analysis is that the referent group in the NECS is smaller than the centenarian group. Finally, our analysis did not include several leading causes of death among older people, such as pulmonary diseases and Alzheimer’s disease.
Although LGP centenarians are on average slightly younger and are of a different genetic and ethnic background, they demonstrate similar compression of morbidity compared to NECS centenarians. These findings suggest that extension of health-span and compression of morbidity is likely generalizable to most centenarian cohorts. Further study into the protective factors that contribute to this longevity phenotype could potentially yield therapies that target the mechanisms responsible for exceptional longevity and may benefit the general aging population. Resulting compression of morbidity can lead not only to less chronic debilitating age-related diseases in individuals, but also to significant reductions in health costs, termed the “longevity dividend”.32
Acknowledgments
Funding Source:
This work was supported by funding from the National Institute on Aging (TP, SA, LN, PS: The Longevity Consortium, U19AG023122). Grants from the National Institutes of Health (P01AG021654) (NB), the Glenn Center for the Biology of Human Aging (Paul Glenn Foundation for Medical Research) (NB), NIH/NIA1 R01 AG 042188-01 (NB). This publication was supported in part by the CTSA Grant UL1 TR001073, TL1 TR001072, KL2 TR001071 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) (SM); by a Medical Student Training in Aging Research (MSTAR) grant administered by AFAR and the National Institute on Aging (KI). NB is the scientific founder of CohBar Inc., stock holder, patent holder, and board member. This company has no relationship to the science discovered and discussed in this paper. The content in this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions:
Ismail, Nussbaum, Sebastiani, Perls, Andersen, Barzilai, Milman: study conception, design, acquisition and interpretation of data, drafting and revising manuscript. All authors approved the final version of the manuscript for submission
Sponsor’s Role: None
| Elements of Financial/Personal Conflicts |
*Khadija Ismail (KI) |
*Lisa Nussbaum (LN) |
Paola Sebastiani (PS) |
Stacy Andersen (SA) |
Thomas Perls (TP) |
Nir Barzilai (NB) |
Sofiya Milman (SM) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | |||||||
| Grants/Funds | X | X | X | X | X | X | X | |||||||
| Honoraria | X | X | X | X | X | X | X | |||||||
| Speaker Forum | X | X | X | X | X | X | X | |||||||
| Consultant | X | X | X | X | X | X | X | |||||||
| Stocks | X | X | X | X | X | X | X | |||||||
| Royalties | X | X | X | X | X | X | X | |||||||
| Expert Testimony | X | X | X | X | X | X | X | |||||||
| Board Member | X | X | X | X | X | X | X | |||||||
| Patents | X | X | X | X | X | X | X | |||||||
| Personal Relationship | X | X | X | X | X | X | X | |||||||
References
- 1.Hoyert D, Xu J. Deaths: Preliminary Data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 2.National Research Council (US), Institute of Medicine (US) U.S. Health in International Perspective: Shorter Lives, Poorer Health. Washington (DC): National Academies Press (US); 2013. [PubMed] [Google Scholar]
- 3.Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66:75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evert J, Lawler E, Bogan H, et al. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 5.Terry D, Sebastiani P, Andersen S, et al. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med. 2008;168:277–283. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen SL, Sebastiani P, Dworkis DA, et al. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 8.Hoover DR, Crystal S, Kumar R, et al. Medical expenditures during the last year of life: findings from the 1992–1996 Medicare current beneficiary survey. Health Serv Res. 2002;37:1625–1642. doi: 10.1111/1475-6773.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan C, Lunney J, Gabel J, et al. Medicare beneficiaries’ costs of care in the last year of life. Health Aff (Millwood) 2001;20:188–195. doi: 10.1377/hlthaff.20.4.188. [DOI] [PubMed] [Google Scholar]
- 10.Cevenini E, Invidia L, Lescai F, et al. Human models of aging and longevity. Expert Opin Biol Ther. 2008;8:1393–1405. doi: 10.1517/14712598.8.9.1393. [DOI] [PubMed] [Google Scholar]
- 11.Willcox DC, Willcox BJ, Wang NC, et al. Life at the extreme limit: phenotypic characteristics of supercentenarians in Okinawa. J Gerontol A Biol Sci Med Sci. 2008;63:1201–1208. doi: 10.1093/gerona/63.11.1201. [DOI] [PubMed] [Google Scholar]
- 12.Engberg H, Christensen K, Andersen-Ranberg K, et al. Cohort changes in cognitive function among Danish centenarians. A comparative study of 2 birth cohorts born in 1895 and 1905. Dement Geriatr Cogn Disord. 2008;26:153–160. doi: 10.1159/000149819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebastiani P, Sun FX, Andersen SL, et al. Families Enriched for Exceptional Longevity also have Increased Health-Span: Findings from the Long Life Family Study. Front Public Health. 2013;1:38. doi: 10.3389/fpubh.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perls TT, Bochen K, Freeman M, et al. Validity of reported age and centenarian prevalence in New England. Age Ageing. 1999;28:193–197. doi: 10.1093/ageing/28.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Desjardins B. Validation of Extreme Longevity Cases in the Past: The French-Canadian Experience. In: Jeune B, Vaupel JW, editors. Validation of Exceptional Longevity. Odense Monographs on Population Aging. Vol. 6. Odense: Odense University Press; 1999. pp. 65–73. [Google Scholar]
- 16.Poulain M, Chambre D, Foulon M. Centenarian Validation in Belgium. In: Jeune B, Vaupel JW, editors. Validation of Exceptional Longevity. Odense Monographs on Population Aging. Vol. 6. Odense: Odense University Press; 1999. pp. 97–118. [Google Scholar]
- 17.Young RD, Desjardins B, McLaughlin K, et al. Typologies of Extreme Longevity Myths. Curr Gerontol Geriatr Res. 2010;2010:1–12. doi: 10.1155/2010/423087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control. Deaths: Final data for 2010. Natl Vital Stat Rep. 2013;62 [PubMed] [Google Scholar]
- 19.Congdon P. Bayesian Statistical Modeling. 2. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 20.CDC WONDER. United States Cancer Statistics. [Accessed October 2, 2014];Centers for Disease Control (online) Available at: http://wonder.cdc.gov/cancer.html.
- 21.Centers for Disease Control and Prevention. Prevalence of coronary heart disease--United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1377–1381. [PubMed] [Google Scholar]
- 22.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 23.Doumas M, Papademetriou V, Faselis C, et al. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15:321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123:1737–1744. doi: 10.1161/CIRCULATIONAHA.110.005645. [DOI] [PubMed] [Google Scholar]
- 25.Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freudenberg-Hua Y, Freudenberg J, Vacic V, et al. Disease variants in genomes of 44 centenarians. Mol Genet Genomic Med. 2014;2:438–450. doi: 10.1002/mgg3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebastiani P, Solovieff N, DeWan AT, et al. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson M, Bae H, Schupf N, et al. Burden of disease variants in participants of the long life family study. Aging (Albany NY) 2015;7:123–132. doi: 10.18632/aging.100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 30.Vergani C, Lucchi T, Caloni M, et al. I405V polymorphism of the cholesteryl ester transfer protein (CETP) gene in young and very old people. Arch Gerontol Geriatr. 2006;43:213–221. doi: 10.1016/j.archger.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Sebastiani P, Bae H, Sun F, et al. Meta-analysis of genetic variants associated with human exceptional longevity. Aging (Albany NY) 2013;5:653–661. doi: 10.18632/aging.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olshansky SJ, Perry D, Miller RA, et al. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]