Abstract
Objectives
To empirically define multimorbidity “classes” based on patterns of disease co-occurrence among older Americans and to examine how class membership predicts healthcare utilization.
Design
Retrospective cohort study
Setting
Nationally representative sample of Medicare beneficiaries in file years 1999–2007
Participants
14,052 participants age ≥65 years in the Medicare Beneficiary Survey who had data available for at least 1 year after index interview
Measurement
Surveys (self-report) assessed chronic conditions; latent class analysis (LCA) was used to define multimorbidity classes based on the presence/absence of 13 conditions. All participants were assigned to a best-fit class. Primary outcomes were hospitalizations and emergency department visits over one year.
Results
Our primary LCA identified six classes. The largest portion of participants (32.7%) was assigned to the ‘Minimal Disease’ Class, in which most persons had ≤ 1 of the conditions. The other five classes represented various degrees and patterns of multimorbidity. Utilization rates were higher in classes with greater morbidity, compared to the Minimal Disease Class. However, many individuals could not be assigned to a particular class with confidence (sample misclassification error estimate = 0.36). Number of conditions predicted the outcomes at least as well as class membership.
Conclusions
Although recognition of general patterns of disease co-occurrence is useful for policy planning, the heterogeneity of persons with significant multimorbidity (≥3 conditions) defies neat classification. A simple count of conditions may be preferable for predicting utilization.
Keywords: health service use, complexity, comorbidity
INTRODUCTION
One in four American adults has multimorbidity, defined as the co-occurrence of at least two chronic conditions1,2. Because the prevalence of many conditions increases with age, multimorbidity is increasingly common throughout the lifespan1. Approximately one-third of Medicare beneficiaries over age 65 have four or more conditions1. Due to demographic trends, the prevalence and severity of multimorbidity is expected to continue rising over the next decades3,4.
Although the majority of older patients exhibit multimorbidity, most treatment plans and clinical guidelines target single diseases5. When the “single-disease paradigm” is rigidly applied to people with significant multimorbidity, the resultant care plans may be impractical or even harmful5,6. An intervention that is good for one disease may be less effective, irrelevant, or deleterious in the presence of co-existing conditions6,7.
Similarly, well-intended policies, such as disease-based quality metrics, can inadvertently incentivize burdensome and inappropriate care plans for patients with multimorbidity5.Due in part to these challenges, multimorbidity is associated with high rates of death, disability, complications, poor quality of life, and healthcare utilization8,9. Thus, it is important for quality surveillance programs and clinical research initiatives to accurately account for multimorbidity10.
At present, there exists little guidance about best practices for treating multimorbid individuals and tracking their health outcomes. It is impractical to devise individualized algorithms for all potential disease combinations, but common approaches such as counting the number of conditions or the number of affected organ systems may be overly simplistic11,12.
The analyses presented here are based on the hypothesis that many common conditions cluster together in the population in predictable patterns. For example, certain disease clusters may be driven by common genetic propensity, lifestyle, or environmental exposures. We hypothesized that: 1) patients can be classified based on which multimorbidity pattern most closely matches their array of comorbidities, and 2) class membership predicts healthcare utilization. Our objective was to empirically define multimorbidity “classes”,based on the pattern of co-occurrence of 13 common chronic conditions. We applied latent class analysis (LCA), a type of structural equation modeling used to identify sub-groups based on a set of observed variables13. The identification and validation of major classes of multimorbidity might help organize specific treatment strategies, research agendas, and system-wide initiatives aimed at improving care for people with various types and degrees of multimorbidity.
METHODS
Data Source
This study is a secondary analysis of data from the Medicare Current Beneficiary Survey (MCBS) Cost and Use files (1999–2007) and linked Medicare claims. The MCBS is a continuous survey of a nationally representative sample of Medicare beneficiaries. The MCBS sample is stratified according to age (with oversampling of persons aged ≥85) and drawn within ZIP code clusters.. Participants are interviewed in person three times per year. If the participant is unable to answer questions, a proxy respondent is designated. Results from the self-report survey are combined with Medicare claims data. Approval for the study was obtained from the institutional review board of Duke University Medical Center.
Sample
MCBS participants who were community-dwelling at their index interview (file years 1999–2006), eligible for Medicare on the basis of age (≥ 65 years), and enrolled in Medicare Fee-for-Service were eligible MCBS operates on a 4-year rotating panel design; subjects enter and leave the survey each year. Participants in this analysis contributed data for at least one year after their index interview to ascertain 12-month utilization outcomes. After applying these criteria, the final analytical sample size was 14,052 individuals.
Measures
Self-reported demographic variables included age, sex, race (white or non-white), highest education level (< high school, high school degree, ≥ college), and marital status. Presence or absence of 13 health conditions were obtained by self-report (“Have you ever been told that you have…?”): hypertension (HTN), arthritis (rheumatoid and/or non-rheumatoid), osteoporosis, diabetes mellitus (DM), non-skin cancer, mental or psychiatric disorder, emphysema or chronic obstructive pulmonary disease (COPD), stroke, Alzheimer’s disease (AD), Parkinson’s disease (PD), heart arrhythmia, congestive heart failure (CHF), and coronary heart disease (CHD), which included myocardial infarction/heart attack, angina pectoris, or CHD.
Dates and types of health service use were identified in CMS standard analytical files. Outcomes of interest were dichotomous measures of any inpatient admission or any emergency department (ED) visit within 12 months.
Analysis
Primary Analysis
In LCA models, variation of observed indicators (e.g., presence or absence of 13 chronic health conditions) is modeled as a function of membership in unobserved (latent) classes. Class membership is probabilistic, with probabilities computed from the estimated model parameters.
First, increasingly complex models (adding more latent classes) were estimated to determine the optimal number of latent classes to fit the data. The Bayesian Information Criterion (BIC) reflects the likelihood function (i.e., how well the model predicts the data) and the number of parameters in the model. Models with smaller BICs are preferable. We compared candidate models’ BICs and applied substantive interpretability and clinical judgment (i.e., Do the classes defined by a given model possess a clinical significance or meaning?).
After selecting a latent class model, we assigned each participant to his or her “best fit” class, meaning the class for which the participant had the highest computed probability of membership.
Finally, regression models were used to examine the relationship between class membership and the dependent variables (hospitalization, ED visit). LCA was performed using the Latent Gold software package (Statistical Innovations, Belmont, MA), which quantifies model entropy and misclassification error. Other analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Secondary Analyses
After reviewing primary analysis results, we pursued secondary, exploratory analyses for two purposes. The first purpose was to determine whether, by altering the observed variable set used to “train” the LCA model, we could derive a superior set of latent multimorbidity classes that provided better data fit and improved entropy, while retaining disease clusters that were clinically meaningful.
The second purpose was to compare the predictive ability of latent class membership to the predictive ability of a simple count of chronic conditions. In regression models in which our utilization outcomes were the dependent variables, we compared models where the primary independent variable was either latent class membership or a simple morbidity count, as well as models that included both independent variables.
RESULTS
Determining the Optimal Number of Latent Classes
The smallest (i.e., most optimal) BIC values were obtained for the 5-class (BIC 151950) and 6-class (BIC 151937) candidate models. Because the difference in BIC values between the 5-class and 6-class models was so small, we considered the merits of both models. We selected the 6-class model for the next steps in our primary analysis (See on-line appendix).
The six classes were labeled based on which conditions exhibited excess prevalence (i.e., prevalence in class exceeds prevalence in full cohort): Minimal Disease Class (prevalence of all conditions is below cohort average), Non-Vascular Class (excess prevalence in cancer, osteoporosis, arthritis, arrhythmia, COPD, and psychiatric disorders), Vascular Class (excess prevalence in HTN, DM, and stroke), Cardio-Stroke-Cancer Class (excess prevalence in CHF, CHD, arrhythmia, stroke and to a lesser extent HTN, DM, cancer), Major Neurologic Disease Class (excess prevalence in AD, Parkinson’s disease, psychiatric disorders), and Very Sick Class (above-average prevalence of all 13 conditions).
Characteristics of Class Members
Every participant was assigned to one of the six classes based on highest calculated probability of membership. Demographics and health status for each class are displayed in Table 1. The Minimal Disease Class comprised the largest group (32.7% of cohort), in which most members had 0 or 1 condition and tended to be younger and more educated. Other classes exhibited varying degrees of multimorbidity, ranging from a mean of 2.8 conditions in the Non-Vascular Class to 6.3 conditions in the Very Sick Class. Age ranges were similar across classes (75.5 to 77.6 years), with the exception of the Major Neurologic Disease Class (mean age 80.7 ± 7.7 years), in which almost 9 in 10 members reported AD. The proportion of males was highest in the Cardio-Stroke-Cancer Class, whereas the proportion of females was highest in the Non-Vascular Class. The Very Sick Class was demographically similar to the Major Neurologic Disease Class, although Very Sick Class members were typically younger (77.0 ± 7.6 years).
TABLE 1.
Characteristics of Persons Assigned to Six Multimorbidity Classes
| Characteristic | Total Sample (N=14,052) |
Minimal Disease (N=4613) 32.8% |
Non- Vascular (N=3509) 25.0% |
Vascular (N=3211) 22.9% |
Cardio- Stroke- Cancer (N=1165) 8.3% |
Neuro. Disease (N=396) 2.8% |
Very Sick (N=1158) 8.2% |
|---|---|---|---|---|---|---|---|
| Age, M ± SDa | 76.4±7.3 | 75.5±7.1 | 77.0±7.3 | 76.0±7.1 | 77.6±7.4 | 80.7±7.7 | 77.0±7.6 |
| Sex, % Male | 43.5% | 52.7% | 23.8% | 46.3% | 64.4% | 39.1% | 38.5% |
| Race, % White | 86.8% | 88.1% | 90.3% | 81.0% | 89.9% | 81.1% | 86.1% |
| Highest Education Level |
|||||||
| Less than High School |
29.7% | 25.7% | 27.7% | 32.1% | 31.2% | 42.4% | 39.2% |
| High School Degree |
50.5% | 50.9% | 53.1% | 49.6% | 48.8% | 43.2% | 47.8% |
| College or Beyond | 19.8% | 23.4% | 19.2% | 18.3% | 19.9% | 14.4% | 13.0% |
| Marital Status, % Married |
53.9% | 61.0% | 47.9% | 53.0% | 59.8% | 45.2% | 44.0% |
| Number of 13 Diagnoses, M ± SD |
2.8±1.8 | 1.1±0.8 | 3.5±1.0 | 2.8±0.9 | 3.8±0.9 | 4.7±1.5 | 6.3±1.1 |
| Prevalence of Condition |
|||||||
| Parkinson’s Disease |
1.3% | 0.9% | 0.9% | 0.9% | 0.6% | 12.9% | 2.6% |
| Alzheimer’s Disease |
3.5% | 1.1% | 0 | 0.1% | 2.4% | 87.9% | 5.4% |
| Psychiatric Disorders |
14.2% | 2.4% | 24.0% | 9.1% | 2.3% | 54.8% | 43.8% |
| Stroke | 11.7% | 2.5% | 6.5% | 15.7% | 20.8% | 38.4% | 35.0% |
| Arthritis | 61.6% | 26.0% | 94.1% | 71.9% | 41.2% | 71.7% | 93.7% |
| Cancer | 18.6% | 14.7% | 28.5% | 10.3% | 21.7% | 15.7% | 24.7% |
| Osteoporosis | 20.6% | 7.1% | 56.8% | 1.6% | 1.8% | 29.8% | 32.7% |
| COPDb | 14.8% | 8.0% | 23.5% | 3.1% | 14.3% | 12.9% | 49.4% |
| Hypertension | 63.9% | 27.8% | 47.1% | 100% | 71.4% | 67.2% | 90.6% |
| Diabetes | 20.7% | 6.0% | 28.8% | 41.7% | 27.7% | 20.2% | 49.7% |
| Arrhythmia | 20.8% | 6.3% | 44.0% | 4.3% | 65.4% | 16.9% | 64.1% |
| CHDc | 25.3% | 5.4% | 15.0% | 25.6% | 74.0% | 33.8% | 83.3% |
| CHFd | 7.3% | 0 | 1.0% | 0% | 31.5% | 3.3% | 52.9% |
SD = standard deviation
COPD = chronic obstructive pulmonary disease
CHD = coronary heart disease
CHF = congestive heart failure
Relationship Between Multimorbidity Class Membership and Outcomes
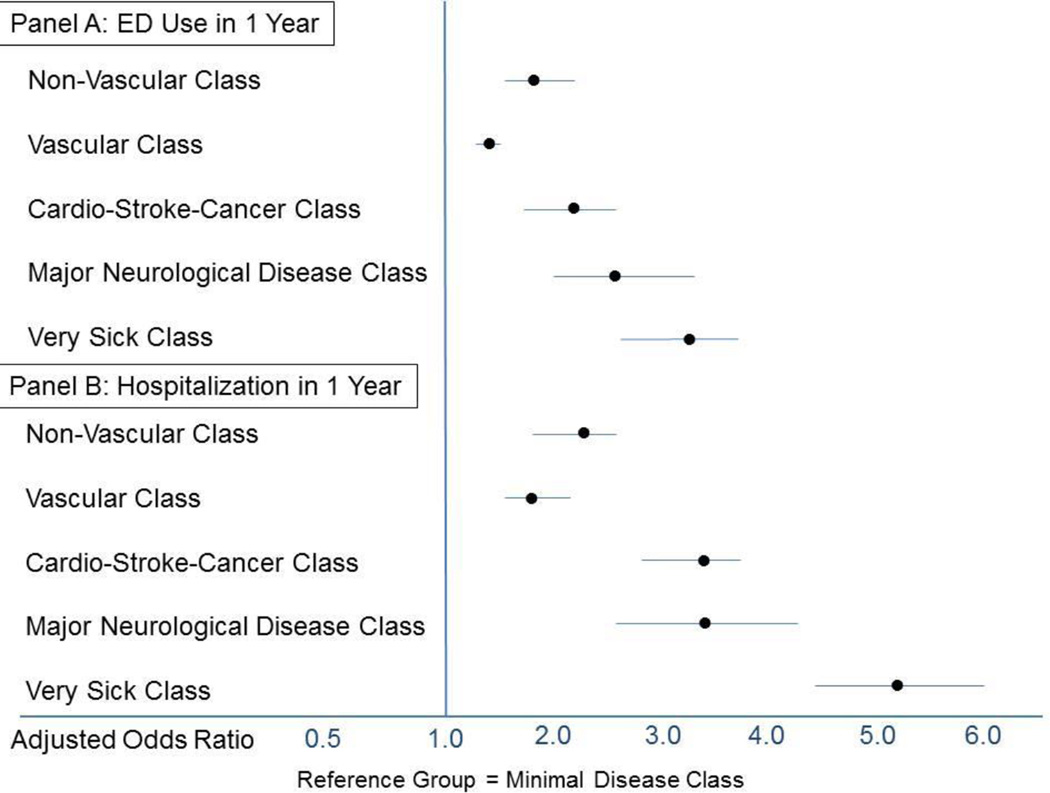
The odds of hospital admission and ED use over a 1-year period, by multimorbidity class, are displayed in Figure 1. The Minimal Disease Class is used as the reference group, because its members had the lowest odds of utilization. Among participants assigned to the Minimal Disease class, the 1-year rate of ED use was 10.9% and the 1-year rate of hospitalization was 8.9%. In analyses that adjusted for age, sex, race, and education, the odds of utilization were higher in classes with higher disease burden. The highest odds of utilization occurred in the Very Sick Class: the adjusted odds ratio for ED use was 3.2 (95% confidence interval [CI] = 2.7 to 3.7) and the adjusted odds ratio for hospitalization was 5.2 (95% CI = 4.4 to 6.1).
FIGURE 1. Odds of Acute Care Utilization, According to Multimorbidity Class.
In logistic regression models that were adjusted for age, race, sex and education level, membership in any one of the “multimorbidity classes” was associated with higher odds of Emergency department (ED) use and hospitalization over 1 year, compared to membership in the Minimal Disease class.
Model fit and misclassification error
Misclassification error was estimated at 0.36 for the sample. In other words, approximately 1 in 3 participants exhibited a pattern of multimorbidity that was a poor fit for any class or was similarly probable in multiple classes. Of the 14052 participants, only 5628 (40.1%) had ≥0.70 calculated probability of membership in the “best-fit” class to which they were assigned; 11781 (83.4%) had ≥0.40 probability. Mean membership probabilities for individuals assigned to the six classes ranged from 0.48 (Cardio-Stroke-Cancer Class) to 0.75 (Minimal Disease Class).
Attempts to Estimate a Better-fit Model
In an effort to reduce misclassification error, we first added “number of chronic conditions” as a 14th observed variable. This strategy produced models with negligible misclassification error (estimate < 0.01), but the latent classes were defined almost entirely by comorbidity count, rather than patterns of disease co-occurrence. Judging by BIC values alone, the 2-class model was optimal and it sorted participants into a low disease prevalence class (0–2 conditions) or a high disease prevalence class (3–11 conditions), with no overlap.
Next, we estimated LCA models using fewer indicator variables. We first eliminated hypertension, osteoporosis, and psychiatric disorders because 1) hypertension and osteoporosis were not strong independent predictors of the outcomes and 2) by eliminating psychiatric disorders, the analysis focused on medical comorbidities. We then eliminated arthritis, cancer, and diabetes, based on relatively weak independent relationship with utilization outcomes. In LCA models based on 7 or 10 indicator variables, misclassification error remained high.
Comparison of Multimorbidity Class vs. Simple Disease Count as Predictors of Outcomes
Table 2 summarizes the predictive power of multiple adjusted logistic regression models predicting 1-year hospitalization or ED use. A comparison of the c-statistic calculated for each model suggests that the models explain similar variance in the utilization outcomes regardless of whether the multimorbidity indicator(s) were multimorbidity class membership (Model 1), a simple count of diseases (Model 2), or both indicators (Model 3). For the outcome of ED utilization, morbidity count remained a significant independent predictor (p<0.001) in Model 3, whereas the significance of class membership as an independent predictor was degraded (p = 0.052).
TABLE 2.
Comparing Regression Models with Different Multimorbidity Indicators as Predictors of Acute Care Utilization
| EDa Visit in 1 Year | Hospital Admission in 1-year | |||||||
|---|---|---|---|---|---|---|---|---|
| Modelb | DFc | Wald χ2 | p value |
C- statisticd |
DF | Wald χ2 | p value |
C- statistic |
| Model 1 | 0.627 | 0.667 | ||||||
| Multimorbidity Class |
5 | 260.5 | <0.001 | 5 | 470.0 | <0.001 | ||
| Model 2 | 0.639e | 0.678e | ||||||
| Morbidity Count |
1 | 325.1 | <0.001 | 1 | 533.3 | <0.001 | ||
| Model 3 | 0.640e | 0.682e | ||||||
| Multimorbidity Class |
5 | 10.9 | 0.05 | 5 | 32.4 | <0.001 | ||
| Morbidity Count |
1 | 74.9 | <0.001 | 1 | 94.4 | <0.001 | ||
ED = emergency department
All models are adjusted for age, race, sex, and education level
DF = degrees of freedom
The c-statistics for Models 2 and 3 were unchanged, regardless of whether “morbidity count” was treated as a continuous vs. class variable. Parameters presented here are taken from analyses that treated morbidity count as a continuous variable (possible range 0–13); multimorbidity class was treated as a nominal class variable with 6 levels.
C-statistic is significantly different (p<0.001) from the c-statistic for Model 1, as assessed by the DeLong test19. C-statistics of Models 2 and 3 were not significantly different from each other (p=0.861 for outcome of ED visits; p=0.170 for outcome of hospital admission).
DISCUSSION
To our knowledge, this is the first study to empirically characterize broad patterns of multimorbidity clusters within the Medicare population. Many tools and indices are available to quantify multimorbidity burden12,14, but this study aimed to define categories of multimorbidity in qualitative terms, based on natural patterns of clustering in the population. The LCA identified six statistically distinct and clinically meaningful classes of multimorbidity, based on the presence or absence of 13 common conditions. However, a key insight is that the application of these empirically-derived classes to individual patients is challenging. The models exhibited high misclassification error, indicating that many participants with multimorbidity could not be confidently assigned to a group. While the recognition of major patterns of disease co-occurrence may help organize prevention and treatment initiatives, a simple count of conditions was an equally informative means of risk-stratifying the population. Considering that many beneficiaries did not fit neatly into a particular group, treatment plans for people with significant multimorbidity demand an individualized approach.
Prior studies have applied LCA, in different populations, to identify patterns of co-occurring conditions. Pugh et al. identified latent classes based on co-occurrence among 32 medical, psychiatric, and deployment-specific conditions in 191,797 Veterans15. Compared to the Medicare beneficiaries, the younger Veteran population exhibited a significantly different spectrum of disease, including prevalent PTSD, pain, traumatic brain injury, and substance abuse. Pugh’s analysis also derived a model with 6 classes, the largest of which exhibited minimal disease burden (53% of cohort)15. In a second study, Islam et al. employed several analytical approaches, including cluster analysis, principal components analysis, and LCA, to describe patterns or clusters of 10 conditions among 4574 older Australians16. The Islam et al. LCA yielded a 4-class model, and the largest group was again a group with minimal disease (55.5% of cohort). The other three groups in Islam’s study resembled our Non-vascular Class (high arthritis, asthma, depression), Vascular Class (high diabetes, hypertension) and Cardio-Stroke-Cancer group (high heart disease, stroke, cancer)16. Neither the Pugh nor the Islam study reported model entropy or misclassification error estimates.
High misclassification error diminishes enthusiasm for the clinical applicability of LCA-derived multimorbidity classes in guiding individual care decisions. Nonetheless, the similarities between the classes that emerged here and in the Islam study16 supports the existence of broad disease clustering patterns in older adults. The patterns reflect plausible disease clusters which share similar underlying etiologies or risk factors. For example, the Vascular group is characterized by diabetes and hypertension, which are part of the metabolic syndrome and are known risk factors for vascular disease17. The older Cardio-Stroke-Cancer group may implicate shared risk factors for cancer and vasculopathy (e.g., smoking).
In a previous study, Kao et al. constructed LCA models based on 27 clinical features to identify phenotypes of people with heart failure18. The Kao analysis identified clinically plausible subtypes of heart failure and class membership was predictive of treatment response. The authors did not discuss misclassification error in class assignment18. Future work should examine whether considering clinical traits aside from comorbidity might improve identification of important phenotypes in persons aging with multimorbidity.
Our study has several limitations. Chronic conditions were identified based on self-report, which may not reflect true disease occurrence and lacks information on disease severity and chronicity. The relationships described may be confounded by factors not controlled for here. Our analysis excluded long-term care residents and does not address health outcomes other than utilization.
Nonetheless, this study is a novel application of LCA to identify patterns of multimorbidity within a representative sample of Medicare beneficiaries. Six classes of disease co-occurrence emerged, and these multimorbidity patterns were clinically recognizable and theoretically plausible. Application to decision-making on individual patients is limited by the fact that many persons with multimorbidity do not fit neatly into one of the six classes. This caveat to the use of LCA-derived groups has not been addressed in prior studies on this topic. Future research that applies LCA to identify sub-groups or phenotypes among older patient populations should consider and report model entropy and misclassification error.
Acknowledgments
Dr. Whitson is supported by the National Institutes of Health (R01AG043438, R24AG045050, P30AG028716) and the Durham VA GRECC and RR&D (I21 RX001721). Dr. Cigolle was supported by the National Institutes of Health (5K08AG031837) and the Ann Arbor VA GRECC. Dr. Hastings is supported by Durham VA HSR&D (IIR 12-052).
Appendix: Selection of Optimal Latent Class Model
We compared increasingly complex models (i.e., models with an increasing number of “latent” classes) in order to select the LCA model that best fit the data. Following standard practice, to select the best model, we compared the Bayesian Information Criterion (BIC) of candidate models and we applied judgment to consider whether different models identified classes made up of morbidity patterns that seemed clinically relevant or meaningful. The 4-class model (BIC=152076) yielded a class with low morbidity (n=4672), a class with mostly non-vascular comorbidities (n=3304), a class with mostly vascular comorbidities (n=3945), and a class with very high morbidity of various types (n=2131). In the 5-class model (BIC = 151950), a new class emerged that was relatively small (n=404) and drew most of its members from the 4-class model’s “non-vascular” group or “very sick/high morbidity” group. This group was clinically meaningful as it was characterized by high clustering of neuro-psychiatric diseases (Alzheimer’s Disease, Parkinson’s Disease, stroke, and psychiatric disorders).
In the 6-class model (BIC=151937), another class emerged, which we labeled the Cardio-Stroke-Cancer class. When compared to the class assignments in either the 4-class model or 5-class model, the new Cardio-Stroke-Cancer class (n=1165) drew its members almost entirely from the “very sick” group (n=676 from 4-class model; n=676 from 5-class model) and the “vascular” comorbidities group (n=446 from the 4-class model; n=436 from the 5-class model). The new Cardio-Stroke-Cancer class had relatively higher morbidity burden than those who remained behind in the “Vascular Class,” which became predominantly characterized by high rates of diabetes and hypertension, as well as higher rates of stroke. In contrast, the new Cardio-Stroke-Cancer class was older, on average, than those left in the Vascular group. Its members had suffered more end-organ cardiac damage (CHD, CHF, arrhythmia), in addition to the higher rates of cancer. The clustering of cancer and serious cardiac disease in the Cardio-Stroke-Cancer group possibly reflects common risk factors such as smoking and poor access to preventative care. The emergent Cardio-Stroke-Cancer group drew many members from the “Very Sick” groups, which meant that in the 6-class model the “Very Sick” group was reserved for those with quite high morbidity burden (≥6 of the 13 conditions), often representing a combination of vascular disease, cancer, and non-vascular conditions. See Table for further comparison of the 5- and 6-class models.
Considering that the 6-class model had the smallest BIC value and that we found the Major Neuro Disease group and the Cardio-Stroke-Cancer group to be clinically relevant, we selected the 6-class model as the most optimal for further analysis.
Appendix Table.
Comparison of 5-class and 6-class Models Estimated by Latent Class Analysis
|
Latent Classes |
Description of Class, Compared to Overall Sample |
5-class Model BICi=151950 |
6-class Model BIC=151937 |
||
|---|---|---|---|---|---|
| N | Number of Conditions (Mean ± SDa) |
N | Number of Conditions (Mean ± SD) |
||
| Minimal Disease | Prevalence of all 13 conditions is below sample prevalence |
4625 | 1.1±0.8 | 4613 | 1.1±0.8 |
| Non-Vascular | Higher prevalence of cancer, psychiatric disorders, osteoporosis, COPDb, arthritis. (In 6- class model, also higher arrhythmia). |
3334 | 3.5±1.0 | 3509 | 3.5±1.0 |
| Vascular | Higher prevalence of HTNc, DMd, stroke. (In 5- class model, also higher CHDe) |
3714 | 2.8±0.9 | 3211 | 2.8±0.9 |
| Cardio-Stroke- Cancer (only present in 6- class modelh) |
Higher prevalence of CHFf, CHD, arrhythmia, stroke. Moderately elevated DM, HTN, cancer. Low prevalence of arthritis, ADg Parkinson’s |
N/A | N/A | 1165 | 3.8±0.9 |
| Major Neurologic Disease |
Very high prevalence of AD, Parkinson’s, stroke, psychiatric disorders. Moderately elevated CHD and osteoporosis |
404 | 4.8±1.6 | 396 | 4.7±1.5 |
| Very Sick | Prevalence of all 13 conditions is above sample prevalence |
1975 | 5.4±1.4 | 1158 | 6.3±1.1 |
SD = standard deviation
COPD = chronic obstructive pulmonary disease
HTN = hypertension
DM = diabetes mellitus
CHD = coronary heart disease
CHF = congestive heart failure
AD=Alzheimer’s Disease
In the 6-class model, participants with CHD tended to be assigned to the “Cardio-Stroke-Cancer Class,” characterized by vascular risk factors (DM, HTN) plus related end organ damage (CHF, CHD) and cancer. In contrast, in the 5-class model, participants with CHD were typically placed into either the “Vascular” Class (characterized by high prevalence of DM, HTN, stroke) or into the “Very Sick” Class, depending on their burden of comorbidities. The emergence of the “Cardio-Vascular-Cancer” Class in the 6-class model tended to restrict the Very Sick Class to those participants with extreme multimorbidity, which was typically both vascular and non-vascular in nature.
BIC = Bayesian Information Criterion (smaller values indicate more optimal models)
Footnotes
| Elements of Conflict |
HEW | KSJ | CC | RS | CFP | LL | SNH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment | X | X | X | X | X | X | X | |||||||
| Grants/Funds | X | X | X | X | X | X | X | |||||||
| Honoraria | X | X | X | X | X | X | X | |||||||
| Speaker Forum |
X | X | X | X | X | X | X | |||||||
| Consultant | X | X | X | X | X | X | X | |||||||
| Stocks | X | X | X | X | X | X | X | |||||||
| Royalties | X | X | X | X | X | X | X | |||||||
| Expert Testimony |
X | X | X | X | X | X | X | |||||||
| Board Member |
X | X | X | X | X | X | X | |||||||
| Patents | X | X | X | X | X | X | X | |||||||
| Personal Relationship |
X | X | X | X | X | X | X | |||||||
Author Contributions:
Study concept and design: HEW, KSJ, SNH, CC
Acquisition of subjects and/or data: SNH
Analysis and interpretation of data: all authors
Preparation of manuscript: all authors
Sponsor’s Role: The sponsors had no role in the design, methods, subject recruitment, data collections, analysis or preparation of paper.
REFERENCES
- 1.Anderson G. Chronic Conditions: Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson Foundation's Partnership for Solutions; 2004. September 2004 update. [Google Scholar]
- 2.Mercer SW, Smith SM, Wyke S, et al. Multimorbidity in primary care: developing the research agenda. Fam Pract. 2009;26:79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AK, Goodman RA, Gordon C, et al. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Pub Health Rep. 2011;126:460–471. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuGoff EH, Canudas-Romo V, Buttorff C, et al. Multiple chronic conditions and life expectancy: a life table analysis. Med Care. 2014;52:688–694. doi: 10.1097/MLR.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 5.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. New Engl J Med. 2011;364:2478–2481. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 8.Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA. 2010;303:1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 10.Parekh AK, Kronick R, Tavenner M. Optimizing health for persons with multiple chronic conditions. JAMA. 2014;312:1199–1200. doi: 10.1001/jama.2014.10181. [DOI] [PubMed] [Google Scholar]
- 11.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 12.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure omorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 13.Formann AK, Kohlmann T. Latent class analysis in medical research. Stat Methods Med Res. 1996;5:179–211. doi: 10.1177/096228029600500205. [DOI] [PubMed] [Google Scholar]
- 14.Chrischilles E, Schneider K, Wilwert J, et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care. 2014;52(Suppl 3):S75–S84. doi: 10.1097/MLR.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 15.Pugh MJ, Finley EP, Copeland LA, et al. Complex comorbidity clusters in OEF/OIF veterans: the polytrauma clinical triad and beyond. Med Care. 2014;52:172–181. doi: 10.1097/MLR.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 16.Islam MM, Valderas JM, Yen L, et al. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PloS one. 2014;9:e83783. doi: 10.1371/journal.pone.0083783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14:160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao DP, Wagner BD, Robertson AD, et al. A personalized BEST: characterization of latent clinical classes of nonischemic heart failure that predict outcomes and response to bucindolol. PloS one. 2012;7:e48184. doi: 10.1371/journal.pone.0048184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or morecorrelated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]