Summary
Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) sense microbial ligands and initiate signaling to induce inflammatory responses. Although the quality of inflammatory responses is influenced by internalization of TLRs, the role of endosomal maturation in clearing receptors and terminating inflammatory responses is not well understood. Here, we report that Drosophila and mammalian Vps33B proteins play critical roles in the maturation of phagosomes and endosomes following microbial recognition. Vps33B was necessary for clearance of endosomes containing internalized PRRs, failure of which resulted in enhanced signaling and expression of inflammatory mediators. Lack of Vps33B had no effect on trafficking of endosomes containing non-microbial cargo. These findings indicate that Vps33B function is critical for determining the fate of signaling endosomes formed following PRR activation. Exaggerated inflammatory responses dictated by persistence of receptors in aberrant endosomal compartments could therefore contribute to symptoms of ARC syndrome, a disease linked to loss of Vps33B.
Keywords: ARC syndrome, TLR signaling, Tolerance, LPS, Phagocytosis
eTOC Blurb
Functional mutations in VPS33B gene lead to a fatal human disease called ARC syndrome. Pasare and colleagues demonstrate that activation of PRRs leads to formation of inflammatory endosomes that require Vps33B for maturation. Loss of Vps33B function causes endosomal accumulation of PRRs and their ligands leading to exaggerated inflammatory responses.
Introduction
Cells of the innate immune system express several different Toll-like receptors (TLRs) that recognize a diverse set of microbial ligands and initiate signaling to generate inflammatory responses. The outcome of TLR signaling depends on the cell type that encounters the pathogen and the specificity of TLR signaling is determined by the downstream adapter proteins used by different TLRs. Endolysosomal compartments play a critical role in determining signaling specificity and strength that leads to inflammatory responses (Kagan et al., 2008; Zanoni et al., 2011). For example, LPS initiates a Myeloid differentiation primary response gene 88(MyD88) and TIR domain containing adapter protein (TIRAP)-dependent TLR4 signaling cascade at the plasma membrane resulting in the induction of pro-inflammatory genes through activation of the NF-κB and AP-1 families of transcriptions factors. In parallel, LPS binding to TLR4 and CD14 leads to rapid receptor endocytosis, which downregulates MyD88 and TIRAP-dependent signaling while synchronously endosomal TLR4 engages a different set of adapters (Kagan and Iwasaki, 2012; Tanimura et al., 2008; Zanoni et al., 2011). The endosomal TRIF and TRAM adaptors enable induction of type I Interferons (IFNs) through activation of Interferon-Regulatory Factor 3 (IRF3) (Kagan et al., 2008; Yamamoto et al., 2003). For other cell surface TLRs, such as TLR2 and TLR5, it is not clear if signaling downstream of these TLRs depends on their endocytosis. By contrast, TLR3, TLR7 and TLR9 primarily reside in endolysosomes and are activated by nucleic acids in endosomes (Kawai and Akira, 2010). Importantly, both TLR7 and TLR9 ecto-domains are cleaved in endolysosomes and this cleavage, at least for TLR9, is essential for signal transduction following ligand recognition (Ewald et al., 2011; Ewald et al., 2008). All endosomal TLRs, in addition to inducing NF-κB dependent genes, induce a type I interferon response by engaging the MyD88 (TLR7 and TLR9) or TRIF (TLR3) downstream adapters (Honda et al., 2005a; Honda et al., 2005b; Yamamoto et al., 2003). Thus, receptor endocytosis and endosomal maturation are critical in determining the spectrum of TLR-induced pro-inflammatory responses.
TLRs also play a critical role in phagocytosis. TLR activation enhances phagocytosis of both gram-positive and gram-negative bacteria while having no demonstrable effects on non-microbial cargo (Blander and Medzhitov, 2004; Underhill et al., 1999). TLRs have also been implicated in regulating phagosomal maturation, but the mechanisms of this regulation are not known (Blander and Medzhitov, 2006; Kagan and Iwasaki, 2012). Endosomal and phagosomal maturation are connected cellular processes that determine the rate of progression of cargo along the endosomal route and its ultimate fate (Kinchen et al., 2008). Since ligands expressed on bacterial cargo activate TLRs, phagosome maturation is likely to play a crucial role in regulating signaling initiated by ligand recognition. Therefore, TLR-facilitated endosomal maturation and signaling by TLRs are intricately linked.
An important step in endosomal maturation is the fusion of late endosomes with lysosomes, which is controlled by the conserved homotypic fusion and vacuole protein sorting (HOPS) complex. To orchestrate lysosomal fusions, HOPS complexes interact with phospholipids, SNARE fusion proteins and small Rab GTP binding proteins (Wickner, 2010). Among HOPS subunits, Vps33 proteins belong to the family SM proteins, named for Sec1 and Munc18, that directly interact with SNAREs during membrane fusions (Lobingier et al., 2014). In Drosophila, loss of Vps33A (encoded by carnation), or its close binding partner Vps16A (Baker et al., 2013), compromises the fusion of lysosomes with late endosomes and autophagosomes (Akbar et al., 2009; Pulipparacharuvil et al., 2005; Takats et al., 2014). By contrast, a fob null mutation blocks the maturation of phagosomes (Akbar et al., 2011). fob encodes the Drosophila ortholog of Vps16B (VIPAS39), the binding partner of Vps33B (Cullinane et al., 2010; Pulipparacharuvil et al., 2005; Tornieri et al., 2013). This defect in phagosome maturation compromises bacterial clearance and renders fob flies susceptible to non-virulent microbial infections (Akbar et al., 2011). Consistent with these observations, the mycobacterial virulence factor PtpA targets Vps33B for dephosphorylation, which contributes to intracellular persistence of Mycobacterium (Bach et al., 2008). Mutations in human Vps33B and VIPAS39 are responsible for arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome (Cullinane et al., 2010; Gissen et al., 2004), a fatal recessive disorder characterized by trafficking defects in multiple organ systems (Gissen et al., 2006; Tornieri et al., 2013) and persistent infections and sepsis (Jang et al., 2009; Seo et al., 2014).
Since the mechanisms of TLR-dependent endosomal maturation and endo-lysosomal fusion are not well understood and our earlier work (Akbar et al., 2011) suggests a role for Vps33B in regulating bacterial clearance in Drosophila hemocytes, we explored the possibility that this SM protein could be responsible for regulating endosomal maturation following TLR activation in mouse macrophages. We have found that functions of Vps33B are conserved between flies and mice, as Vps33B is required in mouse macrophages for normal maturation of phagosomes and specialized endosomes that are induced upon PRR activation. We found that absence of Vps33B caused defects in the degradation of endosomal and phagosomal microbial cargo and their receptors, thus contributing to exaggerated pro-inflammatory responses. These findings suggest that TLR signaling promotes a specialized endosomal pathway and Vps33B is required for ultimate degradation of those endosomes and, importantly, for termination of TLR signaling.
Results
Vps33B has a conserved role in phagosomal clearance
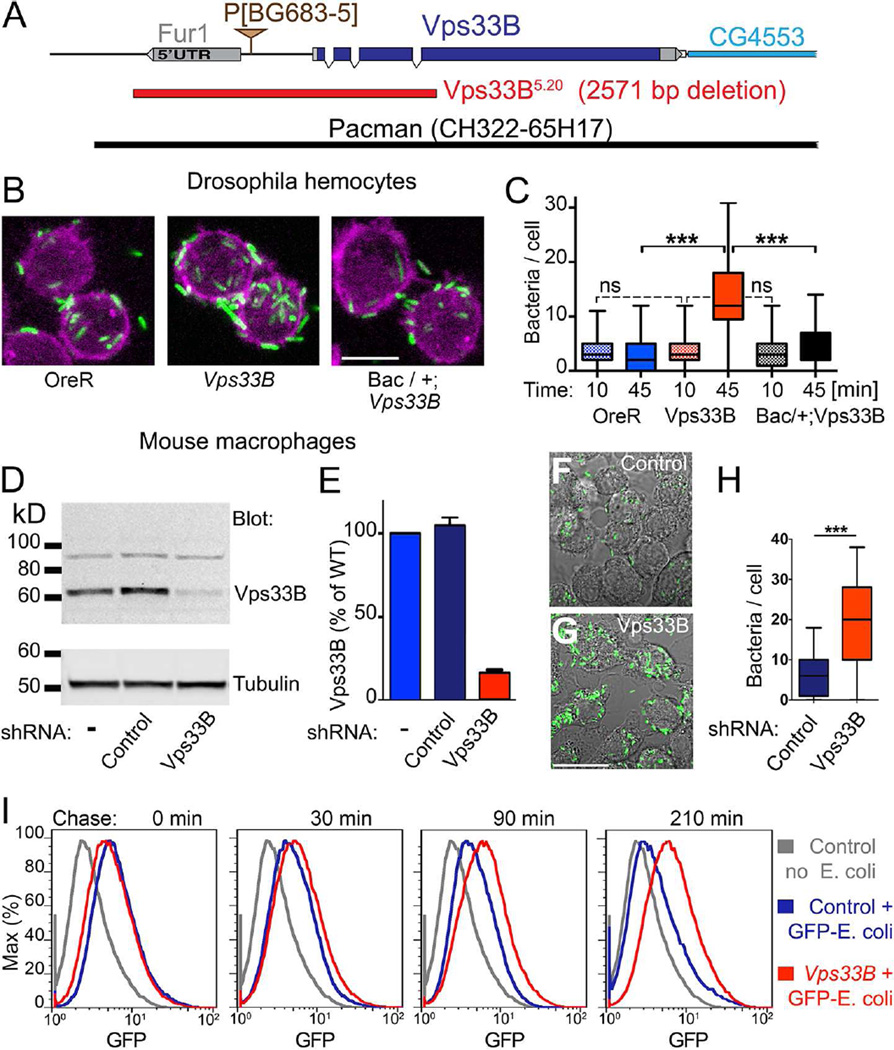
To investigate the role of Vps33B in Drosophila, we generated a null allele (Figure 1A). Similar to fob mutants (Akbar et al., 2011), flies null for Vps33B were viable and fertile. To investigate possible defects in phagosomal maturation, we isolated hemocytes from Vps33B larvae. When challenged with heat-inactivated E. coli, Vps33B hemocytes phagocytosed the bacteria, but, compared to hemocytes from Oregon R wild-type flies (OreR), their phagosomal clearance was inhibited (Figure 1B,C). Phagosomal clearance was restored by a bacterial artificial chromosome (BAC) transgene encompassing the entire Vps33B gene (Figure 1A–C), confirming that, like its binding partner Fob (Akbar et al., 2011), Vps33B is necessary for normal phagosomal maturation.
Figure 1. The requirement of Vps33B for phagosome maturation is conserved.
A) Map of Vps33B flanked by an alternative Fur1 noncoding exon and the neighboring CG4553 gene. The Vps33BB5.20 deletion allele removes the alternative Fur1 noncoding exon, the Vps33B promoter and codons for the first 196 amino acids of Vps33B. A transgene containing P[acman]Ch322-65H17 rescued all phenotypes of Vps33BB5.20 discussed here, but not a linked “blemished wing” phenotype.
B) Micrographs of primary hemocytes that phagocytosed GFP-labeled E. coli for 20 min and were chased for 45 min.
C) Quantification of phagocytosed E. coli detectable after 10-min or 45-min chases. Genotypes in B, C: were OreR, w1118;+/+;Vps33B5.20, and w1118; BACCH322-65H17/+; Vps33B5.20. (***: p<0.001, One way ANOVA)
D) Immuno blots of macrophages expressing none, scrambled control or Vps33B shRNA developed with Vps33B antibodies. Alpha-tubulin served as loading control.
E) Quantification of Vps33B protein from western blots as shown in D (n = 3).
F,G) Micrographs of macrophages expressing scrambled control (F) or Vps33B shRNA (G) allowed to phagocytose GFP-labeled E. coli for 20 min followed by a 45-min chase.
H) Quantification of phagocytosed E. coli detectable after a 45-min chase as shown in F and G (***: p<0.001, Two-tailed t-test).
I) Flow cytometry analysis of macrophages expressing control or Vps33B shRNA allowed to phagocytose GFP-labeled E. coli for 20 min followed by a chase of the indicated times. [All data here are representative of at least 3–5 independent experiments.
Please also see Figure S1.
To test whether this function of Vps33B is conserved between flies and mammals, we used shRNAs to generate immortalized bone marrow-derived macrophages (iBMDMs) (Nagpal et al., 2009) deficient for Vps33B. Five different shRNAs were used to target Vps33B mRNA in macrophages. Expression of shRNA#1 effectively reduced Vps33B protein expression (Figure 1D,E) and was used in all subsequent experiments.
First, we measured the effect of Vps33B silencing on phagocytosis and microbial clearance. Initial phagocytic uptake of dextran beads was unchanged (Figure S1). By contrast, bacterial clearance was significantly reduced in macrophages expressing Vps33B shRNA compared to those expressing scrambled control shRNA (Figure 1F–H). When we quantified phagocytic uptake and subsequent clearance of bacteria by flow cytometry, we found no difference in the ability of wild-type and Vps33B-deficient macrophages to phagocytose bacteria (Figure 1I, 0-min chase). However, subsequent time points revealed drastically reduced ability of Vps33B-deficient macrophages to clear phagocytosed bacteria (Figure 1I). These data indicate that, like its Drosophila ortholog, murine Vps33B is important for clearance of bacterial cargo and suggest a conserved function for Vps33B proteins in the maturation of phagosomes.
Vps33B is critical for regulating inflammatory responses
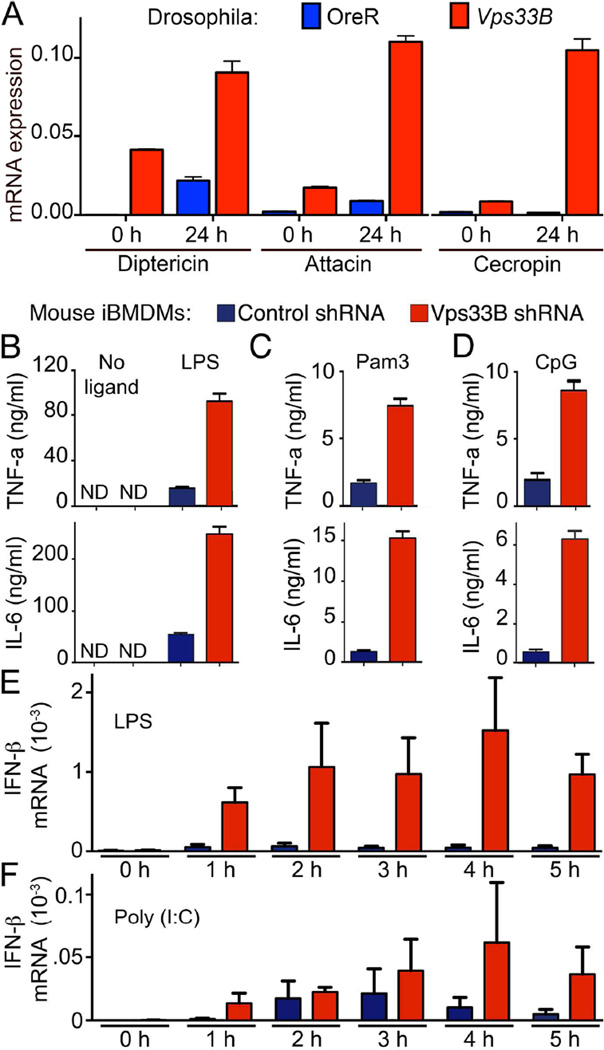
Since Vps33B-deficient macrophages displayed a similar inability to clear phagocytosed bacteria as their Drosophila counterparts, we were interested in understanding the impact of loss of Vps33B function on innate immune responses in both models. In infected flies, activation of the Toll or IMD pathways (Lemaitre and Hoffmann, 2007) induces anti-microbial peptides (AMPs). Consistent with defects in bacterial clearance, Vps33B adult flies injected with heat-killed E. coli exhibited strikingly increased expression of AMPs (Figure 2A).
Figure 2. Vps33B has a conserved function in the regulation of innate immune signaling.
A) Quantification of transcripts encoding the indicated anti-bacterial peptides relative to the ribosomal protein RP49 before or 24 h after injection of E. coli into OreR or Vps33B5.20 mutant adult flies.
B–D) ELISA-quantification of TNFα and IL-6 cytokines after a 12-h stimulation of macrophages expressing control or Vps33B shRNA with no ligand or LPS (B), Pam3CSK4 (C) or CpG (D).
E,F) qRT-PCR quantification of IFNβ expression relative to Histone2A after stimulation of macrophages expressing control or Vps33B shRNA with LPS (E) or Poly:IC (F) for the indicated times. (ND: not detected).
Data in A, E and F are representative of 2 independent experiments and data in B–D are representative of 5 independent experiments.
For macrophages, we decided to focus on the TLR family of PRRs because of the diversity of their ligands, their role in regulating phagocytosis (Barton and Kagan, 2009; Beutler et al., 2006; Kawai and Akira, 2010) and the distinct signaling profiles described for cell surface and endosomal TLRs (Husebye et al., 2006; Kagan et al., 2008). In response to LPS-mediated activation of TLR4, Vps33B silenced macrophages displayed enhanced expression of the pro-inflammatory cytokines TNF-α and IL-6 (Figure 2B). We investigated the status of downstream signaling components, following TLR4 stimulation and did not find any major differences in activation status of NF-κB and MAP Kinases, JNK and ERK (Figure S2). However, p38 phosphorylation was slightly enhanced at early time points (15 and 30 min) in Vps33B-silenced macrophages compared to controls upon LPS stimulation (Figure S2).
Elevated responses were not limited to LPS-induced TLR4 signaling, as activation of TLR2 by Pam3CSK4 (Figure 2C) and TLR9 by CpG (Figure 2D) also led to strongly increased secretion of IL-6 and TNF-α by Vps33B-silenced macrophages. This is consistent with a recent report that TLR2 endocytosis in human monocytes is important for signaling (Brandt et al., 2013). Similarly, the TLR9 data suggest an important role for Vps33B in regulating the function of endosomal TLRs. Furthermore, in response to the TLR4 ligand LPS as well as the TLR3 ligand Poly(I:C), the TRIF-dependent expression of IFN-β was strongly enhanced in macrophages lacking Vps33B (Figures 2E,F). In contrast to the previous understanding that there is spatial segregation of MyD88 (plasma membrane) and TRIF (endosome)-dependent signaling, these data suggest that both MyD88- and TRIF-dependent signaling pathways can be concomitantly active, following TLR4 endocytosis.
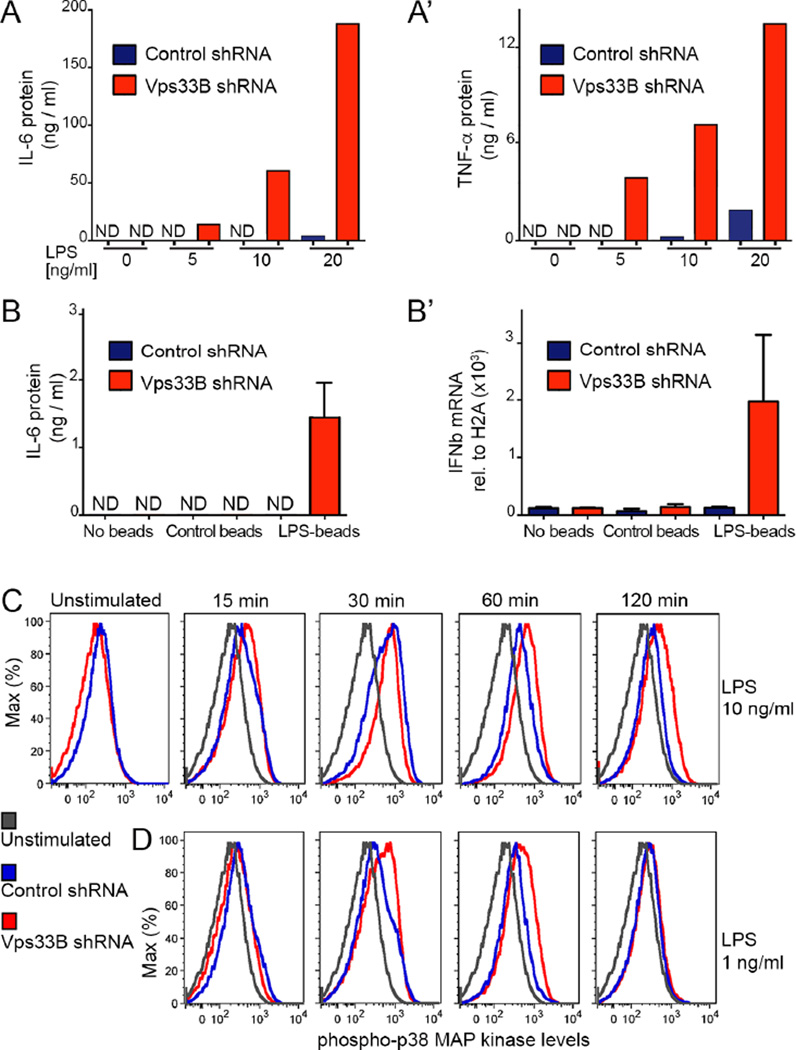
Cytokine production by Vps33B-silenced macrophages was robustly enhanced at low doses of soluble LPS that enter cells by endocytosis (Figure 3A,A’). Similarly, phagocytosis of low concentrations of bead-immobilized LPS induced substantially higher expression of IL-6 and IFN-β (Figure 3B,B’) in Vps33B-deficient macrophages. We further examined the effects of small doses of LPS stimulation on p38 MAP kinase activation in WT and Vps33B-deficient macrophages. At 10 ng/ml of LPS, initial p38 activation was similar (15 and 30 min), but remained elevated in Vps33B-deficient macrophages up to 120 min (Figure 3C). At only 1 ng/ml of LPS, Vps33B-deficient macrophages displayed transiently elevated phospho-p38 at 30 and 60 min compared to control macrophages, but were indistinguishable at 120 min (Figure 3D). It remains to be examined if altered p38 activation contributes to elevated pro-inflammatory responses in both flies and mouse macrophages lacking Vps33B function.
Figure 3. Vps33B silencing sensitizes macrophages to low LPS doses.
A) ELISA measurements of IL-6 (A) and TNF-α (A’) in the supernatants of Vps33B-deficient or control macrophages exposed to the indicated concentrations of LPS for 12 hours.
B) Vps33B-silenced or control macrophages were exposed to control beads or LPS-bound beads for 6 hours. Secreted IL-6 was measured by ELISA (B) or IFNβ transcript relative to Histone2A by qRT-PCR (B’).
(“ND” indicates not detected).
C,D) Flow cytometry analysis of phospho-p38 MAP kinase at indicated time points after stimulation with 10 ng/ml (C) or 10 ng/ml of LPS (D).
Data in this figure here are representative of 3 independent experiments. Please also see Figure S2.
Together, these findings suggest that Vps33B may be important not only for phagosomal maturation, but also for endosomal trafficking of activated TLRs and thereby regulate cytokine production. Since signaling pathways from the plasma membrane and from endosomes are well characterized for TLR4, we focused on TLR4-mediated trafficking for further characterization of the role of Vps33B in regulating signaling outcomes and dynamics of endosomal maturation.
Vps33B modulates endosomal dynamics of pattern recognition receptors
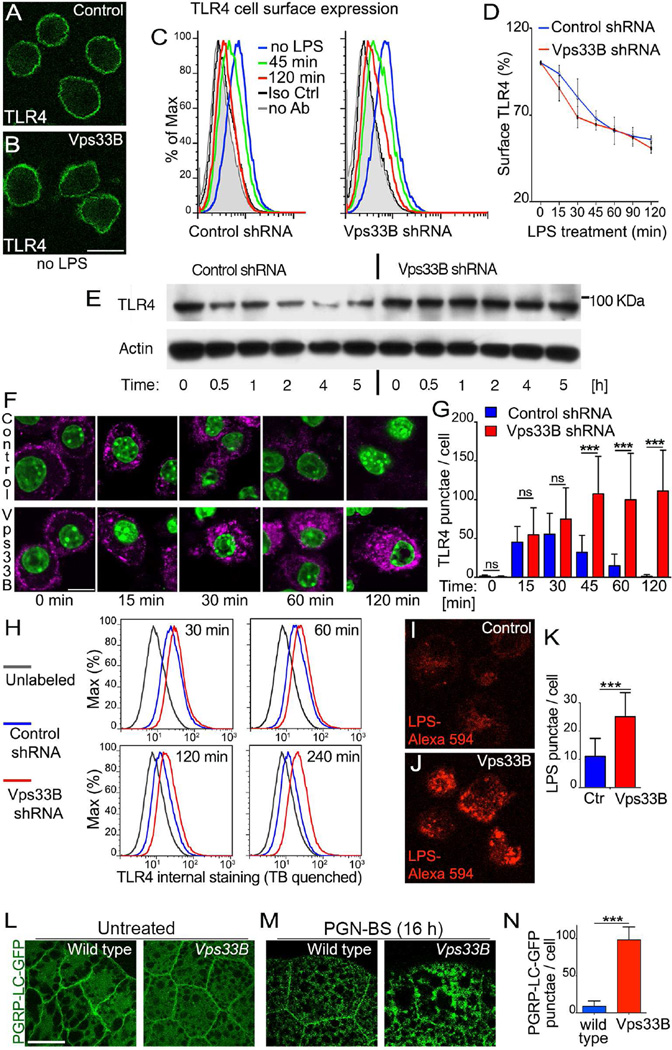
LPS promotes TLR4 internalization and the subsequent distinct signaling from endosomes prior to TLR4 degradation in lysosomes (Husebye et al., 2006; Kagan et al., 2008). To investigate whether Vps33B has a role in TLR4 trafficking after LPS stimulation, we first monitored the loss of TLR4 from the cell surface. Neither immunostaining (Figure 4A,B) nor flow cytometry-based quantification (Figure 4C,D) detected differences in TLR4 surface expression between control and Vps33B-silenced macrophages before LPS treatment. Similarly, LPS-induced downregulation of cell surface TLR4 was unaltered by loss of Vps33B (Figure 4C,D) suggesting that Vps33B does not play a role in the internalization of TLR4 following stimulation. Immuno-blotting revealed a steady decrease of total TLR4 protein in wild-type macrophages, consistent with surface downregulation followed by intracellular degradation. By contrast, despite the downregulation of surface TLR4 following LPS stimulation, there was no reduction in total TLR4 protein in VPS33B-deficient macrophages (Figure 4E). This suggested a possible alteration in intracellular trafficking and accumulation of TLR4 in the absence of Vps33B.
Figure 4. Vps33B is required for degradation of TLR4 and PGRP-LC.
A–D) Surface expression of TLR4 in non-permeabilized macrophages expressing control or Vps33B shRNA detected by immunofluorescence staining (A,B) or measured by flow cytometry (C) at 0, 45 or 120 min after LPS stimulation (1µg/ml). Cells with no antibodies or with isotype control antibodies are shown for comparison. Scale bar: 20 µm. (D) Quantification of flow cytometry data as shown in panels C revealed no significant difference in the decline of TLR4 cell surface expression following LPS stimulation over a 2-h time course.
E) Immuno blot of TLR4 protein in macrophages expressing control or Vps33B shRNA following exposure to LPS (1 µg/ml).
F,G) Intracellular accumulation of TLR4 detected in micrographs (F) of macrophages expressing control or Vps33B shRNA that were surface-labeled with TLR4 antibodies and chased for the indicated times following LPS activation. Scale bar: 10 µm. G) Quantification of internal TLR4 punctae detected in micrographs. (ns: not significant, ***: p<0.001, one-way ANOVA).
H) Flow cytometry analysis of internal TLR4 staining of macrophages expressing control or Vps33B shRNA at indicated times following LPS activation. External staining was quenched with trypan blue (TB).
I–K) Intracellular accumulation of LPS-Alexa-594 (5 µg/ml) after an 18-h exposure of macrophages expressing control (I) or Vps33B shRNA (J). Quantification of fluorescent LPS-Alexa-594 punctae (K) revealed a significant increase in Vps33B-silenced macrophages compared to controls. (***: p<0.001, two-tailed t-test).
L–N) Micrographs visualizing PGRP-LC-GFP localization in wild-type or Vps33B fat bodies cultured without (L) or with PGN-BS (M). Scale bar: 40 µm. Quantification of internal PGRPLC-GFP punctae (N). (***: p<0.001, Two-tailed t-test).
All data in this figure are representative of 4 independent experiments.
To test for changes in the fate of endocytosed receptor, we stained permeabilized macrophages for TLR4. As previously described (Husebye et al., 2010), TLR4 redistributed from the cell surface to endosomes at early time points after LPS stimulation in both control and Vps33B-silenced macrophages (Figures 4F, 15 and 30 min). At later time points, differences emerged between control and Vps33B-silenced cells. At 60 and 120 min after LPS stimulation, the overall amount of intracellular TLR4 was significantly higher in Vps33B-silenced macrophages compared to controls (Figure 4F,G). This difference is most likely due to reduced TLR4 degradation in Vps33B-silenced macrophages, rather than recycling, as cell surface expression did not appreciably differ at these time points (Figure 4C,D). These findings were corroborated by intracellular flow cytometry, which detected high amounts of intracellular TLR4 in VPS33B-deficient macrophages at 2 and 4 hours following LPS stimulation, when TLR4 protein in wild-type cells had substantially declined (Figure 4H).
The intracellular TLR4 accumulation likely represents a subset of receptors that is targeted for LPS-induced degradation in lysosomes of control macrophages, but persists in Vps33B-silenced macrophages. Differences in intracellular trafficking and defects in lysosomal delivery are also consistent with the accumulation of Alexa-594-labeled LPS in Vps33B-silenced macrophages (Figure 4I–K) and point to a role of Vps33B in endo-lysosomal fusion events that result in degradation of TLR4 and its ligand. Consistent with TLR4 accumulation in mouse macrophages, the Drosophila pattern recognition receptor, PGRP-LC, also accumulated in discrete intracellular compartments following stimulation with its ligand PGN-BS in fat bodies of Vps33B, but not wild-type larvae (Figure 4L–N). These data highlight the fundamental similarities in the role of Vps33B in regulating the fate of pattern recognition receptors, following their endocytosis, in both flies and vertebrates.
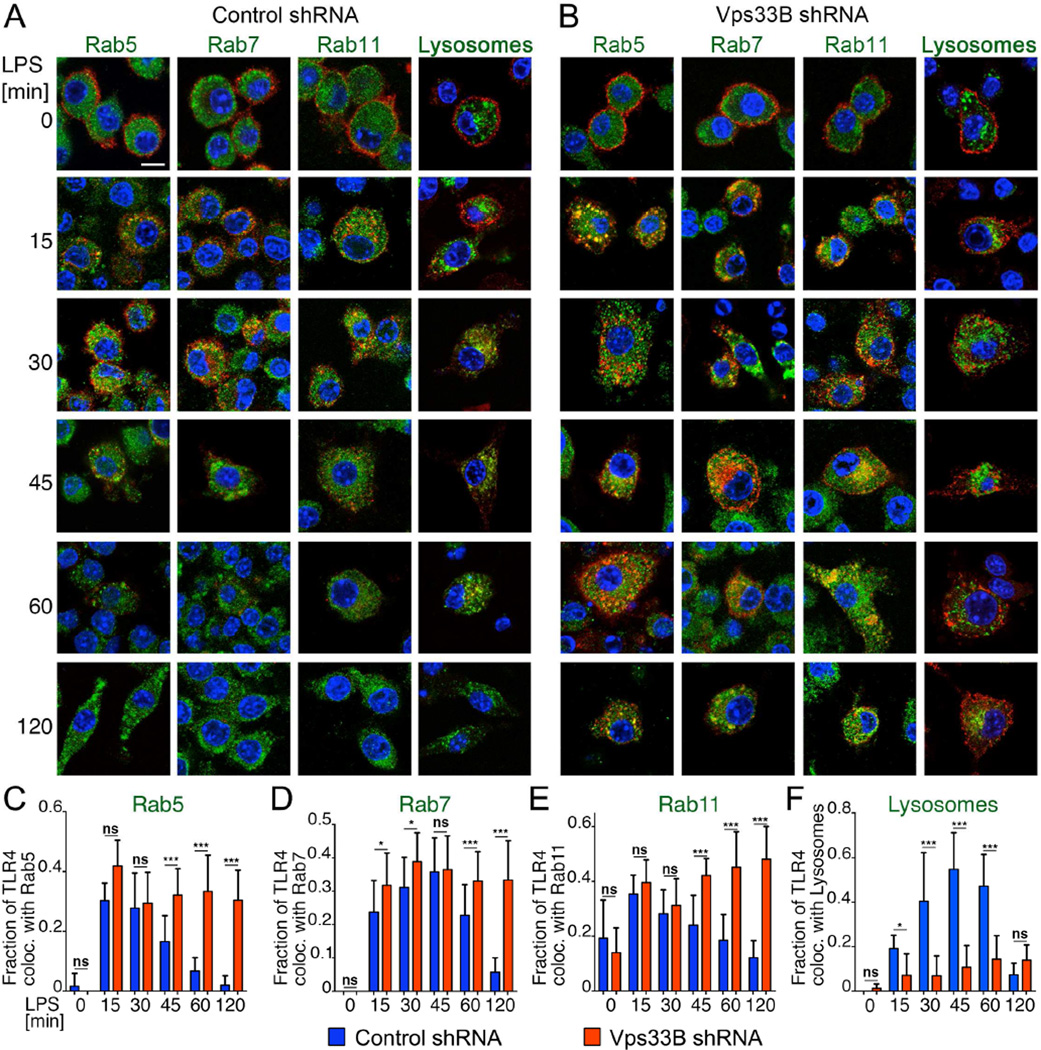
To investigate the dynamics of TLR4 endocytosis and the fate of TLR4 containing endosomes following LPS stimulation, we stained macrophages for Rab proteins associated with various endosomal compartments. Rab5 localizes to early endosomes where it facilitates recruitment of Rab7 and maturation of early endosomes to late endosomes (Poteryaev et al., 2010). Consistent with these roles of Rab5 and Rab7, we found that in both wild-type and Vps33B-deficient macrophages, Rab5 and Rab7 were rapidly recruited to TLR4 containing endosomes at 15 and 30 min following LPS stimulation (Figure 5A–D). In the same time frame, some 30% to 40% of TLR4 colocalized with Rab11a, a marker for recycling endosomes (Figure 5A,B,E), consistent with previous observations (Husebye et al., 2010). Striking differences between control and Vps33B-silenced cells in the endosomal distribution of TLR4 emerged only at later time points. Starting at 45 min after LPS induction, TLR4 was significantly enriched in Vps33B-silenced macrophages compared to controls (Figure 4G) and remained associated with all three endosomal Rabs, whereas their co-localization in wild-type macrophages started to decline (Figure 5A–E), as the majority of TLR4 had reached dextran-labeled lysosomes (Figure 5A,F). In Vps33B-silenced cells, by contrast, TLR4 remained largely excluded from lysosomes (Figure 5B,F). These data suggest that unlike wild-type macrophages, where TLR4-containing endosomes undergo rapid maturation, the TLR4-containing aberrant endosomes in Vps33B-silenced macrophages retain characteristics of early, late and recycling endosomes as their fusion with lysosomes is inhibited.
Figure 5. Vps33B is required for maturation of TLR4-positive endosomes in LPS-stimulated macrophages.
A,B) Micrographs of macrophages expressing control (A) or Vps33B shRNA (B) visualize TLR4-positive endosomes and the indicated Rabs or dextran preloaded into lysosomes, following LPS stimulation chases for the indicated times. Scale bar: 10 µm.
C–F) Quantification of the fraction of TLR4 colocalizing with the indicated Rabs or dextran-labeled lysosomes at different times after LPS stimulation. Note that the lower colocalization of TLR4 with lysosomes at 120 min in control macrophages primarily reflects the low amounts of TLR4 remaining in these cells. All data here are representative of 3 independent experiments.
Vps33B is required for LPS-induced trafficking to lysosomes
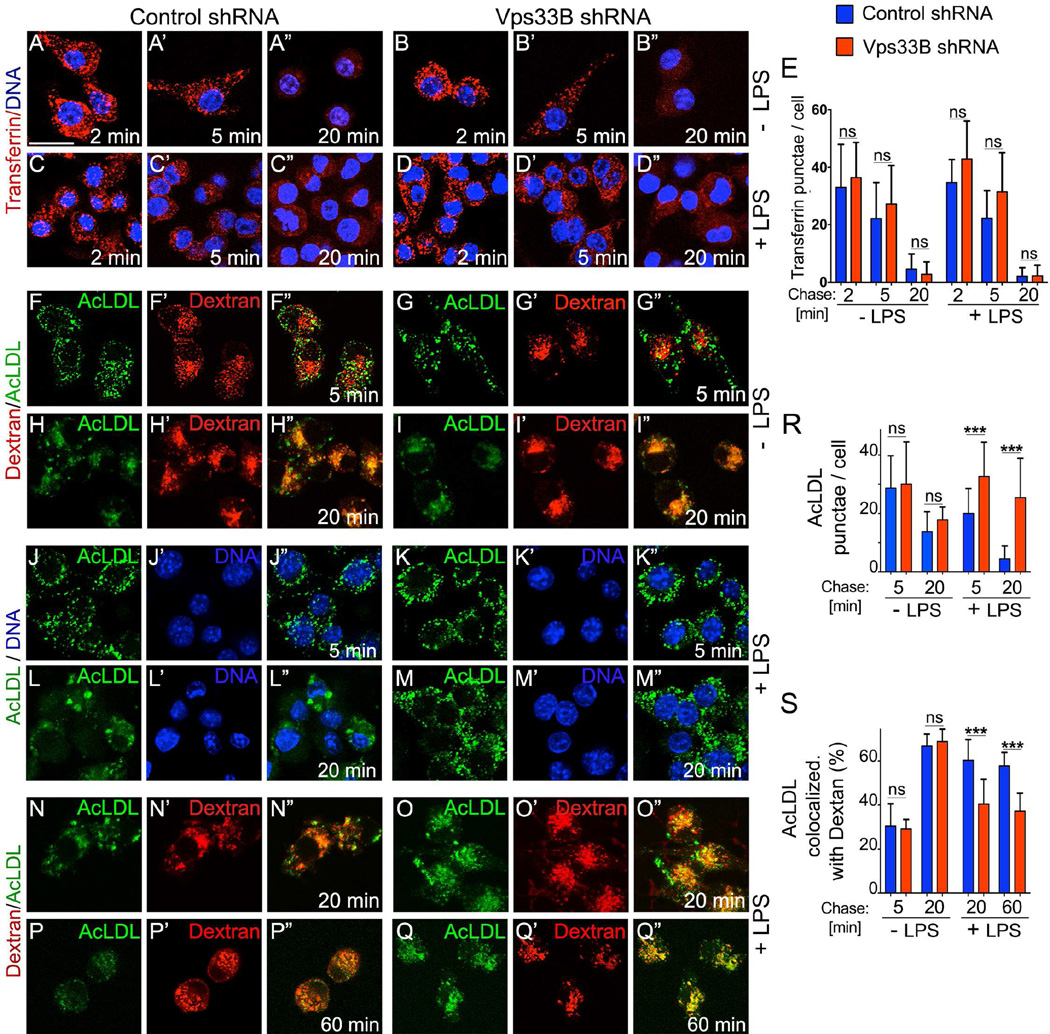
The intracellular accumulation of endocytosed TLR4 and its ligand, LPS, prompted us to examine if Vps33B specifically regulates endosomal trafficking of pattern recognition receptors or also affects other receptors not involved in inflammatory responses. For example, after internalization, Transferrin receptors (TfR) recycle back to the plasma membrane from the common early endosomal network. Unlike TLR4 however, Transferrin internalization and recycling was unaltered by loss of Vps33B in naïve and LPS-stimulated cells (Figure 6A–E).
Figure 6. The requirement of Vps33B for maturation is restricted to only a subset of endosomes.
Micrographs visualize different endosomal trafficking routes in macrophages with or without a 30-min pre-exposure to LPS (1µg/ml) as indicated.
A–E) Transferrin internalization and recycling at the indicated time points in control (A,C) and Vps33B-silenced (B,D) macrophages without (A,B) or with (C,D) LPS treatment. (E) Quantification of Transferrin punctae per cell. Scale bar: 20 µm in A to Q.
F–I) FITC-AcLDL (green) uptake and lysosomal delivery was visualized in control (F,H) or Vps33B-silenced macrophages (G,I) after a chase of 5 min (F,G) or 20 min (H,I). Lysosomes were pre-labeled by internalization and overnight chase of dextran-Alexa594 (red).
J–M) FITC-AcLDL (green) amounts in LPS-treated control (J,L) or Vps33B-deficient (K,M) macrophages after a chase of 5 min (J,K) or 20 min (L,M).
N–Q) FITC-AcLDL (green) lysosomal delivery was visualized in LPS-treated control (N,P) or Vps33B-silenced macrophages (O,Q) after a chase of 20 min (N,O) or 60 min (P,Q). Lysosomes were pre-labeled by internalization and overnight chase of dextran-Alexa594 (red).
R) Quantification of AcLDL-punctae per cell.
(S) Percentage of AcLDL co-localizing with dextran-labeled lysosomes.
Data in this figure are representative of at least 2–4 independent experiments. Please also see Figure S3.
A different endocytic route is taken by cargoes like cholera toxin B subunit (CTxB), which binds to GM1 gangliosides and enters the early endosomal network marked by Transferrin before reaching the Golgi by retrograde transport (Johannes and Popoff, 2008). We observed no difference between Vps33B-silenced and control macrophages in CTxB’s colocalization with early endosomal Transferrin after a 10-min chase (Figure S3A,B) or in its subsequent retrograde transport to the Golgi complex following chases of 15 or 60 min in naïve (Figure S3C–F) or LPS-stimulated cells (Figure S3G–J).
Other cargos, such as acetylated-LDL (AcLDL), are directed toward lysosomal degradation via late endosomes in macrophages (Fukuda et al., 1986). After a 5-min chase, FITC-AcLDL uptake was visible with a typical punctate endosomal distribution in both control and Vps33B-silenced cells (Figure 6F,G,R). After a 20-min chase, more than 60% of Ac-LDL had reached dextran-labeled lysosomes (Figure 6H,I,S). At this time point, regardless of the status of Vps33B FITC fluorescence was reduced (Figure 6R), indicative of AcLDL degradation and quenching of FITC fluorescence by the acidic lysosomal environment.
Since LPS modulates endocytic trafficking of some cargos in macrophages, including AcLDL (Peppelenbosch et al., 1999), we tested whether Vps33B silencing altered trafficking of this scavenger receptor cargo in LPS-stimulated macrophages. As early as 5 min following AcLDL uptake into LPS-stimulated macrophages, Vps33B deficiency resulted in elevated intracellular AcLDL compared to control macrophages (Fig. 6J,K,R). After a 20-min chase in LPS-activated control macrophages (Figure 6L), their FITC fluorescence was 78% reduced (Fig. 6R) consistent with AcLDL degradation and quenching of FITC in lysosomes. Accordingly, the remaining FITC-AcLDL co-localized to 60% with dextran-labeled lysosomes (Fig. 6N,S). By contrast, FITC fluorescence in LPS-activated Vps33B-silenced macrophages was reduced less than 25% after a 20-min chase (Figure 6M,R) and only some 40% of the remaining AcLDL had reached lysosomes (Figure 6O,S). Even after a 60-min chase this fraction did not increase (Fig 6Q,S). Altered maturation of TLR4 and AcLDL-positive endosomes was not due to changes in lysosome structure or distribution as detected by lysotracker or Lamp2 staining (Figure S3K–N). Furthermore, in contrast to lysosomal delivery of AcLDL, recycling of Transferrin (Figure 6C,D,E) and retrograde trafficking of CTxB to the Golgi (Figure S3G–J) were not affected by loss of Vps33B, regardless of TLR stimulation by LPS. Together, these data indicate that LPS stimulation alters only a subset of endosomal trafficking routes in a way that makes them dependent on Vps33B function.
Abrogation of PRR signaling protects Vps33B mutants from death induced by non-pathogenic bacteria
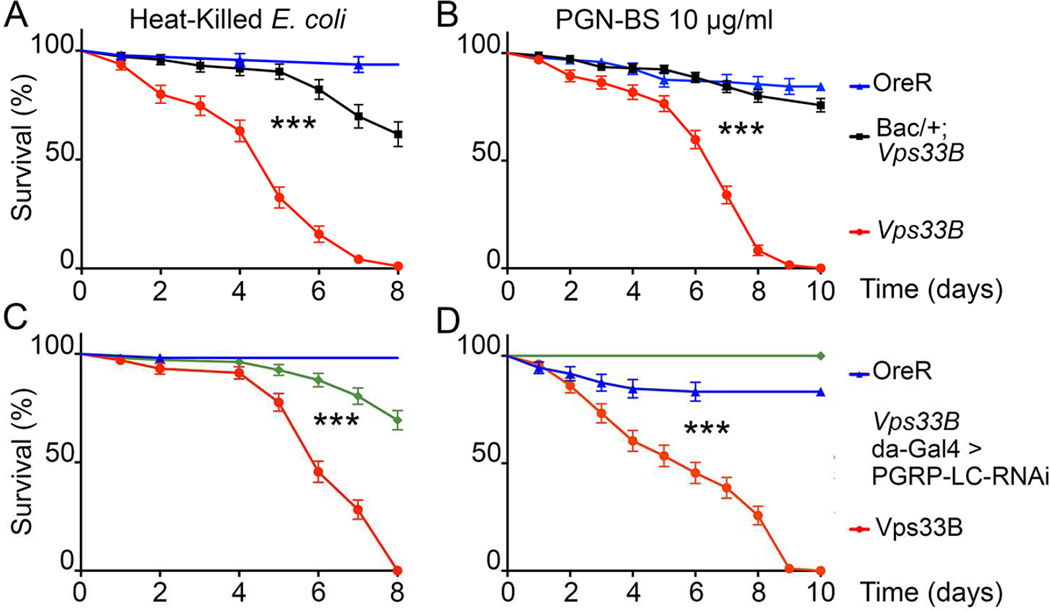
To test the possibility that infected flies might be dying because of exaggerated signaling rather than failure to control bacterial load (Akbar et al., 2011), we injected heat-killed E. coli into Vps33B and fob mutant flies. Unlike wild-type OreR flies, both mutants were susceptible to infections with the dead bacteria (Figures 7A,S4A). Viability after these injections was restored for Vps33B and fob mutants by genomic rescue transgenes confirming that both genes are critical for the normal response to heat-inactivated E. coli (Figures 7A,S4A).
Figure 7. Vps33B is necessary for Drosophila to tolerate non-pathogenic E. coli bacteria.
A–D) Diagrams show the fraction of surviving flies with indicated genotypes after injection with heat-killed E. coli (A, C) or peptidoglycans PGN-BS (B,D). Genotypes: (A,B) OreR, w1118; Vps33B5.2, w1118; BACCH322-65H17/+; Vps33B5.20 (C,D) OreR, w1118; Vps33B5.20, w1118;Vps33B5.20 da-Gal4 / Vps33B5.20 UAS-PGRP-LC-RNAi. (***: p<0.001, log-rank).
Data are representative of 3 independent experiments. Please also see Figure S4.
Bacterial infections trigger activation of the IMD and Toll pathways (Lemaitre and Hoffmann, 2007). These pathways are activated by peptidoglycans (PGN) of bacterial cell walls: DAP-type PGNs, such as PGN-BS isolated from Bacillus subtilis, activate the PGRP-LC receptor in the IMD pathway, whereas Lysine-type PGNs, such as PGN-SA isolated from Staphylococcus aureus, activate the Toll pathway (Kaneko et al., 2004; Leulier et al., 2003). Importantly, activation of these innate immune pathways by either type of PGN was sufficient to compromise survival of Vps33B flies (Figures 7B, Figure S4B). Similar to the response to heat-killed E. coli, this was due to loss of Vps33B function, as a genomic Vps33B transgene restored survival after PGN injection (Figures 7B,S4B). These results indicate that increased susceptibility to bacterial infections in Vps33B mutants is not due the bacterial burden suggesting that instead, exaggerated activation of immune signaling compromises their survival after infections.
To test whether such reduced tolerance to non-pathogenic bacteria was indeed due to cognate interaction between bacterial ligands and specific PRRs, we injected flies in which PGRP-LC receptor was silenced in a Vps33B background. Strikingly, PGRP-LC silencing significantly (p<0.001 log-rank) suppressed lethality of Vps33B mutants after injections with both heat-killed E. coli and PGN-BS (Figure 7C, D). Together these results uncover a role for Vps33B in the regulation of innate immune signaling and reveal its importance in the degradation of microbial cargo and the control of inflammatory responses, which is critical for tolerating microbial burden.
Discussion
Persistent infections and sepsis are recurring symptoms in ARC patients (Jang et al., 2009; Seo et al., 2014). Our findings indicate that, rather than being an indirect consequence of failures in other organ systems, these complications could reflect direct roles of the ARC protein Vps33B in innate immunity. First, we have shown that the requirement of Vps33B for phagosomal maturation was not restricted to Drosophila but conserved in mammalian macrophages. Second, loss of a conserved function of Vps33B in endosomal and phagosomal maturation in macrophages caused accumulation of activated internalized TLR4 receptors and its cargo in aberrant endosomal compartments resulting in elevated pro-inflammatory cytokine production. The importance of this excessive signaling was confirmed by silencing of the PGRP-LC receptor in Drosophila Vps33B mutants, which restored their ability to tolerate non-pathogenic bacteria thereby confirming the potentially important contribution of elevated immune signaling to the symptoms of ARC syndrome.
Members of the Vps33 sub-family of SM proteins mediate fusions with lysosomes and lysosome-related organelles (Solinger and Spang, 2013), but the division of labor between the Vps33A and Vps33B paralogs is not clearly understood. In Drosophila, Vps33A (Carnation) is part of the HOPS complex that mediates formation of pigment granules and the fusion of lysosomes with endosomes and autophagosomes (Akbar et al., 2009; Takats et al., 2014), functions that are conserved in mammals (Jiang et al., 2014; Suzuki et al., 2003). By contrast, loss of Drosophila Vps33B, or its binding partner Fob, does not interfere with endosomal or autophagosomal delivery of cargo to lysosomes, but prevents clearance of phagosomal content this work and (Akbar et al., 2011). Our findings in mouse macrophages suggest that this function is conserved in mammals as well.
Previous work has linked the complex of the ARC proteins, Vps33B and Vipas39, to Rab11a-positive endosomes (Cullinane et al., 2010). Intriguingly, Rab11a-positive endosomes have been identified as intermediates in the delivery of TLRs and the TRAM adaptor to phagosomes (Husebye et al., 2010; Nair-Gupta et al., 2014). Consistent with functions of Vps33B and Rab11a in related trafficking steps, loss of Rab11a alters the intracellular distribution of TLR receptors and compromises the tolerance to TLR ligands (Yu et al., 2014). We show here that following LPS-mediated activation, TLR4 was internalized into Rab5- and Rab7-positive early and late endosomes and there was no difference in the early aspects of endosomal maturation in the presence or absence of Vps33B. Furthermore, TLR4 localized to Rab11a enriched recycling endosomes as reported (Husebye et al., 2010). Our experiments expand on this finding, as we have shown that, although there was no difference in recruitment of Rab5, Rab7 and Rab11a to endocytic compartments that contain TLR4, Vps33B played a critical role in the kinetics of maturation of these endosomes. Absence of Vps33B led to sustained presence of TLR4 in endosomes marked by Rab5, Rab7 and Rab11. Together these findings suggest a role of Vps33B in regulating fusion events of Rab11a-positive endosomes that are critical for degrading activated TLRs and their ligands, thus regulating TLR-induced inflammatory responses in macrophages. In the absence of Vps33B, trapped TLRs induced enhanced inflammatory responses from aberrant endosomal compartments that concomitantly had adopted features of early, late and recycling endosomes.
Trafficking defects due to loss of Vps33B function are not restricted to receptors in the immune system. In ARC patients the altered distribution of the bile salt export protein BSEP in liver cells contributes to cholestasis, a defining feature of ARC, which is also characterized by the intracellular accumulation of CD26 in kidney cells (Cullinane et al., 2010; Gissen et al., 2004). Such defects in intracellular trafficking appear to contrast with our findings that endocytic trafficking appears normal in fob or Vps33B mutant flies (Akbar et al., 2011) and data presented here showing that classic endocytic trafficking routes of scavenger receptors or receptors for transferrin and cholera toxin are unaffected by Vps33B deficiency in naïve macrophages.
A resolution to this paradox may be offered by our analysis of Ac-LDL trafficking. In naïve macrophages, Ac-LDL is internalized and rapidly degraded in lysosomes, regardless of Vps33B function. By contrast, in LPS-stimulated macrophages Ac-LDL appears to be shunted into a different pathway that requires Vps33B for lysosomal delivery. It is important to note here that Vps33B and VIPAS39 are phosphorylated in response to LPS-mediated activation (Chen et al., 2012; Sjoelund et al., 2014) and their activity is regulated by phosphorylation (Bach et al., 2008; Ishii et al., 2012). In particular, dephosphorylation of Vps33B by PtpA of Mycobacteria leads to inhibition of phagolysosomal fusion (Bach et al., 2008). It remains to be tested whether such specific inhibition of Vps33B function is responsible for heightened inflammatory responses that cause pathology during Mycobacterial infections. Ligands of scavenger receptors, such as Ac-LDL, can be internalized by multiple endocytic pathways (Howes et al., 2010), and among them macropinocytosis has been shown to be upregulated in LPS-activated cells of the immune system (West et al., 2004). Other LPS-induced changes in trafficking include altered phagosomal maturation (Blander and Medzhitov, 2006). Changes in the cellular handling of cargo, such as the Ac-LDL ligand of scavenger receptors, are consistent with the intricate link and cross talk between TLRs and scavenger receptors (Erdman et al., 2009). Since LPS has the ability to bind both, scavenger receptors (Hampton et al., 1991) and TLR4 (Poltorak et al., 1998), initiation of signal transduction from TLR4 coincident with scavenger receptor-mediated endocytosis may lead to the formation of Vps33B-dependent endosomes in conjunction with TLR4-induced Vps33B phosphorylation and activation. We propose, thus, that scavenger receptor cargo is trafficked differently in the presence and absence of associated microbial signals; this difference, however, becomes apparent only in the absence of Vps33B.
Notably, our observation that MyD88-dependent and TRIF-dependent genes were upregulated in the absence of VPS33B suggests that both of these signaling events are sustained even after TLR4 is internalized. Endosomes formed in the absence of Vps33B remained positive for Rab5 and Rab7 suggesting that, at least in part, they maintain early endosomal characteristics that may facilitate continued signaling through the MyD88-dependent pathway. Additionally, recruitment of Rab11a might facilitate signaling through the TRIF axis leading to increased production of TRIF-dependent cytokines (Klein et al., 2015). Among the different elements of the downstream signaling cascade, MAP kinase, p38 was appreciably enhanced by the Vps33B-dependent changes in receptor trafficking. In Drosophila, p38 kinase has been implicated in the ability of flies to tolerate non-pathogenic bacteria (Shinzawa et al., 2009), similar to our observations of Vps33B mutant flies. It is therefore plausible that higher p38 phosphorylation at early time points and its sustained activation at later time points might be responsible for increased production of pro-inflammatory cytokines. Alternatively, persistent TLR4 and its ligand in endosomes might lead to other transcriptional and post-transcriptional events (Decque et al., 2016) that enhance inflammatory responses.
Elevated inflammatory responses, in the absence of Vps33B, are not restricted to cell surface TLRs, but extend to endosomal TLRs (TLR3 and TLR9) as well. This suggests that, following ligand recognition, TLR3 or TLR9-containing endosomes depend on Vps33B to undergo further maturation to degrade the respective receptors and their ligands, thus terminating inflammatory responses. Earlier reports suggest a critical role for Rab7b on late endosomes and lysosomes in the degradation of both TLR4 and TLR9 and the termination of their signaling (Wang et al., 2007; Yao et al., 2009). Consistent with this idea, our finding that Vps33B is a major regulator of endosomal and phagosomal maturation following TLR stimulation sheds light on how microbial presence dictates endocytic and phagocytic events and their importance for termination of inflammatory responses.
The intricate interactions between trafficking and signaling may be best illuminated by the extreme susceptibility of fob and Vps33b mutant flies to infections with non-pathogenic E. coli. Our study demonstrates that the cause of death for Vps33B flies is not the bacterial load itself but exaggerated anti-microbial responses to non-pathogenic microbes, similar to sepsis that contributes to death in ARC patients (Jang et al., 2009; Seo et al., 2014). Furthermore, such exaggerated TLR signaling can cause innate immune paralysis (Hotchkiss et al., 2013), where the innate immune system, following a robust response to the first infection, fails to respond to secondary infections, possibly resulting in enhanced susceptibility of ARC patients to pathogenic microbes.
Experimental Procedures
Fly work
Vps33B null allele was generated by mobilizing P-element BG683-5 inserted upstream of the Vps33B start codon as described (Akbar et al., 2009). One line, Vps33B5.20, had a ~2.3 kb-deletion that removes 1161 bp of the Vps33B gene including the first three and part of the fourth exon, a total 736bp of Vps33B coding sequences. This line also deletes 1073 bp in the upstream non-coding region at the 5’ end of the neighboring Fur1 gene. For rescue experiments, the Pacman clone CH322-65H17, which contains the entire genomic region of Vps33B, but not Fur1, was inserted at the 53B2 landing site (Best Gene).
For infection experiments, flies were injected with 80 nl PBS containing a mean of 1,600 heat-killed E. coli (DH5α) and survival and AMP induction were measured (Akbar et al., 2011) with primers listed in supplemental Table I. Peptidoglycans (InvivioGen) PGN-BS (Cat no. tlrl-pgnb3) and PGN-SA (Cat no: tlrl-pgnsa) were injected at 10 µg/ml in PBS (50 nl per fly). All injection experiments were repeated 8 to 10 times.
Gene Silencing in mouse macrophages
Previously generated WT iBMDMs (Nagpal et al., 2009) were used to silence Vps33B as described before (Troutman et al., 2012) by stable expression of MISSION shRNA plasmids containing hairpin sequence generated from different regions of the Vps33B gene according to manufacturer’s instructions (Sigma-Aldrich). Silencing efficiency was tested by RT-PCR and western blotting. Vps33B antibodies (1:500 dilution) were raised in rabbits against a His-fusion protein containing amino acids 341–590 of human Vps33B.
Immunofluorescence Staining
To follow endosomal cargoes, untreated or LPS-treated (1 µg/ml) macrophages were pre-labeled with endocytic tracers or directly used for immunostaining. Imaging experiments were repeated three to five times and typical images are shown. For quantification, sufficient frames were analyzed to sample 50–100 cells. For TLR4 trafficking assays, cells were pre-stained with biotin-labeled TLR4 antibodies (1:100; Novusbio: NBP2-27149B), fixed at indicated times in 4% formaldehyde in PBS and permeabilized on ice with 0.3% saponin in PBS+0.5% BSA. Antibodies were used as follows: GM130 (1:100; BD Bioscience #610822) Rab11a (1:50; Cell Signaling #2413), Rab5a (1:75, Santa Cruz sc-309), Rab7 (1:50, Cell Signaling #9367), LAMP2 (1:500, Santa Cruz #ABL-93). Secondary antibodies were goat anti-rat Alexa488 and goat anti-rabbit Alexa568 or Streptavidin-568 (1:500, Invitrogen). Topro3 was added to visualize DNA before imaging by confocal microscopy.
Western blots
Immuno blots measured TLR4 protein at indicated times after LPS induction (1 µg/ml) as described (Troutman et al., 2012) using TLR4-biotin antibodies (1:1000; Novusbio: NBP2-27149B) and anti-mouse HRP-conjugated secondary antibody (Biorad).
Flow Cytometry
To measure surface TLR4 expression, Vps33B-silenced and control macrophages were stimulated with LPS (1µg/ml) for the indicated times followed by Fc block (Mouse BD Fc #553141) and staining with TLR4 antibody (Novusbio: NBP2-27149B or Biolegend #117609, 145403 and 145402) or isotype control (Biolegend #400507, 400521). Cell-associated fluorescence was analyzed using a FACSCalibur flow cytometer (BD Biosciences).
FlowJo software (Tree Star, inc) was used to analyze flow cytometry data (Hu et al., 2011).
Microscopy and Image analysis
Fluorescence images were captured using a 63×NA1.4 objective (pinhole: one Airey unit) on a Zeiss confocal microscope (LSM510). Images were quantified in ImageJ after using Gaussian blur of 1, auto-thresholding at 0.1–10 for size and 0.05–1 for circularity. 50–100 cells were quantified for each condition. Color images were generated using ImageJ and were adjusted in Adobe Photoshop for contrast.
Supplementary Material
Highlights.
Loss of Vps33B in flies and macrophages leads to enhanced inflammatory responses.
Vps33B is necessary for Drosophila to tolerate non-pathogenic bacteria
Activated TLR4 enters an endosomal trafficking route requiring Vps33B for maturation.
High inflammation due to altered endosomes may contribute to symptoms of ARC syndrome.
Acknowledgments
We thank members of the Pasare and Krämer labs for helpful comments and the Bloomington Stock Center and Dr. Bruno Lemaitre for fly stocks, the Berkeley Drosophila Genome Project for producing DNA clones, the Drosophila Genomics Resource Center for their distribution and Dr. Kate Fitzgerald for wild-type immortalized macrophages. FlyBase provided important information used in this work. This work was supported by grants from the National Institutes of Health (EY010199, EY021922) to H. K. and (AI082265, AI115420 and AI113125) to C. P. and core grant EY020799.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M.A.A., C.P. and H.K. designed the experiments and wrote the manuscript.
M.A.A., C.T. and R.M. performed almost all experiments.
W.H. provided critical advice and did some of the iBMDM experiments.
All authors commented on manuscript, data, and conclusions before submission.
References
- Akbar MA, Ray S, Krämer H. The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol Biol Cell. 2009;20:1705–1714. doi: 10.1091/mbc.E08-03-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar MA, Tracy C, Kahr WH, Krämer H. The full-of-bacteria gene is required for phagosome maturation during immune defense in Drosophila. The Journal of cell biology. 2011;192:383–390. doi: 10.1083/jcb.201008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H, Papavinasasundaram KG, Wong D, Hmama Z, Av-Gay Y. Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell host & microbe. 2008;3:316–322. doi: 10.1016/j.chom.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Baker RW, Jeffrey PD, Hughson FM. Crystal Structures of the Sec1/Munc18 (SM) Protein Vps33, Alone and Bound to the Homotypic Fusion and Vacuolar Protein Sorting (HOPS) Subunit Vps16*. PloS one. 2013;8:e67409. doi: 10.1371/journal.pone.0067409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature reviews Immunology. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annual review of immunology. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nature immunology. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- Brandt KJ, Fickentscher C, Kruithof EK, de Moerloose P. TLR2 ligands induce NF-kappaB activation from endosomal compartments of human monocytes. PloS one. 2013;8:e80743. doi: 10.1371/journal.pone.0080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wu D, Zhang L, Zhao Y, Guo L. Comparative phosphoproteomics studies of macrophage response to bacterial virulence effectors. Journal of proteomics. 2012;77:251–261. doi: 10.1016/j.jprot.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gurakan F, Utine E, Ozkan TB, et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nature genetics. 2010;42:303–312. doi: 10.1038/ng.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decque A, Joffre O, Magalhaes JG, Cossec JC, Blecher-Gonen R, Lapaquette P, Silvin A, Manel N, Joubert PE, Seeler JS, et al. Sumoylation coordinates the repression of inflammatory and anti-viral gene-expression programs during innate sensing. Nature immunology. 2016;17:140–149. doi: 10.1038/ni.3342. [DOI] [PubMed] [Google Scholar]
- Erdman LK, Cosio G, Helmers AJ, Gowda DC, Grinstein S, Kain KC. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. Journal of immunology. 2009;183:6452–6459. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. The Journal of experimental medicine. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Horiuchi S, Tomita K, Murakami M, Morino Y, Takahashi K. Acetylated low-density lipoprotein is endocytosed through coated pits by rat peritoneal macrophages. Virchows Archiv B, Cell pathology including molecular pathology. 1986;52:1–13. doi: 10.1007/BF02889945. [DOI] [PubMed] [Google Scholar]
- Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nature genetics. 2004;36:400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M, Fischler B, et al. Clinical and molecular genetic features of ARC syndrome. Human genetics. 2006;120:396–409. doi: 10.1007/s00439-006-0232-z. [DOI] [PubMed] [Google Scholar]
- Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005a;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005b;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nature reviews Immunology. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Current opinion in cell biology. 2010;22:519–527. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2011;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, et al. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33:583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. The EMBO journal. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Kamimori K, Hiyoshi M, Kido H, Ohta T, Konishi H. Inhibitory effect of SPE-39 due to tyrosine phosphorylation and ubiquitination on the function of Vps33B in the EGF-stimulated cells. FEBS letters. 2012;586:2245–2250. doi: 10.1016/j.febslet.2012.05.051. [DOI] [PubMed] [Google Scholar]
- Jang JY, Kim KM, Kim GH, Yu E, Lee JJ, Park YS, Yoo HW. Clinical characteristics and VPS33B mutations in patients with ARC syndrome. Journal of pediatric gastroenterology and nutrition. 2009;48:348–354. doi: 10.1097/mpg.0b013e31817fcb3f. [DOI] [PubMed] [Google Scholar]
- Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25:1327–1337. doi: 10.1091/mbc.E13-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Iwasaki A. Phagosome as the organelle linking innate and adaptive immunity. Traffic. 2012;13:1053–1061. doi: 10.1111/j.1600-0854.2012.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature immunology. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Goldman W, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, Hengartner MO, Ravichandran KS. A pathway for phagosome maturation during engulfment of apoptotic cells. Nature cell biology. 2008;10:556–566. doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Skjesol A, Kers-Rebel ED, Sherstova T, Sporsheim B, Egeberg KW, Stokke BT, Espevik T, Husebye H. CD14, TLR4 and TRAM Show Different Trafficking Dynamics During LPS Stimulation. Traffic. 2015;16:677–690. doi: 10.1111/tra.12274. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nature immunology. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- Lobingier BT, Nickerson DP, Lo SY, Merz AJ. SM proteins Sly1 and Vps33 co-assemble with Sec17 and SNARE complexes to oppose SNARE disassembly by Sec18. eLife. 2014;3:e02272. doi: 10.7554/eLife.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal K, Plantinga TS, Wong J, Monks BG, Gay NJ, Netea MG, Fitzgerald KA, Golenbock DT. A TIR domain variant of MyD88 adapter-like (Mal)/TIRAP results in loss of MyD88 binding and reduced TLR2/TLR4 signaling. The Journal of biological chemistry. 2009;284:25742–25748. doi: 10.1074/jbc.M109.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, et al. TLR Signals Induce Phagosomal MHC-I Delivery from the Endosomal Recycling Compartment to Allow Cross-Presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi N, Tyra LK, Stenesen D, Krämer H. Acinus integrates AKT1 and subapoptotic caspase activities to regulate basal autophagy. The Journal of cell biology. 2014;207:253–268. doi: 10.1083/jcb.201404028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Poidevin M, Roussel A, Lemaitre B. Tissue- and Ligand-Specific Sensing of Gram-Negative Infection in Drosophila by PGRP-LC Isoforms and PGRP-LE. Journal of immunology. 2012;189:1886–1897. doi: 10.4049/jimmunol.1201022. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch MP, DeSmedt M, ten Hove T, van Deventer SJ, Grooten J. Lipopolysaccharide regulates macrophage fluid phase pinocytosis via CD14-dependent and CD14-independent pathways. Blood. 1999;93:4011–4018. [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Krämer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. Journal of cell science. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- Seo SH, Hwang SM, Ko JM, Ko JS, Hyun YJ, Cho SI, Park H, Kim SY, Seong MW, Park SS. Identification of novel mutations in the VPS33B gene involved in arthrogryposis, renal dysfunction, and cholestasis syndrome. Clinical genetics. 2014 doi: 10.1111/cge.12442. [DOI] [PubMed] [Google Scholar]
- Shinzawa N, Nelson B, Aonuma H, Okado K, Fukumoto S, Miura M, Kanuka H. p38 MAPK-dependent phagocytic encapsulation confers infection tolerance in Drosophila. Cell host & microbe. 2009;6:244–252. doi: 10.1016/j.chom.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Sjoelund V, Smelkinson M, Nita-Lazar A. Phosphoproteome profiling of the macrophage response to different toll-like receptor ligands identifies differences in global phosphorylation dynamics. Journal of proteome research. 2014;13:5185–5197. doi: 10.1021/pr5002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. The FEBS journal. 2013;280:2743–2757. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1146–1150. doi: 10.1073/pnas.0237292100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takats S, Pircs K, Nagy P, Varga A, Karpati M, Hegedus K, Krämer H, Kovacs AL, Sass M, Juhasz G. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell. 2014;25:1338–1354. doi: 10.1091/mbc.E13-08-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochemical and biophysical research communications. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Tornieri K, Zlatic SA, Mullin AP, Werner E, Harrison R, L'Hernault SW, Faundez V. Vps33b pathogenic mutations preferentially affect VIPAS39/SPE-39-positive endosomes. Hum Mol Genet. 2013;22:5215–5228. doi: 10.1093/hmg/ddt378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, Pasare C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:273–278. doi: 10.1073/pnas.1118579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen T, Han C, He D, Liu H, An H, Cai Z, Cao X. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood. 2007;110:962–971. doi: 10.1182/blood-2007-01-066027. [DOI] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annual review of cell and developmental biology. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yao M, Liu X, Li D, Chen T, Cai Z, Cao X. Late endosome/lysosome-localized Rab7b suppresses TLR9-initiated proinflammatory cytokine and type I IFN production in macrophages. Journal of immunology. 2009;183:1751–1758. doi: 10.4049/jimmunol.0900249. [DOI] [PubMed] [Google Scholar]
- Yu S, Nie Y, Knowles B, Sakamori R, Stypulkowski E, Patel C, Das S, Douard V, Ferraris RP, Bonder EM, et al. TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. The EMBO journal. 2014 doi: 10.15252/embj.201487888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.