Abstract
Left ventricular (LV) mass and geometry are associated with risk of cardiovascular disease (CVD). We sought to determine whether LV mass and geometry contribute to risk prediction for CVD in adults ≥ 65 years of the Cardiovascular Health Study. We indexed LV mass to body size (echo-LVMI) and we defined LV geometry as normal, concentric remodeling, and eccentric or concentric LV hypertrophy. We added echo-LVMI and LV geometry to separate 10-year risk prediction models containing traditional risk factors and determined the net reclassification improvement (NRI) for incident CHD, CVD (CHD, heart failure [HF], stroke), and HF alone. Over 10 years of follow up in 2577 participants (64% women, 15% black, mean age 72 years) for CHD and CVD, the adjusted hazards ratios for a 1-SD higher echo-LVMI were 1.25 (95% CI, 1.14–1.37), 1.24 (1.15–1.33) and 1.51 (1.40–1.62), respectively. Addition of echo-LVMI to the standard model for CHD resulted in an event NRI of −0.011 (95% CI, −0.037, 0.028) and non-event NRI of 0.034 (95% CI, 0.008, 0.076). Addition of echo-LVMI and LV geometry to the standard model for CVD resulted in an event NRI of 0.013 (95% CI: −0.0335, 0.0311) and a non-event NRI of 0.043 (95% CI: 0.011,0.09). The non-event NRI was also significant with addition of echo-LVMI for HF risk prediction (0.10, 95% CI: 0.057, 0.16). In conclusion, in adults ≥ 65 years, echo-LVMI improved risk prediction for CHD, CVD, and HF, driven primarily by improved reclassification of non-events.
Keywords: left ventricular mass, risk prediction
INTRODUCTION
Left ventricular hypertrophy is an independent predictor and potentially modifiable risk factor for incident cardiovascular disease (CVD) (1–4). Although previous data demonstrate a strong association between LV mass/hypertrophy and increased cardiovascular risk, other statistical methods may be more useful in determining the utility of a biomarker to aid in clinical decision making. Net reclassification improvement (NRI) determines the number of individuals who are appropriately reclassified into higher or lower risk with addition of a new biomarker to an existing risk algorithm.(5,6) LV mass tends to increase and LV hypertrophy becomes more prevalent with increasing age,(7) so these measures may be particularly important for risk assessment. We sought to determine the extent to which LV mass and geometry determined by echocardiography and ECG improves risk prediction for coronary heart disease (CHD), heart failure (HF), stroke, and global CVD outcomes, beyond models based on traditional risk factors.
METHODS
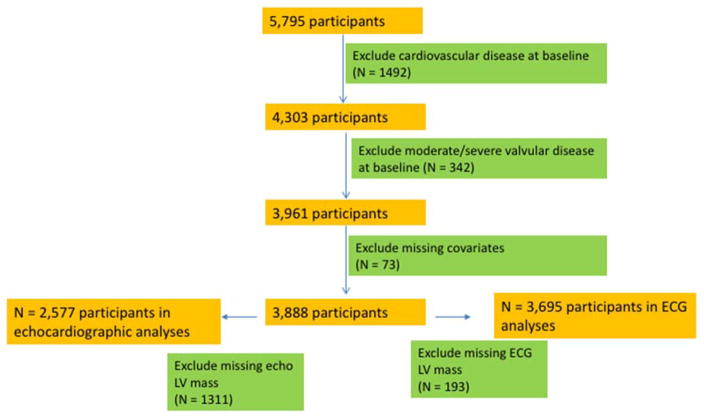
The Cardiovascular Health Study (CHS) is a prospective study sponsored by the National Institutes of Health; details of the study design have been previously published.(8) Participants were recruited from the following four communities: Washington County, MD; Pittsburgh, PA; Forsyth County, NC; and Sacramento County, CA. CHS includes 5,201 community-dwelling men and women aged ≥ 65 years, recruited in 1989–1990; an additional cohort of 687 African-Americans was recruited from 1992–1993. Echocardiograms were recorded in 1989–1990 for the original cohort and 1994–1995 for the African-American cohort. In the present analysis, “baseline” refers to the 1989–90 exam for the initial cohort and the 1994–95 exam for the second cohort. Figure 1 shows the exclusion criteria for the CHD and CVD analyses, which we refer to as Cohort A. For the CHD and CVD analyses, we excluded participants who had a prior diagnosis of CHD, HF, stroke, or atrial fibrillation (total with clinical CVD = 1,529), missing indexed echo LV mass (N = 1,486), missing key covariates at the baseline examination (N = 51), and moderate or severe valvular stenosis or regurgitation (N = 245). After exclusions, 2,577 participants were available for analyses.
Figure 1.
CONSORT diagram for CHS Cohort A (coronary heart disease and cardiovascular disease outcomes)
Figure 2 shows the exclusion criteria for the HF analyses, which we refer to as Cohort B. In Cohort B, we used inclusion criteria that are similar to those previously described by Butler et al in the Health ABC Study.(9) We excluded participants with HF at baseline (N = 297), missing indexed echo-LV mass (N = 1,958), and other missing key covariates or baseline data (N = 82). The final Cohort B included 3551 participants. We then conducted a sensitivity analysis for the HF outcome after additionally excluding participants with LV ejection fraction < 45% (N = 75). Of note, unlike Cohort A, Cohort B does not exclude participants with a history of CHD and stroke.
Figure 2.
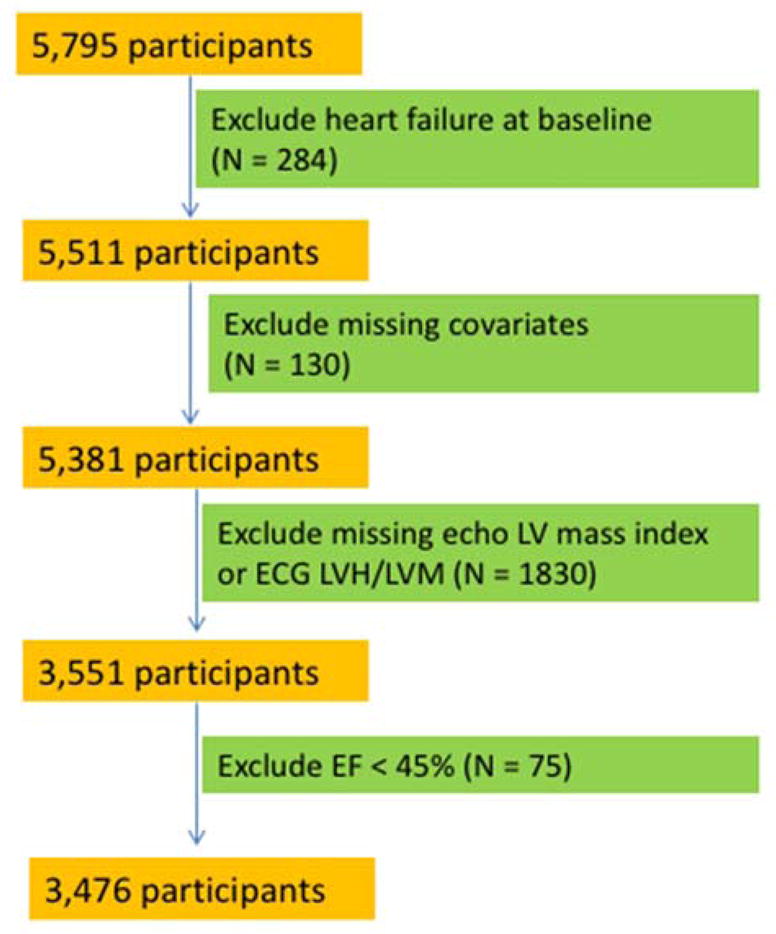
CONSORT diagram for CHS Cohort B (heart failure outcome)
ECG-LVH was considered present if the following Minnesota Codes (10) were present: 3-1, 3-3, 4-1 to 4-3, and 5-1 to 5-3. ECG LV mass (ECG-LVM) was calculated according to the race- and sex-specific formulas and adjusted for body size, as described by Rautaharju and colleagues previously in CHS.(11) Echo-LV mass was calculated from two-dimensionally guided M-mode echocardiograms using a method that has been described in detail previously.(12) We used the following formula described by Devereux(13) to calculate the unadjusted LV mass: LV mass (grams) = 0.80 × 1.04 × [(VSTd + LVIDd + PWTd)3 − (LVIDd)3] + 0.6; where VSTd refers to the ventricular septal thickness in end-diastole, LVIDd refers to LV internal diameter in end-diastole, and PWTd refers to the posterior wall thickness in end-diastole.
The echo-LV mass value obtained from the Devereux equation was adjusted for height, weight, and sex based on a method previously described from the Multi-Ethnic Study of Atherosclerosis.(14) A healthy subgroup (without history of coronary disease, heart failure, stroke, hypertension, diabetes, obesity, or significant subclinical disease by carotid ultrasound and ankle-brachial index) within CHS was used to define reference equations for LV mass and wall thickness, adjusted for height, weight, and sex. We used a linear regression model with log-transformed LV mass as the outcome and gender and log-transformed height and weight as the predictors. We then defined the indexed value of the echo-LV mass (echo-LVMI) for the entire population using the coefficients from the healthy participants. We divided LV mass by height raised to the power of the height coefficient, weight raised to the power of the weight coefficient and the exponentiated intercept for men and the exponentiated sum of the intercept and gender coefficient for women. Indexed wall thickness was created similarly with log-transformed relative wall thickness (RWT) as the outcome variable. We determined the cutoff for LVH to be 1.44 and the cutoff for increased RWT to be 1.31 based on the 95th percentile of these measures on echos from healthy CHS participants. LV geometry was defined as follows: normal: normal LVMI, normal RWT; concentric remodeling: normal LVMI, increased RWT; eccentric LVH: increased LVMI, normal RWT; or concentric hypertrophy: increased LVMI, increased RWT.
Based on the risk prediction model developed by D’Agostino (15), we used the following covariates from the baseline exam for modeling risk associated with CVD, CHD, and stroke: age, sex, race, systolic blood pressure, diabetes status, anti-hypertensive medication, and smoking status (current vs. not). Based on the HF risk prediction model developed by Butler (9), we used the following covariates from the baseline exam for modeling risk associated with HF: age, sex, race, systolic blood pressure, heart rate, smoking status (current, former, never), albumin, fasting glucose, creatinine, and prevalent CHD. Changes to the risk factors in the models established by Butler are that we added race and sex; we also removed ECG LVH from the Butler model in order to evaluate risks associated with echo parameters. Total and high-density lipoprotein cholesterol, albumin, creatinine, and fasting glucose were taken from CHS baseline measurements (1992–1993 for the African-American cohort). Missing data on systolic blood pressure, diabetes status, anti-hypertensive medication, heart rate, and smoking status for the second cohort were imputed with values carried forward from the previous two years, when available. Participants were classified as having diabetes mellitus if any of the following conditions were met: a) use of insulin or an oral hypoglycemic agent, b) fasting glucose level of ≥ 7 mmol/L (126 mg/dL), or c) a non-fasting glucose level of ≥ 11.1 mmol/L (200 mg/dl).
Outcomes were adjudicated by trained physicians, as has been described previously.(16) The CHS Events Committee is comprised of a panel of trained physicians and adjudicated all clinical outcomes. Myocardial infarction was defined based on a combination of clinical history, ECG abnormalities, and cardiac enzymes. The Events Committee classified all deaths into one of the following five groups: 1) atherosclerotic CHD; 2) cerebrovascular disease; 3) atherosclerotic disease other than CHD (e.g., abdominal aortic aneurysm or ischemic bowel); (4) other CVD (such as valvular heart disease or pulmonary embolism); and (5) all other deaths. Only group 1 was included for the CHD outcome and groups 1, 2, 3, and 4 were included for the CVD outcome. Cerebrovascular disease was defined on the basis of clinical symptoms of stroke and imaging findings; both fatal and nonfatal stroke were included in the CVD outcome. We also analyzed heart failure and stroke separately; for the heart failure analyses, we excluded participants who had LV ejection fraction < 45% at the baseline examination as a secondary analysis (N = 75).
Covariates were described as the mean and standard deviation for continuous variables and as N (%) for dichotomous variables, stratified by echo-LVH. In order to use previously validated risk categories for CHD and CVD, our follow up time was 10 years. We used Cox proportional hazards regression to calculate 10-year predicted risk for CHD, heart failure, stroke and all CVD using CHS-specific coefficients for traditional risk factors as described above). The exclusion criteria are shown in Figure 1 for CHD, CVD, and stroke and Figure 2 for HF. Individuals were censored for lack of follow-up, death, or at 10 years. We evaluated the addition of echo-LVMI (denoted continuous echo-LVMI, model 2), ECG-LVM (model 3), echo-LVMI plus LV geometry (model 4), and echo-LVH (model 5) to the base model. The ECG analyses included participants with complete ECG data, even if echocardiographic data was incomplete, so the sample size is greater for the ECG analyses. We tested the proportional hazards assumption using Schoenfeld residuals and found no violations. To assess the discrimination of the models to classify individuals according to event status, we calculated the C-statistic and compared each model to the base model.(17)
We also evaluated the risk classification of these models with net reclassification improvement (NRI) using the following categories of predicted risk: < 10%, 10–20%, and ≥ 20% for CHD similar to prior risk assessment frameworks. The risk categories for all CVD were< 20%, 20–30%, and ≥ 30%, and <5%, 5–10%, 10–20%, and ≥ 20% for HF and stroke separately. The categorical NRI incorporates the user-defined categories and only counts a participant if they are reclassified into a different risk group and was calculated separately for those who experience an event and those who did not. The event NRI was calculated as ([number of events reclassified higher – number of events reclassified lower]/number of events), and similarly the non-event NRI was calculated as([number of nonevents reclassified lower – number of nonevents reclassified higher]/number of nonevents). Due to recent concerns about the validity of the combined categorical NRI, we report the event and non-event NRI separately with bootstrapped confidence intervals.(6) We reported the continuous NRI which incorporates both events and non-events as has been done in previous studies and to facilitate comparisons of different biomarkers.
Sensitivity analyses included repeating the above analyses with further exclusion of those with ejection fraction < 45% (N = 75) for the HF outcome. Statistical significance was established at a two-sided P value < 0.05. Analyses were performed using STATA version 12.1 (Stata Corp, College Station, Texas).
RESULTS
Among the 2577 participants with complete indexed echo data in Cohort A, 11.8% (N = 305) had echo-LVH. As shown in Table 1, participants with LVH were older, more likely to be men, and less likely to be black. Multivariable-adjusted hazards ratios for incident CHD and incident total CVD are shown in Table 2. For a 1-standard deviation increase in echo-LVMI, the hazards ratios for CHD and CVD were 1.25 (95% CI, 1.14–1.37, P < 0.001) and 1.24 (1.15–1.33, P < 0.001), respectively. The hazards ratios for CHD and CVD for a 1-standard deviation increase in ECG-LVM were more modest than for echo-LVMI, but remained statistically significant. Table 2 also shows hazards ratios for incident CHD and CVD as a function of LV geometry. CHS participants with concentric remodeling and eccentric LVH were significantly more likely to experience CHD and CVD during the follow up period. Participants with concentric LVH had an almost 2-fold greater risk for CHD compared to those with normal geometry.
Table 1.
Characteristics of participants (COHORT A) at baseline stratified by presence of echocardiographic left ventricular hypertrophy
| Characteristic | No LVH (N = 2272) | LVH (N = 305) |
|---|---|---|
| Age (years) | 71.9 (5.0) | 73.2 (5.6) |
| Black race (N, %) | 342 (15.1%) | 39 (12.8%) |
| Male sex (N, %) | 779 (34.3%) | 160 (52.5%) |
| Total cholesterol (mg/dl) | 215.2 (38.7) | 204.5 (35.6) |
| Systolic blood pressure (mm Hg) | 135 (20) | 143 (24) |
| High-density lipoprotein cholesterol (mg/dl) | 56.6 (15.8) | 54.0 (15.8) |
| Electrocardiographic left ventricular mass (g) | 147.1 (27.4) | 164.1 (39.4) |
| Echocardiographic left ventricular mass (g) | 134.7 (33.3) | 224.7 (49.8) |
| Anti-hypertensive medication (N, %) | 798 (35.1%) | 146 (47.9%) |
| Current smoker (N, %) | 262 (11.5%) | 47 (15.4%) |
| Diabetes mellitus (N, %) | 264 (11.6%) | 50 (16.4%) |
All values expressed as mean (SD) or N(%)
LVH = left ventricular hypertrophy
Table 2.
Multivariable-adjusted hazards ratios (95% confidence interval) for clinical outcomes by left ventricular mass and geometry
| Parameter | Coronary Heart Disease | Cardiovascular Disease | Heart Failure |
|---|---|---|---|
| Echo-LVH (compared to no LVH) | 1.47 (1.17–1.85)* | 1.50 (1.24–1.81)* | 2.57 (2.14–3.08)* |
| Echo-LVMI (per 1-SD increase) | 1.24 (1.14–1.37)* | 1.26 (1.15–1.33)* | 1.52 (1.41–1.63)* |
| ECG-LVM (per 1-SD increase) | 1.14 (1.04–1.25)* | 1.15 (1.07–1.24)* | 1.38 (1.30–1.47)* |
| GEOMETRY | |||
| Normal geometry | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Concentric remodeling | 1.47 (1.03–2.09)* | 1.37 (1.02–1.85)* | 1.24 (0.86–1.78) |
| Eccentric LVH | 1.44 (1.12–1.85)* | 1.54 (1.26–1.89)* | 2.67 (2.20–3.23)* |
| Concentric LVH | 1.95 (1.17–3.24)* | 1.47 (0.93–2.31) | 2.24 (1.43–3.50)* |
denotes P < 0.05
LVH = left ventricular hypertrophy; LVMI = left ventricular mass index; LVM = left ventricular mass; ECG = electrocardiographic; echo = echocardiographic
All models adjusted for age, race, sex, systolic blood pressure, hypertension medication, total cholesterol, HDL, diabetes, and smoking status
Table 3 shows the cross-tabulations for observed and predicted CHD events with and without continuous echo-LVMI. The C-statistics for the baseline model and with the addition of echo-LVMI were 0.654 and 0.661 respectively (P-value for difference = 0.09). We found that addition of echo-LVMI reclassified 13.6% (N = 351) of participants. The continuous NRI was 0.12 (P = 0.01), the categorical NRI for CHD events was 0.011 (bootstrapped 95% CI: −0.037, 0.028), and the categorical NRI for non-events was 0.034 (bootstrapped 95% CI: 0.008, 0.076). With the addition of categorical echo-LVH to the model based on traditional risk factors, we observed that the continuous NRI was 0.10 (P = 0.03) and the categorical NRI for events and non-events was not significant.
Table 3.
Reclassification for coronary heart disease risk in models with and without continuous echocardiographic left ventricular mass index
| 10-year risk in model with echocardiographic left ventricular mass index added to standard model | ||||
|---|---|---|---|---|
| 10-year risk with standard model | < 10% | 10–20% | ≥ 20% | Total |
| < 10% | 91 | 22 | 0 | 113 |
| Cases (N, %) | 10 (11%) | 1 (5%) | 0 | 11 (10%) |
| 10–20% | 60 | 922 | 116 | 1098 |
| Cases (N, %) | 5 (8%) | 108 (12%) | 27 (23%) | 140 (13%) |
| ≥ 20% | 0 | 153 | 1213 | 1366 |
| Cases | 0 | 29 (19%) | 360 (30%) | 389 (28%) |
| TOTAL (Events, %) | 151 (15, 10%) | 1097 (138, 13%) | 1329 (387, 29%) | 2577 (540, 21%) |
Events net reclassification improvement: −0.011 (95% CI, −0.0369, 0.0277)
Non-events net reclassification improvement: 0.034 (95% CI, 0.0078, 0.0759)
Continuous NRI = 0.12, P = 0.01
The C-statistics with the baseline model for CVD risk prediction including only traditional risk factors and the model including echo-LVMI were 0.659 and 0.666, respectively (p-value for difference = 0.03). Addition of echo-LVMI to the baseline model reclassified 15.2% of participants (N = 391), and the continuous NRI was 0.12 (P = 0.007). The categorical NRI for CVD events and non-events was 0.01 (95% CI: −0.029,0.034) and 0.030 (0.001,0.073), respectively. Table 4 shows observed and predicted CVD events with addition of echo-LVMI and LV geometry to a baseline model containing traditional risk factors. Addition of LVMI and LV geometry to the baseline model reclassified 16.3% (N = 420) of participants and the categorical non-event NRI was statistically significant and the event NRI was not significant.
Table 4.
Reclassification for global cardiovascular disease in models with and without echocardiographic left ventricular mass index and geometry
| 10-year risk in model with echocardiographic left ventricular mass index and geometry | ||||
|---|---|---|---|---|
| 10-year risk in standard model | < 20% | 20–30% | ≥ 30% | Total |
| < 20% | 381 | 59 | 3 | 443 |
| Cases (N, %) | 51 (13%) | 10 (17%) | 0 (0%) | 61 (14%) |
| 20–30% | 98 | 579 | 115 | 792 |
| Cases (N, %) | 9 (9%) | 144 (25%) | 46 (40%) | 199 (25%) |
| ≥ 30% | 0 | 145 | 1197 | 1342 |
| Cases | 0 | 37 (26%) | 505 (42%) | 542 (40%) |
| Total (Events, %) | 479 (60, 13%) | 783 (191, 24%) | 1315 (551, 42%) | 2577 (802, 31%) |
Events net reclassification improvement = 0.013 (95% CI: −0.0335, 0.0311)
Non-events net reclassification improvement = 0.043 (95% CI: 0.011,0.09)
Continuous net reclassification improvement = 0.14, P = 0.001
Additional analyses were conducted to determine reclassification with addition of ECG-LVM to the baseline model. We observed that addition of ECG-LVM did not result in significantly improved reclassification for CHD, but did show modestly improved reclassification for CVD with a continuous NRI = 0.022, P = 0.01). Addition of echo-LVMI to a model containing standard risk factors and ECG-LVM also resulted in improved classification for non-events with statistical significance for HF (0.041, 0.008–0.08) but not for events, similar to the results of the primary analyses.
Among the 3551 participants included in Cohort B, we observed a total of 613 HF events. As shown in Table 2, for a 1-standard deviation increase in the continuous echo-LVMI, the hazards ratio for incident HF was 1.51 (1.40–1.62). When echo-LVH instead of ECG-LVH was added to the baseline risk prediction model, the continuous NRI was 0.23 (P < 0.001). There was no incremental improvement with addition of LV geometry to the model that already contained echo-LVH. Table 5 shows the observed and predicted HF events with addition of continuous echo-LVMI and LV geometry to the model based on Health ABC risk factors. The categorical NRI for non-events and events was 0.10 (0.057,0.16) and −0.02 (−0.046,0.033), respectively. Similar to the echo-LVH model, the improvement in risk prediction was similar for echo-LVMI and echo-LVMI plus LV geometry, indicating that LV geometry does not add to risk prediction when echo-LVMI is in the model. We observed similar results when we additionally excluded participants with LV ejection fraction < 45%.
Table 5.
Reclassification for heart failure (COHORT B) in models with and without echocardiographic left ventricular mass index and geometry
| 10 year risk in model with echocardiographic left ventricular mass index and geometry | |||||
|---|---|---|---|---|---|
| 10 year risk in standard model | 0–5% | 5–10% | 10–20% | >=20% | Total |
| 0–5% | 59 | 16 | 3 | 0 | 78 |
| Cases (N, %) | 0 | 0 | 0 | 0 | 0 |
| 5–10% | 54 | 623 | 103 | 15 | 795 |
| Cases (N, %) | 2 (4%) | 26 (4%) | 11 (11%) | 2 (13%) | 41 (5%) |
| 10–20% | 0 | 311 | 893 | 162 | 1366 |
| Cases (N, %) | 0 | 27 (9%) | 103(12%) | 48 (30%) | 178 (13%) |
| >=20% | 0 | 0 | 255 | 1057 | 1312 |
| Cases (N, %) | 0 | 0 | 46 (18%) | 348 (33%) | 394 (30%) |
| Total (Events, %) | 113 2 (2%) |
950 53 (6%) |
1254 (160, 13%) |
1234 (398, 32%) |
|
Events net reclassification improvement: −0.023, 95% CI: (−0.046,0.033)
Non-events net reclassification improvement: 0.10, 95% CI: (0.057, 0.16)
For the outcome of stroke alone, the C-statistic for the baseline model was 0.70 and there was no significant improvement with the addition of echo-LVMI, ECG LVMI, or LV geometry. The categorical and continuous NRI was also not statistically significant with the addition of these parameters.
DISCUSSION
In CHS participants, we observed that the addition of echocardiographic LV mass and geometry to standard risk prediction models modestly improved risk classification for CHD and CVD, which included CHD, heart failure, and stroke. The findings were driven by improved classification of non-events. These results suggest that LV mass and geometry may be useful in risk prediction of global cardiovascular disease outcomes in adults ≥ 65 years and may be particularly useful in identifying patients who are less likely to benefit from pharmacologic therapy. For HF risk prediction, echo-LVMI offered significantly improved risk prediction beyond risk factors. The further addition of LV geometry to the model did not provide incremental improvement for HF risk prediction.
Risk prediction models such as the Pooled Cohort Equations and the Framingham Risk Score are commonly employed in clinical practice to help determine which individuals may benefit from targeted primary prevention efforts. With increasing age, however, several factors make discrimination more difficult, including a higher prevalence of traditional risk factors such as hypercholesterolemia and hypertension. The identification of additional biomarkers that may improve risk prediction in adults ≥ 65 years could be clinically useful. The recently published Systolic Blood Pressure Intervention Trial (18) showed a reduction in cardiovascular events with tight blood pressure control in all age groups; however, adults older than 65 years may have an increased risk of side effects, including orthostasis and kidney injury. LV mass may serve as a marker of risk that modifies the risk-benefit ratio in favor of or against therapy. Indeed, the categorical NRI was significant for non-events, suggesting that LV mass may identify patients unlikely to benefit from increased therapy, although further study is needed. The magnitude of the NRI was fairly modest but comparable to markers such as C-reactive protein (continuous NRI for CHD = 0.08), ankle-brachial index (0.04), and brachial flow-mediated dilation (0.02).(19) Although not practical for routine screening, echocardiograms are commonly done in clinical practice for a variety of indications, and our study demonstrates that easily derived measures of LV structure are useful for moderately improved risk prediction for HF and CVD.
We observed that echo-LVMI offered significantly improved risk prediction for HF beyond risk factors. The measure of LV mass used in the Health ABC HF risk score was ECG-LVH defined by the Minnesota Code, similar to our analysis;(9) in the present analysis, the addition of ECG-LVH to the Health ABC covariates did not result in improved risk prediction for HF. However, echo-LVH and LVMI offered substantially improved risk prediction for HF when added to risk factors. Compared to echo criteria, ECG criteria for LVH have limited sensitivity;(20) this may partly explain why echo measures performed better than ECG measures. LV geometry, however, did not offer additional improvement beyond LV mass in reclassification for HF, likely due to the fact that LVH is incorporated into the measure of LV geometry. Unlike for atherosclerotic CVD, where a patient may become eligible for statin therapy in a higher risk group, moving to a higher risk category for incident HF is not associated with a clear clinical action. The HF outcome was obtained using different inclusion criteria than the CHD and CVD outcomes. However, the inclusion criteria and covariates for the HF outcome are concordant with the Health ABC study and facilitate comparison across the studies.
One of the major strengths of our study is that the CHS is a biracial, well-phenotyped cohort with well-validated clinical outcomes and internally validated measures of LV mass. One limitation of our analysis is that all patients underwent echocardiography but 34% of the cohort did not have reliable M-mode measurements to calculate LV mass. In previous analyses, the absence of accurate measures of LV mass in CHS participants was associated with greater age, black race, and higher BMI.(21) The aforementioned analysis also demonstrated that CHS participants missing LV mass had higher all-cause mortality but similar rates of CHD, HF, and stroke compared to those who had complete echo data. Missing echo data in blacks could account for our observation that blacks had a lower prevalence of LVH in this analysis than whites; another possible explanation is survivor bias. Another limitation is that the categorical NRI uses thresholds that do not necessarily have a clear action above a specific cutpoint. The continuous NRI provides information that is more easily comparable across different studies and biomarkers, but does not necessarily reflect the degree of improvement. To account for these limitations, we presented the reclassification tables to show clinically meaningful changes in risk categories along with the continuous NRI. We also were not able to classify HF into preserved ejection fraction or reduced ejection fraction.
Acknowledgments
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm. Dr. Desai was supported by Grant T32 HL-69771-8 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kannel WB, Dannenberg AL, Levy D. Population implications of electrocardiographic left ventricular hypertrophy. Am J Cardiol. 1987;60:85I–93I. doi: 10.1016/0002-9149(87)90466-8. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 5.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 6.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014 Jan;25(1):114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–1061. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Annals of Epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 9.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the Health ABC heart failure score. Circulation Heart Failure. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn H. Classification of the electrocardiogram for population studies: Minnesota Code. Journal of Electrocardiology. 1969;2:305–310. doi: 10.1016/s0022-0736(69)80120-2. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju PM, Park LP, Gottdiener JS, Siscovick D, Boineau R, Smith V, Powe NR. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. Journal of Electrocardiology. 2000;33:205–218. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 12.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O’Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reicheck N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 14.Brumback LC, Kronmal R, Heckbert SR, Ni H, Hundley WG, Lima JA, Bluemke DA. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int Journal of Cardiovasc Imaging. 2010;26:459–468. doi: 10.1007/s10554-010-9584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 16.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of Epidemiology. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 18.The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2106. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 21.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]