Abstract
Background
Medical research registries (MRR) are organized systems used to collect, store and analyze patient information. They are important tools for medical research with particular application to the study of rare diseases, including those seen in neuro-ophthalmic practice.
Evidence Acquisition
Evidence for this review was gathered from the writers’ experiences creating a comprehensive neuro-ophthalmology registry and review of the literature.
Results
MRR are typically observational and prospective databases of de-identified patient information. The structure is flexible and can accommodate a focus on specific diseases or treatments, surveillance of patient populations, physician quality improvement, or recruitment for future studies. They are particularly useful for the study of rare diseases. They can be integrated into the hierarchy of medical research at many levels provided their construction is well organized and they have several key characteristics including an easily manipulated database, comprehensive information on carefully selected patients and comply with human subjects regulations. MRR pertinent to neuro-ophthalmology include the UIC neuro-ophthalmology registry, Susac Syndrome Registry, Intracranial Hypertension Registry as well as larger scale patient outcome registries being developed by professional societies.
Conclusion
Medical research registries have a variety of forms and applications. With careful planning and clear goals, they are flexible and powerful research tools that can support multiple different study designs, and through this have the potential to advance understanding and care of neuro-ophthalmic diseases.
Keywords: research methods, registry, neuro-ophthalmology
The Mirriam Webster Dictionary defines registry as “a book or system for keeping an official list or record of items.” It logically flows that a medical research registry is an organized system that is used to collect, store and analyze patient information related to specific diseases, conditions or outcomes for the purposes of research (1). Registries are popular research tools, with over 5000 publications in PubMed that reference “registry” in the last five years. They are usually observational, containing information collected as part of clinical care. They are typically prospective, with data being recorded as it is collected, rather than abstracted from the chart at a future date (2). They can be cross-sectional, with a single snapshot of data for each participant or longitudinal with more data added over time. They can contain de-identified data or be coded to facilitate longitudinal data collection.
Research registries as a research methodology
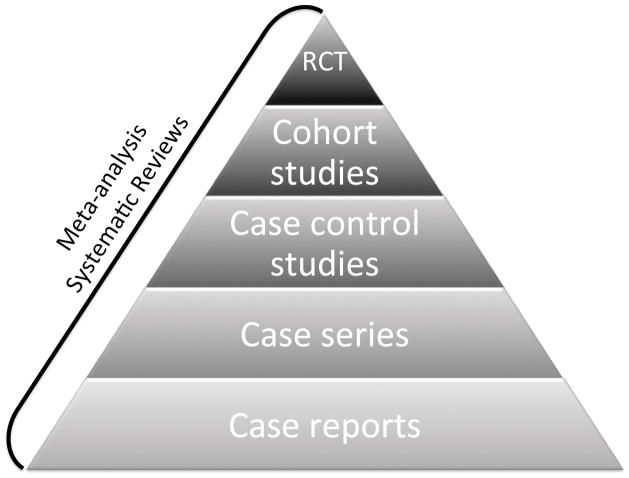
The ubiquitous hierarchy of research study design (Fig 1) ranks the strength of evidence offered by different study designs, with quality of evidence (as it pertains to clinical care) increasing as the apex is approached (3). It should be emphasized that a research registry is not a study design, but rather an important tool that organizes clinical observations for research purposes. As such, it can support the study design of the investigators’ choice.
Figure 1. Research Pyramid.
A representation of the types of human-based medical research studies that contribute knowledge regarding human health and disease. RCT, randomized clinical trials.
With reference to the hierarchy of research studies, (Fig 1), a research registry can be considered a collection of case reports. Combining multiple cases from the registry can create a case series. Registry data can be used in more rigorous study designs by comparing across participants. For example, participants in a registry can be divided into cases and controls based on a particular feature (either baseline or outcome) and outcome or exposure compared between these groups. Alternatively, outcomes of a group of participants in a registry can be compared according to baseline defining features as a cohort study. Research registries can facilitate both of these latter kinds of studies in rare diseases (1). It is important to remember that the quality of these studies depends on the quality of the registry and that any selection bias or data misclassification bias inherent in the registry will impact study validity.
Research registries can support randomized controlled trials (RCT) by providing important baseline data necessary for planning (e.g. sample size calculation). Additionally, registries can be used as a recruitment tool for RCTs if they incorporate a database of patients interested in participating in studies. This can ease the burden of trial recruitment, which is particularly difficult when a rare disease is being studied (4). Some have taken this a step further to use an established registry as the source of participants, baseline and follow up data for a superimposed RCT. In these, so called randomized registries, the cost and logistics of a comparable free standing RCT are dramatically reduced by leveraging existing registry infrastructure and resources (5). Alternatively, a registry can be created specifically for the purpose of conducting a RCT. A registry RCT can be much less expensive than a stand alone RCT, and can accommodate more relaxed enrollment, treatment and data collection parameters than a stand alone RCT. Though not appropriate for all treatment comparisons, it can be particularly useful for comparison of “real world” outcomes between two or more approved treatments (6).
Types of (Research) Registries
Registries are defined by the inclusion criteria applied to the enrolled participants and the type of captured data. They lend themselves to different applications. Although some registries may not be primarily designed for research purposes, many have this as a potential secondary application. Primary registry purposes other than research include surveillance and quality control.
Disease based registries include participants with a certain diagnosis and can be used to study the epidemiology of diseases. These range from small ones, like the European PedNet Haemophilia Registry (1) that contains just over 1000 participants to large, broadly based databases such as the Surveillance, Epidemiology and End-Results (SEER) Program run by the National Cancer Institute that is the most comprehensive cancer registry of its kind. It began collecting data on cancer incidence and survival in 1973 and continues to actively collect data that covers 28% of the U.S. population (7). The SEER program utilizes 18 population based cancer registries across the U.S. (10 state registries, 5 metropolitan registries and 3 Native American registries) and centralized database management software to collect its data. This data is periodically compared to census data to compare baseline characteristics to the general population. The information contained in the registry has been used to publish papers on cancer care/treatment, cancer screening, cancer prevention, genetics, health disparities and other aspects of cancer epidemiology. Investigations can stray far from traditional cancer epidemiological investigations. For example, in 1990 researchers from the SEER program published a paper on the public health impact of the media coverage of colon cancer after Ronald Regan was diagnosed with the disease (8), and in 2002 a SEER related article was published on the over diagnosis of prostate cancer due to prostate specific antigen screening using incidence data of the disease (9).
Surveillance registries include participants who have had certain tests and have particular application to public health as they can be used to monitor patients across care settings and monitor health across a region (10). Maintaining patient privacy is an important consideration in the design of such registries. For example, the New York City Board of Public Health created a mandatory registry for reporting laboratory results of hemoglobin A1C test results as a way to monitor diabetes in a large urban environment (11). Analysis of the registry data allows identification of features associated with poor disease control, which can inform program development with the goal of improving patient care and diabetes outcomes. A secondary capability of such registries is for comparison of care providers and sites, though these can be easily confounded by other factors that vary between sites such as disease severity and patient compliance. Risk adjustment, done through consideration of variables other than site of care that affect outcome is one way to adjust such confounding. There also is a concern for these comparisons to produce legal repercussions for physicians or to impact reimbursement. One example of research that has come out of this registry is a RCT that studied the effectiveness of a telephone intervention improving glycemic control in patients who were in the registry (12).
Provider care or site based registries include participants who received care from certain providers or sites and have application to study of care delivery and quality improvement. While data of this kind is critically important to advance research in the area of health care delivery, many are skeptical that the data will be applied for monitor and reimbursement purposes, which limits voluntary participation. An example of this is the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), which uses a registry of information from patients who had seen particular surgeons to create specific surgeon profiles and assess quality of individual surgeons preforming various operations (13).
Product registries include participants exposed to a certain product (e.g. medication, medical device). Theses have specific application to safety assessment, but also can provide an important source of data for effectiveness evaluation. The Israeli ICD (implantable cardiac-defibrillators) Registry encompasses information on all ICD implants and other ICD operative procedures throughout Israel. This registry has been used to perform research on the rate of appropriate life-saving ICD shock therapies (14).
Recruitment registries are databases of potential subjects and/or potential projects that can be searched to help match eligible participants with appropriate studies. The Research Match program at University of Illinois at Chicago was started in 2009 as a means of helping researchers find participants for their projects (15). Additionally, patients who are interested in participating in research can find projects for which they are eligible. This process also facilitates research by having some of the initial subject registration steps complete before the subject ever meets the research team (16).
The concept of “big health data” or data warehouse is similar to the idea of a research registry. A data warehouse consists of integrated health data from multiple sources on a large scale. One example is merging electronic medical record (EMR), scheduling, billing and prescription filling data at the patient level. Currently, data warehouses are used for retrospective research, which can define real world treatment patterns and responses. With these large databases, it is important to ensure that patient information is being stored and access is being regulated so that patient privacy is not being breached. In the future, data warehouses could integrate financial, social media and other lifestyle data to add further dimensions that may be relevant for medical research and increase privacy concerns (17). A further extension of data warehouses is the Informatics for Integrating Biology and the Bedside (i2b2) database that is run by the NIH. This is a national project that is working to connect multiple data warehouses together to allow researchers to easily access and integrate large amounts of data (18).
Characteristics of effective research registries
A research registry is a collection of research data, and as such, it should be designed and evaluated with the same criteria used to design and evaluate data collection. An effective research registry from a process standpoint is one into which data can be easily entered, safely stored, easily manipulated and easily retrieved. It is also important to carefully plan participant selection and data points to be recorded, being careful to minimize effects of bias on future research applications of the information.
Human subjects regulations must be considered including the Belmont Report principles of respect for persons, beneficence and justice. These issues must be considered during registry design, to be sure that subsequent publications are not rejected for failure to comply with regulations. Informed consent is variably necessary depending on the design of the registry. For a research registry consisting of strictly de-identified data, such as the SEER registry, informed consent may not be required. However, when identifying information is stored, for example to permit linkage with other sources of information or follow up, written informed consent may be required. In the United States institutional review boards and/or human subjects committees can provide clarification and approval.
Participant selection is an important design consideration for a research registry, because this will contribute to both the external validity (generalizability) and internal validity (bias) of any studies based on the registry. If the registry participants do not share similar baseline characteristics with the population they are purported to represent, then the outcome of any resulting research will not be fully generalizable. While it can be difficult to completely eliminate selection bias, for example, one can’t include individuals who haven’t been diagnosed with a disease in a disease based registry, it is important to take steps to minimize this in the planning stages and to characterize/ acknowledge in the research phase. Some registries, like those of very rare conditions, may be based on voluntary reporting and, therefore such as have substantial selection bias. These still have potential to support research, for example, on the range of case features, even if they are less applicable for hypothesis driven projects.
Data management is an important consideration in any research pursuit, and is especially important for medical research registries, which, by definition, contain protected health information. Issues of ease of data management, data security, and cost must be considered. A commonly used platform is the Research Electronic Data Capture (REDCap), a web based system that can be used to build and manage secure forms for data entry and storage that is typically managed centrally (19, 20). This platform includes query tools that facilitate export of de-identified data for desktop analysis (for example using spreadsheet or statistical packages). REDCap is broadly implemented at multiple institutions in the US and abroad (21). Additionally, there are other programs similar to REDCap, which include Medrio (22) and OpenClinica (23)
A research registry needs to contain information that is comprehensive and complete for it to be used effectively as a data set. This must be considered in the planning stages and may require intermediate steps to organize and code the data. A registry containing only clinical data could fail to meet standards for defining certain diagnoses or exam findings in a future research endeavor (24). Furthermore, significant data manipulation is required to extract data points from paper clinical records or free-text electronic records to create a meaningful database. Some investigators may be able to take advantage of their electronic medical record’s collection of “minable” data. Many institutions now have data warehouses that have compiled and integrated patient data from multiple sources to facilitate this. For example, the University of Utah has a Data Resource Center, which electronically houses patient visit, clinical and financial data in one place (25).
Case-Study: The UIC Neuro-ophthalmology Registry
The University of Illinois at Chicago (UIC) Neuro-Ophthalmology Registry was developed in response to two challenges faced in our human subjects research efforts. First, we struggled with identifying research subjects for prospective studies. In particular we found relying on appropriate patients to be seen in clinic to be unpredictable and that many eligible and interested patients often declined due to not planning for a longer visit and not wishing to return for an extra visit. Second, we found billing records to be an unreliable way to identify patients for retrospective studies. Therefore the UIC registry was designed with two parts – a database of potential subjects for future investigational projects, and a database of longitudinal clinical information for use in future retrospective studies. The registry structure and procedures were developed in consultation with our institutional review board to ensure compliance with human subjects regulations. It was determined that because our data collection is prospective and because we record identifying information for the purposes of contacting patients who have expressed interest in future research participation, that prospective, written informed consent was necessary. Due in part to involvement of appropriate experts on the front end, our human subjects protocol was easily approved.
Though the overall population of interest is individuals with neuro-ophthalmic diseases, for practical reasons, the population from which we are sampling is patients receiving neuro-ophthalmic care at our institution. We recognize and acknowledge the selection bias implicit in this design decision. There is a second level of selection bias within our registry because our current requirement for informed consent excludes potential subjects who are unable and/or unwilling to provide informed consent. We assessed the extent of this bias through demographic analysis of the registry participants compared with clinic attendees and found that older and Hispanic patients were underrepresented in the registry compared to the clinic population (26). This bias is attributable to our consent procedures and we are exploring options to enable informed consent in other languages besides English and by designates in the case where the patient lacks capacity to provide consent. There was no demographic bias of patients unwilling to provide informed consent compared with the clinic population.
Our current workflow for enrollment is as follows. A research assistant screens the clinic schedule in advance and flags all non-enrolled patients. At the conclusion of each clinic encounter, the provider discusses the registry with any patient who is flagged, stressing that it is voluntary and separate from clinical care. This takes 1–2 minutes. Interested patients are immediately escorted to a research assistant who obtains written informed consent and collects background data. Strengths of this system include minimal impact on clinic flow and high recruitment yield. Weaknesses of this system include the requirement for an on-site research assistant and space during clinic hours. We found that having the provider completing the informed consent adds an additional 5–10 minutes to each clinic visit, making it impractical from a clinic flow perspective. We also found that distributing an IRB approved recruitment flyer to clinic attendees to solicit volunteers did not generate very many volunteers. On occasion we have given a patient study enrollment materials to take home, and we have found that these rarely convert to an enrollment.
For data management we are using Research Electronic Data Capture (REDCap), a web based system that is hosted centrally at our institution. Multiple research assistants have found the platform to be user friendly for building, maintaining and querying the databases. At this time, many of the fields in our database consist of free-text fields, which facilitates direct copy from our EMR. Following enrollment, data from the initial enrollment form and EMR are copied into REDCap. After follow up visits updated data is copied from the EMR into REDCap. Two shortcomings of our current database are the restriction to clinical data, which is subject to missing data if certain parameters were not recorded clinically, and predominance of free text entries, which limits searchability. To improve our database, we are working to code the free text fields, starting with patient symptoms, signs, tests and diagnoses. This will facilitate database queries. Although Current Procedural Terminology (CPT), Systematized Nomenclature of Medicine-Clinical Terms (SNOMED), Unified Medical Language System (UMLS) codes and International Classification of Disease (ICD) codes associated with the records help to some extent, we have found that they are lacking with respect to many neuro-ophthalmology details. We are developing a list of relevant neuro-ophthalmic codes through an iterative process between the research assistant and provider based on enrolled patient records. Our goal is to compile coding pages that the provider can quickly complete/confirm for each patient at time of enrollment and follow up.
We have begun to use the registry for the purposes of recruitment for prospective studies. To accomplish this. the database is queried for the diagnosis of interest to generate a list of potential subjects. Through phone and e-mail these individuals are contacted. The yield has been higher than it was using our prior strategy of screening clinic patients for potential participation. We attribute this to restricting our efforts to patients who have previously agreed to entertain participation in a study and being able to provide advance notice about time commitment.
Examples of Registries Relevant to Neuro-Ophthalmology
Susac Syndrome Registry
The Susac Syndrome Registry is a two-part disease based registry run by the Cleveland Clinic and designed to study the natural history of Susac Syndrome, a rare disease with approximately 310 cases reported in the literature as of 2014. The registry is composed of both a retrospective/prospective cohort database and a list a patients who are interested in participating in future research endeavors. Patients from across the world can either be referred to the study by their physicians or self-enroll in the study. REDCap is used to manage the databases. The consent process involves the participant contacting the research team, signing a consent form and signing a medical records release form. The research team then obtains the participants’ medical records and transfers them to the secure, de-identified REDCap database (27).
Intracranial Hypertension Registry
The Intracranial Hypertension (IH) Registry was established in 2003 in partnership with the Oregon Health and Science University as an exclusively research oriented, disease based registry designed to further our knowledge of IH and attempt to find more effective treatments for the disease (28). It is an international registry with 2,309 enrolled patients from 26 different counties. It is comprised of both a patient information database and neuro-imaging library with over 8000 imaging studies from patients with both idiopathic IH (90% of enrolled patients) and secondary IH. Patients can either be referred to the registry by their physician or self-enroll in the registry. At the time of enrollment, patients are sent paperwork explaining the registry, a consent form, authorization for medical records and a patient questionnaire. Before enrollment, the research team verifies the diagnosis of IH by reviewing the patient’s medical records. Patients are contacted annually by the registry team with a questionnaire and repeat authorization for updated medical records. The data is managed in a secure, de-identified database with REDCap (E. Tanne, personal communication). As of 2010, information in its database has been used to publish two studies. One study was published in Obesity Review concerning the health care costs associated with IIH (29). Another was published in the Journal of Women’s Health concerning the association between weight gain and the appearance of new visual field defects (30). A recent study examined the association between acetazolamide and nephrolithiasis (31). Again, a registry of this kind is useful as IIH is a relatively rare disease affecting only about 1 in 100,000 people. A similar registry is being instituted in the UK (32).
National Registry of Drug-Induced Ocular Side Effects
The National Registry of Drug-Induced Ocular Side Effects was established in 1976 and is currently funded by the Casey Eye Institute at the University of Oregon and the American Academy of Ophthalmology. Its goal is to create a comprehensive database of information on adverse ocular side effects of drugs, chemicals and herbals through the use case reports in order to identify the earliest signs of adverse reactions. It has an easily accessible website where physicians can submit case reports using a standardized form. No specific identifying patient information is requested on the form, so it appears that individual physicians and their patients must not complete a consent process. The registry also builds its database by monitoring reports sent to the World Health Organization, the Food and Drug Administration and pharmaceutical companies. Information gathered from the registry has been used to publish a book, “Drug Induced Ocular Side Effects” that physicians can purchase for reference (33). The registry website also provides a drug consent form physicians can use to help educate their patients on potential side effects of medications they may be taking and provide a way for physicians to obtain informed consent from a patient before prescribing a specific drug. An example of a research application from this type of registry is a study on the ocular side effects of a drug, such as optic neuritis associated with the use of adalimumab (34) or bortezomib therapy and eyelid chalazia (35).
IRIS and Axon Registries
The American Academy of Ophthalmology (AAO) implemented the Intelligent Research in Sight Registry (IRIS) in 2014 as a comprehensive outpatient clinical registry of ophthalmologic diseases (36). IRIS is a physician-centered registry with an emphasis on physician education and quality improvement. As part of its quality care emphasis, it allows physicians to report to the federal Physician Quality Reporting System (PQRS).
The AAO intends to use information gathered from the registry to plan new continuing medical education (CME) courses for ophthalmologists. The registry also plans to give physicians’ feedback and information about their practices. For example, from the AAO website, “What is the rate of return to the operating room for patients undergoing cataract surgery?” (37). Additionally, it also can be used both to further advances in knowledge on the diagnosis and management of eye diseases, and as a tool to assess physicians and educate them on best practices (38). IRIS is designed as a centralized system where physicians are able to enter information about their patients with little impact on their overall workload (39). For those physicians whose practices use an electronic health records system (EHR), de-identified data can be pulled directly from their EHR to the IRIS database after the proper software has been installed. Because the data is de-identified and a purpose is quality improvement there is no consent process with patients. An early publication from the IRIS database highlights how IRIS data can be combined with Medicare claims records to identify areas of quality improvement; specifically, with regards to cases of endophthalmitis after cataract surgery and monitoring outcomes after less common surgical procedures like cataract surgery combined with vitrectomy (40).
The American Academy of Neurology (AAN) is currently implementing the Axon registry. It will be similar to the IRIS registry as it is a physician-based registry with a main focus on physician education and quality improvement. It also will allow physicians to report to the federal PQRS. The AAN also plans to expand the Axon registry to create CME courses for neurologists. The Axon registry will also be linked with physician’s EHR systems to automatically pull data into the Axon database. A major effort has been the development of outcome measures to include in the registry (41). The AAN will only have access to physician and practice data and will not see any individual patient data, so there is no patient informed consent process (42).
Future Directions
Although there are some disease specific registries relevant to neuro-ophthalmology, not all diseases are represented. The neuro-ophthalmology community would benefit from registries that include more patients and more diseases. One way to minimize the selection bias associated with disease specific registries would be multi-site efforts to construct inclusive registries of neuro-ophthalmic patients. This broader effort would support study of all neuro-ophthalmic disease by facilitating identification of patients with rare diseases for prospective trials and establishing large observational cohorts. Construction of a centralized registry might be logistically prohibitive given privacy concerns of transmitting identified data. An alternative would be to have each institution manage its own registry using a standard coding set to facilitate future combining of de-identified data for study purposes. Coordinated institutional neuro-ophthalmic registries would also facilitate randomized registries trials as a way to compare non-novel treatments in a cost-effective and flexible way.
Conclusions
Medical research registries are a means to gather, store and analyze patient data. While they have typically been used as prospective, observational tools, they have a variety of forms and applications that can fit into the traditional hierarchy of medical research in different ways. They are particularly useful for studying rare diseases, but are by no means limited to these. With careful planning and clear goals, a research registry can be an invaluable tool in medical research.
Acknowledgments
Funding: NIH grants K12 EY 021475, K23 EY 024345, P30 EY 001792, UL1RR029879, unrestricted departmental grant from Research to Prevent Blindness
Footnotes
Conflict of interest: Ms. Blankshain and Dr. Moss have no disclosures related to this manuscript
Statement of Authorship
Category 1:
a. Conception and design
K. Blankshain, H. Moss
b. Acquisition of data
K. Blankshain, H. Moss
c. Analysis and interpretation of data
K. Blankshain, H. Moss
Category 2:
a. Drafting the manuscript
K. Blankshain, H. Moss
b. Revising it for intellectual content
K. Blankshain, H. Moss
Category 3:
a. Final approval of the completed manuscript
K. Blankshain, H. Moss
References
- 1.Fischer K, Ljung R, Platokouki H, Liesner R, Claeyssens S, Smink E, van den Berg HM. Prospective Observational Cohort Studies for Studying Rare Diseases: The European PedNet Haemophilia Registry. Haemophilia. 2014;20:e280–e286. doi: 10.1111/hae.12448. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed November 24, 2015];List of Registries [NIH registry website] 2015 Nov 23; Available at http://www.nih.gov/health-information/nih-clinical-research-trials-you/list-registries.
- 3.Tomlin G, Borgetto B. Research Pyramid: A New Evidence-based Practice Model for Occupational Therapy. Am J Occup Ther. 2011;65:189–196. doi: 10.5014/ajot.2011.000828. [DOI] [PubMed] [Google Scholar]
- 4.Malek AM, Stickler DE, Antao VC, Horton DK. The National ALS Registry: A Recruitment Tool for Research. Muscle Nerve. 2014;50:830–834. doi: 10.1002/mus.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer MS, D’Agostino RB. The Randomized Registry Trial — The Next Disruptive Technology in Clinical Research? N Engl J Med. 2013;369:1579–1581. doi: 10.1056/NEJMp1310102. [DOI] [PubMed] [Google Scholar]
- 6.James S, Rao SV, Granger CB. Registry-based Randomized Clinical Trials – A New Clinical Trial Paradigm. Nat Rev Cardiol. 2015 May;12(5):312–316. doi: 10.1038/nrcardio.2015.33. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed July 12, 2015];Surveillance, Epidemiology, and End Results Program [National Cancer Institute web site] 2015 Apr 10; Available at: http://seer.cancer.gov/about/overview.html.
- 8.Brown MLPA. The Presidential Effect: The Public Health Response to Media Coverage About Ronald Reagan’s Colon Cancer Episode. Public Opin Q. 1990;54:317–329. doi: 10.1086/269209. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni R, Penson D, Legler J, di Tommaso D, Boer R, Gann PH, Feuer EJ. Overdiagnosis Due to Prostate-specific Antigen Screening: Lessons from U.S. Prostate Cancer Incidence Trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 10.Darves B. [Accessed August 8, 2015];ACP Internist Patient Registries: A Key Step to Quality Improvement [American College of Physicians web site] 2005 Sep; Available at: http://www.acpinternist.org/archives/2005/09/patient.htm#help.
- 11.Chamany S, Silver LD, Bassett MT, Driver CR, Berger DK, Neuhaus CE, Kumar N, Frieden TR. Tracking Diabetes: New York City’s A1c Registry. Milbank Q. 2009;87:547–570. doi: 10.1111/j.1468-0009.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamany S, Walker EA, Schechter CB, Gonzalez JS, Davis NJ, Ortega FM, Carrasco J, Basch CE, Silver LD. Telephone Intervention to Improve Diabetes Control: A Randomized Trial in the New York City A1c Registry. Am J Prev Med. 2015;49:832–841. doi: 10.1016/j.amepre.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall BL, Huffman KM, Hamilton BH, Paruch JL, Zhou L, Richards KE, Cohen ME, Ko CY. Profiling Individual Surgeon Performance Using Information from a High-quality Clinical Registry: Opportunities and Limitations. J Am Coll Surg. 2015;221:901–913. doi: 10.1016/j.jamcollsurg.2015.07.454. [DOI] [PubMed] [Google Scholar]
- 14.Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, Glikson M, Beinart R Israeli Working Group of Pacing and Electrophysiology. Contemporary Rates of Appropriate Shock Therapy in Patients Who Receive Implantable Device Therapy in a Real-world Setting: From the Israeli ICD Registry. Heart Rhythm. 2015;12:2426–2433. doi: 10.1016/j.hrthm.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Walsh L. [Accessed November 21, 2015];Discovering ResearchMatch [UIC Center for Clinical and Translational Science web site] 2015 Nov 1; Available at: http://www.ccts.uic.edu/news/discovering-researchmatch?utm_source=CCTS+Winter+2015+Newsletter&utm_campaign=CCTS+Winter+Newsletter+2015&utm_medium=email.
- 16. [Accessed July 18, 2015];ResearchMatch [ResearchMatch web site] 2015 Available at: https://www.researchmatch.org/?route=gard.
- 17.Weber G, Mandl K, Kohane I. Finding the Missing Link for Big Biomedical Data. JAMA. 2014;311:2479–2480. doi: 10.1001/jama.2014.4228. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed January 30, 2106];Informatics for Integrating Biology and the Bedside [i2b2 Website] 2015 Available at: https://www.i2b2.org/index.html.
- 19.Kiplin M, Mare I, Hazelhurst S, Kramer B. The Process of Installing REDCap, A Web Based Database Supporting Biomedical Research. Appl Clin Inform. 2014;5:916–929. doi: 10.4338/ACI-2014-06-CR-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.REDCap. [Accessed August 8, 2015];Research Electronic Data Capture [REDCap web site] 2015 Available at: http://project-redcap.org/
- 21.Pang X, Kozlowski N, Wu S, Jiang M, Huang Y, Mao P, Liu X, He W, Huang C, Li Y, Zhang H. Construction and Management of ARDS/sepsis Registry with REDCap. J Thorac Dis. 2014;6:1293–1299. doi: 10.3978/j.issn.2072-1439.2014.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed October 25, 2015];Medrio [Medrio web site] 2015 Available at: http://medrio.com.
- 23. [Accessed October 25, 2015];Open Clinica: Knowledge Management for Medical Care [Open Clinica web site] 2013 Jul 8; Available at: http://www.openclinica.org/home.html.
- 24.Ewing M, Funk GA, Warren AM, Rapier N, Reynolds M, Bennett M, Mastropieri C, Foreman ML. Improving National Trauma Data Bank coding data reliability for traumatic injury using a prospective systems approach. Health Informatics J. 2015 Oct 29; doi: 10.1177/1460458215610896. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed November 15, 2015];About the Data Resource Center [University of Utah Health Sciences web site] 2005 Jun 29; Available at: http://uuhsc.utah.edu/drc/summary.html.
- 26.Blankshain KD, Park HJ, Moss H. Assessment of Recruitment Patterns in a Neuro-Ophthalmology Registry. North American Neuro-Ophthalmology Society 41st Annual Meeting Syllabus; 2015; p. 315. [Google Scholar]
- 27. [Accessed August 8, 2015];Diseases and Conditions: Susac Syndrome [Cleveland Clinic web site] 2014 Available at: http://my.clevelandclinic.org/health/diseases_conditions/susac-syndrome.
- 28. [Accessed November 28, 2015];The IH Registry [Intracranial Hypertension Research Foundation web site] 2015 Nov 27; Available at: http://ihrfoundation.org/hypertension/info/C36.
- 29.Friesner D, Rosenman R, Lobb BM, Tanne E. Idiopathic Intracranial Hypertension in the USA: The Role of Obesity in Establishing Prevalence and Healthcare Costs. Obes Rev. 2011;12:e372–380. doi: 10.1111/j.1467-789X.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin M, Lobb B, Tanne E, Egan R. Weight and visual field defects in women with idiopathic intracranial hypertension. J Womens Health. 2010;19:1893–1898. doi: 10.1089/jwh.2009.1804. [DOI] [PubMed] [Google Scholar]
- 31.Au JN, Waslo CS, McGwin G, Huisingh C, Tanne E. Acetazolamide-induced nephrolithiasis in idiopathic intracranial hypertension. J Neurophthalmol. 2015 Nov 20; doi: 10.1097/WNO.0000000000000330. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. [Accessed July 2, 2015];Idiopathic Intracranial Hypertension [IIH UK web site] 2012 Available at: http://www.iih.org.uk/research.
- 33. [Accessed August 8, 2015];National Registry of Drug-induced Ocular Side Effects [National Registry of Drug-induced Ocular Side Effects web site] n.d Available at: http://www.eyedrugregistry.com/
- 34.Sing YL, Birnbaum AD, Goldstein DA. Optic Neuritis Associated with Adalimumab in the Treatment of Uveitis. Ocul Immunol Inflamm. 2010;18:475–481. doi: 10.3109/09273948.2010.495814. [DOI] [PubMed] [Google Scholar]
- 35.Fraunfelder FW, Yang HK. Association Between Bortezomib Therapy and Eyelid Chalazia. JAMA Ophthalmol. 2015 doi: 10.1001/jamaophthalmol.2015.3963. EPub ahead of print October 15, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Schneider R. AAO Launches IRIS Registry. Ophthalmol Times. 2014;39:31. [Google Scholar]
- 37. [Accessed September 12, 2015];IRIS Registry [American Academy of Ophthalmology web site] 2015 Nov 21; Available at: http://www.aao.org/iris-registry.
- 38.Academy Plans IRIS Registry to Demonstrate Quality of Care. Rev Ophthalmol. 2013;20:4–5. [Google Scholar]
- 39.Meszaros L, Glasser D. AAO Intelligent Research in Sight Registry Project: A Basic Primer. Ophthal Times. 2014;39:22. [Google Scholar]
- 40.Coleman AL. How Big Data Informs Us About Cataract Surgery: The LXXII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2015;160:1091–1103. doi: 10.1016/j.ajo.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 41. [Accessed November 21, 2015];Quality Measures [American Academy of Neurology web site] 2015 Available at: https://www.aan.com/practice/quality-measures.
- 42. [Accessed November 21, 2015];Axon Registry [American Academy of Neurology web site] 2015 Available at: https://www.aan.com/practice/axon-registry/