Abstract
The pediatric adaptation of the Collaborative Health Outcomes Information Registry (Peds-CHOIR) is a free, open source, flexible learning health care system (LHS) that meets the call by the Institute of Medicine (IOM) for the development of national registries to guide research and precision pain medicine. This report is a technical account of the first application of Peds-CHOIR with three aims: to 1) describe the design and implementation process of the LHS; 2) highlight how the clinical system concurrently cultivates a research platform rich in breadth (e.g., clinic characteristics) and depth (e.g., unique patient and caregiver reporting patterns); and 3) demonstrate the utility of capturing patient-caregiver dyad data in real time, with dynamic outcomes tracking that informs clinical decisions and delivery of treatments.
Technical, financial, and systems-based considerations of Peds-CHOIR are discussed. Cross-sectional, retrospective data from patients with chronic pain (N = 352; 8 – 17 years; M = 13.9 years) and their caregivers are reported, including NIH Patient Reported Outcomes Measurement Information System (PROMIS) domains (mobility, pain interference, fatigue, peer relations, anxiety and depression) and the Pain Catastrophizing Scale. Consistent with the literature, analyses of initial visits revealed impairments across physical, psychological and social domains. Patients and caregivers evidenced agreement in observable variables (mobility); however, caregivers consistently endorsed greater impairment regarding internal experiences (pain interference, fatigue, peer relations, anxiety, depression) than patients’ self-report. A platform like Peds-CHOIR highlights predictors of chronic pain outcomes on a group level and facilitates individually tailored treatment(s). Challenges of implementation and future directions are discussed.
Keywords: pediatric chronic pain, CHOIR, learning health systems, registries, PROMIS
1. Introduction
Epidemiological studies indicate that 1.7 million children in the United States experience moderate to severe chronic pain, with national costs of $19.5 billion annually [23], and associated disability and impaired quality of life [24,31,33]. Pediatric chronic pain often persists into adulthood [2,3,69], and has profound negative impacts on physical, school, social, family, and emotional functioning [21,35,36,47]. Chronic pain etiology is often unknown and without observable disease markers, thereby rendering heavy reliance on self-reported symptoms. In the seminal 2011 report Relieving Pain in America, the Institute of Medicine (IOM) identified the need for better pain data, and called for the development of national registries and learning health systems (LHS) [26]. LHS utilize technology to partner with patients and clinicians, to continuously improve the accuracy of assessment and offer support for clinical decision making [26].
In response to the 2011 IOM report, and supported by the National Institutes of Health (NIH), the Stanford University Division of Pain Medicine and Center for Clinical Informatics developed the Collaborative Health Outcomes Information Registry (CHOIR; http://choir.stanford.edu) [59]. CHOIR is a free, open source, open standard, flexible LHS that runs on a secure Oracle or PostgreSQL database. Using a web-based interface, CHOIR has capabilities to capture data at each clinic visit; display graphical, real-time results that inform point-of-care decisions; and track patient treatment responses longitudinally. The registry emphasizes tracking of patient-generated information as a core component of clinical practice, allowing for individualized improvements in the healthcare delivery process over time [17] and guiding precision pain medicine. In aggregate, CHOIR data can be used to phenotype patients and characterize their response to intervention.
CHOIR is flexible in that each institution can tailor patient-reported-outcome measures to the specific interest of the site, clinic, and condition(s). CHOIR currently incorporates classical testing theory-based measures (e.g. Pain Catastrophizing Scale) [58] and item-response theory measures, such as the NIH Patient Reported Outcomes Measurement Information System (PROMIS)[1]. PROMIS measures are (1) normed and validated in the US, providing a comparative metric across groups; (2) utilize computer adaptive testing [5,28,38] thereby decreasing respondent burden; and (3) enable comparison of results across studies [1]. CHOIR has been shown in previous empirical work to be an effective and useful tool in identifying predictors of functional outcomes in chronic pain conditions [56,57].
The purpose of this study is to describe the first application of CHOIR in a pediatric pain clinic (Peds-CHOIR), with emphasis on the dual-tracking capacity for patient and caregiver reported outcomes. To date, no other reports have been published of open source LHS platforms used in the treatment of pediatric chronic pain that track both child and caregiver reports. This report details the design and implementation process of Peds-CHOIR. The clinical system’s capacity to collect group and dyadic data for informing research is then highlighted via description of clinic characteristics and patient-caregiver reporting patterns. Lastly, a detailed case example highlights the unique functionality and clinical utility of Peds-CHOIR as a flexible LHS that captures patient-caregiver data in real time, helping to augment clinical decisions and treatment delivery.
2. Method
2.1. Participants
This first implementation of Peds-CHOIR took place at Stanford Children’s Health, a moderate-sized, interdisciplinary, pediatric, tertiary referral pain clinic in the United States, which treats children of all ages with mixed etiology chronic pain. Patients and their caregivers complete Peds-CHOIR as a part of their initial assessment and subsequent treatment(s) at the clinic. A retrospective review of data collected from children age 8–17 years old (described as patients in this report) seen for initial evaluation between June 2014 and December 2015 is presented. To underscore Peds CHOIR’s capacity to guide treatment in real time, a case example was selected to exemplify longitudinal tracking of patient-caregiver reported outcomes. Patient information was de-identified to protect confidentiality. The selected patient and primary caregiver completed at least four Peds-CHOIRs, and were seen regularly at the pediatric pain clinic by multiple disciplines (physicians, nursing, and psychologists). This study was approved by the university’s Institutional Review Board.
2.1.1. Setting
The pediatric pain clinic follows a well-documented approach to treating pain and improving function utilizing interdisciplinary assessment and multidisciplinary interventions including medical assessment, physical therapy, and psychological interventions [15,32,39,41,52,70]. Initial evaluation occurs as follows: First, interdisciplinary team members review Peds-CHOIR results, which the patient and caregiver complete either at home or in the clinic before their appointment and discusses possible areas of vulnerability reported by the patient and their caregiver. Second, patient and caregiver(s) meet with the entire team where medical history, presenting concerns, and treatment goals are reviewed. Third, the patient meets with the medical team for a physical exam while the caregiver(s) meets with a psychologist to provide their perspective regarding the impact of pain on the child’s psychosocial functioning. The patient then meets individually with the psychologist for assessment of pain, coping, and overall mental health. Thereafter, the interdisciplinary team confers to formulate the treatment plan. Finally, the interdisciplinary team, family, and patient gather for the feedback portion of the visit and review of a multi-modal treatment plan delineating recommendations. The treatment paradigm often includes weekly: physical therapy for 6–8 weeks, cognitive behavior treatment with the pain psychologist for 6–8 weeks, and acupuncture for 4–6 weeks. Medication treatment and follow-up with the pain physician typically occurs every 2–3 months or sooner when indicated.
2.1.2. Peds-CHOIR Design, Procedure and Implementation
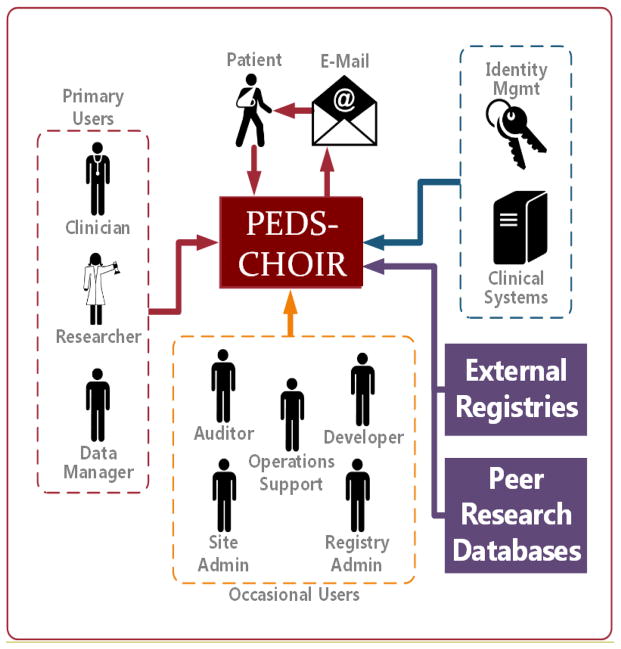
Peds-CHOIR is an open-source, open-standard, LHS with the following characteristics and requirements: (1) accessible project code and documentation, hosted on a free GitHub account (https://github.com/join); (2) code that is installable within a site’s centralized authentication system, which is managed by the site’s information technology (IT) personnel; (3) allows for inclusion or exclusion of any desired measures; (4) accessible email server that accepts SMTP mail; (5) CHOIR code can be customized by individual sites; (6) data acquired by CHOIR is owned by the individual site; and (7) CHOIR code cannot be redistributed or used for commercial purposes (Figure 1). The Peds-CHOIR system was modeled after CHOIR (http://CHOIR.stanford.edu) to ensure comparability and compatibility between the registries to track longitudinal outcomes across developmental phases, and to elucidate correlates and predictors of chronic pain from childhood to adulthood [2,3,18,69]. Similar to CHOIR, Peds-CHOIR was developed with reliable and valid pediatric measures of physical, psychological, and social domains relevant to chronic pain, as well as measures predictive of maladaptive chronic pain coping (described in detail below), affording high efficiency and precision, and decreased patient burden [21,35,36,50]. Beyond the similarities, implementation of Peds-CHOIR necessitated modification of the existing CHOIR technology by adding capabilities for dual tracking to capture both patient and caregiver data longitudinally.
Figure 1.
Overview of Peds-CHOIR infrastructure
Typical operations for Peds-CHOIR include a number of procedural steps (Figure 1). First, operations support staff obtains email consent when patients are scheduled for an initial evaluation at the Pain Clinic, and the primary caregiver’s email is then registered into Peds-CHOIR. Peds-CHOIR receives information from the institution’s clinical system (EPIC in this case) that an appointment has been made. One week prior to the appointment, one URL link of Peds-CHOIR survey is sent to the caregiver’s email through a secure, HIPAA-compliant, university-approved, Oracle database. The database is maintained and administered at this institution by the Stanford Information Resources and Technology department (developer and registry administer in figure 1). Patient and caregiver typically complete the Peds-CHOIR surveys at home prior to the patient’s initial evaluation. If the survey is not completed in advance, patients and their primary caregivers are provided encrypted electronic tablets (e.g., iPad or Android) at clinic check-in before they are seen for their initial interdisciplinary or follow-up appointment. Clinic staff (operations support) then print the Peds-CHOIR output for providers (physician, nurse practitioner, physical therapist, and psychologist) to review prior to seeing the patient. Providers are also able to log into the secure Peds-CHOIR system to view results before and during the appointment with the family. The data are accessible and managed by the research coordinator at our site. Follow-up assessments are administered when patients return for clinic appointments provided that at least 30 days have elapsed since their last completed survey. Refer to Figures 2a and 2b for a sample of the initial assessment and Figures 3a and 3b for a follow-up sample.
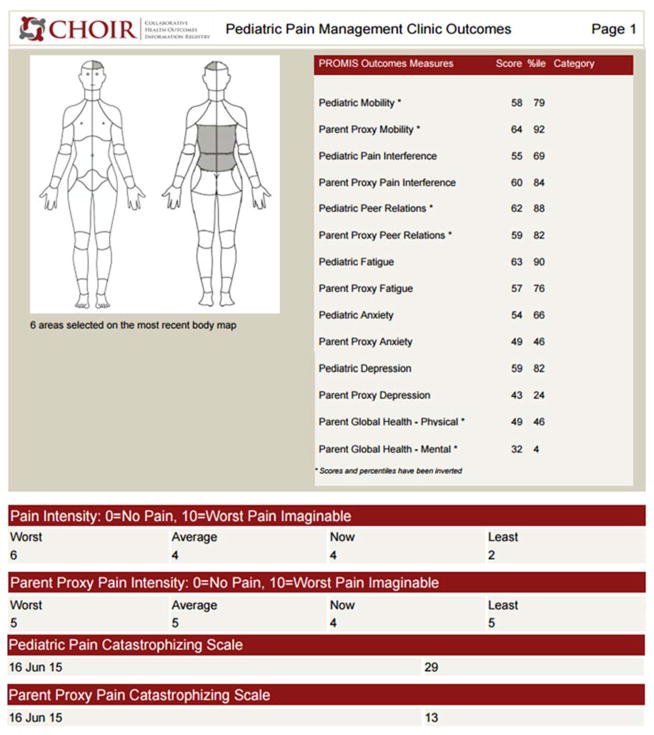
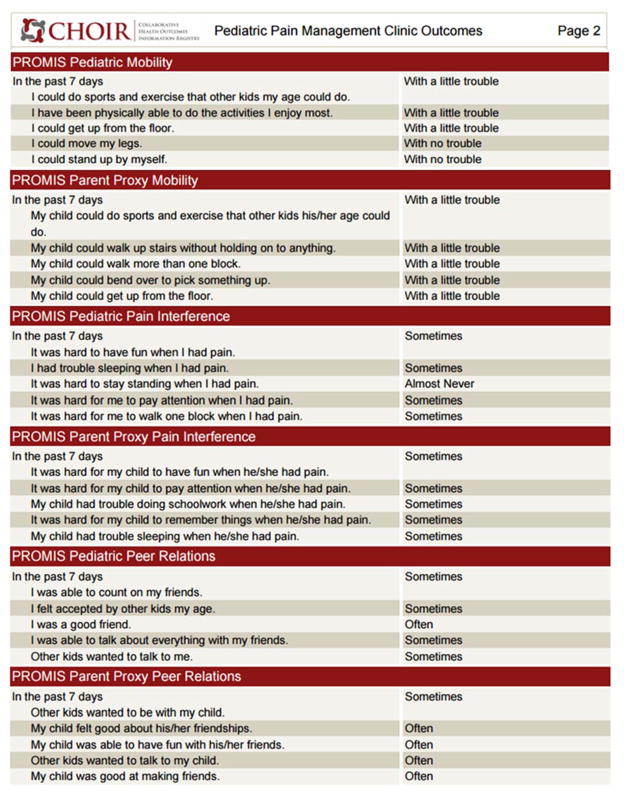
Figure 2.
Figure 2a. Sample first page of Peds-CHOIR initial survey
Figure 2b. Sample second page of Peds-CHOIR initial survey
Figure 3.
Figure 3a. Sample first page of Peds-CHOIR follow-up survey
Figure 3b. Sample second page of Peds-CHOIR follow-up survey
2.1.3. Development of the Assessment Battery
The assessment battery for Peds-CHOIR was informed by collaborations with the adult pain clinic, input from clinicians working in the field of pediatric pain medicine for over 10 years, and current clinical and empirical standards for pediatric pain [12,30,43]. We included pediatric and caregiver PROMIS measures relevant to pediatric chronic pain and consistent with the adult CHOIR.
The initial Peds-CHOIR survey includes our comprehensive Demographic and Pain History Questionnaire, and a graphical body map designed to document pain location(s). The Pain Catastrophizing Scale (PCS), a legacy measure, was added to Peds-CHOIR due to its association with pain intensity and treatment outcomes [8,37,44,61], and to allow for targeting of cognitive patterns that have been associated with impairments in function over time for both patient and caregivers [13,16,18]. We used the Pain Catastrophizing Scale for Children (PCS-C) and Parents (PCS-P) [8,22] and patient- and caregiver-reported PROMIS domains to deeply characterize the patients’ physical, psychological and social functioning including: mobility, pain interference, fatigue, peer relationships, anxiety and depression. Although sleep is an important predictor of outcomes in pediatric chronic pain [48,60,62] the pediatric PROMIS sleep domain had not yet been validated at the time of the launch of Peds-CHOIR. PROMIS Sleep measures will be added in a future version.
At follow-up visits patient and caregiver complete an abbreviated Demographic and Pain History Questionnaire to assess current symptoms, pain intensity, pain location(s) via the body map, all PROMIS measures captured at initial assessment, and the catastrophizing measures (PCS-C and PCS-P).
2.2. Assessment Battery
2.2.1. Demographics and Pain History Questionnaire
The Demographics and Pain History Questionnaire assesses caregiver and patient demographics including age, gender, race, and ethnicity. Although not presented in this paper, information about caregiver demographics (occupation, education, marital status etc.), patient’s medical history, sleep hygiene, medications, PROMIS caregiver global health (comprised of physical and mental health scales), and treatments previously utilized by the patient are also gathered. A trained research assistant extracts primary diagnoses from the medical record following the initial interdisciplinary team evaluation.
2.2.2. Pain Intensity
Patients rate their average, highest, and lowest pain intensity in the last month and current pain intensity on a standard 11-point numeric rating scale from 0 (No pain) to 10 (Worst pain possible) at the time of the assessment [40]. Caregiver perception of the patient’s pain intensity is assessed with the same measurement [67].
2.2.3. Body Map
Body maps are widely used to assess pain location in chronic pain populations [55,66]. They are particularly relevant for studies of patients with multiple locations of pain and play an important role in developing treatment plans and evaluating treatment efficacy [42,66]. At initial and follow-up evaluations, patients complete an electronic body map by either using a touchscreen (if completed in clinic) or by clicking on the body part (if using a desktop at home) to indicate painful body sites. The registry longitudinally tracks the number and location of areas indicated as painful. Our body map includes 74 sites, 36 anterior and 38 posterior, and utilizes a medial line distinguishing right and left head, abdomen, neck, and chest sites (Figure 2a), thereby differing slightly from other body maps [66].
2.2.4. PROMIS Pediatric Patient and Caregiver (Proxy) Outcome Measures
PROMIS utilizes item response theory (IRT) and computer adaptive testing (CAT) to assess patient-reported health outcomes for physical, mental, and social domains of functioning for clinical research and practice. IRT rests on the assumption of invariance, which enables comparison across studies and between reports of similar constructs. CAT works via administration of items from an item bank best suited for the reporter based on responses to earlier items, reducing the number of administered items to optimize patient burden, and improving precision [50,63,65,68]. Thus, although different questions are answered within each construct the final score should still reflect the general construct (e.g. fatigue) allowing for group comparisons within and between reporters. The pediatric PROMIS measures have been utilized in a variety of pediatric chronic health conditions such as asthma, cancer, chronic kidney disease, obesity, rheumatic disease, sickle cell disease, as well as those with chronic pain [4,9,25]. These measures are calibrated with a norming population of patients in the US with chronic conditions and their families, as well as general populations from schools and primary care clinics [51].
PROMIS scores are based on T-score distribution with a mean of 50 and standard deviation (SD) of 10. The pediatric PROMIS measures have been validated to assess physical, psychological, and social functioning domains in children 8 to 17 years of age, while caregivers report their perceptions by proxy [11,65]. The response format is based on a Likert scale (1= “Never/Not able to do” to 5= “Almost always/With no trouble”). Previous analyses of pediatric IRT PROMIS item banks for mobility and pain interference reflected a reliability coefficient of 0.90, while the fatigue item bank evidenced a reliability coefficient of 0.80 [11,34,64]. The anxiety and depressive symptoms item banks have demonstrated a reliability coefficient of 0.85 [27]. No reliability coefficients are currently available for the peer relations domain; however, initial research suggests that the domain is reliable and valid [10]. Further, Kashikar-Zuck et al. presented initial support for the validity of the PROMIS mobility, pain interference, fatigue, peer relationships, anxiety and depression measures in a clinical pediatric chronic pain population and demonstrated that the PROMIS short forms are valid and responsive to change [29].
Mobility
The PROMIS pediatric and proxy mobility item bank assesses activities of physical mobility largely reflective of lower extremity function (e.g., “I could do sports and exercise that other kids my age do;” “My child could stand up without help”). Higher scores indicate a higher level of mobility and are inverted for consistency with measures where higher scores indicate greater dysfunction [11,65].
Pain Interference
The PROMIS pediatric and proxy pain interference item bank assesses the impact of pain on physical, psychological, and social functioning (e.g., “It was hard for me to have fun when I had pain;” “It was hard for my child to run when he/she had pain”). Higher scores reflect greater pain interference (or greater caregiver perceptions of pain interference) in the child’s life [64,65].
Fatigue
The PROMIS pediatric and proxy fatigue item bank assesses a child’s ability to complete daily activities and function at their usual level in the family or in their social roles (e.g., “I was too tired to enjoy the things I like to do;” “My child got tired easily”). Higher scores indicate greater fatigue [34,65].
Peer Relationships
The PROMIS pediatric and proxy peer relationships item bank assesses quality or parental perceived quality of a patient’s relationships with peers (e.g., “Other kids wanted to be my friend;” “My child felt accepted by other kids his/her age”). Higher scores indicate better peer relationships and were inverted for consistency to indicate higher levels of dysfunction [10,65].
Anxiety Symptoms
The PROMIS pediatric and proxy anxiety item bank assesses fears, worries, and nervousness (e.g., “I worried about what could happen to me;” “My child felt nervous”). Higher scores indicate a higher level of anxiety [27,65].
Depressive Symptoms
The PROMIS Pediatric and Proxy Depression item bank assesses negative mood, self-perceptions, and social cognition (e.g., “I could not stop feeling sad;” “My child felt lonely”). Higher scores indicate more symptoms of depression [27,65].
2.2.5. Pain Catastrophizing Scale (PCS-C and PCS-P)
The Pain Catastrophizing Scale for children (PCS-C) assesses child and adolescent catastrophic thinking associated with pain as well as feelings of helplessness [8]. The proxy report (PCS-P) evaluates a caregiver’s catastrophic thinking regarding the child’s pain [22]. Both measures are reliable and valid adaptations of the Pain Catastrophizing Scale [58]. PCS assesses negative cognitive patterns characterized by rumination (e.g., “When I am in pain, I want the pain to go away,” “When my child is in pain, I can’t keep it out of my mind”), magnification (e.g., “When I am in pain, I am afraid that the pain will get worse,” “When my child is in pain, I think of other painful events”), and helplessness (e.g., “When I am in pain, it’s awful and I feel that it overwhelms me,” “When my child is in pain, I keep thinking about how much s/he is suffering”) toward actual or anticipated pain, and is a powerful predictor of maladaptive coping in chronic pain in adults and children. The PCS-C and PCS-P are both 13-item measures that employ a 5-point ordinal scale (0= “Not at all true” to 4 = “Extremely true”). Higher total scores reflect greater catastrophizing tendencies [8,22]. The PCS-C clinical reference point for high catastrophizing is 26 or above [49]. The total PCS-C score and its subscales (magnification, rumination, and helplessness) have internal consistency ranging from α = 0.68 to α = 0.87 [8]. Although there are no published reference points for PCS-P, a cut-off score of 23 and above for high catastrophizing has been used clinically by one of the developers of the scale (Simons, personal communication, October 2015). The PCS-P total score and three subscale scores are also internally consistent with Cronbach’s alpha varying from α = 0.81 to α = 0.93 [22].
2.3. Statistical Analysis
Descriptive statistics were used for presenting demographic information, data were entered and examined to ensure that statistical assumptions for t-tests were met, and analyzed using SPSS version 21.0 (SPSS IBM, New York, NY).
3. Results
3.1. Peds-CHOIR Investment, Design and Implementation Process
Implementation of integrating Peds-CHOIR into this clinic cost approximately $50,000, with $5,000- $7,000 estimated for annual maintenance. The initial startup investment included the cost of technology development efforts to expand the base CHOIR system for dual tracking of patient and caregiver data, incorporating the pediatric assessment battery, and database support. There were multiple additional systems-based considerations (e.g., start-up costs, personnel responsible for registering patients in Peds-CHOIR, faculty response to a novel assessment paradigm), clinical issues (e.g., patient burden, family response), and research considerations (e.g., identification of parsimonious yet meaningful questionnaires for systematic data collection over time) to note in the process of adopting Peds-CHOIR. Anecdotally, implementation of Peds-CHOIR was positively supported by the staff, appreciated by the clinicians, and well tolerated by patients and their families. Patients seemed to appreciate ease of administration, relatively short completion time, and the therapeutically informative visual description (e.g., longitudinal graph tracking) of their progress over time when reviewing outcomes with their treatment providers at follow-up visits. On average, total administration time for the initial assessment was 21.54 minutes (SD=4.91) and follow-up assessment took an average of 18.20 minutes (SD=5.71).
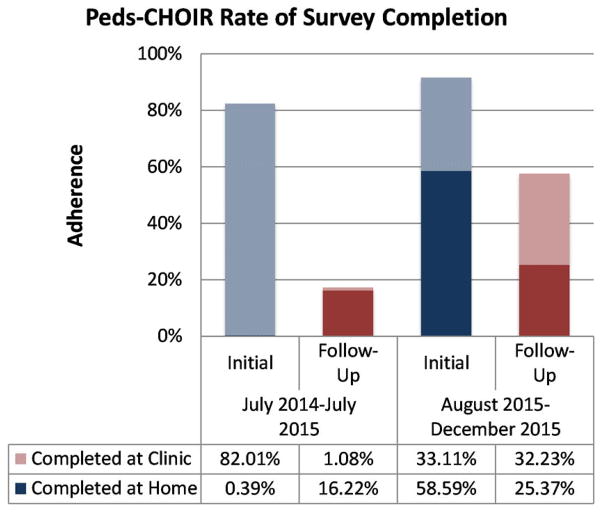
Peds-CHOIR has been utilized for 18 months since its implementation in July 2014. One year after implementing Peds-CHOIR in this clinic, analyses of follow-up completion adherence were low (17.3%) versus the initial surveys (82.4%). This prompted additional staff training, provision of education to clinicians on its utility with the patient during follow-up visits, clinic flow enhancements, and conversations with patients, clinicians, and staff highlighting the benefits of the system for personalizing and thereby optimizing patient care dynamically over time. Data collected between August 2015 and December 2015 indicated significant improvements (57.6%) in completion adherence for completion of follow-up surveys and 91.7% for completion adherence of initial surveys underscoring the importance of education for all stakeholders. Further, more families were completing the surveys at home versus in the clinic which improved clinic flow (Figure 4).
Figure 4.
Peds-CHOIR rates of adherence
3.2. Peds-CHOIR Sample Characteristics
The sample included 352 patients with a mean age of 13.9 years (range = 8–17) and their primary caregivers who presented for initial evaluation at the pediatric pain clinic from June 2014 to December 2015. The sample was predominantly female and Caucasian. The most frequent primary diagnoses were musculoskeletal pain, headaches, and chronic abdominal pain (Table 1). Patients with a psychological diagnosis and whose symptom constellations were secondary to that condition – with no organic etiology – were classified as having a primary psychological diagnosis (e.g., somatic symptom disorder-with predominant pain, functional neurological symptoms disorder).
Table 1.
Demographic and Diagnostic Characteristics of Sample, N=352
| N | % | |
|---|---|---|
| Gender (female) | 264 | 75.0% |
| Race | ||
| Caucasian | 240 | 68.2% |
| Other | 45 | 12. 8% |
| Asian | 25 | 7.1% |
| Unknown | 16 | 4.5% |
| African American | 13 | 3.7% |
| Declines to state | 7 | 2.0% |
| Native Hawaiian or Pacific Islander | 4 | 1.1% |
| American Indian or Alaskan | 2 | 0.6% |
| Primary Pain Diagnoses | ||
| Musculoskeletal pain | 131 | 37.2% |
| Headache | 62 | 17.6% |
| Chronic abdominal pain | 57 | 16.2% |
| Other | 37 | 10.5% |
| Complex regional pain syndrome | 31 | 8.8% |
| Fibromyalgia | 20 | 5.7% |
| Ehlers-Danlos syndrome | 6 | 1.7% |
| Rheumatologic conditions | 3 | 0.9% |
| Primary psychological diagnoses | 3 | 0.8% |
| Missing Diagnosis | 2 | 0.6% |
3.2.1. Descriptive Data from the assessment battery
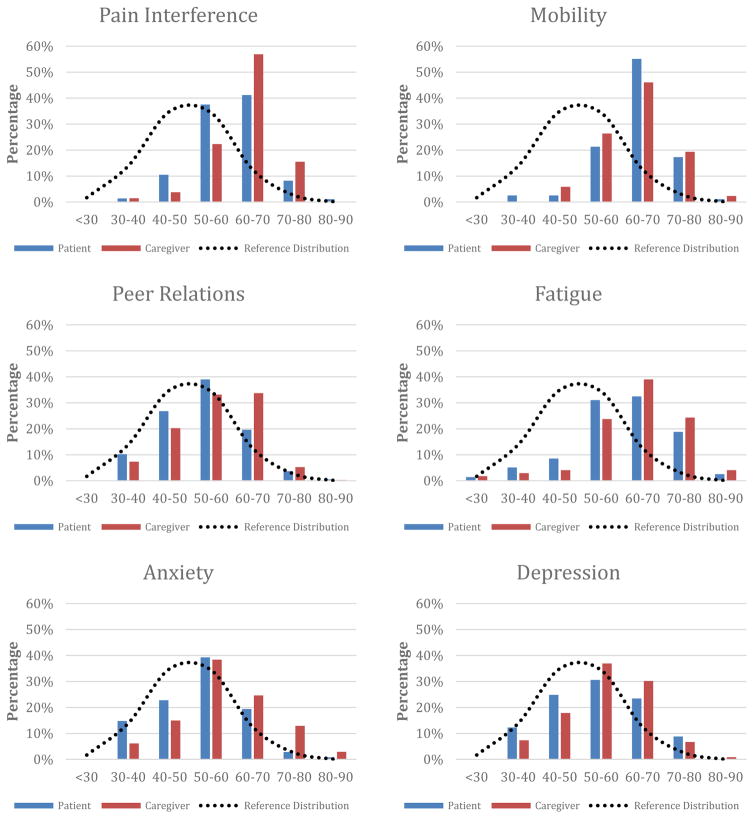
For PROMIS domains, patient self-report means fell within one standard deviation of the norm-referenced population with the exception of mobility and fatigue. Compared to the patients, caregivers reported higher levels of functional impairments across all PROMIS domains except on mobility. Fatigue (patient and caregiver), mobility (patient and caregiver), and pain interference (caregiver) all exhibited mild negative skew, highlighting the high functional impairment reported by patients and caregivers served at this tertiary pediatric pain clinic. Patient and caregiver mean pain intensity ratings were comparable. Table 2 presents means and standard deviations for the PROMIS domains, PCS, and pain ratings while Figure 5 presents a distribution of caregiver and patient self-reported PROMIS scores in comparison to the normal PROMIS distribution curve.
Table 2.
Means and Standard Deviations (SD), Paired Samples T-tests, and Correlations of Patient and Proxy Report across PROMIS Domains, Pain Catastrophizing Scale (PCS), and Pain Intensity
| Measure | Patient | Proxy | Patient/Proxy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| M | (SD) | N | M | (SD) | N | t | 95% CI | Cohen’s d | df | r | |
| PROMIS Mobility | 63.24 | −8.03 | 352 | 63.51 | −8.22 | 341 | −1.19 | −0.93, −0.23 | - | 340 | 0.78** |
| PROMIS Pain Interference | 59.37 | −8.44 | 352 | 62.62 | −7.75 | 341 | −8.12** | −4.12, −2.51 | 0.44 | 340 | 0.56** |
| PROMIS Fatigue | 60.02 | −11.90 | 351 | 62.72 | −11.01 | 341 | −5.70** | −3.69, −1.80 | 0.31 | 340 | 0.70** |
| PROMIS Peer Relations | 52.67 | −9.77 | 351 | 55.91 | −9.49 | 341 | −6.61** | −4.06, −2.20 | 0.36 | 340 | 0.59** |
| PROMIS Anxiety | 52.15 | −10.30 | 351 | 57.46 | −10.99 | 341 | 10.91** | −6.30, −4.37 | 0.59 | 340 | 0.64** |
| PROMIS Depression | 53.89 | −11.10 | 350 | 55.93 | −10.35 | 341 | −4.24** | −3.01, −1.10 | 0.23 | 340 | 0.65** |
| PCS-C/PCS-P | 23.61 | −12.35 | 352 | 21.81 | −11.34 | 343 | 2.41* | −0.33, −3.21 | 0.13 | 342 | 0.34** |
| Pain - Current | 4.54 | −2.67 | 344 | 4.46 | −2.66 | 337 | 1.05 | −0.08, −0.26 | - | 336 | 0.82** |
| Pain-Average | 5.63 | −2.08 | 344 | 5.55 | −2.10 | 337 | 0.53 | −0.13, −0.22 | - | 336 | 0.69** |
| Pain – Highest | 7.76 | −2.06 | 344 | 7.64 | −2.21 | 337 | 1.03 | −0.10, −0.33 | - | 336 | 0.56** |
| Pain – Lowest | 3.18 | −2.28 | 344 | 3.12 | −2.24 | 337 | 0.47 | −0.13, −0.22 | - | 336 | 0.74** |
Note: PROMIS scores are based on T-score distribution with a mean of 50 and SD of 10. Pain intensity score is based on 11-point Numeric Rating Scale.
p<.05 &
p<.001
Figure 5.
Observed distribution of patient-caregiver-reported PROMIS scores compared with reference distribution of PROMIS scores from a US Census population (approximated by a normal distribution) (N=352)
Patient reports of pain catastrophizing on the PCS were normally distributed whereas caregiver report was positively skewed. In order to explore distribution of PCS–C scores, the variable was divided based on identified clinical cut-off score [49]. Such categorization demonstrated that 43.5% of patients were in the clinical range (raw score ≥ 26), 29.3% in the moderate range (scores 15–25) and 27.3% endorsed low levels (0–14) of catastrophizing [16]. Utilizing the recommended clinical cut-off point (≥ 23) for the caregiver, PCS-P demonstrated that 42.9% of caregivers endorsed clinically elevated catastrophizing. Although patients reported slightly higher mean catastrophizing scores compared to caregivers, the percentage of scores in the clinical range was comparable between reporters.
3.2.2. Body Map
All patients in the study completed the body map, and number of painful sites indicated ranged from 0–74. A small subset of patients did not endorse any sites (N = 25; 7.1%) on the body map and one patient reported pain in all sites. The mean number of total body sites endorsed was 10.65 (SD = 12.7), with the majority of patients reporting 10 or fewer sites (N = 247; 70%). The modal number of body sites was 2 (N = 65; 18.5%) and median was 6. The front and back segments of the body map were largely comparable with regard to mean number of sites endorsed (front: M = 4.9, SD = 6.1; back: M = 5.7, SD = 7.1).
3.2.3. Comparison of Patient and Caregiver Report
Analyses revealed moderate to high correlations between patient and caregiver reports across all PROMIS domains, PCS, and pain intensity ratings (Table 2). Paired Samples t-tests allowed for comparison of patient and caregiver reports on PROMIS domains, catastrophizing, and pain intensity ratings. Patient and caregiver reports differed significantly on measures of pain interference, fatigue, peer relations, depression, anxiety, and pain catastrophizing. Calculation of Cohen’s D statistic revealed small to medium effect sizes for these differences (Table 2). There were no statistically significant differences on mobility or measures of pain intensity.
3.3. Point of Care Dynamic Tracking
One unique and meaningful feature of Peds-CHOIR is its ability to track both patient and caregiver perceptions of progress longitudinally. Such data inform assessments, patient and caregiver education needs, and individual- and family-based interventions specific to clinical presentation and patient preferences. Additionally, graphical presentation of progress allows for interactive involvement of the patient and caregiver with the clinician in informing treatment recommendations at point of care.
3.3.1. Sample Case
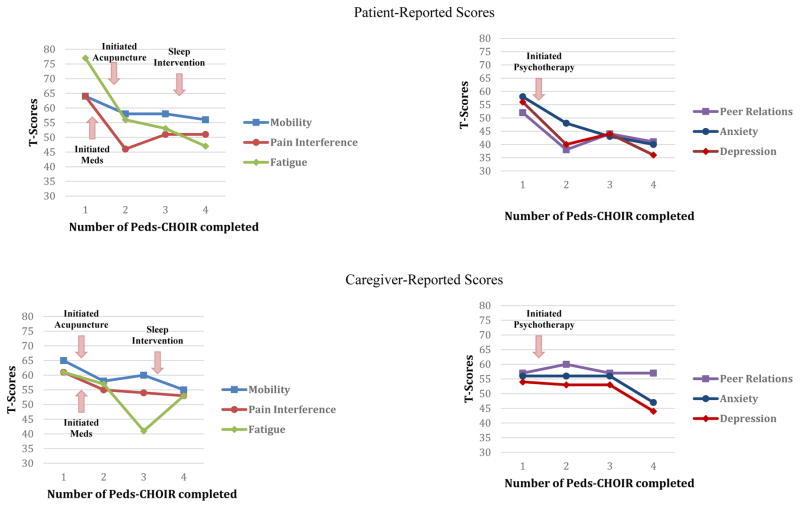
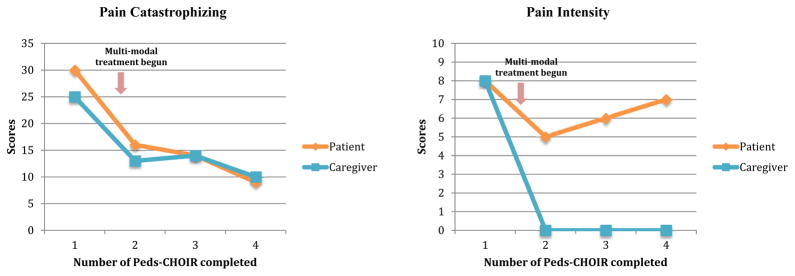
“Jennifer”, is a 12 year-old female with a 6-month history of chronic abdominal pain. She received treatment at the pediatric pain clinic for 9 months (Figure 6 & 7). Her initial assessment, revealed gastritis secondary to an infection, and psychological factors were identified as contributing to her discomfort, as pain symptoms began during a stressful transition to middle school. Both pharmacological and nonpharmacological treatments were consequently recommended, including: probiotics, melatonin, dietary adjustments, acupuncture, and pain psychology. The Peds-CHOIR survey completed at the initial assessment yielded clinically elevated scores across domains, informing the team of her high degree of impairment in functional activities and mental health vulnerability with elevated depression and anxiety symptoms secondary to pain. Jennifer received 8 weekly pain psychology and acupuncture sessions, monthly medical follow-ups where she trialed probiotics and melatonin, and made steady improvements in her function and pain. Thereafter, pain psychology sessions decreased to every 2–4 weeks and acupuncture was discontinued as she sustained functional progress in all domains.
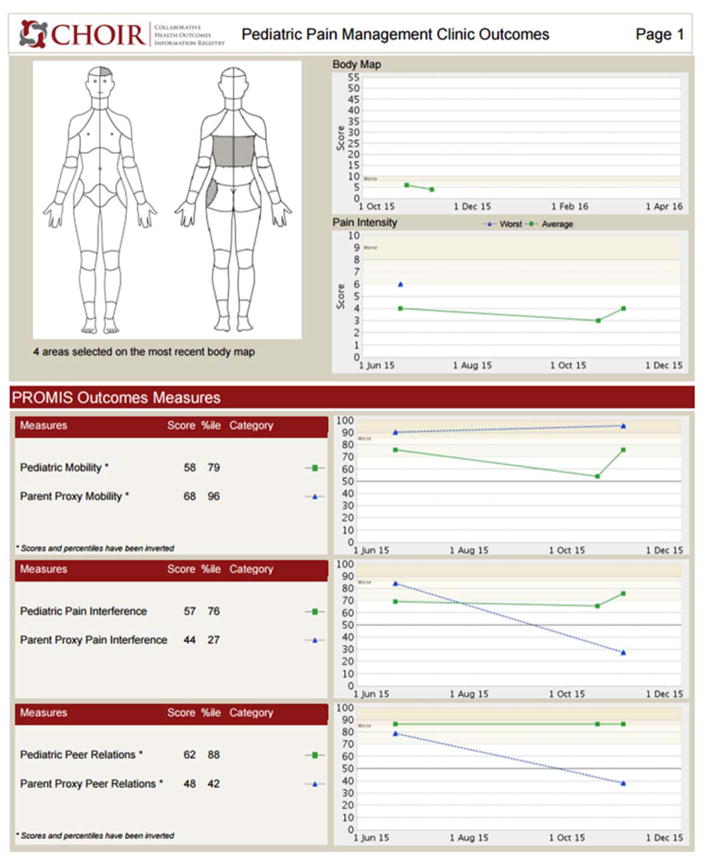
Figure 6.
Sample patient-caregiver PROMIS scores.
Figure 7.
Sample patient-caregiver pain catastrophizing and current pain level scores
Due to the challenges of integrating Peds-CHOIR into the regular follow-ups in the clinic (described in detail above) Jennifer completed her 2nd Peds-CHOIR a few months later, which demonstrated improvements in pain intensity and all domains of functioning. A month later, she completed her 3rd Peds-CHOIR, and she reported increased pain intensity, fatigue, and anxiety, and decreased mobility. These data triggered medical follow-up (where constipation was identified), weekly pain psychology treatment, and another trial of acupuncture.
After completing the 3rd Peds-CHOIR, in a pain psychology session, Jennifer and her mother were presented follow-up graphs that highlighted elevated fatigue, impaired mobility, and increased pain intensity. Reviewing these results and pictorial descriptions of the relationship between fatigue, mobility and pain helped facilitate Jennifer’s motivation to improve sleep hygiene. Thus, a behavioral treatment for sleep was implemented. One month later, the 4th completed Peds-CHOIR surveys revealed that adherence to the treatment had cultivated gradual improvements not only in Jennifer’s fatigue, but also in all domains of PROMIS functioning (Figure 6) including steady improvements in catastrophizing, reflected in their normalization of PCS scores over time. Despite no change reported by the patient on pain intensity, her caregiver reported that her pain had remitted (Figure 7), which underscores the clinical utility of tracking both patient and caregiver perspective to guide treatment.
4. Discussion
The successful and cost-effective long-term management of chronic pain depends on integrated medical, mental health, and physical rehabilitation treatments [20]. It has become standard practice to include multiple informants in pediatric pain research; however, the capability of Peds-CHOIR to collect both patient and caregiver perceptions that dynamically respond to treatment changes in real time is a novel approach [45]. Decisions for patients with chronic pain should be based on valid, reliable and repeated data from multiple reporters across domains of functioning; until now, this approach has been an ideal instead of reality in healthcare. CHOIR and Peds-CHOIR help to provide evidence-based assessment and interventions, and meet the call by the Institute of Medicine (IOM) for the development of national registries to guide research and precision pain medicine.
Pediatric chronic pain is associated with prolonged pain into adulthood [2,3,69], as well as significant impacts on physical, school, social, family, and emotional functioning [21,35,36,47]. Existing literature falls short in informing the developmental indicators that predict protracted pain into adulthood, which motivated the adaptation of CHOIR and the creation of Peds-CHOIR learning healthcare system (LHS). As pain is highly subjective, clinical decisions can be supported by LHS’s for improving the accuracy of pain assessments, and informing clinical decisions and interventions. The main goals of this paper were to describe the platform, showcase how LHS may be leveraged to inform clinical research and treatment, and highlight its dynamic ability to track outcomes longitudinally among patients and caregivers.
4.1. Design and Implementation of Peds-CHOIR: System Challenges and Solutions
LHS’s such as Peds-CHOIR, allow for cross-discipline collaboration between clinicians and researchers with technology experts who build and maintain the platform. Initial cost of implementing the registry was high, primarily due to technological enhancements of the registry. Since the initial investment of building the dual track platform and adding reliable and valid pediatric measures relevant to chronic pain has been completed at this institution, implementation of Peds-CHOIR at other institutions is estimated between $7000 and $5000 annually. In addition, the IT team at this institution is building the capability for patients and caregivers to complete parallel administrations of Peds-CHOIR versus the current sequential administration, which will further reduce patient burden and improve clinic flow. It is at the discretion of individual institutions that choose to adopt Peds-CHOIR to determine which measures to retain, delete, add, and the frequency preferred for tracking follow-up. The platform is set up in such a way that any measure added to the registry or IT enhancements by one institution, are available to all other institutions if desired, without additional cost. Further, the cost of implementation is forecasted to be offset by utilization of the registry, which is predicted to increase efficiency and cost-effectiveness by optimizing treatments.
Deployment of Peds-CHOIR as a LHS to optimize clinical services was well received by providers, clinic staff, and families based on informal feasibility and usability feedback. This was by design; in that CHOIR and Peds-CHOIR were meant to seamlessly integrate into the clinical workflow, as opposed to traditional electronic data capture systems that tend to be more research oriented. Following one year of registry implementation, low completion adherence rates for follow-up surveys necessitated remediation. The following interventions led to measurable improvements: increasing education about utility of the registry for all stakeholders (patients, providers, and staff), offering copies of completed Peds-CHOIR to providers before they see the patient, placing reminder calls to the family to arrive earlier to the appointment if they have not completed the registry, prompting providers whose patients were not completing the registry to discuss its utility with the family and understand barriers to completion, and most importantly utilizing Peds-CHOIR during the session with the patient to increase the patient’s motivation to complete future Peds-CHOIR as well as encourage active engagement in treatment.
4.2. Characteristics of the data and initial outcomes
Adoption of this registry allowed for measurement of prioritized domains of assessment in pediatric pain utilizing evolving measures that harness improved sensitivity to responses [7,13,45]. Patients seen at this tertiary clinic included mostly white, female, school-age children primarily with chronic musculoskeletal pain. Mean PROMIS scores in our clinic sample were clinically elevated, even higher than those reported by a pediatric oncology sample [25]. Such findings highlight the remarkable vulnerability encountered by interdisciplinary pain teams in tertiary care settings, and underscore the need for providers to attune to outcomes.
Concurrent collection of patient and caregiver measures demonstrated that reports of functional impairment were significantly related as assessed by PROMIS domains, which is consistent with previous studies examining correlations between child and caregiver reports [6,71]. Despite denoting comparable pain levels and pain catastrophizing scores, caregivers reported more dysfunction across most PROMIS domains except mobility. Cohen et al. examined adolescent and caregiver concordance and discordance on pain related functioning; while reports were generally related, differences were noted in ratings of internal processes (similar to PROMIS fatigue, anxiety, depression, etc.) but more consistent in observable domains (similar to PROMIS mobility) [7]. It is also possible that concerns regarding social desirability, developmental limitations in insight, or focus on pain as an exclusively medical issue lead patients to underreport psychological vulnerabilities such as anxiety and depression, or to have tendencies to somaticize discomfort and its impact on daily living [18,19,54]. Given the subjective nature of pain-related disability ratings, it is difficult to know if caregivers tend to over-report or if children under-report pain-related disability. For example, caregivers who endorse greater pain catastrophizing themselves may overestimate their child’s emotional discomfort and/or physical limitations based on elevated personal distress. Having greater knowledge of these differences may have a meaningful impact on tailoring clinical interventions to enhance outcomes (e.g., improving caregiver-patient communication, need for additional psychoeducation and/or support for either caregiver or patient, and family based interventions) [14,44,46,53]. Future investigations using Peds-CHOIR should study agreement ratings, and predictors of agreement, between caregiver and patient, as change is tracked over time and in response to treatment.
The case example included in this report of Peds-CHOIR describes how the platform informs the identification of interventions, when they are initiated, and how such intervention(s) impact patient and caregivers reports of outcome indicators during treatment. As highlighted in the case example, Peds-CHOIR longitudinal data tracking may facilitate preventative deployment of treatments that facilitate patient-caregiver communication and commitment to treatment goals, and provides opportunities to examine response to interventions from the perspective of both the patient and caregiver longitudinally. What LHSs such as Peds-CHOIR add to clinical decisions and outcomes over and above the clinical decisions made without the help of the LHS is an important question to pursue to truly understand the value of such tools.
4.3. Future Directions
The Peds-CHOIR system fosters attunement to the clinical needs of patients and their families via outcomes tracking. This LHS allows for dynamic (re)configuration of treatment plans over time for a population who, by its very nature, is continuously evolving. Replication of findings in pediatric pain research is challenged by the variability in measurement and limitations in systems and methodology. Clinical and investigatory work guided by a shared language such as the Peds-CHOIR system across sites would allow for the utilization of a standardized, psychometrically sound, and open-source system of measures. Given Peds-CHOIR’s inclusion of measures (e.g., PROMIS) not yet normed on populations outside the US, validation of such assessment domains in other countries remains indicated as well as the inclusion of validated domains that have been normed on non-English speaking children and their caregivers [1]. Standard adoption of this novel outcome-tracking platform by institutions and clinics worldwide would facilitate opportunities to pool data, unite experts, and yield potentially synergistic scientific collaborations to foster pain management treatment paradigms for even the most complex cases. In short, it would facilitate a novel systems approach to treatment.
Moreover, implementation of Peds-CHOIR at this tertiary care pain clinic will continue to foster dual capture of clinically meaningful changes in pediatric patients with pain and caregiver perceptions, as well as offer empirically sound information to augment treatments by targeting factors associated with greater risk, and identify developmentally relevant variables to assist with prediction of the type of patient who develops prolonged pain into adulthood, to help tailor interventions for those subpopulation of patients. As data collection continues with improving adherence to completion of follow-up surveys, the next vital steps for Peds-CHOIR will be to describe longitudinal outcomes at the group level and analyze sensitivity to change. Translation of the registry into other languages would be an important future goal.
Registries such as Peds-CHOIR are consistent with a paradigm shift toward precision health medicine. Aggregated data may help to highlight patient and caregiver phenotypes associated with long-term wellness versus treatment resistance enabling clinicians to detect and prevent challenges by intervening earlier with at-risk patients. Adoption of the LHS by other sites would also allow for formulation of a larger registry that could help to achieve research goals of identifying patient characteristics predictive of treatment response (e.g. clinical phenotypes) over time, capturing data representative of the populations served, and clinically enhancing treatment for individual patients.
Acknowledgments
Funding: The authors thank Christine Junge at the Department of Anesthesiology, Perioperative, Pain Medicine, for her contributions to the editing of the article as well as the reviewers for their valuable comments and suggestions. We also acknowledge funding from the National Institutes of Health (HHSN 271201200728P, K24 DA029262), as well as the Redlich Pain Endowment and the William and Gretchen Kimball Endowment for Pediatric Pain Management. The authors have no other financial conflicts to disclose.
Footnotes
Disclosures: The authors wish to acknowledge funding from the National Institutes of Health (HHSN 271201200728P, K24 DA029262), as well as the Redlich Pain Endowment and the William and Gretchen Kimball Endowment for Pediatric Pain Management. The authors have no other financial conflicts to disclose.
Contributor Information
Rashmi P. Bhandari, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Amanda B. Feinstein, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Samantha E. Huestis, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Elliot J. Krane, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California.
Ashley L. Dunn, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Lindsey L. Cohen, Department of Psychology, Georgia State University, Atlanta, Georgia
Ming C. Kao, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Beth D. Darnall, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
Sean C. Mackey, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, Stanford, California
References
- 1.Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace F, Leplege A, Lu A, Tulsky DS, Raat H, Ravens-Sieberer U, Revicki D, Terwee CB, Valderas JM, Cella D, Forrest CB, Group PI. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health Qual Life Outcomes. 2013;11:210. doi: 10.1186/1477-7525-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain. 2004;8(3):187–199. doi: 10.1016/j.ejpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Brna P, Dooley J, Gordon K, Dewan T. The prognosis of childhood headache: a 20-year follow-up. Arch Pediatr Adolesc Med. 2005;159(12):1157–1160. doi: 10.1001/archpedi.159.12.1157. [DOI] [PubMed] [Google Scholar]
- 4.Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61(7):1282–1288. doi: 10.1002/pbc.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers CT, Reid GJ, Craig KD, McGrath PJ, Finley GA. Agreement between child and parent reports of pain. Clin J Pain. 1998;14(4):336–342. doi: 10.1097/00002508-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Cohen LL, Vowles KE, Eccleston C. Adolescent chronic pain-related functioning: concordance and discordance of mother-proxy and self-report ratings. Eur J Pain. 2010;14(8):882–886. doi: 10.1016/j.ejpain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 9.DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, Hinds PS, Huang IC, Thissen D, Varni JW. PROMIS((R)) pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015;24(9):2195–2208. doi: 10.1007/s11136-015-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewalt DA, Thissen D, Stucky BD, Langer MM, Morgan Dewitt E, Irwin DE, Lai JS, Yeatts KB, Gross HE, Taylor O, Varni JW. PROMIS Pediatric Peer Relationships Scale: development of a peer relationships item bank as part of social health measurement. Health Psychol. 2013;32(10):1093–1103. doi: 10.1037/a0032670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, Lai JS, Yeatts KB, Dewalt DA. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol. 2011;64(7):794–804. doi: 10.1016/j.jclinepi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eccleston C, Jordan AL, Crombez G. The impact of chronic pain on adolescents: a review of previously used measures. J Pediatr Psychol. 2006;31(7):684–697. doi: 10.1093/jpepsy/jsj061. [DOI] [PubMed] [Google Scholar]
- 13.Eccleston C, McCracken LM, Jordan A, Sleed M. Development and preliminary psychometric evaluation of the parent report version of the Bath Adolescent Pain Questionnaire (BAPQ-P): A multidimensional parent report instrument to assess the impact of chronic pain on adolescents. Pain. 2007;131(1–2):48–56. doi: 10.1016/j.pain.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. The Cochrane database of systematic reviews. 8:CD009660. doi: 10.1002/14651858.CD009660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eccleston C, Palermo TM, Williams AC, Lewandowski Holley A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. The Cochrane database of systematic reviews. 2014;5:CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ennett ST, DeVellis BM, Anne Earp J, Kredich D, Warren RW, Wilhelm CL. Disease Experience and Psychosocial Adjustment in Children with Juvenile Rheumatoid Arthritis: Children's Versus Mothers' Reports. J Pediatr Psychol. 1991;16(5):557–568. doi: 10.1093/jpepsy/16.5.557. [DOI] [PubMed] [Google Scholar]
- 17.Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep. 2013;(Spec No):S16–27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 18.Fearon P, Hotopf M. Relation between headache in childhood and physical and psychiatric symptoms in adulthood: national birth cohort study. BMJ. 2001;322(7295):1145. doi: 10.1136/bmj.322.7295.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber J, Van Slyke DA, Walker LS. Concordance between mothers' and children's reports of somatic and emotional symptoms in patients with recurrent abdominal pain or emotional disorders. J Abnorm Child Psychol. 1998;26(5):381–391. doi: 10.1023/a:1021955907190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–130. doi: 10.1037/a0035514. [DOI] [PubMed] [Google Scholar]
- 21.Gorodzinsky AY, Hainsworth KR, Weisman SJ. School functioning and chronic pain: a review of methods and measures. J Pediatr Psychol. 2011;36(9):991–1002. doi: 10.1093/jpepsy/jsr038. [DOI] [PubMed] [Google Scholar]
- 22.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child's pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006;123(3):254–263. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J Pain. 2014;15(9):925–933. doi: 10.1016/j.jpain.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guite JW, Logan DE, Sherry DD, Rose JB. Adolescent self-perception: associations with chronic musculoskeletal pain and functional disability. J Pain. 2007;8(5):379–386. doi: 10.1016/j.jpain.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, Faulkner C, Liu Y, Cheng YI, Gross HE, Wang J, DeWalt DA. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. doi: 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): 2011. [PubMed] [Google Scholar]
- 27.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao M, Cook K, Olson G, Pacht T, Darnall BD, Weber S, SM Stanford-NIH Pain Registry: Catalyzing the rate limited step of big-data psychometrics with item-response theory and advanced computerized adaptive testing. American Academy of Pain Medicine. 2014;15(3):533. [Google Scholar]
- 29.Kashikar-Zuck S, Carle A, Barnett K, Goldschneider KR, Sherry DD, Mara CA, Cunningham N, Farrell J, Tress J, DeWitt EM. Longitudinal evaluation of patient-reported outcomes measurement information systems measures in pediatric chronic pain. Pain. 2016;157(2):339–347. doi: 10.1097/j.pain.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–1607. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17(4):341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis and rheumatism. 2012;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002;3(5):412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 34.Lai JS, Stucky BD, Thissen D, Varni JW, DeWitt EM, Irwin DE, Yeatts KB, DeWalt DA. Development and psychometric properties of the PROMIS((R)) pediatric fatigue item banks. Qual Life Res. 2013;22(9):2417–2427. doi: 10.1007/s11136-013-0357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewandowski AS, Palermo TM, De la Motte S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151(1):220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: an investigation of moderating effects on the pathway from pain to disability. J Pediatr Psychol. 2005;30(8):698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- 37.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents' pain, functioning, and pain behavior. Clin J Pain. 2013;29(8):681–688. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackey S, Kao M, Cook K, Olson G, Pacht T, Darnall B, Weber S. Collaborative Health Outcomes Information Registry (CHOIR): Open source cloud based platform to generate and support learning healthcare systems. Postgraduate Medicine, Pain Management. 2014;66 [Google Scholar]
- 39.Maillard SM, Davies K, Khubchandani R, Woo PM, Murray KJ. Reflex sympathetic dystrophy: a multidisciplinary approach. Arthritis and rheumatism. 2004;51(2):284–290. doi: 10.1002/art.20249. [DOI] [PubMed] [Google Scholar]
- 40.Miro J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. Eur J Pain. 2009;13(10):1089–1095. doi: 10.1016/j.ejpain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Oerlemans HM, Oostendorp RA, de Boo T, Goris RJ. Pain and reduced mobility in complex regional pain syndrome I: outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain. 1999;83(1):77–83. doi: 10.1016/s0304-3959(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 42.Paananen MV, Auvinen JP, Taimela SP, Tammelin TH, Kantomaa MT, Ebeling HE, Taanila AM, Zitting PJ, Karppinen JI. Psychosocial, mechanical, and metabolic factors in adolescents' musculoskeletal pain in multiple locations: a cross-sectional study. Eur J Pain. 2010;14(4):395–401. doi: 10.1016/j.ejpain.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Palermo TM. Assessment of chronic pain in children: current status and emerging topics. Pain Res Manag. 2009;14(1):21–26. doi: 10.1155/2009/236426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Palermo TM, Eccleston C, Goldschneider KR, McGinn KL, Sethna N, Schechter N, Turner H. AP Society, editor. Assessment and managament of children with chronic pain: a position from the American Pain Society. Glenview, IL: 2012. [Google Scholar]
- 46.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148(3):387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6(4):234–250. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain. 2005;6(3):201–207. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain. 2014;155(11):2360–2367. doi: 10.1016/j.pain.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn H, Thissen D, Liu Y, Magnus B, Lai JS, Amtmann D, Varni JW, Gross HE, DeWalt DA. Using item response theory to enrich and expand the PROMIS(R) pediatric self report banks. Health Qual Life Outcomes. 2014;12:160. doi: 10.1186/s12955-014-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, Group PC. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 Suppl 1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 52.Sherry DD, Wallace CA, Kelley C, Kidder M, Sapp L. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15(3):218–223. doi: 10.1097/00002508-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Sieberg CB, Manganella J. Family Beliefs and Interventions in Pediatric Pain Management. Child Adolesc Psychiatr Clin N Am. 2015;24(3):631–645. doi: 10.1016/j.chc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Simons LE, Logan DE, Chastain L, Cerullo M. Engagement in multidisciplinary interventions for pediatric chronic pain: parental expectations, barriers, and child outcomes. Clin J Pain. 2010;26(4):291–299. doi: 10.1097/AJP.0b013e3181cf59fb. [DOI] [PubMed] [Google Scholar]
- 55.Stinson JN, Stevens BJ, Feldman BM, Streiner D, McGrath PJ, Dupuis A, Gill N, Petroz GC. Construct validity of a multidimensional electronic pain diary for adolescents with arthritis. Pain. 2008;136(3):281–292. doi: 10.1016/j.pain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Sturgeon JA, Darnall BD, Kao MC, Mackey SC. Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain. 2015;16(3):291–298. e291. doi: 10.1016/j.jpain.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sturgeon JA, Dixon EA, Darnall BD, Mackey SC. Contributions of physical function and satisfaction with social roles to emotional distress in chronic pain: a Collaborative Health Outcomes Information Registry (CHOIR) study. Pain. 2015;156(12):2627–2633. doi: 10.1097/j.pain.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 59.Systems Neuroscience and Pain Lab. [Accessed November 4, 2015];Collaborative Health Outcomes Information Registry (CHOIR) Available at: https://choir.stanford.edu.
- 60.Tham SW, Holley AL, Zhou C, Clarke GN, Palermo TM. Longitudinal course and risk factors for fatigue in adolescents: the mediating role of sleep disturbances. J Pediatr Psychol. 2013;38(10):1070–1080. doi: 10.1093/jpepsy/jst051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran ST, Jastrowski Mano KE, Hainsworth KR, Medrano GR, Anderson Khan K, Weisman SJ, Davies WH. Distinct Influences of Anxiety and Pain Catastrophizing on Functional Outcomes in Children and Adolescents With Chronic Pain. J Pediatr Psychol. 2015;40(8):744–755. doi: 10.1093/jpepsy/jsv029. [DOI] [PubMed] [Google Scholar]
- 62.Valrie CR, Bromberg MH, Palermo T, Schanberg LE. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr. 2013;34(2):120–128. doi: 10.1097/DBP.0b013e31827d5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Linden WJ, Hambleton RK. Handbook of Modern Item Response Theory. New York: Springer-Verlag; 1997. [Google Scholar]
- 64.Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, Yeatts K, Dewalt DA. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varni JW, Thissen D, Stucky BD, Liu Y, Gorder H, Irwin DE, DeWitt EM, Lai JS, Amtmann D, DeWalt DA. PROMIS(R) Parent Proxy Report Scales: an item response theory analysis of the parent proxy report item banks. Qual Life Res. 2012;21(7):1223–1240. doi: 10.1007/s11136-011-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Baeyer CL, Lin V, Seidman LC, Tsao JC, Zeltzer LK. Pain charts (body maps or manikins) in assessment of the location of pediatric pain. Pain Management. 2011;1(1):61–68. doi: 10.2217/pmt.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143(3):223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Wainer H, Dorans NJ, Eignor D, Flaugher R, Green BF, Mislevy RJ, Steinberg L, Thissen D. Computerized adaptive testing: a primer. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 69.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150(3):568–572. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White KP, Nielson WR. Cognitive behavioral treatment of fibromyalgia syndrome: a followup assessment. J Rheumatol. 1995;22(4):717–721. [PubMed] [Google Scholar]
- 71.Zhou H, Roberts P, Horgan L. Association between self-report pain ratings of child and parent, child and nurse and parent and nurse dyads: meta-analysis. Journal of advanced nursing. 2008;63(4):334–342. doi: 10.1111/j.1365-2648.2008.04694.x. [DOI] [PubMed] [Google Scholar]