Abstract
Objective
Pancreatic neuroendocrine tumors (PNETs) are the major source of disease-specific mortality in multiple endocrine neoplasia type 1 (MEN1) patients. Chromogranin A (CgA), pancreatic polypeptide (PP), glucagon, and gastrin have some diagnostic value in sporadic PNETs, but there is very little evidence for their efficacy in diagnosing PNETs in MEN1 patients.
Design
We performed a retrospective chart review of the existing MEN1 database in our institution.
Patients
One hundred thirteen patients were eligible for diagnostic value analysis of tumor markers. Patients were excluded if measurement of tumor markers was missing, either 3 months prior to PNETs diagnosis (PNETs patients) or prior to abdominal imaging (non-PNETs patients).
Measurements
Clinicopathologic characteristics and of tumor marker measurements were analyzed.
Results
Of 293 confirmed MEN1 cases, 55 PNETs and 58 non-PNETs met inclusion criteria. The area under the curve (AUC) for CgA, PP, glucagon, and gastrin in MEN1 cases was 59.5%, 64.1%, 77.0%, and 75.9%, respectively. The AUC for the combination of CgA, PP, and gastrin was 59.6%. PP, but not CgA, glucagon or gastrin was significantly associated with both age and PNETs functional status (P=0.0485 and 0.0188, respectively). No markers were significantly associated with sex, PNETs tumor size, tumor number, tumor location, AJCC (American Joint Committee on Cancer) stage, presence of lymph node metastasis, lymphovascular invasion, or overall survival. CgA values were not significantly lower following PNETs resection than pre-operatively (P=0.554).
Conclusions
The value of blood markers for diagnosing PNETs in MEN1 patients is relatively low, even when used in combination.
Keywords: MEN1, Pancreatic neuroendocrine tumors, Chromogranin A, Pancreatic polypeptide, Glucagon
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant syndrome that is caused by a germline mutation mapped to 11q13 in the MEN1 tumor suppressor gene. It is a rare disease, occurring in about one in 30,000 people.1 It mainly affects the parathyroid glands (95%), the pancreas (40%–70%), and the pituitary gland (30%–40%) and less commonly results in tumors in the lungs and bronchus, thymus, and adrenal cortex.2
Although not the most common manifestations of MEN1, pancreatic, bronchial, and thymic neuroendocrine tumors occur and have high malignant potential. Metastatic disease from these primary sites also represents the most common cause of death in MEN1 patients.3 Recent studies showed that pancreatic neuroendocrine tumors (PNETs) development can occur as early as 10–20 years old4, 5. Even with an increased frequency of early diagnosis, PNETs patients continue to experience an average delay in diagnosis of 5–8 years from development of initial signs and symptoms.6, 7 Normally, once a patient is diagnosed with MEN1 on the basis of clinical symptoms or genetic testing results, regular follow-up is recommended to identify and treat MEN1-associated diseases early, especially in those patients with PNETs that are judged to have significant malignant potential.2 It has been shown that with early screening and subsequent treatment, MEN1 patients with PNETs have a better survival and less morbidity.8, 9 Therefore, the early and accurate diagnosis of PNETs in MEN1 patients is of clinical importance.
The most commonly used methods for diagnosing PNETs in MEN1 patients are imaging and biochemical markers. Available imaging techniques include computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), selective arterial angiography, and somatostatin receptor scintigraphy (SRS).2 It is also recommended that MEN1 patients undergo an annual plasma biochemical evaluation of a fasting gastrointestinal tract hormone profile that includes chromogranin A (CgA), pancreatic polypeptide (PP), glucagon, and gastrin levels.2
CgA has been documented to have modest value in the diagnosis of sporadic PNETs10–12, with an overall sensitivity ranging from 53.6% to 77.0% and a specificity between 56.0% and 91.9%. Based on these findings, CgA has been recommended by the recent European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Tumor Society (NANETS) guidelines as the most practical and useful serum tumor marker for sporadic PNETs diagnosis.13, 14 However, the diagnostic value of CgA for PNETs in MEN1 patients has rarely been evaluated.15 Furthermore, there are even fewer studies investigating the diagnostic value of PP, glucagon and gastrin in the setting of MEN1 patients. Thus, the goal of this study was to test the individual and combined diagnostic value of CgA, PP, glucagon, and gastrin in diagnosing PNETs in MEN1 patients.
Materials and methods
Study subjects
The patient cohort for this study consisted of all patients identified in the prospectively maintained MEN1 database from May 1980 to August 2015 in the Department of Surgical Oncology at The University of Texas MD Anderson Cancer Center (Houston, Texas). All patients were diagnosed according to clinical practice guidelines, including clinical and genetic criteria.2 This study was approved by the institutional review board of MD Anderson.
All patients with confirmed MEN1, with or without PNETs, were included. PNETs were diagnosed by pathological examination, or by characteristic findings on CT, MRI, EUS or somatostatin receptor scintigraphy. The absence of PNETs was also determined by at least one of these imaging studies. The following patients were excluded: patients without laboratory results measured within 3 months prior to PNETs diagnosis or prior to the last abdominal imaging, patients without abdominal imaging or with indeterminate PNETs status, and patients with renal dysfunction.
Patients on proton pump inhibitor (PPI) were excluded from CgA and gastrin analysis because of the known association of higher CgA and gastrin levels with chromic PPI administration.16–18 Patients with synchronous thymic, lung, or gastric NETs were also excluded from CgA analysis because of the potential for a higher CgA level in these patients.19
For PNETs patients, the most recent laboratory measurement within 3 months prior to the PNETs diagnosis was chosen. For non-PNETs patients, the most recent laboratory measurement 3 months prior to the last abdominal imaging in which they were confirmed to be PNETs negative was chosen. Only fasting measurements were included. If the most recent result was non-fasting, the next most recent eligible result was used. In addition, as reference ranges differed based on the laboratory performing the testing as well as the age of the patients (only for PP), blood marker data was normalized as the percentage exceeding the upper limit of normal, calculated as follows: [(measurement-upper limit of reference range)/upper limit of reference range]*100.
The primary objective of this study was to detect the individual and combined diagnostic value of CgA, PP, glucagon and gastrin for PNETs in MEN1 patients.
The secondary objective was to detect the correlations between each marker and age, sex, PNETs tumor location, tumor functional status, tumor size, tumor number, AJCC (American Joint Committee on Cancer) stage20, lymph node metastases, lymphovascular invasion and overall survival. In addition, we investigated the impact of PNETs surgery on the values of the serum tumor markers; we included the earliest measurement of post-operative serum markers within one year after the PNETs operation.
Tumor markers
All tumor markers were measured in Mayo Clinic Mayo Medical Laboratories (from Dec 1997 to March 2005) and Quest Diagnostics Laboratories (from March 2005 till now). Chromogranin A (CgA) was tested by immunofluorescent assay at Mayo and by electrochemiluminescent assay at Quest. Pancreatic polypeptide (PP) was tested by radioimmunoassay at both Mayo and Quest. Glucagon was analyzed by immunofluorescent assay at Mayo and by radioimmunoassay at Quest. Gastrin was detected by immunochemiluminescent assay at Mayo and immunoassay at Quest.
Statistical analysis
Receiver operating characteristic (ROC) analysis was performed to assess the ability of each marker individually to predict the likelihood of a PNETs diagnosis. ROC analysis was also performed in together with a logistic regression model that combined all 4 markers. An area under the ROC curve (AUC) between 0.60–0.80 was considered indicative of moderate diagnostic value, and 0.80–1.00 was considered indicative of good diagnostic value.15 Spearman correlation analysis was performed to assess the association between continuous parameters (age, PNETs tumor size and numbers) and each of the 4 markers. Wilcoxon rank-sum tests were performed to assess the association between dichotomous variables (sex, PNETs tumor location, tumor functional status, AJCC stage, lymph node metastases, and lymphovascular invasion) and the markers. Wilcoxon signed-rank tests were performed to compare each of the 4 markers between pre- and post-operative timepoints. A Cox proportional hazards regression analysis was performed to assess the association between overall survival (from the date of PNETs diagnosis) and the markers. Some analyses in some subgroups could not be performed due to the small numbers of patients. All statistical analyses were performed using R software version 3.2.2. All statistical tests used a two-sided significance level of 0.05. Given that this was a retrospective study, no adjustment for multiple testing was made; thus, the few statistically significant results should be interpreted with caution given that several statistical tests were performed.
Results
Study population
We identified 293 MEN1 patients in the database. In total, 180 patients were excluded, including 144 without suitable laboratory results, 26 with uncertain PNETs status, and 10 with renal dysfunction; finally 113 patients were eligible for diagnostic value analysis of tumor markers [38.6% (113 of 293) ] (Figure 1). The follow-up of these patients extented from May 1993 to August 2015. There were 55 PNETs patients and 58 non-PNETs patients; 79, 63, 24, and 52 patients were eligible for the analyses of CgA, PP, glucagon, and gastrin, respectively. Data on CgA, PP, and gastrin were available for 36 patients; 11 of these were PNETs patients.
Figure 1.
Flow chart of patients included in the study. MEN1=multiple endocrine neoplasia syndrome type 1; PNETs=pancreatic neuroendocrine tumors; CgA=chromogranin A; PP=pancreatic polypeptide.
Clinicopathologic characteristics
The clinicopathologic characteristics of both PNETs and non-PNETs patients are shown in Table 1. There were 34 males and 21 females in the PNETs group and 25 males and 33 females in the non-PNETs group, respectively. Patients’ median age at baseline assessment was 41 years (range, 18–76 years) for the PNETs group and 36 years for the non-PNETs group (range, 15–74 years).
Table 1.
Clinicopathologic characteristics of PNETs (n=55) and non-PNETs patients (n=58).
| Clinicopathologic characteristic | Classification | PNETs | Non-PNETs |
|---|---|---|---|
| Sex, n (%) | Male | 34 (61.8) | 25 (43.1) |
| Female | 21 (38.2) | 33 (56.9) | |
| Criteria for MEN1, n (%) | Clinical | 14 (25.5) | 10 (17.2) |
| Genetic | 24 (41.4) | ||
| Genetic with one manifestation | 5 (9.0) | 17 (29.3) | |
| Genetic with two manifestation | 36 (65.5) | 7 (12.1) | |
| Median age (years) at diagnosis (range) | 41 (18–76) | 36 (15–74)* | |
| Other NETs status, n (%) | Yes | 5 (9.1) | 1 (1.7) |
| No | 50 (90.9) | 57 (98.3) | |
| Tumor functional status, n (%) | Functioning | 18 (32.7) | |
| Non-functioning | 37 (67.3) | ||
| Tumor location, n (%) | Pancreatic head and neck | 7 (14.3) | |
| Pancreatic body and tail | 42 (85.7) | ||
| Tumor number, n (%) | 1 | 20 (37.0) | |
| 2 | 9 (16.7) | ||
| 3 | 4 (7.4) | ||
| 4 | 3 (5.6) | ||
| 5 | 3 (5.6) | ||
| >5 | 15 (27.7) | ||
| Median tumor size, cm (range) | 1.5 (0.5–10) | ||
| Operation status, n (%) | Surgery | 28 (50.9) | |
| No surgery | 27 (49.1) | ||
| Lymph node metastases, n (%) | Yes | 7 (30.4) | |
| No | 16 (69.6) | ||
| Lymphovascular invasion, n (%) | Yes | 5 (17.9) | |
| No | 23 (82.1) | ||
| Distant metastases, n (%) | Yes | 5 (9.1) | |
| No | 50 (90.9) | ||
| AJCC staging, n (%) | I | 15 (53.6) | |
| II | 13 (46.4) | ||
| Vital status at last follow-up, n (%) | Alive | 48 (87.3) | |
| Deceased | 7 (12.7) |
PNETs=pancreatic neuroendocrine tumors; MEN1=multiple endocrine neoplasia type 1; NETs=neuroendocrine tumors; AJCC=American Joint Committee on Cancer.
Age of patients when diagnosed with non-PNETs using most recent abdominal imaging.
Diagnostic values of tumor markers for PNETs in MEN1 patients
Figure 2 shows the ROC curves for CgA, PP, glucagon, and gastrin and the combination of CgA, PP, and gastrin.
Figure 2.
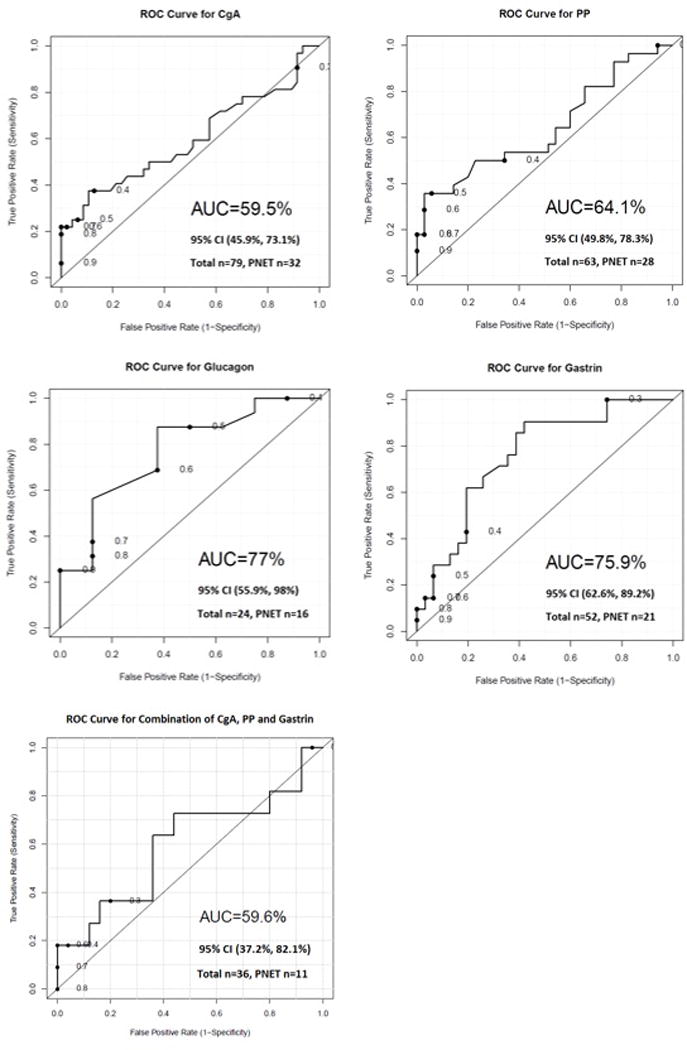
Diagnostic accuracy of the biomarkers for PNETs in MEN1 patients. MEN1=multiple endocrine neoplasia type 1; PNETs=pancreatic neuroendocrine tumors; CgA=chromogranin A; CI=confidence interval; PP=pancreatic polypeptide.
The AUCs for CgA, PP, glucagon, and gastrin were 59.5%, 64.1%, 77.0%, and 75.9%, respectively (the total numbers of patients were CgA=79, PP=63, glucagon=24, and gastrin=52). The AUC of the combination of CgA, PP, and gastrin was 59.6% (number of patients=36).
Correlations between individual tumor markers and age, sex, PNETs tumor size, tumor number, tumor location, tumor functional status, AJCC stage, lymph node metastases, and lymphovascular invasion
For the correlation analysis between the individual tumor markers and age at PNETs diagnosis, tumor size, and tumor number for all PNETs patients, we used a Spearman rank analysis. Only PP was marginally significantly associated with age (P=0.049). This significant result should be interpreted with caution given that several different correlations were tested and no formal adjustment was applied for multiplicity.
Wilcoxon rank-sum tests were used to assess the associations between dichotomous variables (sex, PNETs tumor location, tumor functional status, AJCC stage, lymph node metastases, and lymphovascular invasion) and the markers. Only the serum levels of PP were significantly associated with PNETs functional status, with values of 2765.5 ± 8148.6 in functioning PNETs and −15.0 ± 65.4 in non-functioning PNETs (P=0.019). As noted above, this result should be interpreted with caution given that several statistical tests were performed.
All the results are summarized in the Table 2 and 3.
Table 2.
Correlations between individual tumor marker (CgA and PP) and age, sex, PNETs tumor size, tumor number, tumor location, tumor functional status, AJCC stage, lymph node metastases, and lymphovascular invasion.
| Characteristic | CgA | P value | PP | P value | |
|---|---|---|---|---|---|
| Age | 0.313* | 0.081 | 0.376* | 0.049 | |
|
| |||||
| Tumor size | −0.033* | 0.861 | 0.362* | 0.063 | |
|
| |||||
| Tumor number | 0.126* | 0.500 | 0.125* | 0.535 | |
|
| |||||
| Sex (Mean) (SD) |
Male | 31.6 (191.0) | 0.247 | 2631.4 (8191.2) | 0.260 |
| Female | 70.7 (199.7) | 59.5 (242.1) | |||
|
| |||||
| Tumor location (Mean) (SD) |
Head/neck | −39.8 (45.5) | 0.306 | 132.5 (197.3) | 0.268 |
| Body/tail | 32.4 (153.4) | 1130.4 (5289.1) | |||
|
| |||||
| Tumor functional status (Mean) (SD) |
F | 176.3 (274.7) | 0.059 | 2765.5 (8148.6) | 0.019 |
| NF | 25.5 (158.1) | −15.0 (65.4) | |||
|
| |||||
| AJCC stage (Mean) (SD) |
I | 19.9 (155.1) | 0.420 | 72.6 (330.8) | 0.175 |
| II | 60.4 (171.6) | 99.2 (137.6) | |||
|
| |||||
| LNM (Mean) (SD) |
Yes | 20.0 (80.6) | 0.500 | 107.8 (176.7) | 0.671 |
| No | 20.0 (150.3) | 107.9 (321.0) | |||
|
| |||||
| LVI (Mean) (SD) |
Yes | 14.0 (89.1) | 0.767 | 181.5 (128.0) | 0.120 |
| No | 39.4 (168.0) | 67.8 (273.8) | |||
CgA=chromogranin A; PP=pancreatic polypeptide; PNETs=pancreatic neuroendocrine tumors; AJCC=American Joint Committee on Cancer; F=functioning; NF=non-functioning; LNM=lymph node metastases; LVI=lymphovascular invasion.
Spearman correlation
Table 3.
Correlations between individual tumor marker (glucagon and gastrin) and age, sex, PNETs tumor size, tumor number, tumor location, tumor functional status, AJCC stage, lymph node metastases, and lymphovascular invasion.
| Characteristic | Glucagon | P value | Gastrin | P value | |
|---|---|---|---|---|---|
| Age | 0.428* | 0.098 | 0.297* | 0.191 | |
|
| |||||
| Tumor size | 0.503* | 0.067 | 0.027* | 0.906 | |
|
| |||||
| Tumor number | −0.466* | 0.069 | 0.332* | 0.141 | |
|
| |||||
| Sex (Mean) (SD) |
Male | 2657.5 (2868.6) | 0.051 | −20.3 (53.9) | 0.876 |
| Female | −8.1 (46.7) | 251.4 (942.7) | |||
|
| |||||
| Tumor location (Mean) (SD) |
Head/neck | −14.5 (3.5) | 0.751 | −10.0 (NA) | 0.563 |
| Body/tail | 1135.2 (2244.6) | 4.3 (117.6) | |||
|
| |||||
| Tumor functional status (Mean) (SD) |
F | 389.0 (872.0) | 1.000 | 56.3 (124.7) | 0.393 |
| NF | 1265.4 (2492.6) | 193.3 (864.1) | |||
|
| |||||
| AJCC stage (Mean) (SD) |
I | 1234.0 (2381.1) | 0.914 | −13.3 (46.8) | 0.548 |
| II | 1608.0 (3261.4) | 162.0 (220.2) | |||
|
| |||||
| LNM (Mean) (SD) |
Yes | −22.7 (29.4) | 0.629 | 188.0 (NA) | 0.571 |
| No | 2013.0 (3093.7) | 43.5 (166.9) | |||
|
| |||||
| LVI (Mean) (SD) |
Yes | 3255.5 (4588.4) | 0.267 | −70.0 (NA) | 0.222 |
| No | 915.6 (2097.0) | 59.5 (148.5) | |||
PNETs=pancreatic neuroendocrine tumors; AJCC=American Joint Committee on Cancer; F=functioning; NF=non-functioning; LNM=lymph node metastases; LVI=lymphovascular invasion.
Spearman correlation
Impact of PNETs surgery on the values of tumor markers
As the sample sizes of patients with PP, glucagon, and gastrin were too small, only CgA was eligible for this analysis. The median time of post-operative CgA measurement was 6 months (range, 1–10 months). The CgA values in post-surgery patients were not significantly lower than were those in pre-surgery patients (P=0.554).
Correlation between individual tumor markers and overall survival
Table 4 shows the results from Cox regression models of overall survival in all PNETs patients. Glucagon could not be included due to the small number of patients. Of the other markers, none were significantly associated with survival (P=0.700, 0.731, and 0.427).
Table 4.
Correlation between tumor markers and overall survival.
| Parameter | Number of patients | Number of deaths | Hazard ratio | Lower CI | Upper CI | P value |
|---|---|---|---|---|---|---|
| CgA | 49 | 6 | 1.000 | 1.000 | 1.000 | 0.700 |
| PP | 28 | 3 | 1.002 | 0.991 | 1.013 | 0.731 |
| Gastrin | 32 | 8 | 1.001 | 1.000 | 1.002 | 0.427 |
CI=confidence interval; CgA=chromogranin A; PP=pancreatic polypeptide
Discussion
In this study, we determined the potential role of serum levels of CgA, PP, glucagon, and gastrin, individually and combination, in the diagnosis and follow-up of PNETs in MEN1 patients. Our results suggest that the diagnostic value of these markers is limited; even after combining CgA, PP, and gastrin, the results were still unsatisfactory (AUC=59.6%).
The popularity of genetic testing allows us to detect MEN1 at an early age, but the lack of genotype-phenotype correlation makes it difficult to determine when tumors will develop and progress; 50% of MEN1 develop PNETs21, which are the most important cause of mortality in MEN1 patients3. It remains a clinically important challenge to identify sufficiently sensitive tumor markers for MEN1 patients that can assist in early diagnosis or accurately predict malignant behavior.
The diagnostic value of CgA in sporadic PNETs has been confirmed in a number of studies from different centers and different time periods10, 11, 22; one study recommended that CgA determination be mandatory in the diagnosis of PNETs23. The recent NANETS and ENETS consensus guidelines also suggest that CgA can be used as a marker for PNETs.13, 14 However, very few studies have focused on the clinical significance of other tumor markers, such as PP, glucagon, gastrin, and their combination. Furthermore, in view of the low incidence of MEN1, especially of MEN1 with PNETs, nearly all recent studies have been based on investigation of sporadic PNETs. Although the clinical practice guidelines for MEN1 suggest an annual plasma biochemical evaluation of CgA, PP, glucagon, and gastrin to screen for PNETs, importantly, the recommendation level is only “suggest” and is of “low quality”.2
Considering the above reasons, an investigation into the true usefulness of serum CgA, PP, glucagon, and gastrin for diagnosing PNETs in MEN1 patients is very important. First, biomarkers could potentially allow us to detect and treat PNETs at an early age in the MEN1 patients thereby improving the prognosis, and reassure patients who have not developed PNETs yet. In addition, since MEN1 may be discovered at a very early age through genetic testing, and the biochemical tests will last for lifelong, the financial impact can be impressive.24
Peracchi et al.25 suggested that the finding of very high CgA levels was strongly suggestive of the presence of gastro-entero-pancreatic NET in MEN1 patients, although our results suggest that the value of CgA for diagnosing PNETs in MEN1 patients is low (AUC=59.5%). Matthew et al.26 reported that the presence of a markedly elevated fasting plasma PP level in MEN1 patients was 95% sensitive and 88% specific for the presence of radiographically detectable PNETs. In contrast, our study suggests that the diagnostic value of PP is unsatisfactory, with an AUC of 64.1%. The AUC of glucagon in our study is 77%, which is similar to the result in a study by Laat et al.15 To our knowledge, ours is the first investigation to focus on the diagnostic value of gastrin for PNETs in MEN1 patients; the AUC is 75.9%, which is also not robust.
When trying to investigate the combined diagnostic efficacy of all 4 markers, our sample size was too small to be analyzed. However, we were able to conduct the analysis for the combination of CgA, PP, and gastrin; the diagnostic value of the combination is still very low (AUC=59.6%). These results are consistent with those published by Laat et al.15
Therefore, the value of these blood markers for early diagnosing PNETs in MEN1 patients is not high enough, either individually or in combination. We still rely heavily on the imaging outcomes, especially the EUS, van Asselt et al.27 suggested that EUS is superior to CT/MRI+SRS for pancreatic lesion detection in MEN1 patients, and should be recommended as the first-choice pancreas imaging technique.
We analyzed the correlation between individual tumor markers and important clinicopathologic factors. Due to the small number of patients with distant metastasis who also had eligible serum markers measured at the time of PNETs diagnosis, we were unable to evaluate the potential role of these blood markers in identifying the development of metastatic disease (CgA=1, PP=1, glucagon=2, and gastrin=2).
Qiao et al.11 observed that the serum levels of CgA were decreased in 16 of 17 patients with insulinomas after tumor resection (median, 64.8 ng/ml vs. 50.4 ng/ml; P=0.003). Alaa et al.28 also found that CgA had high sensitivity and specificity for detecting decreases in tumor size after surgical resection. However, we are unable to confirm that PNETs surgery result in significant lowering of the CgA values (P=0.554). The timing of measurements of post-operative CgA values could potentially be the cause for the observed differences in comparison to published literatures.
CgA values were not significantly correlated with tumor number or tumor size, and primary PNETs surgery did not result in lowering of CgA values; thus we speculate that CgA is insufficiently sensitive to provide an accurate predictor of primary PNETs tumor burden. This issue thus remains controversial, as it is correlated with tumor burden in some studies29, 30 but not in others31, 32.
A previous study by Floyd et al.33 showed that serum levels of PP were associated with age; we find similar results in our study, with higher values in older patients (P=0.049). In addition, we found that PP was significantly associated with PNETs functional status, with higher values in functioning PNETs (P=0.019); this is in contrast to the findings of some other investigations31, 34.
In a study of 115 gastro-entero-pancreatic NET patients conducted by Walter et al., it was shown that elevated serum CgA or PP levels were not an independent prognostic factor for overall survival (P=0.86 and 0.48 respectively on univariate analysis).34 Two other investigators have reported that CgA did not appear to be an independent predictor of mortality (P=0.655 and 0.923).35, 36 Similarly, we failed to find an association of CgA, PP, or gastrin with overall survival (P=0.700, 0.731, and 0.427, respectively). Maybe we can use pancreastatin to replace these markers in the future as James et al.37 had shown in their study that higher pancreastatin levels were significantly associated with worse overall survival and progression-free survival.
There are some limitations to our study, including the retrospective design. We accepted characteristic imaging results, even without pathologic confirmation, as sufficient for the diagnosis of the presence of PNETs, which might result in inaccurate categorization of a minority of patients, thus reducing the power of the analyses38. Our study included patients treated over 17 years; different laboratory tests and reference ranges were used during this period, which may introduce limitations in the consistency of the collected data. The low number of patients with available data for all 3 markers might have led to an underestimation of the diagnostic value of the combination of markers. All of these limitations could be resolved in prospective clinical studies with large sample sizes, but such studies would be challenging because of the rarity of the MEN1. A multi-center collaborative investigation could be a solution to this problem.
Conclusions
The results of our study suggest that the value of these markers for diagnosing PNETs in MEN1 patients is relatively low (even when used in combination); thus, they are not sufficient to replace the current imaging methods. There is a need to identify new tumor markers for this condition, including through investigation of novel markers and pathways.
Acknowledgments
This work was supported in part by the Cancer Center Support Grant (NCI grant P30 CA016672).
Footnotes
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.White ML, Doherty GM. Multiple endocrine neoplasia. Surgical oncology clinics of North America. 2008;17:439–459. doi: 10.1016/j.soc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) The Journal of Clinical Endocrinology & Metabolism. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study. Comparison of 106 MEN1/Zollinger-ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 2013;92:135. doi: 10.1097/MD.0b013e3182954af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newey PJ, Jeyabalan J, Walls GV, Christie PT, Gleeson FV, Gould S, Johnson PR, Phillips RR, Ryan FJ, Shine B. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. The Journal of Clinical Endocrinology & Metabolism. 2009;94:3640–3646. doi: 10.1210/jc.2009-0564. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves TD, Toledo RA, Sekiya T, Matuguma SE, Maluf Filho F, Rocha MS, Siqueira SA, Glezer A, Bronstein MD, Pereira MA. Penetrance of functioning and nonfunctioning pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 in the second decade of life. The Journal of Clinical Endocrinology & Metabolism. 2013;99:E89–E96. doi: 10.1210/jc.2013-1768. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. The lancet oncology. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. Journal of gastroenterology. 2014;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 8.Lourenço DM, Jr, Toledo RA, Coutinho FL, Margarido LC, Siqueira SAC, Santos MACGd, Montenegro FLdM, Machado MCC, Toledo SPA. The impact of clinical and genetic screenings on the management of the multiple endocrine neoplasia type 1. Clinics. 2007;62:465–470. [PubMed] [Google Scholar]
- 9.Pieterman C, Schreinemakers J, Koppeschaar H, Vriens M, Rinkes I, Zonnenberg B, Van Der Luijt R, Valk G. Multiple endocrine neoplasia type 1 (MEN1): its manifestations and effect of genetic screening on clinical outcome. Clin Endocrinol (Oxf) 2009;70:575–581. doi: 10.1111/j.1365-2265.2008.03324.x. [DOI] [PubMed] [Google Scholar]
- 10.Hijioka M, Ito T, Igarashi H, Fujimori N, Lee L, Nakamura T, Jensen RT, Takayanagi R. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer science. 2014;105:1464–1471. doi: 10.1111/cas.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao XW, Qiu L, Chen YJ, Meng CT, Sun Z, Bai CM, Zhao DC, Zhang TP, Zhao YP, Song YL. Chromogranin A is a reliable serum diagnostic biomarker for pancreatic neuroendocrine tumors but not for insulinomas. BMC endocrine disorders. 2014;14:64. doi: 10.1186/1472-6823-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik WH, Ryu JK, Song BJ, Kim J, Park JK, Kim YT, Yoon YB. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. Journal of Korean medical science. 2013;28:750–754. doi: 10.3346/jkms.2013.28.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O’Connor J, Pape UF, Plöckinger U. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: biochemical markers. Neuroendocrinology. 2009;90:194–202. doi: 10.1159/000225948. [DOI] [PubMed] [Google Scholar]
- 14.Vinik AI, Woltering EA, Warner RR, Caplin M, O’Dorisio TM, Wiseman GA, Coppola D, Go VLW. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 15.de Laat JM, Pieterman CR, Weijmans M, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B. Low accuracy of tumor markers for diagnosing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 patients. The Journal of Clinical Endocrinology & Metabolism. 2013;98:4143–4151. doi: 10.1210/jc.2013-1800. [DOI] [PubMed] [Google Scholar]
- 16.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas P. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Alimentary pharmacology & therapeutics. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 17.Mosli HH, Dennis A, Kocha W, Asher LJ, Van Uum SH. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. The Journal of Clinical Endocrinology & Metabolism. 2012;97:E1731–E1735. doi: 10.1210/jc.2012-1548. [DOI] [PubMed] [Google Scholar]
- 18.Meng QH, Wagar EA. A Patient with Persistent Lactation and Recurrent Hypercalcemia. Clinical chemistry. 2015;61:1328–1331. doi: 10.1373/clinchem.2014.234849. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–134. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Edge S. American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. Vol. 19. Springer; New York: 2010. p. 718. [Google Scholar]
- 21.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Molecular and cellular endocrinology. 2014;386:2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Igarashi H, Jensen RT. Serum Pancreastatin: The long sought for universal, sensitive, specific tumor marker for neuroendocrine tumors (NETs)? Pancreas. 2012;41:505. doi: 10.1097/MPA.0b013e318249a92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Annals of surgical oncology. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 24.Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3:895–905. doi: 10.1016/S2213-8587(15)00043-1. [DOI] [PubMed] [Google Scholar]
- 25.Peracchi M, Conte D, Gebbia C, Penati C, Pizzinelli S, Arosio M, Corbetta S, Spada A. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. European Journal of Endocrinology. 2003;148:39–43. doi: 10.1530/eje.0.1480039. [DOI] [PubMed] [Google Scholar]
- 26.Mutch MG, Frisella MM, DeBenedetti MK, Doherty GM, Norton JA, Wells SA, Lairmore TC. Pancreatic polypeptide is a useful plasma marker for radiographically evident pancreatic islet cell tumors in patients with multiple endocrine neoplasia type 1. Surgery. 1997;122:1012–1020. doi: 10.1016/s0039-6060(97)90203-8. [DOI] [PubMed] [Google Scholar]
- 27.van Asselt SJ, Brouwers AH, van Dullemen HM, van der Jagt EJ, Bongaerts AH, Kema IP, Koopmans KP, Valk GD, Timmers HJ, de Herder WW. EUS is superior for detection of pancreatic lesions compared with standard imaging in patients with multiple endocrine neoplasia type 1. Gastrointestinal endoscopy. 2015;81:159–167.e152. doi: 10.1016/j.gie.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Saif A, Gibril F, Ojeaburu JV, Bashir S, Entsuah LK, Asgharian B, Jensen RT. Prospective study of the ability of serial measurements of serum chromogranin A and gastrin to detect changes in tumor burden in patients with gastrinomas. Cancer. 2003;98:249–261. doi: 10.1002/cncr.11473. [DOI] [PubMed] [Google Scholar]
- 29.Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin J, Bidart J, Cailleux A, Bonacci R, Ruffie P. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. British journal of cancer. 1998;78:1102. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 31.Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto V, Annibale B, Buonadonna A, Pederzoli P. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. Journal of endocrinological investigation. 2004;27:6–11. doi: 10.1007/BF03350903. [DOI] [PubMed] [Google Scholar]
- 32.Goebel SU, Serrano J, Yu F, Gibril F, Venzon DJ, Jensen RT. Prospective study of the value of serum chromogranin A or serum gastrin levels in the assessment of the presence, extent, or growth of gastrinomas. Cancer. 1999;85:1470–1483. [PubMed] [Google Scholar]
- 33.Floyd J, Jr, Fajans SS, Pek S, Chance R. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent progress in hormone research. 1975;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- 34.Walter T, Chardon L, Chopin-laly X, Raverot V, Caffin AG, Chayvialle JA, Scoazec JY, Lombard-Bohas C. Is the combination of chromogranin A and pancreatic polypeptide serum determinations of interest in the diagnosis and follow-up of gastro-entero-pancreatic neuroendocrine tumours? European Journal of Cancer. 2012;48:1766–1773. doi: 10.1016/j.ejca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Massironi S, Conte D, Sciola V, Spampatti MP, Ciafardini C, Valenti L, Rossi RE, Peracchi M. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. The American journal of gastroenterology. 2010;105:2072–2078. doi: 10.1038/ajg.2010.154. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, Ardill J, Johnston B, Poston G, Rees M. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocrine-related cancer. 2009;16:885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 37.Sherman SK, Maxwell JE, O’Dorisio MS, O’Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Annals of surgical oncology. 2014;21:2971–2980. doi: 10.1245/s10434-014-3728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM. Empirical evidence of design-related bias in studies of diagnostic tests. Jama. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]


