Introduction
Gabapentin has been used as one of the first-line treatments for chronic pain. In various pain models in rodents, gabapentin is remarkably and uniformly effective [4; 7; 10; 16; 17; 29]. However, gabapentin often fails to provide sufficient analgesia in patients [5; 6] and the mechanisms underlying this lack of uniformity in efficacy have not been addressed. We hypothesize that the discrepancy between the clinical and preclinical efficacy of gabapentin may relate in part to the timing of studies in rodents, typically 2–3 weeks after injury.
Gabapentin interacts with α2δ subunits of voltage-gated calcium channels to reduce neuronal excitation [12; 16; 17]. A focus on the spinal cord as a key site of pain transmission and plasticity after nerve injury has led to the theory that gabapentin primarily acts on spinal pain mechanisms. However, a recent clinical study questioned this theory by demonstrating a complete lack of clinical efficacy of intrathecal gabapentin in patients with chronic pain [22], despite the known efficacy of oral gabapentin in this patient population. We and others have proposed a different gabapentin’s action on descending inhibition. As such, systemically administered gabapentin activates descending noradrenergic pathways to reduce hypersensitivity in rodents 2–3 weeks after peripheral nerve injury, and that gabapentin increased lumbar cerebrospinal fluid noradrenaline in patients with chronic orthopedic pain [7; 10; 29]. We and others have posited that gabapentin activates noradrenergic neurons in the locus coeruleus (LC) to induce spinal noradrenaline release that in turn stimulates α2-adorenoceptors, leading to analgesia which relies in part on cholinergic receptor activation [10; 11; 28].
Among various neurochemical inputs in the LC, glutamate is the primary excitatory neurotransmitter on noradrenergic neurons [25]. Extracellular glutamate is classically regulated by two types of astroglial glutamate transporters, primarily glutamate transporter-1 (GLT-1), but also glutamate-aspartate transporter [23; 24]. We previously demonstrated that gabapentin increases extracellular glutamate in the LC by GLT-1 dependent mechanisms to stimulate descending noradrenergic inhibition in rats with normal or early stage neuropathic pain (2–3 weeks after nerve injury) condition [27], suggesting a GLT-1 dependence of gabapentin’s action in the LC at least in the first few weeks after nerve injury. However, we recently demonstrated that GLT-1 is itself down-regulated in the LC 6 weeks after nerve injury [15], leading to our hypothesis that this down-regulation of GLT-1 may result in reduced gabapentin:s efficacy over time after nerve injury. We further propose that restoration of GLT-1 expression by inhibition of histone deacetylase (HDAC) [13; 15; 31] will restore gabapentin efficacy late after injury.
The purpose of this study was to address key gaps in our understanding about how resistance to gabapentin develops in chronic pain after neuropathic injury, focusing on plasticity in GLT-1-dependent gabapentin’s action on the LC, and to test pharmacologic approaches to restore its efficacy. The current study also examined whether later time periods after neuropathic injury also reduces efficacy of the target for norepinephrine release in the spinal cord, α2-adrenoceptors.
Materials and Methods
Animals
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN), weighing 220 to 280 g, were housed under a standard 12-hour light-dark cycle, with free access to food and water. All experiments were approved by the Animal Care and Use Committee at Wake Forest School of Medicine (Winston-Salem, NC).
Surgeries
Unilateral L5 and L6 spinal nerve ligation (SNL) was performed as described previously [14]. Briefly, under anesthesia with 2.0% isoflurane, the right L5 and L6 spinal nerves were tightly ligated using 5-0 silk suture. LC cannula implantation was performed as described previously [10]. Briefly, under anesthesia with 2% isoflurane, a sterile stainless-steel guide cannula (CXG-8 for microdialysis, Eicom Co., Kyoto, Japan, or C315G for small interfering RNA [siRNA] injection, Plastic One, Roanoke, VA) was implanted into the right LC. The coordinates for placement of the tip of the guide cannula were 9.8mm posterior and 1.4mm lateral to the bregma, and 6.5 mm ventral from the surface of the dura mater. Animals were allowed to recover from the surgery at least one week prior to the experiment. After the experiment, all animals received an intra-LC injection of methylene blue (0.5 μl) and were euthanized by an intravenous or intraperitoneal injection of pentobarbital (150 mg/kg). The brain was removed and sectioned, and the placement of the cannula was verified visually. This study included data from only animals (202 of 214 rats) with successful LC cannula placement.
Drugs and small interfering RNA (siRNA) treatments
Gabapentin (Toronto Research Chemicals, Ontario, Canada,) was dissolved in saline and injected intraperitoneally (100 mg/kg) or intravenously (50 mg/kg). These doses of gabapentin were determined from our previous studies [10; 26]. To examine the effect of nerve injury on antihypersensitivity effect of gabapentin, animals received a single injection of intraperitoneal saline or gabapentin (100 mg/kg) at 0, 1, 2, 4, 6, 8, and 10 weeks after SNL surgery. Idazoxan hydrochloride (30 μg/10 μl, Tocris Bioscience, USA), clonidine (1–15 μg/10 μl, Sigma-Aldrich, St. Louis, MO), and atropine (30 μg/10 μl, Sigma-Aldrich) were dissolved in saline and injected intrathecally via the L5–L6 intervertebral space under brief anesthesia using a 30-gauge needle as previously described [15]. Sodium valproate was dissolved in water and orally administered twice a day by a feeding tube (200 mg/kg/day) for 14 days beginning 6 weeks after SNL surgery. The dose of valproate was determined from our previous study [13; 31]. Knock-down of GLT-1 in the LC was performed as previously described [27]. A mixture of siRNAs for rat GLT-1 (SMARTpool #M-091209-02, Thermo Fisher Scientific Inc., Pittsburgh, PA) or a non-targeting siRNA pool (#D-001206-14, Thermo Fisher Scientific Inc.) was dissolved in double distilled water, diluted with the transfection reagent (i-Fect; Neuromics, Edina, MN) to achieve a final concentration of 8.3 pmol/0.5 μl, and injected daily through the LC guide cannula for 5 consecutive days. In all injection studies, animals were randomly allocated to treatment groups.
Behavior test
The person performing the behavioral test (Masafumi Kimura) was blinded to drug and dose. Nociceptive mechanical withdrawal thresholds in the hindpaw were measured with a Randall-Selitto analgesimeter (Ugo Basile, Comerio, Italy) as previously described [21]. A cutoff pressure of 250 g was used to avoid potential tissue injury. All animals were trained for 3 days with this apparatus before baseline values were measured.
Western blotting for GLT-1 in the LC
Western blotting for GLT-1 in the LC was performed as previously reported [27]. Brainstem slices (2 mm thickness) containing the LC were obtained using a precision brain slicer (RBM-4000C, ASI Instruments, Inc.,Warren, MI) and the region of the right LC was carefully dissected under the surgical microscope. The LC and adjacent tissue were homogenized, lysed, and centrifuged for 10 min at 4 °C at 1000G. Protein content in each supernatant was measured using a standard method [2]. Samples (25 μg protein) were placed on 10% gels (Criterion Tris-HCl Gel; Bio-Rad, Hercules, CA), run at 100 V for 1 h, and transferred to nitrocellulose membrane (Bio-Rad). The membrane was blocked with 1% bovine albumin serum in Tris-buffer saline containing 0.1% Tween 20 (TBST), and incubated overnight at 4 °C with a guinea pig anti-GLT-1 (1:5000, AB1783; Millipore, Billerica, MA) or a rabbit α-tubulin (1:5000, 2125S; Cell Signaling, Danvers, MA). After washing with TBST, the membrane was incubated for 1 h at room temperature with a corresponding horseradish peroxidase-conjugated secondary antibody (1:5000; anti-guinea pig or 1:5000; anti-rabbit, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), treated for 1 min with West Pico hemiluminescence substrate (Thermo Fisher Scientific Inc.), and exposed to X-ray film (Kodak BioMax film, Sigma-Aldrich). The density of each specific band was measured using a computer-assisted imaging analysis system (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA).
Immunohistochemistry
Immunostaining for phosphorylated cyclic adenosine monophosphate response element binding protein (pCREB) and dopamine-β-hydroxylase (DβH) was performed as previously described [10]. One hour after intraperitoneal injection of gabapentin or saline, animals were euthanized with an intraperitoneal injection of pentobarbital (150 mg/kg) and perfused with 0.01 M phosphate-buffered saline containing 1% sodium nitrite, followed by 4% formalin in 0.1 M phosphate-buffered saline. The brainstems were dissected out, postfixed in the same fixative, cryoprotected with 30% sucrose in 0.1 M phosphate-buffered saline, and sectioned at a 16 μm thickness. After being pretreated with 1% normal donkey serum (Jackson Immuno Research Laboratories, West Grove, PA), the sections were incubated with a rabbit monoclonal anti–pCREB antibody (1:500, 06-519; Millipore, Billerica, MA) and a mouse monoclonal anti-DβH antibody (1:500, MAB 308; Millipore) overnight. Subsequently, the sections were incubated with Cy2 conjugated anti-mouse IgG (1:200, Jackson Immuno Research Laboratories) and Cy3 conjugated anti-rabbit IgG (1:600, Jackson Immuno Research Laboratories). For quantification, four to five brainstem sections containing the right LC were randomly selected from each rat and digital images were captured using a Nikon Eclipse Ni fluorescent microscope system (Nikon, Tokyo, Japan). All images for each experiment were taken at the same time with the same camera settings, and the persons performing the image analysis (Masafumi Kimura) were blinded to treatment or group. Positive immunostaining for pCREB was defined using a constant threshold across all sections. DβH-immunoreactive (IR) cells with or without pCREB were counted in the LC on the 4–5 sections per rat and reported as % of total DβH-IR cells per rat.
Microdialysis for spinal noradrenaline
Microdialysis in the lumbar spinal dorsal horn was performed as previously described [10]. On the day of the experiment, anesthesia was induced with 2% isoflurane and then maintained with 1.5% isoflurane during the study. A heating blanket was used to maintain rectal temperature 36.5 ± 0.5 °C. The left femoral vein was cannulated for transfusion of saline (1 ml/hr) and gabapentin injection, and the L3–L6 level of spinal cord was exposed by the T13-L1 laminectomy. A microdialysis probe (CX-I-8-01, outer diameter = 0.22 mm, inner diameter = 0.20 mm, length = 1 mm; EICOM Co.) was inserted into the spinal dorsal horn 1 h prior to the experiment and perfused with Ringer’s solution (1.0 μl/min). Then, after two 30-min baseline samples, gabapentin (50mg/kg) or saline was intravenously injected. Noradrenaline content in the microdialysates was measured by high-pressure liquid chromatography with electrochemical detection (HTEC-500, EICOM Co.), as previously described [10].
Statistical Analysis
We determined the sample size for each experiment based on our previous experience and power analysis was not conducted prior to the study to determine appropriate sample sizes. Data are presented as mean ± SD. When appropriate, behavioral and microdialysis data were analyzed by two-way repeated-measures analysis of variance (ANOVA) followed by Tukey post hoc testing using Sigma Plot software (Systat Software Inc, Chicago, IL). Other data from Western blotting, immunohistochemical, and some behavioral experiments were analyzed by one-way or two-way ANOVA followed by Tukey post hoc testing. P < 0.05 was considered significant. To depict likelihood of animals responding to gabapentin as a function of time, we coded each animal at each time dichotomously as responding or not responding to gabapentin and performed logistic regression analysis for likelihood that gabapentin will produce an increase in mechanical withdrawal threshold separately at three levels (Change from baseline: 10, 20, or 30 g), using the Origin 2015 (OriginLab Corporation, Northampton, MA).
Results
Impact of SNL on gabapentin analgesia
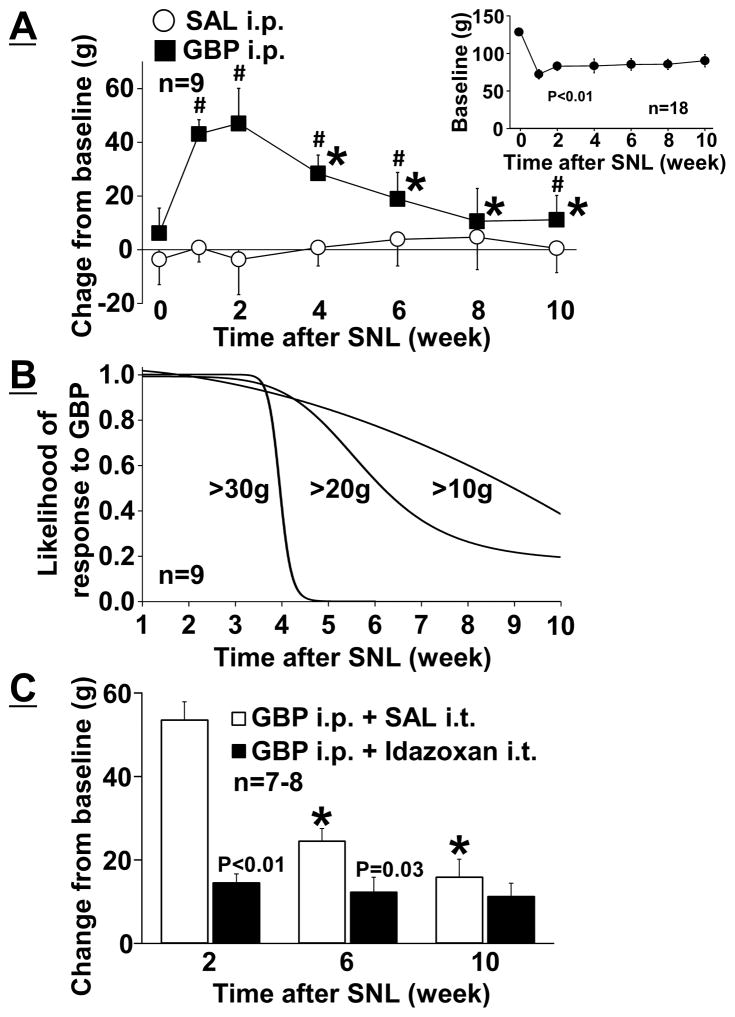
SNL significantly reduced pre-drug baseline withdrawal thresholds in the right hindpaw ipsilateral to surgery and stable mechanical hypersensitivity was observed during the study (Figure 1A - insert, p < 0.01). Animals received a single injection of intraperitoneal saline or gabapentin (100 mg/kg) at 0, 1, 2, 4, 6, 8, and 10 weeks after SNL surgery. There were significant main effects of treatment (F 1, 96 = 163.59; p < 0.01) and time (F 6, 96 = 12.79; p < 0.01), and the treatment × time interaction (F 6, 96 = 15.80; p < 0.01) between gabapentin and saline injected groups (Figure 1A). Post hoc testing revealed that a single intraperitoneal injection of gabapentin significantly increased withdrawal thresholds compared to saline except 8 weeks after SNL (p < 0.05) and that the antihypersensitivity effect of gabapentin was significantly reduced 4 to 10 weeks after SNL compared to 2 weeks after SNL (p < 0.01). Using this time-course data from gabapentin treated SNL rats, we also performed logistic regression analysis to examine likelihood that gabapentin will produce an increase in mechanical withdrawal threshold of three levels (Change from baseline: 10, 20, or 30 g) as a function of time after surgery (Figure 1B). For a large effect size (>30 g), gabapentin uniformly lost antihypersensitivity effect around 5 weeks after injury (R2=0.73). For middle (>20 g, R2=0.49)) and small (>10 g (R2=0.29)) effect sizes, gabapentin lost antihypersensitivity effect in nearly half of the SNL rats 6 and 10 weeks after injury, respectively.
Figure 1.
(A) Effects of SNL on antihypersensitivity effects of gabapentin (GBP). Withdrawal thresholds in the ipsilateral hindpaw to spinal nerve ligation (SNL) were measured prior to (baseline, upper right graph) and one hour after an intraperitoneal injection of saline (SAL) or GBP (100 mg/kg) in rats 0–10 weeks after SNL. Data (mean ± SD) are presented as change from baseline.*P < 0.05 vs. 2 weeks after SNL. #P < 0.05 vs. SAL. (B) Likelihood that gabapentin will produce an increase in mechanical withdrawal threshold of three levels (Change from baseline: 30 g, 20 g, or 10 g) as a function of time after SNL injury. Data are logistic regression analyses of 9 animals. (C) Idazoxan (30 μg/10 μl) or SAL (10 μl) was intrathecally injected 30 minutes after GBP injection (100 mg/kg) in rats at 2, 6, and 10 weeks after SNL. Data (mean ± SD) are presented as change from baseline.*P < 0.01 vs. 2 weeks after SNL.
Whether SNL alters spinal noradrenergic dependency of intraperitoneal gabapentin analgesia was examined in rats 2 to 10 weeks after SNL, using an intrathecal α2-adrenoceptor antagonist idazoxan (Figure 1C). There were significantly main effects of treatment (F 1, 39 = 36.54; p < 0.01) and time (F 2, 39 = 16.47; p < 0.01), and the treatment × time interaction groups (F 2, 39 = 11.73; p < 0.01) between intrathecal saline and idazoxan injected groups. Post hoc testing revealed that the antihypersensitivity effect of gabapentin was significantly reduced 6 and 10 weeks after SNL compared to 2 weeks after SNL in saline treated groups (p < 0.01), but no difference was observed among 2, 6 and 10 weeks after SNL in idazoxan treated groups (p > 0.81), and that intrathecal idazoxan significantly reduced the antihypersensitivity effect from gabapentin 2 (p < 0.01) and 6 (p = 0.03) weeks but not 10 (p = 0.41) weeks after SNL compared to saline. These results suggest that SNL time-dependently impairs the antihypersensitivity effect of gabapentin which relies in part on descending noradrenergic inhibition.
Expression of GLT-1 and gabapentin-induced neuronal activation in the LC
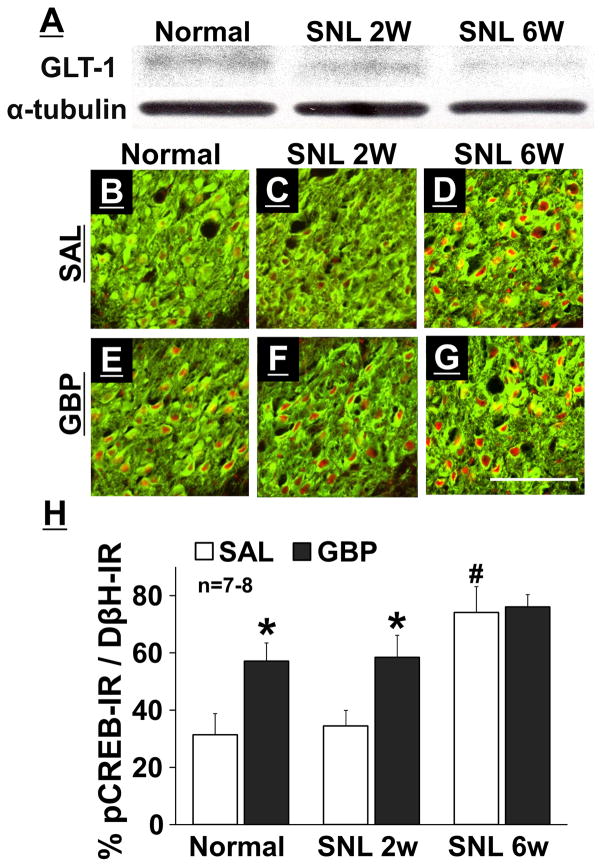
Figure 2A depicts representative immunoblotting images of GLT-1 and α-tubulin in the branstem tissues containing the right LC from normal and SNL rats. Quantitatively, expression of GLT-1 protein in the LC was reduced 6 weeks (SNL 6W, ratio to α-tubulin: 0.43 ± 0.13, n=8, p = 0.03) but not 2 weeks (SNL 2W, ratio to α-tubulin: 0.60 ± 0.20, n = 8, p = 0.67) after SNL surgery compared to normal rats (ratio to α-tubulin: 0.68 ± 0.20, n = 8).
Figure 2.
(A) Representative Western blotting images of GLT-1 and α-tubulin in the right LC from normal, SNL 2W, and SNL 6W rats. (B–G) Photomicrographs and (H) quantification of DβH-IR (green) and pCREB-IR (red) in the right LC collected from normal, SNL 2W, and SNL 6W rats one hour after an intraperitoneal injection of saline (SAL) or gabapentin (GBP, 100 mg/kg). Scale bar = 100 μm. Data (mean + SD) are presented as the percentage of pCREB-IR in DβH-IR neurons. *P < 0.01 vs. saline. #P < 0.01 vs. normal and SNL 2W.
Photomicrographs in Figure 2B–2G depict pCREB-IR in noradrenergic neurons, identified by DβH-IR, in the right LC from normal and SNL rats one hour after an intraperitoneal injection of saline or gabapentin. There were significant main effects of group (F2, 75 = 164.09; p < 0.01) and treatment (F1, 75 = 143.82; p < 0.01), and the group × treatment interaction (F2, 75 = 23.83; p < 0.01) (Figure 2H). Post hoc testing revealed that SNL 6W rats showed an increase in pCREB-IR in LC neurons compared with normal and SNL 2W rats (p < 0.01) in saline treated groups, and that gabapentin increased pCREB-IR in LC neurons compared to saline in normal and SNL 2W rats (p < 0.01), whereas no such increase occurred in SNL 6W rats (p = 0.16).
Role of GLT-1 expression in the LC for antihypersensitivity from gabapentin
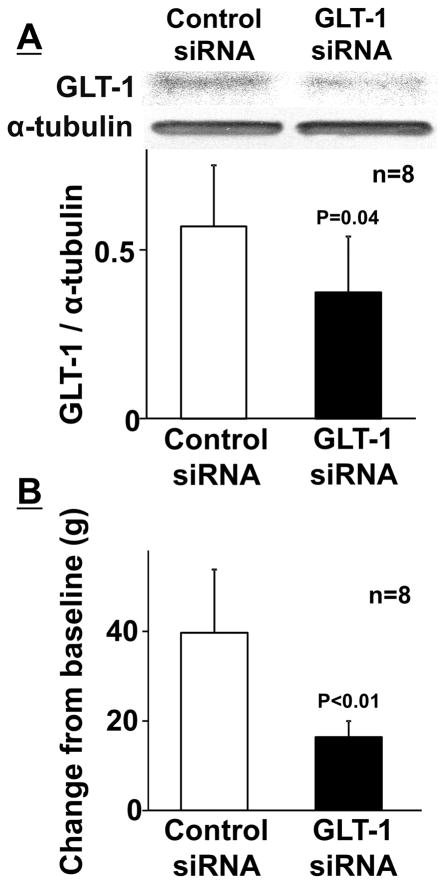
Figure 3A depicts representative immunoblotting images of GLT-1 and α-tubulin in the branstem tissues containing the right LC from SNL 2W rats treated with repeated intra-LC injections of the GLT-1 selective siRNA or non-targeting siRNA (Control) for 5 days beginning 9 days after surgery. Quantitatively, GLT-1 siRNA treatment significantly reduced expression of GLT-1 in the LC (ratio to α-tubulin: 0.37 ± 0.17, n = 8) compared to the control (ratio to α-tubulin: 0.57 ± 0.18, n=8, p = 0.04). GLT-1 siRNA did not alter mechanical withdrawal thresholds in the right hindpaw (80 ± 7 g) compared to the control (85 ± 8 g, p = 0.17), but significantly reduced the antihypersensitivity effect of intraperitoneal gabapentin (Figure 3B, p < 0.01), suggesting that expression of GLT-1 in the LC is essential to antihypersensitivity action of systemic gabapentin in the early phase (2 weeks after nerve injury) of neuropathic pain.
Figure 3.
(A) Representative western blotting images and quantification of GLT-1 and α-tubulin in the right LC from SNL 2W rats treated with repeated intra-LC injections of control (8.3 pmol/0.5 μl) or GLT-1 (8.3 pmol/0.5 μl) siRNA for 5 days. Data (mean + SD) are presented as ratio to α-tubulin. (B) Control and GLT-1 siRNA treated rats received an intraperitoneal injection of gabapentin (100 mg/kg). Withdrawal thresholds in the right hindpaw were measured prior to (baseline) and one hour after gabapentin. Data (mean + SD) are presented as change from baseline.
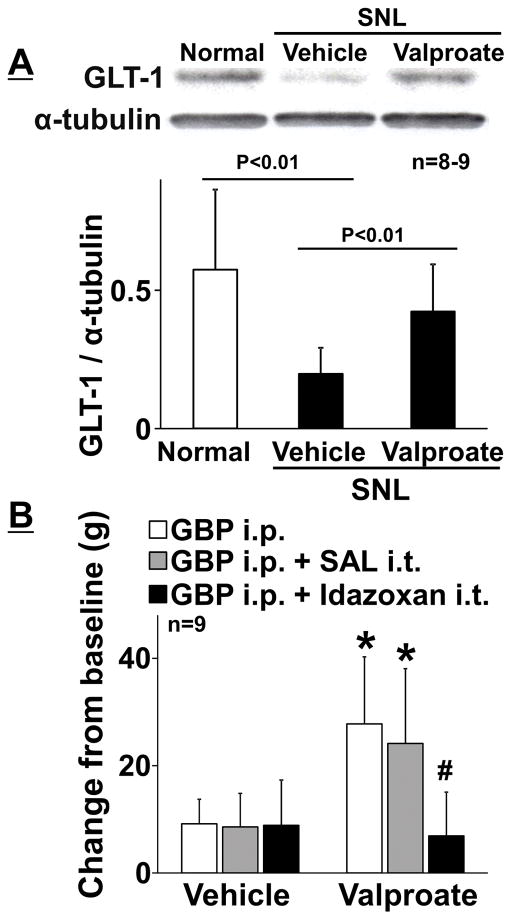
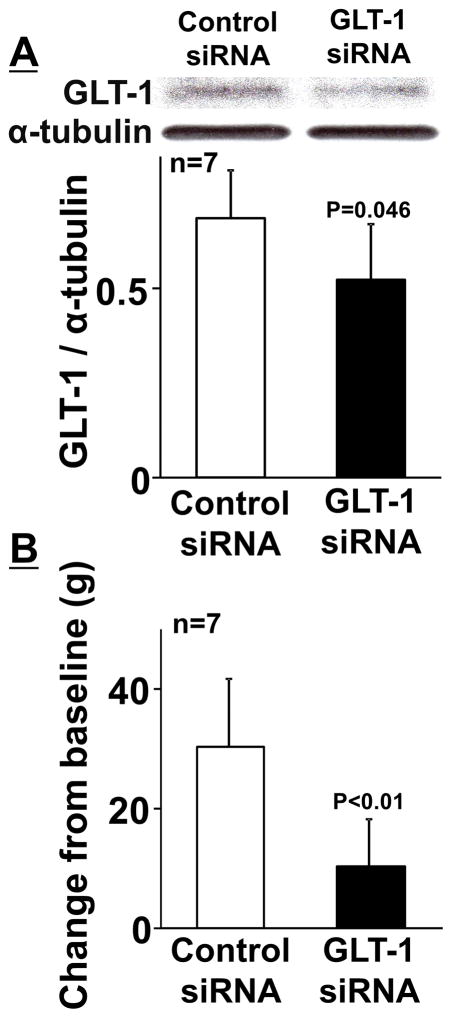
We then examined whether increasing GLT-1 expression in the LC by HDAC inhibition can increase gabapentin’s efficacy later (>6 weeks) after nerve injury. Figure 4A depicts representative immunoblotting images of GLT-1 and α-tubulin in the right LC from normal and SNL rats treated with repeated oral administration of the HDAC inhibitor valproate or vehicle for 2 weeks beginning 6 weeks after nerve injury. Quantitatively, expression of GLT-1 protein in the LC from vehicle-treated SNL rats 8 weeks after nerve injury (SNL 8W, ratio to α-tubulin: 0.20 ± 0.09, n = 9) was significantly lower than that of normal rats (ratio to α-tubulin: 0.57 ± 0.29, n = 8, p < 0.01) and valproate significantly increased GLT-1 expression (ratio to α-tubulin: 0.42 ± 0.17, n=9, p< 0.01) compared to the vehicle in SNL 8W rats, consistent with our previous observation that reduced GLT-1 expression in the LC after SNL was reversed by HDAC inhibition [15]. Oral valproate treatment also significantly increased mechanical withdrawal thresholds in the right hindpaw (102 ± 14 g, n = 9) compared to the vehicle (86 ± 7 g, n = 9, p < 0.01) and restored the antihypersensitivity effect of gabapentin, which was abolished by intrathecal injection of idazoxan, in SNL 8W rats (Figure 4B). Figure 5A depicts representative immunoblotting images of GLT-1 and α-tubulin in the right LC from valproate treated SNL 8W rats with repeated intra-LC injections of the GLT-1 or control siRNA for 5 days. Quantitatively, GLT-1 siRNA treatment significantly reduced expression of GLT-1 in the LC (ratio to α-tubulin: 0.52 ± 0.15, n = 7) compared to the control (ratio to α-tubulin: 0.69 ± 0.13, n = 7, p = 0.046). GLT-1 siRNA did not affect withdrawal thresholds in the right hindpaw (90 ± 10 g) compared to the control (93 ± 9 g, p = 0.50), but significantly reduced gabapentin efficacy after valproate treatment in SNL 8W rats (Figure 5B, p < 0.01). These results suggest that repeated oral administration of valproate increased GLT 1 in the LC to restore the impaired antihypersensitivity effect of gabapentin which relies in part on descending noradrenergic inhibition.
Figure 4.
(A) Representative western blotting images and quantification of GLT-1 and α-tubulin in the right LC from normal and SNL rats treated with vehicle or valproate (200 mg/5 mL/kg/day) for 14 days starting from 6 weeks after SNL surgery. Data (mean + SD) are presented as ratio to α-tubulin. (B) Animals received intraperitoneal gabapentin (GBP, 100 mg/kg) alone or followed by an intrathecal idazoxan (30 μg/10 μl) or saline (SAL, 10 μl). Withdrawal thresholds in the right hindpaw were measured prior to (baseline) and one hour after gabapentin administration. Data (mean + SD) are presented as change from baseline. *P < 0.01 vs. vehicle. #P < 0.01 vs. GBP alone and GBP + SAL.
Figure 5.
(A) Representative western blotting images and quantification of GLT-1 and α-tubulin in the right LC from vehicle or valproate treated SNL rats (see Figure 4) with repeated intra-LC injections of the control (8.3 pmol/0.5 μl) or GLT-1 (8.3 pmol/0.5 μl) siRNA for last 5 days. Data (mean + SD) are presented as ratio to α-tubulin. (B) SNL rats treated with valproate and siRNA received intraperitoneal gabapentin (100 mg/kg). Withdrawal thresholds in the right hindpaw were measured prior to (baseline) and one hour after gabapentin administration. Data (mean + SD) are presented as change from baseline.
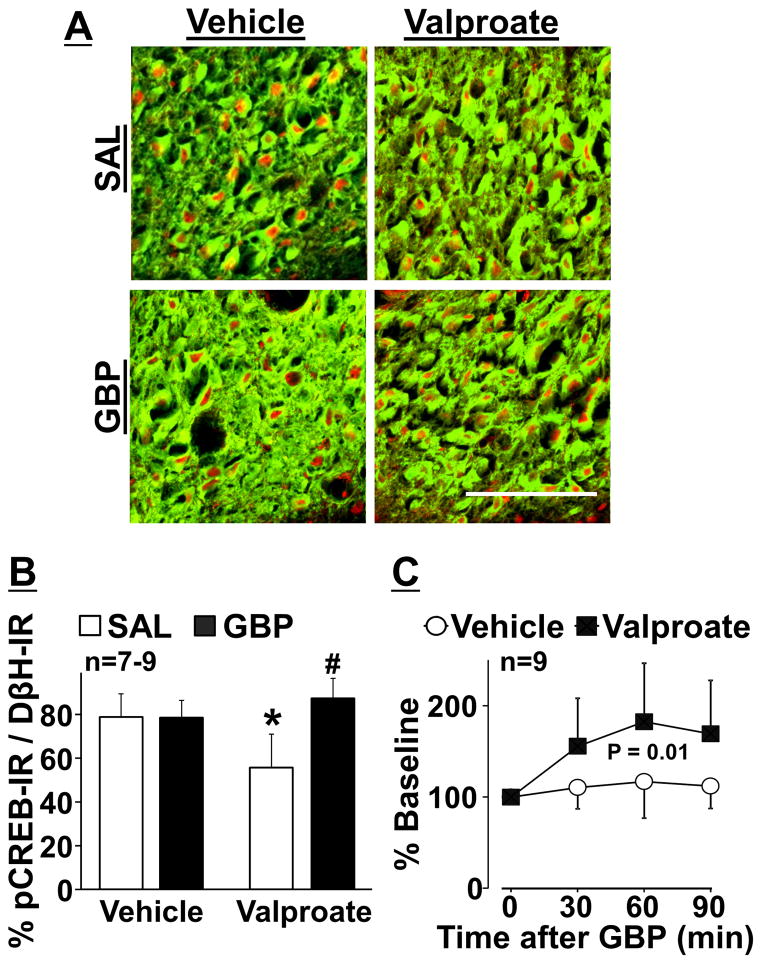
Photomicrographs in Figure 6A depict pCREB-IR in noradrenergic neurons in the right LC from oral vehicle or valproate treated SNL 8W rats one hour after an intraperitoneal injection of saline or gabapentin. Quantitatively, valproate significantly decreased pCREB-IR in the LC neurons compared to the vehicle in saline groups (p < 0.01) and gabapentin increased pCREB-IR in LC neurons compared to saline in valproate treated groups (p < 0.01) (Figure 6B). Based on this result, we examined whether the effects of valproate on basal and gabapentin-induced LC activity reflects to noradrenaline release in the spinal cord (Figure 6C). In microdialysates from the right lumbar spinal dorsal horn, valproate treatment significantly reduced basal noradrenaline concentrations (1.3 ± 0.6 pg/30 μl) compared to the vehicle (2.1 ± 0.9 pg/30 μl, p = 0.048). Animals received a single intravenous injection of saline or gabapentin (50 mg/kg). For gabapentin-induced spinal noradrenaline release, there were significant main effects of group (F1, 48 = 8.00, p = 0.01) and time (F3, 48 = 7.63, p < 0.01), and the group × time interaction (F3, 48 = 3.49, p = 0.02), consistent with current behavioral and LC pCREB results.
Figure 6.
(A) Photomicrographs and (B) quantification of DβH-IR (green) and pCREB- IR (red) in the right LC collected from vehicle or valproate treated SNL rats (see Figure 4) one hour after an intraperitoneal injection of saline (SAL) or gabapentin (GBP, 100 mg/kg). Data (mean + SD) are presented as the percentage of pCREB-IR in DβH-IR neurons. *P < 0.01 vs. vehicle. #P < 0.01 vs. SAL. Scale bar = 100 μm. (C) Microdialysis in the right lumbar spinal dorsal horn was performed in vehicle or valproate treated SNL rats. Changes in noradrenaline concentrations in microdialysates from the right spinal cord following an intravenous GBP injection (50 mg/kg) are presented over the time as percentage of baseline. Data are presented as mean ± SD.
Impact of SNL on spinal α2-adrenoceptor-mediated antihypersensitivity
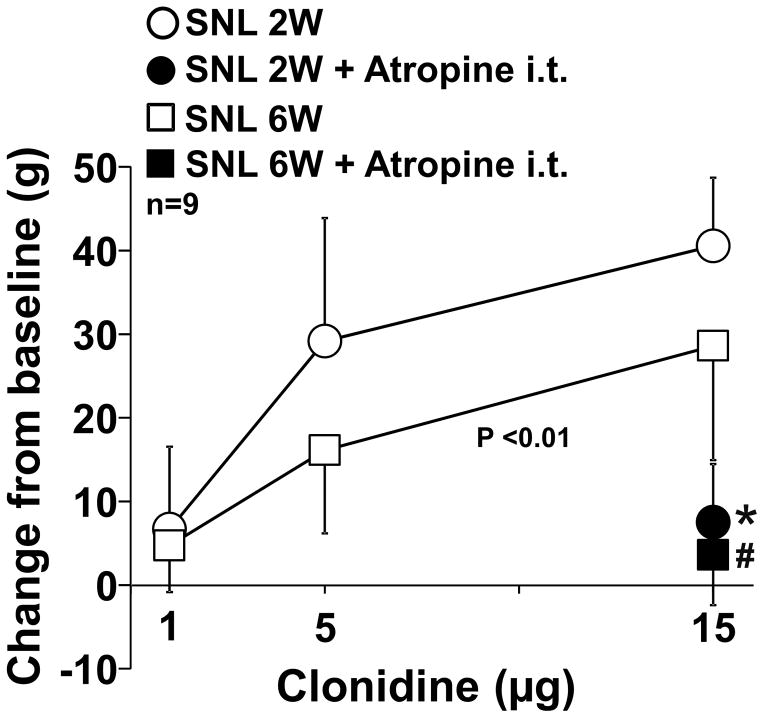
Lastly, we examined whether nerve injury affects the efficacy and cholinergic dependency of α2-adrenoceptor-mediated spinal analgesia by comparing the effects of the intrathecal α2-adrenoceptor agonist clonidine with or without the muscarinic antagonist atropine in SNL 2W and 6W rats (Figure 7). There was a significant main effect of group (F1, 48 = 8.34; P < 0.01) and dose (F2, 48 = 29.05; P <0.01), but not the group × dose interaction (F2, 48 = 1.29; P = 0.29) between clonidine alone groups (Figure 7). In both SNL 2W and 6W rats, intrathecal atropine significantly reduced the antihypersensitivity effect of clonidine (p < 0.01). These results suggest that nerve injury did not affect cholinergic dependency but time-dependently reduced the efficacy of α2-adrenoceptor-mediated antihypersensitivity in the spinal cord after nerve injury.
Figure 7.
Clonidine (1, 5, or 15 μg/10 μl) was intrathecally injected with or without atropine (30 ug) in SNL 2W and 6W rats. Withdrawal thresholds in the right hindpaw were measured prior to (baseline) and one hour after clonidine injection. Data (mean ± SD) are presented as change from baseline. *P < 0.01 vs. clonidine alone in SNL 2W. #P < 0.01 vs. clonidine alone in SNL 6W.
Discussion
Despite being one of the first choice treatments for chronic pain, many patients with chronic pain fail to receive analgesic benefit from gabapentin [5; 6]. In past two decades, pain research has primarily focused on the mechanisms of clinical efficacy of gabapentin, but less attention has paid to understand why all rodents, but only some patients respond to gabapentin. From a public health significant perspective, understanding mechanisms of this heterogeneity in response and how efficacy might be improved is arguably as important as understanding therapeutic mechanisms.
Drawing parallels between behavioral responses in animals, in this case withdrawal responses reflecting hypersensitivity with pressure, to analgesic responses in humans with chronic pain is difficult. Nonetheless, one is struck by the large and homogeneous effect size in response to rats receiving gabapentin after nerve injury compared to the modest proportion of patients with chronic neuropathic pain who receive analgesic benefit from the drug. Although many factors which are different between humans and animals could explain the poor analgesic efficacy of gabapentin, it is conceivable that differences in timing after injury, associated with expression of GLT-1 in the LC, explains in part this discrepancy, and our responder analysis (Figure 1B) suggests that the early uniform effect to gabapentin in rats after injury is lost as time progresses. For a large effect size (>30 g), this is uniformly lost around 5 weeks after injury, and for smaller effect sizes (>10 g), it is lost in nearly half of the animals 10 weeks after injury. We speculate that the heterogeneity in response to gabapentin at later times reflects differences in neurobiological processes of dysfunction at sites of gabapentin action, an hypothesis that could lead to new approaches to restore or uncover gabapentin efficacy in patients. Additionally, these results question the relevance of much of the previously published literature, at least as regards gabapentin, since most of these were performed within the first 2 weeks of surgical injury, and suggest that future studies aimed to better understand individual lack of response to gabapentin should focus on times more remote than 2–4 weeks after injury. Since the current study examined only acute efficacy of gabapentin, further studies are required to clarify whether difference in acute treatment in rodent models and chronic treatment in patients contribute to the discrepancy in gabapentin’s antihypersensitivity effect.
Although gabapentin reduces afferent traffic and response of projection neurons in the spinal cord via interactions with α2δ subunits of voltage-gated calcium channels [12; 16; 17], it is unlikely that gabapentin relies exclusively on spinal actions for its analgesic efficacy. A recent clinical trial failed to show efficacy of intrathecal gabapentin in patients with chronic non-cancer pain [22], in contrast to documented efficacy with systemic administration. We previously suggested that systemic gabapentin acts primarily to produce analgesia by increasing descending noradrenergic inhibition, as witnessed by increased noradrenaline concentration in cerebrospinal fluid in patients with chronic orthopedic pain who received gabapentin compared to placebo [7]. We and others demonstrated in rodents 2–3 weeks after peripheral nerve injury that activation of noradrenergic descending inhibition is essential to antihypersensitivity from gabapentin, by demonstrating that systemic administered gabapentin activates noradrenergic neurons in the LC and induces spinal noradrenaline release to stimulate α2-adorenoceptors and subsequent release of acetylcholine for analgesia [10; 11; 29], consistent with the current results in behavior and LC neuronal activity 1–2 weeks after nerve injury. Surprisingly, this state of affairs is altered beyond the first few weeks after injury, a time rarely studied in rodent experiments.
The current study confirmed our previous observations [15] that basal release of noradrenaline in the spinal cord increased many weeks after nerve injury, yet hypersensitivity remained. Gabapentin, which normally excites LC neurons and leads to a transient increase in noradrenaline release in the spinal cord, did so to a much lesser extent many weeks after neuropathic injury, associated with decreased antihypersensitivity efficacy. These paradoxical findings are consistent with the adaptive gain concept of LC tonic and phasic activity[1], supported by the previous work in animals that, as basal LC activity increases, LC phasic response and its influence on cortical responses to non-noxious sensory stimuli are reduced [3]. However, in contrast to the current pCREB results, our previous study showed no effects of nerve injury on basal and gabapentin-evoked c-fos activity of LC neurons in SNL rats 6 weeks after surgery [26]. This discrepancy in LC neuronal activity may be due to the difference between c-fos and pCREB as different measures of cell activation. The current study did not directly examine whether increased basal LC activity affects gabapentin-evoked LC activity. Future studies should examine neuronal activity more directly via electrophysiological recording in the LC.
We previously demonstrated that gabapentin increases co-transport of Na+ ions and glutamate via glutamate transporters, enhances the glutamate-induced intracellular Ca2+ response via the reverse mode of Na+/Ca2+ exchange, and by this mechanism facilitates glutamate release in cultured astrocytes [30]. Blockade or knock-down of GLT-1 abolishes gabapentin-induced glutamate release in the LC in vivo [27], indicating the role of GLT-1-dependent mechanisms for gabapentin’s action in the LC to stimulate descending inhibition. Consistently, the current study shows that knock-down of GLT-1 in the LC reduces the antihypersensitivity effect of gabapentin in SNL rats 2 weeks after surgery. Similar to the results from exogenous knock-down, GLT-1 expression in the LC decreases 6 weeks after SNL surgery, also resulting in the reduced antihypersensitivity effect of gabapentin. Oral administration of the HDAC inhibitor valproate restores down-regulated GLT-1 expression in the LC, gabapentin-induced LC neuronal activation and subsequent spinal noradrenaline release, and antihypersensitivity effect of gabapentin, and these effects of valproate are reversed by the knock-down of GLT-1 in the LC. These results strongly suggest not only a causal relationship between GLT-1 expression in the LC and the antihypersensitivity effect of gabapentin, but also a possible treatment strategy to restore the impaired gabapentin analgesia using HDAC inhibitors in chronic neuropathic pain. In addition, we recently demonstrated that down-regulation of GLT-1 in the LC also impairs pain-evoked endogenous analgesia in SNL rats 6 weeks after nerve injury [15]. Taken together, these observations suggest that down-regulation of GLT-1 in the LC dysregulates descending noradrenergic inhibition, which is important to endogenous and exogenous analgesia.
Although noradrenergic inhibition exists in the normal state, peripheral nerve injury induces a reliance of α2-adrenoceptors-stimulated analgesia on spinal cholinergic neurons. In the spinal dorsal horn, activation of α2-adrenoceptors, which inhibits acetylcholine release in the normal state, facilitates acetylcholine release after peripheral nerve injury via G protein-mediated mechanisms [8; 9; 18]. This facilitation of spinal acetylcholine release after nerve injury is critical for cholinergic dependent analgesia from activation of α2-adrenoceptors [8; 18–20]. The current results demonstrate that increase in basal descending noradrenergic tone did not affect stable hypersensitivity after SNL injury and that the antihypersensitivity effect of intrathecal clonidine, which is reversed by intrathecal atropine, decreases with time after SNL injury. These results suggest that analgesic efficacy from activation of spinal α2-adrenoceptors but not its cholinergic dependency reduces at chronic phase of neuropathic hypersensitivity. In addition to the LC dysfunction, this reduced spinal noradrenergic inhibition may contribute to in part of impaired gabapentin’s efficacy and may explain in part the paradox of increased basal descending noradrenergic tone yet ongoing hypersensitivity. Further studies should examine whether this reduced α2-adrenoceptor-mediated antihypersensitivity effect is due to the reduction in expression or sensitivity of α2-adrenoceptors.
In summary, the current study demonstrates that down-regulation of astroglial GLT-1 in the LC, occurring more than a month after peripheral nerve injury, is critical to reduced antihypersensitivity efficacy from gabapentin in rats and that reduced spinal noradrenergic inhibition may also contribute to in part of the impaired gabapentin’s efficacy. Oral valproate increases GLT-1 expression in the LC, possibly via HDAC inhibition, to restore the impaired antihypersensitivity effect of gabapentin. Given clinical availability and established safety profiles, valproate should be tested to rescue gabapentin poor-responders.
Acknowledgments
This work was supported by grants DA27690 to K.H. from the National Institutes of Health (Bethesda, Maryland).
Footnotes
Competing interests: Dr. Eisenach has served as a consultant to Adynxx, Inc. and Aerial Biopharma regarding preclinical and early phase clinical novel analgesic drug development.
References
- 1.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Devilbiss DM, Waterhouse BD. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. Journal of neurophysiology. 2011;105(1):69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field MJ, Holloman EF, McCleary S, Hughes J, Singh L. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. The Journal of pharmacology and experimental therapeutics. 1997;282(3):1242–1246. [PubMed] [Google Scholar]
- 5.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106(3):557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Hayashida K, Eisenach JC. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113(2):406–412. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashida K, Kimura M, Yoshizumi M, Hobo S, Obata H, Eisenach JC. Ondansetron reverses antihypersensitivity from clonidine in rats after peripheral nerve injury: role of gamma-aminobutyric acid in alpha2-adrenoceptor and 5-HT3 serotonin receptor analgesia. Anesthesiology. 2012;117(2):389–398. doi: 10.1097/ALN.0b013e318260d381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109(6):1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashida K, Parker R, Eisenach JC. Oral gabapentin activates spinal cholinergic circuits to reduce hypersensitivity after peripheral nerve injury and interacts synergistically with oral donepezil. Anesthesiology. 2007;106(6):1213–1219. doi: 10.1097/01.anes.0000267605.40258.98. [DOI] [PubMed] [Google Scholar]
- 12.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobo S, Eisenach JC, Hayashida K. Up-regulation of spinal glutamate transporters contributes to anti-hypersensitive effects of valproate in rats after peripheral nerve injury. Neuroscience letters. 2011;502(1):52–55. doi: 10.1016/j.neulet.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Suto T, Morado-Urbina CE, Peters CM, Eisenach JC, Hayashida K. Impaired Pain-evoked Analgesia after Nerve Injury in Rats Reflects Altered Glutamate Regulation in the Locus Coeruleus. Anesthesiology. 2015;123(4):899–908. doi: 10.1097/ALN.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 16.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125(1–2):20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. The Journal of pharmacology and experimental therapeutics. 2002;303(3):1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 18.Obata H, Li X, Eisenach JC. alpha2-Adrenoceptor activation by clonidine enhances stimulation-evoked acetylcholine release from spinal cord tissue after nerve ligation in rats. Anesthesiology. 2005;102(3):657–662. doi: 10.1097/00000542-200503000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90(2):509–514. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- 20.Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003;99(1):199–204. doi: 10.1097/00000542-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives internationales de pharmacodynamie et de therapie. 1957;111(4):409–419. [PubMed] [Google Scholar]
- 22.Rauck R, Coffey RJ, Schultz DM, Wallace MS, Webster LR, McCarville SE, Grigsby EJ, Page LM. Intrathecal gabapentin to treat chronic intractable noncancer pain. Anesthesiology. 2013;119(3):675–686. doi: 10.1097/ALN.0b013e3182a10fbf. [DOI] [PubMed] [Google Scholar]
- 23.Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochemistry international. 1998;33(6):479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 24.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 25.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Progress in neurobiology. 1998;56(2):237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 26.Suto T, Eisenach JC, Hayashida K. Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. Pain. 2014;155(10):1935–1942. doi: 10.1016/j.pain.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suto T, Severino AL, Eisenach JC, Hayashida K. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology. 2014;81:95–100. doi: 10.1016/j.neuropharm.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasu K, Honda M, Ono H, Tanabe M. Spinal alpha(2)-adrenergic and muscarinic receptors and the NO release cascade mediate supraspinally produced effectiveness of gabapentin at decreasing mechanical hypersensitivity in mice after partial nerve injury. British journal of pharmacology. 2006;148(2):233–244. doi: 10.1038/sj.bjp.0706731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanabe M, Takasu K, Takeuchi Y, Ono H. Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. Journal of neuroscience research. 2008;86(15):3258–3264. doi: 10.1002/jnr.21786. [DOI] [PubMed] [Google Scholar]
- 30.Yoshizumi M, Eisenach JC, Hayashida K. Riluzole and gabapentinoids activate glutamate transporters to facilitate glutamate-induced glutamate release from cultured astrocytes. European journal of pharmacology. 2012;677(1–3):87–92. doi: 10.1016/j.ejphar.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshizumi M, Eisenach JC, Hayashida K. Valproate prevents dysregulation of spinal glutamate and reduces the development of hypersensitivity in rats after peripheral nerve injury. The journal of pain : official journal of the American Pain Society. 2013;14(11):1485–1491. doi: 10.1016/j.jpain.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]