Abstract
Broadly neutralizing antibodies (bNAbs) represent a new generation of antiviral agents for the prevention and treatment of human immunodeficiency virus 1 (HIV-1) infection. A better understanding of the in vivo efficacy of HIV-1 bNAbs, such as VRC01, in preventing mucosal transmission of HIV-1 has important implications for HIV-1 vaccine design. In this study, we evaluated the efficacy of passively transferred VRC01 antibody in preventing HIV-1 vaginal and rectal transmission in humanized bone marrow/liver/thymus mice (hu-BLT mice). Mice were subcutaneously injected with VRC01 IgG, and 24 hours later, they were challenged intravaginally or intrarectally with HIV-1Ada. All hu-BLT mice receiving VRC01 IgG antibody were aviremic at 2 weeks after intravaginal (n=3) or intrarectal (n=6) challenge as measured by quantitative real-time RT-PCR. In contrast, mice receiving control IgG all became infected. By 5 and 6 weeks post-challenge, some of VRC01 aviremic mice in both the intravaginal and intrarectal challenge groups became viremic. Our results suggest that VRC01 antibody can be protective against HIV-1 vaginal and rectal transmission; however, a single administration of VRC01 cannot completely prevent mucosal infection.
Keywords: VRC01, Hu-BLT mice, HIV-1, vaginal and rectal transmission
Introduction
Neutralizing antibody is a critical protective component in most licensed vaccines; thus, elicitation of such antibodies by a human immunodeficiency virus 1 (HIV-1) vaccine is highly desirable, although it remains elusive. Recently, many broadly neutralizing antibodies (bNAbs) with high potency against HIV-1 have been identified from chronically HIV-1-infected individuals [1–11]. It has been demonstrated that passive transfer of bNAbs can prevent HIV-1 mucosal transmission in the rhesus macaque-chimeric simian/human immunodeficiency virus (SHIV) model [12–19] and in humanized mice generated by injection of CD34+ hematopoietic stem cells [20]. Although these experiments provided important information, these models have certain limitations. There are a limited number of SHIV challenge viruses available for rhesus macaque studies, and macaques cannot be used to test the protection of bNAbs against HIV-1 directly [21, 22]. Humanized mice generated by injection of CD34+ hematopoietic stem cells (hu-HSC mice) have less robust human immune system reconstitution than humanized bone marrow/liver/thymus (hu-BLT) mice [23–26]. hu-BLT mice are a new generation of humanized mice in which human immune cells are reconstituted within mucosal and secondary lymphoid tissues [24–26] and human T lymphocytes undergo positive and negative selection in the human thymic organoid in the context of autologous MHC restriction [24, 25]. Therefore, hu-BLT mice are one of the best available small animal models to study mucosal transmission of HIV-1 and its prevention [23, 26]. VRC01 antibody is the prototype of the VRC antibody class, which can neutralize the CD4 binding site (CD4bs) of HIV-1 gp120 envelope protein, and is the best characterized bNAb in terms of neutralizing profile, structural recognition features, genetic origin, affinity maturation pathways, and lineage evolution [3, 27–30]. Moreover, this bNAb has advanced to clinical trials for the treatment of HIV-1 infection [31, 32]. HIV-1 is primarily transmitted through vaginal and rectal mucosal surfaces, and studying the interaction between bNAb and HIV-1 at these mucosal sites is essential to aid HIV-1 vaccine design. Using hu-HSC mice, the efficacy of vaginal topical administration of VRC01 against HIV-1 intravaginal infection has been demonstrated previously [20]; however, the efficacy of parental administration of VRC01 has not been reported. In this study, we demonstrate, by using the hu-BLT mouse model of HIV-1 transmission, that VRC01 antibody can delay vaginal and rectal transmission of HIV-1; however, a single administration of VRC01 cannot completely prevent mucosal infection. Our results indicate that a combination of broadly neutralizing antibodies may be required to achieve sterilizing protection against HIV-1 mucosal transmission.
Materials and methods
Hu-BLT mice
The hu-BLT mice were generated as described previously [33, 34] at the University of Nebraska-Lincoln Life Sciences Annex. Briefly, 6- to 8-week-old female NSG mice (Cat# 005557, the Jackson Laboratory, Bar Harbor, Maine) received 12 cGy irradiation per gram of body weight with an RS200 X-ray irradiator (RAD Source Technologies, Inc, GA) and were then implanted with two pieces of human fetal liver and one piece of thymic tissue fragments under the left kidney capsule, followed by intravenous injection of 1.5–5 × 105 CD34+ hematopoietic stem cells isolated from human fetal liver. Human fetal liver and thymus tissues were procured from Advanced Bioscience Resources (Alameda, CA). After 12 to 16 weeks, peripheral blood samples were collected for quantification of human immune reconstitution using flow cytometry as described previously [33, 34]. All hu-BLT mice used in VRC01 protection experiments in this study had good human immune reconstitution, with a ratio of peripheral blood hCD45+ cells / (hCD45+ cells plus mCD45+ cells) of 80.7± 9.9% (mean ± SD) (Supplemental Table 1). Mice were randomly assigned to control and VRC01-treated groups with similar immune reconstitution.
VRC01 antibody preparation
VRC01 IgG antibody was produced according to a published protocol [3]. Briefly, VRC01 IgG expression plasmids were provided by the NIH AIDS Reagent Program (Cat# 12035 and 12036), and plasmid DNA was extracted using an endotoxin-free Maxi Kit (QIAGEN). Freestyle 293-F cells (Cat# R790-07, Thermo Fisher Scientific) were co-transfected using 293fectin (Cat# 12347-019, Thermo Fisher Scientific) at a final concentration of 5 µg/ml and 10 µg/ml of vector plasmids expressing the heavy chain and light chain of VRC01, respectively. After culturing for 5 days, cell culture supernatants were harvested and analyzed for the presence of antibody by capture ELISA. Ab-containing culture supernatants were filtered and purified by affinity chromatography using a Protein A column (Pierce) and concentrated using a centrifugal filter (Millipore). The purified VRC01 IgG sample was subjected to SDS-PAGE and stained with Coomassie brilliant blue to check protein size and purity.
Determination of the half-life of VRC01 antibody in HIV-1-naïve hu-BLT mice
To evaluate the half-life of VRC01 antibodies, VRC01 IgG (20 µg/g body weight) was injected subcutaneously into three HIV-1-uninfected hu-BLT mice, and blood was collected 1, 3, 7, 14, 23, and 29 days after antibody administration. To determine the VRC01 IgG concentration in plasma, HIV-1BaL gp120 protein (0.5 µg/ml in PBS, pH 7.4) was adsorbed onto 96-well high-binding ELISA plates (Costar/Corning; Lowell, MA) at 4 °C. After washing five times with PBS-Tween 20 (PBS-T), the plates were blocked with 10% non-fat milk in PBS-T at 37 °C for 2 h. Serial dilutions of plasma and VRC01 standard samples were then incubated in plates at 37 °C for 1 h. After washing with PBS-T, the plates were incubated with secondary antibodies, horseradish peroxidase (HRP)-conjugated goat anti-human IgG1 antibodies (1:4000, Cat# 62-8420, Invitrogen) at 37 °C for 45 min. Finally, the plates were developed by adding streptavidin-coupled peroxidase and 3, 3’, 5, 5’-tetramethylbenzidine substrate (TMB, Sigma, USA), and development was stopped by adding 50 µl of 2 M H2SO4. Finally, the optical density (OD) was read on an ELISA reader at 450 nm, and IgG concentrations were calculated based on standard curves and sample OD values.
Challenge virus preparation
HIV-1Ada for inoculation was obtained from the NIH AIDS Reagent Program (Cat# 416) and was further expanded in pooled PBMC from two healthy human donors. Cell-free supernatant was harvested, filtered, and concentrated by ultracentrifugation, and the viral titer was determined using TZM-bl cells. HIV-1 transmitted/founder (T/F) viruses (HIV-SUMA, HIV-WITO, HIV-1 RHPA, HIV-TRJO and HIV-REJO) were generated by transfection of 293 T cells with infectious molecular clone pSUMA.c/2821, pWITO.c/2474, pRHPA.c/2635, pTRJO.c/2851, and pREJO.c/2864 (cat#11748, 11739, 11744, 11747, and 11746, respectively, from Dr. John Kappes, obtained through the AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH) and expanded by co-culture in pooled PBMCs from two healthy human donors. Cell-free supernatant was harvested, filtered and concentrated by ultracentrifugation. Concentrated virus was resuspended in RPMI, and the viral titer was determined in TZM-bl cells.
In vitro neutralization
VRC01 antibody neutralization activity against five T/F viruses and HIV-1Ada was tested in a luciferase-based assay in TZM-bl cells as described previously [35]. Briefly, fivefold serial dilutions of VRC01 were performed in triplicate in a 96-well flat-bottom plate. One hundred TCID50 of virus and 50 µl of 10% DMEM growth medium containing DEAE-dextran (Sigma) at a final concentration of 11 mg/ml were added to each well and incubated for 1 hour at 37 °C, and TZM-bl cells (1 × 104 per well in a 100-µl volume) were added. Assay controls included TZM-bl cells alone (cell control) and TZM-bl cells with virus (virus control). After a 48-hour incubation at 37 °C, culture medium was removed from each well, and 40 µl of lysis buffer and 60 µl of Galacto-Star luciferase reagents (Applied Biosystems) were added and luminescence was measured. The IC50 was calculated as the antibody dilution that caused a 50% reduction in relative luminescence units (RLUs) compared to the virus control after subtraction of cell control RLUs.
Systemic administration of VRC01 to protect against vaginal and rectal transmission of HIV-1 in hu-BLT mice
To evaluate the efficiency of protection of systemically administered VRC01 against vaginal and rectal transmission of HIV-1, each of the hu-BLT mice in the VRC01 group (vaginal n=3, rectal n=6) and control group (vaginal n=3, rectal n=6) was subcutaneously injected with VRC01 antibody at a dose of 20 mg/kg or the same amount of human control IgG (SIGMA, Cat# I4506). Twenty-four hours later, all of the mice were challenged intravaginally or intrarectally with 2.4 × 104 TCID50 of HIV-1Ada under anesthesia with 10 µl of ketamine/xylazine solution (concentration of 10 mg/ml ketamine and 1.2 mg/ml xylazine) per gram of body weight. After inoculation, mice were immediately kept in an upside-down position for at least 20 minutes.
Viral load quantification
Plasma viral loads in hu-BLT mice were determined at 2 weeks post-virus-challenge using real-time qRT-PCR. Briefly, viral RNA was extracted using a QIAamp Viral RNA Mini Kit (QIAGEN), and HIV RNA was amplified using a TaqMan® One-Step RT-PCR Master M Mix Reagents Kit (Life Technologies) using primers and conditions that have been published previously [36]. The sensitivity of the assay was 800 copies/ml of plasma.
Statistics
The Mann-Whitney test in R was used for statistical analysis.
Results
VRC01 half-life in hu-BLT mice
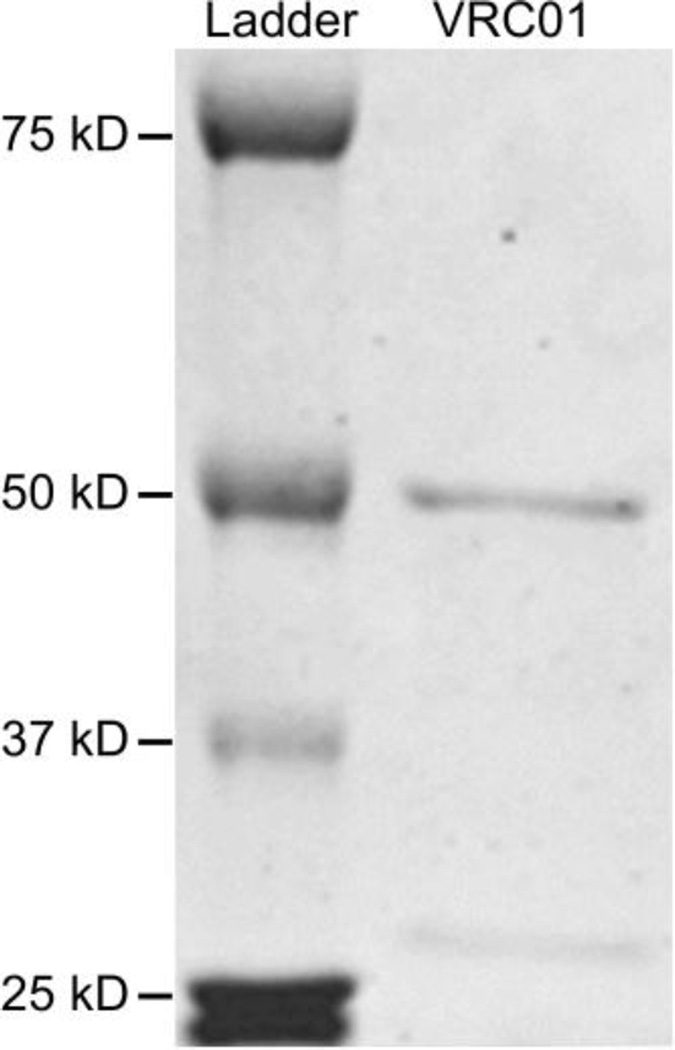
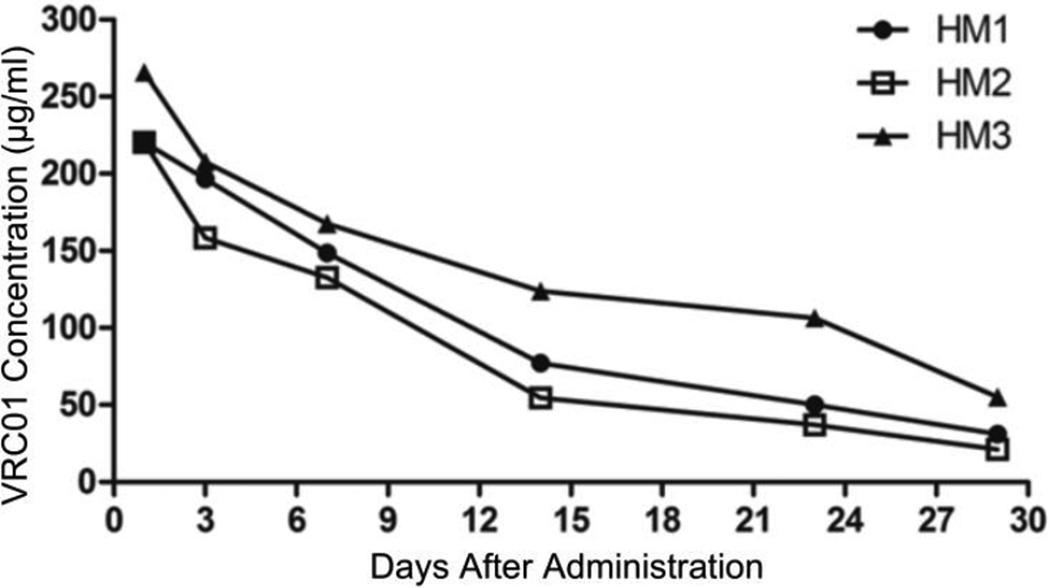
After confirming that VRC01 IgG antibody produced in 293-F cells had good purity and the correct size (Fig. 1), we determined its half-life in vivo. Three HIV-1-naïve hu-BLT mice were subcutaneously injected with VRC01 IgG (20 µg/g body weight), blood was collected at 1, 3, 7, 14, 23 and 29 days post-antibody-administration, and plasma VRC01 levels were quantified using an ELISA assay. As shown in Fig. 2, after administration, the plasma VRC01 levels gradually declined over the time, and the in vivo half-life was calculated to be 8.93 days.
Fig. 1.
Expression and purification of VRC01 IgG antibody. Polyacrylamide gel with Coomassie blue staining. The antibody was purified using protein A as described in Materials and methods. A total of 8 µg of purified protein was loaded onto this SDS-PAGE gel.
Fig. 2.
Kinetics of VRC01 antibody in HIV- 1-naïve hu-BLT mice. VRC01 concentrations in sera of three hu-BLT mice after subcutaneous injection of 400 µg VRC01 IgG antibody
VRC01 neutralizing activity against inoculating virus
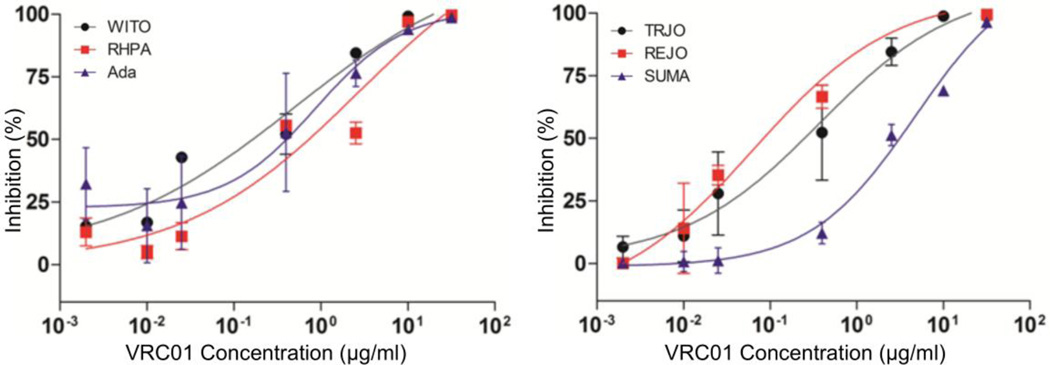
Considering that in vivo bNAb protection against HIV-1 mucosal transmission generally correlates with its in vitro neutralization activity [37–39], we first measured VRC01 neutralization potency against the inoculating virus HIV-1Ada as compared with five transmitted/founder (T/F) HIV-1 viruses in TZM-bl cells (Fig. 3, Table 1). VRC01 at different concentrations achieved 100% neutralization against HIV-1Ada and all five T/F viruses; however, HIV-1Ada only achieved 100% maximum percent inhibition (MPI) at the concentration of 50 µg/ml (Table 1), indicating that it is less sensitive to neutralization than most T/F HIV-1 viruses. We therefore decided to use HIV-1Ada as the challenge virus.
Fig. 3.
Comparison of the neutralizing activity of VRC01 against HIV-1Ada and five transmitted/founder (T/F) HIV-1 viruses. VRC01 IgG antibody inhibited the infection with T/F HIV-1 viruses (TRJO, REJO, SUMA, WITO, and RHPA) and HIV-1Ada in vitro. The neutralization sensitivity of these six viruses to VRC01 IgG antibody was tested. Each curve shows the inhibition of the antibody against the indicated T/F virus or HIV-1Ada.
Table 1.
IC50 and MPI of VRC01 against six HIV-1 strains
| Ada | TRJO | REJO | SUMA | WITO | RHPA | |
|---|---|---|---|---|---|---|
| IC50 (µg/ml) | 1.902 | 1.025 | 0.9438 | 10.49 | 1.559 | 4.058 |
| MPI at 10 µg/ml | 93% | 100% | 100% | 71% | 98% | 95% |
| MPI at 50 µg/ml | 100% | 100% | 100% | 100% | 100% | 100% |
Protection o f VRC01 against vaginal transmission of HIV-1
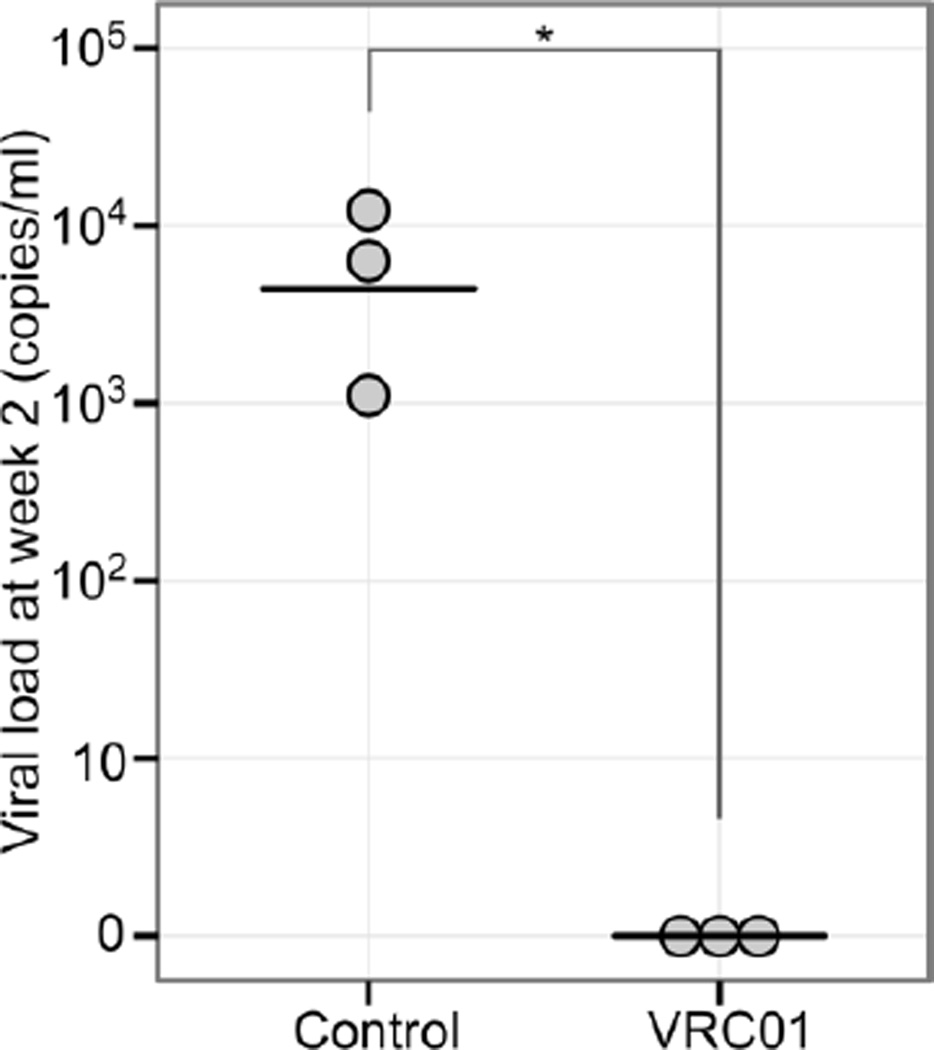
To assess the efficacy of VRC01 in preventing HIV-1 vaginal transmission, we first selected HIV-1Ada as the challenge virus because it was less sensitive to VRC01 neutralization, as revealed in our neutralizing assay. As shown in Fig. 4, all of the mice (n=3) that were subcutaneously injected with 20 mg of VRC01 per kg at 24 hours before intravaginal challenged with 2.4 × 104 TCID50 HIV-1Ada, were aviremic at 2 weeks post-challenge, as assessed by qRT-PCR (sensitivity <800 copies/ml), while all of the mice (n=3) that were injected with the same amount of human control IgG antibody were infected at 2 weeks post-challenge. Two of the VRC01-protected mice were later tested, and the plasma viral load became positive at 5 weeks post-challenge (1.21 × 105 and 1.64 × 105 copies/ml). Additionally, the viral load of the control mice remained positive and showed no significant change.
Fig. 4.
Protection of VRC01 against vaginal transmission of HIV-1. VRC01 IgG antibody (20 µg/g body weight) was administered subcutaneously to three hu-BLT mice (VRC01 treatment group), while the same dosage of human IgG was injected into another three mice (control group), and 24 hours later, all of the mice were challenged intravaginally with 2.4 × 104 TCID50 HIV-1Ada. Peripheral blood was collected 2 weeks after challenge, and viral loads in plasma were quantified by qRT-PCR. *, P < 0.05
Protection of VRC01 against rectal transmission of HIV-1
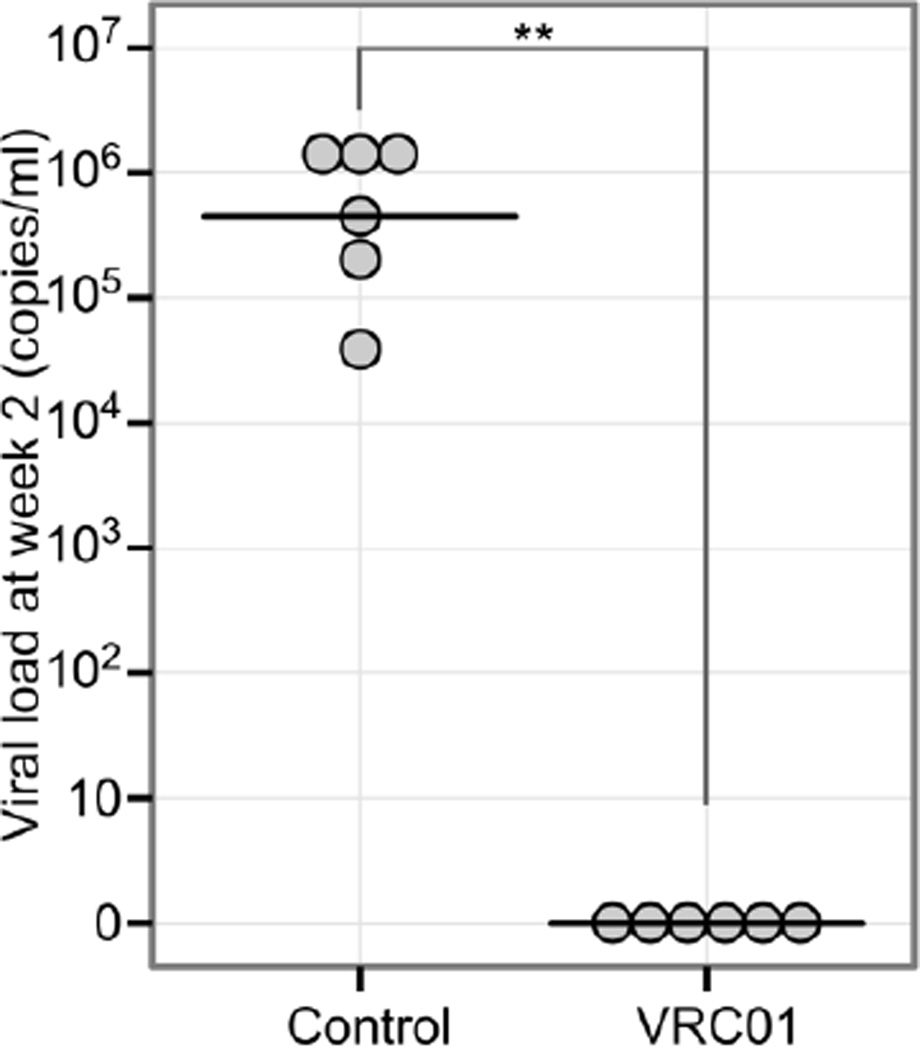
To assess the potency of VRC01 against rectal transmission of HIV-1, each of the hu-BLT mice was subcutaneously injected with 20 mg of of VRC01 antibody per kg (n=6) or the same amount of human control IgG (n=6). Twenty-four hours later, the mice in both groups were challenged intrarectally with 2.4 × 104 TCID50 of HIV-1Ada. All six hu-mice receiving VRC01 antibody were aviremic (Fig. 5) at 2 weeks post-challenge. In contrast, all of the control hu-mice were infected (Fig. 5). Two of the VRC01-protected mice were later tested, and the plasma viral load became positive at 6 weeks post-challenge (7.21 × 104 and 6.07 × 105 copies/ml). Additionally, the viral load of control mice remained positive and showed no significant change.
Fig. 5.
Protection of VRC01 against rectal transmission of HIV-1. VRC01 IgG antibody (20 µg/g body weight) was administered subcutaneously to six hu-BLT mice (VRC01 treatment group), and human IgG was injected into another six mice (control group), and 24 hours later, all of the mice were challenged intrarectally with 2.4 × 104 TCID50 HIV-1Ada. Peripheral blood was collected 2 weeks after challenge, and viral loads in plasma were quantified by qRT-PCR. **, P < 0.01
Discussion
VRC01 antibody is the prototypic bNAb targeting the CD4 binding site (CD4bs) of HIV-1 envelope protein gp120 [3, 27], and it has advanced to clinical trials for the treatment of HIV-1 infection [32]. In this study, we first compared VRC01 neutralizing activity against HIV-1Ada and five T/F viruses by in vitro neutralization assay. VRC01 IgG effectively inhibited infection of all of the viruses tested at 10 µg/ml or 50 µg/ml, and HIV-1Ada was shown to be the most resistant to neutralization (MPI = 100% at 50 µg of VRC01 IgG per ml). Based on these results, we tested the efficacy of VRC01 in preventing vaginal and rectal transmission of HIV-1Ada in hu-BLT mice. VRC01 administered systemically blocked HIV-1Ada vaginal infection at 2 weeks post-challenge: all three VRC01-treated mice were HIV-1 negative, while all three control mice became HIV-1 positive (P < 0.05). While the vaginal route is a major HIV-1 transmission route for women, the anorectal route is an important route of HIV-1 mucosal transmission for men who have sex with men. We then tested VRC01 administered systemically in preventing HIV-1 rectal transmission. All six hu-BLT mice that received VRC01 were aviremic at 2 weeks post-inoculation, while all six hu-BLT mice in the control group were infected (P < 0.01). Previously, it was reported that vaginal topical administration of VRC01 one hour before virus challenge protected against intravaginal infection with HIV-1 Bal in RAG-humanized mice [20]. Furthermore, a previous study showed that a high concentration of plasma VRC01 expressed by adeno-associated virus (AAV) encoding VRC01-IgG protected hu-BLT mice from vaginal infection with HIV-1 [40, 41]. Our study demonstrates that a single administration of VRC01 through the parenteral route can confer some protection against both vaginal and rectal HIV-1 transmission.
Virus neutralization is believed to occur when neutralizing antibody occupies a sufficient number of epitopes on the viral surface. This “occupancy” model, also known as the “multi-hit model” [42], suggests that the most important factor for neutralization is achieving a high enough antibody concentration and density on the virion, leading to inhibition of viral attachment to cellular receptors or the fusion processes [42]. When we tested some of these VRC01-protected aviremic mice at 2 weeks post-challenge after a single administration of VRC01, two VRC01 mice in both the intravaginal and intrarectal challenge group became viremic at 5 and 6 weeks post-challenge, respectively, indicating that a single administration of VRC01 cannot completely prevent mucosal transmission and that a decreased antibody concentration over time may contribute to the reduction of epitope “occupancy” and loss of neutralization capacity.
Taken together, our study demonstrates that VRC01 administered systemically can protect against vaginal and rectal transmission of HIV-1. However, with a single administration of VRC01, some aviremic mice at 2 weeks post-challenge became infected at 5 or 6 weeks post-challenge. While these findings provide further support for the possible use of VRC01 to prevent mucosal transmission of HIV-1 in humans and support the findings that the CD4 binding site is an important target for vaccine development, our study also suggests that “neutralized virus” can regain its infectivity as antibody concentrations decrease over time, and a combination of broadly neutralizing antibodies may be required to achieve sterilizing protection against HIV-1 mucosal transmission.
Supplementary Material
Quantification of human immune reconstitution in mice peripheral blood samples 12–16 weeks post-transplantation in this study using multi-parameter flow cytometry. The percentage of human leukocytes (human CD45+ and mouse CD45−) cells out of total leukocytes (FSC, SSC gating) in hu-BLT mouse blood and percentage of CD4+ T cells and CD8+ T cells out of total human T cells (human CD45+ and CD3+) at weeks 12 to 16 after surgery are also shown in this table.
Acknowledgments
The authors are grateful for the hu-BLT mouse care and maintenance services provided by UNL Life Sciences Annex, and Lance Daharsh for critical reading of this manuscript.
Funding: This work was supported in part by NIH grant R01 AI111862 (to Li Q. and Guo J.) and NIH P30GM103509. Yue Li was supported by a Fogarty International Center grant at the University of Nebraska-Lincoln (D43 TW001429).
Footnotes
Compliance with ethical standards
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 2.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, et al. Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, et al. Rational Design of Envelope Identifies Broadly Neutralizing Human Monoclonal Antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TYK, et al. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012 doi: 10.1038/nature11544. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diskin R, Scheid JF, Marcovecchio PM, West AP, Klein F, Gao H, et al. Increasing the Potency and Breadth of an HIV Antibody by Using Structure-Based Rational Design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5:0008805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 13.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:15. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, et al. Broadly Neutralizing Monoclonal Antibodies 2F5 and 4E10 Directed against the Human Immunodeficiency Virus Type 1 gp41 Membrane-Proximal External Region Protect against Mucosal Challenge by Simian-Human Immunodeficiency Virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, et al. Protection of Macaques against Pathogenic Simian/Human Immunodeficiency Virus 89.6PD by Passive Transfer of Neutralizing Antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 17.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldt B, Rakasz EG, Schultz N, Chan-Hui P-Y, Swiderek K, Weisgrau KL, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders KO, Pegu A, Georgiev IS, Zeng M, Joyce MG, Yang Z-Y, et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. J Virol. 2015;89:5895–5903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veselinovic M, Preston Neff C, Mulder LR, Akkina R. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology. 2012;432:505–510. doi: 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Micro. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8:255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deruaz M, Luster AD. BLT humanized mice as model to study HIV vaginal transmission. J Infect Dis. 2013;208(Suppl 2):S131–S136. doi: 10.1093/infdis/jit318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. The Journal of Experimental Medicine. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brehm MA, Shultz LD, Luban J, Greiner DL. Overcoming Current Limitations in Humanized Mouse Research. Journal of Infectious Diseases. 2013;208:S125–S130. doi: 10.1093/infdis/jit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, et al. Structural Basis for Broad and Potent Neutralization of HIV-1 by Antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Zhang Z, Schramm Chaim A, Joyce MG, Do Kwon Y, Zhou T, et al. Maturation and Diversity of the VRC01-Antibody Lineage over 15 Years of Chronic HIV-1 Infection. Cell. 2015;161:470–485. doi: 10.1016/j.cell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, et al. Multidonor Analysis Reveals Structural Elements, Genetic Determinants, and Maturation Pathway for HIV-1 Neutralization by VRC01-Class Antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clinical & Experimental Immunology. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Science Translational Medicine. 2015;7:319ra206–319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LX, Kang G, Kumar P, Lu W, Li Y, Zhou Y, et al. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc Natl Acad Sci U S A. 2014;111:3146–3151. doi: 10.1073/pnas.1318175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Tso FY, Kang G, Lu W, Li Y, Fan W, et al. Early Initiation of Antiretroviral Therapy Can Functionally Control Productive HIV-1 Infection in Humanized-BLT Mice. J Acquir Immune Defic Syndr. 2015;69:519–527. doi: 10.1097/QAI.0000000000000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 2011;121:2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee FH, Mason R, Welles H, Learn GH, Keele BF, Roederer M, et al. Breakthrough Virus Neutralization Resistance as a Correlate of Protection in a Nonhuman Primate Heterologous Simian Immunodeficiency Virus Vaccine Challenge Study. J Virol. 2015;89:12388–12400. doi: 10.1128/JVI.01531-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, Georgiev IS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88:12669–12682. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola JR. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine. 2002;20:1922–1925. doi: 10.1016/s0264-410x(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 40.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Della-Porta AJ, Westaway EG. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978;38:1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification of human immune reconstitution in mice peripheral blood samples 12–16 weeks post-transplantation in this study using multi-parameter flow cytometry. The percentage of human leukocytes (human CD45+ and mouse CD45−) cells out of total leukocytes (FSC, SSC gating) in hu-BLT mouse blood and percentage of CD4+ T cells and CD8+ T cells out of total human T cells (human CD45+ and CD3+) at weeks 12 to 16 after surgery are also shown in this table.