Abstract
Background
Effective interventions are needed to address the low rate of human papillomavirus vaccination in the United States, particularly among girls and women 16 – 26 years old. Counseling and offering the vaccine to postpartum patients could be an effective strategy to increase uptake among young women who did not complete the 3-dose series at an earlier age.
Objective
The purpose of this evaluation was to assess the effectiveness of a multi-component program designed for postpartum women that used patient navigators and reminders for follow-up visits to improve uptake and completion of the human papillomavirus vaccine series.
Design
As part of standard care, patients ≤26 years of age from Galveston County, Texas who delivered an infant between November 2012 and June 2014 at a public hospital were counseled and offered the human papillomavirus vaccine postpartum. Patient navigators assisted with scheduling follow-up injections during postpartum or well-child visits. A program evaluation was conducted after 20 months.
Results
Of 1,038 patients approached, only 161 (15.5%) had previously completed the vaccine series. Of the 877 patients who had not completed the series, 661 (75.4%) received at least one dose postpartum, with 575 patients receiving their first dose and 86 receiving their second or third doses. By April 2015, initiation rates had increased as a result of this program from 25.4% before the program was initiated to 80.8% and completion rates from 15.5% to 65.1%. Missed appointments for injections were less likely among those who received text message reminders and more likely among those with ≥2 prior pregnancies. Those who were Hispanic or had received an influenza vaccination in the last year were more likely to initiate and complete the series through this program. Patients who missed 1 or more follow-up appointments were less likely to complete the vaccine series.
Conclusions
Offering the human papillomavirus vaccine postpartum dramatically increased initiation rates among postpartum patients. Patient navigation and text messages ensured that a high percentage completed all 3 doses.
Keywords: Human papillomavirus (HPV), HPV vaccine, vaccine uptake, vaccine initiation, vaccine completion, postpartum women, patient navigator
Introduction
The human papillomavirus (HPV) is the cause of almost all cases of cervical cancer as well as many cases of vulvar, vaginal, and anal cancers in women.1 In 2006, the US Food and Drug Administration approved a vaccine that has the potential to markedly decrease the incidence of these diseases. Almost a decade after its introduction, however, uptake of the HPV vaccine in the United States remains below that of several other countries, including England, Scotland, and Australia.2–5 By 2014, only 60% of US girls 13–17 years of age had obtained even one of the 3 required doses, demonstrating the need for catch-up vaccination.6 Between 2008–2010, only 28% of surveyed 18–26 year old females had initiated and 17% had completed the series.7 By 2013, initiation rates rose to only 37% among women 19–26 years of age.8
Although recommended at a younger age, vaccination is effective among women 18–25 years old. A clinical trial demonstrated that it reduces abnormal Pap tests, referral for colposcopy, and treatment related to abnormal cervical cytology when given to women at these ages.9 Thus, vaccination is recommended for women up to 26 years of age not previously vaccinated.10
Surveys of pregnant women demonstrate low rates of HPV vaccination.11 This is especially true among low-income women and women from minority backgrounds. At our institution, only 13% among 500 pregnant patients seen in public clinics in 2012 had initiated the HPV vaccine.12 These low rates may be due to a lack of routine care among low-income adolescents and young adult women.13 Most US women do obtain medical care during pregnancy,14 but the HPV vaccine is not usually discussed during prenatal care as it is not administered during pregnancy. Other barriers facing low-income women include the high cost of HPV vaccination and the challenge of receiving all three doses, even when the vaccine is free of charge. 15,16
Due to low rates of vaccine uptake in the United States, new strategies for increasing opportunities and acceptance for HPV vaccination are needed. The postpartum period could be an opportunity to increase HPV vaccination rates among low-income women. Studies have demonstrated that vaccines are well accepted by women when offered postpartum. For example, 91% of women accepted the hepatitis B vaccine when offered postpartum in one study, while 96% of eligible postpartum women accepted the Tdap vaccine in another.17,18 An examination of the feasibility of postpartum HPV vaccination found an acceptance rate of 95% among 150 women offered the first dose prior to hospital discharge.19 However, only 31% of those patients completed the 3-dose series.
At the University of Texas Medical Branch (UTMB), we surveyed 500 patients attending five prenatal clinics in 2012 to determine whether a postpartum vaccination program would be acceptable among women residing in southeast Texas.12 Over 80% said they were willing to receive a free HPV shot in the hospital after childbirth. Based on these findings, UTMB obtained funding to begin a prevention program that offered counseling for pregnant and postpartum women about HPV and the HPV vaccine. Here, we report on the success of this program during the first 20 months of its implementation.
Methods
Through a grant funded by the Cancer Prevention Research Institute of Texas (CPRIT), UTMB established a program in November of 2012 to offer the quadrivalent HPV vaccine to postpartum women from Galveston County free of charge. Vaccinations were offered prior to hospital discharge. Follow-up doses were given at postpartum visits and, through collaboration with the Department of Pediatrics, at well-child visits for patients’ infants. To reduce missed appointments and increase the rate of series completion, patient navigators (PNs) used multiple reminder methods (texting, mailing reminders, and placing calls) and patient tracking. The Obstetrics & Gynecology department at UTMB serves a low-income population with approximately 88% reporting a family income under $29,900/year and 63% less than $15,000 annually.20 The majority are uninsured, although 40% do qualify for expanded Medicaid coverage during pregnancy. This report describes HPV vaccinations administered on the postpartum unit during the first 20 months (November 2012 to June 2014) of this program with follow-up doses administered through April 2015.
English and Spanish fact sheets about HPV and the HPV vaccine developed by the Centers for Disease Control and Prevention (CDC) 21–23 were distributed in waiting rooms of all UTMB Health prenatal clinics in Galveston County to educate patients. This information was also distributed in the hospital after delivery. Patients then received face-to-face counseling from PNs who had been trained by the first author (ABB). Providers involved in the care of pregnant and postpartum women and their infants were educated through a series of lectures on HPV given across the UTMB campus to attending physicians, residents, medical students, nurses, physician assistants, and staff.24
PNs reviewed the electronic medical records (EMRs) and State of Texas immunization records daily of all patients who delivered a liveborn infant at UTMB in the previous 24 hours to identify those eligible for this program (females ≤26 years old residing in Galveston County who were unvaccinated or incompletely vaccinated against HPV). Eligible patients were then offered written materials and personal counseling about HPV and the vaccine. Non-UTMB medical records were checked, as possible, for those who reported they had already been vaccinated. If it was determined that the patient had not completed the series, she was offered the vaccine postpartum. Patients were informed that one dose could be administered prior to discharge and follow-up doses in conjunction with other scheduled appointments. In addition, patients who agreed to be vaccinated selected the type(s) of reminders (automated phone calls and/or text messages) they wished to receive prior to appointments. Adequate time was given for PNs to address all questions.
Those who agreed to receive a dose of the HPV vaccine postpartum reviewed and signed the State of Texas consent form. For patients under age 18, parental consent was required as mandated by the state. To facilitate tracking, PNs obtained mailing and email addresses and both home and cell phone numbers as well as contact information for up to 3 individuals who could reach the patient if needed. The patient’s obstetric provider was then asked to place an order for HPV vaccination in the EMR.
A month before the next dose was due, PNs reviewed each patient’s and her infant’s EMRs to identify upcoming appointments (eg, postpartum checks or well-child visits) during which the next vaccine dose could be administered. When an appropriately timed appointment was identified, the PN added a HPV vaccination request to the entry for that visit in the EMR for the mother and informed the patient she would receive the next dose at that time. If it was not possible to coordinate the next dose with an already scheduled appointment for the mother or infant at UTMB (ie, the patient selected outside providers), a vaccine-only appointment was scheduled at the closest UTMB facility. Typically, a vaccination-only appointment for the third dose was scheduled at the time the patient received the second dose. To remind patients of their follow-up appointments, automated phone calls or text messages (or both, depending on patient preferences) set up by the PNs through a commercial service were delivered four days, one day, and two hours before the appointment.
If a patient missed an appointment, a PN phoned her the next day to reschedule. If a patient could not be reached by phone, alternative contacts were called when available. Patients who could not be reached by these methods were sent physical letters by mail and, finally, a notice by email to contact UTMB to reschedule the appointment. Patients who missed ≥5 appointments or could no longer be reached were considered inactive. Patients who informed a PN that they were not willing to complete the vaccine series were no longer contacted or tracked.
For billing purposes, UTMB personnel obtained information to determine if the patient had current coverage for vaccinations through Medicaid or another insurance provider. If not, CPRIT paid the costs of all vaccines. Women were excluded from this evaluation if they 1) were minors whose parents did not sign the vaccination consent form, or 2) did not receive the vaccine postpartum due to hospital error. With approval from the UTMB Institutional Review Board, records were reviewed to evaluate the project.
Statistical Analysis
Bivariate analyses compared characteristics between patients who initiated the HPV vaccine postpartum with those who declined. In addition, adjusted multivariable logistic regression analysis was conducted to examine correlates of HPV vaccine initiation and completion among all enrolled patients, series completion among postpartum initiators and series completion within 6 months of postpartum initiation (the timeframe recommended by the CDC).22 We only included characteristics that had an association with the outcome with a p value <0.2 value in initial analyses of the associations. Age at first sexual intercourse and number of lifetime sexual partners were not included in the multivariable model due to excessive missing data. We also examined associations with missing an appointment for follow-up vaccine doses using adjusted logistic regression analysis. Statistical tests were based on 2-tail and a p value <0.05 was considered as statistically significant. All statistical analyses were conducted using Stata Statistical Software (Stata 14, College Station, Texas, USA).
Results
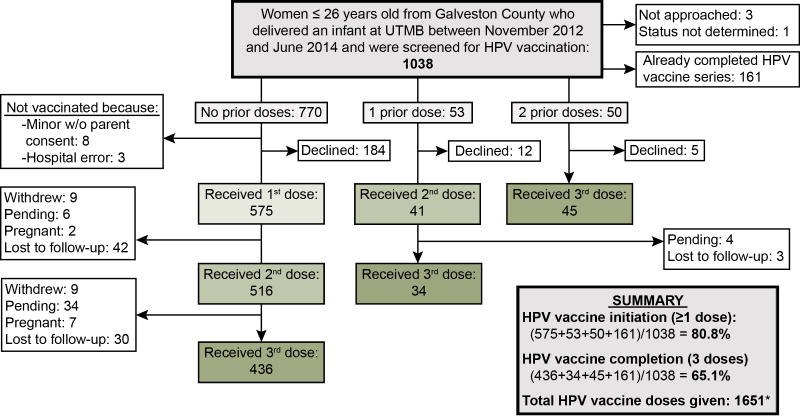
From November 1, 2012 through June 30, 2014, 1,038 eligible patients ≤26 years of age who delivered an infant at UTMB were screened and those eligible were invited to participate in this project (Figure 1). Among them, 264 (25.4%) had previously initiated the vaccine and 161 (15.5%) had completed the 3-dose series prior to this pregnancy. Of the 877 patients eligible for 1 or more HPV vaccine doses, 575 (65.6%) received the first dose postpartum (postpartum initiators) and 86 (9.8%) received their second or third dose postpartum. Among the previously unvaccinated patients, 184 declined (21.0%) vaccination. Of 11 other women who did not receive the vaccine, 8 were minors whose parents did not sign the vaccination consent form and 3 were not approached due to hospital error. Overall, 75.8% (436/575) of new initiators completed the series by April 2015.
Figure 1.
Patient participation. Flow chart showing number of patients approached and number of doses given.
*Four women received a 4th dose in error as we could not confirm their HPV vaccination status prior to injection. These four doses are included.
UTMB, University of Texas Medical Branch; w/o, without.
Among those screened on the postpartum unit, only 25.4% had previously received any HPV vaccines. At the time we conducted this assessment, 80.8% of patients had received at least 1 vaccine, and completion rates rose from 15.5% at baseline to 65.1% as a result of this program. Among new initiators who completed the series (n=436), 265 (60.8%) patients completed the series within 6 months as recommended by the CDC. The average time between the first and second dose was 2.3±2.1 months and the average time between the second and third dose was 5.6±2.5 months. A total of 1,651 vaccine doses were provided for the 575 new initiators and 103 incompletely vaccinated patients who participated in this program. Almost 59% of doses were funded by Medicaid, 36% by CPRIT, and 5% by private insurance. CPRIT funded 2%, 42% and 78% of the first, second, and third doses, respectively.
When asked their reason for declining the HPV vaccine postpartum, 111 of the 184 previously unvaccinated patients who refused vaccination stated that they were not interested in receiving the vaccine (60.3%). It is unknown whether these patients were referring to the postpartum period only or at any time. Other reasons for declining included wanting to discuss it with their primary physician (12.5%), a belief that they did not need it because they were married (7.6%), opposition to this vaccine by the patient or family member (6.0%), and fear that the injection would be too painful (3.8%).
Patients who were postpartum vaccine initiators were more likely to be Hispanic and to have received the influenza vaccine during this pregnancy compared to decliners (Table 1). Age at first sexual intercourse, history of prior sexually transmitted disease, number of lifetime sexual partners, history of tobacco use, obstetric complications, delivery method, neonatal intensive care unit (NICU) admission, and breastfeeding did not differ between postpartum initiators and decliners.
Table 1.
Socio-demographic characteristics of patients eligible to participate in the postpartum HPV vaccination program, and who had not received at least 1 HPV vaccine
| Characteristics | Enrolled in program, initiated vaccination (n=575) | Declined to participate (n=184) | P value |
|---|---|---|---|
| Age (years) | n(%) | .688 | |
| 14–20 | 130 (22.6) | 39 (21.2) | |
| 21–26 | 445 (77.4) | 145 (78.8) | |
| Race/ethnicity | <.001 | ||
| Non-Hispanic white | 141 (24.5) | 62 (33.7) | |
| Non-Hispanic Black | 120 (20.9) | 43 (23.4) | |
| Hispanic | 307 (53.4) | 66 (35.9) | |
| Non-Hispanic others | 7 (1.2) | 13 (7.1) | |
| Gravidity | .459 | ||
| 1 | 195 (34.0) | 68 (37.0) | |
| 2 or more | 379 (66.0) | 116 (63.0) | |
| Age at 1st sexual intercourse | .087 | ||
| ≤14 years old | 91 (15.8) | 29 (15.8) | |
| ≥15 years old | 328 (57.0) | 90 (48.9) | |
| Unknown | 156 (27.1) | 65 (35.3) | |
| Number of lifetime sexual partners | .160 | ||
| 1 | 94 (16.4) | 24 (13.0) | |
| 2–5 | 194 (33.8) | 54 (29.4) | |
| 6 or more | 101 (17.6) | 30 (16.3) | |
| Unknown | 186 (32.4) | 76 (41.3) | |
| STD history | .173 | ||
| No | 313 (58.8) | 105 (64.8) | |
| Yes | 219 (41.2) | 57 (35.2) | |
| History of tobacco use | .478 | ||
| No | 429 (76.5) | 136 (79.1) | |
| Yes | 132 (23.5) | 36 (20.9) | |
| History of marijuana use | .699 | ||
| No | 503 (90.8) | 156 (91.8) | |
| Yes | 51 (9.2) | 14 (8.2) | |
| History of alcohol use | .046 | ||
| No | 491 (88.2) | 159 (93.5) | |
| Yes | 66 (11.9) | 11 (6.5) | |
| Received influenza vaccine during this pregnancy | <.001 | ||
| No | 241 (42.7) | 105 (60.3) | |
| Yes | 324 (57.4) | 69 (39.7) | |
| Received any other vaccine postpartum | .238 | ||
| No | 376 (65.4) | 129 (70.1) | |
| Yes | 199 (34.6) | 55 (29.9) | |
| Obstetric complicationa | |||
| No | 376 (65.4) | 133 (72.3) | .083 |
| Yes | 199 (34.6) | 51 (27.7) | |
| Cesarean delivery | |||
| No | 406 (70.6) | 124 (67.4) | .408 |
| Yes | 169 (39.4) | 60 (32.6) | |
| Infant admitted to NICU | |||
| No | 505 (86.8) | 160 (87.0) | .755 |
| Yes | 70 (12.2) | 24 (13.0) | |
| Intent to breastfeed | |||
| No | 92 (16.1) | 23 (12.6) | .243 |
| Yesb | 478 (83.9) | 160 (87.4) |
Boldface indicates significant results.
Had any of the following complications recorded in the electronic medical records by a physician during this pregnancy: pregnancy induced hypertension, chronic hypertension, diabetes mellitus, chorioamnionitis, endometritis, preterm labor, preterm delivery, premature rupture of membranes (PROM), preterm PROM, postpartum hemorrhage, and oligohydramnios
Includes women who used both bottle and breast milk HPV, human papillomavirus; NICU, neonatal intensive care unit; STD, sexually transmitted disease.
We also examined factors associated with postpartum vaccine initiation, completion among all program participants, completion among postpartum initiators, and timely completion among postpartum initiators (Table 2). After adjusting for possible confounders, Hispanic women were more likely to receive a vaccine postpartum and complete the vaccine series compared to non-Hispanic white patients. Vaccine series completion within 6 months among postpartum initiators was also higher among Hispanic patients. Uptake of the influenza vaccine during pregnancy was associated with postpartum HPV vaccine receipt and completion. Older women were more likely to complete but not initiate the vaccine series than girls and younger women. Older postpartum initiators were more likely to complete the series and more likely to complete within 6 months than girls and younger women. Patients with 2 or more previous pregnancies were less likely to complete the series and complete the series within 6 months compared to those reporting a first pregnancy.
Table 2.
Correlates of HPV vaccine initiation, completion, completion among initiators and completion within 6 months of initiation among 14–26–year-old postpartum patients
| Vaccine administration, entire sample (≥ 1 dose) (n=759) | Vaccine completion, entire sample (3-dose) (n=759) | Vaccine completion among initiators (n=575) | Vaccine completion within 6 months among initiators (n=575) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age (years) | ||||||||
| 14–20 | Reference | Reference | Reference | Reference | ||||
| 21–26 | 1.04 (0.64–1.68) | .872 | 1.62 (1.08–2.42) | .019 | 2.25 (1.34–3.77) | .002 | 2.30 (1.44–3.66) | <.001 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | Reference | Reference | Reference | Reference | ||||
| Non-Hispanic black | 1.24 (0.73–2.11) | .431 | 1.41 (0.88–2.59) | .150 | 1.44 (0.77–2.69) | .255 | 0.90 (0.52–1.57) | .716 |
| Hispanic | 2.33 (1.46–3.72) | <.001 | 2.08 (1.39–3.10) | <.001 | 1.57 (0.93–2.66) | .093 | 1.63 (1.03–2.57) | .037 |
| Gravidity | ||||||||
| 1 | Reference | Reference | Reference | Reference | ||||
| 2 or more | 0.93 (0.60–1.43) | .732 | 0.72 (0.50–1.05) | .087 | 0.59 (0.35–0.97) | .039 | 0.59 (0.39–0.89) | .012 |
| Received influenza vaccine during this pregnancy | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 2.11 (1.43–3.12) | <.001 | 1.82 (1.32–2.52) | <.001 | 1.36 (0.89–2.09) | .155 | 1.03 (0.71–1.49) | .868 |
| Received any other vaccine postpartum | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.50 (0.98–2.29) | .061 | 0.99 (0.70–1.40) | .960 | 0.70 (0.45–1.08) | .106 | 0.79 (0.54–1.15) | .223 |
| History of tobacco use | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.69 (0.41–1.18) | .178 | 0.77 (0.50–1.21) | .257 | 0.88 (0.49–1.57) | .659 | 0.92 (0.56–1.50) | .727 |
| History of alcohol use | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.58 (0.27–1.25) | .164 | 0.84 (0.47–1.48) | .544 | 1.16 (0.58–2.34) | .676 | 1.30 (0.71–2.38) | .401 |
| STD history | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.29 (0.84–1.96) | .241 | 0.98 (0.69–1.39) | .925 | 0.74 (0.47–1.16) | .190 | 0.92 (0.63–1.36) | .682 |
| Received automated text message | -a | -a | ||||||
| No | Reference | Reference | ||||||
| Yes | 1.23 (0.73–2.08) | .434 | 1.10 (0.70–1.73) | .671 | ||||
| Received automated phone call | -a | -a | ||||||
| No | Reference | Reference | ||||||
| Yes | 0.89 (0.47–1.68) | .722 | 1.12 (0.66–1.90) | .676 | ||||
Multivariable logistic regression analysis was used. Adjusted by the variables listed in this Table
These variables were not included in the two multivariable logistic regression models as some women declined the vaccine and thus did not receive automated phone call or text message reminders.
HPV, human papillomavirus; STD, sexually transmitted disease.
A higher proportion of those that did not miss follow-up appointments completed the vaccine series compared to those that missed at least 1 appointment (87.9% vs. 70.2%, p<0.001). Among 575 postpartum HPV vaccine initiators, 182 (31.7%) attended all appointments while 393 (68.3%) missed at least one follow-up appointment. Further analysis of missed appointment data showed that non-Hispanic blacks and patients with two or more prior pregnancies were more likely to miss one or more follow-up appointments (Table 3). Women ≥21 years of age and those who received automated text messages were less likely to miss an appointment.
Table 3.
Correlates of missing one or more appointment for follow-up vaccine doses
| Characteristics | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Age (years) | ||
| 14–20 | Reference | |
| 21–26 | 0.51 (0.31–0.85) | .010 |
| Race/ethnicity | ||
| Non-Hispanic white | Reference | |
| Non-Hispanic black | 2.00 (1.07–3.76) | .031 |
| Hispanic | 0.91 (0.56–1.47) | .698 |
| Gravidity | ||
| 1 | Reference | |
| 2 or more | 2.00 (1.30–3.07) | .002 |
| STD history | ||
| No | Reference | |
| Yes | 1.04 (0.69–1.58) | .853 |
| History of tobacco use | ||
| No | Reference | |
| Yes | 1.13 (0.66–1.92) | .659 |
| History of alcohol use | ||
| No | Reference | |
| Yes | 0.74 (0.39–1.43) | .376 |
| Received influenza vaccine during this pregnancy | ||
| No | Reference | |
| Yes | 0.85 (0.57–1.26) | .414 |
| Received any other vaccine postpartum | ||
| No | Reference | |
| Yes | 1.11 (0.74–1.66) | .623 |
| Received automated phone call reminders | ||
| No | Reference | |
| Yes | 0.95 (0.54–1.65) | .843 |
| Received automated text message reminders | ||
| No | Reference | |
| Yes | 0.42 (0.25–0.72) | .002 |
Boldface indicates significant results.
STD, sexually transmitted disease
Automated phone calls and text messages were received by 87.0% and 78.8% of patients, respectively, prior to any appointments. The median number of times PNs called a patient due to missed appointments was 4 (interquartile range 1–8). Overall, 12.7% of women in the project received letters and 6.1% received emails because they missed appointments and could not be reached by phone. Of the 72 patients that initiated the HPV vaccine postpartum and were lost to follow-up, 34 patients moved out of Galveston County (5.9% of vaccine initiators), 29 patients (5.0%) changed their telephone number or address and could not be found, and 9 (1.6%) were lost to follow-up for other reasons.
Comment
Among women residing in the southern portion of the U.S., cervical cancer diagnoses and mortality rates continue to exceed those of the rest of the country.25 Women with low incomes and minority backgrounds are at highest risk of this deadly, HPV-linked disease.26,27 In fact, those living below the poverty line are 3 times more likely to contract a high-risk strain of HPV than women who are not poor.27 Thus, it is critical to develop and implement effective interventions to increase HPV vaccine initiation and completion rates among low-income women, including those from minority backgrounds.
Ideally, the HPV vaccine should be administered at 11–12 years of age as it is most effective if given prior to the onset of sexual activity and vaccination at ≤15 years of age results in a higher antibody levels than vaccination of older patients. 28 However, it still provides some benefit if given at a later age as it is unlikely that the patient would have contracted all the HPV types for which the vaccine provides protection. In fact, a study of 3,276 sexually active 18–26-year-old women found that only 9% tested positive for 1 or more of the 4 HPV types targeted by the quadrivalent HPV vaccine and none tested positive for all 4 types.29 The American College of Obstetricians and Gynecologists states that HPV DNA testing is not recommended before vaccine administration and that eligible women should be offered the vaccine regardless of prior exposure to HPV as infection with all vaccine types is unlikely.30 Furthermore, administering the vaccine to women who are already positive for one or more HPV types has not been demonstrated to result in harm to the patient. Therefore, we did not examine patients’ histories of abnormal Pap tests or HPV tests before administering the vaccines.
HPV vaccination rates among low-income, postpartum patients from minority backgrounds are very low in the southeastern region of Texas. In our population, only 25.4% had received at least one dose and only 15.5% of patients eligible for this vaccine had already received all 3 doses. Through this program, UTMB offered HPV vaccination postpartum free of charge which increased the percentage of women who received at least 1 dose to 80.8%. This exceeds national averages for both 13–17 year olds and 18–26 year olds. Moreover, Hispanic patients accepted the vaccine twice as often as white, non-Hispanic women. Many of those patients were likely new immigrants to the United States who may not have had the opportunity to be vaccinated at a young age. For example, in 2012 we found that 14% of pregnant women attending UTMB prenatal clinics had moved to the United States in the prior five years.12 Thus, postpartum vaccination may offer a way to identify and vaccinate this hard-to-reach population of new immigrants who are at increased risk of cervical cancer.31
It is likely that this program’s success was due to the fact that multiple barriers were addressed. One major barrier among those over age 18 is the high cost of the vaccine as this age group is no longer eligible for free vaccines through the Vaccines for Children program. By the time all 3 doses are administered, the HPV vaccine is the most expensive routine vaccine in the United States.15 The high cost of this vaccine has been shown to be a particular concern to Hispanics,32 who comprise a large part of UTMB’s patient population. A postpartum vaccination program solves part of this issue as pregnant low-income patients often have Medicaid coverage, which remains active for 8 weeks after delivery, and will cover HPV vaccination. This allowed time to administer the first 2 injections. In this program, the majority of all injections were covered by Medicaid. Due to the fact that CPRIT funds were required for 36% of injections, primarily for the third injection, it is important to identify how this would be funded in other settings. States other than Texas may offer Medicaid for a longer period of time postpartum, and this may be one source of payment. Compensation may also be available through the Affordable Care Act. Such coverage is currently available for those who purchase insurance through the exchange or apply for insurance subsidies.
Our program also addressed other barriers, such as lack of access to a site that provides vaccination. Impoverished women typically only access emergency care, where vaccines are rarely administered, with limited participation in preventive care where the majority of vaccines are given. Problems with access may be solved by offering the vaccine where individuals are already receiving care. Hospitalization immediately following delivery is one such opportunity, as most women stay in the hospital at least 24 hours. Moreover, most women have extensive contact with health care personnel during pregnancy, 14 creating multiple opportunities for providers to discuss the HPV vaccine. Discussing the vaccine at prenatal appointments may have had an impact on the rates of acceptance in our program, as physician recommendation has been shown to be a strong predictor HPV vaccine acceptance.33 In addition, women may be more receptive during the postpartum period to messages about cervical cancer prevention, as a serious illness would interfere with their ability to care for their child.
Another problem with HPV vaccination, particularly among young women, is a low rate of vaccine series completion.34 In our postpartum vaccination program, however, most patients received all 3 doses within the CDC’s recommended time frame of 6 months. We achieved this by timing HPV vaccine injections with existing doctor visits for the mother or her infant. Following delivery, women typically have at least one postpartum visit and frequently visit their child’s pediatrician over the next 12 months. If the HPV vaccine is administered postpartum, these visits can be used to complete the series. This requires coordination with other providers, but is feasible in settings where multiple specialties and clinic locations see patients within the same health system.
Our program differs considerably from a study by Wright et al carried out among postpartum women in another region of the United States.19 First, we evaluated the results of a program in which patients were offered the HPV vaccine as part of standard care. We employed patient navigators as well as text reminders and coordinated vaccination with pediatric appointments for the infants of the mothers. Wright et al’s program used phone and mail reminders and scheduled vaccine-only visits for the third vaccine doses, which can be difficult for new mothers to add to their schedule when they are caring for children or returning to work.19
Moreover, nearly 80% of patients who initiated the HPV vaccine while on the postpartum ward in our program received text message reminders about their follow-up appointments for the second and third vaccine dose. This reminder service may partially explain why we achieved a high percentage of vaccine completion among this group (75.8%) as patient reminder and recalls systems have been shown to improve immunization rates.35 Another reason our team may have achieved high completion rates was the availability of counseling and navigation services in English and Spanish. Many patients at UTMB are Spanish-speaking and availability of services in a preferred language has been shown to improve the timeliness with which Spanish-speaking patients acquire cancer care.36 Finally, personalized phone calls by the PNs likely improved completion rates as this has been shown to be the most effective reminder method for improving immunization rates.35 It should be noted that the amount of effort to recall patients represents a significant time commitment that would not normally be spent in a typical healthcare setting; however, programs that utilize PNs may be warranted when they substantially increase adherence to preventive care.
HPV vaccine initiation could be improved in the United States through implementation of similar programs at other institutions. Series completion is more difficult, but it could also be improved by coordinating vaccination appointments for postpartum women with pediatricians. This could be facilitated by the PNs. Currently, PNs are available primarily for cancer patients. Funding of the patient navigators thus remains a barrier to implementation of this program in many hospitals.
Some barriers remained, including the challenge of completing the relatively lengthy HPV-vaccine series among patients who moved outside of Galveston County or changed their telephone number. Additionally, we also found that patients who had 2 or more previous pregnancies were less likely to complete the series or complete it within the CDC recommended timeframe. This suggests that intense family care duties may be a barrier for some women.
Overall, this project markedly improved HPV vaccination rates among patients from Galveston County who delivered an infant at UTMB. This supports offering the HPV vaccine postpartum and coordinating follow-up doses with postpartum and well-child visits as an effective strategy for catch-up HPV vaccination among low-income and Hispanic patients.
Acknowledgments
Financial Support: Support for this vaccination program and evaluation was provided by a prevention grant from the Cancer Prevention & Research Institute of Texas (PP120150, awarded to ABB); the Institute for Translational Sciences at the University of Texas Medical Branch, though a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH); and the Building Interdisciplinary Research Careers in Women’s Health Program (K12HD052023, awarded to ABB) from the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Office of the Director (OD) of the NIH.
Kimberly S. Carlson, RN, Maria D. Garcia, MS, Margarita Morgado, MA, and Didi Rivas assisted with data collection (UTMB). Karry K. McCarty, BS, assisted with data management (UTMB). Susan Y. Rojahn, PhD, assisted with manuscript preparation (UTMB).
Footnotes
Disclosure statement: The authors report no conflict of interest.
Presentation: The findings of this program evaluation were presented at the 30th International Papillomavirus Conference & Clinical and Public Health Workshops (HPV 2015), Lisbon, Portugal - September 17–21, 2015.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of CPRIT, NIAID, NICHD, OD, or the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monk BJ, Tewari KS. The spectrum and clinical sequelae of human papillomavirus infection. Gynecol Oncol. 2007;107(2 suppl 1):S6–13. doi: 10.1016/j.ygyno.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 2.Drolet M, Benard E, Boily M, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(5):565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks R, Copas A, Wilkinson D, Robinson A. Uptake of the HPV vaccination programme in England: a cross-sectional survey of young women attending sexual health services. BMJ. 2014;90:315–321. doi: 10.1136/sextrans-2013-051179. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh K, Pollock K, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. British J of Cancer. 2014;110:2804–2811. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canfell K, Egger S, Velentzis L, et al. Factors related to vaccine uptake by young adult women in the catch-up phase of the National HPV Vaccination Program in Australia: Results from an observational study. Vaccine. 2015;33(20):2387–2394. doi: 10.1016/j.vaccine.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman M, Laz T, Berenson A. Geographic variation in human papillomavirus vaccination uptake among young adult women in the United States during 2008–2010. Vaccine. 2013;47:5495–5499. doi: 10.1016/j.vaccine.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams W, Lu P, O’Halloran A, et al. Vaccination coverage among adults, excluding Influenza vaccination – United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;64(04):95–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez A, Solomon D, Herrero R, et al. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. Am J Epi. 2013;178(5):752–760. doi: 10.1093/aje/kwt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz L, Dunne E, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Imunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63(RR05):1–30. [Google Scholar]
- 11.Heyman K, Worley M, Frey M, Kessler R, Bodurka D, Slomovitz B. Willingness of pregnant women to vaccinate themselves and their newborns with the HPV vaccine. Vaccine. 2011;29(28):4618–4622. doi: 10.1016/j.vaccine.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 12.Berenson A, Male E, Lee T, et al. Assessing the need for and acceptability of a free-of-charge postpartum HPV vaccination program. Am J Obstet Gynecol. 2014;210(3):213–217. doi: 10.1016/j.ajog.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams SH, MJP, CEI Adolescent and Young Adult Preventive Care: Comparing National Survey Rates. Am J Prev Med. 2015;49(2):238–247. doi: 10.1016/j.amepre.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Hamilton B, Sutton P, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58(24):1–85. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. [Accessed September 1, 2015];CDC Vaccine Price List. http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
- 16.Harper D, Verdenius I, Harris G, et al. The influence of free quadrivalent human papillomavirus vaccine (HPV4) on the timely completion of the three dose series. Prev Med. 2014;61:20–25. doi: 10.1016/j.ypmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Healy C, Rench M, Castagnini L, Baker C. Pertussis immunization in a high-risk postpartum population. Vaccine. 2009;27(41):5599–5602. doi: 10.1016/j.vaccine.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Stringer M, Ratcliffe S, Gross R. Acceptance of hepatitis B vaccination by pregnant adolescents. MCN Am J Matern Child Nurs. 2006;31(1):54–60. doi: 10.1097/00005721-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Wright J, Govindappagari S, Pawar N, et al. Acceptance and compliance with postpartum human papillomavirus vaccination. Obstet Gynecol. 2012;120(4):771–782. doi: 10.1097/AOG.0b013e31826afb56. [DOI] [PubMed] [Google Scholar]
- 20.Davlin S, Berenson A, Rahman M. Correlates of HPV knowledge among low-income minority mothers with a child 9–17 years of age. J Pediatr Adolesc Gynecol. 2015;28(1):19–23. doi: 10.1016/j.jpag.2014.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Accessed September 1, 2015];Genital HPV Infection - CDC Fact Sheet. http://www.cdc.gov/std/hpv/hpv-factsheet-march-2014.pdf.
- 22.Centers for Disease Control and Prevention. [Accessed September 1, 2015];HPV Vaccine Gardasil What You Need to Know. http://www.cdc.gov/vaccines/hcp/vis/vis-statements/hpv-gardasil.pdf.
- 23.Centers for Disease Control and Prevention. [Accessed September 1, 2015];HPV Vaccines for Boys and Girls. http://www.cdc.gov/vaccines/vpd-vac/hpv/downloads/dis-hpv-color-office.pdf.
- 24.Berenson A, Rahman M, Hirth J, Rupp R, Sarpong K. A brief educational intervention increases providers' human papillomavirus vaccine knowledge. Hum Vaccin Immunother. 2015;11(6):1331–1336. doi: 10.1080/21645515.2015.1022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(10 Suppl):2855–2864. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 26.Singh G, Miller B, Hankey B, Edwards B. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 27.Kahn J, Lan D, Kahn R. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol. 2007;110(1):87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 28.Block S, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey A, Gebremariam A, Koutsky L, Manhart L. Using risk factors to predict human papillomavirus infection: implications for targeted vaccination strategies in young adult women. Vaccine. 2008;26(8):1111–1117. doi: 10.1016/j.vaccine.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 30.The American College of Obstetricians and Gynecologists. Human Papillomavirus Vaccination. Obstet Gynecol. 2015;126:e38–43. [Google Scholar]
- 31.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 32.Downs L, Scarinci I, Einstein M, Collins Y, Flowers L. Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol Oncol. 2010;117(3):486–490. doi: 10.1016/j.ygyno.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal S, Weiss T, Zimet G, Ma L, Good M, Vichnin M. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician's recommendation. Vaccine. 2011;29(5):890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 34.Richards M, Peters M, Sheeder J. Human Papillomavirus Vaccine: Continuation, Completion and Missed Opportunities. J Pediatr Adolesc Gynecol. 2015 doi: 10.1016/j.jpag.2015.08.003. In press. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson Vann J, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;3:CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charlot M, Santana M, Chen C, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477–1483. doi: 10.1002/cncr.29221. [DOI] [PMC free article] [PubMed] [Google Scholar]