Abstract
Background
Although metabolic surgery was originally performed to treat hypercholesterolemia, the effects of contemporary bariatric surgery on serum lipids have not been systematically characterized.
Methods and Results
MEDLINE, EMBASE and Cochrane databases were searched for studies with ≥20 obese adults undergoing bariatric surgery [Roux-en-Y Gastric Bypass (RYGBP), Adjustable Gastric Banding, Bilio-Pancreatic Diversion (BPD), or Sleeve Gastrectomy]. The primary outcome was change in lipids from baseline to one-year after surgery.
The search yielded 178 studies with 25,189 subjects (pre-operative BMI 45.5±4.8kg/m2) and 47,779 patient-years of follow-up. In patients undergoing any bariatric surgery, compared to baseline, there were significant reductions in total cholesterol (TC; -28.5mg/dL), low density lipoprotein cholesterol (LDL-C; -22.0mg/dL), triglycerides (-61.6mg/dL) and a significant increase in high density lipoprotein cholesterol (6.9mg/dL) at one year (P<0.00001 for all). The magnitude of this change was significantly greater than that seen in non-surgical control patients (eg LDL-C; -22.0mg/dL vs -4.3mg/dL). When assessed separately, the magnitude of changes varied greatly by surgical type (Pinteraction<0.00001; eg LDL-C: BPD -42.5mg/dL, RYGBP -24.7mg/dL, Adjustable Gastric Banding -8.8mg/dL, Sleeve Gastrectomy -7.9mg/dL). In the cases of Adjustable Gastric Banding (TC and LDL-C) and Sleeve Gastrectomy (LDL-C), the response at one year following surgery was not significantly different from non-surgical control patients.
Conclusions
Contemporary bariatric surgical techniques produce significant improvements in serum lipids, but changes vary widely, likely due to anatomic alterations unique to each procedure. These differences may be relevant in deciding the most appropriate technique for a given patient.
Keywords: bariatric surgery, cholesterol, lipids, meta-analysis, obesity
Introduction
The original impetus for the development of metabolic surgery was the treatment of hypercholesterolemia. 2015 marked the fiftieth anniversary of Buchwald’s report of partial-ileal bypass as an effective intervention for treating hypercholesterolemia,1 and the twenty-fifth anniversary of the publication of the initial results of the randomized trial testing the procedure’s efficacy – Program On the Surgical Control of the Hyperlipidemias (POSCH).2 This procedure achieved marked reduction of cholesterol absorption and, correspondingly, serum cholesterol in the pre-statin era,3 eventually resulting in reduced cardiovascular death in patients with history of myocardial infarction.2
Bariatric surgery has since evolved to four dominant procedures (Bilio-pancreatic Diversion (BPD), Roux-en-Y Gastric Bypass (RYGBP), Adjustable Gastric Banding, Sleeve Gastrectomy), ranging from largely malabsorptive to completely restrictive, regarded as the most effective therapies for treating obesity. The procedures have shown significant beneficial effects beyond weight reduction, including resolution of dyslipidemia.4 Although thousands of reports of contemporary bariatric surgical techniques have been published, changes in serum lipids beyond the period of early, rapid weight loss are not well characterized. With this meta-analysis, we sought to describe the effects of contemporary bariatric surgical procedures on serum lipids of obese patients at one year and more after surgery.
Methods
Data Sources and Searches
Following PRISMA guidelines, we searched PubMed/MEDLINE, EMBASE and Cochrane databases from inception through April, 2015. With a trained librarian, we created comprehensive searches for the concepts of bariatric surgery and lipid profiles (eTable 1), without language, publication date, or study design limitations.
Study Selection
Studies 1) involving ≥20 obese adults undergoing RYGBP, Adjustable Gastric Banding, Sleeve Gastrectomy, or BPD; 2) reporting data on lipid profile at baseline and follow-up; and 3) reporting follow-up for at least one year, were included. Studies including non-obese subjects or combining data from subjects undergoing disparate or novel procedures were excluded.
Data Extraction and Quality Assessment
Using standardized data forms, two authors (SH, AP) assessed the full text of initially non-excluded articles to determine if average total cholesterol (TC), Low Density Lipoprotein-cholesterol (LDL-C), triglycerides, and/or High Density Lipoprotein-cholesterol (HDL-C) values, and measures of variance, were presented for subjects at baseline and at least one year following surgery. Average lipid values lacking variance were not included in analyses. If subject duplication was discovered, the report containing the largest number of subjects was included.
Outcomes
Our principal summary measure was difference in mean lipid value (TC, LDL-C, triglycerides, HDL-C) of the sample before and after (one year and last reported follow-up value) bariatric surgery.
Data Synthesis and Statistical Analysis
Using RevMan version 5.3 (Copenhagen), we performed random effects modeling of mean differences in a factorial fashion by lipid component and surgical technique/control subjects not undergoing surgery.5 Data was analyzed at one year and longest reported follow-up beyond one year. Heterogeneity was assessed using I2 statistics.6 Funnel plots were visually assessed for suggestion of publication bias.
Our primary analysis was a comparison of mean difference in lipid values from baseline at one year in patients undergoing bariatric surgery. Our secondary analyses included comparisons of mean change of each lipid parameter in 1) the surgical cohort and non-surgical controls at one year and beyond one year, and 2) the four individual contemporary bariatric surgical techniques at one year and beyond one year.
Sensitivity analyses included assessments of mean change in lipid parameters 1) by procedure, stratified by study design (randomized, prospective, retrospective); and 2) in studies excluding versus those not excluding the use of lipid-lowering medications. Differences in subgroups were assessed using a test for interaction with p-value<0.05 considered significant.
Results
Of 2,908 articles identified from our database search, 180 articles (178 separate studies - 11 randomized trials, 93 prospective observational studies, 74 retrospective analyses) met inclusion criteria (Figure 1).
Figure 1.
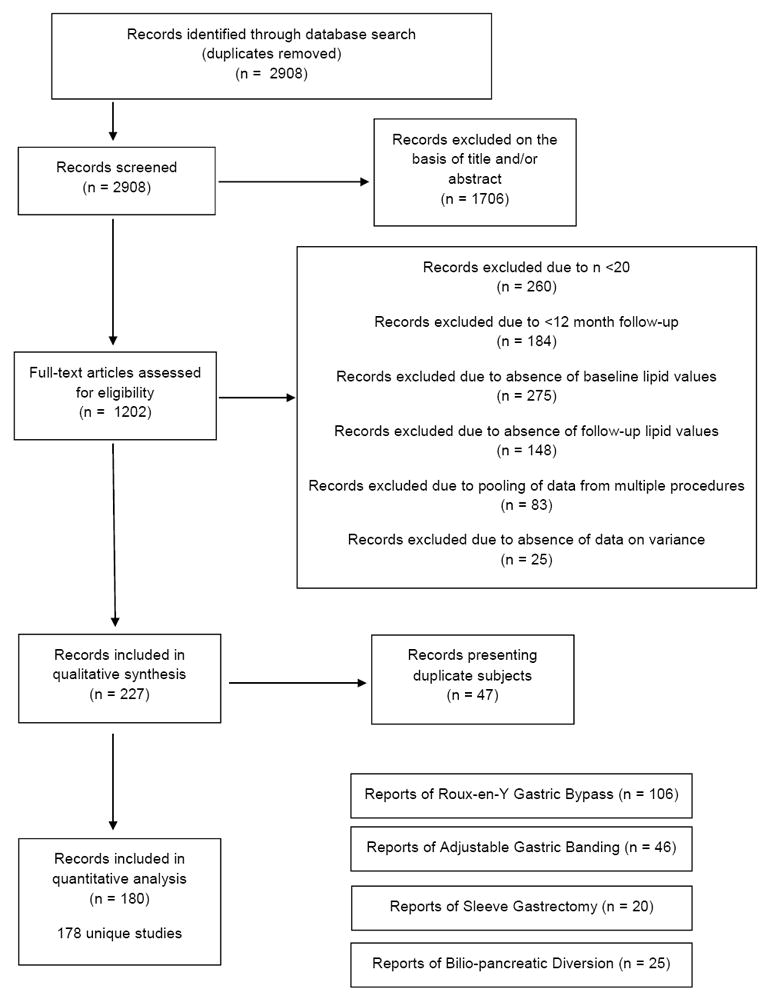
PRISMA flowchart of study selection
The 178 studies reported on 25,189 patients with 47,779 patient-years of follow-up. At the time of surgery, subjects were 40.7±4.8 years and BMI was 45.5±4.8kg/m2. Mean follow-up across all studies was 27.9 months. Eighty-eight studies reported data beyond one year (mean 47.5 months). eTable 2 presents composite characteristics of subjects described in reports of each surgical procedure. eTable 3 lists each study included in the meta-analysis.
Total Cholesterol
In surgical subjects, there was a significant reduction in mean TC at one year compared with baseline (p<0.00001, Figure 2), and in comparison to non-surgical obese control subjects (p<0.00001, eFigure 1a). There were considerable differences in the magnitude of reduction by surgical procedure (pinteraction<0.00001), with overall non-significant changes following Sleeve Gastrectomy (p=0.11). Although significantly lower than baseline, reductions observed following Adjustable Gastric Banding did not differ from those of control subjects at one year. Changes in TC between all subjects undergoing bariatric surgery and non-surgical controls were not statistically different beyond one year (p=0.08, eFigure 1b), although when assessed independently, RYGBP and BPD produced significantly greater reductions than controls (eFigure 2).
Figure 2.
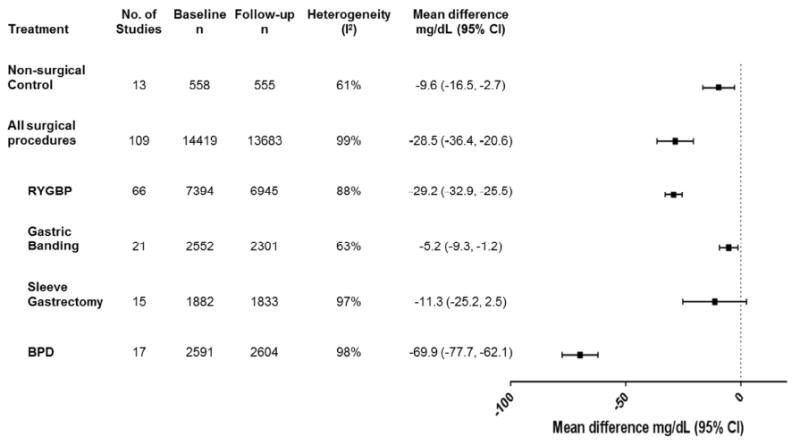
Changes in total cholesterol (mg/dL) from baseline to one year in obese subjects with or without bariatric surgery.
BPD = Bilio-pancreatic diversion, CI = confidence interval, RYGBP = Roux-en-Y gastric bypass
LDL-Cholesterol
In the surgical cohort, there was a significant reduction in mean LDL-C at one year compared to baseline (p<0.00001, Figure 3), whereas the non-surgical cohort did not experience significant changes in mean LDL-C at one year or more after baseline sampling (eFigures 3a & 3b). The degree of change in mean LDL-C at one year from baseline varied considerably by procedure (pinteraction<0.00001; Figure 3a), with non-significant reductions following Sleeve Gastrectomy (p=0.13). Further, changes at both one year and beyond one year after Adjustable Gastric Banding and Sleeve Gastrectomy did not differ from those of non-surgical controls. At last follow-up beyond one year, each procedure, except for BPD, was associated with more modest changes in LDL-C (eFigure 4), and the change in the entire surgical cohort was not significantly different from non-surgical controls (p=0.21, eFigure 3b).
Figure 3.
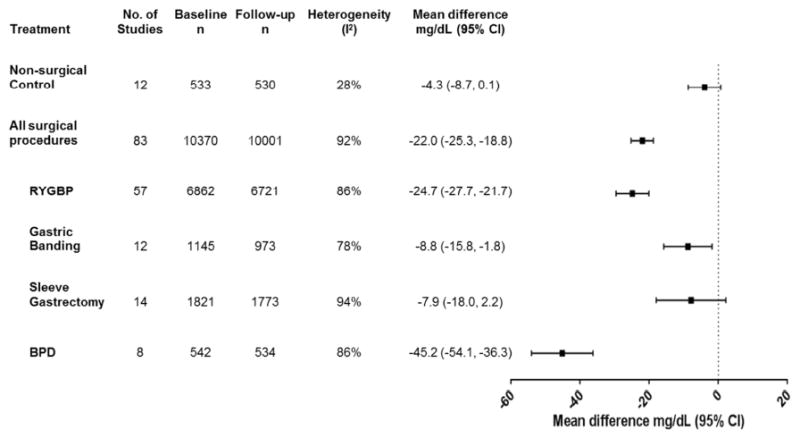
Changes in LDL-cholesterol (mg/dL) from baseline to one year in obese subjects with or without bariatric surgery.
BPD = Bilio-pancreatic diversion, CI = confidence interval, RYGBP = Roux-en-Y gastric bypass
Triglycerides
In surgical subjects, there was a significant reduction in average triglycerides at one year compared to both baseline and to changes in non-surgical controls (p<0.00001, Figure 4), but there were sizable differences by procedure (pinteraction<0.00001). Similar reductions were observed across procedures in studies reporting data beyond one year (eFigure 5). Non-surgical control subjects also experienced significant, although relatively modest, reductions in triglycerides at one year follow–up, but changes did not reach statistical significance beyond one year (eFigure 5).
Figure 4.
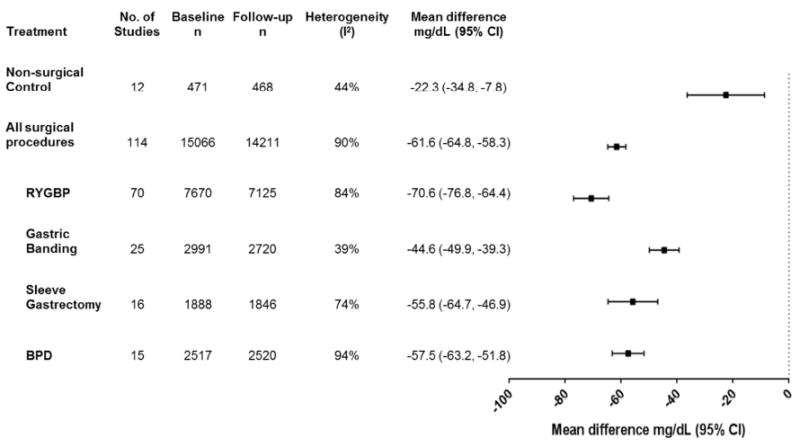
Changes in triglycerides (mg/dL) from baseline to one year in obese subjects with or without bariatric surgery.
BPD = Bilio-pancreatic diversion, CI = confidence interval, RYGBP = Roux-en-Y gastric bypass
HDL-Cholesterol
In the surgical cohort, there was a significant increase in mean HDL-C at one year compared to baseline (p<0.00001, Figure 5), whereas there was no change in the control cohort (p=0.24). There were significant differences in magnitude of change by procedure (pinteraction<0.00001), with non-significant reductions following BPD (p=0.17). Beyond one year, all procedures were associated with greater increases in average HDL-C compared to baseline (eFigure 6), including BPD, although this procedure did not differ from non-surgical treatment.
Figure 5.
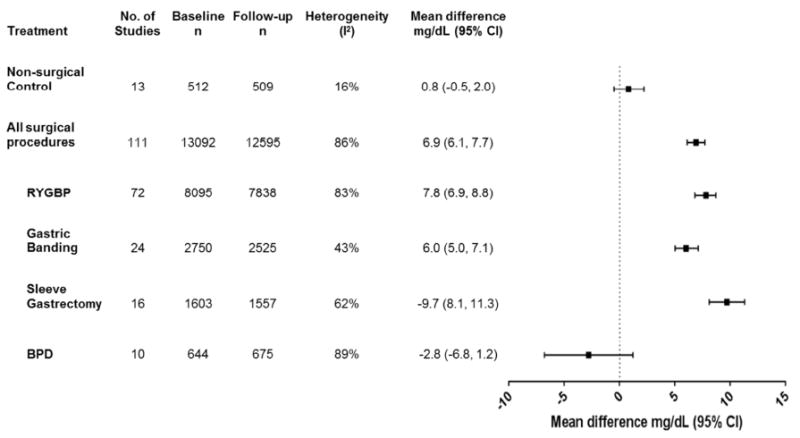
Changes in HDL-cholesterol (mg/dL) from baseline to one year in obese subjects with or without bariatric surgery.
BPD = Bilio-pancreatic diversion, CI = confidence interval, RYGBP = Roux-en-Y gastric bypass
Sensitivity analyses
Study type
Our results were largely consistent for RYGBP, Adjustable Gastric Banding and Sleeve Gastrectomy in sensitivity analyses based on study type (randomized, prospective, retrospective; eFigures 9–14). There were three exceptions: 1) reductions in TC and triglycerides at one year following RYGBP were greatest in randomized studies; 2) retrospective studies of Adjustable Gastric Banding exhibited more modest increases in HDL-C at last follow-up beyond one year than did prospective and randomized studies; and 3) prospective observational studies of Sleeve Gastrectomy reported significantly greater reductions in LDL-C at one year than did randomized and retrospective studies.
In contrast, there were several differences in lipid profile response to BPD by study type (eFigures 15-16). Retrospective studies of BPD reported greater reductions in TC and LDL-C, but more modest reductions in triglycerides and increases in HDL-C at one year, than did randomized reports. The differences between study types were similar for TC and LDL-C beyond one year.
Lipid-lowering medications
Sensitivity analyses of studies excluding versus not excluding patients taking lipid-lowering medications demonstrated persistent improvements in all lipid parameters in both groups (eFigures 17-18). However, the test for interaction (lipid-lowering medication exclusion status and changes in lipid parameter) was significant for a small number of comparisons. Studies of RYGBP excluding the use of lipid-lowering medications reported significantly greater average reductions in LDL-C than studies not excluding their use (-31.7mg/dL vs -23.8mg/dL at one year, pinteraction=0.02; eFigure 19b; -34.3mg/dL vs -17.5mg/dL, beyond one year, pinteraction=0.009; eFigure 20b). There were more modest changes in average triglycerides at one year following RYGBP in studies disallowing lipid medication use (-53.7mg/dL vs -64.7mg/dL; pinteraction=0.0009; eFigure 19c), but not beyond one year (eFigure 20c). Increases in HDL-C at one year were significantly greater following RYGBP in studies excluding lipid medications (9.0mg/dL vs 7.6mg/dL, pinteraction=0.03; eFigure 19d). There were no differences between mean changes in lipid parameters following Adjustable Gastric Banding in studies excluding or allowing lipid medications, other than a more modest increase in HDL-C at one year following Adjustable Gastric Banding in studies excluding lipid medication use (4.0mg/dL vs 6.8mg/dL, p=0.0001; eFigures 21-22).
Discussion
In this meta-analysis of 178 studies of contemporary bariatric surgical techniques enrolling over 25,000 subjects with nearly 48,000 patient-years of follow-up, we observed significant improvements in TC, LDL-C, triglycerides and HDL-C at one year following surgery. These changes were of greater magnitude than the modest changes experienced by non-surgical obese control patients, and were sustained beyond one year. However, changes in lipids from baseline differed greatly by procedure, with only RYGBP resulting in improvements in each lipid parameter relative to controls at both one year and last follow-up beyond one year. The results were consistently seen in sensitivity analyses, including studies stratified by exclusion of lipid-lowering medication use.
Dyslipidemia is the foremost cause of atherosclerotic cardiovascular disease (CVD),7 the leading cause of death worldwide,8 and obesity is an independent risk factor for both dyslipidemia and CVD.9 The dyslipidemia most commonly associated with obesity includes elevated triglycerides and small, dense LDL particles, with subnormal HDL-C. This is a manifestation of insulin resistance common in obesity which contributes to increased free fatty acid delivery to the liver, stimulating very low density lipoprotein (VLDL) production, and, in turn, increasing circulating triglycerides.10 As insulin sensitivity improves with weight loss, VLDL and triglyceride levels generally fall. While we observed reduced average triglycerides following bariatric surgery, there was significant variation between procedures in the degree of triglyceride reduction, as well as the magnitude of change of other lipid parameters. It is likely that the unique anatomical alterations and resulting digestive and endocrinologic changes occurring with each procedure explain these differences.
Contemporary bariatric surgical procedures are categorized as hybrid restrictive/malabsorptive (BPD, RYGBP) or wholly restrictive (Adjustable Gastric Banding, Sleeve Gastrectomy) procedures.11 BPD (which is most similar to Buchwald’s early partial-ileal bypass) is performed by removing the majority of the stomach and forming anastomoses between the stomach and distal jejunum, and between the proximal jejunum and ileum. In addition to reduced stomach capacity, there is marked reduction in digestive enzyme exposure time following BPD. RYGBP involves creation of a small proximal gastric pouch that is anastomosed to the jejunum. The excluded, but retained, stomach and duodenum are anastomosed to the distal jejunum. Like BPD, RYGBP produces weight loss secondary to reduced stomach capacity (restriction) and impaired nutrient absorption (malabsorption). Adjustable gastric banding involves placement of a fluid-containing band around the proximal stomach. The band is connected to a subcutaneous port that allows for injection or removal of fluid. This procedure does not alter the gastrointestinal tract other than to restrict proximal stomach size. Sleeve gastrectomy consists of the stomach volume-reducing portion (removal of the gastric fundus and most of the gastric body) of BPD. Although this procedure is considered restrictive, gastric fundus removal results in reduced gastric lipase secretion and altered gastrointestinal transit time, as well as loss of the majority of ghrelin-producing cells. The impact of the latter is minimally understood, but may play a role in improved insulin sensitivity.12
Differences in triglyceride reduction among procedures were most evident at more than one year after surgery, when reductions in subjects undergoing RYGBP and BPD were nearly 50% greater than in Adjustable Gastric Banding and Sleeve Gastrectomy, despite similar baseline BMI in RYGBP and Adjustable Gastric Banding cohorts. Part of this difference may be secondary to greater weight loss and improvements in insulin sensitivity achieved following RYGBP and BPD versus restrictive procedures.13-16 However, the anatomic alterations of RYGBP and BPD may also play important roles. While studies of restrictive procedures have demonstrated correlations of triglyceride change with reductions in body weight, adiposity or insulin resistance,17-19 the associations are much weaker in hybrid procedures.20-23 The impacts on incretin hormones, in particular the exaggerated post-prandial GLP-1 response characteristic of RYGBP and BPD, yield improvements in insulin sensitivity beyond those expected due to weight loss alone, and beyond that seen with restrictive procedures.24 Improved insulin sensitivity results in lower VLDL levels and, in turn, triglycerides, in patients undergoing RYGBP and BPD.
As described above, low HDL-C is characteristic of dyslipidemia frequently seen in obesity. RYGBP, Sleeve Gastrectomy and Adjustable Gastric Banding all resulted in increased HDL-C at one year and beyond. Despite rapid insulin sensitivity improvement and the greatest degree of weight loss associated with BPD, the procedure did not significantly change HDL-C levels at one year and increased levels only very modestly with longer term follow-up. This is likely due to the degree of fat and cholesterol malabsorption unique to this procedure.25 While increased HDL-C was infrequently achieved in studies of BPD, the return to pre-surgical levels is presumably a manifestation of improved insulin sensitivity and decreased turnover that eventually balances the reduced cholesterol absorption characteristic of BPD.
It is this fat malabsorption that likely accounts for the markedly greater reductions in LDL-C and TC following BPD versus other bariatric surgical procedures. RYGBP also results in malabsorption, although not to the degree of BPD, and this may explain the differences in changes in these parameters between RYGBP and the wholly restrictive Adjustable Gastric Banding.26, 27 We did not find significant changes in LDL-C or TC following Sleeve Gastrectomy. Although the removal of the majority of the stomach could presumably result in impaired fat absorption secondary to reduced gastric lipase secretion, if present, this was not manifest in the studies included in this analysis. To our knowledge, despite the fact that Sleeve Gastrectomy has become the most commonly performed bariatric surgical procedure over the past five years,28, 29 there are currently no published reports on cholesterol absorption in humans following Sleeve Gastrectomy.
Influence of lipid lowering medications
Our findings of significant improvements in lipid parameters at both one year and longer following surgery persisted in our sensitivity analyses comparing studies excluding and not excluding lipid-lowering medications, although we did observe greater reductions in LDL-C following RYGBP in studies in which lipid medications were excluded. Interestingly, the studies excluding subjects on lipid-lowering medications exhibited less robust reductions in triglycerides than did the remainder of studies in the meta-analysis involving RYGBP. We hypothesize that this is due to relatively fewer diabetics in these studies, given the exclusion of statin use.
Limitations
The inclusion of non-randomized prospective and retrospective studies introduces a risk for bias. Given knowledge of differing metabolic and weight loss outcomes with each procedure, non-randomized studies could be predisposed to achieve “expected” results if patients with particular comorbidities selected a certain procedure. The absence of significant differences between randomized, prospective and retrospective subgroups for nearly every outcome following RYGBP, Adjustable Gastric Banding and Sleeve Gastrectomy suggests a limited effect of bias on our overall findings. However, it should be noted that in studies reporting data beyond one year, retrospective studies tended to display the largest effect size, potentially due to loss to follow-up of subjects who had less positive outcomes, or selective reporting bias.
Further, there was significant heterogeneity among the studies included in this meta-analysis. This could partially be due to varying use of lipid-lowering medications among study participants, particularly in relation to the outcomes of LDL-C and TC. Our sensitivity analyses of studies excluding subjects not on lipid-lowering therapy support this conclusion. However, the significant improvements seen in lipid parameters in both arms of these sensitivity analyses suggest that the heterogeneity does not influence our overall findings.
Conclusions
In this meta-analysis of the effects of contemporary bariatric surgical techniques on serum lipids at least one year post-operatively, we observed significant improvements in TC, LDL-C, triglycerides and HDL-C compared to both baseline values and to changes seen in non-surgical controls, but the magnitude of changes differed greatly by procedure. These differences suggest that pre-operative lipid profile should be taken into context with other metabolic abnormalities and obesity-related comorbidies in an individual contemplating surgery. Our findings highlight the need for additional prospective studies and mechanistic research on the specific metabolic effects of different bariatric surgical techniques, particularly Sleeve Gastrectomy, the most common, but least studied contemporary bariatric procedure.
Clinical Significance.
Contemporary bariatric surgery improves lipid parameters at one year following surgery compared to baseline and non-surgical controls
Changes in total cholesterol and LDL-cholesterol do not differ from those of non-surgical controls at last follow-up beyond one year
Changes in all major serum lipid parameters differ greatly by procedure type
There is little data on the effects on lipid profile of the currently most commonly performed bariatric procedure – sleeve gastrectomy
Acknowledgments
We thank Coen van Solingen and Eugenia Gianos for assistance in translation of non-English articles.
Funding Source: This was an unfunded study.
Dr. Heffron was supported in part by the T32 HL 098129 and KL2 TR001446-01 training grants.
Footnotes
All authors had access to the data and contributed sufficiently to writing the manuscript to be included as authors.
Disclosures: Dr. Christine Ren-Fielding is a consultant for Apollo Endosurgery. Other authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchwald H. Lowering of cholesterol absorption and blood levels by ileal exclusion. Experimental basis and preliminary clinical report. Circulation. 1964;29:713–20. doi: 10.1161/01.cir.29.5.713. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD, Jr, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH) The New England journal of medicine. 1990;323:946–55. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Moore RB, Varco RL. Surgical treatment of hyperlipidemia. 3. Clinical status of the partial ileal bypass operation. Circulation. 1974;49:I22–37. [PubMed] [Google Scholar]
- 4.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart (British Cardiac Society) 2012;98:1763–77. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 5.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 6.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in medicine. 1988;7:889–94. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. Journal of clinical lipidology. 2014;8:473–88. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. British medical bulletin. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 9.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 10.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, Gonzalez-Campoy JM, Jones SR, Kumar R, La Forge R, Samuel VT. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. Journal of clinical lipidology. 2013;7:304–83. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Baker MT. The history and evolution of bariatric surgical procedures. The Surgical clinics of North America. 2011;91:1181–201, viii. doi: 10.1016/j.suc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. The Journal of clinical endocrinology and metabolism. 2013;98:4391–9. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 13.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. The American journal of medicine. 2008;121:885–93. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. The American journal of medicine. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Hedberg J, Sundstrom J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15:555–63. doi: 10.1111/obr.12169. [DOI] [PubMed] [Google Scholar]
- 16.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. The Cochrane database of systematic reviews. 2014;8 doi: 10.1002/14651858.CD003641.pub4. Cd003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon JB, O’Brien PE. Lipid profile in the severely obese: changes with weight loss after lap-band surgery. Obesity research. 2002;10:903–10. doi: 10.1038/oby.2002.124. [DOI] [PubMed] [Google Scholar]
- 18.Frige F, Laneri M, Veronelli A, Folli F, Paganelli M, Vedani P, Marchi M, Noe D, Ventura P, Opocher E, Pontiroli AE. Bariatric surgery in obesity: changes of glucose and lipid metabolism correlate with changes of fat mass. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2009;19:198–204. doi: 10.1016/j.numecd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Busetto L, Pisent C, Rinaldi D, Longhin PL, Segato G, De Marchi F, Foletto M, Favretti F, Lise M, Enzi G. Variation in lipid levels in morbidly obese patients operated with the LAP-BAND adjustable gastric banding system: effects of different levels of weight loss. Obes Surg. 2000;10:569–77. doi: 10.1381/096089200321594192. [DOI] [PubMed] [Google Scholar]
- 20.Pontiroli AE, Laneri M, Veronelli A, Frige F, Micheletto G, Folli F, Adami G, Scopinaro N. Biliary pancreatic diversion and laparoscopic adjustable gastric banding in morbid obesity: their long-term effects on metabolic syndrome and on cardiovascular parameters. Cardiovascular diabetology. 2009;8:37. doi: 10.1186/1475-2840-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Diaz Jde D, Lozano O, Ramos JC, Gaspar MJ, Keller J, Duce AM. Changes in lipid profile after biliopancreatic diversion. Obesity surgery. 2003;13:756–60. doi: 10.1381/096089203322509345. [DOI] [PubMed] [Google Scholar]
- 22.Gleysteen JJ, Barboriak JJ. Improvement in heart disease risk factors after gastric bypass. Archives of surgery (Chicago, Ill : 1960) 1983;118:681–4. doi: 10.1001/archsurg.1983.01390060003001. [DOI] [PubMed] [Google Scholar]
- 23.To VT, Huttl TP, Lang R, Piotrowski K, Parhofer KG. Changes in body weight, glucose homeostasis, lipid profiles, and metabolic syndrome after restrictive bariatric surgery. Exp Clin Endocrinol Diabetes. 2012;120:547–52. doi: 10.1055/s-0032-1323738. [DOI] [PubMed] [Google Scholar]
- 24.Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–11. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- 25.Scopinaro N, Marinari GM, Pretolesi F, Papadia F, Murelli F, Marini P, Adami GF. Energy and nitrogen absorption after biliopancreatic diversion. Obesity surgery. 2000;10:436–41. doi: 10.1381/096089200321594309. [DOI] [PubMed] [Google Scholar]
- 26.Pihlajamaki J, Gronlund S, Simonen M, Kakela P, Moilanen L, Paakkonen M, Pirinen E, Kolehmainen M, Karja V, Kainulainen S, Uusitupa M, Alhava E, Miettinen TA, Gylling H. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism: clinical and experimental. 2010;59:866–72. doi: 10.1016/j.metabol.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Benetti A, Del Puppo M, Crosignani A, Veronelli A, Masci E, Frige F, Micheletto G, Panizzo V, Pontiroli AE. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes care. 2013;36:1443–7. doi: 10.2337/dc12-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen NT, Nguyen B, Gebhart A, Hohmann S. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. Journal of the American College of Surgeons. 2013;216:252–7. doi: 10.1016/j.jamcollsurg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Reames BN, Finks JF, Bacal D, Carlin AM, Dimick JB. Changes in bariatric surgery procedure use in Michigan, 2006-2013. Jama. 2014;312:959–61. doi: 10.1001/jama.2014.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]


