Abstract
3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) enhances desire to socialize and feelings of empathy, which are thought to be related to increased oxytocin levels. Thus, variation in the oxytocin receptor gene (OXTR) may influence responses to the drug. Here, we examined the influence of a single OXTR nucleotide polymorphism (SNP) on responses to MDMA in humans. Based on findings that carriers of the A allele at rs53576 exhibit reduced sensitivity to oxytocin-induced social behavior, we hypothesized that these individuals would show reduced subjective responses to MDMA, including sociability. In this three-session, double blind, within-subjects study, healthy volunteers with past MDMA experience (N = 68) received a MDMA (0, 0.75 mg/kg, and 1.5 mg/kg) and provided self-report ratings of sociability, anxiety, and drug effects. These responses were examined in relation to rs53576. MDMA (1.5 mg/kg) did not increase sociability in individuals with the A/A genotype as it did in G allele carriers. The genotypic groups did not differ in responses at the lower MDMA dose, or in cardiovascular or other subjective responses. These findings are consistent with the idea that MDMA-induced sociability is mediated by oxytocin, and that variation in the oxytocin receptor gene may influence responses to the drug.
Keywords: MDMA, oxytocin, OXTR, social behavior
Introduction
3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) is an amphetamine analogue used recreationally in social contexts. It is widely known for its ability to enhance mood and distinctly social feelings, such as empathy and a sense of connection with others (Bravo, 2001; Sumnall, Cole, & Jerome, 2006; Ter Bogt & Engels, 2005). The “prosocial” effects of MDMA appear to contribute not only to its recreational use and abuse potential (McGregor, Callaghan, & Hunt, 2009; Ter Bogt & Engels, 2005), but may also contribute to its promise as an adjunct in psychotherapy (Doblin, 2002; Mithoefer, Wagner, Mithoefer, Jerome, & Doblin, 2011; Mithoefer et al., 2013). The mechanism by which MDMA produces these uniquely prosocial effects is not yet entirely understood.
Like other amphetamine derivatives, MDMA gives rise to its primary behavioral effects through release of dopamine and norepinephrine. However, unlike other prototypical “stimulant” drugs, MDMA also acts on 5HT-1A receptors in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus, leading to an increase in oxytocin levels in the brain and periphery (Han & Gu, 2006; Thompson, Callaghan, Hunt, Cornish, & McGregor, 2007; Verrico, Miller, & Madras, 2006). Oxytocin is a neuropeptide involved in affiliative behavior and bonding. It is synthesized in the SON and PVN and released during positive social interactions and physical contact with others (Grewen, Girdler, Amico, & Light, 2005). Exogenous administration of oxytocin in humans produces prosocial effects such as reduced responses to threat and stress (Norman et al., 2011) and increased trust and positive communication (Ditzen et al., 2009; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005). Interestingly, MDMA induces oxytocin release in rodents, and the prosocial effects of MDMA can be blocked by oxytocin receptor antagonists (Thompson et al., 2007; Thompson, Hunt, & McGregor, 2009). Similarly, in humans, MDMA increases plasma oxytocin levels (Hysek, Domes, & Liechti, 2012; Hysek et al., 2013; Kirkpatrick, Francis, Lee, de Wit, & Jacob, 2014a), and these increases were correlated with enhanced sociability (Dumont et al., 2009). Other studies, however, have suggested that the empathogenic effects of MDMA are unrelated to changes in peripheral oxytocin (Kuypers et al., 2014). The role of oxytocin in producing the prosocial effects of MDMA is still unclear.
Additional insight into the potential mechanisms of the prosocial effects of MDMA has come from neuroimaging, psychophysiological, and behavioral studies. One study showed that MDMA (1.5 mg/kg) reduces amygdala responses to angry faces, while enhancing ventral striatal responses to socially rewarding, happy faces (Bedi, Phan, Angstadt, & de Wit, 2009). These findings are in line with studies showing that MDMA enhances emotional responses to and ability to identify positive facial expressions, and impairs identification of negative ones (Hysek et al., 2012, 2013; Kirkpatrick, Lee, Wardle, Jacob, & de Wit, 2014b; Wardle & de Wit, 2014). The role of oxytocinergic signaling in producing these effects is not yet known.
Several single nucleotide polymorphisms (SNPs) in the oxytocin receptor gene (OXTR) have been associated with differences in socio-emotional behavior and social processing. Here, we focus on rs53576, in intron 3 of the OXTR gene. Individuals homozygous for the G/G allele at this locus are more empathic and report more positive emotions and greater sociability (Bakermans-Kranenburg & van Ijzendoorn, 2008; Lucht et al., 2009; Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Tost et al., 2010). By contrast, carriers of the A allele at the rs53756 polymorphism show deficits in social processing (Kim et al., 2010; Rodrigues et al., 2009), self-report greater loneliness (Lucht et al., 2009), and are judged by others to be less social (Kogan et al., 2011), although there is some evidence to suggest the effects of this polymorphism on social behavior may be overestimated (Jostins, Pickrell, MacArthur, & Barrett, 2012). In addition to influencing social behavior, this allele affects individual responses to intranasal oxytocin. One recent study showed that the effects of oxytocin on preferences for infant faces are only observed in individuals homozygous for the G allele (Marsh et al., 2012). Another study showed that the G allele is associated with increased sensitivity to the emotion-recognition- enhancing effects of intranasal oxytocin (Chen et al., 2015). Although it has been argued that the effects the effects of OXTR genotype may have been overestimated, this literature nevertheless suggests that rs53576 influences social processing, and may also affect sensitivity to exogenously administered oxytocin.
Despite the evidence above, there have been no investigations of how this SNP contributes to individual differences in responses to prosocial drugs. The aim of this study is to examine the influence of rs53576 on subjective responses to MDMA. Here, healthy adults with prior MDMA experience received placebo and single oral doses of the drug (0.75 mg/kg and 1.5 mg/kg) under double blind conditions. They rated their subjective or mood responses, and cardiovascular measures were obtained. Based on the evidence that MDMA’s prosocial effects are mediated by oxytocin, and the known involvement of this SNP in sensitivity to oxytocin, we hypothesized that A/A individuals would experience reduced prosocial drug effects compared to individuals carrying the G allele.
Methods and materials
Study design
For this analysis, we combined data from two similar studies examining behavioral and subjective effects of MDMA using within-subjects, placebo-controlled designs (Kirkpatrick et al., 2014b; Wardle & de Wit, 2014). Healthy, occasional MDMA users attended three sessions, separated by at least 5 days, during which they received placebo, 0.75 mg/kg, or 1.5 mg/kg MDMA under double blind conditions. At all sessions, measures of subjective and cardiovascular responses were assessed over 5 h. Subjects provided saliva samples for genetic analysis during the first session.
Participants
Healthy adults (39 male and 29 female), ages 18–35 years, were recruited through flyers, online advertisements, and word of mouth referrals. They underwent psychiatric and medical screening, including a physical examination, electrocardiogram, modified structured clinical interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1996), and provided self-reported drug and health histories. Inclusion criteria were 4–40 times self-reported ecstasy use with no adverse responses; high school education; English fluency; body mass index between 19 and 30. Exclusion criteria were regular medication except oral contraceptives; medical conditions contraindicating MDMA; past year DSM-IV Axis I diagnosis; lifetime history of substance dependence; women who were pregnant or planning a pregnancy, or use of more than 25 cigarettes per week.
Procedure
Participants first attended an orientation session in which they provided informed consent and were acquainted with laboratory procedures and study protocol. They were instructed to consume normal amounts of caffeine and nicotine the morning of each session, and to fast for 2 h prior to the session. They were also asked to refrain from alcohol and over-the-counter drugs for 24 h before and 12 h after the session, marijuana for seven days before and 24 h after the 2 A. K. BERSHAD ET AL. Downloaded by [University of Chicago Library] at 10:35 21 March 2016 session, and from all other recreational drugs for 48 h before and 24 h after the session. Compliance was verified using breath (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA). To minimize the influence of hormones on drug responses, female participants not using hormonal contraceptives were scheduled during the follicular phase of their menstrual cycles (White, Justice, & de Wit, 2002). Women were tested for pregnancy at the beginning of each session. Participants were told that the purpose of the study was to investigate individual differences in drug responses, and that during the session they might receive a stimulant (e.g., amphetamine or ecstasy), a sedative (e.g., Valium), hallucinogen (e.g., LSD), cannabinoid (e.g., marijuana), or a placebo. All procedures were carried out in accordance with the Declaration of Helsinki and approved by the University of Chicago Institutional Review Board.
Sessions were conducted from 9:00 am to 2:00 pm. Upon arrival, participants provided breath and urine samples for drug and pregnancy testing and consumed a light breakfast to standardize drug absorption. At 9:15 am, they completed baseline measures of subjective and cardiovascular effects, and at 9:30 am ingested a capsule containing MDMA powder (0.75 and 1.5 mg/kg, maximum dose of 125 mg, with lactose filler) or placebo (lactose only). Capsules were prepared for each participant, based on body weight, in 00 opaque capsules by the University of Chicago Hospitals Investigational Pharmacy. Between scheduled measures, participants relaxed, read or watched a neutral movie (e.g., no thrillers). They were not allowed to work or study. At 10:00 am and every subsequent 30–60 min, subjective and cardiovascular effects were assessed. At 2:00 pm participants completed an end of session questionnaire and were discharged, provided their subjective and cardiovascular measures had returned to baseline.
Measures
Subjective responses
Subjective effects were assessed using visual analogue scales ranging from 1 to 100 (not at all – extremely). Participants rated the degree to which they felt “anxious,” “restless,” “dizzy,” “lonely,” “sedated,” “sociable,” “confident,” “loving,” “playful,” and “friendly,” which are adjectives that were used to assess MDMA effects in previous studies (Kirkpatrick et al., 2014a; Wardle & de Wit, 2014; Wardle, Kirkpatrick, & de Wit, 2014). They also completed a drug effects questionnaire (DEQ; Fischman & Foltin, 1991) in which they rated on the same scale the degree to which they “feel high,” “feel drug,” “like drug,” “dislike drug,” and “want more.” At the end of each session, participants were asked which drug they thought they received; a stimulant (e.g., amphetamine or ecstasy), sedative (e.g., valium), cannabinoid (e.g., marijuana), hallucinogen (e.g., LSD), or placebo.
Cardiovascular measures
Blood pressure and heart rate were measured every 30 min throughout the sessions using portable monitors (Life Source, A&D Company, Tokyo, Japan).
Genotyping
DNA samples were genotyped at oxytocin receptor SNP rs53576 (A/G) using the ABI TaqMan Assay and a Step One PCR machine in accordance with the manufacturer’s instructions (Applied Biosystems, Foster City, CA).
Data analysis
To obtain a summary measure for the subjective effects of MDMA across the session, we calculated area-under-the-curve (AUC) for each of the VAS measures using the trapezoidal method. To reduce the data into factors appropriate for analysis with genotype, we calculated change-from-placebo scores for each MDMA dose (0.75 and 1.5 mg/kg), and conducted single sample t-tests on the resulting scores. The drug had no effect on “sedation” or “loneliness” so these were excluded from further analysis.
The change scores were entered into principal components analyses (promax rotation, eigenvalue = 1), with separate analyses conducted for each active dose. Outcome measures that either: (1) did not load greater than 0.5 on any single factor, or (2) cross-loaded on multiple factors (i.e., measures that loaded greater than 0.5 on more than one factor) were removed and the remaining outcome measures were subjected to further principal component analyses. For each dose, this process was repeated until all remaining outcome measures loaded greater than 0.5 on a single factor. This process resulted in four distinct factors, which we named sociability, anxiety, euphoria, and dizziness (Table1). Following principal components analyses, we calculated final factor scores using the average AUC values for outcome measures that loaded on the same factor at each active dose.
Table 1.
Factor loadings from the principal component analysis (PCA) of subjective effect measures.
| MDMA dose | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0.75 mg/kg | 1.5 mg/kg | |||||||
| Euphoria | Sociability | Anxiety | Dizziness | Euphoria | Sociability | Anxiety | Dizziness | |
| VAS scale | ||||||||
| High | 0.80 | 0.86 | ||||||
| Feel | 0.82 | 0.82 | ||||||
| Like | 0.86 | 0.78 | ||||||
| More | 0.81 | 0.69 | ||||||
| Friendly | 0.81 | 0.95 | ||||||
| Sociable | 0.89 | 0.83 | ||||||
| Confident | 0.86 | 0.80 | ||||||
| Playful | 0.88 | 0.79 | ||||||
| Loving | 0.63 | 0.79 | ||||||
| Restless | 0.98 | 0.79 | ||||||
| Anxious | 0.74 | 0.85 | ||||||
| Dizzy | 0.94 | 0.97 | ||||||
We grouped our participants into three groups by genotype at the OXTR SNP rs53576. These groups were then compared on the four derived subjective effect factor scores (i.e., euphoria, anxiety, dizziness, and sociability) using a two-way repeated measures ANOVA with a within-subjects factor (MDMA change from placebo score for 0.75 and 1.5 mg/kg) and a between-subjects factor (OXTR genotype). Significant interactions were followed-up with post hoc comparisons using Bonferroni corrections at each dose level. Using the same data analysis strategy, we also examined the relationship between genotype and MDMA-related cardiovascular effects. For all analyses, p values were considered statistically significant at less than 0.05.
Results
Participant characteristics
Twenty-eight participants possessed the G/G genotype, 30 A/G, and 10 A/A. This distribution did not deviate from Hardy–Weinberg equilibrium (χ2 = 0.18, p = 0.67). No differences in age, gender distribution, education, BMI, or drug use history were observed among the genotype groups (Table 2). All participants were Caucasian, primarily in their twenties (M = 23.8 years, SD = 4.1), with some college education (M = 14.7 years, SD = 1.3), and moderate recreational drug use. The participants had consumed MDMA recreationally an average of 14 times before (SD = 11.0).
Table 2.
Demographics and substance use characteristics.
| A/A (N = 10) |
A/G (N = 30) | G/G (N = 28) | |
|---|---|---|---|
| Demographic variables n (%) or M (SD) | |||
| Sex (male/female) | 3/7 | 20/10 | 16/12 |
| Race (Caucasian) | 10 (100%) | 30 (100%) | 28 (100%) |
| Age | 24.8 (4.5) | 23.9 (4.0) | 23.4 (3.8) |
| BMI | 22.5 (2.3) | 23.0 (2.8) | 22.9 (2.4) |
| Education in years | 14.8 (1.7) | 14.9 (1.5) | 14.5 (1.0) |
| Substance use | |||
| Typical alcoholic drinks/week | 7.8 (8.0) | 9.3 (8.0) | 10.6 (8.4) |
| Cigarettes/week | 2.2 (4.9) | 2.8 (5.0) | 1.2 (2.2) |
| MDMA (total number of times used) | 14.3 (12.0) | 13.7 (11.3) | 14.7 (10.6) |
| Baseline measures | |||
| Sociability | 46.5 (13.8) | 40.0 (14.5) | 44.8 (17.9) |
| Euphoria | 0.51(0.7) | 2.4 (6.2) | 2.8 (5.3) |
| Anxiety | 17.5 (14.7) | 13.6 (10.9) | 16.7 (15.8) |
| Dizziness | 4.5 (5.5) | 4.2 (6.3) | 6.5 (11.6) |
To verify that baseline measures of anxiety and sociability did not differ among the three genotypes, we averaged precapsule measures of euphoria, anxiety, sociability, and dizziness across the three sessions and conducted between-subjects ANOVAs on these measures. We observed no significant differences among genotypes on these baseline measures (F(2, 66) = 0.71, F(2, 66) = 0.48, F(2, 66) = 0.93, F(2, 66) = 0.59).
Drug identifications
The participants were asked which drug they thought they had received. Some data were missing due to experimenter error. On the placebo session, 35/64 correctly identified placebo (55%). The rest believed they had received a sedative (e.g., Valium; 17/64), stimulant (e.g., MDMA; 3/64), cannabinoid (e.g., marijuana; 8/64), or hallucinogen (e.g., LSD; 1/64). On the 0.75 mg/kg session, 39 correctly identified the drug (59%). The rest believed they had received placebo (5/66), a sedative (18/66), a cannabinoid (1/66), or a hallucinogen (3/66). On the 1.5 mg/kg session 47 correctly identified the drug (76%). The rest thought they received placebo (3/62), a sedative (5/62), a hallucinogen (5/62), or a cannabinoid (2/62).
Effects of MDMA
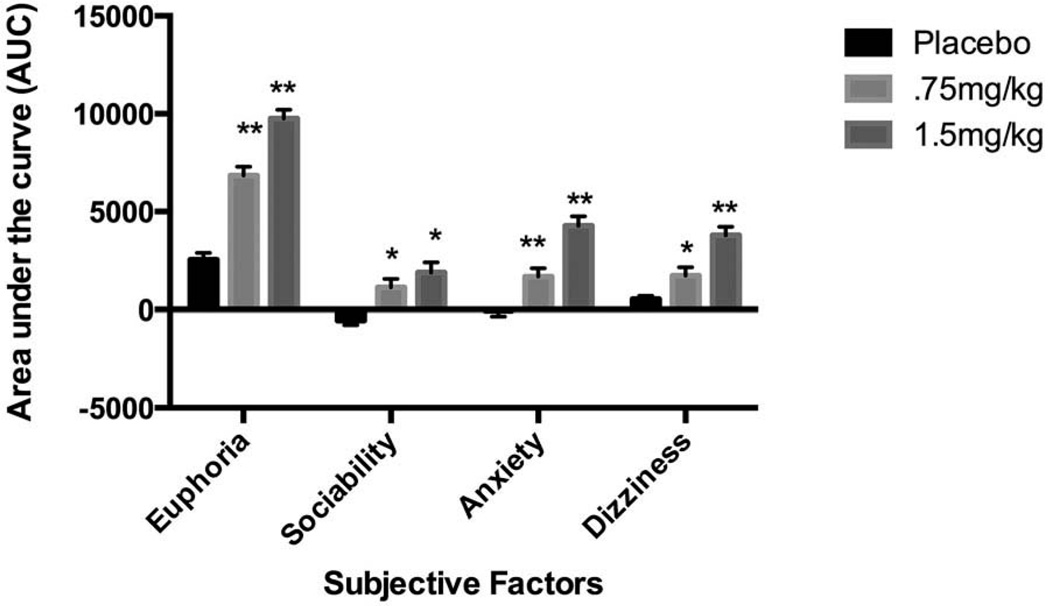
Both MDMA doses increased ratings of subjective measures of sociability (main effect of dose F(2, 66) = 8.10, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.01, 0 vs. 1.5 mg/kg, p < 0.01), anxiety (dose F(2, 66) = 28.84, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.01, 0 vs. 1.5 mg/kg, p < 0.001), euphoria (dose F(2, 66) = 80.21, p < .001; 0 vs. 0.75 mg/kg, p < 0.001, 0 vs. 1.5 mg/kg, p < 0.001), and dizziness (dose F(2, 66) = 21.34, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.05, 0 vs. 1.5 mg/kg, p < 0.001) (Figure 1). Additionally, both doses of the drug increased heart rate (dose F(2, 66) = 52.0, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.001, 0 vs. 1.5 mg/kg, p < 0.001), systolic blood pressure (dose F(2, 66) = 51.4, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.001, 0 vs. 1.5 mg/kg, p < 0.001), and diastolic blood pressure (dose F(2, 66) = 26.9, p < 0.001; 0 vs. 0.75 mg/kg, p < 0.01, 0 vs. 1.5 mg/kg, p < 0.001).
Figure 1.
Subjective responses to single oral doses of MDMA (0.75 and 1.5 mg/kg) and placebo on four factors summarizing the effects of the drug. The subjective factors were derived from a principal component analysis of drug-related visual analogue scales, and AUC was calculated using the change from precapsule. Bars depict mean ± SEM. * indicates significant difference from placebo, p < 0.05; ** indicates significant difference from placebo, p < 0.01.
Effects of genotype on responses to MDMA
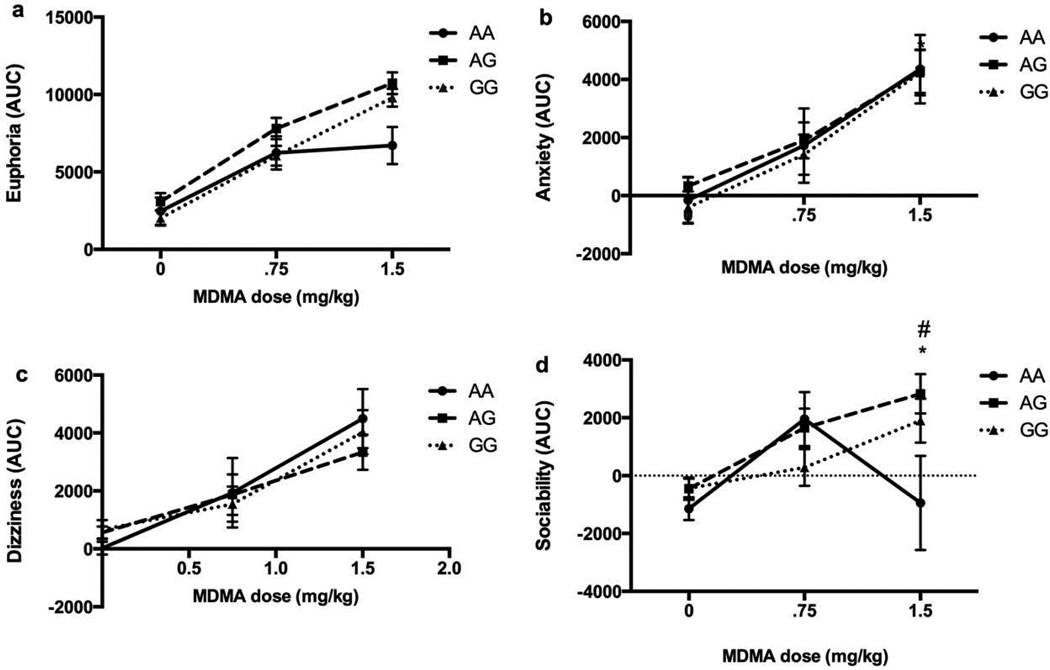
MDMA differentially affected ratings of sociability across the genotypic groups (Sociability; Dose*Genotype F(2, 65) = 2.81, p = 0.03). Individuals possessing the A/A genotype reported no significant increase in feelings of sociability at the higher dose of 1.5 mg/kg, while individuals carrying the G allele reported increased sociability at 1.5 mg/kg MDMA. There was a trend toward differential ratings of euphoria across genotype groups, but the interaction did not reach significance (Euphoria; Dose*Genotype F(2, 66) = 2.20, p = 0.07). Individuals with the A/A genotype also reported smaller increases in euphoria compared to those with the G allele at the same 1.5 mg/kg dose (Figure 2). The genotypic groups did not differ in MDMA-induced dizziness, anxiety, or cardiovascular effects.
Figure 2.
Mean ratings of euphoria, anxiety, dizziness, and sociability after MDMA or placebo, by genotype (AA: N = 10; AG: N = 30, GG: N = 28). AUC was calculated using the change from precapsule. Bars depict mean ± SEM. * indicates significant difference between A/A and A/G; # indicates significant difference between A/A and G/G, p < 0.05.
Discussion
Here we report that a variation in OXTR genotype affects subjective responses to MDMA in healthy adults. MDMA (0.75 and 1.5 mg/kg) produced expected increases in feelings of sociability, euphoria, anxiety, and dizziness, as well as heart rate and blood pressure in a sample of 68 healthy young adults. Consistent with the hypothesis that oxytocin mediates some of the prosocial effects of MDMA, individuals with the A/A genotype at rs53576 reported lower subjective ratings of sociability and euphoria after the higher dose compared to individuals carrying the G allele at this locus. The differences were not evident at the lower dose, and the differences were not observed on other measures of the drug’s effects (e.g., blood pressure and self-reported dizziness). The association between this polymorphism and certain psychosocial responses to MDMA supports the hypothesis that some of the prosocial effects of the drug are mediated through release of oxytocin.
Several previous studies have found that A allele carriers exhibit differences in social behavior, consistent with our results showing reduced sociability in response to the prosocial drug MDMA. A allele carriers are less sensitive to social cues (Rodrigues et al., 2009; Tops, van IJzendoorn, Riem, Boksem, & Bakermans-Kranenburg, 2011), less trusting (Krueger et al., 2012), and judged by others to be less social (Kogan et al., 2011). In our study, we observed no differences in baseline self-reported sociability, as has been previously shown with sensitive social processing tasks. However, the genotype influenced MDMA-induced sociability at the higher dose of the drug. Interestingly, MDMA has been shown to increase plasma oxytocin levels in humans, which may contribute to its prosocial effects, but only at higher doses (Kirkpatrick et al., 2014a). It is possible that our measure of sociability was not sensitive enough to detect subtle baseline differences, and that the high dose of the drug magnified potential differences in sociability via an oxytocinergic mechanism.
Our observed genetically based individual differences in sensitivity to acute doses of MDMA may contribute to variability in reactions to the drug in recreational settings as well as variability in its possible psychotherapeutic effects. The prosocial effects of MDMA are believed to contribute to its use and abuse (Sumnall et al., 2006; Ter Bogt & Engels, 2005). These findings suggest that carriers of the G allele at this locus in the OXTR may be particularly susceptible to these prosocial effects, and thus to repeated use of the drug. In this study, we limited our sample to MDMA users who reported using the drugs between 4 and 40 times, and a sample with a greater range of MDMA use would be required to address this question. Beyond differences in susceptibility to recreational use, individuals may differ in the benefit derived from MDMA in therapeutic settings. MDMA has long been considered a promising potential adjunct to psychotherapy due to its prosocial effects (Greer & Tolbert, 1986). The drug, when administered therapeutically at 75–250 mg (within the range of doses used in this study), may improve the therapeutic relationship by increasing trust (Grinspoon & Bakalar, 1986; Riedlinger & Montagne, 2001) and reduce the negative feelings of fear during difficult treatment protocols, such as exposure therapy for posttraumatic stress disorder (PTSD) (Doblin, 2002; Mithoefer et al., 2011, 2013). Some of these effects of MDMA may be mediated through the release of oxytocin, and so OXTR variation may also contribute to individual differences in the therapeutic efficacy of the drug. While one recent study found that A/A individuals may be less sensitive to social ostracism (McQuaid, McInnis, Matheson, & Anisman, 2015), they may also be less likely to derive psychological benefits from social support in general. These individuals are less likely to seek social support (Kim et al., 2010), and for A/A homozygotes, social support during stress does not reduce cortisol responses (Chen et al., 2011). More work is needed to determine whether and how these genetic differences impact the beneficial effects of the MDMA when used therapeutically.
There are some limitations to the present study. First, the overall sample size was small. Moreover, the A/A genotype group had the smallest N and also reported the lowest MDMA effect on sociability and euphoria. It has been suggested that the failure to observe drug effects in rare, homozygote genotype groups is indicative of insufficient power rather than an actual lack of drug effect (Hart, de Wit, & Palmer, 2013). Therefore, it may be the case that the lower ratings of sociability and euphoria in the A/A genotype group are due to the size of the group. However, this is less likely given that other subjective drug effects with comparable effect sizes (dizziness, anxiety) did not differ in the A/A group. Still, future studies may benefit from using prospective genotyping to replicate the current findings in larger, more balanced genotype groups.
Another limitation is that because this study was a first-look at MDMA effects and OXTR genotype interactions, we did not explore potential interactions between genotype and the timecourse of MDMA effects. We did not have a specific hypotheses regarding differential timecourse effects across genotypes, and in an effort to minimize the number of hypotheses tested, we chose to conduct analyses on the summary measure of area under the curve scores. The use of summary variables here, such as area under the curve scores and PCA factor scores, also carries an increased risk of type I errors. The results presented here should be considered preliminary and should be replicated in future studies with larger sample sizes, in which timecourse effects could also be more specifically addressed.
The sample of individuals tested in this study was comprised of healthy adults with a very specific MDMA use history (4–40 times) and no psychiatric symptomatology. It will be important for future studies to examine the relationship between OXTR variation and subjective MDMA responses across a wider range of MDMA users, as inclusion of heavier MDMA users could help determine whether the G allele on the rs53756 SNP is associated with increased use and/or abuse of the drug. Finally, the current findings suggest that individuals may differ in their sensitivity to MDMA’s therapeutic effects. This possibility can be further explored by examining the genotypes of patients who vary in their responses to MDMA-augmented treatment for various psychiatric disorders, including PTSD.
In conclusion, individuals with the A/A genotype on this OXTR SNP reported less sociability and euphoria in response to 1.5 mg/kg MDMA compared to G allele carriers. The genotypic groups did not differ in response to the lower dose of the drug, or to any other drug effects. These findings provide additional evidence that oxytocin mediates the distinct social effects of MDMA. Together, these findings suggest that genetic variation in OXTR may influence social responses to MDMA, which can influence both the recreational use and therapeutic efficacy of the drug.
Acknowledgments
The authors would like to thank Celina Joos, Charles Frye, Jon Solamillo, and Aoibhin Curran for help with data collection, and the University of Chicago Investigational Pharmacy service for preparing the drug capsules. Additionally, the authors are grateful to Katharina Domschke and Abraham Palmer for assistance with genotyping.
Funding
This work was supported by grants from the National Institute on Drug Abuse [grant numbers R01 DA002182 and R21 DA026570] to H. de Wit. Anya K. Bershad was supported by a grant from the National Institute of General Medical Sciences [2T32GM007281].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207(1):73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo GL. What does MDMA feel like? In: Holland J, editor. Ecstasy: The complete guide: A comprehensive look at the risks and benefits of MDMA. Rochester, VT: Park Street Press; 2001. [Google Scholar]
- Chen FS, Kumsta R, Dvorak F, Domes G, Yim OS, Ebstein RP, Heinrichs M. Genetic modulation of oxytocin sensitivity: A pharmacogenetic approach. Translational Psychiatry. 2015;5(10):e664. doi: 10.1038/tp.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Doblin R. A clinical plan for MDMA (Ecstasy) in the treatment of posttraumatic stress disorder (PTSD): Partnering with the FDA. Journal of Psychoactive Drugs. 2002;34(2):185–194. doi: 10.1080/02791072.2002.10399952. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, Sweep FCGJ, van der Steen R, Hermsen R, Donders ART, Touw DJ, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Social Neuroscience. 2009;4(4):359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders. New York, NY: Biometrics Research Department; 1996. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective effects measurements in assessing abuse liability of drugs in humans. British Journal of Addiction. 1991;86(12):1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. Journal of Psychoactive Drugs. 1986;18(4):319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Can drugs be used to enhance the psychotherapeutic process. American Journal of Psychotherapy. 1986;40(3):393–404. doi: 10.1176/appi.psychotherapy.1986.40.3.393. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: Failure to replicate. Neuropsychopharmacology. 2013;38(5):802–816. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012;222(2):293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst161. nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, Pickrell JK, MacArthur DG, Barrett JC. Misuse of hierarchical linear models overstates the significance of a reported association between OXTR and prosociality. Proceedings of the National Academy of Sciences. 2012;109(18):E1048–E1048. doi: 10.1073/pnas.1202539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences. 2010;107(36):15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology. 2014a;46:23–31. doi: 10.1016/j.psyneuen.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and Intranasal Oxytocin on Social and Emotional Processing. Neuropsychopharmacology. 2014b;39(7):1654–1663. doi: 10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rodrigues Saturn S. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition’. Proceedings of the National Academy of Sciences. 2011;108(48):19189–19192. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krueger F, Parasuraman R, Iyengar V, Thornburg M, Weel J, Lin M, Lipsky R. Oxytocin receptor genetic variation promotes human trust behavior. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers KP, Torre Fornell RDL, Farré Albadalejo M, Lahoz Y, Dziobek I, Van Den Bos W, Ramaekers JG. No evidence that MDMA-induced enhancement of emotional empathy is related to peripheral oxytocin levels or 5-HT1a receptor activation. PLoS ONE. 2014;9(6):e100719. doi: 10.1371/journal.pone.0100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Kroemer H. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(5):860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Henry HY, Pine DS, Gorodetsky EK, Goldman D, Blair R. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224(4):469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor I, Callaghan P, Hunt G. From ultrasocial to antisocial: A role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British Journal of Pharmacology. 2009;154(2):358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, Anisman H. Distress of ostracism: Oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsu166. nsu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer M, Wagner M, Mithoefer A, Jerome L, Martin S, Yazar-Klosinski B. Durability of improvement in PTSD symptoms and absence of harmful effects or drug dependency after MDMA-assisted psychotherapy: A prospective long-term follow-up study. Journal of Psychopharmacology (Oxford, England) 2013;27(1):28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of±3, 4-methylenedioxymethamphetamine- assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology. 2011;25(4):439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, DeVries AC, Berntson GG. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology. 2011;25(10):1313–1319. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedlinger J, Montagne M. Using MDMA in the treatment of depression. In: Holland J, editor. Ecstasy: The complete guide. Rochester, VT: Park Street Press; 2001. [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: An exploration of the subjective experiences of ecstasy. Journal of Psychopharmacology. 2006;20(5):670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Ter Bogt TF, Engels RCME. “Partying” hard: Party style, motives for and effects of MDMA use at rave parties. Substance Use & Misuse. 2005;40(9–10):1479–1502. doi: 10.1081/JA-200066822. [DOI] [PubMed] [Google Scholar]
- Thompson M, Callaghan P, Hunt G, Cornish J, McGregor I. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3, 4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146(2):509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson M, Hunt G, McGregor I. Neural correlates of MDMA (“Ecstasy”)-induced social interaction in rats. Social Neuroscience. 2009;4(1):60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- Tops M, van IJzendoorn MH, Riem MM, Boksem MA, Bakermans-Kranenburg MJ. Oxytocin receptor gene associated with the efficiency of social auditory processing. Frontiers in Psychiatry. 2011;(2) doi: 10.3389/fpsyt.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107(31):13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: Implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology. 2006;189(4):489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology. 2014;231(21):1–11. doi: 10.1007/s00213-014-3570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Kirkpatrick MG, de Wit H. ‘Ecstasy’ as a social drug: MDMA preferentially affects responses to emotional stimuli with social content. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu035. nsu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacology, Biochemistry and Behavior. 2002;73(4):729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]