Abstract
Advanced age is the greatest risk factor for the majority of human ailments, including spine-related chronic disability and back pain, which stem from age-associated intervertebral disc degeneration (IDD). Given the rapid global rise in the aging population, understanding the biology of intervertebral disc aging in order to develop effective therapeutic interventions to combat the adverse effects of aging on disc health is now imperative. Fortunately, recent advances in aging research have begun to shed light on the basic biological process of aging. Here we review some of these insights and organize the complex process of disc aging into three different phases to guide research efforts to understand the biology of disc aging. The objective of this review is to provide an overview of the current knowledge and the recent progress made to elucidate specific molecular mechanisms underlying disc aging. In particular, studies over the last few years have uncovered cellular senescence and genomic instability as important drivers of disc aging. Supporting evidence comes from DNA repair-deficient animal models that show increased disc cellular senescence and accelerated disc aging. Additionally, stress-induced senescent cells have now been well documented to secrete catabolic factors, which can negatively impact the physiology of neighboring cells and ECM. These along with other molecular drivers of aging are reviewed in depth to shed crucial insights into the underlying mechanisms of age-related disc degeneration. We also highlight molecular targets for novel therapies and emerging candidate therapeutics that may mitigate age-associated IDD.
Keywords: intervertebral disc, aging, oxidative damage, inflammation, cellular senescence, DNA repair
I. INTRODUCTION
Life expectancy has dramatically increased over the past century largely due to advances in medicine, control of infectious diseases, and improved nutrition. There were an estimated 0.5 billion people 65 years and older worldwide in 2010, and this number is projected reach a staggering 1.5 billion by 20501. Emerging within the older population are numerous age-associated chronic diseases, including heart disease, cancer, and diabetes, which are the leading causes of death in developing countries2. Low back pain, which also increases with age, is the leading cause of physical disability3,4. Age-associated chronic diseases, including those of the musculoskeletal system, impose the greatest burden on global health presently and in the future.
One of the largest age-dependent chronic disorders is degeneration of the joints, resulting in enormous socioeconomic and health impacts. Intervertebral disc degeneration (IDD) and osteoarthritis and are the most common underlying causes of joint-related chronic disability and debilitating pain in the older adults5–7. Unfortunately, decreased mobility is a validated predictor of loss of independence and mortality in the elderly8,9. Preserving healthy joints, particularly intervertebral discs in the spine, is vital for maintaining mobility in old age10. Individuals over 60 years old are more likely to suffer from pain stemming from IDD. As such, there is now great impetus to understand healthy disc aging in order to preserve mobility and fitness in the elderly population.
Organismal aging results from time-dependent accumulation of molecular and cellular damage that leads to impaired tissue homeostasis and eventual physiological and functional decline. Numerous types of damage are implicated in driving aging: accumulation of damaged proteins, mitochondrial damage and dysfunction, telomere shortening, DNA damage, attrition of quality control mechanisms (autophagy, DNA repair, etc.), and the loss of tissue-specific progenitor cells and tissue regenerative capacity11–14. The consequences of these different types of damage have recently been categorized into key aging hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication15. Remarkably little is known about the contribution of these aging hallmarks to disc degeneration.
The purpose of this perspective is to provide an overview of disc aging characteristics and to organize the biochemical cascade of disc aging into three phases: (1) accumulation of damage to biomolecules, (2) aberrant cellular response to damage, and (3) loss of biologic structure and function. This perspective will focus particularly on cell senescence, inflammation, and oxidative damage as underlying mechanisms driving disc aging. Other important hallmarks of aging that have not been explored in the disc will be also highlighted in order to stimulate research that identifies potential causes and therapies for age-associated IDD.
II. AGING CHARACTERISTICS OF INTERVERTEBRAL DISCS
A. Disc anatomy and composition
Intervertebral discs are polyaxial cartilaginous joints that function primarily to provide support and flexibility to the otherwise rigid spine16. Situated between two adjacent vertebrae, discs consist of an outer, fibrous annulus fibrosus (AF) that circumferentially encloses a central, gelatinous nucleus pulposus (NP). Discs are constrained within and connected to adjacent vertebral bodies by superior and inferior cartilaginous end plates (CEP). The AF is composed of highly organized lamellae of predominantly type I collagen fibrils with alternating fiber angles of approximately 30°. AF functions mainly to bear circumferential stresses required to restrain NP swelling and tensile forces generated during bending or twisting. Conversely, the NP contains loose randomly organized networks of collagen type II and elastin fibers that encase proteoglycan aggregates. NP functions mainly to counteract and distribute compressive loads with large swelling pressures17. Discs consist mostly of extracellular matrix (ECM) sparsely populated by cells that are fibrochondrocytic in AF and chondrocyte-like and notochordal in NP. Discs are predominantly avascular, aneural tissues that exchange nutrients and metabolites primarily by diffusion to and from micro-vessels in the CEP and outer AF18. The restricted transport and low cellularity of the discs limit repair and make the disc particularly susceptible to injury and the aging-associated accumulation of tissue damage.
B. Features of disc aging
Intervertebral discs appear to undergo age-related degenerative changes earlier in life than other tissues19,20. Based on studies of humans and different animal models (Table 1), these age-related changes include increased number and size of tissue fissures, the presence of granular debris, and neovascularization from the outer aspect of the annulus inwards19. The NP becomes more fibrous as its proteoglycan content and hydration diminish with age, leading to fissures and progressive loss of NP size and pressurization and overall disc height21,22. Age-related accumulation of oxidized matrix proteins transforms the clear, gelatinous NP in youth to yellow, fibrous tissue in older individuals due to deposition of a brown ‘age pigment’ known as lipofusin, which originates from the slow peroxidation of lipids23 (Fig. 1). Ossification and thinning of the CEP, microfractures in the adjacent subchondral bone, bone sclerosis, and drastic reduction in the number of vascular channels in the CEP are also found with increasing age24. Reduced CEP vascular flow might contribute to a further decreased in nutrient supply to the disc, accumulation of cellular waste products, and an increasingly acidic environment (pH 6.3–6.6) that together with other stress factors can negatively impact cell function25. As such, elucidating disc aging-induced mechanisms will provide new opportunities for ameliorating age-related IDD.
Table 1.
Animal models used to investigate age-associated intervertebral disc degeneration
| Mouse models | |||||
|---|---|---|---|---|---|
| Gene symbol | Gene name/description | Protein function | Model | Age-related disc degenerative changes | Ref |
| Ercc1 | Excision repair cross-complementing rodent repair 1 | DNA repair | M | Systemic ERCC1-deficiency accelerated age-dependent disc degeneration | 28 |
|
| |||||
| n/a | Senescence-accelerated mouse (SAM) | Gene not yet identified | Derived from AKR/J | Amyloid deposition in disc AF, articular cartilage | 221 |
|
| |||||
| DmdMdx | X-chromosome-linked muscular Dystrophy | Dystrophin-skeletal muscle integrity | M | Age-related loss of disc proteoglycan | 222 |
|
| |||||
| Cnn2 | Connective tissue growth factor (CTGF or CCN2) | Matricellular protein involved in cellular adhesion, migration, ECM synthesis | TG | Notochord-specific CCN2 deletion accelerated age-dependent disc degeneration | 223 |
|
| |||||
| Bgn | Biglycan | Small leucine repeat proteoglycans ECM | TG | Age-related early onset of disc degeneration | 224 |
|
| |||||
| Ky | Kyphoscoliosis peptidase | Cytoskeleton-associated protease required for normal muscle growth | ARM | Degenerative changes in cervical and thoracic discs; kyphosis | 225 |
|
| |||||
| Skt | Sickle tail | Linked to Danforth’s short tail locus | TG | Abnormal development of the intervertebral disc | 226 |
|
| |||||
| n/a | Danforth’s short tail locus (Sd) | Gene not yet identified | M, SD | Aberrant patterns of vertebrae and disc development | 227 |
|
| |||||
| C57Bl6 | Wildtype mice | Natural aging mice | Natural aging-related disc degeneration | 228 | |
| Other animal models | |||||
|---|---|---|---|---|---|
| Animals | Scientific name | Key features & analysis methods | Age-related disc degenerative changes | Ref | |
| Hamster | Cricetinae | Chinese hamsters prone to development of spontaneous diabetes. Spines of age 10–33 months histologically analyzed | Aging changes in intervertebral discs Spondylosis was present at an earlier age in diabetic than in nondiabetic hamster. | 229 | |
|
| |||||
| Rat | Rattus | Male Fisher 344 rats. μCT of spines of age 3, 12, 18, 30 months | Age-associated morphometric and degenerative disc changes | 230 | |
|
| |||||
| Sand rat | Psammomys obesus | Spontaneous development of diabetes. Age 1–46 months analyzed radiographically and histologically | Age-related and diabetes-related spontaneous development of lumbar disc degeneration | 231 | |
|
| |||||
| Rabbit | Oryctolagus cuniculus | New Zealand White rabbits. Age 1–30 months studied by MRI, histology, gene expression | Natural aging-related disc degeneration | 232 | |
|
| |||||
| Pig | Sus scrofa domesticus | Porcine discs of age 2–3 weeks, 6–9 month, 2–3 years analyzed by histology, GAG assay, cell density assay, gene expression | Disc MMP-1 increased with age. GAG and collagen I, II, aggrecan decreased with age. | 219 | |
|
| |||||
| Dog | Canis lupus familiaris | Discs of chondrodystrophic (CD) and nonchondrodystrophic(NCD) dogs of age 1–7 years were analyzed by histology, GAG, and MMP activity | CD dogs showed early age onset of disc degenerative changes compared to NDC dogs. | 233 | |
|
| |||||
| Sheep | Ovis aries | Discs of new-born, 3, 12, and >36 months were analyzed mechanically and microscopically to assess nucleus pulposus-endplate integration | Rapid increase in NP-EP insertion nodes between birth and 3 months, after which this integration remained constant. | 234 | |
|
| |||||
| Alpaca | Vicugna pacos | Young (2–6 years) and older (>10 years) alpaca underwent MRI evaluation to detect cervical spine degeneration | No cervical disc degeneration in young alpacas. Increased disc degeneration incidence and severity at lower cervical levels in older alpacas | 235 | |
|
| |||||
| Rhesus Macaques | Macaca mulatta (Macaques monkeys) | Longitudinal study of macques 11–32 years of age to assess disc space narrowing (DSN) by radiography. | Age-associated disc space narrowing, osteophytosis, increased disc tissue stiffness | 236 | |
Genetic models: Mutation (M), Transgenic (TG), Autosomal recessive mutation (ARM), Truncation mutation (TM). N/A: not applicable.
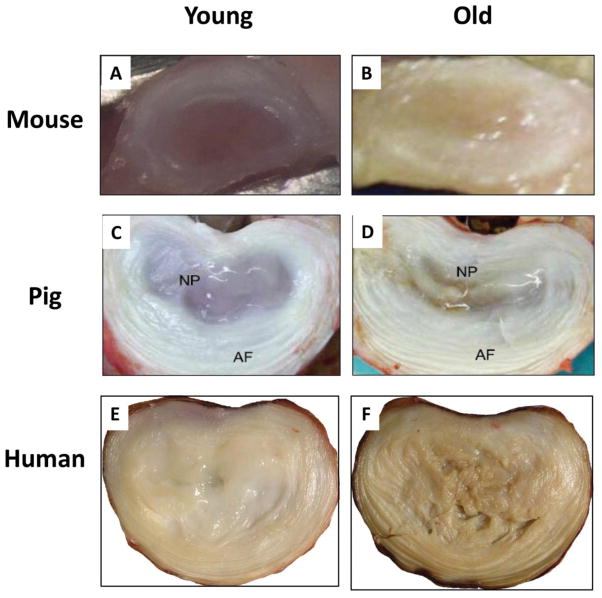
Figure 1. Gross features of the aged mammalian discs.
Axial sections of young and aged lumbar discs of 3 month- vs. 24 month-old mice (A, B), 3 week- vs. 3 year-old pigs (C, D) 219, and 16 year- vs. 55 year-old humans (E,F, courtesy of Dr. Ian Stokes), are shown. Old discs exhibit an overall loss of hydration, loss of demarcation between the AF and NP boundary, and tissue discoloration (old disc more yellowish). Average lumbar disc cross-sectional diameters are approximately 2–3mm for mice, 24–30 mm for pigs, and 45–55mm for humans 220.
C. Distinguishing disc aging from disc degeneration
Disc aging can be distinguished from disc degeneration by several defining characteristics. First, disc degeneration refers to the structural and functional failure of the disc as a result of aberrant, pathological cellular and ECM changes26. Disc degeneration may be caused by genetic predisposition, injury, aging, and environmental factors such as smoking, or any combination thereof27–33. Unlike disc aging, disc degeneration is not exclusive to the older population, i.e., disc degeneration can be present in a younger person due to injury or faulty genetics32–34. Conversely, disc aging is systemic and occurs in all spinal discs of all older individuals. In other words, a degenerated disc, but never an aged disc, can be found among the other young healthy discs and body organs in a young individual. However, the specific differences between an aged disc and a degenerated disc have not been clearly defined as both appear to share a number of similar changes19,20. Importantly, future work to identify the features common to aging and degeneration that are most pathologic to disc function will be critical in guiding novel treatments.
D. Impact on disc aging by the surrounding spinal structures
Because aging is systemic, age-related changes in spinal structures can greatly impact disc health (Fig. 2). Osteoporosis and osteopenia are commonly observed in the aging spine, predisposing it to vertebral compression fractures35 and correlating with increased IDD36. Moreover, age-dependent endplate thinning and fracture create abnormal stress distributions and injury propagation to the adjacent disc, which increases the risk of IDD34,37,38–40. Modic changes in the vertebral body and/or endplate identified by MRI have been associated with aging along with loss of disc height and signal intensity41. Vertebral endplate sclerosis is predicted to reduce bulk fluid movement in to and out of the disc, which could limit ancillary nutrient transport42,43,44. Together with reduced endplate pore density and size seen with aging, these changes could significantly impact disc nutrient supply and thus disc cell survival18. Age-related facet cartilage erosion can impose abnormal load on disc, altering local mechanobiological responses45. Aging of the posterior and anterior spinal ligaments, important dynamic stabilizers of the spine, alters their material properties (increased stiffness) which conceivably could also influence mechanical strain and stability of the discs35,46. Finally, age-driven changes in the spinal muscle, e.g., fatty deposit or infiltration, could also affect the overall stability of the spine and hence the biology of the disc47. In summary, disc aging is a complex systemic process that is intimately modulated by the interactions among the different aging spinal structures (Fig. 2).
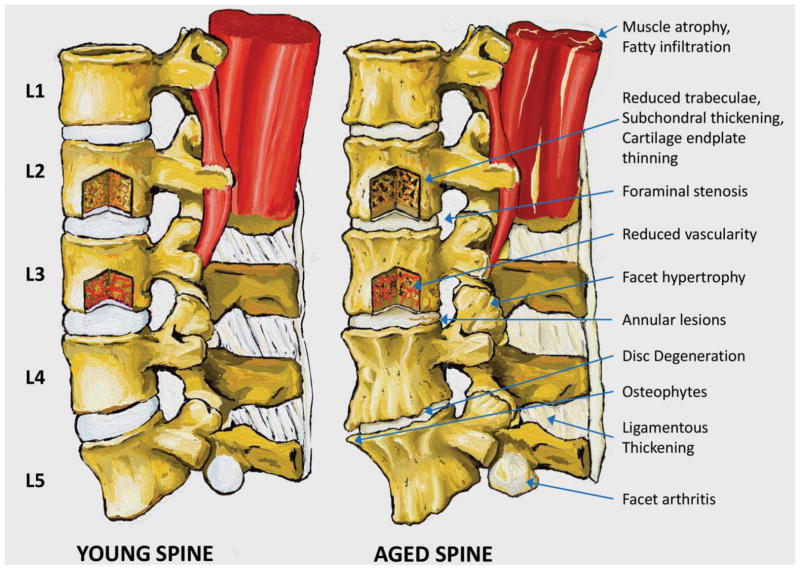
Figure 2. Gross features of the aging spine.
Young and aged lumbar spines are visually compared to illustrate the wide range of tissues and processes involved in aging of the spine. Muscle atrophy and fatty infiltration is evident at L1-2 in the aged spine. 25Similarly, a window into L3 depicts reduced vascularity and fewer capillaries reaching the endplate. Foraminal stenosis is shown at L2-3 (arrow), L3-4, and L4-5, and facet hypertrophy is evident at L3-4 (arrow) and L4-5. An annular lesion is present in the posterior portion of the L3-4 disc. Disc degeneration with evident loss of disc height and prominent anterior osteophytes occur at L4-5. Ligamentous thickening is indicated in the interspinous and supraspinous ligament; thickening of the ligamentum flavum occurs with aging but is not observable in the sagittal view. Finally, facet cartilage arthritis is revealed on the inferior facet of L5.
III. BIOCHEMICAL CASCADE OF INTERVERTEBRAL DISC AGING
The biochemical process of disc aging can be organized into three distinct phases (Fig. 3A). First, there is damage to biomolecules such as DNA and proteins that results from exposure to inflammatory and oxidative stress. Second, aberrant cellular responses to damage exacerbates tissue damage when the responses become dysregulated. Third, accumulated damage leads to loss of biologic structure and function of disc tissue, as discussed in details below. Aging of the other spinal structures (Fig. 2) probably follows this same biochemical cascade.
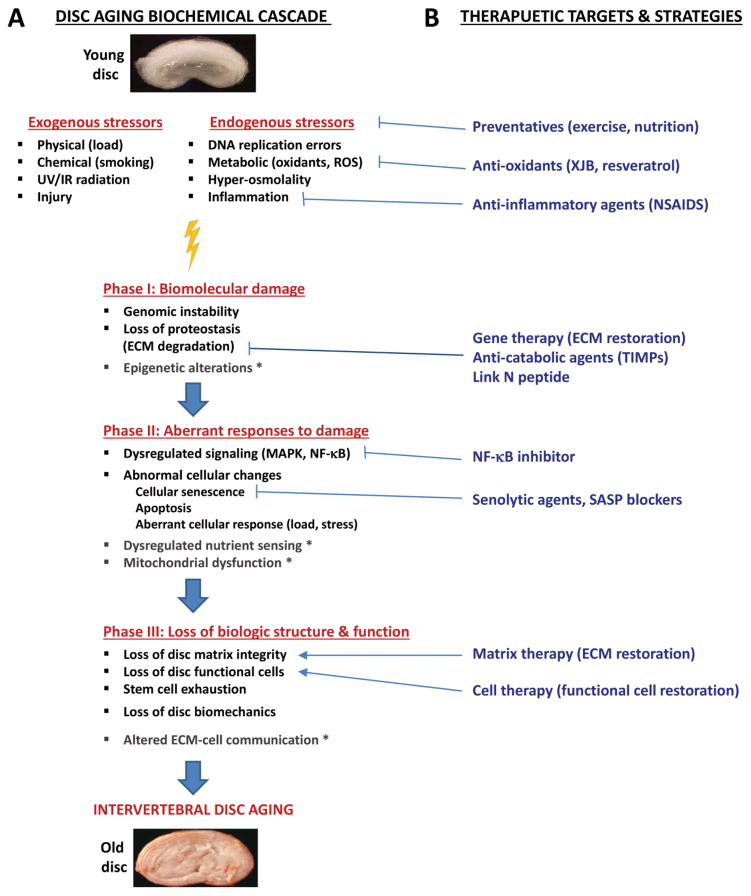
Figure 3. Proposed biochemical cascade in disc aging process and potential therapeutic targets.
(A) With aging, there is time-dependent accumulation of biomolecular damage (Phase I), most importantly, DNA damage, in the disc due to exogenous and endogenous factors. Cellular responses to accumulated damage over time become dysregulated (Phase II) leading to more damage and eventual loss of disc biologic structure and function (Phase III). This results in degenerative changes observed in aged discs. * Observed in other tissues but not yet investigated in disc tissue. (B) Potential therapeutic targets to delay or ameliorate age-related degeneration. Oxidative and inflammatory stress can be reduced with anti-oxidants and anti-inflammatory drugs. Reducing chronic activation of NF-κB signaling by pharmacologic intervention may be efficacious in delaying age-related degeneration. Moreover, removal of senescent cells or blocking formation of SASP could potentially mitigate disc matrix catabolism. Finally, protein-, gene- and cell-based therapy could also conceivably delay or help restore age-related loss of disc matrix and functional cells.
A. Phase I of Biochemical Cascade of Disc Aging: Biomolecular Damage
A solid body of research supports time-dependent accumulation of stochastic damage to biological macromolecules as a driver of aging48. Well documented in aged tissue are damaged proteins and genetic materials which lead to genomic instability, epigenetic alterations, and loss of proteostasis. Proteostasis refers to protein homeostasis regulated by a dynamic equilibrium of protein synthesis and degradation. ECM damage resulted from loss of proteostasis during the course of age-related IDD has been extensively documented. The role of DNA damage in disc aging has also been recently reported. However, epigenetic alterations in disc aging have not yet been explored.
Damage and Perturbation of ECM Integrity in Aged Disc
Accumulation of molecular damage in the ECM of the aging disc has been well recognized (Fig. 4A) and reviewed49. Disc proteoglycan aggregates, consisting primarily of aggrecan core protein, link protein, and hyaluronan encased within the collagenous fiber network, provide the osmotic properties that create swelling pressure necessary to counteract compressive loading. In the aged disc the majority of the aggrecan exists in a non-aggregated form and contains decreased glycosaminoglycan (GAG) chain length, which is thought to be derived from proteolytic damage49. Link protein levels decrease with age50 while total disc hyaluronan levels increase with age, possibly as a response to its own proteolysis51. Versican is another major hyaluronan-binding proteoglycan in disc tissue which undergoes extensive degradative damage with aging52. The resulting non-aggregating proteoglycans may not have the same functional ability as that of intact aggregates, as their size, charge density, spatial rigidity and matrix interactions are diminished49,51,53,54.
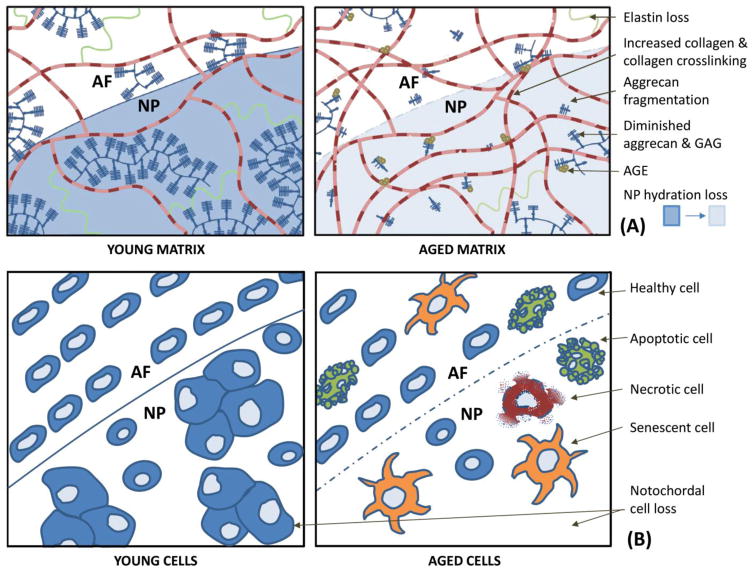
Figure 4. Molecular and cellular features of the aging disc.
Young and aged extracellular matrix (A) and cells (B) are schematically compared to summarize important changes that occur during disc aging. Panel A, young matrix is rich in elastin (green, coiled fiber), aggregated aggrecan (dark blue, bottle-brush aggregate), and collagen fibers (banded fibers). Aged matrix shows loss of elastin, increased collagen and collagen crosslinking, fragmented aggregan, diminished GAG quality, reduced aggregan aggregates, increased accumulation of advanced glycation end-products (AGEs) along with lower hydration49. Panel B, young AF cells are elongated fibrochondrocytes and NP cells are a mixture of large, clustering, notochordal cells and smaller, chondrocyte-like cells. Aged cells show reduced cellularity, loss of notchordal cells, and incidence of senescence, apoptosis, and necrosis.
The disc also contains the small leucine-rich repeat family of proteoglycans (SLRPs), which are characterized by their interaction with collagen fibers. Biglycan and decorin, the dermatan sulfate proteoglycans which interact with collagen type VI and type I/II, respectively, lose their GAG content with age due to proteolytic damage55. The keratin sulfate-containing fibromodulin and lumicans are two other disc collagen fibril-associated SLRPs. Whereas fibromodulin abundance decreases with age and exists mostly in non-glycated form in adult discs, lumican increases in aged discs and exists as a glycoprotein throughout life49,56. Since dermatan sulfate and keratin sulfate mediate intermolecular interaction, their age-related alterations most likely affects disc matrix structure. Age-related changes in other disc matrix constituents, e.g., elastin, fibronectin, have also been documented with unclear consequences on disc matrix organization and cell structure and function49,57.
Disc tissue contains three broad categories of fibrillar, fibril-associated, and pericellular collagens whose relative abundance changes with age. The disc collagen network consists predominantly of Type I and II fibrillar collagen which accounts for approximately 80% of the total disc collagen. Type VI pericellular collagen accounts for 10–20% of the disc collagen49. Age-associated changes in disc collagen structure include proteolytic damage in fibrillar collagen as a result of dysregulated collagenase activity58. Damaged fibrillary collagen weakens the mechanical strength of disc tissue and leads to the formation of non-enzymatic crosslinks between basic amino acids of collagen and reducing sugars59. This results in advanced glycation end-products (AGEs) which elicit oxidative stress. AGEs are present throughout the disc and increase in abundance with age60 and may impair collagen fibril formation61,62. In mice, oxidative stress was shown to increase loss of disc elasticity and alter the secondary and tertiary conformation of collagen molecules, which increases their susceptibility to cleavage by MMPs63. Hence age-related ECM molecular alterations can result in a decline in the structural integrity and biomechanical function of the disc.
DNA Damage in Aged Disc
Besides ECM damage, aged discs also exhibit cellular damage. In particular, damaged DNA, unlike damaged proteins or other macromolecules that generally can be degraded and replaced by new synthesis, is especially harmful and requires repair in order to maintain normal cellular function. Each cell in an organism is subjected to tens of thousands of DNA lesions each day due to the inherent chemical instability of DNA structure, metabolic byproducts, and environmental mutagens and genotoxins11. Despite elaborate repair mechanisms, cells still amass DNA damage over time. Inherited defects in genome maintenance mechanisms invariably lead to a variety of diseases characterized by accelerated aging of one or more organ systems12. For example, deficiency in humans of certain genes involved in repair of DNA damage, such as ERCC1-XPF, leads to dramatic progeroid, or accelerated aging syndrome64. Indeed, DNA repair-deficient Ercc1−/Δ mice exhibit premature onset of key disc aging features, including loss of matrix proteoglycan, reduced disc height, and increased cellular senescence28,65 (Table 1). DNA damage as a driver of disc aging is further supported by exposure studies of human and mice to genotoxic stress, including ionizing radiation and tobacco smoking, which in mice dramatically accelerated disc degenerative changes66,67,68.
Stressors Driving Disc Biomolecular Damage
Exogenous and endogenous stressors causing molecular damage in aging discs are thought to be predominantly oxidative and inflammatory in nature (Fig. 3A)65,69. Evidence of oxidative damage in aged disc include accumulation advanced glycation end products (AGEs), e.g., pentosidine and carboxymethyl-lysine, produced by nonenzymatic glucosylation and oxidation of proteins and lipids60,70. Pentosidine, which cross-links collagen molecules, might play an important role in increased collagen stiffness and fragility to weaken cartilage biomechanics with old age59,60,71. Redox proteomic analysis also revealed oxidative post-translational modifications, e.g., protein carbonylation, in disc matrix isolated from aging mice; this change was associated with protein fragmentation and aggregation and increased disc stiffness63.
The source of reactive oxygen species (ROS) driving oxidative damage includes free radicals generated from radiation, by-products of oxidative phosphorylation, cellular response to chronic inflammatory stress exposure, and decreased synthesis of ROS-scavenging enzymes72. Although residing in a low oxygen tension environment, resident disc cells, especially AF cells, are capable of oxidative phosphorylation which can generate ROS. In addition, aged discs acquire fissures and associated neovascularization which exposure the otherwise hypoxic resident cells to higher oxygen tension and thus oxidative stress73. Increased ROS contribute to aging changes in cells and tissues by damaging proteins, lipids, and DNA. One key marker of protein oxidation is nitrotyrosine that is formed by the reaction of protein tyrosine residues with peroxynitrite (ONOO−)74. Peroxynitrite, formed by rapid reaction of nitric oxide (NO) with oxygen radical superoxide (O2−), is a potent cytotoxic damaging nitrating and oxidizing agent.
Intriguingly, hyperosmolality is recently demonstrated as a non-classical inducer of DNA damage. Hyperosmolality induces DNA double strand breaks, which activate the ATM-p53-p21WAF1 axis leading to the hypophosphorylation of the pRb protein and cell cycle arrest in the G1 phase of the cell cycle75. NP cells are continuously exposed to hyperosmolality, up to 500mOsm/kg H2O in vivo as compared with <300mOsm/kg H2O in the majority of the other tissues76. Increased osmolality in NP cells was found to provoke chromatin changes and DNA damage75. It is still unclear what level of hyperosmolality is needed to overwhelm NP cell DNA repair capacity to introduce DNA damage.
Abnormal mechanical loading represents another major potential stressors that can promote disc tissue damage. Cohort analyses point to associations between long-term physical loading and loss of spinal mobility and disc height77, and other age-associated IDD78,79,80. In animal studies, rats with imposed upright stance for up to 11 months showed increased disc senescence, presumably due to altered magnitude and mode of disc loading81. Modest age-related IDD features in rat discs were observed following compressive overloading for eight weeks82,83. However, more studies are needed to establish role of abnormal mechanical loading in promoting disc degenerative changes, with or without age associations.
Last but not least, nutritional stress can also promote perturbation in disc tissue. The avascular nature of disc tissue results in an environment of low oxygen and glucose concentrations and high lactate concentration84,85. Despite low physiologic concentrations of oxygen85 and glucose84 and high concentrations of lactate (>10x plasma concentrations)86, which acidify the inner disc environment, disc cells can remain viable and functional in this hostile environment. Yet low nutrition and pH are also the very factors that reduce the disc’s resilience to additional nutritional and environmental stresses87,88; this is because disc nutrient supply barely hovers above the cellular requirements in the NP89. This precarious balance may expose disc cells to nutritional deprivation due age-related processes. For instance, disc cell death is initiated if glucose concentrations drop below critical thresholds (<0.5 mM) 90. Acidic conditions (pH < 6.7) can also lower cell viability91. Low O2 and pH conditions have been shown to diminish proteoglycan and collagen synthesis86,92.
B. Phase II of Biochemical Cascade of Disc Aging: Aberrant Responses to Damage
In an attempt to repair damage, cellular responses may become dysregulated over time, which exacerbate cellular and ECM damage. Aberrant molecular signaling, abnormal changes in cell fate, dysregulated nutrient sensing, and mitochondrial dysfunction all have been reported in non-disc tissues15. However, only abnormal alterations in cell fate (e.g., cellular senescence) and dysregulated signaling (e.g., NF-κB pathway) have been reported recently in studies on disc aging. These two areas of research are discussed below.
Cell Functional and Phenotypic Changes in Disc Aging
AF and NP differ in their developmental origin, with AF developing from the mesenchyme and the NP from the notochord. The cells in the outer AF are elongated and fibroblast-like, whereas the inner AF and NP are populated by more spheroidal, chondrocyte-like cells. While cell types in the outer AF appear to change little during lifetime, NP cell subpopulations undergo a substantial change whereby clusters of large vacuolated notochordal cells in young NP are replaced by smaller chondrocyte-like cells in older NP93. Cell density decreased from 0 to 16 years, and no significant variation occurred thereafter in human lumbar discs 94. Cell density in normal, mature disc is approximately 4x106 cells/cm3 in the NP and 9x106 cells/cm3 in the AF 49. During aging disc cells undergo a number of phenotypic changes, including a switch from an anabolic to a catabolic phenotype95–99. Cells isolated from aged discs exhibit reduced collagen and proteoglycan matrix anabolism95–97,100. Moreover, found in aged discs are elevated levels matrix proteoglycan degradative products and certain matrix metalloproteinases (MMPs), including MMP-3 and ADAMTS-5101,98,99,102,103. The pro-inflammatory cytokine TNF-α is also shown to be more highly expressed in older adult discs than young adult discs104. These observations suggest imbalanced matrix homeostatic phenotype as a consequence of age-associated changes in disc cells. Additionally, other cell phenotypic changes such as elevated necrosis, apoptosis, and senescence have been reported105,106 (Fig. 4B). These phenotypic and functional changes are likely consequences of aberrant cellular responses to accumulated biomolecular damage in disc tissue. Such changes also likely contribute to loss of functional cells, leading to age-related depletion of disc matrix proteoglycan, tissue dehydration, and altered load distribution that may increase risk of injury87,107.
The Role of Cellular Senescence in Disc Aging
Cell senescence, originally described as a process that limits cell proliferation108,109, is an important mechanism for preventing the proliferation of potential cancer cells. This type of senescence is known as replicative senescence, characterized by cessation of cell proliferation due to critical shortening of telomere length after successive replicative cell cycles. Another type of cellular senescence was discovered relatively recently, which was termed, “stress-induced premature senescence (SIPS)”. SIPS is formed as a result of accumulate genomic and mitochondrial DNA damage. SIPS cells also acquire a senescence associated secretory phenotype (SASP), a unique feature in which they secrete high amounts of numerous inflammatory cytokines and matrix proteinases, which can have profound catabolic effects on neighboring cells and ECM110,111. Because senescent cells accumulate in various tissues and organs with aging112, SASP is currently believed to disrupt tissue structure and function and promote aging113. This theory is supported by a seminal study demonstrating that clearance of senescent cells delays aging-associated disorders114.
An increased number of senescent cells were observed in both aged and degenerated discs105,115–118, as measured by increased expression of senescent markers, including senescence-associated β-galactosidase (SA-βgal), p16INK4A, and decreased telomere length. Thus, cellular senescence is a potential driver of both disc degeneration and disc aging, possibly through the SASP mechanism that promotes pathologic disc matrix catabolism. Evidence supporting this idea originally came from a study showing a positive correlation between the senescent marker p16INK4A and matrix proteases expression (MMP-13 and ADAMTs-5) in human disc tissue, implicating senescent disc cells as a source of these catabolic enzymes105. In vitro cell culture studies using H2O2 to simulate oxidative DNA damage that induces SIPS also revealed an altered, catabolic phenotype119. These H2O2-induced senescent disc cells exhibited SASP, as characterized by their secretion of high levels of MMPs and pro-inflammatory cytokines. These senescent disc cells also showed growth arrest and perturbed matrix homeostasis, i.e., reduced matrix synthesis capacity (anabolism) and increased matrix degradation (catabolism)120. However, to determine the causative role of cellular senescence in driving disc aging, genetic and pharmacological in vivo strategies are needed to study the effects of ablation of senescent cells on age-associated IDD.
Possible causes of disc cellular senescence
DNA damage is the underlying cause of cellular senescence, but how cells become senescent in disc tissue is not fully understood. Current evidence supports the existence of both replicative senescence and SIPS in discs. Telomere length shortening, a marker of replicative senescence, is observed in aged and degenerated human disc tissue, as is increased p16Ink4a immunopositivity, a marker of SIPS105,121. Elevated cellular senescence was observed in discs of DNA repair-deficient Ercc1−/Δ mice28 as well as in genotoxin-exposed mice 66,67, suggesting that DNA damage is a key driver of disc cellular senescence. Other potential sources of oxidative DNA damage include oxidative stress induced by inflammation and high glucose-induced oxidative stress, e.g., in diabetes122. IL-1, a predominant cytokine implicated in the pathogenesis of disc degeneration123,124, has been suggested to promote SIPS in NP cells. Indeed, spontaneous age-related IDD with associated senescent phenotype is seen in an IL-1Ra knockout mouse model 125. Finally, activation of WNT/β-catenin signaling was also reported to promote cellular senescence in rat disc cells126, but it is unclear in disc tissue how this signaling is influenced by oxidative or inflammatory stress that drives senescence. In summary, disc senescence phenotype appears to be specific aberrant cell response to DNA damage which can cause further tissue perturbation and damage during the course of disc aging (Fig. 3A).
Aberrant Molecular Signaling in Disc Aging
Biomolecular damage, e.g., DNA damage, can initiate aberrant signaling cascade, which then, if left unchecked, acts to cause further molecular damage. It is now well known that in addition to environmental factors and behavior traits, genetics greatly influences lifespan. Most longevity genes identified starting from the early 1980s in various models (worms, fruit flies, mice, monkeys … etc.) implicate one of three major signaling pathways in cells: insulin/IGF-1, sirtuins, or mTOR13,127,128. These three pathways regulate a variety of cellular processes, including cell growth, cell proliferation and survival, protein synthesis, and transcription mechanisms. The roles of these signaling pathways in disc aging, however, have not been explored although it is known that Insulin-like growth factor-1 (IGF-1) induces disc anabolic activity129 while SIRT1 is expressed by human NP cells and acts to suppress NP cell matrix metabolism and proliferation130. However, signaling other than these three pathways have been reported to influence age-related IDD, including NF-κB, MAPK, and HIF-1α signaling which are known to be involved in stress responses and SIPS, which are discussed below.
NFkB signaling in age-related disc degeneration
NF-κB signaling is central to the cellular response to inflammation, stress and damage. NF-κB is comprised of a family of structurally related transcription factors, which in mammals consists of five protein subunits, RelA or p65, c-Rel, RelB, p50 and p52. NF-κB exists as a homodimer or a heterodimer, with the p50–p65 heterodimer being the most common form which controls the expression of the majority of NF-κB-regulated genes131. Chronic activation of NF-κB has been linked to tissue aging and many age-related degenerative diseases, including musculoskeletal disorders such as muscular dystrophy, osteoarthritis, and osteoporosis132,133,134.
NF-κB is also implicated age-associated IDD135. In disc tissue, NF-κB activity was shown to correlate with accumulated oxidative stress and increase with age and degeneration70. Systemic inhibition of NF-κB activity by pharmacologic and genetic means has been shown to ameliorate age-associated IDD in a mouse model of accelerated aging136. Most other studies focus on the role of NF-κB in mediating degenerative and inflammatory disc disease. Increased NF-κB activity is found in degenerative discs135. Compared to asymptomatic discs, symptomatic discs have higher levels of pro-inflammatory cytokines that are considered typical NF-κB target genes, e.g. TNF-α, IL-1β, IL-6 and IL-8104,123,124,137. Together, these findings support the role of dysregulated NF-κB chronic activation in promoting IDD and age-related IDD.
MAPK signaling in disc biology and disc aging
Mitogen-Activated Protein Kinases (MAPKs) are a family of signal transduction pathways, allowing the cells to respond to multiple extracellular inputs, such as hormones, growth factors, inflammatory cytokines, and environmental stresses such as ionizing radiation or osmotic stress138,139. In mammals, these diverse signals activate at least three major subfamilies of MAPKs, the extracellular signal-regulated kinases (ERK), c-Jun NH2-terminal kinases (JNKs), and p38 isoforms (p38MAPKs)140,141. Activation of MEK/ERK and JNK are involved in the induction of cellular senescence142,143. On the other hand, p38 MAPK activation is a marker of senescence and plays a vital role in establishing SASP which probably affects local tissue homeostasis144. Consistent with in vitro data, up-regulated p38 MAPK expression has been reported in senescent AF cells isolated by laser capture microdissection145.
Multiple components of the catabolic machinery (e.g. MMPs, ADAMTSs, COX-2, PGE2, iNOS, etc) are regulated by MAPK family members. Many of these catabolic genes are also regulated by NF-κB signaling, highlighting the cross-talks between the components of MAPK and NF-κB pathways135. In disc tissue, the major pro-inflammatory cytokines, i.e., IL-1β and TNF-α, activate ERK and/or p38 and consequently catabolic molecules such as ADAMTs-4, MMP-3 or syndecan-4 146–149. Inhibition of MAPK activation by specific synthetic compounds or naturally occurring molecules such as glucosamine prevent these processes, indicating that MAPK regulation may represent a promising tool to mitigate disc degeneration and aging147,150.
Increased disc cell proliferation and formation of cell clusters is a characteristic feature of disc degeneration151. This is thought to be partly due the over-expression of growth factors and their receptors152. Growth factors such as PDGF, IGF-I or bFGF stimulate cell proliferation via ERK activation153,154, indicating an additional role of MAPK in disc degeneration. Conversely, hyperosmotic conditions inhibit ERK, arrest cell cycle in the G2/M phase via p38 MAPK activation and restrain the mitogenic effect of growth factors, indicating that the reduced osmolality prevailing in the degenerated disc may boost cell proliferation155. Whether disc aging involves these same signaling pathways as they are related to cell proliferation and clustering awaits further investigation.
HIF-1α signaling in disc biology and disc aging
For any meaningful discussion of aging of disc at the molecular level, it is important to consider the unique niche and physiology of the cells of NP and inner AF. NP resides in a hypoxic and hyperosmotic environment156,157 to which they have adapted a novel hypoxia signaling governed by the activities of the transcription factors hypoxia-inducible factor-1α and -2α (HIF-1/2). Unlike other cell types in which HIF-1α protein stability and activity are enhanced under hypoxia and abolished under normoxia, NP cells constitutively express both HIF-1α and HIF-2α, even under normoxic conditions158. In fact, NP cells are either partially or wholly refractory to propyl-hydroxylases (PHD)-dependent degradation159,160. Similarly, Factor Inhibiting HIF-1 (FIH-1) does not control HIF-1α transcriptional activity in NP cells161 as typically seen in other cell types. The constitutive HIF-1 expression has important metabolic consequences for NP cells which are obligate glycolytic and rely very little on aerobic respiration even when oxygen is abundant 162. In addition to this metabolic adaptation, HIFs promote survival and function of NP cells through upregulation of crucial genes including, aggrecan162, galectin-3163, β-1,3-glucuronyltransferase 1164, and VEGF-A165. In fact HIF-1 is indispensable for NP cell survival as demonstrated by conditional knockout of HIF-1α in mouse notochord by Foxa2-Cre which results in smaller, non-vacuolated cells in the NP at E15.5 and massive apoptotic cell death in the NP at birth166. Thus, the important role of HIF signaling in physiological adaptation and function of NP cells suggests that its dysregulation may contribute to cellular aging and degeneration.
The role of HIF signaling in organismal aging was recently elucidated. Dysregulated increase in HIF-1α activities due to age-dependent decline of nuclear NAD+ was found to interfere with the coordination between nuclear and mitochondrial activities, resulting in mitochondrial dysfunction and accelerated aging167. However, other studies suggested a role of HIF-1 in suppressing cellular senescence168,169. These conflicting results require further investigation in order to elucidate how dysregulated HIF signaling contributes to disc aging, given that robust and stabilized HIF-1α expression is vital for NP cell survival and function162. One possible mechanism involves cross-talk between HIF and NF-kB pathways under pathological conditions170,171. TNF-α controls expression of PHD2 and 3 through NF-κB pathway, and both PHDs in turn serve to control p65/RelA of NF-κB transactivation. Importantly, PHDs partially control a broader TNF-dependent inflammatory response by promoting expression of several cytokines and chemokines170,171. More studies are needed to detangle the complex interaction and possible dysregulation between HIF and NF-kB pathways that might predispose disc tissue to accelerated aging.
C. Phase III of Biochemical Cascade of Disc Aging: Loss of Biologic Structure and Function
Loss of disc biologic structure and function (phase III) is an end consequence of time-dependent accumulation of damage resulting from biomolecular changes (phase I) and associated aberrant signaling (phase II) (Fig. 3A). Damage-induced apoptosis, cellular senescence, and pathologic alterations in matrix metabolism all likely contribute to the loss of disc functional cells, stem cell pool172–175, and ECM structural integrity (Fig. 4). These changes are expected to alter ECM-cell communication and eventually the normal physiology and biomechanical function of the disc (Fig. 3A).
Loss of disc ECM integrity and altered cell mechanobiology
Loss of disc matrix proteoglycans decreases the negatively charged ionic environment, reducing hyperosmotic loading ionic flux176. Loss of hydration alters fluid pressurization and fluid flows in the disc177. Age-related changes to the solid matrix, such as increased non-enzymatic collagen cross-linking59,178, protein glycation179, or protein denaturation180 can increase structural stiffness 180 and alter disc strains181. Moreover, AF damage increases with age182, which can alter tensile stiffness and annular local strains183,184. These changes perturb the cellular microenvironment and compromise normal disc function.
Cell clustering and interactions with the pericellular matrix also appear to change with age185, which suggest altered cellular mechanical properties186. Indeed, disc cells from young and middle-aged bovine respond differently to the same compression regimes, with older cells showing diminished capacity for matrix repair187. Similarly, AF cells from mature and aged pigs subjected to loading exhibit differences in anabolic and catabolic gene expression, further suggesting changes to cells or mechanotransduction with aging188. Cells isolated from injury-induced degenerative discs also demonstrated differences in response to mechanical loading189, but the effect of physiologic aging was not evaluated.
Mechanical features of aging discs: loss of physiological biomechanics
The complex structure of the disc allows multiaxial motion while maintaining stability190,191. NP and AF interact to support compressive loading and facilitate segmental motion. Compression increases swelling pressure in the NP192,193 which is constrained by the surrounding, distensible AF and the adjacent CEPs194. In distraction, the AF limits vertebral body motion181. The organized, concentric lamellae of the AF resist torsion through circumferential tensile stress in its collagen and elastin fibers195–197. The physiological function of the spine thus depends on these integrated interactions among the different disc regions198,199. A number of mechanical features specific to aging have been identified within the disc. Hydration levels within the NP decrease in aging, which, along with GAG depletion200, reduces swelling pressure in the NP192,201 and increases the shear modulus of NP with age202. On the other hand, hydration in the AF remains relatively unchanged with aging192, and annular tensile properties change only modestly with aging183,203. The net result of these age-related changes, is a loss of elasticity and increased stiffness63,180, which overlaps with mechanical changes in non-age related disc degeneration.
POTENTIAL THERAPIES TO DELAY AGE-ASSOCIATED IDD
The events within disc aging cascade present potential therapeutic targets for treating or delaying age-related IDD (Fig. 3B). In theory, treatments that reduce free radical production may ameliorate macromolecular damage. Indeed, accelerated aging Ercc1−/Δ mice treated with systemic administration of XJB-5-131, a mitochondria-targeted ROS scavenger, resulted in improved disc GAG content and proteoglycan synthesis204. This demonstrates that mitochondria-derived ROS drives aging-related IDD and that radical scavengers may play a promising role in slowing disc aging. Anti-oxidants such as curcumin and resveratrol have also been reported to be therapeutic for treating IDD, as have a number of other anti-inflammatory agents such as NSAIDS and IL-1Ra205–210. Whether these same agents are therapeutic against age-related IDD await further research.
Minimizing the aberrant damage responses that exacerbate tissue damage is another strategy for treating age-related IDD. One promising candidate is the NF-κB pathway whose chronic activation has been closely linked to age-related diseases. Previous studies demonstrated that blocking NF-κB activity pharmacologically and genetically in the Ercc1−/Δ rodent model of accelerated aging delayed the onset of age-dependent disc proteoglycan loss and other degenerative changes136,211. Moreover, intra-discal injection of ‘naked’ NF-κB decoy oligonucleotides proved effective in partially restoring disc height in an animal model of IDD212, indicating that dysregulated activation of NF-κB is involved in disc matrix loss.
Other potential strategies involve the use of protein-, gene-, and cell-based therapy to counter age-related changes to the matrix and cells, respectively213–215 (Fig. 3B). The goal of most gene therapy approaches is to replace loss of disc ECM via increased matrix synthesis or to inhibit catabolic factors that degrade the matrix216. Cell-based therapy frequently aims to restore loss of functional disc cells with possible anti-inflammatory effects217,218. These interventions aim at either preventing damage, ensuring optimal cellular response to damage, or restoring tissue loss associated with aging. Indeed, such strategies exist and have been extensively investigated in the context of disc degeneration. To be effective, however, therapeutic interventions need to target the early phases of the disc aging cascade prior to the occurrence of functional failure.
CONCLUSIONS AND PERSPECTIVES
A large number of studies dating back to the early 1950s describe various age-related disc degenerative changes. However, the molecular mechanisms which initiate and mediate disc aging are still poorly understood. Recent advances in the aging research field are beginning to reveal important insights into the mechanisms of organismal aging. Based on these insights, we proposed to organize the complex multi-step process of disc aging into three distinct phases to guide future research: (1) biomolecular damage, (2) aberrant damage responses, and (3) loss of biologic structure and function. While genomic instability, cellular senescence, and dysregulated NF-κB signaling have been recently uncovered in disc aging, research is still lagging to elucidate the roles of other important aging hallmarks such as mitochondrial dysfunction, stem cell exhaustion, altered intercellular communication, and epigenetic alterations in disc tissue. The unique disc biological niche, i.e., mechanically loaded, nutrient-poor, acidic and hypoxic environment, offers an excellent opportunity to discover novel disc aging mechanisms that might be distinct from those driving aging in other tissues. Importantly, disc aging is a systemic process that does not occur in isolation and is likely influenced by the aging processes of neighboring spinal structures (Fig. 2), circulating factors, and remote body tissues and organs. Hence, aging research of the whole spine, not just the disc, is an important, immediate future direction. The broad, multi-tiered approach is necessary to provide the basic information needed for the development of effective therapies and interventions to delay the onset of age-related spinal disorders. This is imperative given aging is a big risk factor for IDD-associated chronic pain and disability, the prevalence of which will undoubtedly be amplified with the growing aging population.
Acknowledgments
This work was made possible in part by the Public Health Service grant R01 AG044376-01 (NVV), R01-AR055655, R01-AR064733 (MVR) from the National Institute of Health and UPMC Rehabilitation Institute Pilot Grant 2015 (RAH).
LIST OF ABBREVIATIONS
- AF
Annulus Fibrosus
- ADAMTS
A Disintegrin And Metalloproteinase with Thrombospondin Motifs
- AGES
Advanced Glycation End products
- CEP
Cartilaginous Endplate
- ECM
Extracellular Matrix
- GAG
Glycosaminoglycan
- IDD
Intervertebral Disc Degeneration
- MMP
Matrix Metalloproteinase
- MRI
Magnetic Resonance Imaging
- NP
Nucleus Pulposus
- ROS
Radical Oxygen Species
- SA-βgal
Senescence Associated Beta Galactosidase
- SASP
Senescence Associated Secretory Phenotype
- SIPS
Stress-induced Premature Senescence
- SLRP
Small Leucine-rich Repeat family of Proteoglycans
References
- 1.World Population Prospects The 2015 Revision. at < http://esa.un.org/unpd/wpp/Publications/Files/Key_Findings_WPP_2015.pdf>.
- 2.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Burden of Musculoskeletal Diseases in the United States. American Academy of Orthopaedic Surgeons; 2008. at < http://www.boneandjointburden.org/docs/TheBurden of Musculoskeletal Diseases in the United States (BMUS)1st Edition(2008).pdf>. [Google Scholar]
- 4.Derby R, Lee SH, Kim BJ. Discography. In: Slipman CW, Derby R, Simeone FA, Mayer TG , editors. Interventional Spine: an algorithmic approach. Elsevier; 2008. pp. 291–302. [Google Scholar]
- 5.Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey. 1991–1994 at < http://www.jrheum.org/content/33/11/2271.full.pdf>. [PubMed]
- 6.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 7.Aging Theories of Primary Osteoarthritis: From Epidemiology to Molecular Biology. doi: 10.1089/1549168041552964. at < http://online.liebertpub.com/doi/pdf/10.1089/1549168041552964>. [DOI] [PubMed]
- 8.Cesari M, et al. Oxidative Damage, Platelet Activation, and Inflammation to Predict Mobility Disability and Mortality in Older Persons: Results From the Health Aging and Body Composition Study. Journals Gerontol Ser A Biol Sci Med Sci. 2012;67A:671–676. doi: 10.1093/gerona/glr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–8. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 10.Morone NE, et al. Impact of chronic musculoskeletal pathology on older adults: a study of differences between knee OA and low back pain. Pain Med. 10:693–701. doi: 10.1111/j.1526-4637.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- 13.Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–6. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 15.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50:771–6. doi: 10.1016/j.bone.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roughley PJ, Alini MAJ. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 18.Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 19.Boos N, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976) 1988;13:173–8. [PubMed] [Google Scholar]
- 21.Kumaresan S, Yoganandan N, Pintar FA, Macias M, Cusick JF. Morphology of young and old cervical spine intervertebral disc tissues. Biomed Sci Instrum. 2000;36:141–6. [PubMed] [Google Scholar]
- 22.Prescher A. Anatomy and pathology of the aging spine. Eur J Radiol. 1998;27:181–95. doi: 10.1016/s0720-048x(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 23.Hormel SE, Eyre DR. Collagen in the ageing human intervertebral disc: an increase in covalently bound fluorophores and chromophores. Biochim Biophys Acta. 1991;1078:243–50. doi: 10.1016/0167-4838(91)90565-h. [DOI] [PubMed] [Google Scholar]
- 24.Edelson JG, Nathan H. Stages in the natural history of the vertebral end-plates. Spine (Phila Pa 1976) 1988;13:21–6. doi: 10.1097/00007632-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Bibby SRS, Jones DA, Ripley RM, Urban JPG. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 26.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 27.Nasto LA, et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. Spine J. 2014;14:416–23. doi: 10.1016/j.spinee.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo N, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan D, Song Y, Sham P, Cheung KMC. Genetics of disc degeneration. Eur Spine J. 2006;15(Suppl 3):S317–25. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon CH, et al. Part 2: Quantitative proton T2 and sodium magnetic resonance imaging to assess intervertebral disc degeneration in a rabbit model. Spine (Phila Pa 1976) 2012;37:E1113–9. doi: 10.1097/BRS.0b013e3182583447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samartzis D, Karppinen J, Chan D, Luk KDK, Cheung KMC. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum. 2012;64:1488–96. doi: 10.1002/art.33462. [DOI] [PubMed] [Google Scholar]
- 32.Livshits G, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–5. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–72. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17:289–99. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003;12(Suppl 2):S97–S103. doi: 10.1007/s00586-003-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada A, Okuizumi H, Miyagi N, Genda E. Correlation between bone mineral density and intervertebral disc degeneration. Spine (Phila Pa 1976) 1998;23:857–61. doi: 10.1097/00007632-199804150-00003. discussion 862. [DOI] [PubMed] [Google Scholar]
- 37.Dudli S, Haschtmann D, Ferguson SJ. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res. 2012;30:809–816. doi: 10.1002/jor.21573. [DOI] [PubMed] [Google Scholar]
- 38.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 39.Holm S, Holm AK, Ekström L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004 doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Dolan P, et al. Intervertebral disc decompression following endplate damage: implications for disc degeneration depend on spinal level and age. Spine (Phila Pa 1976) 2013;38:1473–81. doi: 10.1097/BRS.0b013e318290f3cc. [DOI] [PubMed] [Google Scholar]
- 41.Määttä JH, Wadge S, MacGregor A, Karppinen J, Williams FMK. ISSLS Prize Winner: Vertebral Endplate (Modic) Change is an Independent Risk Factor for Episodes of Severe and Disabling Low Back Pain. Spine (Phila Pa 1976) 2015;40:1187–93. doi: 10.1097/BRS.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 42.Gullbrand SE, et al. Low rate loading-induced convection enhances net transport into the intervertebral disc in vivo. 2015;15:1028–1033. doi: 10.1016/j.spinee.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Galbusera F, Mietsch A, Schmidt H, Wilke HJ, N-WC Effect of intervertebral disc degeneration on cell viability: a numerical investigation. Comput Methods Biomech Biomed Engin. 2013;16:328–337. doi: 10.1080/10255842.2011.619184. [DOI] [PubMed] [Google Scholar]
- 44.Ayotte DC, Ito K, Perren SM, Tepic S. Direction-dependent constriction flow in a poroelastic solid: the intervertebral disc valve. J Biomech Eng. 2000;122:587–93. doi: 10.1115/1.1319658. [DOI] [PubMed] [Google Scholar]
- 45.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976) 2005;30:167–73. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 46.Sairyo K, et al. Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine (Phila Pa 1976) 2007;32:E340–7. doi: 10.1097/01.brs.0000263407.25009.6e. [DOI] [PubMed] [Google Scholar]
- 47.D’hooge R, et al. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther. 2012;17:584–8. doi: 10.1016/j.math.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Campisi J, Vijg J. Does Damage to DNA and Other Macromolecules Play a Role in Aging? If So, How? Journals Gerontol Ser A Biol Sci Med Sci. 2009;64A:175–178. doi: 10.1093/gerona/gln065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–9. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 50.Tengblad A, Pearce RH, Grimmer BJ. Demonstration of link protein in proteoglycan aggregates from human intervertebral disc. Biochem J. 1984;222:85–92. doi: 10.1042/bj2220085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott JE, Bosworth TR, Cribb AM, Taylor JR. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184(Pt 1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 52.Sztrolovics R, et al. The characterization of versican and its message in human articular cartilage and intervertebral disc. J Orthop Res. 2002;20:257–266. doi: 10.1016/S0736-0266(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 53.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977;16:22–9. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- 55.Roughley PJ, et al. Non-proteoglycan forms of biglycan increase with age in human articular cartilage. Biochem J. 1993;295(Pt 2):421–6. doi: 10.1042/bj2950421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sztrolovics R, Alini M, Mort JS, Roughley PJ. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine (Phila Pa 1976) 1999;24:1765–71. doi: 10.1097/00007632-199909010-00003. [DOI] [PubMed] [Google Scholar]
- 57.Silberberg F, Meier-Ruge W, Odermatt B. Age-related changes in fibronectin in annulus fibrosus of the sand rat (Psammomys obesus) Exp Cell Biol. 1989;57:233–237. doi: 10.1159/000163532. [DOI] [PubMed] [Google Scholar]
- 58.Hollander A. Enhanced denaturation of the a1(II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. Journal of Orthopaedic Research. 1996 doi: 10.1002/jor.1100140111. at < http://repository.liv.ac.uk/1774860/>. [DOI] [PubMed]
- 59.Pokharna HK, Phillips FM. Collagen crosslinks in human lumbar intervertebral disc aging. Spine (Phila Pa 1976) 1998;23:1645–8. doi: 10.1097/00007632-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 60.Sivan SS, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gautieri A, Redaelli A, Buehler MJ, Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014;34:89–95. doi: 10.1016/j.matbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Verzijl N, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 63.Scharf B, et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem Biol. 2013;20:922–34. doi: 10.1016/j.chembiol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niedernhofer LJ, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–43. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 65.Vo N, et al. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831–7. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasto LA, et al. Genotoxic stress accelerates age-associated degenerative changes in intervertebral discs. Mech Ageing Dev. 134:35–42. doi: 10.1016/j.mad.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellor FE, Breen AC. Ionizing radiation exposure and the development of intervertebral disc degeneration--no case to answer. Spine J. 2013;13:224–6. doi: 10.1016/j.spinee.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 69.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nerlich AG, et al. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci. 2007;1096:239–48. doi: 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- 71.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. J Biochem. 1998;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiku ML, Liesch JB, Robertson FM. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990;145:690–6. [PubMed] [Google Scholar]
- 73.Ali R, Le Maitre CL, Richardson SM, Hoyland JA, Freemont AJ. Connective tissue growth factor expression in human intervertebral disc: implications for angiogenesis in intervertebral disc degeneration. Biotech Histochem. 2008;83:239–45. doi: 10.1080/10520290802539186. [DOI] [PubMed] [Google Scholar]
- 74.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275:32460–6. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 75.Mavrogonatou E, Kletsas D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair (Amst) 2009;8:930–943. doi: 10.1016/j.dnarep.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30:858–64. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 77.Raty HP, Battie MC, Videman T, Sarna S. Lumbar mobility in former elite male weight-lifters, soccer players, long-distance runners and shooters. Clin Biomech. 1997;12:325–330. doi: 10.1016/s0268-0033(97)00011-9. [DOI] [PubMed] [Google Scholar]
- 78.Videman T, et al. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine (Phila Pa 1976) 1995;20:928–935. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 79.Videman T, Levälahti E, Battié MC. The effects of anthropometrics, lifting strength, and physical activities in disc degeneration. Spine (Phila Pa 1976) 2007;32:1406–1413. doi: 10.1097/BRS.0b013e31806011fa. [DOI] [PubMed] [Google Scholar]
- 80.Galbusera F, Brayda-Bruno M, Wilke HJ. Is post-contrast MRI a valuable method for the study of the nutrition of the intervertebral disc? Journal of Biomechanics. 2014 doi: 10.1016/j.jbiomech.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 81.Xing QJ, et al. Leg amputation accelerates senescence of rat lumbar intervertebral discs. Spine (Phila Pa 1976) 2010;35:E1253–E1261. doi: 10.1097/BRS.0b013e3181e7d087. [DOI] [PubMed] [Google Scholar]
- 82.Wuertz K, et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27:1235–1242. doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.latridis JC, Godburn K, Wuertz K, Alini M, Roughley PJ. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine (Phila Pa 1976) 2011;36:203–209. doi: 10.1097/BRS.0b013e3181cec247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 85.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982:296–302. [PubMed] [Google Scholar]
- 86.Ohshima H, Urban JPG. The effect of lactate and pH on proteoglycan. Spine (Phila Pa 1976) 1992;17:1079–82. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 87.WG, QZ, XG, MDB Simulation of the progression of intervertebral disc degeneration due to decreased nutritional supply. Spine (Phila Pa 1976) 2014;39:E1411–7. doi: 10.1097/BRS.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shirazi-Adl A, Taheri M, Urban JPG. Analysis of cell viability in intervertebral disc: Effect of endplate permeability on cell population. J Biomech. 2010;43:1330–1336. doi: 10.1016/j.jbiomech.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 89.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 90.Bibby SRS, Urban JPG. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bibby SRS, Jones Da, Ripley RM, Urban JPG. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 92.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 93.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liebscher T, Haefeli M, Wuertz K, Nerlich AG, Boos N. Age-related variation in cell density of human lumbar intervertebral disc. Spine (Phila Pa 1976) 2011;36:153–9. doi: 10.1097/BRS.0b013e3181cd588c. [DOI] [PubMed] [Google Scholar]
- 95.Cole TC, Ghosh P, Taylor TK. Variations of the proteoglycans of the canine intervertebral disc with ageing. Biochim Biophys Acta. 1986;880:209–19. doi: 10.1016/0304-4165(86)90082-6. [DOI] [PubMed] [Google Scholar]
- 96.Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine (Phila Pa 1976) 2000;25:166–9. doi: 10.1097/00007632-200001150-00005. [DOI] [PubMed] [Google Scholar]
- 97.Singh K, Masuda K, Thonar EJMA, An HS, Cs-Szabo G. Age-Related Changes in the Extracellular Matrix of Nucleus Pulposus and Anulus Fibrosus of Human Intervertebral Disc. Spine (Phila Pa 1976) 2009;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearce RH, Mathieson JM, Mort JS, Roughley PJ. Effect of age on the abundance and fragmentation of link protein of the human intervertebral disc. J Orthop Res. 1989;7:861–7. doi: 10.1002/jor.1100070612. [DOI] [PubMed] [Google Scholar]
- 99.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J 326 (Pt. 1997;1:235–41. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okuda S, et al. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine (Phila Pa 1976) 2001;26:2421–6. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 101.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine (Phila Pa 1976) 1997;22:2781–95. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 102.Haidong Xu, Mei Qiang, Xu Bin, Gang Liu JZ. Expression of matrix metalloproteinases is positively related to the severity of disc degeneration and growing age in the East Asian lumbar disc herniation patients. Cell Biochem Biophys. 2014;70:1219–25. doi: 10.1007/s12013-014-0045-y. [DOI] [PubMed] [Google Scholar]
- 103.Zhao CQ, et al. ADAMTS-5 and intervertebral disc degeneration: The results of tissue immunohistochemistry and in vitro cell culture. J Orthop Res. 2011;29:718–725. doi: 10.1002/jor.21285. [DOI] [PubMed] [Google Scholar]
- 104.Bachmeier BE, et al. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- 105.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–61. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Zhu Q, Gao X, Gu W. Temporal changes of mechanical signals and extracellular composition in human intervertebral disc during degenerative progression. J Biomech. 2014;47:3734–3743. doi: 10.1016/j.jbiomech.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 109.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 111.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15(Suppl 3):S312–6. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gruber HE, Ingram JA, Davis DE, Hanley EN. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009;9:210–5. doi: 10.1016/j.spinee.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 117.Kim KW, Chung HN, Ha KY, Lee JS, Kim YY. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009;9:658–66. doi: 10.1016/j.spinee.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 118.Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther. 2008;10:R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89–103. doi: 10.22203/ecm.v030a07. [DOI] [PubMed] [Google Scholar]
- 120.Ngo Kevin. Master’s Thesis. University of Pittsburgh; 2015. CHARACTERIZATION OF SENESCENT INTERVERTEBRAL DISC CELLS AND THEIR ROLE IN PERTURBATION OF MATRIX HOMEOSTASIS. [Google Scholar]
- 121.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park JS, Park JB, Park IJ, Park EY. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int Orthop. 2014;38:1311–20. doi: 10.1007/s00264-014-2296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phillips KLE, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23:1165–77. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 124.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phillips KLE, Jordan-Mahy N, Nicklin MJH, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72:1860–7. doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- 126.Hiyama A, et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–47. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–12. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pawlikowska L, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–72. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang CY, Travascio F, Gu WY. Quantitative analysis of exogenous IGF-1 administration of intervertebral disc through intradiscal injection. J Biomech. 2012;45:1149–55. doi: 10.1016/j.jbiomech.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Z, et al. Expression of silent mating type information regulator 2 homolog 1 and its role in human intervertebral disc cell homeostasis. Arthritis Res Ther. 2011;13:R200. doi: 10.1186/ar3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 132.Acharyya S, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim HJ, et al. Antioxidant α-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-κB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-κB ligand and tumor necrosis factor-α. Free Radic Biol Med. 2006;40:1483–1493. doi: 10.1016/j.freeradbiomed.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 134.Adler AS, et al. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–57. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases. Eur Cell Mater. 2012;23:103–19. doi: 10.22203/ecm.v023a08. discussion 119–20. [DOI] [PubMed] [Google Scholar]
- 136.Nasto LA, et al. ISSLS prize winner: inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976) 2012;37:1819–25. doi: 10.1097/BRS.0b013e31824ee8f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Le Maitre C, Hoyland J, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang P, Han J, Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218–26. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 140.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 141.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 142.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–19. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maruyama J, Naguro I, Takeda K, Ichijo H. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem. 2009;16:1229–35. doi: 10.2174/092986709787846613. [DOI] [PubMed] [Google Scholar]
- 144.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gruber HE, Hoelscher GL, Ingram JA, Zinchenko N, Hanley EN. Senescent vs. non-senescent cells in the human annulus in vivo: cell harvest with laser capture microdissection and gene expression studies with microarray analysis. BMC Biotechnol. 2010;10:5. doi: 10.1186/1472-6750-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, et al. Tumor necrosis factor-α- and interleukin-1β-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-κB axis: implications in inflammatory disc disease. Am J Pathol. 2014;184:2560–72. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mavrogonatou E, Angelopoulou MT, Kletsas D. The catabolic effect of TNFα on bovine nucleus pulposus intervertebral disc cells and the restraining role of glucosamine sulfate in the TNFα-mediated up-regulation of MMP-3. J Orthop Res. 2014;32:1701–7. doi: 10.1002/jor.22725. [DOI] [PubMed] [Google Scholar]
- 148.Tian Y, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182:2310–21. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang W, et al. Interleukin-1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–272. doi: 10.1016/j.cca.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 150.Studer RK, et al. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008;26:991–998. doi: 10.1002/jor.20604. [DOI] [PubMed] [Google Scholar]
- 151.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 152.Pratsinis H, Kletsas D. Growth Factors in Intervertebral Disc Homeostasis. Connect Tissue Res. 2015;49:273–276. doi: 10.1080/03008200802147951. [DOI] [PubMed] [Google Scholar]
- 153.Pratsinis H, Constantinou V, Pavlakis K, Sapkas G, Kletsas D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30:958–64. doi: 10.1002/jor.22017. [DOI] [PubMed] [Google Scholar]
- 154.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–66. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mavrogonatou E, Kletsas D. Effect of varying osmotic conditions on the response of bovine nucleus pulposus cells to growth factors and the activation of the ERK and Akt pathways. J Orthop Res. 2010 doi: 10.1002/jor.21140. [DOI] [PubMed] [Google Scholar]
- 156.Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–83. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. Matrix Biol. 2014;40:10–6. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Risbud MV, et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–9. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 159.Fujita N, Chiba K, Shapiro IM, Risbud MV. HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2012;27:401–12. doi: 10.1002/jbmr.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fujita N, et al. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J Biol Chem. 2012;287:16975–86. doi: 10.1074/jbc.M111.334466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hirose Y, et al. FIH-1-Mint3 axis does not control HIF-1 transcriptional activity in nucleus pulposus cells. J Biol Chem. 2014;289:20594–605. doi: 10.1074/jbc.M114.565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agrawal A, et al. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 163.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–61. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 164.Gogate SS, Nasser R, Shapiro IM, Risbud MV. Hypoxic regulation of β-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: role of hypoxia-inducible factor proteins. Arthritis Rheum. 2011;63:1950–60. doi: 10.1002/art.30342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Agrawal A, et al. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008;58:3798–808. doi: 10.1002/art.24073. [DOI] [PubMed] [Google Scholar]
- 166.Merceron C, et al. Loss of HIF-1α in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One. 2014;9:e110768. doi: 10.1371/journal.pone.0110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–38. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Welford SM, et al. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006;20:3366–71. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kilic Eren M, Tabor V. The role of hypoxia inducible factor-1 alpha in bypassing oncogene-induced senescence. PLoS One. 2014;9:e101064. doi: 10.1371/journal.pone.0101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fujita N, et al. Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-α (TNF-α) on cells of the nucleus pulposus through co-activation of nuclear factor κB (NF-κB)/p65 signaling. J Biol Chem. 2012;287:39942–53. doi: 10.1074/jbc.M112.375964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li J, et al. Prolyl-4-hydroxylase domain protein 2 controls NF-κB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J Biol Chem. 2015;290:7195–207. doi: 10.1074/jbc.M114.611483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Blanco JF, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–65. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 173.Feng G, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675–85. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Risbud MV, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–44. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 175.Sakai D, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Setton La, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–7. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 177.Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2710–2723. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- 178.Chuang SY, Popovich JM, Lin LC, Hedman TP. The effects of exogenous crosslinking on hydration and fluid flow in the intervertebral disc subjected to compressive creep loading and unloading. Spine (Phila Pa 1976) 2010;35:E1362–E1366. doi: 10.1097/BRS.0b013e3181e68695. [DOI] [PubMed] [Google Scholar]
- 179.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39:1021–1029. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 180.Scharf B, et al. Age-related carbonylation of fibrocartilage structural proteins drives tissue degenerative modification. Chem Biol. 2013;20:922–934. doi: 10.1016/j.chembiol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.O’Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res. 2011;29:547–55. doi: 10.1002/jor.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Osti OL, Vernon-Roberts B, Moore R, Fraser RD. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678–682. doi: 10.1302/0301-620X.74B5.1388173. [DOI] [PubMed] [Google Scholar]
- 183.Cortes DH, Han WM, Smith LJ, Elliott DM. Mechanical properties of the extra-fibrillar matrix of human annulus fibrosus are location and age dependent. J Orthop Res. 2013;31:1725–1732. doi: 10.1002/jor.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Iatridis JC, Michalek AJ, Purmessur D, Korecki CL. Localized intervertebral disc injury leads to organ level changes in structure, cellularity, and biosynthesis. Cellular and Molecular Bioengineering. 2009;2:437–447. doi: 10.1007/s12195-009-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211:444–452. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cao L, Guilak F, Setton LA. Pericellular Matrix Mechanics in the Anulus Fibrosus Predicted by a Three-Dimensional Finite Element Model and In Situ Morphology. Cell Mol Bioeng. 2009;2:306–319. doi: 10.1007/s12195-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800–6. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cho H, Seth A, Warmbold J, Robertson JT, Hasty KA. Aging affects response to cyclic tensile stretch: Paradigm for intervertebral disc degeneration. Eur Cells Mater. 2011;22:137–146. doi: 10.22203/ecm.v022a11. [DOI] [PubMed] [Google Scholar]
- 189.Sowa GA, et al. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli: a pilot study. Am J Phys Med Rehabil. 2012;91:846–55. doi: 10.1097/PHM.0b013e31825f145a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stokes IAF, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976) 2004;29:2724–32. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abumi K, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142–7. doi: 10.1097/00007632-199011010-00011. [DOI] [PubMed] [Google Scholar]
- 192.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 1988;13:179–87. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 193.Iatridis JC, MacLean JJ, O’Brien M, Stokes IAF. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32:1493–7. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–72. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 195.Krismer M, Haid C, Rabl W. The contribution of anulus fibers to torque resistance. Spine (Phila Pa 1976) 1996;21:2551–7. doi: 10.1097/00007632-199611150-00004. [DOI] [PubMed] [Google Scholar]
- 196.Michalek AJ, Iatridis JC. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. Spine J. 2012;12:425–432. doi: 10.1016/j.spinee.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu J, Fairbank JCT, Roberts S, Urban JPG. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976) 2005;30:1815–20. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- 198.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine (Phila Pa 1976) 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 199.Hartman RA, Bell KM, Debski RE, Kang JD, Sowa GA. Novel ex-vivo mechanobiological intervertebral disc culture system. J Biomech. 2012;45:382–5. doi: 10.1016/j.jbiomech.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Muller-Lutz A, et al. Age-dependency of glycosaminoglycan content in lumbar discs: A 3T gagCEST study. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.24945. [DOI] [PubMed] [Google Scholar]
- 201.Iatridis JC, Setton LA, Weidenbaum M, Mow VC. The viscoelastic behavior of the non-degenerate human lumbar nucleus pulposus in shear. J Biomech. 1997;30:1005–1013. doi: 10.1016/s0021-9290(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 202.Iatridis JC, Setton LA, Weidenbaum M, Mow VC. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15:318–322. doi: 10.1002/jor.1100150224. [DOI] [PubMed] [Google Scholar]
- 203.Acaroglu ER, et al. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 204.Nasto LA, et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res. 2013;31:1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klawitter M, et al. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm. 2012;9:29. doi: 10.1186/1476-9255-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma T, et al. The effect of curcumin on NF-κB expression in rat with lumbar intervertebral disc degeneration. Eur Rev Med Pharmacol Sci. 2015;19:1305–14. [PubMed] [Google Scholar]
- 207.Li X, et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine (Phila Pa 1976) 2008;33:2586–95. doi: 10.1097/BRS.0b013e3181883883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krupkova O, et al. Epigallocatechin 3-gallate suppresses interleukin-1β-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372–86. doi: 10.22203/ecm.v028a26. [DOI] [PubMed] [Google Scholar]
- 209.Horii M, et al. Direct application of the tumor necrosis factor-α inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine (Phila Pa 1976) 2011;36:E80–5. doi: 10.1097/BRS.0b013e3181d4be3c. [DOI] [PubMed] [Google Scholar]
- 210.Le Maitre CL, Freemont AJ, Hoyland JA. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol. 2006;87:17–28. doi: 10.1111/j.0959-9673.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tilstra JS, et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 2012;122:2601–12. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Okuma M, Miyamoto K, Chujo T, Kitahara S, Masuda A new gene therapy approach: In vivo transfection of “naked” NF-kB decoy oligonucleotide restored disc degeneration in the rabbit annular needle puncture model. Orthopedic Research Society, translator. 2005 at < http://www.ors.org/Transactions/51/0045.pdf>.
- 213.Nishida K, et al. Gene therapy approach for disc degeneration and associated spinal disorders. Eur Spine J. 2008;17(Suppl 4):459–66. doi: 10.1007/s00586-008-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Feng C, Liu H, Yang Y, Huang B, Zhou Y. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem. 2015;35:1–16. doi: 10.1159/000369670. [DOI] [PubMed] [Google Scholar]
- 215.Mwale F, Wang HT, Roughley P, Antoniou J, Haglund L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng Part A. 2014;20:2942–9. doi: 10.1089/ten.tea.2013.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Leckie SK, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Clarke LE, Richardson SM, Hoyland JA. Harnessing the Potential of Mesenchymal Stem Cells for IVD Regeneration. Curr Stem Cell Res Ther. 2015;10:296–306. doi: 10.2174/1574888x10666141202112638. [DOI] [PubMed] [Google Scholar]
- 218.Leckie SK, et al. Injection of human umbilical tissue derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13:263–272. doi: 10.1016/j.spinee.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cho H, et al. Snapshot of degenerative aging of porcine intervertebral disc: a model to unravel the molecular mechanisms. Exp Mol Med. 2011;43:334–40. doi: 10.3858/emm.2011.43.6.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Busscher I, Ploegmakers JJW, Verkerke GJ, Veldhuizen AG. Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J. 2010;19:1104–14. doi: 10.1007/s00586-010-1326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shimizu K, et al. Amyloid deposition in intervertebral discs of senescence-accelerated mouse. Arthritis Rheum. 1982;25:710–2. doi: 10.1002/art.1780250618. [DOI] [PubMed] [Google Scholar]
- 222.Isaac C, et al. Dystrophin and utrophin ‘double knockout’ dystrophic mice exhibit a spectrum of degenerative musculoskeletal abnormalities. J Orthop Res. 2013;31:343–9. doi: 10.1002/jor.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bedore J, et al. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 2013;65:2634–44. doi: 10.1002/art.38075. [DOI] [PubMed] [Google Scholar]
- 224.Furukawa T, et al. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine (Phila Pa 1976) 2009;34:E911–7. doi: 10.1097/BRS.0b013e3181b7c7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mason RM, Palfrey AJ. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2:333–8. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- 226.Semba K, et al. A novel murine gene, Sickle tail, linked to the Danforth’s short tail locus, is required for normal development of the intervertebral disc. Genetics. 2006;172:445–56. doi: 10.1534/genetics.105.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Paavola LG, Wilson DB, Center EM. Histochemistry of the developing notochord, perichordal sheath and vertebrae in Danforth’s short-tail (sd) and normal C57BL/6 mice. J Embryol Exp Morphol. 1980;55:227–45. [PubMed] [Google Scholar]
- 228.Holguin N, Aguilar R, Harland RA, Bomar BA, Silva MJ. The aging mouse partially models the aging human spine: lumbar and coccygeal disc height, composition, mechanical properties, and Wnt signaling in young and old mice. J Appl Physiol. 2014;116:1551–60. doi: 10.1152/japplphysiol.01322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Silberberg R, Gerritsen G. Aging changes in intervertebral discs and spondylosis in Chinese hamsters. Diabetes. 1976;25:477–83. doi: 10.2337/diab.25.6.477. [DOI] [PubMed] [Google Scholar]
- 230.Laing AC, Cox R, Tetzlaff W, Oxland T. Effects of advanced age on the morphometry and degenerative state of the cervical spine in a rat model. Anat Rec (Hoboken) 2011;294:1326–36. doi: 10.1002/ar.21436. [DOI] [PubMed] [Google Scholar]
- 231.Gruber HE, Gordon B, Williams C, Norton HJ, Hanley EN. Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross-sectional analyses of a large male and female population. Spine (Phila Pa 1976) 2007;32:2529–36. doi: 10.1097/BRS.0b013e318158cd69. [DOI] [PubMed] [Google Scholar]
- 232.Sowa G, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33:1821–8. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 233.Bergknut N, et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976) 2012;37:351–8. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 234.Wade KR, Robertson PA, Broom ND. Influence of maturity on nucleus-endplate integration in the ovine lumbar spine. Eur Spine J. 2014;23:732–44. doi: 10.1007/s00586-014-3181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stolworthy DK, et al. MRI evaluation of spontaneous intervertebral disc degeneration in the alpaca cervical spine. J Orthop Res. 2015 doi: 10.1002/jor.22968. [DOI] [PubMed] [Google Scholar]
- 236.Duncan AE, Colman RJ, Kramer PA. Longitudinal study of radiographic spinal osteoarthritis in a macaque model. J Orthop Res. 2011;29:1152–60. doi: 10.1002/jor.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]