Abstract
Increased vascular permeability is a common pathogenic feature in many inflammatory diseases. For example in acute lung injury (ALI) and its most severe form, the acute respiratory distress syndrome (ARDS), lung microvessel endothelia lose their junctional integrity resulting in leakiness of the endothelial barrier and accumulation of protein rich edema. Increased reactive oxygen species (ROS) generated by neutrophils (PMNs) and other inflammatory cells play an important role in increasing endothelial permeability. In essence, multiple inflammatory syndromes are caused by dysfunction and compromise of the barrier properties of the endothelium as a consequence of unregulated acute inflammatory response. This review focuses on the role of ROS signaling in controlling endothelial permeability with particular focus on ALI. We summarize below recent progress in defining signaling events leading to increased endothelial permeability and ALI.
Graphical Abstract
Introduction
Inflammatory syndromes, as an example - acute lung injury (ALI) and its agonal variant acute respiratory distress syndrome (ARDS) are the result of severe disturbances on the endothelial barrier[1, 2]. The worsening loss of endothelial barrier function is thought to be the result of an unregulated acute inflammatory response following an initiating event, such as sepsis. If this unchecked it leads to activation of the acute inflammatory response at a systemic level affecting the permeability of multiple vascular barriers, including lungs. One of the earliest manifestations is activation of pulmonary endothelial cells (EC) and macrophages (MΦ), upregulation of adhesion molecules, and production of cytokines and chemokines that induce a massive sequestration of PMNs within the pulmonary microvasculature, resulting from inappropriate adhesion of PMNs and other leukocytes with the hyper-adherent endothelium. These inflammatory cells transmigrate across the endothelium into tissue and release a variety of cytotoxic and proinflammatory compounds, including reactive oxygen species (ROS), proteolytic enzymes, and nitrogen species, cationic proteins, lipid mediators, and additional inflammatory cytokines[3]. Other cells remain sequestered in the microvessel where they produce the same toxic substances that also induce endothelial injury. In this short review, we will focus on the role of ROS-mediated signaling in disrupting the barrier properties of the endothelium in the context of ALI (used here as a paradigm for other inflammatory disease associated with inflammation). We will focus on recent progress in studies on the signaling event underline the interaction of PMN and EC and its consequences on endothelial barrier function.
Constitution of endothelial barrier and regulation of EC permeability
The endothelium functions as a semipermeable barrier regulating tissue fluid homeostasis and transmigration of leukocytes and providing essential nutrients across the vessel wall (see reviews [4, 5]). Transport of plasma proteins and solutes or transmigration of leukocytes across the endothelium involves two different routes: one transcellular, via caveolae-mediated vesicular transport, and the other paracellular, through interendothelial junctions[5]. Briefly, the transcellular or transcytosis pathway is responsible for the transport of albumin across the endothelial barrier via fission of plasma membrane macrodomains enriched with caveolin-1 (Cav-1), caveolae, from the luminal surface of the endothelial cell followed by transport of caveolar vesicles to the basal surface. This finely regulated process is essential for transport of albumin, albumin-bound ligands, and hormones and for control of tissue oncotic pressure in the normal continuous endothelium and the interstitial space[5]. The paracellular permeability of the endothelial barrier is maintained by the interendothelial junctions, the structures that by connecting adjacent endothelial cells into the monolayer restrict the transport of plasma proteins of the size of albumin from the vessel lumen to stroma[5]. Two general types of interendothelial junctions present in the endothelium, tight junctions (TJs) and adherence junctions (AJs), contribute to maintenance of the endothelial barrier. The molecule primarily responsible for AJs is the transmembrane hemophilic adhesion molecule, vascular endothelial (VE)-cadherin[6]. Homotypic formation of firm EC–EC junctions is maintained by VE cadherin with its cytoplasmic domain binding to β-catenin, and α-catenin which is also linked to the actin cytoskeleton[6]. The linkage between VE-cadherin-based adheren junctional complex and the actin cytoskeleton contributes to the strong adhesion[7]. While the transcellular permeability is mainly regulated by caveolae-mediated transcytosis, there are two independent mechanisms involved in regulating paracellular endothelial permeability: destabilization of AJs via phosphorylation of constituents of AJs, which in most cases leads to VE-cadherin internalization, and activation of acto-myosin contractility accompanied by reorganization of the actin cytoskeleton into stress fibers, thus applying mechanical forces to AJs that break apart the junctions (see more details in[5]). Below we describe the paracellular permeability of lung EC regulated by ROS-mediated signaling as there is far more known about this pathway than the transcytosis/transcellular which remain enigmatic despite its potential importance in regulating tissue fluid balance and pathophysiological relevance in inflammatory diseases.
Participation of ROS in regulating lung EC barrier function
At physiological concentrations, reactive oxygen species (ROS) play an important role as regulatory mediators in signaling processes including regulation of vascular tone, monitoring of oxygen tension in the control of ventilation and erythropoietin production, and signal transduction from membrane receptors in various physiological processes [8]. Many of the ROS-mediated responses protect the cells against oxidative stress and reestablish “redox homeostasis” [8]. Increased reactive oxygen species (ROS) generated in tissue during inflammation play an important role in the development of not only the full-blown inflammatory disease but also its chronicity; this case has been strongly made for ALI and its progression to ARDS[9-13]. Biologically important ROS include superoxide anion radical (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH−), and hypohalous acids such as HOCl[3]. There are many potential sources of ROS, including phagocytes (PMN and MΦ) and non-professional phagocytes including endothelium, epithelium, fibroblasts, and smooth muscle cells that express NADPH oxidase (NOX) are capable of generating physiologically important amounts of ROS[3]. Other sources of ROS include mitochondrial electron transport chain, cytochrome P450, and xanthine oxidase[3]. Leukocytes, principally PMN and MΦs, are generally considered to be the most prodigious source of ROS in the context of ALI/ARDS[3] (Figure 1). PMN and MΦ express NOX2 that can generate ROS in substantial amounts[14]. The large numbers of activated PMN in the lung in ALI has focused attention on these phagocytes as a major source of ROS and disease progression. While major mechanisms of scavenging of ROS exist and are important, these are overwhelmed in the face of intense barrage of ROS generated during the inflammatory crisis. To add to the “toxic soup”, diverse pro-inflammatory mediators lipopolysaccharide (LPS), cytokines, chemokines, complement fragments, clotting fragments, and lipid mediators are released setting up multiple amplification loops and further activating PMNs and other inflammatory cells to generate ROS[3]. The ECs are also involved in this amplification process. There are four isoforms, NOX1, NOX2, NOX4 and NOX5, expressed in endothelial cells[14-16]. A critical in vivo role of endothelial NOX2[17] in oxidant-mediated endothelial barrier dysfunction has been described using transgenic mice overexpressing NOX2 in the endothelium[15, 18-22]. In response to inflammatory mediators, endothelial overexpression of NOX2 led to increased ROS production compared to wild type, whereas knockdown of NOX2 with siRNA reduced this effect [23, 24].
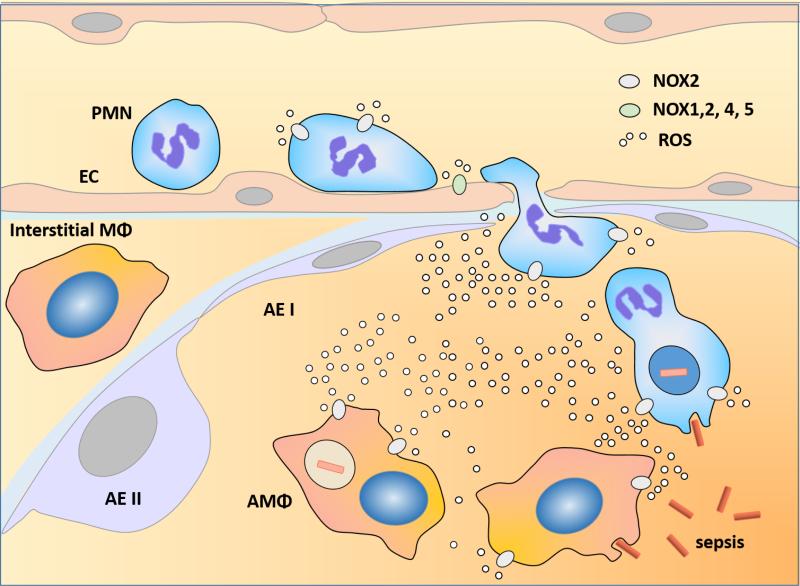
Figure 1. NOX (NADPH oxidase) mediated production of ROS (reactive oxygen species).
In response to an inciting event such as a result of a bacterial infection, the pulmonary macrophages (MΦ) and endothelial cells (EC) become activated and upregulate surface expression of adhesion molecules, leading to polymorphonuclear leukocytes (PMN) adhesion and subsequent transmigration from the intravascular space into the alveolus[3].. Activated alveolar MΦ (AMΦ), PMN and EC produce reactive oxygen species (ROS), however, PMN is the major source of ROS production in the context of acute lung injury and acute respiratory distress syndrome (ARDS) induced by sepsis[3]. AEI: alveolar epithelial type I; AEII: alveolar epithelial type II. NOXs are divided into two major groups here according to its distribution in cells: NOX2 group expressed in phagocytes (PMN, MΦ)[14]; NOX1,2,4,5 group expressed in ECs[14-16].
In addition, post translational mechanisms are important in the generation of ROS and likely participate in the loss of endothelial barrier function. A key step in NOX2-derived ROS production is the phosphorylation on serines by PKC isoforms such as PKCδ[25]. NOX4 and NOX5 isoforms, abundant in ECs[15], their roles has not been well investigated. Interesting recent evidence shows that NOX5, also present in ECs, can be activated by Ca2+ [15], raising the intriguing possibility that Ca2+ entry may be important in activation of ROS signaling through this isoform; thus, Ca2+-mediated signaling pathway and ROS-mediated signaling pathway may interact to synergistically increase EC permeability.
ROS-mediated signaling in controlling EC paracellular permeability
ROS mediated EC barrier dysfunction by directly attacking on various cellular components including membrane, cytosolic, and nuclear lipids and proteins through oxidative modifications [3]. Below we discuss the signals activated by ROS that underlie the increased permeability response.
1. Role of ROS in initiating Ca2+ signaling in ECs
ROS generation by activated MΦ, PMNs, ECs, and other inflammatory cells sequestered in lungs is a critical factor mediating increased lung EC permeability in the setting of sepsis [9-13]. An increase in cytosolic Ca2+ precedes changes in endothelial cell shape and the opening of AJs [26-29]. ROS production from endothelium also increases cytosolic Ca2+ mediating increased lung EC permeability[30, 31]. However, precisely how oxidants influence Ca2+ signaling remains unclear. With the discovery of oxidant-sensitive transient receptor potential (TRP) channels including TRPC3,4,5,6, TRPM2, TRPMV1, TRPA1which are also permeable to Ca2+ [32-41], we now have better understanding of the mechanisms underlying the link between oxidant stress and EC permeability. TRP family has emerged as a predominant regulator of non-selective cation channels that mediate Ca2+ entry in endothelial cells[26, 42-44]. Of these TRP channels, TRPC, TRPM and TRPV are more studied in endothelial cells[30, 42, 45-47]. Like other TRP channels, these channels also contains six transmembrane domains with a pore forming unit located between transmembrane domain 5 and 6[44]. TRPC and TRPM family members also contain proline -rich sequences in the C-terminal region of TRP domain designated as TRP box 2 which binds phosphatidyl insositol phosphates, such as PI (4, 5) P2 [4, 44, 48, 49]. TRPC and TRPV family members also contain 3-4 ankyrin repeats at their N-terminus[44, 50]. The functional TRP channel is a tetramer, and may be composed of homo- or hetero- tetramers formed within the same sub-family[44]. Recent studies in our laboratory along with other studies strongly indicate that TRPC (including TRPC1, TPPC3, TRPC4 and TRPC6) act as store operated Ca2+ (SOC) entry or receptor-operated calcium (ROC) entry channels that participate in vascular permeability regulation[51-55]. The important association between calcium release from the endoplasmic reticulum (ER) and calcium entry across the plasma membrane had been recognized for about 30 years[56]. But the nature of coupling between ER and plasma membrane that underlies SOC entry has been poorly understood until the recent identification of ORAI (calcium release-activated calcium channel protein 1), the pore of Ca2+ release-activated Ca2+ channel (CRAC) and stromal interaction molecule 1 (STIM1), a ROS sensor[57], which also senses the depletion of Ca2+ from the ER, and then oligomerizes, translocates to junctions adjacent to plasma membrane, helps to organize ORAI or TRPC channels in the membrane[58]. The following section we will focus on the roles of the most-studied TRPC6, TRPM2 and STIMI on ROS-mediated Ca2+ signaling in ECs.
TRPC6 is highly expressed in human and mouse lung endothelial cells[27, 29, 59-61] and can be activated by ROS (H2O2)[62, 63]. In TRPC6-expressing HEK293T cells, H2O2 significantly stimulated Ca2+ entry in a dose-dependent manner[62]. Electrophysiological experiments showed that H2O2 significantly increased TRPC6 channel open probability and whole-cell currents[62]. Additionally, H2O2 stimulated a dose-dependent constriction of the aortas from wild type but not from the vessels of TRPC6 deficient mice[63]. These results suggest a new signaling pathway mediated by ROS-TRPC6 in controlling vessel contraction [63]. TRPC6 is also activated by DAG independently of store depletion and is therefore referred to as receptor-operated channels (ROC) [27, 29, 52, 64]. Several studies showed that TRPC6 plays an important role in regulating endothelial permeability[29, 59, 60]. Initially, flufenamic acid, a TRPC6 activator, was shown to increase water conductivity in frog mesenteric vessels[59, 65]. Subsequent studies showed that siRNA-induced suppression of TRPC6 channel in human pulmonary artery endothelial cells decreased endothelial cell permeability in response to thrombin[27]. Recently we showed that TRPC6 plays a key role in signaling both LPS-induced lung vascular permeability and inflammation[29]. Tauseef et al showed that LPS, which is well-known to induce ROS, resulted in DAG production which activated TRPC6 [29]. Activated TRPC6 induced MLCK (myosin light chain kinase) activity that by stimulating actomyosin cross-bridging mediates endothelial cell contraction leading to increased lung vascular permeability[29]. Additionally, activated MLCK promoted the interaction of myeloid differentiation primary response gene (MyD88) with inteterleukin receptor −1 associated kinase 4 which is involved in triggering NF-kB signaling and pulmonary inflammation downstream of TLR4 [29]. Thus, TRPC6 appears to be at the center of the signaling pathways mediating ALI owing to its dual role in increasing lung vascular permeability and mediating TLR4 signaling[29]. In a recent study we also showed that TRPC6 was required for transendothelial migration i.e. diapedesis downstream of PECAM (Platelet endothelial cell adhesion molecule-1) homophilic interactions[66]. We showed that TRPC6 colocalized with PECAM and regulated PMN transendothelial migration (TEM). Expression of dominant-negative TRPC6 or shRNA knockdown in endothelial cells arrested PMN apically over the junction while selective activation of endothelial TRPC6 with hyperforin 9 induced TEM even in the absence of PECAM[66]. Consistently, in a croton oil-mediated acute ear inflammation model, mice lacking TRPC6 exhibited a profound defect in neutrophil TEM with no effect on leukocyte trafficking[66] (Figure 2). It is interesting that ROS activates TRPC6 channels via modification of thiol groups of intracellular proteins and this cysteine oxidation-dependent pathway not only stimulates the TRPC6 channel by itself but also sensitizes the channels to DAG [62]. Thus, ROS may enhance LPS-TRPC6 pathway in a DAG dependent manner in regulating EC permeability.
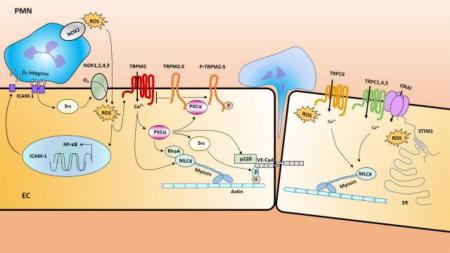
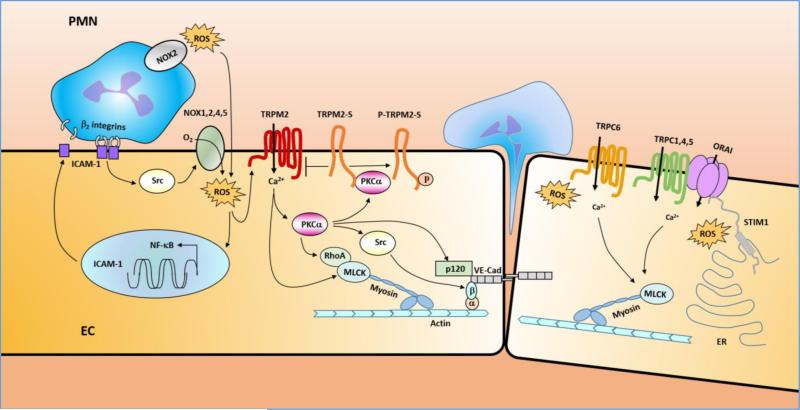
Figure 2. Model of ROS-signaling regulating endothelium permeability.
Upon ROS challenge, endothelial cells (EC) become activated and upregulate surface expression of adhesion molecules such as ICAM1(intercellular adhesion molecule 1) through a NF-κB dependent mechanism[103-105]. The binding of the increased ICAM1 surface expression of EC with β2 integrin of PMN induces the activation of endothelial NOXs (NOX1,2,4,5) through Src-dependent mechanism[95],[125]. Thus, activation of the NOX isoforms above and resulting endothelial ROS production stimulates NF-κB-dependent upregulation of ICAM-1 expression in ECs setting-up a positive feedback loop leading to ROS production. ROS released from both PMN NOX2 and EC NOX1,2,4,5 activated the redox sensitive Ca2+ permeable channel TRPM2 in the endothelium and lead to Ca2+ entry into EC and promote PKCα activation. Activated PKCα may regulate EC permeability via three major pathways: (1). Activated PKCα phosphorylates p120-catenin to induce dissociation of p120-catenin from VE-cadherin[91] leading to disruption of adherent junctions (AJs) and transmigration of PMN; (2). Activated PKCα mediates activation of cSrc [92, 93] which directly or indirectly leads to tyrosine phosphorylation of VE-cadherin and β-catenin, resulting in AJ destabilization[94, 95]; (3). Activated PKCα cooperates with MLCK to elicit a coordinated spatial activation of RhoA and global reorganization of the actin cytoskeleton, resulting in endothelial barrier dysfunction[5]. Activated PKCα also interacts with TRPM2-S and release its interaction with TRPM2 and thereby promotes TRPM2 channel activity[30, 46]. Thus, TRPM2/TRPM2-S interaction serves as a critical switch responsible for increased lung vascular permeability and PMN transmigration. On the other hand, ROS may also participate in regulating Ca2+ influx by inducing activation of redox-sensitive TRPC6 [29, 62, 63] and TRPC1,4,5[51-55] and ORAI[58] trough redox-sensitive STIM1[57, 69, 70]. The rise in intracellular Ca2+ through these TRPC channels or ORAI may activate key signaling pathways, resulting in MLC-dependent endothelial cell contraction and disassembly of vascular endothelial cadherin (VE-cadherin) at the adherens junctions and lead to increased vascular permeability [64, 73].
STIM1, a type 1A single transmembrane protein originally identified as a tumor suppressor protein, has been established as a Ca2+ sensor within the ER stores[57, 67]. STIM1 contains N-terminal EF hand domain, a Ca2+ binding domain, a sterile α motif or SAM domain, a single transmembrane domain (TM), an ezrin-radixin-moesin (ERM) domain, a serine-proline rich region (S/P-region) and a lysine rich region (E-region)[57, 58, 68]. STIM1 dimerizes before clustering and the activation spices is the dimer. When bound with Ca2+ in ER lumen, STIM1 exists as an individual unit or a monomer. Upon store depletion the EF hand domains and SAM domains from different STIM1 protein aggregate and form multimeric puncta which leads to its interaction with store operated Ca2+ channel and activation of SOC entry[28, 58, 67]. STIM1 has been shown to regulate TRPC channels such as TRPC1, 4,5, and ORAI channels which are the important constituents of SOC and CRAC respectively[69, 70]. STIM1 has been shown to act as ROS sensor[57]. ROS were shown to induce STIM1 aggregation, STIM translocation to ER–plasma membrane junctions and activation of ORAI channels without Ca2+ store depletion[57, 71]. ROS-induced S-glutathionylation of Cys56 in the amino terminus of STIM1 decreases Ca2+ binding by the EF-hand domain and triggers STIM1 activation[57, 71]. Oxidant stress led to a phenotypic shift in Ca2+ mobilization from an oscillatory to a sustained elevated pattern via CRAC-mediated capacitive Ca2+ entry, and STIM1- and ORAI-deficient cells are resistant to oxidant stress[71]. These experiments reveal that Cys56 is a sensor for oxidant-dependent activation of STIM1 and demonstrate a molecular link between oxidant stress and Ca2+ signaling via the CRAC channel[71]. A subsequent study report an alternative role of Cys56 in the mechanism of STIMI activation [72]. It was discovered that STIM1 oligomerization and SOC were modulated by the endoplasmic reticulum (ER) oxidoreductase ERp57 which interacts with the ER luminal domain of STIM1[72]. The interaction involving two conserved cysteine residues, C49 and C56. SOC is inhibited in C56 mutants of STIM1[72]. Thus, the role of Cys56 in activationg STIM1 is not completely clear. Our laboratory showed that Cav-1 forms an important link between TRPC1 and inositol 1,4,5-trisphosphate receptor 3 (IP3R3) [53]. We found that Cav-1 scaffold domain (CSD) interacts with both TRPC1 and IP3R3, and thereby regulates SOC-mediated entry [53]. The rise in intracellular Ca2+ through these TRPC channels may activate key signaling pathways, resulting in MLC-dependent endothelial cell contraction and disassembly of vascular endothelial cadherin (VE-cadherin) at the AJs and lead to increased vascular permeability [64, 73]. Thus, oxidant-sensitive STIMI1 may form a complex with TRPCs and/or ORAI to regulate ROS-mediated Ca2+ signaling in regulating EC permeability (Figure 2).
Recently we have described the important role of the redox-sensitive Ca2+ permeable cation channel TRPM2 (transient receptor potential melastatin 2) in regulating EC permeability following oxidative stress[30, 46]. The method of TRPM2 activation and its role is described in Figure 2. TRPM2 is an oxidant-sensitive cation channel expressed in endothelial and phagocytic cells[30, 35, 47, 74-78]. Channel opening after exposure to oxidants is induced by binding of the intracellular second messenger adenosine diphosphoribose (ADP-ribose) or related molecules to the Nudix box sequence[32, 78-80] in the carboxyl-terminal sequence of TRPM2[77]. The mechanism of TRPM2 channel activation involves H2O2, produced during oxidative stress, which activates the nuclear and mitochondrial production of ADP-ribose [32, 35, 80] that binds to the TRPM2 Nudix box sequence, and signals TRPM2-mediated Ca2+ entry[32, 35, 78-81]. We observed that suppressing endogenous TRPM2 expression or activity by small interfering RNAs, a specific anti-TRPM2 blocking antibody, overexpression of TRPM2-S isoform, and poly-ADP-ribose polymerase inhibitors prevented the generation of ADP-ribose[30, 35, 82, 83] and abolished the H2O2-induced Ca2+ influx via TRPM2 channel and, importantly, prevented the increase in endothelial permeability[30].
In addition to the full-length Ca2+ permeable channel protein TRPM2 (i.e., TRPM2-L), several TRPM2 isoforms have been identified, potentially the most important of these is the short splice variant (TRPM2-S)[84]. TRPM2-S lacks the carboxyl terminus of the longer forms including the putative Ca2+-permeable pore, and thus does not function directly as a Ca2+ channel but interacts with TRPM2 in the plasma membrane[30, 46], negatively regulates Ca2+-channel activity of TRPM2[46, 82, 84]. We observed that overexpression of TRPM2-S isoform inhibited the H2O2-induced Ca2+ influx via TRPM2 channel and prevented the increase in endothelial permeability[30]. Thus, TRPM2/TRPM2-S interaction is pathophsyiologically important and play a key role in the mechanism of increased lung vascular permeability and PMN tissue infiltration under oxidant stress.
2. ROS-Ca2+-PKCα pathway in signaling EC permeability
An earlier review well summarized potential mechanisms underlying ROS-mediated increased endothelial cell permeability that involved a serine and threonine-specific protein kinase family protein kinase C (PKC) such as PKCζ[4]. Among PKC family, PKCα is a member activated by Ca2+ and the second messenger DAG[85]. Ca2+ binding to the “Ca2+-binding loops” of PKCα is required for its translocation from the cytoplasm to the plasma membrane and for PKCα activation [86, 87]. We showed that TRPC6-induced Ca2+-entry was required for inducing PKCα activity [27]. Intriguingly, we found that PKCα has an important role in signaling the observed oxidant-induced TRPM2 activation[30, 46] raising the possibility that TRPC6 may induce TRPM2. We identified a high-affinity binding site for PKCα in the N-terminal domain of TRPM2, which regulated the interaction of PKCα with a TRPM2 isoform. H2O2 induced rapid co-localization of PKCα with TRPM2-S (the short-splice variant of TRPM2) that was not observed when TRPM2 was knocked down25,37; it is therefore likely that PKCα regulates TRPM2-induced Ca2+ entry and endothelial permeability through PKCα phosphorylation of TRPM2-S (Figure 2).
PKCα activation is known to increase microvascular permeability as first described by us in 1990 [88]. PKCα was found to change its intracellular distribution on H2O2 exposure [89] and signal H2O2-induced increase in endothelial permeability[12, 90] through mediating the phosphorylation of p120-catenin [91] (Figure 2). On the other hand, PKCα mediate activation of cSrc [92, 93] which directly or indirectly leads to tyrosine phosphorylation of VE-cadherin and β-catenin, resulting in adherens junction destabilization[94, 95]. PKCα also modulates RhoA GTPase activation by phosphorylation of the upstream regulators of RhoA, the Rho guanosine diphosphate (GDP) dissociation inhibitor GDI-1 and p115RhoGEF [5, 96]. PKCα phosphorylates GDI-1 at Ser96, thus reducing GDI-1 affinity for RhoA that favors the exchange of GDP to GTP by p115RhoGEF[5]. RhoA facilitates phosphorylation-induced inhibition of myosin light chain phosphatase (MLCP) by activating Rho kinase (ROCK)[97, 98]. The inhibition of MLCP accompanied by the Ca2+/calmodulin-dependent activation of MLCK leads to phosphorylation of MLC and induces acto-myosin contraction in response to proinflammatory mediators such as thrombin and histamine [5, 99-101]. In this regard, PKCα cooperates with MLCK to elicit a coordinated spatial activation of RhoA and global reorganization of the actin cytoskeleton, resulting in endothelial barrier dysfunction[5] (Figure 2). Thus, ROS-TRPM2-PKCα may form an important pathway in ROS-mediated controlling of EC permeability (Figure 2).
3. ROS -ICAM-1-Src circuit in controlling EC permeability
It is well known that oxidative stress is also initiated by activated PMNs [3, 12] following the adhesion and sequestration of PMNs. The production of oxygen metabolites such as H2O2 increases endothelial adhesivity of PMNs and lung vascular endothelial permeability[12, 82-84], both critical factors governing formation of tissue edema and PMN extravasation. Endothelial cell surface expression of ICAM-1, the counter-receptor for PMN β2-integrins, results in adhesion of activated PMNs to ECs and subsequently, PMN transmigration into tissue[102] (Figure 2). ROS stimulate the activation of NF-κB[103], which in turn controls the expression of key genes involved in mediating lung vascular inflammation and injury[103-105]. In resting cells, NF-κB proteins are sequestered in the cytoplasm through their tight association with IκB proteins. NF-κB activation relies on IκB phosphorylation and degradation, such that the freed NF-κB proteins translocate into the nucleus and regulate the expression of multiple inflammatory target genes[106]. As NOX2 is an essential regulator of oxidative stress-induced NF-κB activation[107], NOX2 activation induces upregulation of ICAM-1 expression through NOX-dependent NF-κB activation, it is possible that increased ICAM-1 expression promotes additional PMN/EC interaction, and hence amplifies TRPM2-mediated Ca2+-entry activating a feed-forward mechanism and leading to severe lung injury (Figure 2). In endothelial cells, the increase in intracellular Ca2+ was shown to induce exocytosis of Weibel-Palade bodies (WPB) [108-111] (specialized secretory vesicles containing preformed proteins such as P-selectin which are released upon cell stimulation) [112-117]. P-selectin (CD62P) is a member of the selectin family of cell adhesion molecules[118] and play a key role in PMN rolling adhesion to activated EC [119]. But the effect of ROS in regulating exocytosis of WBP remain unclear. One group reported that the expression of P-selectin on the surface of endothelial cells was accompanied by qualitatively parallel increases in ROS generation [120]. Both P-selectin expression and ROS generation were inhibited, dose dependently, by the exogenous administration of disparate cell-permeable antioxidants and also by the inhibition of either of the known membrane-associated ROS-generating enzymes NADPH oxidase or xanthine oxidase [120]. In contrast, it was found that H2O2 inhibit thrombin-induced exocytosis of granules from endothelial cells by inhibiting N-ethylmaleimide sensitive factor (NSF), a protein that regulates membrane fusion [121]. It would be important to determine whether ROS-mediated Ca2+ signaling up-regulates membrane surface expression of P-selectin through exocytosis of WPB and therefore up-regulated interaction of PMN and EC and form another pathway in amplifying TRPM2 signaling.
Recent studies have pointed to a key signaling role of ICAM-1, beyond its function as an adhesive protein regulating leukocyte adhesion and transmigration [122-124]. ICAM-1 engagement was shown to lead to activation of tyrosine kinase Src [95]. PMN binding to ICAM-1 or direct ICAM-1 cross-linking induced Src activation[125]. c-Src is an important upstream kinase that regulates NADPH oxidase-induced ROS production [7, 126] and the cytoplasmic tyrosine kinase c-Src induces NADPH oxidase activation [127, 128]. Exposure of cultured ECs to LDL stimulated ROS formation, which was prevented by Src kinase inhibitor PP1 [129]. Src mediates phosphorylation of p47phox and its translocation to the membrane in hyperoxia-induced activation of NADPH oxidase in lung ECs [130]. Src may also activate NADPH oxidase indirectly through another kinase such as PKCζ[131, 132]. Bearing in mind that the PMN β2-integrin interaction with ICAM-1 in ECs can activate ICAM-1 signaling [122-124], thus, NOXICAM-1-Src may form a key circuit in controlling EC permeability through ROS-mediated TRPM2 activation (Figure 2). On the other hand, the cytoplasmic tyrosine kinase c-Src may directly regulate EC permeability by transducing signals that mediate AJ destabilization and acto-myosin contractility [5, 94, 133-137]. c-Src activation directly or indirectly leads to tyrosine phosphorylation of VE-cadherin and β-catenin, resulting in AJ destabilization as mentioned above[94, 95]. Transmigration of leukocytes is associated with c-Src dependent phosphorylation of VE-cadherin at Tyr658 and Tyr731 and reduces VE-cadherin binding to p120-catenin and β-catenin[95]. Thus, ROS -ICAM-1-Src circuit may control lung EC permeability through both promoting the interaction of PMN and EC and initiating TRPM2 activation (Figure 2).
Role of ROS signaling in controlling EC transcellular permeability
There is little information about the role of ROS in controlling the transcellular permeability of EC[4, 5]. As mentioned earlier, the increase in intracellular Ca2+ in EC was shown to induce exocytosis of WPB including P-selectin upon cell stimulation[112-117], whether caveolar transcytosis of albumin requires a similar exocytic stimulus is unknown[4, 5]. It is important to clarify whether ROS control EC paracellular permeability via activating Ca2+ signal through redox-sensitive channel such as TRPM2.
Concluding Remarks
Increased ROS generated in tissue plays an important role in increasing endothelial permeability that progresses to inflammatory syndromes such as ALI/ARDS. Although in essence, these syndromes are caused by dysfunction and compromise of the barrier properties of endothelium as a consequence of an unregulated acute inflammatory response, the precise mechanisms of oxidant-mediated disruption of endothelial barrier remain elusive. With the discovery of oxidant-sensitive Ca2+ permeable TRP channels such as TRPM2 and TRPC6, and sensors for both Ca2+ and ROS such as STIM1, the mechanism underlying ROS mediated Ca2+ signaling in regulating EC permeability become clearer. Our most recent observations imply the potentially crucial role of endothelial redox sensitive TRPM2 channel activation in mediating the PMN activation-induced endothelial permeability and PMN infiltration as well as the role of PKCα – TRPM2-S pathway and ICAM1- c-Src pathway in the regulation of TRPM2 activation and TRPM2-induced increase in endothelial permeability under oxidant stress. Because both isoforms of TRPM2 (TRPM2-L and TRPM2-S) are expressed in endothelial cells, the mechanisms that regulate their expression or control of their interaction could be targeted to pharmacologically suppress endothelial permeability under oxidant stress. On the other hand, redox sensitive TRPC6 and other TRPCs such as TRPC1 which may form CRAC by connecting with ORAI and ROS sensor STIM1, may server as another important regulator in controlling EC permeability upon oxidant stress resulting from challenges such as sepsis. Thus, mechanisms that regulate these factors such as their expression or control of their interaction could be targeted to pharmacologically suppress endothelial permeability during ALI/ARDS result from oxidant stress.
Research Highlights.
Increased reactive oxygen species (ROS) generated in tissue during inflammation play an important role in the development of ALI and its progression to ARDS.
ROS initiated Ca2+ signaling in tissue plays an important role in increasing endothelial permeability.
Redox sensitive TRPM2 channel and TRPC6 and other TRPCs such as TRPC1 which form CRAC by connecting with ORAI and ROS sensor STIM1, are key players in mediating ROS-induced Ca2+ signaling in regulating endothelial permeability upon oxidant stress.
Acknowledgments
This work was supported by NIH grant P01HL77806. Tomohiro Kiya was supported by Saiseikai Otaru Hospital, Japan.
List of Abbreviations
- AJs
adherence junctions
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- Cav-1
caveolin-1
- CRAC
calcium-release-activated calcium channel
- CSD
Cav-1 scaffold domain
- DAG
diacylglycerol
- EC
endothelial cells
- ER
endoplasmic reticulum
- ERM
ezrin-radixin-moesin
- GDI
GDP dissociation inhibitor
- GDP
guanosine diphosphate
- HEK293T
- ICAM-1
Intercellular Adhesion Molecule 1
- IP3R3
inositol 1,4,5-trisphosphate receptor 3
- LPS
lipopolysaccharide
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- MyD88
myeloid differentiation primary response gene 88
- MΦ
macrophages
- NF-kB
nuclear factor kappa-B
- NSF
N-ethylmaleimide sensitive factor
- NOX
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
- ORAI
calcium release-activated calcium channel protein
- PECAM
platelet endothelial cell adhesion molecule-1
- PKC
protein kinase C
- PMN
neutrophils (polymorphonuclear leukocytes)
- ROC
receptor-operated calcium
- ROCK
Rho kinase
- ROS
reactive oxygen species
- SAM
sterile alpha motif
- SOC
store operated calcium
- S/P-region
serine-proline rich region
- STIM1
stromal interaction molecule 1
- TEM
transendothelial migration
- TJs
tight junctions
- TLR4
toll-like receptor 4
- TM
transmembrane domain
- TRP
transient receptor potential
- TRPM2-L
transient receptor potential (TRP) subfamily M2, long form
- TRPM2-S
transient receptor potential (TRP) subfamily M2, short form
- VE
vascular endothelial
- WPB
Weibel-Palade bodies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Lesur O, Berthiaume Y, Blaise G, Damas P, Deland E, Guimond JG, Michel RP. Acute respiratory distress syndrome: 30 years later. Can Respir J. 1999;6:71–86. doi: 10.1155/1999/812476. [DOI] [PubMed] [Google Scholar]
- 3.Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 4.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 5.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 6.Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- 7.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Johnson A, Phillips P, Hocking D, Tsan MF, Ferro T. Protein kinase inhibitor prevents pulmonary edema in response to H2O2. Am J Physiol. 1989;256:H1012–1022. doi: 10.1152/ajpheart.1989.256.4.H1012. [DOI] [PubMed] [Google Scholar]
- 10.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 11.Barnard ML, Matalon S. Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol. 1992;72:1724–1729. doi: 10.1152/jappl.1992.72.5.1724. [DOI] [PubMed] [Google Scholar]
- 12.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 13.Moskovitz J, Yim MB, Chock PB. Free radicals and disease. Arch Biochem Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 14.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 16.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 18.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 19.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 20.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 21.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 22.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 23.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 25.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 26.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 27.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 28.Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38beta mitogen-activated protein kinase. J Biol Chem. 2013;288:17030–17041. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 31.Siflinger-Birnboim A, Lum H, Del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol. 1996;270:L973–978. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn FJ, Heiner I, Luckhoff A. TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose. Pflugers Arch. 2005;451:212–219. doi: 10.1007/s00424-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 33.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 35.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto S, Takahashi N, Mori Y. Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels. Prog Biophys Mol Biol. 2010;103:18–27. doi: 10.1016/j.pbiomolbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–287. doi: 10.1016/j.ceca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium. 2011;50:407–423. doi: 10.1016/j.ceca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Simon F, Varela D, Cabello-Verrugio C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell Signal. 2013;25:1614–1624. doi: 10.1016/j.cellsig.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Knowles H, Li Y, Perraud AL. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res. 2013;55:241–248. doi: 10.1007/s12026-012-8373-8. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu S, Takahashi N, Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters) Handb Exp Pharmacol. 2014;223:767–794. doi: 10.1007/978-3-319-05161-1_3. [DOI] [PubMed] [Google Scholar]
- 42.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal. 2009;11:765–776. doi: 10.1089/ars.2008.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecquet CM, . Ahmmed GU, Malik AB. TRPM2 channel regulates endothelial barrier function. Adv Exp Med Biol. 2009;661:155–167. doi: 10.1007/978-1-60761-500-2_10. [DOI] [PubMed] [Google Scholar]
- 46.Hecquet CM, Zhang M, Mittal M, Vogel SM, Di A, Gao X, Bonini MG, Malik AB. Cooperative interaction of trp melastatin channel transient receptor potential (TRPM2) with its splice variant TRPM2 short variant is essential for endothelial cell apoptosis. Circ Res. 2014;114:469–479. doi: 10.1161/CIRCRESAHA.114.302414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hecquet CM, Malik AB. Role of H(2)O(2)-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost. 2009;101:619–625. [PMC free article] [PubMed] [Google Scholar]
- 48.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 49.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 51.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 52.Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–142. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- 53.Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 55.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1303–1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- 56.Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 57.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pocock TM, Foster RR, Bates DO. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1015–1026. doi: 10.1152/ajpheart.00826.2003. [DOI] [PubMed] [Google Scholar]
- 60.Kini V, Chavez A, Mehta D. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem. 2010;285:33082–33091. doi: 10.1074/jbc.M110.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, Brandes RP, Grimminger F, Meissner M, Freichel M, Offermanns S, Veit F, Pak O, Krause KH, Schermuly RT, Brewer AC, Schmidt HH, Seeger W, Shah AM, Gudermann T, Ghofrani HA, Dietrich A. Activation of TRPC6 channels is essential for lung ischaemiareperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem. 2010;285:23466–23476. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem. 2011;286:31799–31809. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 65.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 66.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–1899. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Putney JW. Pharmacology of store-operated calcium channels. Molecular interventions. 2010;10:209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels. 2009;3:221–225. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 70.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 71.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prins D, Groenendyk J, Touret N, Michalak M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011;12:1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 74.Dietrich A, Gudermann T. Another TRP to endothelial dysfunction: TRPM2 and endothelial permeability. Circ Res. 2008;102:275–277. doi: 10.1161/CIRCRESAHA.107.170548. [DOI] [PubMed] [Google Scholar]
- 75.Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 76.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 77.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 78.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 79.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 80.Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 81.Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, Barber DL, Stahl R, Carey DJ, Cheung JY, Miller BA. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol. 2006;290:C1146–1159. doi: 10.1152/ajpcell.00205.2005. [DOI] [PubMed] [Google Scholar]
- 83.Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
- 85.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci (Landmark Ed) 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase C-alpha. Differential roles of Ca2+ ligands and membrane binding residues. J Biol Chem. 1998;273:17544–17552. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- 87.Evans JH, Murray D, Leslie CC, Falke JJ. Specific translocation of protein kinase Calpha to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol Biol Cell. 2006;17:56–66. doi: 10.1091/mbc.E05-06-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest. 1990;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li PF, Maasch C, Haller H, Dietz R, von Harsdorf R. Requirement for protein kinase C in reactive oxygen species-induced apoptosis of vascular smooth muscle cells. Circulation. 1999;100:967–973. doi: 10.1161/01.cir.100.9.967. [DOI] [PubMed] [Google Scholar]
- 90.Siflinger-Birnboim A, Goligorsky MS, Del Vecchio PJ, Malik AB. Activation of protein kinase C pathway contributes to hydrogen peroxide-induced increase in endothelial permeability. Lab Invest. 1992;67:24–30. [PubMed] [Google Scholar]
- 91.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285:L434–442. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- 92.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 93.Li D, Shatos MA, Hodges RR, Dartt DA. Role of PKCalpha activation of Src, PI-3K/AKT, and ERK in EGF-stimulated proliferation of rat and human conjunctival goblet cells. Investigative ophthalmology & visual science. 2013;54:5661–5674. doi: 10.1167/iovs.13-12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 95.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 96.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- 97.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 98.Barandier C, Ming XF, Rusconi S, Yang Z. PKC is required for activation of ROCK by RhoA in human endothelial cells. Biochem Biophys Res Commun. 2003;304:714–719. doi: 10.1016/s0006-291x(03)00668-5. [DOI] [PubMed] [Google Scholar]
- 99.Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79:477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 100.Zheng HZ, Zhao KS, Zhou BY, Huang QB. Role of Rho kinase and actin filament in the increased vascular permeability of skin venules in rats after scalding. Burns. 2003;29:820–827. doi: 10.1016/j.burns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q, Doerschuk CM. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J Immunol. 2000;164:6487–6494. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 103.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kB transcription factor and HIV-1. EMBO Journal. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 105.Xue J, Thippegowda PB, Hu G, Bachmaier K, Christman JW, Malik AB, Tiruppathi C. NF-kappaB regulates thrombin-induced ICAM-1 gene expression in cooperation with NFAT by binding to the intronic NF-kappaB site in the ICAM-1 gene. Physiol Genomics. 2009;38:42–53. doi: 10.1152/physiolgenomics.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 107.Fink K, Duval A, Martel A, Soucy-Faulkner A, Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J Immunol. 2008;180:6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 108.Weibel ER, Palade GE. New Cytoplasmic Components in Arterial Endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–110. [PubMed] [Google Scholar]
- 110.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 112.Birch KA, Ewenstein BM, Golan DE, Pober JS. Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. J Cell Physiol. 1994;160:545–554. doi: 10.1002/jcp.1041600318. [DOI] [PubMed] [Google Scholar]
- 113.Fu J, Naren AP, Gao X, Ahmmed GU, Malik AB. Protease-activated receptor-1 activation of endothelial cells induces protein kinase Calpha-dependent phosphorylation of syntaxin 4 and Munc18c: role in signaling p-selectin expression. J Biol Chem. 2005;280:3178–3184. doi: 10.1074/jbc.M410044200. [DOI] [PubMed] [Google Scholar]
- 114.Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res. 2003;92:272–278. doi: 10.1161/01.res.0000057386.15390.a3. [DOI] [PubMed] [Google Scholar]
- 115.Zupancic G, Ogden D, Magnus CJ, Wheeler-Jones C, Carter TD. Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. J Physiol. 2002;544:741–755. doi: 10.1113/jphysiol.2002.027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erent M, Meli A, Moisoi N, Babich V, Hannah MJ, Skehel P, Knipe L, Zupancic G, Ogden D, Carter T. Rate, extent and concentration dependence of histamine-evoked Weibel-Palade body exocytosis determined from individual fusion events in human endothelial cells. J Physiol. 2007;583:195–212. doi: 10.1113/jphysiol.2007.132993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 119.Kakkar AK, Lefer DJ. Leukocyte and endothelial adhesion molecule studies in knockout mice. Curr Opin Pharmacol. 2004;4:154–158. doi: 10.1016/j.coph.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Takano M, Meneshian A, Sheikh E, Yamakawa Y, Wilkins KB, Hopkins EA, Bulkley GB. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am J Physiol Heart Circ Physiol. 2002;283:H2054–2061. doi: 10.1152/ajpheart.01001.2001. [DOI] [PubMed] [Google Scholar]
- 121.Matsushita K, Morrell CN, Mason RJ, Yamakuchi M, Khanday FA, Irani K, Lowenstein CJ. Hydrogen peroxide regulation of endothelial exocytosis by inhibition of N-ethylmaleimide sensitive factor. J Cell Biol. 2005;170:73–79. doi: 10.1083/jcb.200502031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem Int. 2008;52:14–25. doi: 10.1016/j.neuint.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 123.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal. 2002;4:39–47. doi: 10.1089/152308602753625843. [DOI] [PubMed] [Google Scholar]
- 124.Hordijk P. Endothelial signaling in leukocyte transmigration. Cell Biochem Biophys. 2003;38:305–322. doi: 10.1385/cbb:38:3:305. [DOI] [PubMed] [Google Scholar]
- 125.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 127.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–4298. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O'Donnell RW, Johnson DK, Ziegler LM, DiMattina AJ, Stone RI, Holland JA. Endothelial NADPH oxidase: mechanism of activation by low-density lipoprotein. Endothelium. 2003;10:291–297. doi: 10.1080/10623320390272280. [DOI] [PubMed] [Google Scholar]
- 130.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez EM, Dunham EE, Martin GS. Atypical protein kinase C activity is required for extracellular matrix degradation and invasion by Src-transformed cells. J Cell Physiol. 2009;221:171–182. doi: 10.1002/jcp.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zang Q, Frankel P, Foster DA. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 1995;6:1367–1373. [PubMed] [Google Scholar]
- 133.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin CH, Cheng HW, Hsu MJ, Chen MC, Lin CC, Chen BC. c-Src mediates thrombin-induced NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells. J Immunol. 2006;177:3427–3438. doi: 10.4049/jimmunol.177.5.3427. [DOI] [PubMed] [Google Scholar]
- 135.Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- 136.Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, Gorgoulis VG, Malliri A. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell. 2009;33:639–653. doi: 10.1016/j.molcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 137.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]