Abstract
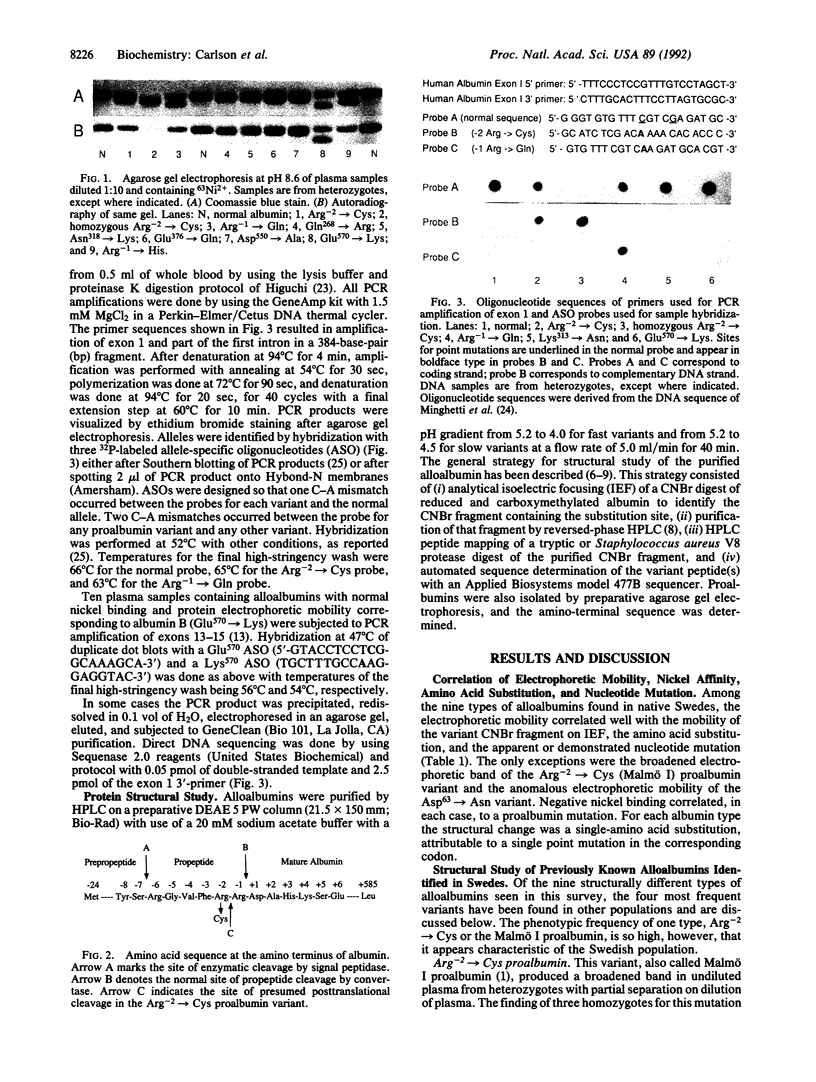
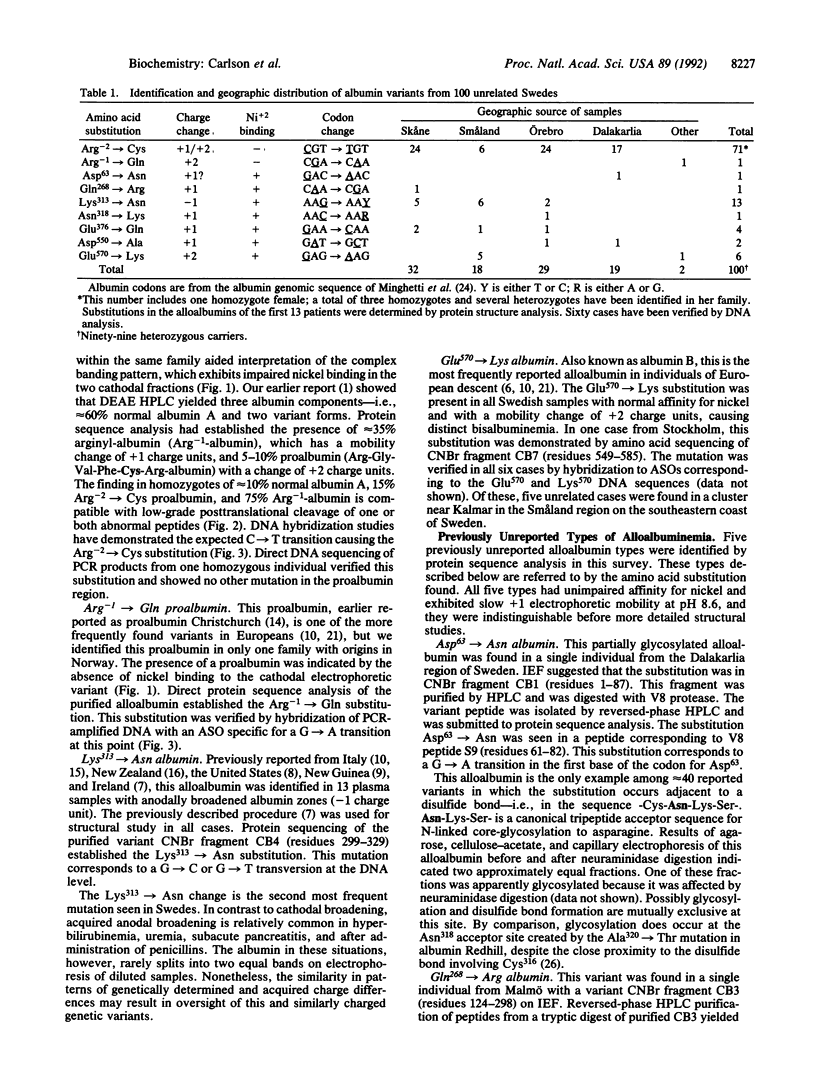
Plasma samples exhibiting alloalbuminemia on electrophoresis at pH 8.6 were requested from clinical laboratories throughout Sweden. Nine variants, each representing a different single point mutation, were found in 100 apparently unrelated Swedes. The overall prevalence of alloalbuminemia was estimated at 1:1700. Mutations were identified by protein-structural analysis followed by allele-specific DNA hybridization to verify the most common types. Slightly retarded (+1) mobility was seen in 80 cases. Of these, 71 had the Arg(-2)----Cys proalbumin variant previously called Malmö I proalbumin. Thirteen examples of the second most frequent type, the substitution Lys313----Asn and a mobility change of -1 charge unit, were found, as well as six cases of Glu570----Lys (albumin B) and a single case of Arg-1----Gln (proalbumin Christchurch). Five previously unreported types of alloalbuminemia were identified: four instances of Glu376----Gln, which is the second known mutation at this site; two examples of Asp550----Ala, the second mutation reported at this site; and one example each of Asp63----Asn, Gln268----Arg, and Asn318----Lys. Other mutations were identified among eight subjects of foreign descent. The high frequency and relatively uniform geographic distribution of the Arg-2----Cys mutation suggest that it may have occurred in a founder individual many generation ago in Sweden.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Huss K., Madison J., Putnam F. W., Salzano F. M., Franco M. H., Santos S. E., Freitas M. J. Amino acid substitutions in albumin variants found in Brazil. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1821–1825. doi: 10.1073/pnas.86.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Ishioka N., Huss K., Madison J., Putnam F. W. Identical structural changes in inherited albumin variants from different populations. Proc Natl Acad Sci U S A. 1989 Jan;86(2):434–438. doi: 10.1073/pnas.86.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Madison J., Huss K., Ishioka N., Satoh C., Fujita M., Neel J. V., Sakurabayashi I., Putnam F. W. Point substitutions in Japanese alloalbumins. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6092–6096. doi: 10.1073/pnas.86.16.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Madison J., Shimizu A., Putnam F. W. Point substitutions in albumin genetic variants from Asia. Proc Natl Acad Sci U S A. 1990 Jan;87(1):497–501. doi: 10.1073/pnas.87.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Arai K., Madison J., Laurell C. B., Galliano M., Watkins S., Peach R., Myles T., George P., Putnam F. W. Hypermutability of CpG dinucleotides in the propeptide-encoding sequence of the human albumin gene. Proc Natl Acad Sci U S A. 1990 May;87(10):3909–3913. doi: 10.1073/pnas.87.10.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O., Carrell R. W. Functional abnormality of proalbumin Christchurch. Biochim Biophys Acta. 1980 Jan 24;621(1):83–88. doi: 10.1016/0005-2795(80)90064-1. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., Herbert P. Albumin Canterbury (313 Lys----Asn). A point mutation in the second domain of serum albumin. Biochim Biophys Acta. 1987 Apr 8;912(2):191–197. doi: 10.1016/0167-4838(87)90088-4. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., Myles T., Peach R. J., Donaldson D., George P. M. Albumin Redhill (-1 Arg, 320 Ala----Thr): a glycoprotein variant of human serum albumin whose precursor has an aberrant signal peptidase cleavage site. Proc Natl Acad Sci U S A. 1990 Jan;87(1):26–30. doi: 10.1073/pnas.87.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. O. The molecular abnormality of albumin Parklands: 365 Asp----His. Biochim Biophys Acta. 1985 Aug 23;830(3):320–324. doi: 10.1016/0167-4838(85)90289-4. [DOI] [PubMed] [Google Scholar]
- Fine J. M., Marneux M., Rochu D. Human albumin genetic variants: an attempt at a classification of European allotypes. Am J Hum Genet. 1987 Mar;40(3):278–286. [PMC free article] [PubMed] [Google Scholar]
- Galliano M., Minchiotti L., Iadarola P., Stoppini M., Ferri G., Castellani A. A. The molecular defect of albumin Tagliacozzo: 313 Lys----Asn. FEBS Lett. 1986 Nov 24;208(2):364–368. doi: 10.1016/0014-5793(86)81050-x. [DOI] [PubMed] [Google Scholar]
- Galliano M., Minchiotti L., Porta F., Rossi A., Ferri G., Madison J., Watkins S., Putnam F. W. Mutations in genetic variants of human serum albumin found in Italy. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8721–8725. doi: 10.1073/pnas.87.22.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss K., Putnam F. W., Takahashi N., Takahashi Y., Weaver G. A., Peters T., Jr Albumin Cooperstown: a serum albumin variant with the same (313 Lys----Asn) mutation found in albumins in Italy and New Zealand. Clin Chem. 1988 Jan;34(1):183–187. [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Franzén B. Agarose gel electrophoresis. Clin Chem. 1979 Apr;25(4):629–638. [PubMed] [Google Scholar]
- Madison J., Arai K., Sakamoto Y., Feld R. D., Kyle R. A., Watkins S., Davis E., Matsuda Y., Amaki I., Putnam F. W. Genetic variants of serum albumin in Americans and Japanese. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9853–9857. doi: 10.1073/pnas.88.21.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti P. P., Ruffner D. E., Kuang W. J., Dennison O. E., Hawkins J. W., Beattie W. G., Dugaiczyk A. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11-22 of chromosome 4. J Biol Chem. 1986 May 25;261(15):6747–6757. [PubMed] [Google Scholar]
- Neel J. V. Rare variants, private polymorphisms, and locus heterozygosity in Amerindian populations. Am J Hum Genet. 1978 Sep;30(5):465–490. [PMC free article] [PubMed] [Google Scholar]
- Petersen K. B., Kølvraa S., Bolund L., Petersen G. B., Koch J., Gregersen N. Detection of alpha 1-antitrypsin genotypes by analysis of amplified DNA sequences. Nucleic Acids Res. 1988 Jan 11;16(1):352–352. doi: 10.1093/nar/16.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Davis E., Madison J., Watkins S., McLaughlin H., Leahy D. T., Putnam F. W. Purification and structural study of two albumin variants in an Irish population. Clin Chim Acta. 1991 Dec 31;204(1-3):179–187. doi: 10.1016/0009-8981(91)90229-6. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Blumberg B. S., Putnam F. W. Amino acid substitutions in genetic variants of human serum albumin and in sequences inferred from molecular cloning. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4413–4417. doi: 10.1073/pnas.84.13.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Isobe T., Putnam F. W., Fujita M., Satoh C., Neel J. V. Amino acid substitutions in inherited albumin variants from Amerindian and Japanese populations. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8001–8005. doi: 10.1073/pnas.84.22.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Structural changes and metal binding by proalbumins and other amino-terminal genetic variants of human serum albumin. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7403–7407. doi: 10.1073/pnas.84.21.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tárnoky A. L. Genetic and drug-induced variation in serum albumin. Adv Clin Chem. 1980;21:101–146. doi: 10.1016/s0065-2423(08)60087-6. [DOI] [PubMed] [Google Scholar]
- Watkins S., Madison J., Davis E., Sakamoto Y., Galliano M., Minchiotti L., Putnam F. W. A donor splice mutation and a single-base deletion produce two carboxyl-terminal variants of human serum albumin. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5959–5963. doi: 10.1073/pnas.88.14.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]





